Abstract
Background:
In April 2015, the government of Georgia (country) initiated the world’s first national hepatitis C elimination program. An analysis of blood donor infectious screening data was conducted to inform a strategic plan to advance blood transfusion safety in Georgia.
Study design and methods:
Descriptive analysis of blood donation records (2015–2017) was performed to elucidate differences in demographics, donor type, remuneration status, and seroprevalence for infectious markers (hepatitis C virus antibody [anti-HCV], human immunodeficiency virus [HIV], hepatitis B virus surface antigen [HBsAg], and Treponema pallidum). For regression analysis, final models included all variables associated with the outcome in bivariate analysis (chi-square) with a p value of less than 0.05.
Results:
During 2015 to 2017, there were 251,428 donations in Georgia, representing 112,093 unique donors; 68.5% were from male donors, and 51.2% of donors were paid or replacement (friends or family of intended recipient). The overall seroprevalence significantly declined from 2015 to 2017 for anti-HCV (2.3%−1.4%), HBsAg (1.5%−1.1%), and T. pallidum (1.1%−0.7%) [p < 0.0001]; the decline was not significant for HIV (0.2%−0.1%). Only 41.0% of anti-HCV seropositive donors underwent additional testing to confirm viremia. Infectious marker seroprevalence varied by age, sex, and geography. In multivariable analysis, first-time and paid donor status were associated with seropositivity for all four infectious markers.
Conclusion:
A decline during the study period in infectious markers suggests improvement in blood safety in Georgia. Areas that need further improvement are donor recruitment, standardization of screening and diagnostic follow-up, quality assurance, and posttransfusion surveillance.
Keywords: Hepatitis C, blood transfusion, public health, Georgia (Country)
INTRODUCTION
Hepatitis C virus (HCV) is a virulent, bloodborne pathogen and a major cause of chronic hepatitis worldwide. In 2015, over 71 million people were infected with HCV, and nearly 400,000 deaths were ascribed to hepatitis C.1 Although infection may be subclinical, a high proportion (75%‐85%) of infected individuals will develop chronic hepatitis, of whom 10%‐20% will proceed to cirrhosis and/or hepatocellular carcinoma.2 The advent of combination treatment with ledipasvir (an inhibitor of nonstructural protein 5A thus affecting HCV replication) and sofosbuvir (a nucleotide polymerase inhibitor affecting RNA synthesis) has revolutionized treatment of HCV infection, attaining sustained virologic response ([SVR] undetectable HCV RNA ≥12 weeks following completion of treatment), representing virologic cure for the overwhelming majority (95%‐99%) of those who are treated.3–6
Hepatitis C is a major public health challenge in the country of Georgia. With dissolution of the Soviet Union, economic and social hardship followed,7 contributing to a rise in injection drug use (IDU), a major mode of HCV transmission.8 In 2015, a national serosurvey found that an estimated 5.4% of the adult population of Georgia (approximately 150,000) had chronic HCV infection, of whom nearly two‐thirds were unaware of their infection.9 While IDU has been shown to be the major mode of HCV transmission in Georgia,10, 11 blood transfusion is an independent risk factor for HCV infection.9, 12
Given the high prevalence of hepatitis C, the government of Georgia, in partnership with Gilead and with technical assistance provided by the US Centers for Disease Control and Prevention, initiated a national public health program to eliminate hepatitis C in Georgia by 2020.13 The program, launched in April 2015, combines hepatitis C screening and provision of antiviral treatment with a goal of identifying 90% of HCV‐infected individuals, treating 95% of those with chronic HCV infection, and curing 95% of those who undergo treatment. In addition to IDU, this comprehensive program has sought to address all modes of transmission, including unsafe medical or dental procedures as well as blood transfusion.14 The latter is a well‐established mode of transmission for HCV.9 While mandatory blood donor screening for hepatitis C virus antibody (anti‐HCV) has been in effect in Georgia since 1997, a 1998 analysis found the anti‐HCV seroprevalence in blood donors to be 6.9%, reflecting the high background prevalence of HCV infection coupled with deficient blood donor selection.15 In the same survey, the respective donor seroprevalences of hepatitis B virus surface antigen (HBsAg), human immunodeficiency virus (HIV), and Treponema pallidum were 3.4, 0.06 and 2.3%, suggesting additional transfusion safety risks.15 We sought to characterize the epidemiology of the major transfusion‐transmitted infections (TTIs) in Georgia as a general measure of national blood transfusion safety. The data are able to inform development of a strategic plan to improve blood safety while also benefiting the hepatitis C elimination program.
METHODS
Setting and overview of blood transfusion services
The country of Georgia, a former Soviet Bloc country, is situated in the Caucasus region of Eurasia.9 Following dissolution of the Soviet Union, blood collection facilities in Georgia were privatized. Donations from paid as well as replacement (friends or family of the intended transfusion recipient) donors are permissible in Georgia. Similar to donor screening practices in other countries, prospective blood donors are assessed before donation, using a donor history questionnaire, to determine their eligibility to donate. A major function of the questionnaire is to identify sociodemographic and medical risk factors for TTIs. While the use of a donor history questionnaire is mandated by ministerial decree in Georgia, there is some variation in the questionnaires that are used by the individual blood centers. If no high‐risk behaviors are elicited, then samples are collected from the donor for infectious screening and a blood product is collected. The blood product is maintained in quarantine until the results of the infectious screening results are known. Only blood products that are negative for all screened infectious agents are allowed to be transfused.
A State Safe Blood Program has been in operation in Georgia since 1997. The State Safe Blood Program strives to improve national standards of blood collection and transfusion services, so as to ensure a safe and affordable blood supply that is able to meet the countryʼs transfusion needs. The programʼs functions include reimbursement of blood centers for serology‐based blood donor screening (i.e., for anti‐HCV, HBsAg, HIV, and T. pallidum), external quality assurance (EQAS) of TTI testing, administration of a Unified Electronic Blood Donor Database, and expansion of efforts to increase voluntary nonremunerated donors (VNRBDs). In 2017, 20 blood establishments held state licenses for blood collection, 12 of which participated in the State Safe Blood Program; only two were not for‐profit organizations.14 Concerted efforts to reform the health care system in Georgia over the past decade include expanded support of vertical programs, such as the hepatitis C elimination program and the State Safe Blood Program.
Source of data and analysis
An analysis was conducted using Georgiaʼs Unified Electronic Blood Donor Database. In the database, unique donor identification numbers are assigned to donors and donations, providing access to donor demographics (age, sex, and geographic region of collection); date(s) of donations; mode of remuneration (i.e., VNRBD, replacement and paid); donor status (i.e., first time vs. repeat); and seroreactivity for anti‐HCV, HBsAg, HIV, and T. pallidum as reported by the collecting blood center. Information on the blood banks that participated in the State Safe Blood Program was also available for evaluation.
The analysis was confined to blood donation data from January 1, 2015, through December 31, 2017. The minimum age of eligibility for blood donation in Georgia is 18 years. Repeat donor data were included for each year; donors who screened positive for any of the four infectious markers (i.e., HIV, HBsAg, anti‐HCV, or T. pallidum) in any of their donations within the study period were reported as positive for that marker. When we analyzed overall findings for the combined 3 years, 2015 through 2017, repeat donor data were counted once and seroreactive results for any donation were prioritized for reporting. The overall 3‐year (2015‐2017) results that are shown represent cumulative infection prevalence rates over the 3‐year period. Thus, the “overall” data reported are not a simple summation of the individual years. If donors had multiple donations within the study period with different levels of remuneration, paid donor status was prioritized, followed by VNRBD and finally replacement. Final remuneration status was assigned accordingly. Individuals with more than one blood donation were classified as repeat donors. Age and region of donation were reported based on the donorʼs first/earliest donation in the study period.
For this analysis, paid donors refers to individuals who received monetary compensation for their donation. Replacement donors comprised friends or family of the intended transfusion recipient; replacement donors were either recruited to donate specifically for the index recipient or to donate with a view to restore the blood bank inventory following the transfusion of the intended recipient. By contrast, VNRBDs have no direct knowledge of transfusion recipients and receive no financial compensation for their donation.
Data management and statistical analysis
Descriptive analysis of donation records was performed to elucidate differences in demographics, donor type, remuneration status, and infectious marker prevalence. Variables with missing values for more than 10% of the sample are shown in the tables. Statistically significant associations in bivariate analysis were determined using chi‐square tests with a significance level of p less than 0.05. For regression analysis, final models included all variables available for bivariate analysis (age, sex, region of donation, donor type, and remuneration status), which were tested for goodness of fit and collinearity among predictors. For donors screening positive for anti‐HCV, analysis of their continuum of care, including treatment for hepatitis C, was obtained from Georgiaʼs national hepatitis C screening registry as well as treatment records from the countryʼs national hepatitis C elimination program, with results through December 31, 2018. Computer software (SAS version 9.4, SAS Institute) was used for all statistical analyses.
RESULTS
A total of 252,019 donations were recorded during the study period; 591 were excluded if the associated donorʼs age was either missing or listed as less than 18 years. The final result of 251,428 donations represents 112,093 unique adult donors, corresponding to an average of 83,809 collections per year (Table 1). Of those donors, 68.5% were male (n = 76,389), 44.7% (n = 50,098) were aged 18 to 29 years, and 51.2% were either paid (n = 30,806) or replacement (n = 26,570). Missing values were noted for sex (0.5%; n = 567) and remuneration (13.2%; n = 14,835). The majority were donated in Tbilisi (54.8%), followed by the regions of Imereti (15.1%) and Kvemo Kartli (11.5%). The overall donor prevalences for anti‐HCV, HBsAg, HIV, and T. pallidum were 2.4, 1.7, 0.2 and 1.1%, respectively.
Table 1.
Demographic characteristics of donor population and blood product collections in Georgia, 2015–2017
| Overall n (%)* | Blood Donation Year | |||
|---|---|---|---|---|
| 2015 | 2016 | 2017 | ||
| Donations | 251,428 | 79,191 | 84,503 | 87,734 |
| Unique Donors | 112,093 | 48,634 | 50,893 | 51,689 |
| Sex† | ||||
| Female | 35,137 (31.5) | 14,494 (29.9) | 15,956 (31.6) | 16,330 (31.6) |
| Male | 76,389 (68.5) | 33,931 (70.1) | 34,592 (68.4) | 35,277 (68.4) |
| Age (years) | ||||
| 18–29 | 50,098 (44.7) | 22,584 (46.4) | 22,668 (44.5) | 22,959 (44.4) |
| 30–39 | 30,492 (27.2) | 12,659 (26.0) | 13,651 (26.8) | 13,877 (26.8) |
| 40–49 | 19,794 (17.7) | 8,170 (16.8) | 9,015 (17.7) | 9,384 (18.2) |
| 50+ | 11,709 (10.4) | 5,221 (10.7) | 5,559 (10.9) | 5,469 (10.6) |
| Region of Donation | ||||
| Tbilisi | 61,457 (54.8) | 28,289 (58.2) | 28,196 (55.4) | 27,704 (53.6) |
| Adjara | 11,572 (10.3) | 3,075 (6.3) | 4,594 (9.0) | 5,575 (10.8) |
| Imereti | 16,935 (15.1) | 6,902 (14.2) | 7,240 (14.2) | 6,639 (12.8) |
| Kvemo Kartli | 12,919 (11.5) | 5,687 (11.7) | 6,581 (12.9) | 7,396 (14.3) |
| Samegrelo | 4,273 (3.8) | 1,655 (3.4) | 1,681 (3.3) | 1,585 (3.1) |
| Shida Kartli | 4,937 (4.4) | 3,026 (6.2) | 2,601 (5.1) | 2,790 (5.4) |
| Donor Type | ||||
| Repeat | 55,116 (49.2) | 30,267 (62.2) | 31,822 (62.5) | 32,071 (62.0) |
| First Time | 56,977 (50.8) | 18,367 (37.8) | 19,071 (37.5) | 19,618 (38.0) |
| Remuneration | ||||
| Volunteer | 39,882 (35.6) | 13,747 (28.3) | 17,703 (34.8) | 16,573 (32.1) |
| Paid | 30,806 (27.5) | 18,705 (38.5) | 18,640 (36.6) | 16,964 (32.8) |
| Replacement | 26,570 (23.7) | 9,088 (18.7) | 11,003 (21.6) | 9,896 (19.1) |
| Missing | 14,835 (13.2) | 7,094 (14.6) | 3,547 (7.0) | 8,256 (16) |
| HBsAg Results | ||||
| - | 110,165 (98.3) | 47,892 (98.5) | 50,280 (98.8) | 51,115 (98.9) |
| + | 1,928 (1.7) | 742 (1.5) | 613 (1.2) | 574 (1.1) |
| Anti-HCV Results | ||||
| - | 109,348 (97.6) | 47,495 (97.7) | 50,011 (98.3) | 50,962 (98.6) |
| + | 2,745 (2.4) | 1,139 (2.3) | 882 (1.7) | 727 (1.4) |
| HIV | ||||
| - | 111,862 (99.8) | 48,559 (99.8) | 50,807 (99.8) | 51,619 (99.9) |
| + | 231 (0.2) | 75 (0.2) | 86 (0.2) | 70 (0.1) |
| T.Pallidum | ||||
| - | 110,831 (98.9) | 48,122 (98.9) | 50,519 (99.3) | 51,313 (99.3) |
| + | 1,262 (1.1) | 512 (1.1) | 374 (0.7) | 376 (0.7) |
The overall 3 years, 2015‐2017, results shown represent cumulative infection prevalence rates over the 3 years period
Missing values not shown
anti‐HCV = hepatitis C virus antibody; HBsAg = hepatitis B surface antigen; HIV = human immunodeficiency virus.
HCV
For anti‐HCV, significant differences were seen by sex, age, and region in bivariate analysis (all p < 0.0001; Table 2). The highest rates were seen among male donors (2.8%), those aged 40 to 49 years (4.5%), and in the regions of Samegrelo (5.0%) and Shida Kartli (3.3%; Table 2). After adjusting for covariates, first‐time donors were more likely to be anti‐HCV positive than repeat donors (odds ratio [OR], 7.95; 95% confidence interval [CI], 7.12‐8.88), as were paid (OR, 3.59; 95% CI, 3.21‐4.02) and replacement donors (OR, 1.17; 95% CI, 1.02‐1.34) as compared to VNRBDs (Table 3). Male (as compared to female) donors (OR, 2.37; 95% CI, 2.15‐2.61) and age groups 30 years or older (as compared to those aged 18‐29 years) were also more likely to be anti‐HCV positive in the adjusted model. Anti‐HCV positivity prevalence declined in donors from 2.3% in 2015 to 1.4% in 2017, an overall decline of 39.9% (p < 0.0001; Table 1).
Table 2.
Bivariable analysis of infectious marker seropositivity by demographic characteristics of blood donors in Georgia, 2015–2017
| Infectious Markers n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBsAg + | HBsAg − | P-value | Anti-HCV + | Anti-HCV − | P-value | HIV + | HIV − | P-value | T.Pallidum + | T.Pallidum − | P-value | |
| Overall | 1,928 (1.7) | 110,165 (98.3) | 2,745 (2.4) | 109,348 (97.6) | 231 (0.2) | 111,862 (99.8) | 1,262 (1.1) | 110,831 (98.9) | ||||
| Sex | ||||||||||||
| Female | 423 (1.2) | 34,714 (98.8) | <.0001 | 596 (1.7) | 34,541 (98.3) | <.0001 | 50 (0.1) | 35,087 (99.9) | 0.002 | 406 (1.2) | 34,731 (98.8) | 0.56 |
| Male | 1,492 (2.0) | 74,897 (98.0) | 2,135 (2.8) | 74,254 (97.2) | 179 (0.2) | 76,210 (99.8) | 852 (1.1) | 75,537 (98.9) | ||||
| Age (years) | ||||||||||||
| 18–29 | 570 (1.1) | 49,528 (98.9) | <.0001 | 577 (1.2) | 49,521 (98.8) | <.0001 | 103 (0.2) | 49,995 (99.8) | 0.70 | 208 (0.4) | 49,890 (99.6) | <.0001 |
| 30–39 | 828 (2.7) | 29,664 (97.3) | 824 (2.7) | 29,668 (97.3) | 69 (0.2) | 30,423 (99.8) | 273 (0.9) | 30,219 (99.1) | ||||
| 40–49 | 386 (2.0) | 19,408 (98.0) | 890 (4.5) | 18,904 (95.5) | 35 (0.2) | 19,759 (99.8) | 450 (2.3) | 19,344 (97.7) | ||||
| 50+ | 144 (1.2) | 11,565 (98.8) | 454 (3.9) | 11,255 (96.1) | 24 (0.2) | 11,685 (99.8) | 331 (2.8) | 11,378 (97.2) | ||||
| Region of Donation | ||||||||||||
| Tbilisi | 784 (1.3) | 60,673 (98.7) | <.0001 | 1,323 (2.2) | 60,134 (97.8) | <.0001 | 103 (0.2) | 61,354 (99.8) | <.0001 | 491 (0.8) | 60,966 (99.2) | <.0001 |
| Adjara | 395 (3.4) | 11,177 (96.6) | 245 (2.1) | 11,327 (97.9) | 53 (0.5) | 11,519 (99.5) | 267 (2.3) | 11,305 (97.7) | ||||
| Imereti | 416 (2.5) | 16,519 (97.5) | 521 (3.1) | 16,414 (96.9) | 29 (0.2) | 16,906 (99.8) | 286 (1.7) | 16,649 (98.3) | ||||
| Kvemo Kartli | 159 (1.2) | 12,760 (98.8) | 280 (2.2) | 12,639 (97.8) | 20 (0.2) | 12,899 (99.8) | 77 (0.6) | 12,842 (99.4) | ||||
| Samegrelo | 117 (2.7) | 4,156 (97.3) | 213 (5.0) | 4,060 (95.0) | 17 (0.4) | 4,256 (99.6) | 74 (1.7) | 4,199 (98.3) | ||||
| Shida Kartli | 57 (1.2) | 4,880 (98.8) | 163 (3.3) | 4,774 (96.7) | 9 (0.2) | 4,928 (99.8) | 67 (1.4) | 4,870 (98.6) | ||||
| Donor Type | ||||||||||||
| Repeat | 243 (0.4) | 54,873 (99.6) | <.0001 | 462 (0.8) | 54,654 (99.2) | <.0001 | 87 (0.2) | 55,029 (99.8) | 0.0005 | 403 (0.7) | 54,713 (99.3) | <.0001 |
| First Time | 1,685 (3.0) | 55,292 (97.0) | 2,283 (4.0) | 54,694 (96.0) | 144 (0.3) | 56,833 (99.7) | 859 (1.5) | 56,118 (98.5) | ||||
| Remuneration | ||||||||||||
| Volunteer | 574 (1.4) | 39,308 (98.6) | <.0001 | 672 (1.7) | 39,210 (98.3) | <.0001 | 67 (0.2) | 39,815 (99.8) | 0.12 | 215 (0.5) | 39,667 (99.5) | <.0001 |
| Paid | 400 (1.3) | 30,406 (98.7) | 879 (2.9) | 29,927 (97.1) | 63 (0.2) | 30,743 (99.8) | 354 (1.1) | 30,452 (98.9) | ||||
| Replacement | 727 (2.7) | 25,843 (97.3) | 836 (3.1) | 25,734 (96.9) | 66 (0.2) | 26,504 (99.8) | 496 (1.9) | 26,074 (98.1) | ||||
| Missing | 227 (1.5) | 14,608 (98.5) | 358 (2.4) | 14,477 (97.6) | 35 (0.2) | 14,800 (99.8) | 197 (1.3) | 14,638 (98.7) | ||||
anti‐HCV = hepatitis C virus antibody; HBsAg = hepatitis B surface antigen; HIV = human immunodeficiency virus.
Table 3.
Multivariable analysis of risk factors for infectious marker seroreactivity in blood donors in Georgia, 2015–2017
| Infectious Markers | ||||||||
|---|---|---|---|---|---|---|---|---|
| HBsAg Positive | Anti-HCV Positive | HIV Positive | T. Pallidum Positive | |||||
| Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.64 (1.47, 1.82) | 1.67 (1.52, 1.83) | 1.65 (1.20, 2.56) | 0.97 (0.86, 1.09) | ||||
| Age (years) | ||||||||
| 18–29 | Ref | Ref | Ref | Ref | ||||
| 30–39 | 2.43 (2.18, 2.70) | 2.38 (2.14, 2.65) | 1.10 (0.81, 1.49) | 2.17 (1.81, 2.60) | ||||
| 40–49 | 1.73 (1.52, 1.97) | 4.04 (3.63, 4.49) | 0.86 (0.59, 1.26) | 5.58 (4.73, 6.58) | ||||
| 50+ | 1.08 (0.90, 1.30) | 3.46 (3.06, 3.92) | 1.00 (0.64, 1.56) | 6.98 (5.86, 8.31) | ||||
| Region of Donation | ||||||||
| Tbilisi | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Adjara | 2.74 (2.42, 3.09) | 2.37 (2.07, 2.71) | 0.98 (0.86, 1.13) | 0.90 (0.77, 1.04) | 2.74 (1.97, 3.82) | 2.89 (2.00, 4.17) | 2.93 (2.52, 3.41) | 2.60 (2.19, 3.07) |
| Imereti | 1.95 (1.73, 2.20) | 1.44 (1.23, 1.68) | 1.44 (1.30, 1.60) | 1.13 (0.99, 1.29) | 1.02 (0.68, 1.54) | 0.98 (0.60, 1.60) | 2.13 (1.84, 2.47) | 1.31 (1.09, 1.57) |
| Kvemo Kartli | 0.96 (0.81, 1.15) | 1.14 (0.96, 1.36) | 1.01 (0.88, 1.15) | 1.17 (1.03, 1.35) | 0.92 (0.57, 1.49) | 0.85 (0.52, 1.39) | 0.74 (0.59, 0.95) | 0.75 (0.58, 0.96) |
| Samegrelo | 2.18 (1.79, 2.65) | 1.52 (1.21, 1.90) | 2.39 (2.06, 2.77) | 1.84 (1.53, 2.21) | 2.38 (1.42, 3.98) | 2.39 (1.30, 4.38) | 2.19 (1.71, 2.80) | 1.27 (0.96, 1.68) |
| Shida Kartli | 0.90 (0.69, 1.19) | 1.08 (0.82, 1.42) | 1.55 (1.32, 1.83) | 1.32 (1.10, 1.57) | 1.09 (0.55, 2.15) | 0.93 (0.45, 1.94) | 1.71 (1.32, 2.21) | 1.09 (0.83, 1.42) |
| Donor Type | ||||||||
| Repeat | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| First Time | 6.88 (6.01, 7.88) | 7.67 (6.66, 8.84) | 4.94 (4.47, 5.46) | 7.95 (7.12, 8.88) | 1.60 (1.23, 2.09) | 1.66 (1.25, 2.21) | 2.08 (1.85, 2.34) | 2.43 (2.13, 2.78) |
| Remuneration | ||||||||
| Volunteer | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Paid | 0.90 (0.79, 1.02) | 2.00 (1.74, 2.30) | 1.71 (1.55, 1.90) | 3.59 (3.21, 4.02) | 1.22 (0.86, 1.72) | 1.90 (1.29, 2.79) | 2.14 (1.81, 2.54) | 3.86 (3.20, 4.67) |
| Replacement | 1.93 (1.73, 2.15) | 1.25 (1.08, 1.44) | 1.90 (1.71, 2.10) | 1.17 (1.02, 1.34) | 1.48 (1.05, 2.08) | 1.19 (0.78, 1.81) | 3.51 (3.00, 4.12) | 2.39 (1.97, 2.89) |
Final models included age, sex, region of donation, donor type, and remuneration status.
HBsAg = Hepatitis B surface antigen; Anti‐HCV = hepatitis C virus antibody; HIV = human immunodeficiency virus.
Overall, 2.4% (n = 2745) of adult donors tested anti‐HCV positive over the 3‐year period. Of those, 41.0% (n = 1126) had an HCV nucleic acid amplification test (NAT) or HCV core antigen test to determine viremia (Fig. 1). Of those who underwent viremia testing, 78.6% (n = 885) had evidence of active infection, and 83.3% (n = 737) of those completed the additional diagnostic workup and evaluation necessary for enrollment in the national hepatitis C elimination program. After enrollment, 98.1% (n = 723) initiated treatment and 94.5% (n = 683) of those completed their treatment regimen. SVR, indicative of a cure, was ultimately achieved in 98.2% of those who were tested for SVR (n = 494/503).
Figure 1.
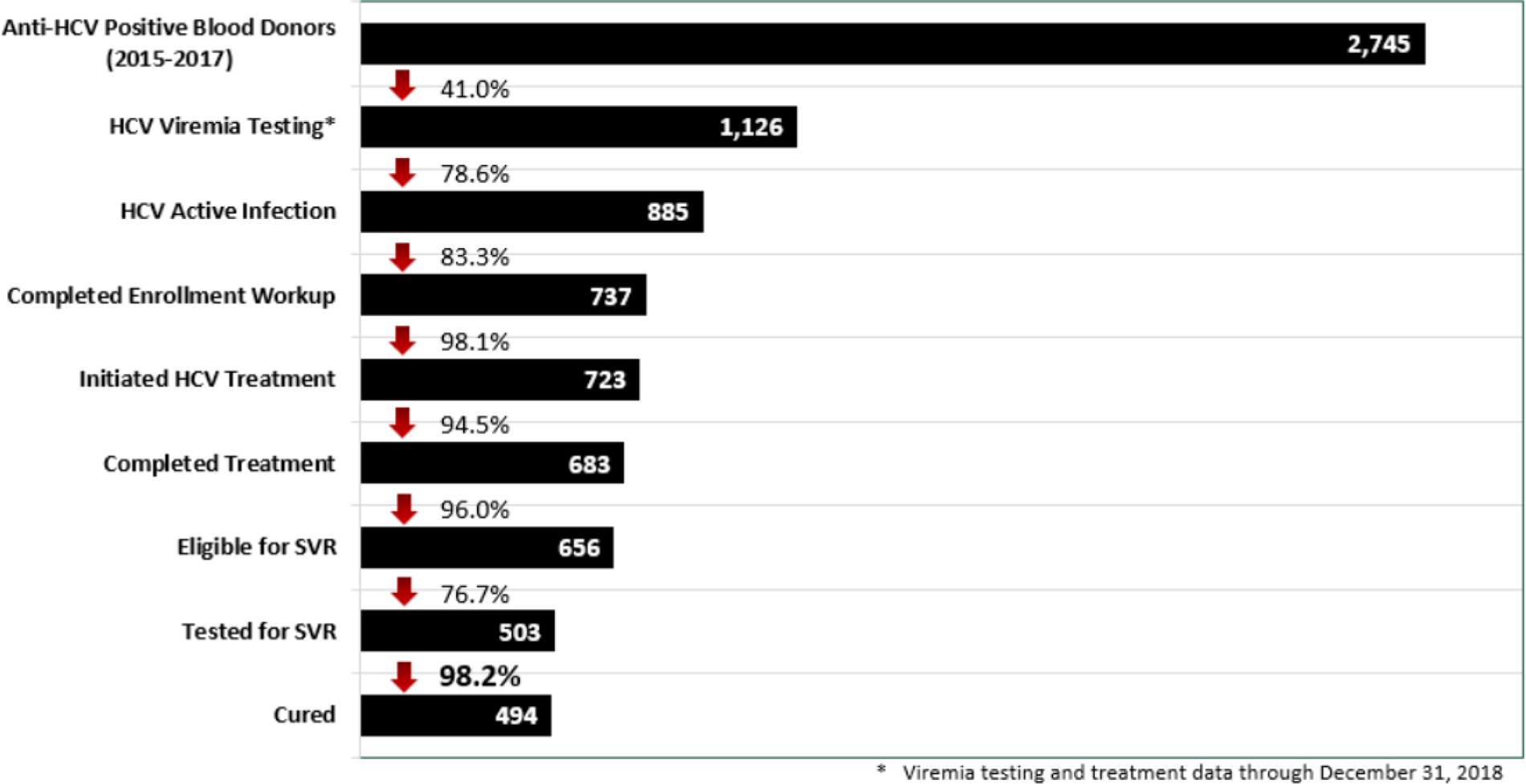
Hepatitis C care cascade among anti-HCV positive blood donors in Georgia
HBV
In bivariate analysis, HBsAg positivity prevalence differed by sex, age, and region of donation (all p < 0.0001), and was highest in males (2.0%), donors aged 30 to 39 (2.7%), and in the regions of Adjara (3.4%), Samegrelo (2.7%), and Imereti (2.5%; Table 2). After adjusting for covariates, first‐time donors (OR, 7.67; 95% CI, 6.66‐8.84) were more likely to be HBsAg positive as compared to repeat donors, as were paid (OR, 2.00; 95% CI, 1.74‐2.30) and replacement (OR, 1.25; 95% CI, 1.08‐1.44) donors as compared to volunteers, males as compared to females (OR, 1.79; 95% CI, 1.60‐2.00) and age groups 30 years or older as compared to those aged 18‐29 years (Table 3). Donor hepatitis B prevalence declined by 27.2% from 1.5% in 2015 to 1.1% in 2017 (p < 0.0001; Table 1).
HIV
HIV prevalence differed by sex (p = 0.002) and region (p < 0.0001), with the highest rates among males (0.2%) and donors in the regions of Adjara (0.5%) and Samegrelo (0.4%; Table 2). Rates were similar among all age groups at 0.2%. In multivariable analysis, first‐time versus repeat (OR, 1.66; 95% CI, 1.25‐2.21), paid versus volunteer (OR, 1.90; 95% CI, 1.29‐2.79) and male as compared to female (OR 2.37; 95% CI, 2.15‐2.61) donors were more likely to be HIV positive (Table 3). A significant decline over time was not observed.
T. pallidum
Prevalence of T. pallidum increased with age from 0.4% among 18‐ to 29‐year‐old donors to 2.8% among those aged 50 years or older (p < 0.0001). Region of collection was significant in bivariate analysis (p < 0.0001); prevalence was highest in Adjara (2.3%), Imereti (1.7%), and Samegrelo (1.7%; Table 2). Prevalence did not differ significantly by sex in bivariate analysis. In multivariable analysis, first‐time donors were more likely than repeat donors (OR, 2.08; 95% CI, 1.85‐2.34) to be positive as were paid (OR, 3.86; 95% CI, 3.20‐4.67) and replacement (OR, 2.39; 95% CI, 1.97‐2.89) donors as compared to VNRBDs (Table 3). Also, males versus females (OR, 1.34; 95% CI, 1.19‐1.52), and age groups 30 years or older versus those aged 18 to 29 years were more likely to be T. pallidum positive. T. pallidum rates declined in donors by 30.9%, from 1.1% in 2015 to 0.7% in 2017 (p < 0.0001; Table 1).
Coinfections
Over the 3 years of analysis, 5933 (5.3%) of 112,093 blood donors tested positive for at least one infectious marker. Of those, 223 (3.8%) were coinfected with two markers; the most common coinfection was hepatitis B virus/HCV among 90 donors, followed by 79 HCV/T.Pallidum coinfected, and 35 with HBV/T. pallidum coinfection. Five donors tested positive for three infectious markers.
DISCUSSION
The findings indicate ongoing challenges surrounding blood transfusion safety in Georgia. Over a 3‐year period, there was a high proportion of male, first‐time, and paid or replacement blood donors, characteristics that were significantly associated with TTI seropositivity. Nonetheless, a significant decline in anti‐HCV, HBsAg, and T. pallidum, with rates that were lower than those of the general population, suggest improved donor selection. Blood transfusion was identified as a risk factor for anti‐HCV positivity in a 2015 serosurvey,9 prompting its inclusion as a key strategy in the national hepatitis C elimination program. Therefore, resources have been directed to improve blood safety, which is an area of need that might not otherwise have received the same attention. Further, blood donors found to be anti‐HCV positive were referred to the hepatitis C treatment program for confirmatory testing. Pairing hepatitis C elimination with a blood transfusion safety initiative has been mutually beneficial.
Despite their public health role, blood centers deliberately separate themselves from provision of care given the potential incentive for test‐seeking behavior, which confers risk of TTIs. The findings in Georgia challenge this dogma, as evidenced by a significant decline in anti‐HCV, HBsAg, and T. pallidum positivity in blood donors, while still advancing the national hepatitis C elimination program. Georgia has also had a long‐standing state‐sponsored HIV program, which predates the national hepatitis C elimination program, whereby blood donors who screen positive are referred for confirmatory testing and treatment (free of charge) if positive. Donor HIV seroprevalence remains low in Georgia, suggesting that absolute separation of blood collection from public health screening may not be necessary.
Donor recruitment and predonation evaluation (i.e., use of the donor history questionnaire) play an important role in the prevention of TTIs. Specifically, risk‐based deferral reduces reliance on laboratory‐based screening.16 Pertinent to our study, both first‐time and paid blood donors are considered higher risk for TTIs than VNRBDs.17–20 By contrast, repeat donation selects for individuals of lower infectious risk, given that those who screened positive during an initial donation would have been permanently deferred from blood donation.21 In Georgia, the odds of anti‐HCV seroreactivity were almost eightfold higher in first‐time as compared to repeat donors. Given that over half of donors in Georgia are first‐time donors, there is a need to bolster recruitment, with renewed focus on transitioning first‐time donors to a stable pool of repeat donors.
Paid donation is actively discouraged in most high‐income countries,22–24 given that remuneration serves as a disincentive to admit any high‐risk behavior during predonation screening. Consequently, the World Health Organization (WHO) advocates exclusively for VNRBDs.25 Early evidence of risk includes a 1962 study that observed the incidence of posttransfusion viral hepatitis to be fourfold higher in recipients of blood from paid donors (i.e., as compared to those who received blood from VNRBDs).24 Paid donation still remains common in former Soviet Union countries, where its risk has not been well characterized. Available data, including those from our study, corroborate the high risk;19 paid donation in Georgia was associated with increased odds (e.g., over 3.5‐fold for anti‐HCV) of infectious marker seropositivity. Ultimately, there is a need to convert the donor pool to a volunteer base. Such a complex undertaking requires an infrastructure to recruit donors, educate the general population and ultimately change human behavior.
Recent examples of countries that have transitioned to voluntary blood donor bases are few. China is one example that transitioned from paid to nonremunerated (albeit compulsory), and subsequently VNRBDs, under a broad blood safety initiative.26 The latter included legislative changes coupled with a massive investment in infrastructure with reorganization and centralization of transfusion services, expanded quality management, and adoption of NAT. The transition in China took almost 15 years (1998‐2012), which may be ascribed to the absence of voluntary donors at the start of the blood safety initiative.
There are other factors besides donor selection that are likely contributing to TTI risk in Georgia. Foremost is nearly exclusive reliance on antibody‐based methods for donor screening. Incidence data are lacking for the major TTIs, but the high prevalence in the general population, particularly given suboptimal donor selection, raises concern of preseroconversion infections that are being missed.20 HCV, in particular, has a long preseroconversion window period (approx. 70 days), which can otherwise be minimized (approx. 10 days) with donor HCV NAT.27, 28 Adoption of HCV core antigen testing and ultimately HCV NAT screening of blood donations would reduce the window period, thus minimizing the risk of transfusion‐transmitted HCV.20 It would also confer other benefits to transfusion safety through addition of infrastructure and quality oversight. In the case of hepatitis B, NAT has the added benefit of being able to capture occult HBV infections (i.e., DNA+/HBsAg−), which otherwise go undetected in the absence of HBV core antibody testing. A counterpoint is that NAT is high cost and NAT yield (DNA/RNA+/Ab or Ag−) rates are highly variable.27, 29–32 Assessment of the incremental benefit (i.e., above extant serological testing) is needed. In the case of Georgia, classic incidence modeling33, 34 is an unlikely substitute for rigorous laboratory surveillance given incomplete data on key input variables for a determination of transmission risk.
The hepatitis C elimination program in Georgia has benefited blood transfusion safety. Similarly, blood donor screening has identified seroreactive individuals, enabling those donors to enter the cascade of care with confirmatory testing and treatment (when indicated), thus benefiting the elimination program directly. Nevertheless, only 41% of anti‐HCV seropositive blood donors underwent further testing for viremia, indicating a need to improve linkage to care. While linkage to care is a challenge of the elimination program in general, the rates of donors who underwent follow‐up testing after being identified through donor screening was below those in the general population (71.5%).35 Given that in Georgia, SVR has been achieved in 98.2% of those who completed a standard hepatitis C antiviral regimen,35 this merits investigation given the scope for improvement. While beyond the analysis, routine HCV confirmatory testing to ascertain the presence of viremia was initiated in 2018 for blood donors who are found to be HCV seroreactive during screening. This further highlights the reciprocal benefits of the hepatitis C elimination program.
The study had limitations. Foremost was the absence of confirmatory testing coupled with a lack of consistency in testing algorithms used (i.e., whether reactive samples underwent repeat and necessary additional diagnostic testing), heterogeneity in the assays (i.e., manufacturers of the screening kits) and variability in their level of automation (i.e., spanning rapid testing to fully automated platforms). This lack of standardization impeded interpretation of some of the results. For instance, anti‐HCV positivity alone is not evidence of active infection; approximately 15% to 25% of infected individuals will clear the virus spontaneously.2 For another, T. pallidum results were reported qualitatively without knowledge of whether treponemal‐specific versus nontreponemal tests were used.36 Nontreponemal tests have a risk of false positivity. While this detracts from the T. pallidum findings, we still believe that its presentation is important and supports the hypothesis that HCV elimination had broader benefits beyond HCV testing alone. Although not included in this analysis, there has been significant work in EQAS of donor screening. Such has served to document the variability in performance coupled with the diversity of testing platforms and methods in use for each TTI marker. As one example, of 12 laboratories that were evaluated during a round of EQAS, six assays were in use for anti‐HCV alone. Second, nonuniform capture of data pertaining to remuneration (missing in 13% of donors) could impact the findings. Similarly, risk factors for infection, such as IDU or history of blood transfusion, were not available for analysis, which could bias results. Third, there is uncertainty surrounding the extent to which the seroprevalence findings are generalizable to the nondonor population. While blood donors offer a convenient population for infectious disease surveillance, blood donation selects for a healthier subset of the population.37 Indeed, similar prevalence findings in a given donor and general population would suggest deficient selection and predonation screening. Fourth, differences concerning the predonation questionnaire could have introduced variations in seroprevalence by both blood center and the period of blood collection. Further, the absence of a postdonation questionnaire precluded identification of risks factors for TTIs, which could have helped to modify the predonation selection process. Finally, one cannot claim direct causal effect: The decline in donor HCV seroprevalence could reflect a general decline in prevalence stemming from the broader HCV elimination program.
In conclusion, the study highlights collateral benefit of a national hepatitis C elimination program on blood safety in Georgia. Investment in blood safety has afforded dual benefit, serving to contend with risks associated with blood transfusion, a highly efficient mode of transmission for HCV, while aligning with the national program goals to enroll people with hepatitis C into treatment. Ongoing challenges in Georgia span donor recruitment, testing, quality assurance, and posttransfusion surveillance. Finally, the findings further show replacement and paid donation to be relatively unsafe with respect to infectious risk. Acknowledging the challenges surrounding donor mobilization in low‐ and low‐middle‐income countries to meet transfusion demand, the findings support a long‐standing position by the WHO that favors blood collection from volunteer donors.25
ACKNOWLEDGMENTS
The authors are grateful to all the clinicians and laboratory staff who are participating in the Georgia HCV Elimination Program, especially Tengiz Tsertsvadze (Infectious Diseases, AIDS, and Clinical Immunology Research Center, Tbilisi, Georgia), Maia Butsashvili (Neolab, Tbilisi, Georgia), David Metreveli, (Medical Center Mrcheveli, Tbilisi, Georgia) and Lali Sharvadze (Joint Georgian‐French Hepatology Clinic Hepa, Tbilisi, Georgia). Further, we wish to acknowledge all partners on the HCV Elimination Program: Georgian Harm Reduction Network, Tbilisi, Georgia; World Health Organization, Geneva, Switzerland; Extension for Community Healthcare Outcomes (ECHO), University of New Mexico, New Mexico, USA; Liver Institute and Foundation for Education and Research (LIFER), Boston, Massachusetts, USA; Foundation for Innovative Diagnostics (FIND), Geneva, Switzerland; Médecins du Monde (MDM), Paris, France; Abbott Laboratories, Chicago, Illinois, USA; Bristol University, Bristol, UK; Georgia State University, Atlanta, Georgia, USA; The Global Fund to Fight AIDS, Tuberculosis and Malaria, Geneva, Switzerland; Vitalant Research Institute, San Francisco, California, USA; Johns Hopkins University, Maryland, USA; and Gilead Sciences, Foster City, California, USA.
ABBREVIATIONS:
- anti-HCV
hepatitis C virus antibody
- EQAS
external quality assurance
- HBsAg
hepatitis B virus surface antigen
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
injection drug use
- NAT
nucleic acid amplification test
- SVR
sustained virologic response
- TTIs
transfusion-transmitted infections
- VNRBDs
voluntary nonremunerated donors
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and not necessarily the official position of the US Centers for Disease Control and Prevention. Dr. Bloch is a member of the United States Food and Drug Administration (FDA) Blood Products Advisory Committee. Any views or opinions that are expressed in this manuscript are that of the author’s, based on his own scientific expertise and professional judgement; they do not necessarily represent the views of either the Blood Products Advisory Committee or the formal position of FDA, and also do not bind or otherwise obligate or commit either Advisory Committee or the Agency to the views expressed.
CONFLICT OF INTEREST
EK, SS, MA, LG, TK, NC, SMK, AG, AT, VG, MN, FA, MI, and BS have disclosed no conflicts of interest. EMB is an investigator on trials to evaluate pathogen reduction technology funded by the US government. EMB has received education speaker fees for Grifols Diagnostics Solutions.
References
- 1.WHO. Hepatitis C: key facts [monograph on the Internet]. 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
- 2.Liang TJ, Rehermann B, Seeff LB, et al. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000; 132: 296‐ 305. [DOI] [PubMed] [Google Scholar]
- 3.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370: 1879‐ 88. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889‐ 98. [DOI] [PubMed] [Google Scholar]
- 5.Lee YA, Friedman SL. Reversal, maintenance or progression: what happens to the liver after a virologic cure of hepatitis C? Antiviral Res 2014; 107: 23‐ 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011; 52: 889‐ 900. [DOI] [PubMed] [Google Scholar]
- 7.Skarbinski J, Walker HK, Baker LC, et al. The burden of out‐of‐pocket payments for health care in Tbilisi, Republic of Georgia. JAMA 2002; 287: 1043‐ 9. [DOI] [PubMed] [Google Scholar]
- 8.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345: 41‐ 52. [DOI] [PubMed] [Google Scholar]
- 9.Nasrullah M, Sergeenko D, Gvinjilia L, et al. The Role of screening and treatment in national progress toward hepatitis C elimination ‐ Georgia, 2015‐2016. MMWR Morb Mortal Wkly Rep 2017; 66: 773‐ 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stvilia K, Tsertsvadze T, Sharvadze L, et al. Prevalence of hepatitis C, HIV, and risk behaviors for blood‐borne infections: a population‐based survey of the adult population of Tʼbilisi, Republic of Georgia. J Urban Health 2006; 83: 289‐ 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stvilia K, Spradling PR, Asatiani A, et al. Progress in testing for and treatment of hepatitis C virus infection among persons who inject drugs ‐ Georgia, 2018. MMWR Morb Mortal Wkly Rep 2019; 68: 637‐ 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagan LM, Kasradze A, Salyer SJ, et al. Hepatitis C prevalence and risk factors in Georgia, 2015: setting a baseline for elimination. BMC Public Health 2019; 19: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitruka K, Tsertsvadze T, Butsashvili M, et al. Launch of a nationwide hepatitis C elimination program–Georgia, April 2015. MMWR Morb Mortal Wkly Rep 2015; 64: 753‐ 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Labour HaSA, National Center for Disease Control and Public Health (NCDC); US Centers for Disease Control and Prevention. National Hepatitis C Elimination Report: Georgia, 2015‐2017. U.S.A: Ministry of Labour HaSA; 2017. [Google Scholar]
- 15.Butsashvili M, Tsertsvadze T, McNutt LA, et al. Prevalence of hepatitis B, hepatitis C, syphilis and HIV in Georgian blood donors. Eur J Epidemiol 2001; 17: 693‐ 5. [DOI] [PubMed] [Google Scholar]
- 16.Fridey JL, Townsend MJ, Kessler DA, et al. A question of clarity: redesigning the American Association Of Blood Banks ablood donor history questionnaire–a chronology and model for donor screening. Transfus Med Rev 2007; 21: 181‐ 204. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen M, Lelie N, Sykes W, et al. Impact of individual‐donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion 2009; 49: 1115‐ 25. [DOI] [PubMed] [Google Scholar]
- 18.Walsh JH, Purcell RH, Morrow AG, et al. Posttransfusion hepatitis after open‐heart operations. Incidence after the administration of blood from commercial and volunteer donor populations. JAMA 1970; 211: 261‐ 5. [DOI] [PubMed] [Google Scholar]
- 19.Kalibatas V Payment for whole blood donations in Lithuania: the risk for infectious disease markers. Vox Sang 2008; 94: 209‐ 15. [DOI] [PubMed] [Google Scholar]
- 20.Busch MP, Bloch EM, Kleinman S. Prevention of transfusion‐transmitted infections. Blood 2019; 133: 1854‐ 64. [DOI] [PubMed] [Google Scholar]
- 21.Bloch EM, Vermeulen M, Murphy E. Blood transfusion safety in Africa: a literature review of infectious disease and organizational challenges. Transfus Med Rev 2012; 26: 164‐ 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunin CM. Serum hepatitis from whole blood: incidence and relation to source of blood. Am J Med Sci 1959; 237: 293‐ 303. [DOI] [PubMed] [Google Scholar]
- 23.Grady GF, Bennett AJ. Risk of posttransfusion hepatitis in the United States. A prospective cooperative study. JAMA 1972; 220: 692‐ 701. [DOI] [PubMed] [Google Scholar]
- 24.Grady GF, Chalmers TC. Risk of post‐transfusion viral hepatitis. N Engl J Med 1964; 271: 337‐ 42. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Towards 100% voluntary blood donation: a global framework for action [monograph on the Internet]. Switzerland: WHO Press; 2010. Available from: https://www.who.int/bloodsafety/publications/9789241599696/en/ [Google Scholar]
- 26.Wang Y, Wu Y, Chen Y, et al. The journey toward safer and optimized blood service in China: national strategy and progress. Transfusion 2016; 56: 3112‐ 20. [DOI] [PubMed] [Google Scholar]
- 27.Dodd RY, Notari EP, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window‐period risk in the American Red Cross blood donor population. Transfusion 2002; 42: 975‐ 9. [DOI] [PubMed] [Google Scholar]
- 28.Tsertsvadze T, Sharvadze L, Chkhartishvili N, et al. The natural history of recent hepatitis C virus infection among blood donors and injection drug users in the country of Georgia. Virol J 2016; 13: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brojer E, Grabarczyk P, Liszewski G, et al. Characterization of HBV DNA+/HBsAg‐ blood donors in Poland identified by triplex NAT. Hepatology 2006; 44: 1666‐ 74. [DOI] [PubMed] [Google Scholar]
- 30.Manzini P, Girotto M, Borsotti R, et al. Italian blood donors with anti‐HBc and occult hepatitis B virus infection. Haematologica 2007; 92: 1664‐ 70. [DOI] [PubMed] [Google Scholar]
- 31.Velati C, Romano L, Fomiatti L, et al. Impact of nucleic acid testing for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus on the safety of blood supply in Italy: a 6‐year survey. Transfusion 2008; 48: 2205‐ 13. [DOI] [PubMed] [Google Scholar]
- 32.Crowder LA, Steele WR, Notari EP, et al. Prevalence, incidence, and risk factors of human immunodeficiency virus infection in blood donors in the Southeastern United States. Transfusion 2017; 57: 404‐ 11. [DOI] [PubMed] [Google Scholar]
- 33.Weusten JJ, van Drimmelen HA, Lelie PN. Mathematic modeling of the risk of HBV, HCV, and HIV transmission by window‐phase donations not detected by NAT. Transfusion 2002; 42: 537‐ 48. [DOI] [PubMed] [Google Scholar]
- 34.Kleinman S, Busch MP, Korelitz JJ, et al. The incidence/window period model and its use to assess the risk of transfusion‐transmitted human immunodeficiency virus and hepatitis C virus infection. Transfus Med Rev 1997; 11: 155‐ 72. [DOI] [PubMed] [Google Scholar]
- 35.Tsertsvadze T, Gamkrelidze A, Chkhartishvili N, et al. Three years of progress towards achieving hepatitis C elimination in the country of Georgia, April 2015–March 2018. Clin Infect Dis 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev 1995; 8: 1‐ 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golding J, Northstone K, Miller LL, et al. Differences between blood donors and a population sample: implications for case‐control studies. Int J Epidemiol 2013; 42: 1145‐ 56. [DOI] [PMC free article] [PubMed] [Google Scholar]


