Abstract
Pheochromocytomas (Pheos) and paragangliomas (PGLs) are neuroendocrine tumors. Approximately 30–40% of Pheos/PGLs are due to germline mutations in one of the susceptibility genes, including those encoding the succinate dehydrogenase subunits A-D (SDHA-D). Up to 2/3 of patients affected by SDHB mutated Pheo/PGL develop metastatic disease with no successful cure at present. Here, for the first time, we evaluated the effects of SDHB silencing in a three dimension (3D) culture using spheroids of a mouse Pheo cell line silenced or not (wild type/wt/control) for the SDHB subunit. We investigated the role of the microenvironment on spheroid growth and migration/invasion by co-culturing SDHB-silenced or wt spheroids with primary cancer-activated fibroblasts (CAFs). When spheroids were co-cultured with fibroblasts, SDHB-silenced cells showed a significant increase in matrigel invasion as demonstrated by the computation of the migratory areas (P < 0.001). Moreover, cells detaching from the SDHB-silenced spheroids moved collectively, unlike the cells of wt spheroids that moved individually. Additionally, SDHB-silenced spheroids developed long filamentous formations along which clusters of cells migrated far away from the spheroid, whereas these structures were not present in wt spheroids. We found that lactate, largely secreted by CAFs, plays a specific role in promoting migration only of SDHB-silenced cells. In this study, we demonstrated that SDHB silencing per se increases tumor cell migration/invasion and that microenvironment, as represented by CAFs, plays a pivotal role in enhancing collective migration/invasion in Pheo SDHB-silenced tumor cells, suggesting their role in increasing the tumor metastasizing potential.
Keywords: SDHB, tumor migration, spheroids, tumor microenvironment, pheochromocytoma/paraganglioma
Introduction
Germline mutations in nuclear genes encoding succinate dehydrogenase (SDH), or mitochondrial complex II, are related with the occurrence of pheochromocytoma/paraganglioma (Pheo/PGL).
SDH is a tetrameric protein composed by two catalytic subunits (SDHA and SDHB), and two structural subunits (SDHC and SDHD) that anchor the complex to the inner mitochondrial membrane. Despite Pheo/PGL are mostly benign, mutations in the B subunit are highly associated with malignancy.
Although several hypotheses have been proposed, the precise mechanisms by which the impaired SDH activity leads to tumorigenesis and SDHB mutations to malignancy remain unknown (Baysal & Maher 2015). This scenario is even more complicated by the demonstration that tumor microenvironment plays a pivotal role in modulating cell metabolism, tumor growth and progression (Rapizzi et al. 2015).
Solid tumors are very complex tissues, comprising of not only cancer cells but also non-malignant stromal cells such as endothelial cells, fibroblasts, immune cells, and extracellular matrix. Together these cells form so-called tumor microenvironment. Over the last few years, it has become more and more evident that the continual interplay between cancer and stromal cells generates a positive loop that leads cancer cells to survive the hostile environment, grow and spread metastases to healthy tissues (Hu & Polyak 2008, Hanahan & Weinberg 2011, Fiaschi et al. 2012, Hanahan & Coussens 2012, Karagiannis et al. 2012, Zhang & Liu 2012, Santi et al. 2013, Taddei et al. 2013, Quail & Joyce 2013). Thus, the tumor microenvironment has become a potential target for the therapy of certain tumors (for a recent reviews, see Sounni & Noel 2013, Klemm & Joyce 2014).
Single cell and collective migration are hallmarks of cancer invasion and collective migration differs from single cell migration in that the cells remain connected as they move (Frield & Gilmour 2009, Wang et al. 2016). Particularly, tumor microenvironment plays a central role in the induction of collective migration by different mechanisms including soluble factors secreted by stromal and tumor cells (Frield & Gilmour 2009).
We recently demonstrated that the microenvironment, represented by fibroblasts, induces metabolic changes and increases proliferation in SDHB-silenced neuroblastoma cells, suggesting that fibroblast-derived factors play an important role in neuroblastoma progression (Rapizzi et al. 2015).
Cancer cell lines grown as two-dimensional (2D) cultures are a widely used model for studying cancer biology and testing new anti-cancer drugs. However, 2D cultures have major limitations, such as artificial cell-to-cell interactions and conditions of cell growth, so that they do not closely mimic the heterogeneity and tissue context of in vivo tumors. Developing three-dimensional (3D) cell cultures, such as multicellular tumor spheroids, has the potential to overcome some of these limitations (Fennema et al. 2013).
In this study, we investigated the changes induced by tumor microenvironment, here represented by primary mouse fibroblasts, on growth and invasiveness of spheroids generated by wt and SDHB-silenced mouse Pheo cells (MPCs).
Materials and methods
Cell culture and clone selection for SDHB silencing
Fibroblasts were obtained from legs of newborn mice by enzymatic digestion with trypsin. The digestion was plated for 1h at 37°C then adherent cells, mostly fibroblasts, were washed twice in PBS and let grow in DMEM + 10% FCS. Co-cultures were performed in multiplates with inserts leading to a separation of tumor cells and fibroblasts by a permeable membrane (3μm pores, Greiner Bio-One International, Germany).
SDHB was stably knocked down by viral transduction with MISSION lentiviral particles (Sigma-Aldrich) containing short hairpin RNA (shRNA) against murine SDHB or a non-targeting shRNA construct as control. Cultures were treated with puromycin to select for vector integration. Mouse Pheo cell line mouse tumor tissue (MTT) derived was grown in DMEM supplemented with 10% FCS, 5% horse serum, 2 mM l-glutamine and 100 U/mL penicillin. Mouse primary fibroblasts were grown in DMEM supplemented with 10% FCS, 2mM L-glutamine and 100U/mL penicillin. All cells were cultured at 37°C in a 5% CO2 humidified atmosphere.
Spheroid generation
Spheroids of a consistent cell number and size were generated in non-adhesive round bottomed 96-well plates (Nunclon Sphera, Thermo Fisher). Tumor cells were grown as adherent monolayer cultures until confluency and dissociated into single cells. To generate cancer spheroids, 5 × 103 tumor cells were added into each well of the round bottomed 96-well plates. The round bottomed plates were then centrifuged at 220g at room temperature for 10 min to initiate cell-cell interaction and incubated at 37°C, 5% CO2. After 48 h, spheroids were easily transferred using a regular pipette without dissociating. This procedure generated spheroids with homogeneous size and geometry (diameter ≥350μm). Inverted microscopic analyses were routinely performed before each experiment to verify spheroid diameter. Only those with a diameter ≥350μm were used.
Conditioned medium
To obtain conditioned medium, we performed 2D cultures of mixed fibroblasts and tumor cells. Mouse primary fibroblasts and tumor cells were seeded in co-culture in a 2:1 ratio, and allowed to adhere overnight. In doing so, fibroblasts were activated by tumor cells, and thus named in this study as activated fibroblasts. Then, the culture medium was replaced with fresh DMEM containing 10% FCS. Following incubation for 72h, the conditioned medium was collected, centrifuged for 5 min at 1200rpm and used for further experiments. Therefore, ‘medium conditioned by activated fibroblasts’ means the medium is conditioned not only by the presence in culture of fibroblasts and tumor cells but also by the presence of tumor cells. The conditioned medium was used for growth studies.
Lactate concentration measurement
Lactate was measured using the Lactate Colorimetric/Fluorometric Assay Kit (Biovision, Milpitas, USA) according to the manufacturer’s protocol. Briefly, mouse primary fibroblasts were seeded into 12-well plates in single culture or in co-culture with spheroids. They were left to grow for 72 h in DMEM serum-free. Then, the inserts of the co-cultures were separated from fibroblasts, and the medium was replaced with fresh one. Fibroblasts were left with the fresh medium for 24h. Samples were then diluted 1:5 with buffer provided in the kit to 50 μL and then mixed with 50 μL of mix solution in a 96-well plate. The plate was then incubated at 37°C for 30 min. The absorbance was measured at a wavelength of 570nm with a microplate reader and normalized on number of cells.
3D migration assays
To determine the effects of mouse primary fibroblasts on spheroid cell migration, spheroids were co-cultured with mouse primary fibroblasts using culture inserts (Greiner Bio-one) (see Fig. 1). Fibroblasts were seeded in 12-well plates (15 × 104 cells/well) and the next day they were activated with conditioned media (see above) for 24h. At the same time, the matrigel solution was prepared according to the manufacturer’s instructions. Briefly Corning Matrigel Basement Membrane Matrix (BD Biosciences, concentration 9.7mg/mL) was mixed in a ratio of 1:1 with DMEM to obtain the final solution of 0.3% matrigel. The solution was added to the growth surface of culture inserts for multiwell plates (transparent membrane with 3μm pores), and let it to be hydrated overnight at 37°C.
Figure 1.
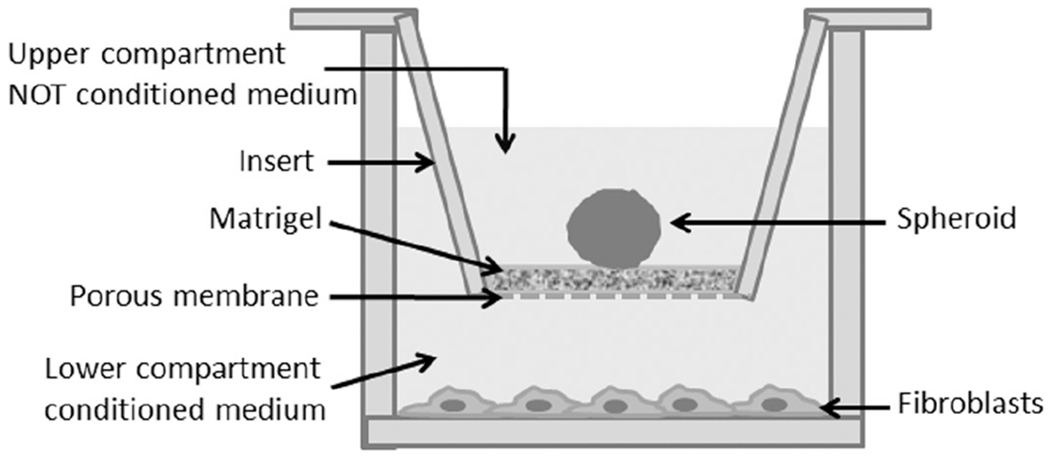
Schematic representation of 3D migration assay. The spheroid were laid on 0.3% matrigel in the upper compartment of the transwell insert. For co-culture experiments activated fibroblasts were plated in the lower compartment and grown in conditioned medium, while for single culture experiments the lower compartment was without fibroblasts and filled with not conditioned medium.
Then, spheroids were selected and individually laid on the matrigel in the transwell insert and placed in the multiwell plates with fibroblasts. As controls, we used spheroids in single culture (without fibroblasts in the bottom well plate). Bright field images of the spheroids, stained or not with crystal violet, were acquired at different times by an AxioCam MRc digital camera for an inverted microscope Axiovert25 (Zeiss). Using ImageJ software, areas were calculated by drawing circles around the spheroids at different times. Cell migration areas were calculated as the difference between areas at day 0 and days 5 and 8 (Stein et al. 2007, Guiet et al. 2011).
To determine the effects of lactate on spheroid cell migration, spheroids were cultured in culture inserts laid on the matrigel in DMEM without serum, in the lower chamber DMEM without serum plus 10mM lactate was added. After 5 days of culture, spheroids were fixed, stained with crystal violet and the migration areas were measured as mentioned above.
Electron microscopy
Monolayer culture cells and spheroids of 15 days were washed with PBS and were directly fixed in cold 2.5% glutaraldehyde and 2% formaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) overnight at 4°C and postfixed in cold 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4) for 1 h at room temperature. The samples were dehydrated in graded acetone, passed through propylene oxide and embedded in epoxy resin. Ultrathin sections were stained with gadolinium acetate and alkaline bismuth subnitrate and examined under a JEM 1010 electron microscope (Jeol, Tokyo, Japan) at 80 kV. Photomicrographs were taken with a MegaView III (Soft Imaging System, Muenster, Germany) digital camera connected with a personal computer with dedicated software (AnalySIS, Soft Imaging Software, Muenster, Germany).
Immunofluorescent staining
The matrigel solution was added to the growth surface of chambers slides (Nunclon Sphera, Thermo Fisher) and let to be hydrated for 1h at 37°C; then a single spheroid was laid on the matrigel. After 3h, DMEM + 10% FCS (used as control) or DMEM + 10% FCS conditioned by fibroblasts were added. After 5 days the samples were fixed with 4% paraformaldehyde for 10 min, followed by permeabilization in PBS with 0.1% Triton X-100 for 45 min at room temperature. The spheroids were then incubated for 1h at 37°C with phalloidin fluorescein isothiocyanate labeled (Sigma-Aldrich) to visualize actin filaments, and with TO-PRO-3 iodide (Life Technologies) for nuclei staining.
Confocal images were acquired with a Leica SP2-AOBS, as follows. HC PL fluotar 20× 0.5NA objective, voxel size x = 0.732 μm, y = 0.732 μm, z = 0.814μm; HCX PL APO 63× 1.4NAobjective, voxel size x = 0.232μm, y = 0.232μm, z = 0.244μm. Images were prepared for publication using the Fiji software (Schindelin et al. 2012) and are shown as maximum intensity projection along the z-axis.
Statistical analysis
Data analysis was performed by the computer program GraphPad Prism Version 5.0 for Windows (GraphPad Software). The statistical significance of value differences was evaluated by one-way ANOVA followed by Bonferroni’s multiple comparison test using GraphPad Prism Version 5.0 for Windows; Studen’s t-test was used for comparing the two classes of data. A P value of less than 0.05 was considered significant.
Results
In the present study we used MTT cells that represent a more aggressive derivative of MPC (Martiniova et al. 2009). We previously established a stable SDHB-silenced MTT cell line in which ratio of succinate:fumarate and catecholamine content were significantly increased (not shown).
Spontaneous 3D structures in SDHB silencing cells, spheroid formation and growth
We observed that in 2D culture, MTT cells aggregate and tend to grow in clusters. After about 10 days, aggregates were visible and clear in both wt and SDHB-silenced cultures. Interestingly, only SDHB-silenced aggregates, but not wt ones, were surrounded by clusters of cells as indicated by arrows (Fig. 2A).
Figure 2.
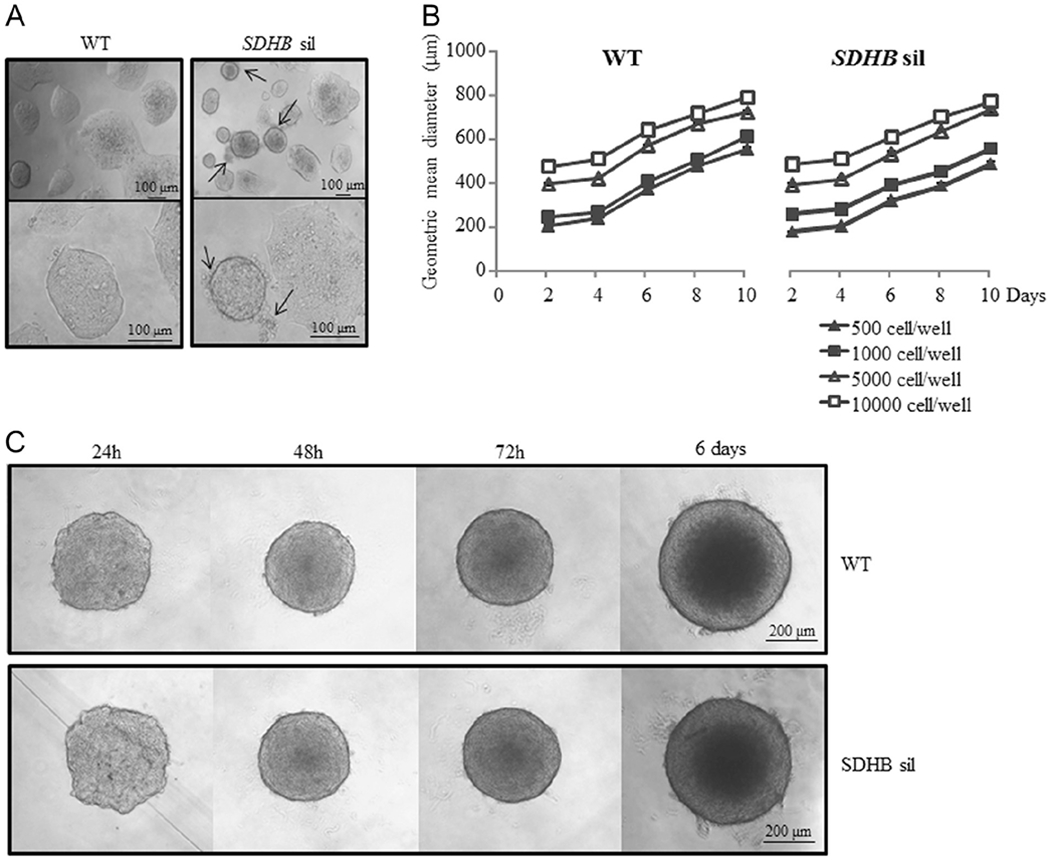
Effects of SDHB silencing on cell and spheroid growth. (A) SDHB-silenced cells in monolayer culture form aggregates surrounded by clusters of cells (indicated by arrows), not visible in control wt aggregates. (B) The spheroid diameter is used to measure spheroid growth. Spheroid growth, is cell number and time dependent, with no differences between SDHB-silenced and wt spheroids. (C) The images are representative of SDHB-silenced and wt control spheroid formation followed in time, starting from 5 × 103 cells/well. After 48 h, both SDHB-silenced and wt control spheroids assume a compact and rigid discoid shape with clear edges. Images are representative of more than three independent experiments. Graphs are the means of three independent experiments, each performed in duplicates±s.e.m.
As these cells were inclined to grow in clusters, and 3D structures resemble small tumor masses, we decided to work with spheroids. At first, we induced spheroid formation starting from different initial cell concentrations (ranging from 500 cells/well to 10,000 cells/well). As shown in Fig. 2C, after 48 h, spheroids assume a compact and rigid discoid shape with clear edges, and their growth was almost linear in time (Fig. 2B), with no significant differences between wt and SDHB-silenced spheroids.
SDHB silencing causes mitochondria swelling and junctional laxity
It has been demonstrated that PGL cells from SDHD mutation carriers present an increased number of swollen mitochondria (Douwes Dekker et al. 2003). Using electron microscopy, we observed that in SDHB-silenced spheroids mitochondria were swollen, poorly organized and with reduced cristae. Moreover, SDHB-silenced spheroids showed higher laxity at their peripheral sites when compared to wt spheroids, indicating a laxity in cell junctions (Fig. 3).
Figure 3.
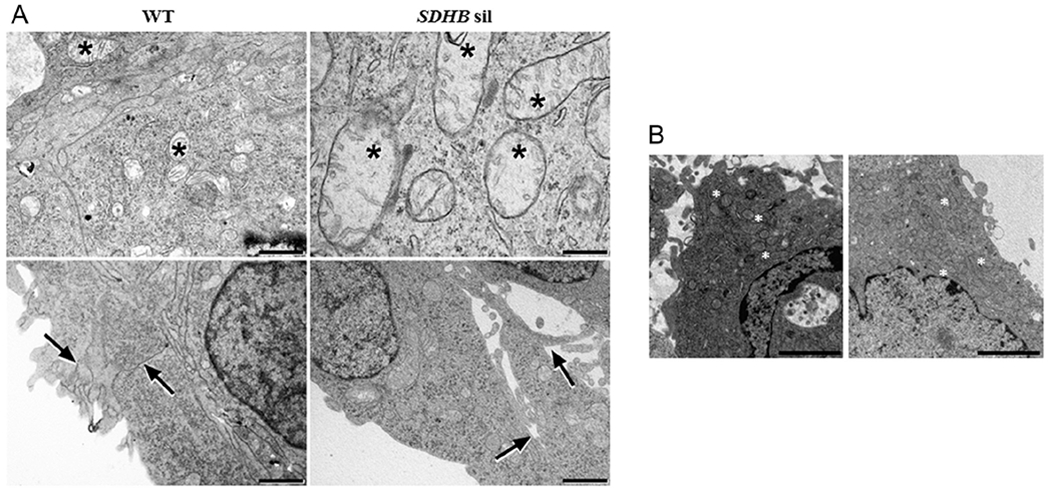
Effects of SDHB silencing on mitochondria morphology and cellular junctions. Ultrathin sections of SDHB-silenced and control spheroids or cells were examined under a JEM 1010 electron microscope. Micrographs were taken using a MegaView III digital camera (SIS-Soft Imaging System, Munster, Germany). (A) In SDHB-silenced spheroids mitochondria (asterisks) are swollen with impaired internal cristae, and the cell junctions at the spheroid edge (arrows) are lax. Images are representative of at least 20 microscopic fields for single spheroid. Electron microscopy; scale bar = 400 nm for the upper right panel, 1 μm for the other three panels. (B) When cells are cultured in monolayer, no significant differences in mitochondria (asterisks) morphology could be appreciated between SDHB-silenced cells and wt cells. Images are representative of at least 20 microscopic fields for single spheroid. Electron microscopy; scale bar = 2 μm.
Role of tumor microenvironment
Effects of tumor microenvironment on spheroid growth
We studied the effects of microenvironment on spheroid growth. Spheroids were cultivated in round bottom, low adherence plates and treated with different media. We observed a rapid and very similar spheroid growth both in wt and in SDHB-silenced not conditioned spheroids (Fig. 4A). Surprisingly, we obtained a considerable different result when we treated the spheroids with medium conditioned by activated fibroblasts. In this culturing condition, both in wt and in SDHB-silenced spheroids, the spheroid diameters, considered as a growing parameter, were significantly lower than those of spheroids not conditioned by the microenvironment (Fig. 4A). In contrast, in this culturing condition we found lot of cells surrounding spheroids (Fig. 4B). These cells were viable, as demonstrated by their ability to attach and grow in normal flat culturing plates (not shown). These results suggest that microenvironment induces massive cell migration.
Figure 4.
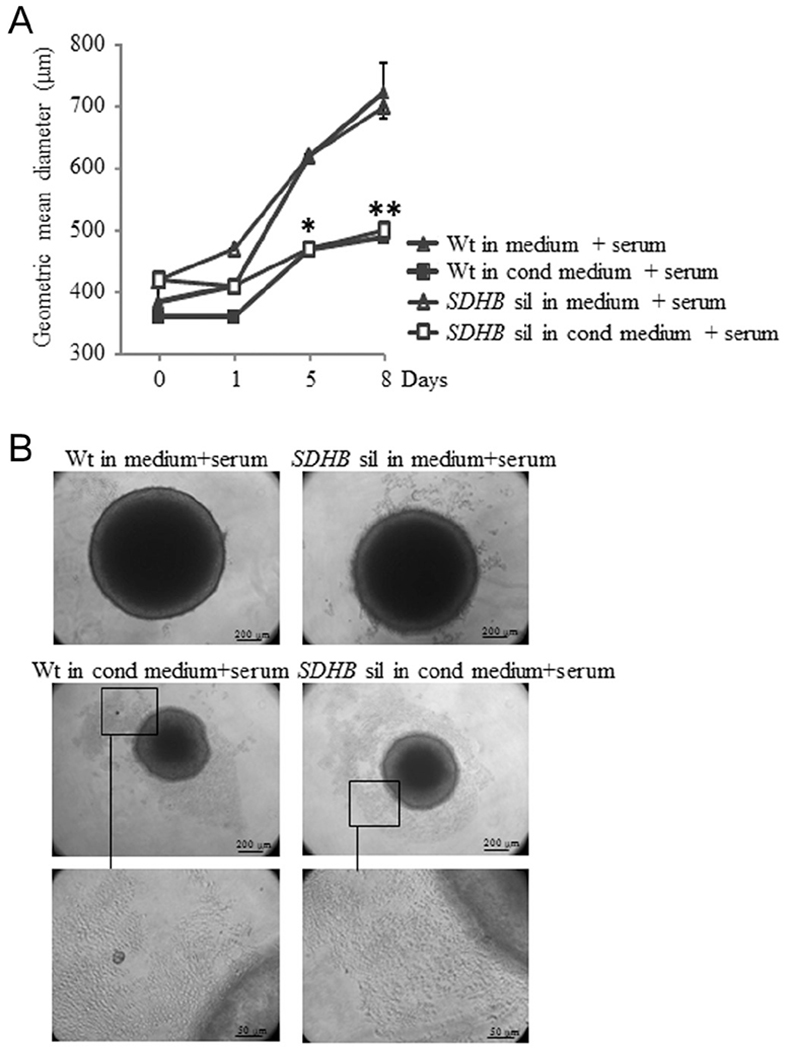
Effects of the microenvironment on tumor spheroid growth. (A) Spheroids were cultivated in round bottom, low adherence plates and treated with conditioned or not conditioned medium. Spheroid growth, treated with not conditioned medium, is almost linear and very similar in both wt control and SDHB-silenced spheroids. On the contrary, spheroid growth, treated with conditioned medium, is significantly lower than those of not conditioned spheroids. Also in this case with no differences between SDHB-silenced and control ones. (B) In these representative images is shown that the number of spheroid surrounding cells are considerably higher if conditioned medium is used, compared with not conditioned medium. The microscopic fields in the black highlighted boxes are shown at higher magnification. Images are representative of more than three independent experiments. Graphs are the means of three independent experiments, each performed in duplicates ± s.e.m., *P<0.05, **P<0.01.
Tumor microenvironment increases spheroid migration/invasion
The next step was to investigate if tumor microenvironment is able to modulate spheroid migration/invasion of extracellular matrix, here represented by matrigel. Interestingly, we observed a higher trend in migratory pattern in single cultured SDHB-silenced spheroids compared with single cultured wt spheroids, but migrating cells remained attached to the spheroids, forming a crown around them. In this culturing condition, a significant increase between SDHB-silenced spheroids and wt ones was found only after 8 days of culture (Fig. 5B). When the spheroids were co-cultured activated fibroblast conditioned medium, using the transwell inserts, we observed an evident detachment of clusters of viable cells in the surrounding space in both wt and SDHB-silenced spheroids compared to their single culture counterparts (Fig. 5A), but SDHB-silenced cells showed a significant greater migratory capability than wt cells, as demonstrated by the computation of the migratory areas (Fig. 5B). Using the confocal microscopy, and acquiring the images at higher magnification, it was possible to notice that SDHB-silenced cells invaded the surrounding space moving collectively, unlike the wt spheroids, where cells tended to move individually. In particular, SDHB-silenced spheroids develop long filamentous formations along which cells migrate far away from the spheroid in clusters, whereas wt spheroids develop less filaments, but most importantly, cells move along them in a single cell manner (Fig. 5C).
Figure 5.
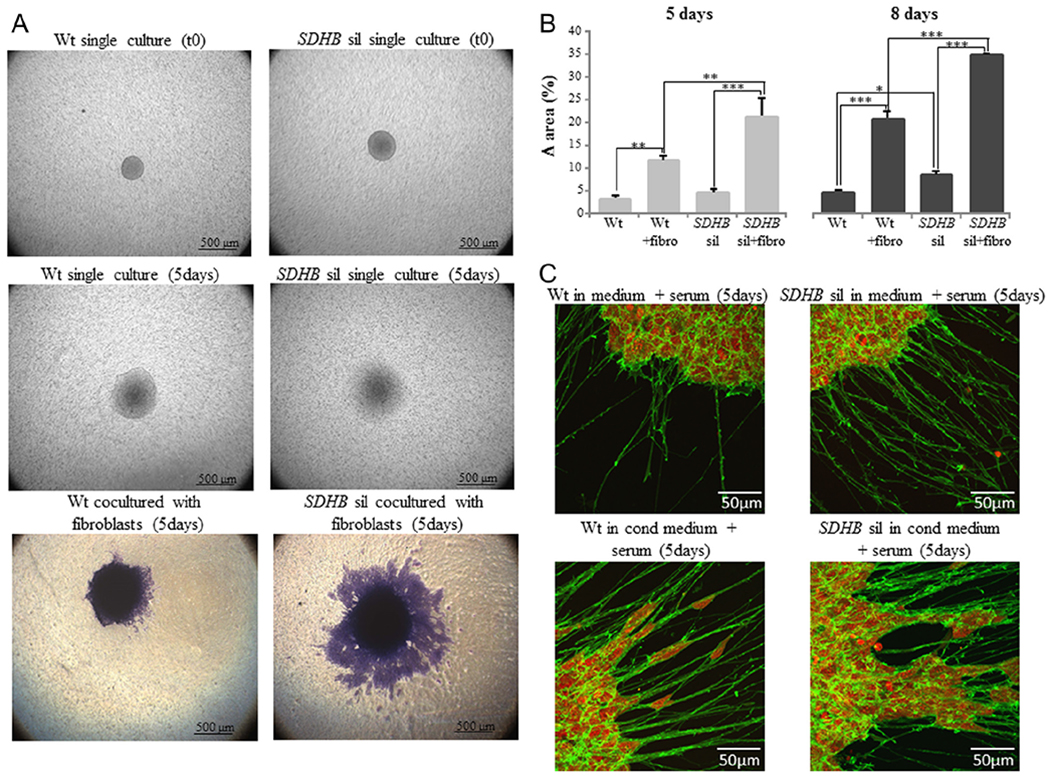
Effects of the microenvironment on tumor spheroid migration. (A) The spheroids were laid on 0.3% matrigel in the upper compartment of transwell insert and the migration capability was observed after 5 and 8 days. At time 0, both control and SDHB-silenced spheroids are very similar in size and show clear edges, while after 5days of culture migration/invasion process is evident. In single cultured spheroids migrating cells remain around the spheroids, forming a crown. If spheroids are influenced by the presence of activated fibroblasts plated in the lower compartment, an evident detachment of clusters of viable cells in the surrounding space is observed. (B) Spheroid migration areas were calculated as the difference between areas at day 0 and day 5 or day 8. By the computation of the migratory areas, it is shown that the migration process is significantly increased if spheroids are co-cultured with fibroblasts in both SDHB-silenced and control spheroids. Nevertheless, the effect of the microenvironment is significantly more evident in SDHB-silenced spheroids compared with wt control. (C) Spheroids were labeled with phalloidin fluorescein isothiocynate to visualize actin filaments (in green), and with TO-PRO-3 iodide for nuclei staining (in red). Confocal images were acquired with a Leica SP2-AOBS, processed using Fiji software, and shown as maximum intensity projection along the z-axis. It is possible to notice that SDHB-silenced spheroids develop long filamentous formations along which clusters of cells migrate far away from the spheroid, whereas wt spheroids develop less filaments, along which, cells move in a single cell manner. Images are representative of more than three independent experiments. Bars are the means of three independent experiments, each performed in duplicates± s.e.m., *P<0.05, **P<0.01, ***P<0.001.
Effects of fibroblast released lactate on spheroid migration
We observed that spheroid-activated fibroblasts released significant higher amounts of lactate into the culture medium compared to not activated fibroblasts (Fig. 6A). We then investigated the possible role of lactate in modulating tumor cell motility. As shown in Fig. 6B, after 5 days of culture, lactate alone was able to significantly increase motility only in SDHB-silenced, but not in wt control spheroids, as demonstrated by the computation of the migratory areas (Fig. 6B).
Figure 6.
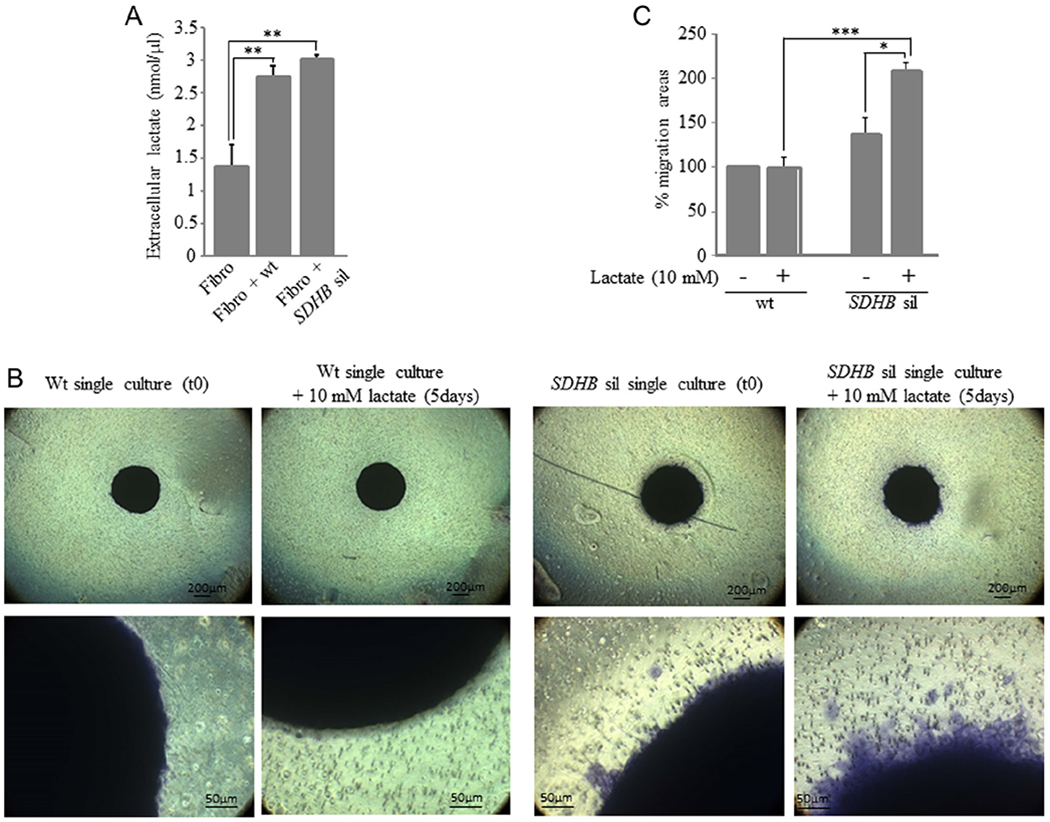
Effects of microenvironment lactate production on tumor spheroid migration. (A) MTT conditioned fibroblasts significantly increase lactate release in the extracellular medium. (B) Spheroids were laid on 0.3% matrigel in the upper compartment of transwell insert meanwhile in the lower chamber serum-free DMEM containing 10 mM lactate was added. Migration capability was observed after 5 days. Only SDHB-silenced spheroids are influenced by the presence of lactate in the lower compartment. When spheroids are observed at higher magnification, only in SDHB-silenced spheroids it is possible to notice migrating cells surrounding the spheroid, data confirmed by the computation of the migratory areas. Spheroid migration areas were calculated as the difference between areas at day 0 and day 5, expressed in percentage (C). Images are representative of more than three independent experiments. Bars are the means of three independent experiments, each performed in duplicates±s.e.m., *P<0.05, **P<0.01, ***P< 0.001.
Discussion
In this study, for the first time, we used tumor spheroids of a mouse Pheo cell line silenced or not for the catalytic SDHB subunit. We demonstrated that the microenvironment, here represented by primary CAFs, plays a pivotal and specific role in increasing the migration/invasion properties of spheroid cells and that this effect is extraordinarily enhanced in SDHB-silenced ones. Eventually, we found that lactate, largely secreted by CAFs, plays a specific role in promoting migration only of SDHB-silenced cells.
In the recent years, in vitro cell cultures have seen significant evolution by the introduction of three-dimensional (3D) culture systems and 3D cell cultures are at present accepted as excellent models for cancer research, which surpass the traditional monolayer cultures (Maddaly et al. 2017). Despite cancer research has already taken benefits from 3D cell cultures, the potential of these culture systems are not still completely exploited. The distribution on the external part of the spheroids of the actively dividing cells and the formation of the necrotic core leads, as a consequence, to nutrition and oxygen gradients. This is probably why only this 3D cell spatial arrangement allowed us to visualized swollen mitochondria in SDHB-silenced cells, resembling those found in human SDHx mutated Pheos/PGLs, whereas in 2D cultures, SDHB-silenced cell mitochondria did not differ from the ones in control cells (Fig. 3).
The use of spheroids does not just mean using a system that more closely resembles the tumor mass, but also it gives the possibility to better investigate other features, such as cell-cell interactions. Once again, only in 3D spheroid it was possible to reveal that cell junctions in the proliferating layer of SDHB-silenced cells were loose compared to controls, suggesting the predisposition of SDHB-silenced cells to migrate away from the spheroid. Nevertheless, a significant increase in SDHB-silenced cell migration, compared to controls, occurs only after the onset of other favorable factors such as the presence of fibroblasts in culture.
It is very well known that tumor microenvironment plays a crucial role in tumor progression. CAFs are able to modulate and rewire the metabolic changes and requirements of tumor cells, by releasing a large amount of molecules (for a recent review see Chiarugi & Cirri 2016). In this study, we have shed some light on the open question of why SDHB mutated Pheos/PGLs are much more aggressive and prone to metastasize than tumors mutated in other susceptibility genes, despite the fact that the loss of SDH activity affects cellular energy production and impair mitochondria function.
What is really impressive in our results is the specific pro-migratory effect of fibroblasts. This effect is particularly pronounced in SDHB mutated spheroids. Usually, to study the effect of a drug or a substance, cells are kept in a serum-free medium, so as not to have any interference or cross reactivity between the serum and the compounds. In our experiments, we can observe the fibroblast pro-migratory effect also in the presence of serum. When spheroids are grown in low adherence round bottom plates, and in medium containing serum, we can observe an almost linear growth in time. In this culture condition, proliferating cells belonging to the outer layer of the spheroids remain attached to the spheroid contributing to the increase of the spheroid masses. On the contrary, in presence of fibroblasts, the dividing cells of the outer layer do not increase the spheroid mass, but they move apart from the spheroid into the well. This phenomenon is even more clearer when spheroids are laid on a matrigel layer, where SDHB-silenced spheroid cells massively migrate in a collective manner, whereas wt spheroid cells migrate significantly less and in a single cell manner.
In a recent work, we have demonstrated that primary CAFs and human neuroblastoma cells in co-cultures establish reciprocal metabolic changes with an increased lactate production by fibroblasts, and an increased lactate uptake and proliferation in tumor cells (Rapizzi et al. 2015). Interestingly, we found that lactate is one of the factors that have a pivotal role in contributing specifically to SDHB-silenced spheroid cell migration. We hypothesized that lactate is particularly important in SDHB-silenced cells because these cells are metabolically deficient.
Another advantage in the use of the 3D spheroids is that it is possible to embed or lay them on the extracellular matrix, here represented by matrigel, mimicking the in vivo conditions. In vivo the extracellular matrix contributes in modulating the signaling of some growth factors and cytokines, whose function depends on their interaction with their receptors, but also in shaping the tumor mass (Maddaly et al. 2017).
In this study, we found that only SDHB-silenced spheroids form long filamentous structures that seem to be used by migrating cells as binaries to collectively migrate far from the spheroids. In many types of human malignant tumors, the cells are often connected to form groups that are able to migrate, and thus this migration is called collective migration (Frield & Gilmour 2009, Wang et al. 2016). In view of our results, we speculate that SDHB-silenced cells are more sensitive than control cells to some pro-migration drivers produced by fibroblasts. It is also possible that, due to other still unknown factors, collective invasion into the surrounding stroma might provide some advantages for SDHB-silenced tumor cells in comparison to single cell spreading.
In conclusion, in this paper 3D cultures were used for the first time as an experimental model to study the effects of SDHB silencing as well as of fibroblasts on Pheo/PGL cell migration/invasion. Most importantly, we demonstrated that CAFs enhance collective migration/invasion specifically in Pheo SDHB-silenced tumor spheroids, suggesting that the discovery of the factors responsible for this effect may reveal potential targets for new drugs aimed at reducing tumor malignancy.
Acknowledgement
We wish to thank Dr Arthur Tischler, who generated mouse pheochromocytoma cells (MPC) from the same mouse from which mouse tumor tissue derived (MTT) cells were derived.
Funding
This work was supported by the Paradifference Foundation and by the Fondazione Cassa di Risparmio di Pistoia e Pescia (Prot. 2016.0241/gi) to M M; E R, R F, L C and M M are members of the ENS@T (European Network for the Study of Adrenal Tumors). This research was supported, in part, by the Intramural Research Program of the NIH, NICHD.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Baysal BE & Maher ER 2015. 15 YEARS OF PARAGANGLIOMA: Genetics and mechanism of pheochromocytoma-paraganglioma syndromes characterized by germline SDHB and SDHD mutations. Endocrine-Related Cancer 22 T71–T82. (doi: 10.1530/ERC-15-0226) [DOI] [PubMed] [Google Scholar]
- Cerqueira OL, Truesdell P, Baldassarre T, Vilella-Arias SA, Watt K, Meens J, Chander H, Osorio CA, Soares FA, Reis EM, et al. 2015. CIP4 promotes metastasis in triple-negative breast cancer and is associated with poor patient prognosis. Oncotarget 6 9397–9408. (doi: 10.18632/oncotarget.3351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi P & Cirri P 2016. Metabolic exchanges within tumor microenvironment. Cancer Letters 380 272–280. (doi: 10.1016/j.canlet.2015.10.027) [DOI] [PubMed] [Google Scholar]
- Douwes Dekker PB, Hogendoorn PC, Kuipers-Dijkshoorn N, Prins FA, van Duinen SG, Taschner PE, van der Mey AG & Cornelisse CJ 2003. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. Journal of Pathology 201 480–86. (doi: 10.1002/path.1461) [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Stull R, Reiser HR, Sunderland T, Murphy DL & Kopin IJ 1986. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clinical Chemistry 32 2030–2033. [PubMed] [Google Scholar]
- Fennema E, Rivron N, Rouwkema J, van Blitterswijk C & de Boer J 2013. Spheroid culture as a tool for creating 3D complex tissues. Trends in Biotechnology 31 108–115. (doi: 10.1016/j.tibtech.2012.12.003) [DOI] [PubMed] [Google Scholar]
- Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P & Chiarugi P 2012. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Research 72 5130–5140. (doi: 10.1158/0008-5472.CAN-12-1949) [DOI] [PubMed] [Google Scholar]
- Frield P & Gilmour D 2009. Collective migration in morphogenesis, regeneration and cancer. Nature Reviews Molecular Cell Biology 10 445–457. (doi: 10.1038/nrm2720) [DOI] [PubMed] [Google Scholar]
- Greene LA & Tischler AS 1976. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. PNAS 73 2424–2428. (doi: 10.1073/pnas.73.7.2424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiet R, Van Goethem E, Cougoule C, Balor S, Valette A, Al Saati T, Lowell CA, Le Cabec V & Maridonneau-Parini I 2011. The process of macrophage migration promotes matrix metalloproteinase-independent invasion by tumor cells. Journal of Immunology 187 3806–3814. (doi: 10.4049/jimmunol.1101245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D & Weinberg RA 2011. Hallmarks of cancer: the next generation. Cell 144 64–V674. (doi: 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- Hanahan D & Coussens LM 2012. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21 309–322. (doi: 10.1016/j.ccr.2012.02.022) [DOI] [PubMed] [Google Scholar]
- Hu M & Polyak K 2008. Microenvironmental regulation of cancer development. Current Opinion in Genetics & Development 18 27–34. (doi: 10.1016/j.gde.2007.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH & Diamandis EP 2012. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Molecular Cancer Research 10 1403–1418. (doi: 10.1158/1541-7786.MCR-12-0307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm F & Joyce JA 2015. Microenvironment regulation of therapeutic response in cancer. Trends in Cell Biology 25 198–213. (doi: 10.1016/j.tcb.2014.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaly R, Subramaniyan A & Balasubramanian H 2017. Cancer cytokines and the relevance of 3D cultures for studying those implicated in human cancers. Journal of Cellular Biochemistry 118 2544–2558. (doi: 10.1002/jcb.25970) [DOI] [PubMed] [Google Scholar]
- Martiniova L, Lai EW, Elkahloun AG, Abu-Asab M, Wickremasinghe A, Solis DC, Perera SM, Huynh TT, Lubensky IA, Tischler AS, et al. 2009. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clinical and Experimental Metastasis 26 239–250. (doi: 10.1007/s10585-009-9236-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG & Stelzer EHK 2007. The third dimension bridges the gap between cell culture and live tissue. Nature Reviews Molecular Cell Biology 8 839–845. (doi: 10.1038/nrm2236) [DOI] [PubMed] [Google Scholar]
- Powers JF, Evinger MJ, Tsokas P, Bedri S, Alroy J, Shahsavari M & Tiscler AS 2000. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell and Tissue Research 302 309–320. (doi: 10.1007/s004410000290) [DOI] [PubMed] [Google Scholar]
- Qin N, de Cubas AA, Garcia-Martin R, Richter S, Peitzsch M, Menschikowski M, Lenders JW, Timmers HJ, Mannelli M, Opocher G, et al. 2014. Opposing effects of HIF1α and HIF2α on chromaffin cell phenotypic features and tumor cell proliferation: insights from MYC-associated factor X. International Journal of Cancer 135 2054–2064. (doi: 10.1002/ijc.28868) [DOI] [PubMed] [Google Scholar]
- Quail DF & Joyce JA 2013. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine 19 1423–1437. (doi: 10.1038/nm.3394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapizzi E, Fucci R, Giannoni E, Canu L, Richter S, Cirri P & Mannelli M 2015. Role of microenvironment on neuroblastoma SK-N-AS SDHB-silenced cell metabolism and function. Endocrine-Related Cancer 22 409–117. (doi: 10.1530/ERC-14-0479) [DOI] [PubMed] [Google Scholar]
- Richter S, Peitzsch M, Rapizzi E, Lenders JW, Qin N, de Cubas AA, Schiavi F, Rao JU, Beuschlein F, Quinkler M, et al. 2014. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. Journal of Clinical Endocrinology and Metabolism 99 3903–3911. (doi: 10.1210/jc.2014-2151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi A, Caselli A, Paoli P, Corti D, Camici G, Pieraccini G, Taddei ML, Serni S, Chiarugi P & Cirri P 2013. The effects of CA IX catalysis products within tumor microenvironment. Cell Communication and Signaling 11 81. (doi: 10.1186/1478-811X-11-81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9 676–682. (doi: 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE & Noel A 2013. Targeting the tumor microenvironment for cancer therapy. Clinical Chemistry 59 85–93. (doi: 10.1373/clinchem.2012.185363) [DOI] [PubMed] [Google Scholar]
- Stein AM, Demuth T, Mobley D, Berens M & Sander LM 2007. A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophysical Journal 92 356–365. (doi: 10.1529/biophysj.106.093468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei ML, Giannoni E, Comito G & Chiarugi P 2013. Microenvironment and tumor cell plasticity: an easy way out. Cancer Letters 341 80–96. (doi: 10.1016/j.canlet.2013.01.042) [DOI] [PubMed] [Google Scholar]
- Tischler AS & Favier J 2015. Models of pheochromocytoma: what;s on the horizon? International Journal of Endocrine Oncology 2 171–174. (doi: 10.2217/ije.15.14) [DOI] [Google Scholar]
- Wang X, Enomoto A, Asai N, Kato T & Takahashi M 2016. Collective invasion of cancer: perspectives from pathology and development. Pathology International 66 183–192. (doi: 10.1111/pin.12391) [DOI] [PubMed] [Google Scholar]
- Zhang J & Liu J 2012. Tumor stroma as targets for cancer therapy. Pharmacology and Therapeutics 137 200–215. (doi: 10.1016/j.pharmthera.2012.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]


