Abstract
OBJECTIVE
Estimate (1) prevalence of major depressive disorder (MDD) diagnosis; (2) risk factors associated with MDD diagnosis; (3) time at which MDD is diagnosed post-spinal cord injury (SCI) and interaction of inferred mobility status (IMS) in a commercially insured population over three years.
DESIGN
Retrospective longitudinal cohort design.
SETTING
A commercial insurance claims database from January 1, 2010 – December 31, 2013.
PARTICIPANTS
Individuals with an index cervical or thoracic SCI in 2011 or 2012, without history of MDD ≤30 days pre-SCI (n=1,409).
INTERVENTION
Not applicable.
MAIN OUTCOMES
Prevalence of, risk factors associated with, and time to MDD diagnosis post-SCI. A stratified survival analysis using IMS, based upon durable medical equipment (DME) claims, was also completed.
RESULTS
Post-SCI, 20.87% of the sample was diagnosed with new-onset MDD. Significant (p<0.05) risk factors included: employed, length of index hospitalization, discharged from index hospitalization with healthcare services, rehabilitation services post-SCI, and two of five IMS comparisons. Median time to MDD was 86 days. Survival analysis demonstrated a significant difference between six of ten IMS comparisons. Regarding new-onset or recurring MDD, 30.66% of the sample was diagnosed post-SCI. Significant risk factors included: female, employed, length of index hospitalization, discharge from index hospitalization with healthcare services, rehabilitation services post-SCI, MDD >30 days pre-SCI, catheter claims, and two of five IMS comparisons. Median time to MDD was 74 days. Survival analysis demonstrated a significant difference between four of ten IMS comparisons.
CONCLUSIONS
Prevalence of MDD post-SCI is greater than the general population. Stratification by IMS illustrated that individuals with greater inferred reliance on DME are at a greater risk for MDD and have shorter time to MDD diagnosis post-SCI.
Keywords: Spinal Cord Injury, Depression, Cluster Analysis, Survival Analysis, Durable Medical Equipment
Introduction
Approximately 17,000 new spinal cord injuries (SCI) occur every year1 and 18.7%–26.3% of individuals post-SCI meet criteria for major depressive disorder (MDD).2 Depressive symptoms post-SCI predict reduction in leisure activities,3 spending more time in bed,4 increased healthcare utilization,5,6 and early mortality.7 Specifically among community dwellers post-SCI, those with probable MDD have an 86% greater risk of death compared to those without MDD.7
Studies have reported the prevalence of and risk factors associated with MDD post-SCI and have explored cost and/or treatment utilization,5,8–13 but no studies have reported when MDD is diagnosed post-SCI. The time at which MDD is diagnosed post-injury is important, since depression has been noted to impact recovery.14,15
To understand current clinical practice patterns for the time to MDD diagnosis post-SCI, insurance claims data eliminates of some biases in survey or clinical research but sacrifices clinical details (ie. severity of depressive symptoms, severity of injury, and functional mobility). Clinical details are important factors in research regarding MDD post-SCI, since mobility impairments4,16,17 and injury severity13,18 are identified risk factors of MDD. Therefore, a proxy is needed when utilizing insurance claims data. Kumar et al. explored using comorbidity indexes in a 100% Medicare sample in individuals with stroke, lower extremity (LE) joint replacements, and LE fractures at both discharge to and from inpatient rehabilitation to predict functional mobility.19,20 Unfortunately, comorbidity indexes were not suitable proxies for functional mobility at either time point.19,20 Thus, different methods to identify a proxy for functional mobility are needed.
This study explored the diagnosis of MDD post-SCI over a 3-year period among privately insured individuals using commercial insurance claims data. To overcome the lack of clinical detail regarding mobility impairment, we developed a new measure utilizing durable medical equipment (DME) claims to stratify our sample based on inferred mobility status (IMS).
We aimed to answer the following questions: (1) What is the prevalence of MDD diagnosis post-SCI?; (2) What are the risk factors associated with MDD diagnosis post-SCI?; (3) When is MDD diagnosed post-SCI and does it vary by IMS? We hypothesized that the prevalence of MDD diagnosis would be similar to previously published literature of 20–30%. Hypothesized risk factors associated with MDD diagnosis included, age, female sex, history of MDD, IMS, and proxies of injury severity such as length of index hospitalization. Based on prior studies assessing depressive symptoms post-SCI, the median time to MDD diagnosis was hypothesized to be one year, but would vary by IMS.
Methods
A retrospective longitudinal cohort design was applied using MarketScan® Commercial Claims and Encounters Database (Truven Health Analytics, Ann Arbor, MI). This database includes de-identified inpatient, outpatient, and pharmacy claims from employer sponsored insurance (ESI) plans. Individuals enrolled in ESI plans include employees, those on long-term disability and COBRA, spouses, and dependents. Within MarketScan® database, data are available on the patient level for the duration of an individual’s coverage.
The dataset included inpatient, outpatient, and pharmacy claims and encounters from January 1, 2010 through December 31, 2013. SAS 9.4 (SAS Institute, Cary, NC) was utilized for dataset construction and statistical analyses. Statistical significance was determined at the 0.05 level. The Institutional Review Board classified this study as “Not Human Research.”
Dataset Construction
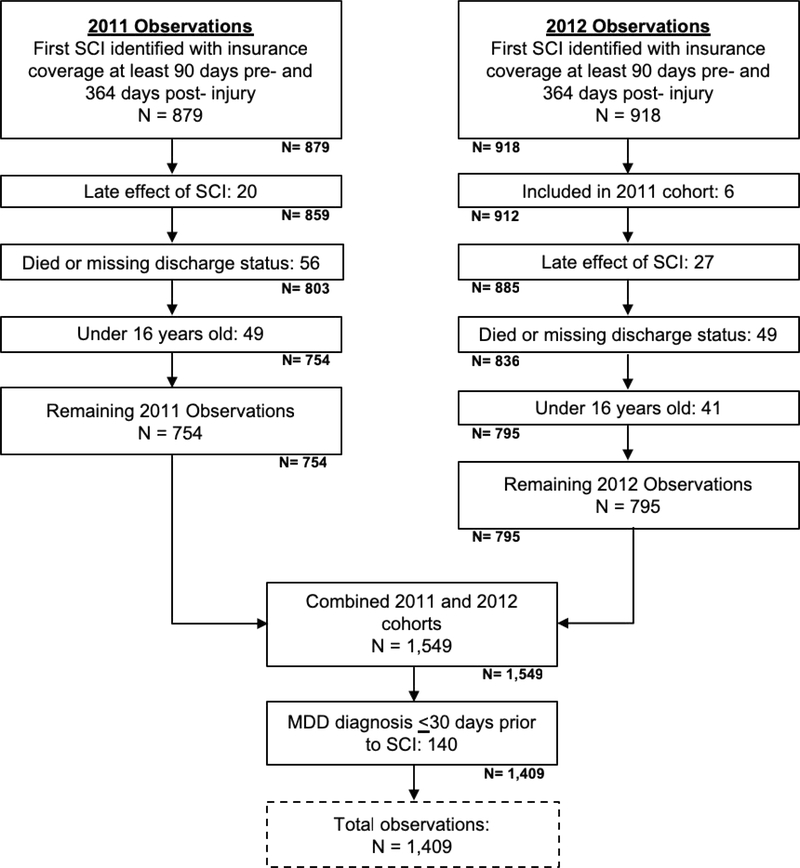
Two inception cohorts, 2011 and 2012, were extracted by identifying individuals with an acute, inpatient diagnosis of cervical or thoracic traumatic SCI, who had coverage ≥90 days pre-injury and ≥364 days post-injury. Cases were identified as traumatic, based upon the International Classification of Diseases, 9th Revision (ICD-9) codes from the Barell injury diagnosis matrix (Supplemental Table 1).21 All claims from January 1, 2010 through December 31, 2013 were extracted. Individuals were excluded if any of the following were noted: diagnosis of late effect of SCI (ICD-9: 907.2), which is indicative of a prior SCI; died or missing discharge status; <16 years old at time of injury; or MDD diagnosis ≤30 days pre-injury.
Individuals were classified as having MDD diagnosis if ICD-9 codes for major depressive disorder, single (296.2) or recurrent (296.3), were identified within inpatient or outpatient encounters or at least one outpatient pharmacy claim for antidepressants was filed.
Date of every diagnosis code and pharmacy claim was noted in relation to SCI index date. Dichotomous variables were created using the first date, diagnosis or pharmacy claim, for the following variables with respect to index date: (1) MDD any time pre-SCI, (2) MDD ≤30 days pre-SCI, (3) MDD post-SCI without an identified history of MDD pre-SCI (new-onset), (4) MDD post-SCI regardless of diagnosis pre-injury (new-onset or recurring). Individuals with MDD diagnosis ≤30 days pre-SCI were excluded, based on the assumption they would maintain the diagnosis and treatment immediately post-SCI.
Dichotomous variables were created to identify patient characteristics. Employment status was classified as employed (part- or full-time) versus other, including spouses, dependents, retired employees, COBRA participants, and those on long-term disability. Discharge location after index hospitalization was identified as either discharged with medical services (i.e. inpatient rehabilitation, skilled nursing, and home health) or discharged home without services. To ascertain index SCI injury severity, dichotomous variables were created for cervical level of injury, mechanical ventilation, tracheostomy, and spinal surgery using ICD-9 diagnosis and procedure codes (Supplemental Table 2). ICD-9 diagnosis and procedure, Healthcare Common Procedure Coding System (HCPCS), and Current Procedural Terminology (CPT) codes from inpatient and outpatient claims and encounters were analyzed to identify those with at least one claim for any rehabilitation service (physical, occupational, or speech therapy) post-SCI (Supplemental Table 3).
Since MarketScan® does not contain information regarding functional status, HCPCS codes were used to identify DME claims any time post-SCI. Dichotomous variables were created for the following equipment: power wheelchairs, manual wheelchairs, walkers, crutches, canes, mechanical lifts, transfers boards, LE orthotics, and catheters (Supplemental Table 4). From these nine DME variables, eight were selected (power wheelchairs, manual wheelchairs, walkers, crutches, canes, mechanical lifts, transfers boards, and LE orthotics) to use in a k-means cluster analysis (Proc Fastclus) to infer mobility status (ie. IMS groups).
The k-means cluster analysis uses a partitional algorithm to divide the sample into k clusters (“groups”) based upon identified variables.22,23 The k value is a specified number of clusters.22,23 Through the algorithm, k observations are selected and identified as cluster seeds.22,23 Remaining observations are assigned to the nearest seed based upon Euclidean distance.22,23 The seeds are then replaced by a value for the cluster center (mean or other location estimate).22,23 This step is repeated n times or until the value of the cluster seed is near or equal to zero.22,23 The final clusters are created by assigning the observations to the nearest seed.22,23 Final number of clusters is determined through a trial and error process in which the output meets a determined criterion.23
Statistical Analysis
Means and standard deviations or medians were used to describe continuous data and frequencies and percentages were used for categorical data.
Two multiple logistic regressions were estimated to identify risk factors associated with MDD diagnosis. Dichotomous outcomes were used for new-onset and new-onset or recurring MDD diagnosis post-SCI. Sex, age, length of index hospitalization, and total number of days insured were used to control for potential confounding factors. IMS was also included in the models. Variables not found to be significant were removed one at a time and models were refit. Model calibration was assessed using c-static. No significant interactions were identified between sex and age categories and the models’ main effects.
To evaluate trends in time (days) from index hospitalization to MDD diagnosis, a Kaplan-Meier survival analysis was completed for both outcomes, new-onset and new-onset or recurring MDD diagnosis post-SCI. Survival analyses were estimated with and without IMS stratification. A Wilcoxon test with Bonferroni correction was used to analyze IMS differences for p<0.05.
Results
Demographics
The final sample included 1,409 individuals that met criteria for index SCI in 2011 or 2012 (Figure 1). The population was primarily male (67.85%) and most had a cervical injury (68.35%) (Table 1). Mean age was 43.29±15.33 years (range 16–64) and the average length of index hospitalization was 10.35±16.62 days. About half of the sample had spinal surgery (53.58%) and were discharged home without healthcare services (56.07%), but 80.13% received rehabilitation services. After excluding individuals with MDD ≤30 days pre-SCI, 12.63% had a history of MDD pre-SCI with the first identified diagnosis date ranging from 31 to 700 days (median 398 days) pre-injury. Post-SCI 20.87% of the sample was diagnosed with new-onset MDD diagnosis and 30.66% had new-onset or recurring MDD.
Fig 1.
Identification of spinal cord injury cohort.
Table 1.
Demographics and characteristics of those hospitalized with index SCI in 2011 and 2012
| Characteristics | Overall (N=1,409) |
|---|---|
| n (%) | |
| Female sex | 453 (32.15) |
| Highest level of SCI | |
| Cervical | 963 (68.35) |
| Thoracic | 446 (31.65) |
| Employment status | |
| Employed (full- or part- time) | 548 (38.89) |
| Retired | 116 (8.23) |
| Long term disability | 6 (0.43) |
| Other | 125 (8.87) |
| Dependent (spouse or child) | 614 (43.58) |
| Discharge location from index hospitalization | |
| Home | 790 (56.07) |
| Inpatient rehabilitation | 273 (19.38) |
| Home with home health | 109 (7.74) |
| Skilled nursing facility | 60 (4.26) |
| Other | 177 (12.56) |
| Procedures | |
| Mechanical ventilation | 81 (5.75) |
| Tracheostomy | 0 (0.00) |
| Spinal surgery | 755 (53.58) |
| Catheter claims | 247 (17.53) |
| Any rehabilitation services post-SCI | 1,129 (80.13) |
| Mean ± SD | |
| Age (years) | 43.29 ± 15.33 |
| Length of index hospital stay (days) | 10.25 ± 16.62 |
| Insurance coverage | |
| Days of coverage pre-SCI | 599.76 ± 273.17 |
| Days of coverage post-SCI | 627.38 ± 191.29 |
| Total days of coverage | 1,227.14 ± 278.69 |
Inferred Mobility Status
Five IMS groups were identified: IMS-power wheelchair users (IMS-PWC), IMS-manual wheelchair users with LE orthotics (IMS-MWC-LEO); IMS-manual wheelchair users without LE orthotics (IMS-MWC); IMS-walker users (IMS-W); IMS-no assistive devices or LE orthotics (IMS-NAD). The five IMS groups are consistent with observed clinical presentations post-SCI, ranging from the greatest inferred impairment (IMS-PWC) consisting of primarily cervical lesions, highest cost of index hospitalization, and greater need for medical support post-discharge, to the least inferred impairment (IMS-NAD) consisting of both cervical and thoracic level injuries, lowest cost of index hospitalization, and least need for medical support post-discharge. The frequency of MDD diagnosis within the IMS groups followed a similar pattern with the greatest percentage of individuals with MDD diagnosis noted within IMS-PWC and least within IMS-NAD (Table 2 and Supplemental Section 5).
Table 2.
Characteristics of sample stratified by inferred mobility status
| IMS n = 1,409 |
|||||
|---|---|---|---|---|---|
| IMS-PWC n = 84 (5.96%) | IMS-MWC-LEO n = 86 (6.10%) | IMS-MWC n = 95 (6.74%) | IMS-W n = 223 (15.83%) | IMS-NAD n = 921 (65.37%) | |
| Demographics | Mean (±SD) | ||||
| Age | 41.32 (±16.56) | 35.57 (±15.37) | 38.80 (±16.23) | 47.38 (±14.74) | 43.67 (±14.88) |
| Index hospitalization | |||||
| Length of stay (days) | 27.30 (±23.49) | 16.83 (±15.26) | 17.20 (±17.38) | 9.25 (±8.52) | 7.60 (±16.13) |
| Total net cost | $212,881 (±170,013) | $133,755 (±124,180) | $119,524 (±107,214) n (%) | $69,585 (±80,058) | $58,385 (±118,834) |
| Females sex | 17 (20.24) | 27 (31.40) | 30 (31.58) | 87 (39.01) | 292 (31.70) |
| Cervical lesion | 69 (82.14) | 29 (33.72) | 47 (49.47) | 132 (59.19) | 686 (74.48) |
| Trach. or mechanical ventilation | 17 (20.24) | 4 (4.65) | 11 (11.58) | 7 (3.14) | 42 (4.56) |
| Spinal surgery | 61 (72.62) | 60 (69.77) | 60 (63.16) | 123 (55.16) | 451(48.97) |
| Wheelchair | |||||
| Power wheelchair | 80 (95.24) | 1 (1.16) | 4 (4.21) | 6 (2.69) | 0 (0.00) |
| Manual wheelchair | 2 (2.38) | 85 (98.84) | 95 (100.00) | 42 (18.83) | 0 (0.00) |
| Ambulation devices | |||||
| Cane | 0 (0.00) | 1 (1.16) | 9 (9.47) | 12 (5.38) | 27 (2.93) |
| Crutches | 0 (0.00) | 7 (8.14) | 2 (2.11) | 5 (2.24) | 8 (0.87) |
| Walker | 3 (3.57) | 19 (22.09) | 0 (0.00) | 223 (100.00) | 0 (0.00) |
| Transfer assistance | |||||
| Transfer board | 19 (22.62) | 51 (59.30) | 0 (0.00) | 5 (2.24) | 5 (0.54) |
| Lift | 54 (64.29) | 9 (10.47) | 4 (4.21) | 3 (1.35) | 6 (0.65) |
| Lower extremity orthotics | 9 (10.71) | 58 (67.44) | 0 (0.00) | 24 (10.76) | 32 (3.47) |
| D/C from index hospitalization with healthcare services | 68 (80.95) | 69 (80.23) | 72 (75.79) | 127 (56.95) | 280 (30.40) |
| Rehabilitation post-SCI | 82 (97.62) | 84 (97.67) | 88 (92.63) | 202 (90.58) | 673 (73.07) |
| MDD diagnosis post-SCI | |||||
| New-onset | 44 (52.38) | 37 (43.02) | 28 (29.47) | 52 (23.32) | 133 (14.44) |
| New-onset or recurring | 52 (61.90) | 40 (46.51) | 41 (43.16) | 79 (35.43) | 220 (23.89) |
| Time to MDD diagnosis post-SCI | Median | ||||
| New-onset (days) | 97.50 | 57.00 | 74.50 | 80.00 | 96.00 |
| New-onset or recurring (days) | 98.00 | 57.00 | 71.00 | 65.00 | 68.50 |
Abbreviations: Trach., tracheostomy; D/C, discharge.
Risk Factors of MDD Diagnosis
Table 3 summarizes multiple logistic regressions results. For new-onset MDD diagnosis post-SCI, significant factors included employed (OR, 1.73; CI95%, 1.31–2.30), length of index hospitalization (OR, 1.01; CI95%, 1.00–1.02), discharged with healthcare services (OR, 1.98; CI95%, 1.46–2.69), and rehabilitation services post-SCI (OR, 1.96; CI95%, 1.23–3.13). When compared to IMS-NAD, the IMS groups IMS-PWC (OR, 3.37; CI95%, 2.03–5.59) and IMS-MWC-LEO (OR, 2.68; CI95%, 1.63–4.42) were significant. This model had acceptable discrimination (c-statistic=0.72).
Table 3.
Results of logistic regressions predicting MDD diagnosis post-SCI for those hospitalized with index SCI in 2011 and 2012
| OR (95% CI) | χ | p | |
|---|---|---|---|
|
New-onset MDD post-SCI |
|||
| Female | 1.03 (0.77 – 1.38) | 0.04 | 0.85 |
| Less than 35 years old | 1.11 (0.82 – 1.51) | 0.46 | 0.50 |
| Total number of days insured | 1.00 (1.00 – 1.00) | 1.22 | 0.27 |
| Employed | 1.73 (1.31 – 2.30) | 14.51 | 0.0001* |
| Length of index hospitalization | 1.01 (1.00 – 1.02) | 5.72 | 0.0168* |
| D/C from index hospitalization with healthcare services | 1.98 (1.46 – 2.69) | 19.28 | <0.0001* |
| Rehabilitation services post-SCI | 1.96 (1.23 – 3.13) | 8.00 | 0.0047* |
| Inferred mobility status (Ref = IMS-NAD) | |||
| IMS-PWC | 3.37 (2.03 – 5.59) | 22.04 | <0.0001* |
| IMS-MWC-LEO | 2.68 (1.63 – 4.42) | 15.01 | 0.0001* |
| IMS-MWC | 1.48 (0.89 – 2.46) | 2.26 | 0.13 |
| IMS-W | 1.37 (0.94 – 2.00) | 2.70 | 0.10 |
|
New-onset or recurring MDD post-SCI |
|||
| Female | 1.40 (1.06 – 1.85) | 5.46 | 0.02* |
| Less than 35 years old | 0.88 (0.65 – 1.18) | 0.74 | 0.39 |
| Total number of days insured | 1.00 (1.00 – 1.00) | 0.40 | 0.53 |
| Employed | 1.76 (1.34 – 2.32) | 16.58 | <0.0001* |
| Length of index hospitalization | 1.01 (1.00 – 1.02) | 5.27 | 0.0217* |
| D/C from index hospitalization with healthcare services | 1.75 (1.30 – 2.35) | 13.65 | 0.0002* |
| Rehabilitation services post-SCI | 1.83 (1.21 – 2.75) | 8.26 | 0.004* |
| MDD diagnosis >30 days pre-SCI | 12.63 (8.42 – 18.93) | 150.59 | <0.0001* |
| Catheter claims | 1.62 (1.04 – 2.53) | 4.49 | 0.03* |
| Inferred mobility status (Ref = IMS-NAD) | |||
| IMS-PWC | 2.63 (1.40 – 4.94) | 9.10 | 0.0026* |
| IMS-MWC-LEO | 1.83 (1.03 – 3.25) | 4.27 | 0.0388* |
| IMS-MWC | 1.42 (0.83 – 2.42) | 1.62 | 0.20 |
| IMS-W | 1.36 (0.94 – 1.95) | 2.70 | 0.10 |
Abbreviations: OR, odds ratio; D/C, discharge
Significant value (p <0.05).
For the outcome new-onset or recurring MDD diagnosis post-SCI, significant factors included female (OR: 1.40, CI95%, 1.06–1.85), employed (OR, 1.76; CI95%, 1.34–2.32), length of index hospitalization (OR, 1.01; CI95%, 1.00–1.02), discharged with healthcare services (OR, 1.75; CI95%, 1.30–2.35), rehabilitation services post-SCI (OR, 1.83; CI95%, 1.21–2.75), catheter claims (OR, 1.62; CI95%, 1.04–2.53) and MDD >30 days pre-SCI (OR, 12.63; CI95%, 8.42–18.93). When compared to IMS-NAD, the IMS groups IMS-PWC (OR, 2.63; CI95%, 1.40–4.94) and IMS-MWC-LEO (OR, 1.83; CI95%, 1.03–3.25) were significant. This model had acceptable discrimination (c-statistic=0.79).
Time to MDD Diagnosis
Median time to new-onset MDD diagnosis post-SCI was 86.0 days and 74.0 days for new-onset or recurring MDD. See Table 2 for median time to MDD diagnosis by IMS.
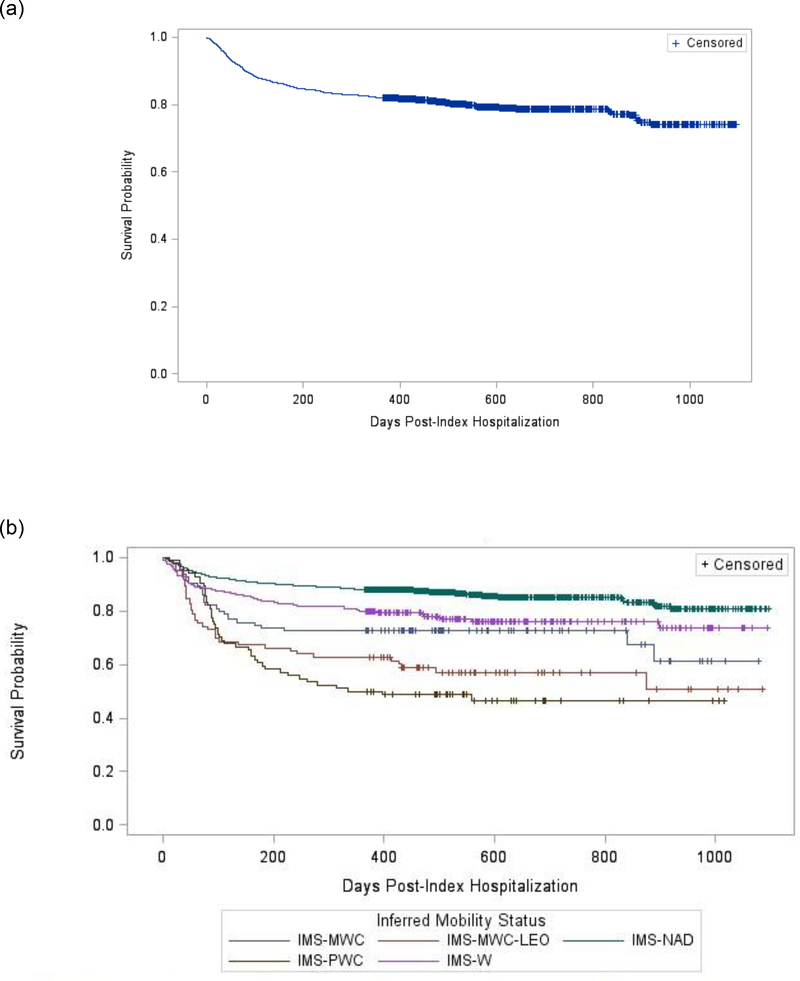
Figure 2(a) and (b) shows the simple and stratified survival analyses of time to new-onset MDD diagnosis post-SCI. Test for equality over strata was significant (χ2=116.79, p=<0.0001). Six of the ten stratified survival functions comparisons were significant (adjusted p<0.05): IMS-NAD was significantly different than the other four IMS groups and IMS-PWC was significantly different than IMS-MWC and IMS-W.
Fig 2.
Survival analysis for time to new-onset MDD diagnosis post-SCI: (a) unstratified analysis; (b) stratified analysis by inferred mobility status.
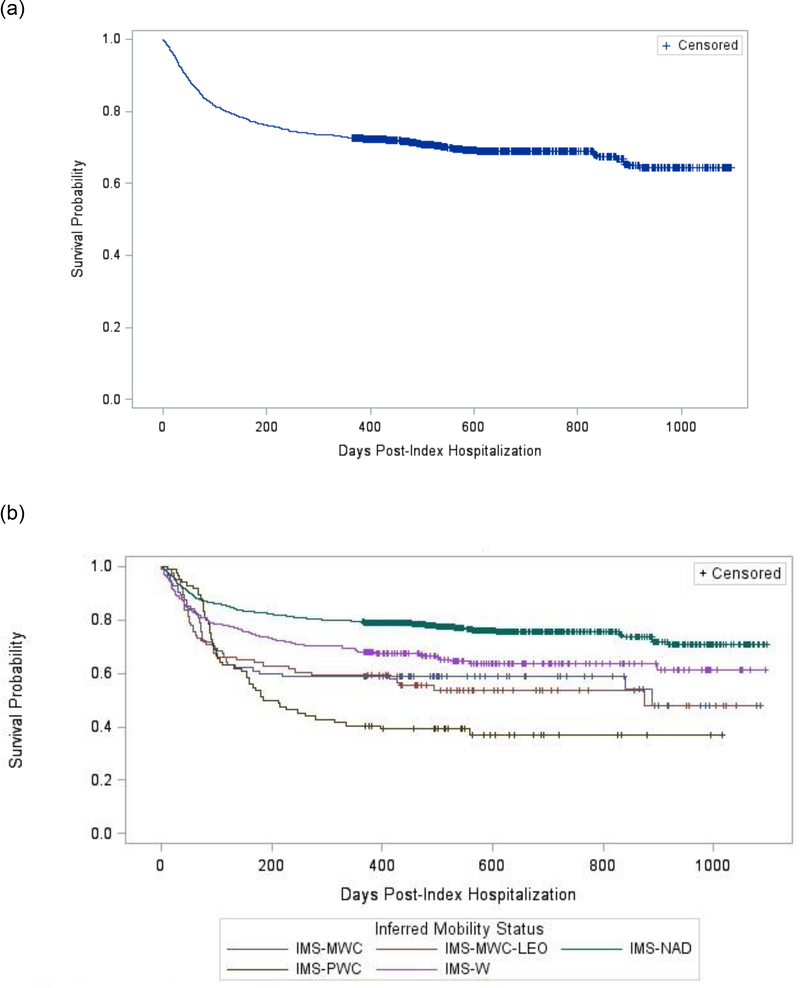
Figure 3(a) and (b) illustrates the simple and stratified survival analyses of time to new-onset or recurring MDD diagnosis post-injury. Test for equality over strata was significant (χ2=72.81, p=<0.0001). Four of the ten stratified survival functions comparisons were significant (adjusted p<0.05): IMS-NAD was significantly different than the other four IMS groups.
Fig 3.
Survival analysis for time to new-onset or recurring MDD diagnosis post-SCI: (a) unstratified analysis; (b) stratified analysis by inferred mobility status.
Discussion
This study offers several unique contributions to the growing body of knowledge of MDD post-SCI and suggests a new method to control for possible mobility impairments within commercial insurance claims data. Although not without limitations, our approach provided a novel opportunity to explore clinically relevant questions in a large group of individuals that would otherwise be extremely difficult using typical sampling approaches.
The identified prevalence of MDD diagnosis post-SCI fell within our hypothesized range of 20–30%. Methods used to assess depressive symptoms or diagnose depressive disorder(s) vary widely within the literature. We identified two studies that retrospectively analyzed veteran claims data for depressive disorders post-SCI.5,12 While the reported prevalence was similar, these studies included depressive disorder diagnosis codes beyond MDD and did not include antidepressant medication claims.5,12 We wanted to assess the diagnosis of MDD, thus we exclusively used ICD-9 codes for MDD. We also opted to include antidepressant pharmacy claims as an indicator of MDD based on the recommendations of two systematic reviews24,25 and with the goal of possibly improving the sensitivity of identifying MDD while diminishing positive predicative values.24 Regardless of the method(s) used to identify MDD or depressive disorder(s) post-SCI, the prevalence is greater than the general population at 7.1%.26 The high prevalence of MDD post-SCI coupled with the negative prognosis for recovery suggests the importance of a greater focus on identifying and effectively treating MDD post-SCI to improve long-term outcomes.
In addition to prevalence, we explored risk factors associated with MDD diagnosis post-SCI. Finding that employment increased risk of MDD was surprising as previous studies reported employment decreased odds of depression27 and unemployment was associated with depression post-SCI.18,28 This discrepancy could be due to our unique sample of acutely injured individuals insured though ESI plans. To retain ESI coverage, employees (33.98% of our sample) must maintain employment; therefore, the burden falls upon the employee. Higher rates of insurance discontinuation have been noted among individuals who sustained trauma with severe spine injury compared to the uninjured population within MarketScan® data.29 Thus, the fear of losing coverage post-SCI could place additional stress on employees, thereby increasing their risk of MDD diagnosis. The differing conclusions regarding the nature of the relationship between employment status and the presence of MDD likely reflect a variety of factors that contribute to the complexity in the etiology of MDD as well as the influence that personal factors play in its genesis.
To our knowledge, this study is the first to use DME HCPCS codes to control for inferred mobility status. Our novel use of a DME claims based cluster analysis stemmed from the work of Faurot et al. who used Medicare Claims Data and Medicare Current Beneficiaries Survey to predict activities of daily living dependency as a proxy for frailty in the elderly.30 This study indicated possible merit of using DME equipment as a proxy for mobility impairment(s).30 When applied to our sample, the analysis created a strong clinical representation of observed mobility impairments post-SCI.
The inclusion of IMS in our logistic regressions and survival analyses led to several unique findings: individuals with DME claims that are indicative of increased impairment, such as wheelchair claims, had a greater risk of MDD diagnosis post-SCI and their time to MDD diagnosis was shorter compared to those with limited to no relevant DME claims. While we are unable to draw any conclusions regarding the causation, we feel these findings are important.
First, these results contribute to inconclusive literature regarding the associations of depression post-SCI with index injury severity and mobility impairment.16–18,31 We found that proxies for index injury severity and mobility impairment(s) were significant risk factors for MDD diagnosis. While our findings are supported by other studies,16–18,31 definitive conclusions regarding these associations in the post-SCI cohort as a whole cannot be made. More precise descriptors of injury severity and/or functional ability are likely required to more conclusively determine the extent to which these variables influence MDD presence, severity and impact on individuals post-SCI.
Second, time to MDD diagnose post-SCI has not previously been explored. This study offers initial insight into clinical practice regarding the timing of MDD diagnosis post-SCI. Contrary to our hypothesis, we found that among those with an MDD diagnosis half were diagnosed within three months of their index injury and the timing varied by IMS. Again, conclusions regarding causation cannot be made, but we believe this information justifies the need for further research to explore the timing of MDD diagnosis and treatment of MDD in individuals post-SCI.
Study Limitations
Five primary limitations could have resulted in misidentification of MDD. First, MDD diagnosis or antidepressant claims could have occurred outside the study time frame. Second, only ICD-9 diagnosis codes for MDD were used. Third, antidepressants can be prescribed for off-label use, such as pain;32 therefore, it is possible that some individuals were erroneously identified as having MDD. Fourth, depressive symptoms were not assessed; therefore, those with undiagnosed and/or untreated depression were misclassified. Additionally, the presence of a MDD diagnosis does not mean the individual met criteria for MDD, though concordance between clinical criteria of depression and Medicare claims has shown the positive predictive value of a claim to be 66%, with a negative predictive value of 77%.33 Fifth, treatment techniques for MDD beyond antidepressant medication claims were not captured.
HCPCS codes for DME have not been previously utilized to infer mobility status, thus, the validity of this method needs to be explored. We were only able to capture DME claims, not what equipment was issued, used, or obtained without insurance coverage. We attempted to account for possible functional changes by including multiple types of DME and not setting a specific cutoff time post-SCI by which DME claims had to be filed.
There are additional limitations, secondary to using an administrative database. Maximum age of inclusion in MarketScan® is 64 years, since Medicare is primary for those ≥65 years old. Further, insurance databases were designed for billing, not clinical research. Coding errors and changes in coding practices over time could have impacted our results. Lastly, external validity of this study is limited to individuals insured through ESI plans.
Conclusions
Our findings demonstrate a substantial increased prevalence of MDD diagnosis post-SCI relative to the non-injured population. Identified risk factors included being female, employment, proxies for index injury severity, and history of MDD. Risk of and time to MDD diagnosis post-SCI also appears to be associated with DME claims and thus inferred mobility impairment(s). We believe these factors are important to recognize as predictors of depression post-SCI, but future work describing the complexity and potential interactions of these, and other, variables to the presence and severity of MDD would have significant clinical value. Given the associated consequences of depression on long-term health and function in individuals following SCI, the more fully developed our understanding is of this relationship, the better prepared we will be to address the significant impact of this condition on patients, families and caregivers.
Supplementary Material
Acknowledgements
We sincerely thank the anonymous reviewers for their time and thoughtful comments that enabled us to greatly improve the clarity and quality of our manuscript.
Funding: This work was partially supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina (MUSC), through NIH - NCATS Grant Number UL1 TR001450, and in part by the Foundation for Physical Therapy Research through Promotion of Doctoral Studies (PODS I) Scholarship. Data for the study was provided through support for the CEDAR core funded by the MUSC Office of the Provost.
ABBREVIATIONS
- CPT
Current Procedural Terminology
- DME
Durable Medical Equipment
- ESI
Employer Sponsored Insurance
- HCPCS
Healthcare Common Procedure Coding System
- ICD-9
International Classification of Diseases, 9th Revision
- IMS
Inferred Mobility Status
- IMS-MWC
Inferred Mobility Status-Manual Wheelchair without Lower Extremity Orthotics
- IMS-MWC-LEO
Inferred Mobility Status-Manual Wheelchair with Lower Extremity Orthotics
- IMS-NAD
Inferred Mobility Status-No Assistive Devices or Orthotics
- IMS-PWC
Inferred Mobility Status-Power Wheelchair
- IMS-W
Inferred Mobility Status-Walker
- LE
Lower Extremity
- MDD
Major Depressive Disorder
- SCI
Spinal Cord Injury
Footnotes
Disclosure of Conflicts of Interest: The authors declare no conflicts of interest.
Catherine J. VanDerwerker: Received a Promotion of Doctoral Studies (PODS I) Scholarship from the Foundation for Physical Therapy Research.
Chris M. Gregory: Nothing to disclose.
Kit N. Simpson: Nothing to disclose.
Device Statement: The manuscript submitted does not contain information about medical device(s).
Clinical Trial Number: Not applicable
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Dataset: MarketScan® Commercial Claims and Encounters Database. Truven Health Analytics, Ann Arbor, MI. January 1, 2010 – December 31, 2013.
- 1.National Spinal Cord Injury Statistical Center. Spinal Cord Injury (SCI) Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2016. [Google Scholar]
- 2.Williams R, Murray A. Prevalence of depression after spinal cord injury: A meta-analysis. Arch Phys Med Rehabil. 2015;96(1):133–140. [DOI] [PubMed] [Google Scholar]
- 3.Elliott TR, Shewchuk RM. Social support and leisure activities following severe physical disability: Testing the mediating effects of depression. Basic Appl Soc Psych. 1995;16(4):471–487. [Google Scholar]
- 4.Tate D, Forchheimer M, Maynard F, Dijkers M. Predicting depression and psychological distress in persons with spinal cord injury based on indicators of handicap. Am J Phys Med Rehabil. 1994;73(3):175–183. [DOI] [PubMed] [Google Scholar]
- 5.Ullrich PM, Smith BM, Blow FC, Valenstein M, Weaver FM. Depression, healthcare utilization, and comorbid psychiatric disorders after spinal cord injury. J Spinal Cord Med. 2014;37(1):40–45.24090156 [Google Scholar]
- 6.Dryden DM, Saunders LD, Rowe BH, et al. Utilization of health services following spinal cord injury: A 6-year follow-up study. Spinal Cord. 2004;42(9):513–525. [DOI] [PubMed] [Google Scholar]
- 7.Krause JS, Carter RE, Pickelsimer EE, Wilson D. A prospective study of health and risk of mortality after spinal cord injury. Arch Phys Med Rehabil. 2008;89(8):1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weeks DL, Greer CL, Bray BS, Schwartz CR, White JR Jr. Association of antidepressant medication therapy with inpatient rehabilitation outcomes for stroke, traumatic brain injury, or traumatic spinal cord injury. Arch Phys Med Rehabil. 2011;92(5):683–695. [DOI] [PubMed] [Google Scholar]
- 9.Rabadi MH, Vincent AS. Do vascular risk factors contribute to the prevalence of pressure ulcer in veterans with spinal cord injury? J Spinal Cord Med. 2011;34(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Findley PA, Banerjea R, Sambamoorthi U. Excess mortality associated with mental illness and substance use disorders among veteran clinic users with spinal cord injury. Disabil Rehabil. 2011;33(17–18):1608–1615. [DOI] [PubMed] [Google Scholar]
- 11.Banerjea R, Findley PA, Smith B, Findley T, Sambamoorthi U. Co-occurring medical and mental illness and substance use disorders among veteran clinic users with spinal cord injury patients with complexities. Spinal Cord. 2009;47(11):789–795. [DOI] [PubMed] [Google Scholar]
- 12.Smith BM, Weaver FM, Ullrich PM. Prevalence of depression diagnoses and use of antidepressant medications by veterans with spinal cord injury. Am J Phys Med Rehabil. 2007;86(8):662–671. [DOI] [PubMed] [Google Scholar]
- 13.Dryden DM, Saunders LD, Rowe BH, et al. Depression following traumatic spinal cord injury. Neuroepidemiology. 2005;25(2):55–61. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy P, Lude P, Elfstrom ML, Smithson EF. Psychological contributions to functional independence: A longitudinal investigation of spinal cord injury rehabilitation. Arch Phys Med Rehabil. 2011;92(4):597–602. [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Sattar AB. Predictors of functional outcome in patients with traumatic spinal cord injury after inpatient rehabilitation: in Saudi Arabia. NeuroRehabilitation. 2014;35(2):341–347. [DOI] [PubMed] [Google Scholar]
- 16.Monin JK, Schulz R, Martire LM, Connelly D, Czaja SJ. The personal importance of being independent: associations with changes in disability and depressive symptoms. Rehabil Psych. 2014;59(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riggins MS, Kankipati P, Oyster ML, Cooper RA, Boninger ML. The relationship between quality of life and change in mobility 1 year postinjury in individuals with spinal cord injury. Arch Phys Med Rehabil. 2011;92(7):1027–1033. [DOI] [PubMed] [Google Scholar]
- 18.Arango-Lasprilla JC, Ketchum JM, Starkweather A, Nicholls E, Wilk AR. Factors predicting depression among persons with spinal cord injury 1 to 5 years post injury. NeuroRehabilitation. 2011;29(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Graham JE, Resnik L, et al. Examining the association between comorbidity indexes and functional status in hospitalized medicare fee-for-service beneficiaries. Phys Ther. 2016;96(2):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Graham JE, Resnik L, et al. Comparing comorbidity indices to predict post-acute rehabilitation outcomes in older adults. Am J Phys Med Rehabil. 2016;95(12):889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barell V, Aharonson-Daniel L, Fingerhut L, et al. An introduction to the Barell body region by nature of injury diagnosis matrix. Inj Prev. 2002;8(2):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SAS Institute Inc. SAS/STAT© User’s Guide. 8 ed. Cary, NC: SAS Institute Inc.; 1999. [Google Scholar]
- 23.Frades I, Matthiesen R. Chapter 5: Overview on Techniques in Cluster Analysis In: (eds) MR, ed. Bioinformatics Methods in Clinical Research. Methods in Molecular Biology. Vol 593 New York City, NY: Humana Press; 2010. [DOI] [PubMed] [Google Scholar]
- 24.Townsend L, Walkup JT, Crystal S, Olfson M. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:163–173. [DOI] [PubMed] [Google Scholar]
- 25.Fiest KM, Jette N, Quan H, et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Substance Abuse and Mental Health Services Administration and Policy in Mental Health. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H-53; Rockville, MD: 2018. [Google Scholar]
- 27.Kalpakjian CZ, Albright KJ. An examination of depression through the lens of spinal cord injury. Comparative prevalence rates and severity in women and men. Womens Health Issues. 2006;16(6):380–388. [DOI] [PubMed] [Google Scholar]
- 28.Bombardier CH, Fann JR, Tate DG, et al. An exploration of modifiable risk factors for depression after spinal cord injury: Which factors should we target? Arch Phys Med Rehabil. 2012;93(5):775–781. [DOI] [PubMed] [Google Scholar]
- 29.Kastenberg ZJ, Hurley MP, Weiser TG, et al. Adding insult to injury: Discontinuous insurance following spine trauma. J Bone Joint Surg Am. 2015;97(2):141–146. [DOI] [PubMed] [Google Scholar]
- 30.Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bombardier CH, Richards JS, Krause JS, Tulsky D, Tate DG. Symptoms of major depression in people with spinal cord injury: Implications for screening. Arch Phys Med Rehabil. 2004;85(11):1749–1756. [DOI] [PubMed] [Google Scholar]
- 32.Mehta S, Guy S, Lam T, Teasell R, Loh E. Antidepressants are effective in decreasing neuropathic pain after SCI: A meta-analysis. Top Spinal Cord Inj Rehabili. 2015;21(2):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang S, Jayadevappa R, Zee J, et al. Concordance between clinical diagnosis and medicare claims of depression among older primary care patients. Am J Geriatr Psychiatry. 2015;23(7):726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.