Abstract
Electroconductive hydrogels (ECHs) are highly hydrated 3D networks generated through the incorporation of conductive polymers, nanoparticles, and other conductive materials into polymeric hydrogels. ECHs combine several advantageous properties of inherently conductive materials with the highly tunable physical and biochemical properties of hydrogels. Recently, the development of biocompatible ECHs has been investigated for various biomedical applications, such as tissue engineering, drug delivery, biosensors, flexible electronics, and other implantable medical devices. Several methods for the synthesis of ECHs have been reported, which include the incorporation of electrically conductive materials such as gold and silver nanoparticles, graphene, and carbon nanotubes, as well as various conductive polymers (CPs), such as polyaniline, polypyrrole, and poly(3,4-ethylenedioxyythiophene) into hydrogel networks. Theses electroconductive composite hydrogels can be used as scaffolds with high swellability, tunable mechanical properties, and the capability to support cell growth both in vitro and in vivo. Furthermore, recent advancements in microfabrication techniques such as three dimensional (3D) bioprinting, micropatterning, and electrospinning have led to the development of ECHs with biomimetic microarchitectures that reproduce the characteristics of the native extracellular matrix (ECM). In addition, smart ECHs with controlled structures and healing properties have also been engineered into devices with prolonged half-lives and increased durability. The combination of sophisticated synthesis chemistries and modern microfabrication techniques have led to engineer smart ECHs with advanced architectures, geometries, and functionalities that are being increasingly used in drug delivery systems, biosensors, tissue engineering, and soft electronics. In this review, we will summarize different strategies to synthesize conductive biomaterials. We will also discuss the advanced microfabrication techniques used to fabricate ECHs with complex 3D architectures, as well as various biomedical applications of microfabricated ECHs.
Keywords: Electroconductive Hydrogel, Conductivity, Biomedical Application, Tissue Engineering, Biosensors, Drug Delivery
Graphical abstract
1. Introduction
Hydrogels are three dimensional (3D) networks of hydrophilic polymeric networks that can be formed through different mechanisms such as physical entanglement, electrostatic interactions, or covalent chemical crosslinking [1]. Hydrogels are remarkably suitable for a wide range of applications such as drug delivery, tissue engineering, and soft electronics for biomedical devices, due to their high hydration, tunable physical properties, and porous architecture [2, 3]. These characteristics also enable the diffusion of biomolecules, oxygen, and metabolic waste across the 3D structure of hydrogels, which is an important trait for substrates used in the physiological context [4, 5]. Hydrogels can also be tuned to mimic biochemical, mechanical, and topographical cues from the native extracellular matrix (ECM), in order to modulate physiological responses in cells and tissues [6]. Therefore, several naturally-derived and synthetic-based polymers, as well as various fabrication methods have been reported for the design and manufacture of hydrogels with different physicochemical properties [7, 8]. These polymers may be used individually or in combination with other polymers to yield composite hydrogels with increased functionality. Moreover, hydrogels may be further modified through the incorporation of chemical or biological active moieties such as growth factors, cell binding and protease-sensitive sites, or other stimuli-responsive molecules to promote their functions [9]. Although hydrogels have been demonstrated to be highly versatile platforms for different biomedical applications, their insulating nature often limits their potential for the modulation of electrically-sensitive cells and tissues such as cardiac and neural tissues [10].
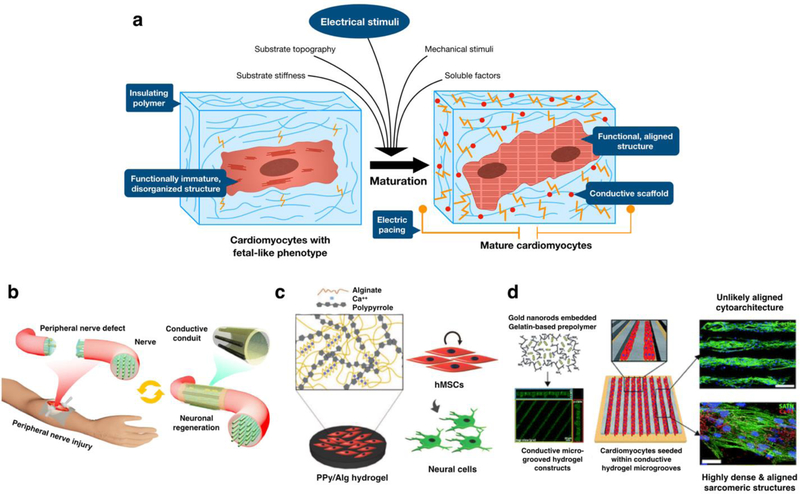
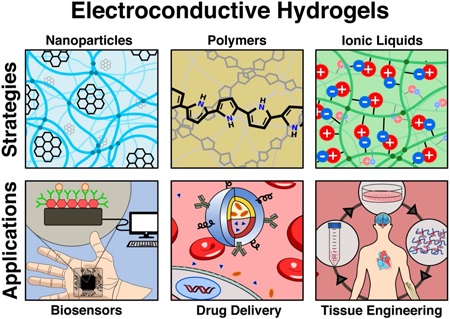
In recent years, the development of advanced biomaterials and chemistries combined with micro- and nanotechnologies have improved the ability to control the properties and functionality of hydrogels for a wide range of applications (Figure 1) [6]. For instance, the incorporation of inherently conductive materials to hydrogels via blending, doping or chemical modification have led to the development of new class of electroconductive hydrogels (ECHs) [11]. ECHs are composite biomaterials that combine the electroconductive capabilities of different materials with the intrinsic properties of crosslinked hydrogel networks [12]. Several strategies for the synthesis of ECHs have been reported such as the incorporation of conductive polymers (CPs) (e.g. polyaniline (PANi), polypyrrole (PPy), polythiophene (PTh), and poly(3,4-ethylenedioxythiophene) (PEDOT)) within a hydrogel network [12–16]. The organic nature of CPs greatly facilitates their chemical modification to incorporate different bioactive functional motifs into ECHs and provide them with high conductivity and processability [11]. ECHs can also be engineered by the in situ reduction of metal ions within the polymer network to form metallic nanoparticles (NPs) [17]. In this regard, different types of NPs have been used for the engineering of nanocomposite ECHs with tunable electrical, mechanical and optical properties [18]. For example, the incorporation of one dimensional carbon nanotubes (CNTs) and two dimensional (2D) graphene has been shown to impart high electrical conductivity and increased mechanical strength to hydrogels [19]. In addition, our group has recently demonstrated the engineering of ECHs with intrinsic electrical conductivity through the functionalization of different hydrogels with a choline-based bio-ionic liquid (Bio-IL) [20]. This diverse range of synthesis methodologies has led to the development of ECHs with distinct physical and biochemical properties, which offer unique advantages for different biomedical applications, such as tissue engineering, drug delivery, and engineering biosensors and medical devices [13]. Strategies for designing these therapeutic and diagnostic ECH-based technologies often includes electrical characteristics biomimetic to that of the native tissue (Table 1).
Figure 1. Synthesis and applications of ECHs.
ECHs can be formed by using different conductive materials including conductive nanoparticles, conductive polymers, or ionic liquids. Engineered ECHs have been utilized for different biomedical applications, including biosensors, drug delivery, and tissue engineering.
Table 1:
ECHs can be tailored to mimic the electrical properties of native tissues when used for cardiac and neural tissue engineering.
Previous studies have demonstrated the ability of ECHs to mediate the adhesion, proliferation, migration, and differentiation of different cell types including cardiomyocytes (CMs), neurons, fibroblasts, endothelial cells, human mesenchymal stem cells (hMSCs), and preosteoblasts [21]. In addition, recent advances in hydrogel synthesis and fabrication techniques have led to the engineering of multifunctional ECHs that are able to sense and respond to different physicochemical stimuli [11]. This new class of smart ECHs have been increasingly used for a variety of applications, ranging from stimulating and recording electrodes, tissue engineered constructs, and electrically controlled drug release devices and biosensors [13]. Furthermore, the development of advanced microengineering techniques have allowed the accurate recapitulation of the complex microarchitectural features of physiological tissues [22]. In this regard, different patterning and templating approaches have been used to fabricate micro-scale structures of ECHs using a broad range of biocompatible and biodegradable materials [23]. With the advent of microengineering techniques such as 3D printing, electrospinning, and other lithography-based approaches, it is possible to exert precise control over the composition, geometry, and spatial arrangement of cells and biomolecules within ECHs. This unprecedented degree of customization holds remarkable potential for the engineering of smart interfaces and biomimetic scaffolds for fundamental research and clinical applications.
While previous review papers have mainly focused on either natural [12] or synthetic [13, 27, 28] systems to form ECHs, our review is inclusive to all major biomaterials used for the synthesis of ECHs. Here, we describe the most significant conductive materials incorporated into hydrogels to impart electroconductivity. Furthermore, while recent review articles have detailed different fabrication strategies to form hydrogels with specific architectures [29–31], here we review the latest in advanced microfabrication techniques used specifically in the fabrication of ECHs, as well as their implementation in a wide range of biomedical applications. These techniques include 3D printing, electrospinning, micropatterning, and self-assembly. We discuss the significance of these new and developing fabrication methods, and their ability to impart ECHs with new properties, such as self-healing, complex architecture, strong adhesion to native tissues, and the ability to respond to different stimuli. As these properties make ECHs attractive for use in biomedical-related applications, we will lastly review the strategies involved to tailor ECHs to specific biomedical applications, such as tissue engineering, drug delivery, and biosensing. In summary, this review explicitly elaborates on a) the key role of ECHs in the engineering of therapeutic and diagnostic systems, b) extensively reports natural and synthetic materials used to develop ECHs, and c) state of the art biofabrication methods for engineering ECHs.
2. Conductive Nanoparticle-incorporated ECHs
The design of ECHs for biomedical applications may include conductive nanoparticles, such as graphene or CNTs, conductive polymers, or ionic liquids to impart electroconductivity. These conductive biomaterials have unique advantages and disadvantages to their application, as well as expected conductivity ranges for these systems (Table 2). These points must be carefully considered when tailoring an ECH for a specific biomedical application.
Table 2.
Advantages and disadvantages of the incorporation of common conductive biomaterials to form ECHs and the conductivity of these systems.
| Conductive materials | Advantages | Disadvantages | Conductivity (S/cm) | Refs |
|---|---|---|---|---|
| AuNPs/polymer | • Tunable conductivity • Generally biocompatible |
• AuNP cytotoxicity is not fully understood • Synthesis of AuNPs may be difficult depending on target particle size • Possible generation of ROS |
8.0 × 10−4 – 1.0 × 10−2 | [34–37, 60, 61] |
| AgNPs/polymer | • High conductivity • Highly antibacterial |
• AgNPs increase brittleness of ECH • Possible generation of ROS |
1.0 × 10−4 – 5.8 × 10−1 | [51, 62–67] |
| Graphene/polymer | • High conductivity • Robust mechanical strength • Generally biocompatible |
• Complicated fabrication method for GO • rGO frequently aggregates during ECH synthesis • Cytotoxicity of GO, rGO is not fully understood |
4.0 × 10−5 – 5.8 × 10−1 | [58, 68–71] |
| CNTs/polymer | • High conductivity • Robust mechanical strength |
• CNTs frequently show aggregation during ECH synthesis • CNTs increase brittleness of ECHs • Cytotoxicity not fully understood |
5.0 × 10−5 – 9.0 | [59, 70, 72, 73] |
| PANi/polymer | • Facile synthesis • Antimicrobial • Highly conductive • Facilitates cell proliferation |
• Fabrication requires harsh chemical environment | 5.0 × 10−4 – 1.2 × 10−2 | [13, 74, 75] |
| PPy/polymer | • Facile synthesis • Biocompatible • Environmentally Stable |
• Poor solubility in polar solvents • Poor mechanical strength, brittle |
1.2 × 10−3 – 1.2 × 102 | [76–79] |
| PEDOT/polymer | • High conductivity • Facilitates cell proliferation • Biocompatible • High stability |
• Poor mechanical strength, brittle • Cytotoxicity not fully understood |
6.7 × 10−4 – 1.0 × 10−1 | [80–84] |
| Bio-IL/polymer | • High conductivity • Biocompatible |
• Variable cytotoxicity | 1.4 × 10−4 – 1.0 × 10−2 | [20, 85–88] |
2.1. Gold Nanoparticles
Metallic NPs are colloids ranging from 1 to 100 nanometers in size featuring a high surface area-to-volume ratio [32], which exhibit chemical and physical properties much different from that of bulk metals [33]. Gold NPs (AuNPs) and silver NPs (AgNPs) are of particular interest to engineer ECHs for applications requiring electroactive properties. In recent years, AuNPs have gained significant interest due to their unique conductive [34], optical [35–37], and magnetic properties [38, 39]. These characteristics have been shown to be particularly advantageous for the development of biosensors [40], and drug delivery systems [41], as well as various tissue engineering applications [42]. Apart from their ease of synthesis, their high stability [40] and biocompatibility [36–38], and tunable properties of AuNPs by varying their structural size and shape make them attractive candidates for the synthesis of ECHs for biomedical applications. Some of the drawbacks of incorporating AuNPs into ECHs is their tendency to generate reactive oxygen species (ROS). ROS are oxygen-derived small molecules that are naturally produced endogenously from several sources including through cellular respiration in the mitochondria and from an incomplete reduction of oxygen and NADPH in the plasma membrane [43]. At moderate concentrations, ROS play critical roles in the regulation of cell function, such as cell growth, migration, or apoptosis, however, high concentrations of these molecules can result in damage of proteins, lipids, and DNA potentially leading to diseases and necrosis [44, 45]. Recently, ECHs have been designed that incorporate AuNPs for the purpose of generating high concentrations of ROS in order to eradicate diseased cells [46, 47]. However, when developing ECHs for regeneration of damaged tissues, it is important to note that due to their small size, AuNPs are capable of penetrating cell membranes and cause cellular dysfunction. Therefore, significant efforts have been conducted to determine the ideal size and shape of AuNPs, and to optimize their in vivo pharmacokinetics for therapeutic and clinical applications [48].
AuNPs incorporated ECHs have been used as drug delivery vehicles, by mediating the release of hydrophilic drugs encapsulated in their matrix. This is primarily due to their thermally responsive capabilities, which allow for dramatic phase changes through local changes in temperature that do not affect the surrounding tissues [49]. AuNPs also feature high absorption capacity and scattering power, which are highly advantageous for the development of drug delivery systems [50]. Current research has focused on studying the effect of the size and shape of AuNPs on drug release time, water absorbance, surface properties, as well as chemical and physical behaviors of AuNP containing biomaterials. AuNP-incorporated ECHs may also possess the ability to generate heat through the absorption of visible to near infrared (NIR) light, a trait which may be utilized in drug delivery systems to initiate the collapse of ECHs [51]. Drug delivery strategies may take advantage of this phenomenon by loading drugs in AuNP-incorporated ECHs and stimulating a local region with light for controlled release of therapeutic molecules. For example, in a recent study, Strong et al. designed an ECH system composed of the thermally responsive polymer poly(N-isopropylacrylamide-co-acrylamide) (NIPAAm-co-AAm) and NIR absorbing silica-gold nanoshells with a lower critical solution temperature (LCST) just above physiological temperature at 40 °C [52]. The LCST refers to the temperature at which smart hydrogels will physically shrink from a swollen to a collapsed state. The role of AuNPs in this context was to absorb NIR irradiation through external stimulation, which resulted in ECH deswelling and subsequent release of chemotherapeutic drugs. Their study investigated the ability for this AuNP-incorporated ECH system to initiate pulsatile drug release of either doxorubicin, or a DNA duplex. Experimental analysis into the efficacy of this drug delivery system was evaluated by culturing colon carcinoma cells on the surface of ECHs and comparing samples irradiated with light to non-irradiated samples. AuNP-integrated ECHs that were irradiated with NIR resulted in a 30% decrease in cell proliferation as compared with ECHs that had not been exposed with NIR [52]. It was, therefore, proposed that these AuNP-integrated ECHs would be able to rapidly deliver chemotherapeutic drugs to the site of a tumor while minimizing the exposure of the drugs to viable tissue.
In another study, Das et al. developed an ECH as a drug delivery system by grafting hydroxypropyl methyl cellulose (HPMC) on polyacrylamide (PAM), and then coating the hydrogel with AuNPs [50]. In vitro biodegradation analysis carried over 21 days in phosphate buffered saline (PBS) demonstrated the enzymatic biodegradation of ECHs over a constant rate. Furthermore, in vitro studies using hMSCs seeded on ECHs demonstrated the cytocompatibility and non-toxic nature of the engineered nanocomposites. Lastly, in vitro drug release kinetics for 5-ASA and ornidazole, showed that the amount of drug released was lower in ECHs with a higher concentration of AuNPs. This behavior highlighted the ability of AuNP-incorporated ECHs to be used for time-release strategies. Tissue regenerative strategies have also utilized AuNPs combined with hydrogels owing to their enhanced electrical properties, which may provide adequate coupling between adjacent cells [34]. ECHs formed based on AuNPs have been used for cardiac, bone, and nerve tissue engineering, due to their biocompatibility, high mechanical strength, and conductivity [53, 54].
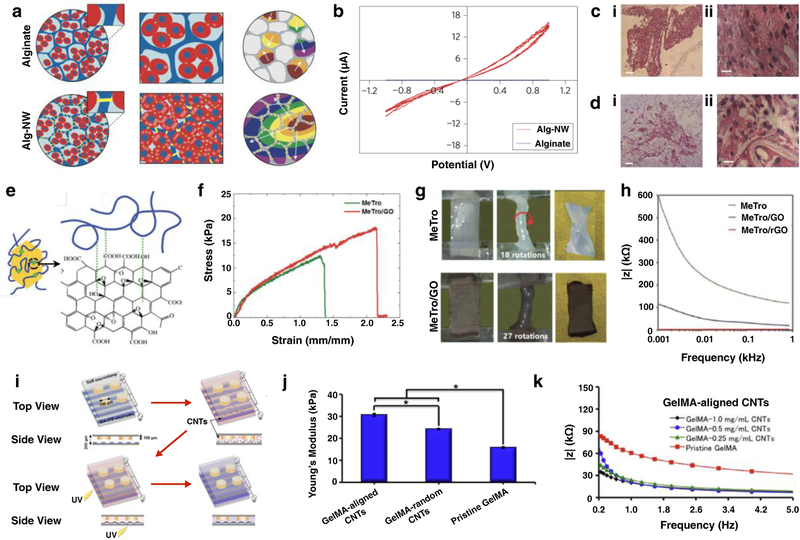
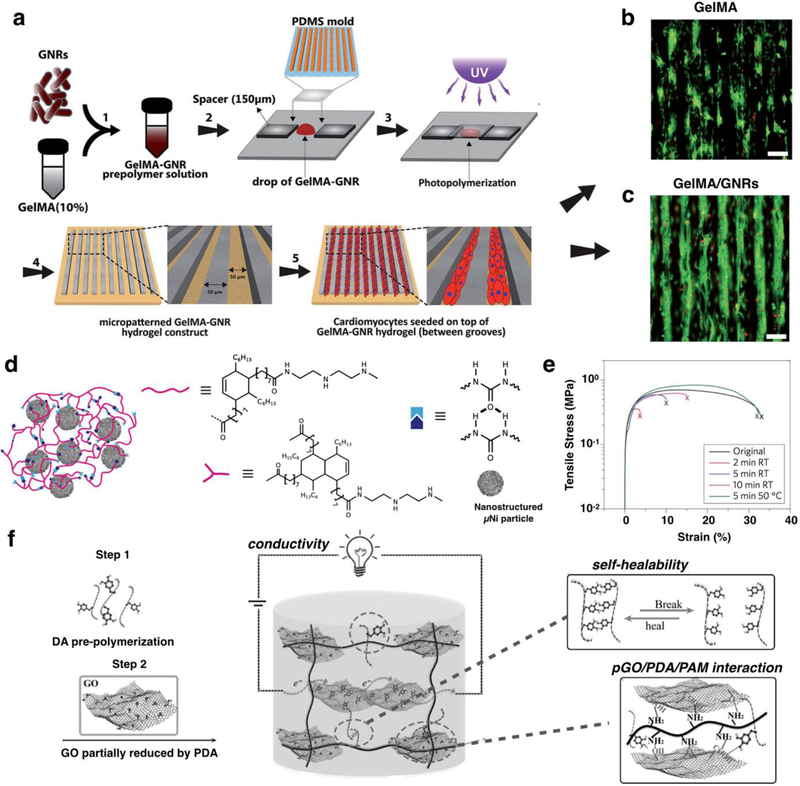
Gelatin methacryloyl (GelMA) is a photocrosslinkable biopolymer that has been widely used for tissue engineering applications as it is capable of supporting cell adhesion due to the presence of Arg-Gly-Asp (RGD) motifs [55]. A recent study conducted by Navaei et al. demonstrated the effectiveness of gold nanorods (GNRs) embedded in GelMA hydrogels to develop cardiac tissue constructs [38]. Hydrogels containing a higher concentration of GNRs showed lower electrical impedance, compared with control samples containing GelMA only. This lower impedance reflected the high electrical conductivity of hybrid hydrogels embedded with GNR, which in turn facilitated electrical propagation and promotes CM coupling [38]. The incorporation of GNRs also had a significant effect on the swelling ratio and the porosity of the hydrogels, which are key in the ability of a hydrogel to mediate nutrient and gas exchange [38, 56]. Furthermore, in vitro studies demonstrated that CMs seeded on the surface of GNR-embedded ECHs exhibited homogeneous distributions, as compared to control hydrogels without GNRs. Gold nanowires (GNWs) have also been incorporated into polymeric scaffolds for the synthesis of ECHs for tissue engineering applications. For instance, Dvir et al. reported the addition of GNWs to alginate scaffolds, to improve electrical communication between cells for engineering functional cardiac patches (Figure 2a) [57]. By incorporating GNWs, these conductive scaffolds demonstrated increased electrical conductivity, enhancing the function of cardiac tissue constructs (Figure 2b). CMs and fibroblasts were also seeded onto scaffolds under static and electrically stimulated conditions before implantation. Hematoxylin and eosin (H&E) staining revealed better-aligned CMs within GNW embedded scaffolds after 8 days of culture (Figure 2c i,ii), compared to a pure alginate matrix (Figure 2d i,ii) [57]. These results demonstrated the potential of GNW-loaded hydrogels in tissue engineering, for the development of materials that modulate excitable cells.
Figure 2. Synthesis and applications of ECHs formed by using conductive NPs.
Schematic for the formation of alginate hydrogels and gold nanowires (NW)-alginate ECHs. Cardiomyocytes are cultured in alginate hydrogels formed small clusters and beated asynchronously. However, cardiomyocytes cultured in alginate-NW ECHs formed organized cardiac-like tissue and beat synchronously. Components of engineered cardiac tissue are shown: cardiac cells (red), alginate pore walls (blue), NW (yellow) (a). Current/Potential graph of alginate hydrogels and alginate-NW ECHs showing higher electrical conductivity exhibited by the ECHs (b). Hematoxylin and eosin (H&E) staining images based on in vitro studies showed thick tissue in the NW-alginate ECHs (ci, cii), whereas the samples containing pure alginate showed non-continuous tissue separated by pore walls (di, dii). [57] Synthesis of ECHs by coating graphene oxide (GO) with methacryloyl-substituted tropoelastin (MeTro) (e). Elastic modulus of MeTro hydrogels and MeTro/GO ECHs demonstrating that the addition of GO significantly increases the elastic modulus (f). Torsion test on MeTro hydrogel and MeTro/GO ECH was conducted by twisting scaffolds for multiple rounds. Significant deformation was observed in MeTro hydrogel, however, MeTro/GO ECHs did not display any deformation (g). Overall impedance of MeTro hydrogels, MeTro/GO ECHs, and MeTro/reduced GO (rGO) ECHs shows that electrical resistance was the lowest for ECHs fabricated with rGO (h). [58] Schematic for the fabrication of CNT-embedded GelMA ECHs using dielectrophoresis force to align CNTs in GelMA. Highly aligned CNTs were observed in under 1 min, and the GelMA prepolymer was photocrosslinked using UV light (i). Young’s modulus of GelMA hydrogels, and ECHs containing GelMA and both randomly arranged and aligned CNTs. Results showed that the alignment of CNTs in GelMA-based ECHs resulted in a stiffer material as compared to ECHs fabricated with randomly dispersed CNTs (j). Electrical evaluation of these ECHs demonstrated that the incorporation of CNTs into hydrogels resulted in lower impedance compared to pristine GelMA hydrogels. Further the conductivity of these ECHs could be finely tuned by adjusting the concentration of CNTs in the system (k). [59] Scale bar= 200 µm (ci, di), 20 µm (cii, dii).
Sources:
[57], Copyright 2011. Reproduced with permission from Springer Nature
[58], Copyright 2016. Reproduced with permission from John Wiley and Sons
[59], Copyright 2016. Reproduced with permission from Elsevier Inc.
AuNPs have been used to engineer ECHs for a wide range of biomedical applications including biosensing, bio-imaging, and tissue engineering owing to their high electrical conductivity, distinct optical behavior, low cytotoxicity, and large surface area. These properties make AuNPs a useful tool as recognition elements for detection of specific biological analytes. AuNP-incorporated ECHs have also been used to develop drug delivery systems with the capability to trigger the release of loaded molecules. Further, these versatile biomaterials have demonstrated the ability to improve cell-cell coupling in ECHs developed for cardiac and neural tissue engineering. However, challenges still exist in the fabrication of AuNP-incorporated ECHs for biomedical applications. For instance, while widely considered to be noncytotoxic, their slow rate of clearance from the physiological environment needs to be considered when designing implantable ECHs containing AuNPs. In addition, the preparation of AuNPs is time consuming and costly, and multistep reactions may result in cytotoxicity. Another consideration is that AuNPs tend to aggregate together during synthesis of ECHs due to their large surface area. Nevertheless, AuNP-incorporated ECHs have shown promise to be successfully applied for future therapeutic and diagnostic innovations.
2.2. Silver Nanoparticles
AgNPs have also been used in combination with several types of polymers and biomaterials to engineer ECHs with enhanced electrical conductivity, as well as antimicrobial properties. The ability to eliminate bacteria in the clinical setting is the main driving force behind the success of AgNPs in the biomedical field. The antimicrobial properties of AgNPs are mainly due to the oligodynamic effect, a biocidal effect characterized by the binding of small metal ions to reactive groups, which results in the denaturing of cellular proteins in bacteria [62]. AgNPs are highly active against many types of gram-positive and gram-negative bacteria, including antibiotic-resistant bacterial strains [62–64, 89, 90]. However, similar to AuNPs, AgNPs have the potential to generate ROS that are capable of damaging protein, lipids, and DNA [91]. Therefore, it is necessary to determine cytotoxic effects of ECHs fabricated with AgNPs.
Recently, AgNPs have also been incorporated into hydrogels to impart electrical conductivity into these systems. The combination of antimicrobial and conductive properties makes AgNPs an attractive material for use in biomedical applications such as wound and burn dressings [92], coatings for surgical instruments [93], and biosensors [94]. In addition, AgNPs have also been used in a wide variety of industrial applications related to commercial sanitization, including areas of food packaging, textiles, plastics, soaps, and water treatment [95].
The incorporation of AgNPs into polymeric networks have been shown to influence the mechanical and swelling properties of ECHs. Previous studies have reported that the addition of AgNPs to polyvinylpyrrolidone (PVP) and polyvinylalcohol (PVA) hydrogels led to increased mechanical stiffness [65]. In addition, previous studies have shown that AgNP-loaded ECHs often exhibited lower swelling ratios when compared to control hydrogels without AgNPs [51, 66]. These lower swelling ratios associated with the incorporation of AgNPs into ECHs have been directly correlated to increased electrical conductivity. When water uptake into a polymeric network occurs, this will increase the distance between AgNPs, resulting in decreased conductivity. Therefore, polymer networks loaded with AgNPs must be designed to maintain adequate swelling to prevent loss of conductivity. In one study, Lee, W. et al. found that the impedance of AgNP-loaded ECHs decreased 2 orders of magnitude compared to control hydrogels, reflecting the electroactive properties provided through the incorporation of AgNPs [66].
AgNP/polymer composite ECHs are also particularly advantageous to the field of biosensors. For example, Xiang et al. developed an AgNP-loaded ECH with homogeneous dispersion of nanoparticles for biosensor applications [96]. These ECHs were rendered conductive by immersing swollen poly(HEMA-PEGMA-MAA) (PHPM) hydrogels in aqueous 0.01 M AgNO3, then, reducing Ag+ into the polymer network by submerging the hydrogels in 0.02 M NaBH4. These Ag-loaded ECHs were then evaluated for electrical conductivity. It was determined that conductivity in these ECHs was remarkably higher than that of control PHPM hydrogels, with values just under 600 µS cm−1 [96]. Further, because the AgNPs had been anchored to deprotonated -COOH functional groups, the pH of these ECHs had a significant effect on the conductivity. The pH responsiveness to these ECHs make them a smart material that could be further developed into biosensors or other biomedical applications.
Like AgNPs, silver nanowires (AgNWs) are another type of biocompatible material that have been used to develop ECHs for flexible bioelectronics. Recently, Ahn et al. developed an ECH by incorporating AgNW-based microelectrodes into a PAM-based hydrogel using a photolithographic process [97]. These materials displayed excellent electrical properties, where the conductivity could be accurately tuned by controlling the AgNW width, and spin coating speed [97]. Further, they possessed robust mechanical properties and excellent flexibility necessary for use with biological tissues. This study described a process to develop AgNW-based ECHs that are promising materials for use as flexible bioelectronic devices.
While incorporation of AgNPs and AgNWs into hydrogels is a noteworthy strategy to engineer ECHs, progress of these systems tailored for biomedical applications has been slow compared that of AuNPs. Most of the research involving AgNPs is greatly interested with their antimicrobial properties. Nonetheless, AgNPs have been found to possess excellent conductive and optical properties, and incorporation of AgNPs into polymer-based hydrogels has little effect on their mechanical properties. Taken together, the properties of AgNP incorporated ECHs make them suitable materials for a wide range of biomedical applications, including drug delivery systems, biosensors, and flexible electronics.
2.3. Graphene
Graphene is a 2D hexagonal lattice of carbon that possesses unique mechanical and conductive properties. Although pure graphene is extremely difficult to produce due to its atomic thickness, several methods have been reported for the synthesis of graphene derivatives [68–70]. One common derivative is graphene oxide (GO), where oxygen atoms form bonds with the carbon lattice and create small imperfections in the lattice structure [68–70]. However, oxidized graphene exhibits poor electrical conductivity, and must be deoxidized to be used in the synthesis of ECHs. Oxidized graphene can be deoxidized through the repair of the sp2 carbon bonds, which yields a conductive reduced graphene oxide (rGO) [68]. In addition, hydrothermal reduction of high concentration GO solutions can also be used to produce chemically converted graphene [69]. While graphene has been incorporated into many synthesis strategies to develop ECHs with robust electrical and mechanical properties, cytotoxicity remains to be a concern when these nanoparticles used for biomedical applications. The toxic effects demonstrated by graphene can be influenced by their size, shape, surface charge, surface area, and functional groups [98]. Future studies involving graphene nanoparticles must investigate the in vitro and in vivo biocompatibility as well as the mechanisms of cytotoxicity before seeking approval from the Food and Drug Administration (FDA).
Conventional methods for the fabrication of ECHs using rGO are limited due to the poor water solubility of rGO NPs and their tendency to aggregate in solution [68]. This affects the homogeneity of ECHs, which could lead to anomalies in the conductivity of the hydrogel. Jo et al. reported that hydrogels containing homogenous rGO networks can be formed by first creating hydrogels containing GO, and then reducing it to rGO in situ [68]. In this study, ECHs consisting of GO and polyacrylamide (GO/PAAm) were reduced in an L-ascorbic acid solution. Scanning electron microscope (SEM) analysis of the reduced GO/PAAm ECHs revealed no significant clusters of rGO, which suggested that rGO was uniformly distributed [68]. Furthermore, in vitro studies using C2C12 myoblasts revealed that cell adhesion and proliferation was improved for GO/PAAm and rGO/PAAm ECHs, when compared to control PAAm hydrogels. In another recent study, we incorporated GO NPs inside a highly elastic methacryloyl-substituted tropoelastin (MeTro) hydrogel to form ECHs for cardiac tissue engineering applications [58]. The covalent bonds between polymeric chains, along with hydrophobic and electrostatic interactions between MeTro and GO yielded ECHs with excellent mechanics, electrical conductivity, and biocompatibility (Figure 2e) [58]. ECHs containing GO nanoparticles showed longer elongation at their breaking point as compared to pure MeTro hydrogels (Figure 2f). As a MeTro-based ECH, this material possessed robust mechanical flexibility making them ideal for various tissue engineering applications where dynamic movement is required (Figure 2g). In addition, MeTro/GO ECHs displayed excellent electrical properties with significantly lower electrical resistance compared with pure MeTro hydrogels (Figure 2h). Furthermore, these scaffolds supported the growth and function of CMs in vitro and elicited no inflammatory response when implanted in rats [58].
There has also been an increase interest for the use of GO-loaded ECHs in drug delivery systems owing primarily to their remarkable conductive [99] and magnetic [100] properties. In one recent study, Servant et al. took advantage of these conductive properties when synthesizing stimuli-responsive hydrogels composed of poly(methacrylic acid) (PMMA) and ball-milled graphene (GBM) to control the release of small molecules in vivo. PMMA/GO ECHs exhibited remarkable electrical properties. It was shown that the bulk resistance of these materials decreased with an increasing concentration of GBM. These smart ECHs were evaluated for their ability to control the release of a small molecule drug, 14C-sucrose, in vivo through subcutaneous implantation into CD-1 mice. Mice were then electrically stimulated at 10V for 1 min periods at 2 h intervals, which is a relatively low voltage and short time period. It was found that PMMA/GO ECHs containing 14C-sucrose greatly outperformed control PMMA hydrogels with 5.5% 14C-sucrose released in the blood 8 min following electrical stimulation [99]. The development of advanced drug delivery systems that are able to control the release of molecules is a vastly important biomedical field. This work demonstrates that GO can be used to develop smart ECHs for controlled release of biomolecules.
GO-incorporated ECHs have demonstrated their potential for different tissue engineering applications such as cardiac and neural tissue engineering, owing to their excellent mechanical, and conductive properties. The amphiphilic nature of GO provides it with a structure that is deemed as biocompatible; however, more in vitro and in vivo investigations are required to evaluate the reaction these materials elicit to living tissues. In addition, the superior fluorescence quenching, and surface functionalization observed by GO make them excellent materials for use in biosensors and drug delivery systems. The unique chemical and physical properties of GO make it and excellent candidate for development into future biotechnological and biomedical applications.
2.4. Carbon Nanotubes
CNTs are nanostructures comprised of a cylindrical lattice of carbon atoms and are characterized either as single-walled or multi-walled nanotubes. CNTs have gained significant interest due to their unique properties, including high compressive and tensile strength, as well as high electrical conductivity [70, 72, 73, 101–103]. CNTs have been shown to be useful for engineering ECHs due to their ability to reduce brittleness and significantly increase electrical conductivity. However, they can also be difficult to incorporate into ECHs due to unique chemical structure, which is characterized by strong Van der Waals forces, high hydrophobicity, and low entropy [72]. The combination of these factors often leads to the aggregation of CNTs in solution, resulting in the formation of non-homogenous mixtures [72], which can be a challenge for the formation of ECHs.
Previous studies have described different methods for CNT dispersion into polymer solutions to engineer ECHs [72]. For instance, CNT-incorporated ECHs were formed by using pH-sensitive microgel particles containing high concentrations of –COOH functional groups [72]. When the pH of a solution containing CNTs and pH-responsive microgel particles approached the pKa value of the microgel particles, the swelling and attractive forces between the microgels and the CNTs could facilitate CNTs dispersion and minimize aggregation. These engineered microgel/CNT ECHs exhibited elastic moduli suitable for tissue engineering applications, such as intervertebral disk repair [72]. In addition, these microgel/CNT composites exhibited a conductivity of 0.031 S cm−1, which was significantly higher than other ECHs, particularly polyacrylamide/CNTs [72]. In addition, the engineered microgel/CNTs ECHs were shown to be highly biocompatible in vitro by seeding adipose-derived hMSCs on the surface of these ECHs for 7 days.
The field of cardiac tissue engineering has also greatly benefited from the synthesis of CNT-embedded ECHs. A recent study conducted by Ahadian et al. used dielectrophoresis to align CNTs in a GelMA-based hydrogel (Figure 2i), which enhanced the cardiac differentiation of embryoid bodies cultured in the microwells of patterned ECHs [59]. In addition, CNT-loaded ECHs exhibited higher elastic moduli (Figure 2j), lower electrical resistance, and supported increased beating of the cells as compared to pure GelMA hydrogels (Figure 2k). These results demonstrated that the engineered materials could be suitable for broad applications within regenerative medicine and cell therapy where conductivity of the matrix plays an important role [59].
CNTs have also been incorporated into ECHs to form drug delivery systems. In a recent study, Cirillo et al. developed gelatin/acrylamide//polyethylene dimethacrylate ECHs containing varying concentrations of CNTs and investigated their effectiveness in modulating the delivery of Curcumin (Cur). As expected, ECHs containing higher concentrations of CNTs exhibited lower electrical resistivity and provided a means to electrically stimulate these ECHs and initiate the release of Cur. In vitro drug releasing studies conducted on ECHs with varying CNT concentrations showed that samples containing 1.25% (w/v) CNTs best met the therapeutic needs for Cur given as a topical wound dressing. Further, the rate of Cur released could be modulated by controlling the external voltage applied to these ECHs. This versatility demonstrates the potential for CNT-based ECHs to be used in future drug delivery systems.
Incorporation of CNTs into polymeric networks to develop ECHs has become a reliable strategy to produce scaffolds with excellent conductivity and mechanical strength. These traits make them suitable biomaterials for use in tissue engineering applications. In addition, CNTs possess excellent stability, and magnetic properties making them excellent candidates for a wide range of other biomedical applications, such as biosensors or drug delivery systems. However, the future direction of CNTs incorporated ECHs should focus on in vivo models that address biocompatibility concerns, especially those that arise from health effects with the respiratory system. If CNTs can be incorporated into biocompatible ECHs without toxicological or inflammatory issues, these NPs will be excellent candidates for modern biomedical applications in the future.
3. Electroconductive Polymer-incorporated ECHs
3.1. Polyaniline
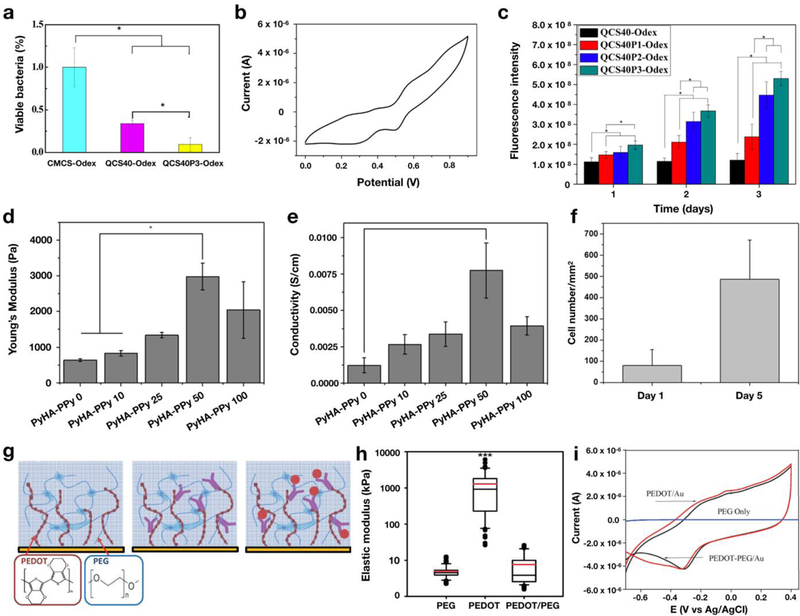
PANi is an electrically conductive polymer with a conjugated backbone that has been widely used for the synthesis of ECHs due its mechanical stability, ease of fabrication, and low manufacturing costs [74, 104]. Previous studies have reported the use of several derivatives of PANi, including its most reduced form (leucoemeraldine), its fully oxidized form (pernigranalline), and a partially oxidized form (emeraldine) [28, 105] for the formation of ECHs. Many natural and synthetic polymers have been used in conjunction with PANi to engineer ECHs. For instance, Xia et al. described the development of ECHs using PANi and polyacrylic acid (PAA), by reacting positively charged aniline monomers with the negatively charged COO– functional groups in PAA [75]. Their results showed that the addition of PANi increased the electrical conductivity, as well as compressive and elastic moduli of the resulting ECHs. In particular, this improved electrical conductivity was likely due to PANi fibers filling in small pores of the PAA hydrogel [75]. A similar study conducted by Zhao et al. described the engineering of an injectable ECH using quaternized chitosan and PANi for tissue engineering applications [79]. The engineered ECH was shown to possess high antibacterial activity against Gram-negative and Gram-positive bacteria (Figure 3a), as well as increased electrical conductivity (Figure 3b) and swellability. In addition, in vitro studies using C2C12 myoblasts demonstrated that ECHs containing higher concentrations of PANi showed significantly increased cell proliferation as compared to pure chitosan hydrogels grafted with oxidized dextran as a control group (Figure 3c). This study introduced a new class of bioactive scaffolds that may be tailored for a variety of tissue engineering applications, such as scaffolds for cardiac and nerve tissue regeneration [79].
Figure 3. Structure and properties of ECHs formed by using conductive polymers.
In vivo antibacterial activity test using hydrogels formed with carboxymethyl chitosan (CMCS-Odex), quaternized chitosan (QCS40-Odex), and quaternized chitosan with 3% (w/v) polyaniline (PANi). Oxidized dextran (Odex) was used as a crosslinker to form these ECHs. Results showed that engineered PANi-incorporated ECHs exhibited high antimicrobial properties (a). PANi-incorporated ECHs also exhibited high electrical conductivity (b). These ECHs demonstrated increased C2C12 cell proliferation with an increasing concentration of PANi, after 3 days of culture (c). [79] ECH developed by crosslinking polypyrrole (PPy) with hyaluronic acid (HA). Mechanical testing of HA-based ECHs with varying concentrations of PPy demonstrated that higher concentrations of PPy results in higher elastic modulus (d). In addition, higher concentrations of PPy increased electrical conductivity up to samples containing 50 mM (e). Plot of the number of attached 3T3 cells seeded on the surface of HA-based ECHs containing 50 mM PPy. These results showed that HA/PPy ECHs supported cell adhesion and the proliferation of 3T3 cells up to 5 days (f). [107] Schematic for the synthesis of ECHs by adding PEDOT (red) and PEG (blue) to the surface of an electrode. Biorecognition molecules (purple) are added into the gel by attaching to -COOH groups of PEDOT. Capture of B-IFN-γ molecules (red dot) resulted in a change in the electrical signal of the ECH (g). Assessment of the mechanical properties of PEG hydrogels, as well as PEDOT and PEDOT/PEG ECHs. Here the results showed that by adding PEDOT to PEG did not significantly change the elastic modulus compared to pure PEG hydrogels (h). However, the addition of PEDOT resulted in a significant increase in electrical conductivity (i), which is necessary for application in the field of biosensors. [108]
Sources:
[79], Copyright 2015. Reproduced with permission from Elsevier Inc.
[107], Copyright 2016. Reproduced with permission from Springer Nature.
[108], Copyright 2016. Reproduced with permission from John Wiley and Sons Inc.
As a conductive polymer, PANi has gained the most attention for the development of ECHs designed for biomedical related applications. This is due to the high electrical conductivity, stability, and unique redox properties found in PANi, as well as ease of synthesis and low cost [106]. However, some limitations have slowed the development of PANi-incorporated ECHs for biomedical applications, such as harsh processing conditions, toxicity, and non-biodegradability. Future investigations should evaluate PANi combined with other monomers to develop ECHs that possess high conductivity, as well as biodegradability, and biocompatibility.
3.2. Polypyrrole
PPy is an electroconductive polymer that has been used for the synthesis of ECHs due to its facile synthesis, environmental stability, tunable mechanical properties, and biocompatibility [109–111]. PPy has also been used for other purposes, including drug delivery systems [78, 112] and bio-electrodes [113, 114], as well as the engineering of cardiac tissue constructs [115, 116] and artificial muscles [117]. A recent study conducted by Yang et al., reported the combination of PPy with hyaluronic acid (HA) by conjugating N-(3-aminopropyl) pyrrole onto HA polymer chains yielding ECHs with enhanced electrical conductivity (Figure 3d) [107]. Their results showed that the elastic modulus and electrical conductivity of the engineered ECHs increased concomitantly with increasing concentrations of PPy up to 50mM (Figure 3e). The highest electrical conductivity obtained by these PPy-incorporated ECHs was approximately 7.3 mS/cm, when fabricated with a 0.5 mM PPy concentration (Figure 3f) [107]. In vitro cell studies conducted by seeding 3T3 cells on PPy-ECHs showed increased cell adhesion and proliferation, as compared to pure HA hydrogels. Taken together, these results demonstrated that PPy-incorporated ECHs could be used to develop tissue engineering scaffolds in conjunction with excitable cell types, as well as for future prosthetic devices.
The excellent redox properties of PPy have also made them attractive materials for use in the development of patterned electrodes for the skin. In a recent study, Hur et al. developed a PPyincorporated smart ECH using agarose as the polymeric network [76]. This strategy yielded remarkable ECHs that were not only highly conductive, but also contained thermoplastic properties, which enabled thermal or light-assisted healing of the network. These ECHs exhibited mechanical properties that were similar to that of human skin, with a Young’s modulus of 27–46 kPa, while also possessing an electrical conductivity in the same range as other ECHs fabricated using CPs (0.35 S cm−1) [76]. By developing ECHs with similar mechanical flexibility and self-healing properties, as well as high electrical conductivity, these materials have demonstrated ideal physical and chemical properties for applications involving flexible electronics and biosensors.
PPy has become a very popular CP for the fabrication of ECHs suitable for biomedical applications. Importantly, these CPs exhibit good in vitro and in vivo biocompatibility and excellent stimulus-responsive properties, as well as high thermal stability [28]. These traits make them excellent biomaterials for use in biomedical related applications, such as biosensors, drug delivery, and flexible electronics. PPy may also be used for tissue engineering applications where excitable cell types are found, such as neural [118] and cardiac tissue [119]. Some challenges in fabrication of high quality PPy-incorporated ECHs is overcoming their poor solubility in polar solvents as well as their brittleness. Future research into PPy-incorporated ECHs may focus on incorporation natural polymers with good biodegradation profiles and cell adhesion to be used for biomedical applications.
3.3. Polythiophenes / PEDOT
Polythiophenes (PThs) are another class of innately electroconductive polymer that has been used to impart electrical conductivity to ECHs due to their solubility, high thermal stability, and excellent electrical conductivity when in a doped state [80]. However, the use of PThs for biomedical applications is often limited due to the increased weight and rigidity of PThs, which can potentially decrease the mechanical and electroactive performance of resulting ECHs [80, 81]. Unlike PThs, its derivative poly(3,4-ethylenedioxythiophene) (PEDOT) is commonly used in the synthesis of ECHs due to its biocompatibility, cost-effectiveness [120], and electrochemical stability in aqueous solutions [121–125]. While PEDOT itself is conductive, increased electrical conductivity as well as cationic conductivity is rendered when PEDOT is combined with poly(styrene sulfonate) (PSS) [126]. PEDOT:PSS also maintains suitable conductivity in the body and may be cleared by the kidneys, which has resulted in significant attention in a variety of biomedical fields [82, 83].
Recent studies have shown that PEDOT-incorporated ECHs can be used to closely mimic the conductive and mechanical properties of certain tissues, making them suitable to use as scaffolds for tissue engineering. A study by Kim et al. reported that native cardiac tissue has an elastic modulus within the range of 10–100 kPa, and has a conductivity ranging from 10−3 S/cm to 10−2 S/cm. In attempt to engineer biomimetic biomaterials for cardiac tissue regeneration, Kim et al. reported the formation of an ECH based on a RGD-modified polyethylene glycol (PEG) containing PEDOT with an elastic modulus of 21 ± 4 kPa and a conductivity of 1.69 × 10−2 S/cm [82]. In vitro cell studies using electro-responsive H9C2 cells were conducted to determine the effect of PEDOT on cell attachment and proliferation. Their results showed that PEDOT-containing ECHs supported cell adhesion and proliferation without compromising the electrical and physiochemical properties of the hydrogel, and thus was found to be a promising candidate for tissue engineering applications [82].
Another study that investigated PEDOT-incorporated ECHs for potential use in tissue engineering was recently conducted by our group. In this study, Spencer et al. developed ECHs containing GelMA and various concentrations of PEDOT:PSS up to 0.3% (w/v) [83]. Results showed that an increase in the concentration of PEDOT:PSS up to 0.3% did not change the mechanical properties of the ECHs; however, the swelling ratio significantly decreased with higher concentrations of PEDOT. Furthermore, electrical conductivity of the engineered ECHs was also characterized using electrochemical impedance spectroscopy (EIS). The results showed that ECHs that containing 0.3% PEDOT:PSS had significantly lower electrical resistance (261.0 kOhm) when compared to pure GelMA samples (449.0 kOhm) [83]. The biocompatibility of PEDOT:PSS-incorporated ECHs was investigated in vitro by 3D encapsulating of C2C12 cells into these scaffolds and cultured for up to 5 d. Our results showed high cell viability for engineered ECH containing up to 0.1% PEDOT:PSS concentration [83]. Taken together, PEDOT has demonstrated the ability to impart conductivity while remaining biocompatible and having little effect on mechanical properties. Therefore, PEDOT:PSS containing ECHs show potential to be used in future tissue engineering applications.
PEDOT has also investigated for use to develop ECHs for biosensors. Recently, Shin et al. developed a PEDOT/PEG-based ECH to detect specific antigen molecules in the physiological context. The outer layers of these sensors were coated in the ECH loaded with a cytokine-specific antibody, which is attached to a PEDOT-COOH group. Once these biosensors come in contact with the IFN-γin analyte, the ECHs undergo a quantifiable decrease in electrical conductivity that would serve as the detection mechanism (Figure 3g). Despite the high mechanical strength of PEDOT, PEDOT/PEG ECHs exhibited an elastic modulus of 7.58 ± 0.84 kPa, which was similar to that of pure PEG hydrogels (Figure 3h). In contrast with non-conductive PEG hydrogels, PEDOT/PEG ECHs exhibited remarkable electrical conductivity that was highly distinguishable from control samples (Figure 3i). These ECHs demonstrated a system that was successfully able to transfer the detection of an analyte into an electrochemical signal. These results suggest that PEDOT is an effective material to impart electrical conductivity for future biosensor applications.
Advancements in the development of PEDOT-incorporated ECHs have led to promising innovations in areas including biosensors, implantable electrodes, and drug delivery systems. Investigators are able to take advantage of the high electrical conductivity, as well as chemical and environmental stability exhibited by PEDOT. Further, PEDOT has been widely reported as a biocompatible material with many in vitro investigations confirming no cytotoxicity. However, due to high mechanical stiffness of this material, the foreign body response may disrupt performance when PEDOT-incorporated ECHs are implanted in soft tissues, such as in the brain. While these ECHs have displayed excellent properties for biomedical applications, long term in vitro and in vivo investigations are required to determine their effects on surrounding tissues before they may be transitioned into the clinical setting. Taken together, PEDOT-incorporated ECHs are a promising biomaterial for future biomedical related therapeutic and diagnostic strategies.
4. Ionic Liquid (IL) Conjugated ECHs
ILs have been implemented in a wide range of industrial applications, including fuel cells [85], solar cells [86], batteries [87], and sensors [127] due to their many unique properties such as low volatility, non-flammability, high thermal stability, and high ionic conductivity [128–132]. ILs are liquids comprised completely of ions from salts, which have a melting point below 100°C. There are many sub-categories of ILs, including room temperature ILs, task-specific ILs, polyionic liquids, and supported IL membranes [131]. Previous studies have focused on the characterization of the physical structure and properties, nano-organization and self-assembly, and advanced chemical transformations of ILs [133]. Recently, it has been demonstrated that ECHs may be engineered by combining ILs with polymers for applications in tissue engineering, electrochemical biosensors, electro-stimulated controlled drug release systems, and neural prosthetics [134, 135].
For example, ECHs can be formed by polymerizing monomers in ILs to increase the electrical conductivity of these scaffolds. This approach was demonstrated by Liang et al., using microcrystalline cellulose and PPy as polymers, to form a network in 1-butyl-3-methylimidazolium chloride (BMIMCl) IL [135]. BMIMCI was used in this study to provide a solvent that could dissolve cellulose while protecting its structure from degradation. The swelling of ECHs was found to be dependent on the microcrystalline cellulose concentration, with higher concentrations exhibiting lower swelling ratios. Furthermore, the mechanical properties were substantially improved with the incorporation of PPy, increasing from a maximum stress of 1.53 MPa for control samples, to 26.25 MPa for PPy-containing ECHs [135]. Their results also demonstrated that the composite hydrogels synthesized using ILs were suitable for the development of biological and semiconducting materials, as well as drug delivery systems and neural prosthetics [135]. In another study, Robinson et al. develop a synthetic sensing skin by 3D printing two inks; one that was ionically conductive, and one that was electrically insulating [134]. For this, they used the conductive 1-decyl-3-methylimidazolium chloride IL, while the insulating material was a silicone elastomer. This approach was used to print a micropatterned material that could act as stretchable capacitive sensors. Both inks were extruded and polymerized in situ to form a layer of ECHs, and a layer of insulating silicone. The resulting capacitive skin demonstrated excellent adhesion to actuation chambers, as well as the ability to detect a compressive force of approximately 2 N. The combination of an ECH with the insulating silicone led to the development of the first printable skin to demonstrate enabled tactile sensing and kinesthetic feedback [134].
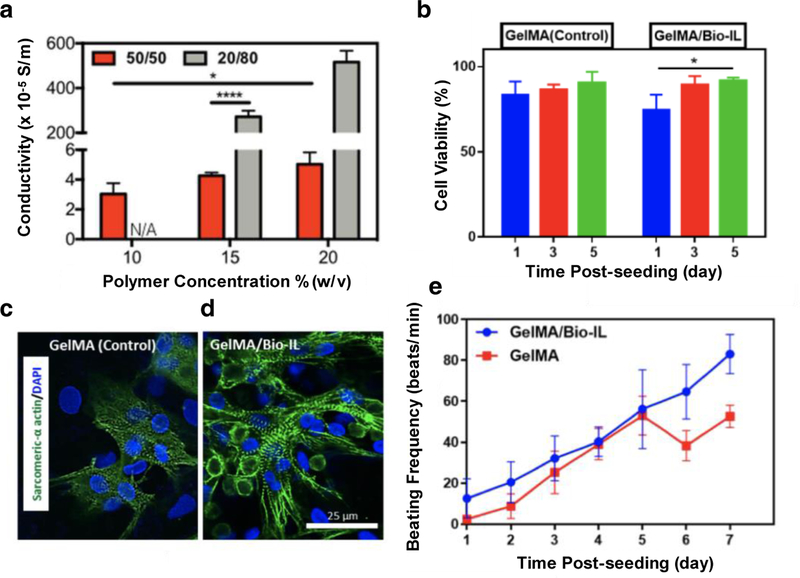
Recently, our team demonstrated the conjugation of a choline-based bio-ionic liquid (Bio-IL) to a hydrogel network to form ECHs with controlled conductivity and physical properties for cardiac tissue engineering applications [20]. The difference between Bio-ILs and ILs are that Bio-ILs exhibit enhanced biocompatibility and thus may be attractive materials for use in biomedical applications. ECHs with tunable properties were synthesized by photocrosslinking various ratios of GelMA prepolymer to acrylated Bio-IL in the presence of Eosin Y photoinitiator and 120 s visible light. Our results showed that these composite hydrogels demonstrated tunable electrical conductivity that was in the range of native cardiac tissue (Figure 4a). Biocompatibility of GelMA and GelMA/Bio-IL hydrogels were assessed by seeding CMs on the surface of these materials and culturing for 5 days. Both GelMA hydrogels and GelMA/Bio-IL ECHs demonstrated excellent cell viability at day 5 of cell culture (Figure 4b). Further, immunofluorescent staining of sarcomeric α-actinin showed that GelMA control hydrogels exhibited an intermittent pattern of sarcomeric α-actinin (Figure 4c), while GelMA/Bio-IL ECHs exhibited a homogeneous distribution of the protein (Figure 4d). As these ECHs were designed to assist in electrical propagation generated by cardiac tissue for synchronous heart beating, characterization of CM beating when seeded on GelMA and GelMA/Bio-IL hydrogels was evaluated. Results showed that by day 7 of cell culture, CMs seeded on GelMA/Bio-IL ECHs had a significantly higher beating frequency as compared to control GelMA hydrogels (Figure 4e). This can be attributed to the high electrical conductivity observed in GelMA/Bio-IL ECHs. Taken together, these results demonstrate the approach to conjugate Bio-ILs to polymeric networks as an effective means to impart electrical conductivity to otherwise insulating hydrogels.
Figure 4. Synthesis and characterization of ECHs engineered using Bio-ILs.
Electrical conductivity assessment of ECHs formed with GelMA and bio-ionic liquid (Bio-IL) demonstrating that scaffolds fabricated with higher concentrations of Bio-IL exhibited greater electrical conductivity (a). Biocompatibility assessment using CMs seeded on the surface of GelMA hydrogels and GelMA/Bio-IL ECHs in vitro demonstrated that the incorporation of Bio-IL into hydrogels had no significant effect on cell viability after 5 days (b). Immunofluorescent staining of sarcomeric α-actinin (green) and DAPI (blue) in CMs seeded on control GelMA hydrogels (c) and GelMA/Bio-IL (d) ECHs at day 7. Characterization of synchronous beating of CMs seeded on GelMA hydrogels and GelMA/Bio-IL ECHs over 7 days of culture showing that the conductive GelMA/Bio-IL hydrogel exhibited higher beating frequencies compared to non-conductive GelMA hydrogels (e). [20], Copyright 2017. Reproduced with permission from Nature Publishing Group. Scale bars= 25 µm (d).
5. Microfabrication of ECHs
5.1. 3D Printing
3D printing technology has significantly improved over the past few decades, enabling the creation of complex 3D structures that might otherwise be impossible to fabricate with traditional molding techniques or top-down milling procedures [136]. Not surprisingly, this technology has made its way into the biomedical field to form complex structures which have great potential to contribute to our understanding of healthy and diseased tissue states [137], expand our treatment options for those diseases [138], or fabricate medical devices [139]. In addition, the turnover time for these structures or devices is appreciably less than many molding or fabrication processes, which can reduce lead time and accelerate products to patient timelines.
For many biomedical applications, engineering biomaterials with biomimetic mechanical properties has been a challenge. This mechanical mismatch has inspired widespread interest in the field of flexible electronics and electroactive tissue engineering [140]. To this end, the design of soft and conductive materials has been an emerging area of research in recent decades. Since the hydrostatic and mechanical properties of hydrogels are very similar to human tissues, they are an obvious choice as a material for fabricating complex 3D bioprinted structures for biomedical applications. In this section, we will review recent work in the field of 3D printing of ECHs and the applications targeted for the formulations and technology.
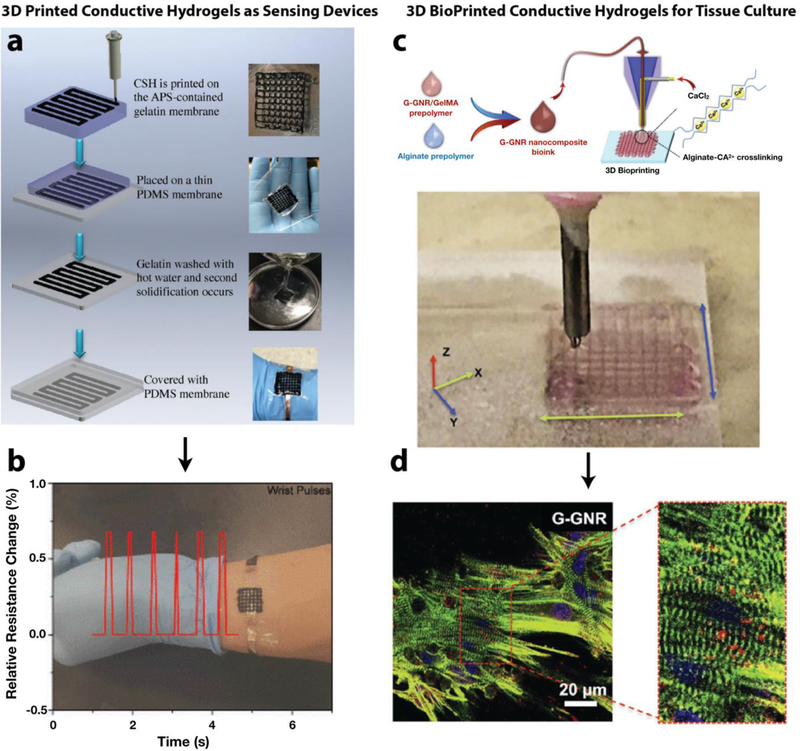
One of the common applications of 3D printed ECHs is as pressure/motion sensors or as biosensors [141]. For these applications, tuning the geometrical or chemical structure of the ECHs can modulate its electrical properties and causes a proportional response, signaling the occurrence of an event. In the case of pressure or motion sensors, the compression or extension of the hydrogel causes a corresponding change in electrical resistance and thus the generation of an electrical signal [142]. For biosensors, an analyte of interest binds to the conductive material and induces a change in its electrical properties, causing the signal to be generated [108]. Normally these sensors require advanced fabrication techniques to achieve the complex landscape for the sensor design. 3D printing can eliminate the need for costly tooling and equipment that is typically required to form these patterns and enables rapid formation of multiple designs at minimal cost and significantly reduced lead time. In some cases, elastomeric polymers, such as polydimethylsiloxane (PDMS), are utilized as printing substrates onto which the conductive hydrogel can be patterned with the printing device [143]. The PDMS helps to maintain the overall stability of the printed structure and provides a template for the sensing function of the device. While non-hydrogel conductive elastomers can be used for pressure or motion sensors [144], detection of water-soluble analytes might not be as sensitive or accurate if non-porous hydrophobic materials, such as PDMS, are used. For example, inkjet printing was used to pattern a nanostructured PANi hydrogel for multiplex detection of glucose, lactate, and triglycerides in real time selectively and with high sensitivity [145]. The use of printing for the devices enabled them to fabricate pages of sensor arrays with 96 electrodes in minutes. In comparison, traditional photolithography patterning methods would require pre-fabrication of photomasks and careful substrate preparation and washing and cleaning steps before the device could be realized. Pressure sensors were fabricated by printing a conductive self-healing hydrogel composed of polyacrylic acid and PPy (Figure 5a), wherein changes in pressure caused a corresponding change in electrical resistance [146]. The devices were coated with a thin layer of PDMS to contain the conductive gel and pressed onto the wrist for taking blood pulse readings. These printed devices could be used as wearable sensors that conformed to the shape of the arm, finger, or wrist (Figure 5b). One group designed a photocurable conductive and elastic hydrogel that could be printed using digital light processing (DLP) stereolithography (SLA) [147]. The printed constructs were not tested for specific applications, but the formulation and printing technology combined are promising for applications where a transparent, elastic, and conductive hydrogel is required, such as for optogenetics [148]. Careful deliberation of the materials and methods will undoubtedly improve the performance and capabilities of 3D printed conductive hydrogel-based devices in the near future.
Figure 5. 3D printing of conductive hydrogels for different biomedical applications.
Pressure sensors were fabricated by 3D printing a conductive self-healing (CSH) hydrogel composed of PPy-grafted chitosan and poly(acrylic acid), and coating them with a thin layer of PDMS (a). When applied to the wrist, the sensor could detect blood flow pulses in the veins below the skin in real-time (b). [146] Hydrogel prepolymer solution containing sodium alginate, gold nanorods, and GelMA was bioprinted via a co-axial nozzle, in which the alginate prepolymer solution in the core was crosslinked by calcium ions flown in the sheath (c). Lattice-like structures could be printed, and hydration was maintained by the continuous flow of the aqueous sheath solution. Cardiomyocytes printed in these structures expressed higher levels of Cxn43 cardiac junction protein and exhibited higher contraction rates compared to controls (d). [152]
Sources:
[146], [152] Copyright 2018, 2017, respectively. Reproduced with permission from John Wiley and Sons Inc.
Recapitulation of complex tissues in vitro is becoming increasingly more possible as advanced microfabrication strategies evolve. 3D bioprinting has enabled the generation of features in an additional dimension compared to 2D patterning methods and has provided scientists with the capacity to form biomimetic tissue structures in vitro. Ultimately, the goal of this technology is to generate full-scale tissues or organs that can replace organ donation and transplants, thus reducing issues with donor availability and chronic organ rejection [149]. Bioprinted hydrogels with improved electrical conductivity have been shown to improve the function of electroactive tissues, such as cardiac and neural tissue [57, 150]. For example, PEGDA hydrogels mixed with various concentrations of amine functionalized multi-walled carbon nanotubes (MWCNTs) was 3D printed into grid-like structures for nerve regeneration [151]. Results showed that neural stem cells (NSCs) proliferated more and differentiated early on scaffolds containing MWCNTs compared to controls without conductive nanotubes. In addition, exogenous electrical stimulation in the form of biphasic pulses enhanced neuronal maturity for structures containing the MWCNTs as confirmed by quantitative polymerase chain reaction (qPCR). A similar study targeting cardiac tissue utilized a bioink containing GNRs, GelMA, and sodium alginate with a co-axial printing system, where sodium alginate was used as a structural material that was rapidly crosslinked as aqueous calcium chloride was extruded through the shell of the nozzle (Figure 5c) [152]. CMs encapsulated in the 3D printed GelMA/GNR hydrogel structure expressed higher levels of Connexin 43 (Cxn43) cardiac junction protein and exhibited higher contraction rates than GelMA controls (Figure 5d). These results show promise as materials that can help us design cell-laden 3D printed structures with enhanced tissue function that may enable tissue replacement in the future.
3D printing has been widely used in the biomedical field. Its simplicity, low cost, minimization of waste, and ever-advancing capability have made this technique an extremely powerful tool that will revolutionize our ability to iterate designs in ways previously not possible. ECHs have proven utility for applications where an electrically active, flexible, and hydrated material is required, such as in sensing devices and for tissue culture. The combination of these approaches has provided researchers with the means to create complex structures with unprecedented speed and precision. For 3D printed conductive hydrogel motion sensors, the capabilities tested thus far were limited to simple events, such as hand or arm movement [143]. More sophisticated devices capable of sensing motion and responding could be used as medical devices, such as a film that can sense heart beat rhythms and provide an electrical stimulation impulse if arrhythmia is detected. An ongoing challenge in biosensors is improving selectivity [153]. Hydrogel compositions could be chemically modified to improve selectivity and prevent false positive or false negative responses. For 3D bioprinting, designing ECHs bioink with biocompatibility and printability is a major limitation, especially in the case of printing complex cell-laden 3D structures. In addition, the ability to directly print integrated electrochemical probes into 3D printed devices would enable interrogation and stimulation of electroactive cells encapsulated in the structure [154]. Finally, the resolution of 3D printing is rapidly improving, and decreased feature size can shrink the overall size of devices or sensors and increase the fidelity of bioprinted structures that mimic native tissues. These improvements will enable 3D printing combined with ECHs to contribute even further to multiple facets of the biomedical field.
5.2. Electrospinning
The primary goal for the design of tissue engineered scaffolds is to develop materials that structurally and functionally mimics the native ECM. The ECM is a complex network of proteins, proteoglycans, and glycosaminoglycans that provides physical support for cells [155, 156]. Furthermore, the ECM is responsible for the promotion of cell adhesion and migration, as well as proliferation, and function [157]. This is achieved, in part, due to the complex nanostructure of protein fibers, such as collagen and elastin [157], and the presence of specific ligands and growth factors [158, 159]. Protein fibers may range in diameter from several tens to several hundred nanometers. In this regard, electrospinning has been increasingly used for the engineering of polymer fibers that resemble the fibrous architecture of the ECM.
Electrospinning systems are comprised of three main components; a high voltage supplier capable of generating 10–20 kV of potential, a small diameter metal nozzle, and a metal collector (Figure 6a) [160–162]. The generated electrical field drives the polymer feed to the grounded metal collector in a process that is referred to as a whipping mode, which is characterized by increased acceleration and oscillation [160, 163, 164]. The solvent used in the polymer feed is mainly evaporated or solidified in the electrospinning process, and trace amounts of solvent in the fibers may be removed using vacuum after synthesis.
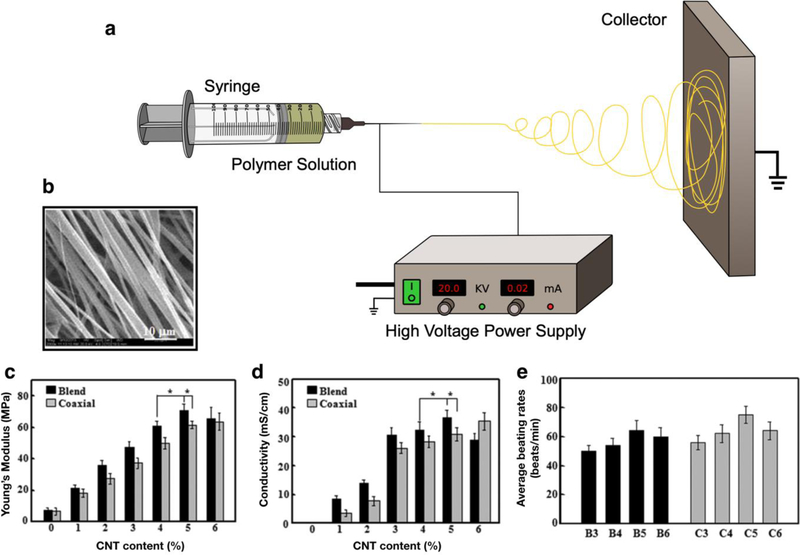
Figure 6. Electrospinning of ECHs for different biomedical applications.
Schematic for an electrospinning set up consists of a polymer dissolved in solvent being injected out of a metal nozzle (a). A high voltage power supply is connected to a metal nozzle and a metal collector creating an electrical field. A polymeric solution is then slowly pumped out of the syringe and spun onto the metal collector. Representative SEM image of a blend electrospun PELA/CNT fibrous scaffold with a 5% CNT concentration showing high alignment of fibers and 2 µm fiber diameter (b). Mechanical study of electrospun PELA/CNTs demonstrated that ECHs fabricated with higher concentrations of CNTs resulted in a higher Young’s modulus (c). Electrical evaluation of electrospun ECHs showed that these fibrous scaffolds exhibited higher conductivity when fabricated with a higher concentration of CNTs in both blended and coaxial electrospun PELA/CNT scaffolds (d). Beating rate of CMs when seeded on PELA/CNT fibrous scaffolds and cultured for 710 days. The beating rate for ECHs coaxially electrospun with a 5% CNT concentration (C5) achieved an average beating rate of 70–80 times/min, which is similar to that rate of CMs seeded on other non-conductive hydrogels (e). [171], Copyright 2016. Reproduced with permission from Elsevier Inc.
Electrospun conductive fibrous scaffolds have been widely used in tissue engineering and biomedical applications due to the promotion of favorable cellular responses, such as increased adhesion and proliferation [165–167]. CPs such as PANi, PPy, and PTh have been electrospun into composite fibers with non-conductive polymers to form hydrophilic scaffolds for cardiac, neural, and skeletal regenerative tissue engineering [166, 168]. The combination of CPs with natural polymers provides good electrical conductivity while also improving the swellability, biodegradation, and biocompatibility of these hydrophilic scaffolds. For example, an electrospun PANi-gelatin fiber blend has recently been engineered for cardiac and neural tissue engineering [169]. These PANi-gelatin scaffolds demonstrated a uniform distribution of polymers, which exhibited high biocompatibility, and were able to promote the adhesion and proliferation of cardiac myoblasts [169]. Furthermore, mechanical characterization of PANi-gelatin fibers showed an increase in the elastic modulus, and a reduction in fiber size with increasing PANi concentrations.
Another recent study by Malki et al. investigated ECHs fabricated by electrospinning of albumin and then absorbing AuNRs into the fibers to engineer cardiac patches capable of cardiac tissue regeneration following myocardial infarction (MI) [170]. Through the electrospinning of albumin, a porous scaffold composed of ribbon-like fibers with a thickness of 0.5 µm, was developed that closely resembled the structure of cardiac tissue [170]. Further, the incorporation of AuNRs to these cardiac patches improved electrical conductivity that would assist in the cell-cell interactions that are responsible to synchronous heart beating. The AuNR-incorporated cardiac patches were evaluated in vitro through CM encapsulation within these hybrid scaffolds to analyze the expression of the gap junction protein Cxn43. Their results also showed that after 7 days of cell culture there was a significant expression of Cxn43, as well as pronounced actinin striation, suggesting the ability of cells to contract synchronously [170]. The electrical enhancement of these electrospun scaffolds yielded cardiac patches which were able to support CM contraction and may be used to improve the function of cardiac tissue following MI.
In another study, Liu et al. developed ECHs with architecture that closely resembled the cardiac microenvironment through electrospinning poly(ethylene glycol)-poly(??,??-Lactide) (PELA) copolymers with CNTs onto a spinning mandrel [171]. Their approach investigated the efficacy of both blend electrospinning and coaxial electrospinning techniques. Blend electrospinning involved mixing CNTs and PELA into a polymer solution. Coaxial electrospinning followed the same principles, however, two separate solutions, one containing PELA and the other CNTs, were coaxially and simultaneously electrospun through different capillary tubes into the same nozzle. Electrospinning PELA/CNT onto a rotating mandrel resulted in aligned fibers with diameters between 2–3 µm (Figure 6b). These blended and coaxial electrospun scaffolds demonstrated that higher CNT concentrations resulted in higher mechanical stiffness, concomitantly (Figure 6c). Further, scaffolds fabricated with higher concentrations of CNTs yielded higher electrical conductivity (Figure 6d). The beating strength of CMs when seeded on the engineered fibrous mats was evaluated for up to 10 days. Results showed that CMs seeded on electrospun scaffolds fabricated with 5% CNTs had significantly higher average beating rates (70–80 beats/min) when compared to scaffolds fabricated with 3%, 4%, and 6% CNT concentrations (Figure 6e). CMs seeded on all PELA/CNT electrospun scaffolds showed synchronous beating, except for those fabricated with 6% CNTs. The engineered fibrous scaffolds in this study could mimic the organized cardiac muscle fibers of the heart.
Electrospinning is a facile method that allows precise control over many parameters of the synthesis process, such as voltage applied, distance from the nozzle to collector, and flow rate. In addition, metal collectors may be static, generating randomly arranged fibers, or rotating, which results in highly aligned fibers. By adjusting these parameters, it is possible to engineer ECHs with finely tuned fiber morphology and geometry. Using advanced electrospinning set-ups, ECHs with enhanced microarchitecture, porosity, mechanical properties, and electrical conductivity have been fabricated with exceptional properties for biomedical applications. Further, electrospinning provides a method to optimize the patterning of cells or bioactive ligands in a way that is biomimetic to the structure and morphology of native tissues. Further characterization including in vivo studies, however, will be required for electrospun ECHs before they may be used for clinical applications. In addition, future studies should seek to improve cost-effectiveness and increase the yield of fibrous scaffolds. Nonetheless, modern electroactive and fibrous scaffolds, with excellent scalability, reproducibility, and consistency show promise for use in future biomedical applications such as cardiac and neural tissue engineering.
5.3. Micropatterning of ECH
Micropatterned hydrogels have been used in a wide range of biomedical applications, such as drug delivery [172, 173], and tissue engineering [174]. Some of the most commonly used techniques for micropatterning of hydrogels include microfluidics [175], magnetic [176] and acoustics guided hydrogel assembly [177]. However, micropatterned non-conductive hydrogels could impede electrical cell-cell coupling and lead to signal deferment inside the scaffolds [57]. This, in turn, could potentially limit their application in the context of physiological environments where excitable cell types are present such as nerve and cardiac tissues. Therefore, micropatterning of ECHs has gained significant attention, and different techniques have been investigated to generate unique architectures of micropatterned ECHs for biomedical applications [178]. For example, in a study by Kim et al., conductive PEDOT-incorporated ECHs were patterned to develop flexible electrodes [179]. Briefly, a PEDOT film was prepared by reacting a liquid phase PEDOT monomer casting with the oxidant Fe(III) tosylate. A UV-induced photopolymerization of PEG was then performed using a photolithography technique at the PEG/PEDOT interface using a photomask. The PEG hydrogel was then peeled off removing the region of PEDOT film that was exposed to UV irradiation. Finally, a second PEG gelation step was performed on the remaining patterned PEDOT film, which left it embedded in the hydrogel. Micropatterned PEDOT-embedded ECHs exhibited high electrical conductivity coupled with flexible mechanical properties, which demonstrate their potential for biomedical applications such stimuli-responsive drug delivery systems and growth factor delivery systems [179].
In another study, Wu et al. reported the engineering of conductive GelMA-PANi ECHs in patterned hexagonal geometries, by utilizing digital projection stereolithography [180]. This was achieved by injecting GelMA-PANi precursor solution in a chamber and crosslinking this ECH in organized patterns using a computer-aided design-based digital mask. GelMA/PANi ECHs demonstrated remarkably lower impedance (2.9 ± 0.3 kΩ) compared to control GelMA hydrogels (6.9 ± 0.7 kΩ), at physiologically relevant frequencies. In addition, in vitro cell studies were used to investigate the effect that these micropatterned ECHs on the morphology of adhered cells. This was done using C3H/10T½ murine mesenchymal progenitor cells (10T½s) cells seeded on the surface of GelMA and GelMA/PANi samples and cultured for 5 days. Results showed that 10T½s cells seeded on GelMA/PANi ECHs demonstrated better adhesion and viability after 5 days compared to pristine GelMA [179]. Further, while 10T½s cells adhered exclusively to GelMA in control samples, migration between the hexagonal patterns was observed in GelMA/PANi ECHs.
More recently, Navaei et al. described a technique for micropatterning CMs onto ECHs by incorporating conductive GNRs into GelMA hydrogels to create microgrooved architectures [181]. Their approach utilized a micro-mold composed of PDMS with a microgrooved topography. A prepolymer solution containing GelMA and GNRs was pipetted on a 3-(Trimethoxysilyl)propyl methacrylate (TMSPMA)-coated slide, and the PDMS micromold was placed on top. (Figure 7a). The solution was then photopolymerized through exposure to UV irradiation. The resulting ECH constructs possessed microgrooves that were 50 µm in width. GelMA/GNR ECH constructs also exhibited significantly lower impedance (1.35 Ω 0.36 kΩ) as compared with pristine GelMA (15.58 Ω 9.18 kΩ) samples. In addition, cellular viability and morphology was investigated in vitro by seeding CMs between the microgrooves of GelMA-GNR and GelMA constructs. Their results showed there was an enhanced organization and spreading of CMs on GelMA/GNR ECHs (Figure 7b), when compared to pure GelMA hydrogels (Figure 7c) after 7 days of culture. This study demonstrated the efficacy of micropatterning techniques to fabricate ECHs with and highly organized structures that could be used to develop native-like cardiac tissues.
Figure 7. Micropatterning and self-assembling of ECHs for different biomedical applications.
Schematic of GelMA-GNR ECHs that were photocrosslinked in the presence of UV light in a microgrooved PDMS mold. The resulting ECH was seeded with CMs, which aligned within the microgrooves (a). Live/dead assay performed using CMs seeded on pure GelMA hydrogels (b) and GelMA-GNR ECHs (c). Live cells were stained in green, while dead CMs were stained in red, confirming high cell viability after 7 days of cell culture. [181] Schematic of supramolecular organic-inorganic composite capable of self-healing via interactions between oligomer chains (pink lines) with micro nickel and urea groups (blue and purple shapes) (d). Representative stress-strain curves for ECHs containing 31% micro nickel volume ratio under different healing conditions showing that healing efficiency was found to be a function of time (e). [183] Schematic of composite ECHs constructed with partially reduced GO using polydopamine, crosslinked with polyacrylamide (f). [184] Scale bars= 100 µm (b, c). Scale bars= 100 µm (b, c).
Sources:
[181], Copyright 2017. Reproduced with permission from the Royal Society of Chemistry.
[183], Copyright 2012. Reproduced with permission from Springer Nature.
[184], Copyright 2017. Reproduced with permission from John Wiley and Sons Inc.
Micropatterning of ECHs has also been performed to enhance therapies designed to stimulate nerve tissue regeneration. In a recent study, Lee et al. developed an ECH composed of PED and AgNWs with parallel microridges using a standard soft lithography process [182]. Briefly, a microgrooved PDMS stamp was fabricated and placed on a polyethylene terephthalate (PET) film. A prepolymer solution containing PEG and AgNWs was then injected between the grooves of the stamp and the PET film. The prepolymer solution was then photopolymerized using UV irradiation, and the PDMS stamp was peeled from the ECH. Electrical characterization of these ECHs was conducted using Comsol Multiphysics software to simulate the current at the wall of PEG/AgNW and PEG samples when a voltage of 10 V was applied. Results of this simulation showed that PEG/AgNW exhibited a significantly higher current as compared to pure PEG hydrogels due to the higher conductivity [182]. To determine if these materials could support the differentiation of NSCs into neurons, and guide neurite outgrowth, NSCs were incubated and grown into neurospheres, then seeded on PEG/AgNW and PEG samples. Following 1 day of culture, an intermediated electrical stimulus of 5, 10, and 20 V was applied. Results showed that neurite growth was much higher in the PEG/AgNW ECHs compared to PEG hydrogels [182]. Further, the neurite growth followed the microgrooves of the ECH, while neurite growth on PEG hydrogels appeared more randomly dispersed. The combination of high conductivity, robust mechanical properties, and micropatterns that support the differentiation of NSCs into neurons suggests that these ECHs are suitable for applications involving excitable cell types, such as neuronal and cardiac tissue engineering.
Overall, micropatterning of ECHs is a facile and precise method to control the 2D arrangement in a way that mimics the ECM of natural tissues. Advanced micropatterning techniques can also be used to guide cellular interactions thus influencing the function of tissues. However, the use of micropatterning ECHs does have some limitations. For example, one challenge is determining an appropriate UV exposure time that would photocrosslink hydrogels, but not affect cellular viability. Another limitation is the inability to micropattern 3D constructs that closely represent the microenvironment in vivo. However, ECHs with micropatterned surfaces shows potential in developing future 2D modeling systems that emulate human physiological functions. Taken together, micropatterned ECHs holds great potential for the development of stimuli-responsive systems for biomedical innovations, and to facilitate the development of new therapeutics aimed towards cardiac and neural tissue regeneration, as well as biosensors and drug delivery systems.
5.4. Self-Assembly / Self-Healing
Directly combining conductive components such as CPs and NPs into polymeric networks has been regarded as the most straightforward approach to synthesize ECHs [185–187]. However, conventional approaches to fabricating ECHs yields matrices with randomly suspended conducting components, which limits the ability to impart morphologies that enhance cell-material interactions [188]. To address this issue, self-assembly approaches have been introduced as an efficient strategy to develop a new class of smart materials with the ability to spontaneously form complex structures without external participation [189, 190]. Self-assembled hydrogels are characterized by the interaction of weak noncovalent bonds to form networks, such as hydrogen bonds, ionic interactions, van der Waals forces, and π-π stacking [191]. However, the absence of covalent bonds in these networks may result in weak mechanical properties making them unsuitable for biomedical applications. Therefore, it is often necessary to engineer self-assembling ECHs with either strong noncovalent bonds (e.g. ionic interactions, metal-ligand coordination), or multiple weak interaction sites (e.g. hydrogen bonds, VDW forces) [192].
Due to its unique structure, graphene has become a popular biomaterial capable of imparting selfassembling properties to ECHs. Self-assembled graphene hydrogels (SGH) have been increasingly used in multiple applications such as drug delivery systems [193], supercapacitors [194], and devices for human motion detection [195]. One study conducted by Xu et al., engineered SGHs with 3D networks that were fabricated using a single-step hydrothermal approach [189]. Briefly, this was achieved by heating 2 mg/mL of a homogenous GO solution to 180 °C for 12 h using an autoclave. The effect of this treatment hydrothermally reduced GO creating graphene sheets, which self-assembled into ECHs via π-π stacking interactions. Characterization of these SGHs showed that these materials exhibited high electrical conductivity (5 × 10−3 S/cm), high storage modulus (450–490 kPa), and high thermal stability [189]. It was therefore proposed that these self-assembling ECHs were suitable for biomedical applications, such as drug delivery systems, and tissue engineering.
ECHs with self-healing properties have also gained significant attention based on their ability to spontaneously restore the original functionality of ECHs after being damaged, which can prolong service life and avoid failure in therapeutic or diagnostic biomaterials [196]. This new class of smart material relies on dynamic and reversible noncovalent bonds, such as hydrophobic interactions, host-guest interactions, or hydrogen bonding to autonomous crosslink polymeric networks following physical, chemical, or mechanical damage to the ECHs [184]. Advanced self-healing smart materials, used for biomedical applications such as wound healing, where cuts, scratches, and breaks are common, would improve performance and lower the overall cost of these systems [196].
A study by Tee et al., described the development of biomimetic electronic skin sensors with enhanced mechanical sensing and self-healing properties [183]. This composite ECH was formed by using a supramolecular polymer capable of forming hydrogen bonds with itself, coupled with micro-nickel particles with nanoscale surface modifications (Figure 7d) [183]. The resulting ECH was able to self-heal by forming new hydrogen bonds in response to mechanical trauma, which in turn restores the structural integrity of the hydrogel. An important feature of supramolecular polymeric networks is their glass transition below room temperature, which allows for better movement of polymer chains after damage. These ECHs exhibited high electrical conductivity (40 S/cm), and increasingly higher elastic moduli with increasing concentrations of micro nickel particles. In addition, the engineered ECHs were able to electrically heal within 15 seconds after damage occurred (Figure 7e), which could enable the engineering of electric skin sensors, as well as flexible biosensors.
In another study, Han et al. developed a mussel-inspired self-adhesive, self-healing, stretchable ECH to be used as implantable and wearable bioelectronics [184]. These ECHs were fabricated in three steps. Briefly, dopamine was prepolymerized to form polydopamine (PDA). Next, partially reduced GO (pGO), where both GO and rGO were present, was achieved by exposure to PDA. Lastly, pGO and PDA were polymerized in the presence of acrylamide monomers, forming PDA/pGO/PAM composite ECHs (Figure 7f). The resulting pGO and PDA chains interacted with PAM forming hydrogen bonds and π-π stacking between catechol groups [184]. This ECH exhibited high conductivity, the ability to electronically and mechanically heal after damage, as well as high stretchability and toughness. Furthermore, the ECH possessed excellent attachment to native skin without the use of additional adhesives. Both in vitro studies using bone marrow stem cells and in vivo studies in a rabbit model demonstrated the high biocompatibility of the engineered ECHs. These studies demonstrate that selfhealing ECHs hold great potential for the development of implantable and flexible bioelectronics.
Smart ECHs capable of self-assembling have been utilized as scaffolds for various biomedical applications. Further, self-healing ECHs have been engineered to autonomously adapt to dynamic environments and serve to lengthen the service life of these implantable gels. Both of these classes of materials rely on weak noncovalent bonds to assemble and repair polymeric networks. However, due to the absence of stronger covalent bonds, these self-assembling and self-healing ECHs often lack the proper mechanical stiffness necessary for use as tissue regenerative therapies or drug delivery systems. In addition, the rational design of such complex structures is often a challenge and prediction of ECH behaviors is often based on empirical observations. Regardless, the benefits of these smart ECHs make them attractive materials for many biomedical related applications where self-regulation and durability are required.
6. Biomedical Applications of ECHs
ECHs constitute an emergent class of multifunctional and smart materials that possess technologically relevant properties for different biomedical applications. These properties stem mainly from their mechanical strength, high water uptake, tunable microarchitecture, and electrical conductivity, which are highly advantageous in the engineering of electrochemical biosensors, drug delivery systems, and excitable tissue constructs [11]. In addition, ECHs can be engineered to possess physicochemical characteristics that favor their implantation into the body and minimize the foreign body response (FBR), which could lead to fibrous encapsulation and implant failure [197]. These characteristics have allowed the engineering of biomedical devices that sense and modulate a wide array of electrically active physiological tissues such nerve, as well as cardiac and skeletal muscle.
6.1. Biosensors and electrode coatings
The engineering of low-cost, versatile, and scalable biosensors for the accurate and rapid detection and quantification of different metabolites and biomarkers, is of great interest for the pharmaceutical and healthcare industries [198]. One of the most widely reported application for ECHs is the engineering of electrochemical biosensors for the detection and quantification of different clinically relevant molecules. Electrochemical biosensors are composed of biological sensing elements (e.g., enzymes) that interact with a given analyte, producing a signal that is transduced by the ECH and transformed into an electrical signal (Figure 8a) [199]. Despite the remarkable potential of CPs as coatings for biomedical devices, they are often brittle when deposited as thin films, and are susceptible to the unspecific deposition of cells and proteins (i.e., biofouling) [197]. Their incorporation into ECHs effectively addresses these limitations since hydrogels can be molecularly engineered to prevent biofouling and possess increased mechanical resilience [108]. The mechanism by which ECHs transduce different physicochemical stimuli, as well as the different types of electroactive polymers used for this application have been reviewed elsewhere [14, 200–203].
Figure 8. ECHs for tissue engineering applications.
The addition of electrically conductive materials (red circles) can be used to provide conductive properties to intrinsically insulating hydrogels (a). These ECHs could be used to deliver relevant biophysical stimuli to cardiomyocytes in vitro, such as mechanical and biological cues, or electrical stimulation/pacing. These maturation cues have been shown to trigger phenotypical changes that ultimately lead to fully mature and functional cardiomyocytes. ECHs have been used to develop conductive nerve conduits as an alternative to nerve autografts for nerve regeneration and repair (b). [220]. ECHs could provide physiological stimuli that mimic native nerve tissues and induce the differentiation of progenitor cell types to neural lineages (c). [224] ECHs deliver biomimetic topographical and electrical cues in vitro to form highly oriented cellular constructs with tissue-level functionality (d). [181] Scale bars= 100 µm (d upper image), 20 µm (d lower image).
Sources:
[220], Copyright 2017. Reproduced with permission from the American Chemical Society.
[224], Copyright 2016. Reproduced with permission from John Wiley and Sons Inc.
[181], Copyright 2017. Reproduced with permission from the Royal Society of Chemisty.
Monoclonal antibodies (mAbs) could also be bound to ECHs to engineer immunosensing interfaces for the detection of biomarkers associated with different pathologies or cancer (Figure 8b). Wang et al. recently reported the engineering of an ECH composed of 1,3,5-benzenetricarboxylic acid and Fe3+, and electrochemically deposited AuNPs [204]. This ECH was then deposited on a glassy carbon electrode (GCE) to develop a highly sensitive label-free electrochemical immunosensor for the detection of neuron-specific enolase (NSE), a biomarker associated with lung cancer. In another recent study, Shin et al. reported the engineering of a biosensor based on the incorporation of mAbs to a PEG and PEDOTECH. This approach was used for the detection of bovine-interferon-γ, an inflammatory cytokine that can be used for diagnosing tuberculosis in cows [108]. In addition to enzymes and antibodies, biomolecules such as DNA (Figure 8c) and even whole cells (Figure 8d) can also be employed as biological recognition elements. For instance, Gao et al. reported the engineering of an electrochemical biosensor based on immobilized Saccharomyces cerevisiae cells on a chitosan hydrogel film with boron-doped nanocrystalline diamond particles, electrodeposited onto a GCE [205]. Their results demonstrated that this whole-cell biosensor could be used to assess the acute toxicity of waste water samples in a highly sensitive, integrated, and miniaturized fashion. Taken together, these studies demonstrate the potential of ECHs to engineer conductive and anti-fouling interfaces for biosensors, which allow electrochemical detection in complex media such as whole blood.
A different class of biosensors have been engineered through molecularly imprinted polymers (MIPs), which are artificial macromolecular networks with high affinity towards a specific template molecule (Figure 8e) [206]. This approach was used by Bayer et al. for the detection of specific protein biomarkers, since charged amino acids cause changes in the electroconductivity of an MIP upon binding of the target protein [206]. Furthermore, ECHs have also been explored for the engineering of stimuli-responsive devices that can sense changes in temperature. For instance, Shi et al. recently reported the combination of conductive PANi and PPy CPs, with a thermally responsive poly(N-isopropylacrylamide) (PNIPAM) hydrogel [207]. Using this approach, they engineered a smart material with high thermo-responsive sensitivity and electrical conductivity, as well as enhanced mechanical properties for stimuli-responsive electronic devices, and self-adaptive and flexible bioelectronics [207].
The use of ECHs as electrode coatings has also been explored for the engineering of tissue interfaces with enhanced biocompatibility for bionic implants (Figure 8f). The engineering of biomimetic coatings could theoretically reduce the effect of strain mismatch at the tissue interface, which would minimize the deposition of fibrous scar tissue and improve device function [14]. In this regard, Mario Cheong et al. described the covalent incorporation of bioactive molecules within a conducting poly(vinyl alcohol) (PVA)-heparin hydrogel with PEDOT [211]. To improve the biological interaction of the engineered ECH, sericin and gelatin were covalently attached to the matrix via methacrylate crosslinking. Their results showed that the composites could be used to deliver nerve growth factor (NGF) to target cells and could be further modified to incorporate bioactive motifs and deliver water-soluble drug molecules. A similar approach was recently reported by Goding et al., for the engineering of PEDOT/PVA-taurine composites as soft, hydrogel-based electrodes for low impedance neuroprosthetic devices [212]. In this study, they demonstrated the development of a novel ECH system that enabled the controlled incorporation of covalently attached conductive polymer dopants directly to the polymer backbone. Their results provided insight into the role of immobilized dopants in the fabrication of interpenetrating networks in ECHs, as well as in the morphological, electrochemical, and mechanical properties of the composites [212].
Conventional environmentally responsive hydrogels can respond to various cues such as temperature, pH, and ionic strength. More recently, stimulus-responsive ECHs have emerged as a new class of smart materials with high affinity towards specific physicochemical cues [200]. This high specificity is achieved through the incorporation of biomolecules with intrinsic molecular recognition ability (e.g., peptides, nucleic acids, mAbs, enzymes, etc.) into the polymer network. They can be engineered to possess different mechanisms of molecular recognition, and to trigger different responsive behaviors upon activation. In addition, the conductive matrixes provided by ECHs allowed the collection and rapid propagation of electric charge across large surface areas with high biocompatibility. Therefore, biosensors based on smart ECHs hold significant potential in different areas of clinical diagnostics and therapeutics, as implantable interfaces to sense and modulate different electroactive tissues.
6.2. Drug delivery systems
Recent advancements in nanotechnology and smart biomaterials have led to the engineering of systems that not only sense physiological stimuli, but could also respond to deliver a therapeutic output [201]. The delivery of therapeutic biomolecules to the implant site could, in turn, trigger specific tissue responses, or prevent the development of infection or inflammation [203]. Despite their intrinsic insulating nature, the porous architecture and high-water content of hydrogels provide the unique ability to load bioactive molecules or drugs into the polymer network. Moreover, ECHs respond to applied electrical fields either by swelling, shrinking, bending, or other structural changes due to minor differences in the electric potential across the hydrogel [200]. Previous studies demonstrated that HA-, alginate-, and chitosan-based hydrogels possess intrinsic electroactive properties due to the presence of acidic or basic ionic groups throughout their polymer networks [41]. However, their low electrical responsiveness and poor mechanical performance limit their application for implantable smart systems for long-term drug delivery [12]. More recently, the engineering of composite ECHs with enhanced mechanical and electroconductive properties has allowed the development of electrically controlled drug release devices (EDRDs) (Figure 8g) [208]. Moreover, the modulation of the electrical stimulus could be used to achieve different release profiles (e.g., burst release, slow-elution, etc.) due to combinatorial electrophoretic and electroosmotic effects [12]. The different mechanisms by which small biomolecules could be loaded into ECHs, as well as the various strategies used to trigger and modulate their release have been reviewed previously in the literature [200, 201, 208].
ECHs have also been used to deliver antimicrobial agents to prevent the development of infection at the implant site. For instance, Paradee et al., reported the engineering of a benzoic acid-loaded PEDOT-alginate composite for transdermal drug delivery [213]. Their results demonstrated that the release profile and the rate of diffusion of the drug were dependent on the crosslinking ratio, PEDOT polymer size, strength of the electric field, and the polarity of electrodes. ECHs could also be used to deliver anti-inflammatory drugs to improve the long-term biocompatibility of implanted biomedical devices. In this regard, Heo et al. recently reported the incorporation of cyclosporine A (CsA)-loaded PLGA microspheres into a PEDOT:PSS/PEG hydrogel (Figure 8h) [209]. The formation of microwells and chemical treatment of the surface of the electrode led to increased surface area for loading of high doses of the drug, as well as enhanced adhesion of the hydrogel to the electrode. Moreover, in vivo experiments showed that delivery of CsA resulted in a significant decrease in fibrous tissue deposition and increased axonal density [209]. In another study by Pairatwachapun et al., the diffusion coefficient of acetylsalicylic acid from carrageenan hydrogels was shown to be significantly enhanced by incorporating the CP PTh, followed by an applied electric field [214]. Another potential application for ECHs in EDRDs, is the development of platforms for transdermal patient-controlled analgesia. In recent years, the engineering of patch-like devices that possess both the microelectronic-processing mechanism along with the drug of interest in a small wearable device has been actively pursued (Figure 8i) [210, 215]. The engineering of iontophoretic patches for transdermal delivery would eliminate the need for conventional intravenous or epidural administration, resulting in lower costs and improved patient acceptability [210]. However, the passive diffusion of the drug into the aqueous physiological environment, as well as the precise modulation of the release profile and pharmacokinetics of the drug still constitute significant technical challenges [12]. Recent advancements in material science and other emerging areas such as gene editing technologies, could be integrated into EDRDs to engineer bioelectronics with enhanced therapeutic function [216]. These hybrid systems could one day monitor multiple parameters continuously and respond with the precise release of therapeutic molecules in real time.
6.3. Tissue engineering
Polymeric hydrogels have been extensively used as scaffolds to mimic biological functionality and induce the proliferation, differentiation, and migration of cells, both in vitro and in vivo (Figure 9a). Cells in physiological environments are exposed to various types of physicochemical cues that modulate the development and regeneration of tissues, including endogenous electric fields from excitable cell types. Therefore, ECHs have been increasingly explored for the engineering of functional tissue constructs, to control the behavior and promote the regeneration of excitable tissues [12]. To this end, the use of ECHs for the engineering of scaffolds for neural cell encapsulation, nerve conduits, and electrode coatings for neural tissue interfaces has been widely reported (Figure 9b). Previous studies have shown that neuroblastic PC-12 cells under electrical stimulation in vitro exhibit increased proliferation and differentiation [217–220], as well as a higher number of neurites with a greater overall length [28]. This behavior has been mainly associated to enhanced fibronectin adsorption onto the ECHs, coupled with the direct effect of the electrical field on the integral membrane proteins of these cells [221, 222]. In a different study by Yow et al., hMSCs growing on PPy-collagen hydrogels exhibited neuronal-like morphology and upregulation of noggin, MAP2, neurofilament, ß tubulin III, and nestin neural markers [223]. Using a similar approach, Yang et al. recently demonstrated that PPy-alginate hydrogels promoted the adhesion and growth of hMSCs, as well as overexpression of Tuj1 and MAP2 neural differentiation markers (Figure 9c) [224]. In addition, the engineered PPy-alginate hydrogels were shown to possess high biocompatibility in vivo, as demonstrated by subcutaneous implantation experiments. These studies demonstrate that ECHs are remarkably advantageous to study the effects of electrical fields on stem cells and/or neural cells in vitro, and to engineer heterocellular neural tissue constructs and interfaces for in vivo implantation.
Figure 9. ECHs for tissue engineering applications.
The addition of electrically conductive materials (red circles) can be used to provide conductive properties to intrinsically insulating hydrogels (a). These ECHs could be used to deliver relevant biophysical stimuli to cardiomyocytes in vitro, such as mechanical and biological cues, or electrical stimulation/pacing. These maturation cues have been shown to trigger phenotypical changes that ultimately lead to fully mature and functional cardiomyocytes. ECHs have been used to develop conductive nerve conduits as an alternative to nerve autografts for nerve regeneration and repair (b). [220], Copyright 2017. Reproduced with permission from American Chemical Society. ECHs could provide physiological stimuli that mimic native nerve tissues and induce the differentiation of progenitor cell types to neural lineages (c). [224], Copyright 2016. Reproduced with permission from John Wiley and Sons. ECHs deliver biomimetic topographical and electrical cues in vitro to form highly oriented cellular constructs with tissue-level functionality (d). [181], Copyright, 2017. Reproduced with permission from the Royal Society of Chemisty. Scale bars= 100 µm (d upper image), 20 µm (d lower image).
The complex organization and microarchitecture of the myocardium, along with the electrical and mechanical coupling between CMs, are critical for the maintenance of the synchronous contractility of the heart [225, 226]. In recent years, the engineering of cardiac tissue constructs using hydrogel-based biomaterials with biomimetic physicochemical cues have demonstrated great promise for cardiac tissue regeneration and repair [6, 227]. For instance, Dong et al. recently described the engineering of an injectable, self-healing ECHs based on chitosan-graft-aniline tetramer (CS-AT) and dibenzaldehyde-terminated poly(ethylene glycol) (PEG-DA) [228]. Their results demonstrated that the engineered ECH could be used to deliver C2C12 and H9c2 myoblasts in vivo, and that they possessed high biocompatibility and biodegradability. In another study by Baei et al., thermosensitive chitosan-AuNPs ECHs were shown to support the proliferation, migration, and differentiation of hMSCs, as shown by the expression of the α-myosin heavy chain (α-MHC) and Nkx-2.5 cardiac markers [34]. Furthermore, in addition to electrical stimulation, the delivery of mechanical and topographical cues could also be incorporated into multifunctional ECHs. In this regard, Gelmi et al. recently reported the use of a PLGA-PPy hydrogel as an electromechanically active scaffold to promote the engraftment, proliferation, and differentiation of induced pluripotent stem cells (iPSCs) into CMs [229]. The mechanical actuation of the scaffold enabled individual microfiber actuation to provide encompassing, coherent physiological strain to individual cells, which mimicked the native mechanical flow and force in the heart. In another study by Navaei et al., 50 µm microgrooves were incorporated into gelatin GelMA hydrogels with electrically conductive GNRs (Figure 9d) [181]. Fluorescent images revealed uniform, dense, and highly aligned cellular organization, as well as enhanced cytoskeletal alignment and cellular connectivity.
In addition to cardiac and neural cell types, ECHs have also been shown to modulate the proliferation and differentiation of preosteoblasts MC3T3-E1 for bone tissue engineering [230], as well as human primary skin fibroblasts for wound healing [231, 232]. However, conventional methods for engineering ECHs through the incorporation of conductive nanomaterials and polymers are often associated with poor solubility, processability, biodegradability, and biocompatibility [233]. To address these limitations, our group recently reported a new method to engineer electroconductive materials from inherently non-conductive polymers, through the conjugation of a choline-based Bio-IL [20]. Our results demonstrated that GelMA/Bio-IL ECHs could be used to modulate the proliferation and contractile function of primary rat CMs in vitro. Furthermore, as current understanding of biological processes increases, and new multifunctional smart biomaterials are developed, novel applications of ECHs in tissue engineering will be explored. For instance, ECHs could be used to engineer bioactuators that transduce electrical stimuli into mechanical force, which in turn could be used as biomimetic muscle fibers [203]. ECHs could also be used to develop transparent and deformable touch sensors for emerging applications, such as bionic skin, as well as flexible/wearable biomedical devices [234].
Concluding Remarks and Future Perspectives
Herein, we have discussed several strategies used to impart electrical conductivity into hydrogels, such as the incorporation of metal NPs, graphene, or CNTs, as well as several innately CPs such as PANi, PPy, or PEDOT. The combination of these synthesis methods and biomaterials has enabled the development of ECHs that demonstrate remarkable potential for use in clinically relevant biomedical applications, including tissue engineering, drug delivery systems, flexible electronics, and biosensors. However, some of the major obstacles for these biomaterials lies in addressing issues with their processability, and cytotoxicity. Further, research has primarily focused on the in vitro biocompatibility of these materials. Future research must investigate the biocompatibility of these materials in vivo using animal studies before clinical trials. In addition, merging ECHs with advanced microfabrication techniques have allowed the accurate reproduction of the native structural properties of the ECM, which in turn enhances the functionality of ECHs in physiological environments. Future research on ECHs will focus on addressing the limitations of current fabrication techniques, such as producing self-healing ECHs with more robust mechanical properties for tissue engineering. In addition, 3D bioprinting technologies will continue to improve the resolution, speed, and compatibility with other biomaterials. It will be essential to develop ECH-based bioinks that may be 3D printed into constructs with suitable mechanical and electrical properties, and integrity for biomedical applications. In addition to 3D printing, other microfabrication techniques have been used to develop ECHs with precise structure and function and will undoubtedly be instrumental in the development of future translational biomedical technologies.
Acknowledgements
N.A. acknowledges the support from the American Heart Association (AHA, 16SDG31280010), and National Institutes of Health (R01- EB023052; R01HL140618).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Li W, Li T, Li G, An L, Li F, Zhang Z. Electrospun H4SiW12O40/cellulose acetate composite nanofibrous membrane for photocatalytic degradation of tetracycline and methyl orange with different mechanism. Carbohydr Polym 2017;168:153–62. [DOI] [PubMed] [Google Scholar]
- [2].Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J 2015;65:252–67. [Google Scholar]
- [3].Ullah F, Othman MB, Javed F, Ahmad Z, Md Akil H. Classification, processing and application of hydrogels: A review. Mater Sci Eng C 2015;57:414–33. [DOI] [PubMed] [Google Scholar]
- [4].Jen AC, Wake MC, Mikos AG. Review: Hydrogels for cell immobilization. Biotechnol Bioeng 1996;50:357–64. [DOI] [PubMed] [Google Scholar]
- [5].Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res 2015;6:105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guan X, Avci-Adali M, Alarcin E, Cheng H, Kashaf SS, Li Y, et al. Development of hydrogels for regenerative engineering. Biotechnol J 2017;12:1600394/1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buwalda SJ, Boere KW, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release 2014;190:254–73. [DOI] [PubMed] [Google Scholar]
- [8].Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater 2014;26:85–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sood N, Bhardwaj A, Mehta S, Mehta A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv 2016;23:758–80. [DOI] [PubMed] [Google Scholar]
- [10].Green RA, Hassarati RT, Goding JA, Baek S, Lovell NH, Martens PJ, et al. Conductive hydrogels: mechanically robust hybrids for use as biomaterials. Macromol Biosci 2012;12:494501. [DOI] [PubMed] [Google Scholar]
- [11].Mawad D, Lauto A, Wallace GG. Conductive Polymer Hydrogels. In: Kalia S, editor. Polymeric Hydrogels as Smart Biomaterials Cham: Springer International Publishing, 2016. p. 19–44. [Google Scholar]
- [12].Shi Z, Gao X, Ullah MW, Li S, Wang Q, Yang G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016;111:40–54. [DOI] [PubMed] [Google Scholar]
- [13].Guiseppi-Elie A Electroconductive hydrogels: synthesis, characterization and biomedical applications. Biomaterials 2010;31:2701–16. [DOI] [PubMed] [Google Scholar]
- [14].Green RA, Baek S, Poole-Warren LA, Martens PJ. Conducting polymer-hydrogels for medical electrode applications. Sci Technol Adv Mater 2010;11:014107/1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hardy JG, Lee JY, Schmidt CE. Biomimetic conducting polymer-based tissue scaffolds. Curr Opin Biotechnol 2013;24:847–54. [DOI] [PubMed] [Google Scholar]
- [16].Guo B, Glavas L, Albertsson A-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog Polym Sci 2013;38:1263–86. [Google Scholar]
- [17].Das S, Chakraborty P, Mondal S, Shit A, Nandi AK. Enhancement of Energy Storage and Photoresponse Properties of Folic Acid-Polyaniline Hybrid Hydrogel by in Situ Growth of Ag Nanoparticles. ACS Appl Mater Interfaces 2016;8:28055–67. [DOI] [PubMed] [Google Scholar]
- [18].Zare Y, Shabani I. Polymer/metal nanocomposites for biomedical applications. Mater Sci Eng C 2016;60:195–203. [DOI] [PubMed] [Google Scholar]
- [19].Kong L, Chen W. Carbon nanotube and graphene-based bioinspired electrochemical actuators. Adv Mater 2014;26:1025–43. [DOI] [PubMed] [Google Scholar]
- [20].Noshadi I, Walker BW, Portillo-Lara R, Shirzaei Sani E, Gomes N, Aziziyan MR, et al. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci Rep 2017;7:4345/1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Q, Wang Q, Teng W. Injectable, degradable, electroactive nanocomposite hydrogels containing conductive polymer nanoparticles for biomedical applications. Int J Nanomed 2016;11:131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li Y, Kilian KA. Bridging the Gap: From 2D Cell Culture to 3D Microengineered Extracellular Matrices. Adv Healthc Mater 2015;4:2780–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee GH, Lee JS, Wang X, Lee SH. Bottom-Up Engineering of Well-Defined 3D Microtissues Using Microplatforms and Biomedical Applications. Adv Healthc Mater 2016;5:56–74. [DOI] [PubMed] [Google Scholar]
- [24].Qazi TH, Rai R, Dippold D, Roether JE, Schubert DW, Rosellini E, et al. Development and characterization of novel electrically conductive PANI-PGS composites for cardiac tissue engineering applications. Acta Biomater 2014;10:2434–45. [DOI] [PubMed] [Google Scholar]
- [25].Stout DA, Yoo J, Santiago-Miranda AN, Webster TJ. Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular application. Int J Nanomed 2012;7:5653–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brocker DT, Grill WM. Principles of electrical stimulation of neural tissue. Handb Clin Neurol 2013;116:3–18. [DOI] [PubMed] [Google Scholar]
- [27].Zarrintaj P, Bakhshandeh B, Saeb MR, Sefat F, Rezaeian I, Ganjali MR, et al. Oligoaniline-based conductive biomaterials for tissue engineering. Acta Biomater 2018;72:16–34. [DOI] [PubMed] [Google Scholar]
- [28].Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater 2014;10:2341–53. [DOI] [PubMed] [Google Scholar]
- [29].Duan B State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann Biomed Eng 2017;45:195–209. [DOI] [PubMed] [Google Scholar]
- [30].Al-Enizi AM, Zagho MM, Elzatahry AA. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018;8:259/1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li J, Zhang K, Huang N. Engineering Cardiovascular Implant Surfaces to Create a Vascular Endothelial Growth Microenvironment. Biotechnol J 2017;12:1600401/1–8. [DOI] [PubMed] [Google Scholar]
- [32].Thomas V, Namdeo M, Murali Mohan Y, Bajpai SK, Bajpai M. Review on Polymer, Hydrogel and Microgel Metal Nanocomposites: A Facile Nanotechnological Approach. J Macromol Sci Part A 2007;45:107–19. [Google Scholar]
- [33].Li F, Lu J, Kong X, Hyeon T, Ling D. Dynamic Nanoparticle Assemblies for Biomedical Applications. Adv Mater 2017;29:1605897/1–30. [DOI] [PubMed] [Google Scholar]
- [34].Baei P, Jalili-Firoozinezhad S, Rajabi-Zeleti S, Tafazzoli-Shadpour M, Baharvand H, Aghdami N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater Sci Eng C 2016;63:131–41. [DOI] [PubMed] [Google Scholar]
- [35].Kim JH, Lee TR. Hydrogel-templated growth of large gold nanoparticles: synthesis of thermally responsive hydrogel-nanoparticle composites. Langmuir 2007;23:6504–9. [DOI] [PubMed] [Google Scholar]
- [36].Jiang XM, Wang LM, Wang J, Chen CY. Gold nanomaterials: preparation, chemical modification, biomedical applications and potential risk assessment. Appl Biochem Biotechnol 2012;166:1533–51. [DOI] [PubMed] [Google Scholar]
- [37].Cobley CM, Chen J, Cho EC, Wang LV, Xia Y. Gold nanostructures: a class of multifunctional materials for biomedical applications. Chem Soc Rev 2011;40:44–56. [DOI] [PubMed] [Google Scholar]
- [38].Navaei A, Saini H, Christenson W, Sullivan RT, Ros R, Nikkhah M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater 2016;41:133–46. [DOI] [PubMed] [Google Scholar]
- [39].Shevach M, Maoz BM, Feiner R, Shapira A, Dvir T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J Mater Chem B 2013;1:5210–7. [DOI] [PubMed] [Google Scholar]
- [40].Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev 2012;112:2739–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Culver HR, Clegg JR, Peppas NA. Erratum: Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc Chem Res 2018;51:2600–0. [DOI] [PubMed] [Google Scholar]
- [42].Vial S, Reis RL, Oliveira JM. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr Opin Solid State Mater Sci 2017;21:92–112. [Google Scholar]
- [43].Kim KS, Lee D, Song CG, Kang PM. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 2015;10:2709–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev 2015;24:1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu Q, He C, Xiao C, Chen X. Reactive Oxygen Species (ROS) Responsive Polymers for Biomedical Applications. Macromol Biosci 2016;16:635–46. [DOI] [PubMed] [Google Scholar]
- [46].Khaing Oo MK, Yang Y, Hu Y, Gomez M, Du H, Wang H. Gold nanoparticle-enhanced and size-dependent generation of reactive oxygen species from protoporphyrin IX. ACS Nano 2012;6:1939–47. [DOI] [PubMed] [Google Scholar]
- [47].Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 2014;22:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 2011;40:1647–71. [DOI] [PubMed] [Google Scholar]
- [49].Strong LE, West JL. Thermally responsive polymer-nanoparticle composites for biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011;3:307–17. [DOI] [PubMed] [Google Scholar]
- [50].Das R, Das D, Ghosh P, Dhara S, Panda AB, Pal S. Development and application of a nanocomposite derived from crosslinked HPMC and Au nanoparticles for colon targeted drug delivery. RSC Adv 2015;5:27481–90. [Google Scholar]
- [51].Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ. Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv Sci 2015;2:1400010/1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Strong LE, Dahotre SN, West JL. Hydrogel-nanoparticle composites for optically modulated cancer therapeutic delivery. J Control Release 2014;178:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhu Q, Bao J, Huo D, Yang M, Hou C, Guo J, et al. 3D Graphene hydrogel – gold nanoparticles nanocomposite modified glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Sens Actuators B 2017;238:1316–23. [Google Scholar]
- [54].Bodelón G, Costas C, Pérez-Juste J, Pastoriza-Santos I, Liz-Marzán LM. Gold nanoparticles for regulation of cell function and behavior. Nano Today 2017;13:40–60. [Google Scholar]
- [55].Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015;73:254–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K, et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 2012;33:9009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol 2011;6:720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Annabi N, Shin SR, Tamayol A, Miscuglio M, Bakooshli MA, Assmann A, et al. Highly Elastic and Conductive Human-Based Protein Hybrid Hydrogels. Adv Mater 2016;28:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ahadian S, Yamada S, Ramon-Azcon J, Estili M, Liang X, Nakajima K, et al. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater 2016;31:134–43. [DOI] [PubMed] [Google Scholar]
- [60].Pan Y, Bartneck M, Jahnen-Dechent W. Cytotoxicity of Gold Nanoparticles. In: Düzgüneş N, editor. Methods in Enzymology Vol 509 Oxford: Academic Press, 2012. p. 225–42. [DOI] [PubMed] [Google Scholar]
- [61].Zhao X, Ding X, Deng Z, Zheng Z, Peng Y, Long X. Thermoswitchable electronic properties of a gold nanoparticle/hydrogel composite. Macromol Rapid Commun 2005;26:1784–7. [Google Scholar]
- [62].Varaprasad K, Mohan YM, Ravindra S, Reddy NN, Vimala K, Monika K, et al. Hydrogel-silver nanoparticle composites: A new generation of antimicrobials. J Appl Polym Science 2010;115:1199–207. [Google Scholar]
- [63].Reithofer MR, Lakshmanan A, Ping AT, Chin JM, Hauser CA. In situ synthesis of size-controlled, stable silver nanoparticles within ultrashort peptide hydrogels and their anti-bacterial properties. Biomaterials 2014;35:7535–42. [DOI] [PubMed] [Google Scholar]
- [64].Varaprasad K, Mohan YM, Vimala K, Mohana Raju K. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J Appl Polym Science 2011;121:784–96. [Google Scholar]
- [65].Zhao F, Yao D, Guo R, Deng L, Dong A, Zhang J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015;5:2054–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee W-F, Tsao K-T. Preparation and properties of nanocomposite hydrogels containing silver nanoparticles by ex situ polymerization. J Appl Polym Science 2006;100:3653–61. [Google Scholar]
- [67].Devaki SJ, Narayanan RK, Sarojam S. Electrically conducting silver nanoparticle–polyacrylic acid hydrogel by in situ reduction and polymerization approach. Mater Lett 2014;116:135–8. [Google Scholar]
- [68].Jo H, Sim M, Kim S, Yang S, Yoo Y, Park JH, et al. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater 2017;48:100–9. [DOI] [PubMed] [Google Scholar]
- [69].Yang X, Qiu L, Cheng C, Wu Y, Ma ZF, Li D. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films. Angew Chem Int Ed 2011;50:7325–8. [DOI] [PubMed] [Google Scholar]
- [70].Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng 2014;111:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang L, Shi G. Preparation of highly conductive graphene hydrogels for fabricating supercapacitors with high rate capability. J Phys Chem C 2011;115:17206–12. [Google Scholar]
- [72].Cui Z, Zhou M, Greensmith PJ, Wang W, Hoyland JA, Kinloch IA, et al. A study of conductive hydrogel composites of pH-responsive microgels and carbon nanotubes. Soft Matter 2016;12:4142–53. [DOI] [PubMed] [Google Scholar]
- [73].Sang L, Liu Y, Hua W, Xu K, Wang G, Zhong W, et al. Thermally sensitive conductive hydrogel using amphiphilic crosslinker self-assembled carbon nanotube to enhance neurite outgrowth and promote spinal cord regeneration. RSC Adv 2016;6:26341–51. [Google Scholar]
- [74].Li W, Gao F, Wang X, Zhang N, Ma M. Strong and Robust Polyaniline-Based Supramolecular Hydrogels for Flexible Supercapacitors. Angew Chem Int Ed 2016;55:9196–201. [DOI] [PubMed] [Google Scholar]
- [75].Xia Y, Zhu H. Polyaniline nanofiber-reinforced conducting hydrogel with unique pH-sensitivity. Soft Matter 2011;7:9388–93. [Google Scholar]
- [76].Hur J, Im K, Kim SW, Kim J, Chung DY, Kim TH, et al. Polypyrrole/Agarose-based electronically conductive and reversibly restorable hydrogel. ACS Nano 2014;8:10066–76. [DOI] [PubMed] [Google Scholar]
- [77].Carquigny S, Lakard B, Lakard S, Moutarlier V, Hihn J-Y, Viau L. Investigation of pharmaceutically active ionic liquids as electrolyte for the electrosynthesis of polypyrrole and active component in controlled drug delivery. Electrochim Acta 2016;211:950–61. [Google Scholar]
- [78].Samanta D, Meiser JL, Zare RN. Polypyrrole nanoparticles for tunable, pH-sensitive and sustained drug release. Nanoscale 2015;7:9497–504. [DOI] [PubMed] [Google Scholar]
- [79].Zhao X, Li P, Guo B, Ma PX. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater 2015;26:236–48. [DOI] [PubMed] [Google Scholar]
- [80].Pattavarakorn D, Youngta P, Jaesrichai S, Thongbor S, Chaimongkol P. Electroactive performances of conductive polythiophene/hydrogel hybrid artificial muscle. Energy Procedia 2013;34:673–81. [Google Scholar]
- [81].Chen L, Kim B, Nishino M, Gong JP, Osada Y. Environmental responses of polythiophene hydrogels. Macromolecules 2000;33:1232–6. [Google Scholar]
- [82].Kim YS, Cho K, Lee HJ, Chang S, Lee H, Kim JH, et al. Highly conductive and hydrated PEG-based hydrogels for the potential application of a tissue engineering scaffold. React Funct Polym 2016;109:15–22. [Google Scholar]
- [83].Spencer AR, Primbetova A, Koppes AN, Koppes RA, Fenniri H, Annabi N. Electroconductive Gelatin Methacryloyl-PEDOT:PSS Composite Hydrogels: Design, Synthesis, and Properties. ACS Biomater Sci Eng 2018;4:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dai T, Qing X, Lu Y, Xia Y. Conducting hydrogels with enhanced mechanical strength. Polymer 2009;50:5236–41. [Google Scholar]
- [85].Yang L, Deng W, Zhang Y, Tan Y, Ma M, Xie Q. Boosting current generation in microbial fuel cells by an order of magnitude by coating an ionic liquid polymer on carbon anodes. Biosens Bioelectron 2017;91:644–9. [DOI] [PubMed] [Google Scholar]
- [86].Huang X, Guo H, Wang K, Liu X. Ionic liquid induced surface trap-state passivation for efficient perovskite hybrid solar cells. Organic Electrs 2017;41:42–8. [Google Scholar]
- [87].Romann T, Anderson E, Pikma P, Tamme H, Möller P, Lust E. Reactions at graphene|tetracyanoborate ionic liquid interface – New safety mechanisms for supercapacitors and batteries. Electrochem Commun 2017;74:38–41. [Google Scholar]
- [88].Viswanathan G, Murugesan S, Pushparaj V, Nalamasu O, Ajayan PM, Linhardt RJ. Preparation of biopolymer fibers by electrospinning from room temperature ionic liquids. Biomacromolecules 2006;7:415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Murthy PS, Murali Mohan Y, Varaprasad K, Sreedhar B, Mohana Raju K. First successful design of semi-IPN hydrogel-silver nanocomposites: a facile approach for antibacterial application. J Colloid Interface Sci 2008;318:217–24. [DOI] [PubMed] [Google Scholar]
- [90].Bajpai SK, Chand N, Mahendra M. In situformation of silver nanoparticles in poly(methacrylic acid) hydrogel for antibacterial applications. Polym Eng Sci 2013;53:1751–9. [Google Scholar]
- [91].Zhang W, Li Y, Niu J, Chen Y. Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir 2013;29:4647–51. [DOI] [PubMed] [Google Scholar]
- [92].Rattanaruengsrikul V, Pimpha N, Supaphol P. In vitro efficacy and toxicology evaluation of silver nanoparticle-loaded gelatin hydrogel pads as antibacterial wound dressings. J Appl Polym Science 2012;124:1668–82. [Google Scholar]
- [93].Grade S, Eberhard J, Neumeister A, Wagener P, Winkel A, Stiesch M, et al. Serum albumin reduces the antibacterial and cytotoxic effects of hydrogel-embedded colloidal silver nanoparticles. RSC Adv 2012;2:7190–6. [Google Scholar]
- [94].Garcia-Astrain C, Chen C, Buron M, Palomares T, Eceiza A, Fruk L, et al. Biocompatible hydrogel nanocomposite with covalently embedded silver nanoparticles. Biomacromolecules 2015;16:1301–10. [DOI] [PubMed] [Google Scholar]
- [95].Haider A, Kang I-K. Preparation of silver nanoparticles and their industrial and biomedical applications: a comprehensive review. Adv Mater Sci Eng 2015;2015:165257/1–16. [Google Scholar]
- [96].Xiang Y, Chen D. Preparation of a novel pH-responsive silver nanoparticle/poly(HEMA– PEGMA–MAA) composite hydrogel. Eur Polym J 2007;43:4178–87. [Google Scholar]
- [97].Ahn Y, Lee H, Lee D, Lee Y. Highly conductive and flexible silver nanowire-based microelectrodes on biocompatible hydrogel. ACS Appl Mater Interfaces 2014;6:18401–7. [DOI] [PubMed] [Google Scholar]
- [98].Gurunathan S, Kim JH. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int J Nanomed 2016;11:1927–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Servant A, Leon V, Jasim D, Methven L, Limousin P, Fernandez-Pacheco EV, et al. Graphene-based electroresponsive scaffolds as polymeric implants for on-demand drug delivery. Adv Healthc Mater 2014;3:1334–43. [DOI] [PubMed] [Google Scholar]
- [100].Yang X, Zhang X, Ma Y, Huang Y, Wang Y, Chen Y. Superparamagnetic graphene oxide–Fe 3 O 4 nanoparticles hybrid for controlled targeted drug carriers. J Mater Chem 2009;19:2710–4. [Google Scholar]
- [101].Chen C, Yang C, Li S, Li D. A three-dimensionally chitin nanofiber/carbon nanotube hydrogel network for foldable conductive paper. Carbohydr Polym 2015;134:309–13. [DOI] [PubMed] [Google Scholar]
- [102].Ahadian S, Ramon-Azcon J, Estili M, Liang X, Ostrovidov S, Shiku H, et al. Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Sci Rep 2014;4:4271/1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chen Z, To JWF, Wang C, Lu Z, Liu N, Chortos A, et al. A Three-Dimensionally Interconnected Carbon Nanotube-Conducting Polymer Hydrogel Network for High-Performance Flexible Battery Electrodes. Adv Energy Mater 2014;4:1400207/1–12. [Google Scholar]
- [104].Szollosi A, Hoschke A, Rezessy-Szabo JM, Bujna E, Kun S, Nguyen QD. Formation of novel hydrogel bio-anode by immobilization of biocatalyst in alginate/polyaniline/titanium-dioxide/graphite composites and its electrical performance. Chemosphere 2017;174:58–65. [DOI] [PubMed] [Google Scholar]
- [105].Dispenza C, Presti CL, Belfiore C, Spadaro G, Piazza S. Electrically conductive hydrogel composites made of polyaniline nanoparticles and poly(N-vinyl-2-pyrrolidone). Polymer 2006;47:961–71. [Google Scholar]
- [106].Pillay V, Tsai TS, Choonara YE, du Toit LC, Kumar P, Modi G, et al. A review of integrating electroactive polymers as responsive systems for specialized drug delivery applications. J Biomed Mater Res A 2014;102:2039–54. [DOI] [PubMed] [Google Scholar]
- [107].Yang J, Choe G, Yang S, Jo H, Lee JY. Polypyrrole-incorporated conductive hyaluronic acid hydrogels. Biomater Res 2016;20:31/1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Shin DS, Matharu Z, You J, Siltanen C, Vu T, Raghunathan VK, et al. Sensing Conductive Hydrogels for Rapid Detection of Cytokines in Blood. Adv Healthc Mater 2016;5:659–64, 27. [DOI] [PubMed] [Google Scholar]
- [109].Brahim S, Narinesingh D, Guiseppi-Elie A. Polypyrrole-hydrogel composites for the construction of clinically important biosensors. Biosens Bioelectron 2002;17:53–9. [DOI] [PubMed] [Google Scholar]
- [110].Kim DH, Abidian M, Martin DC. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J Biomed Mater Res A 2004;71:577–85. [DOI] [PubMed] [Google Scholar]
- [111].Ketabat F, Karkhaneh A, Mehdinavaz Aghdam R, Hossein Ahmadi Tafti S. Injectable conductive collagen/alginate/polypyrrole hydrogels as a biocompatible system for biomedical applications. J Biomater Sci Polym Ed 2017;28:794–805. [DOI] [PubMed] [Google Scholar]
- [112].Liu M, Xu N, Liu W, Xie Z. Polypyrrole coated PLGA core–shell nanoparticles for drug delivery and photothermal therapy. RSC Adv 2016;6:84269–75. [Google Scholar]
- [113].Kim SY, Palmore GTR. Conductive hydrogel for bio-electrocatalytic reduction of dioxygen. Electrochem Commun 2012;23:90–3. [Google Scholar]
- [114].Shi Y, Pan L, Liu B, Wang Y, Cui Y, Bao Z, et al. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J Mater Chem A 2014;2:6086–91. [Google Scholar]
- [115].Spearman BS, Hodge AJ, Porter JL, Hardy JG, Davis ZD, Xu T, et al. Conductive interpenetrating networks of polypyrrole and polycaprolactone encourage electrophysiological development of cardiac cells. Acta Biomater 2015;28:109–20. [DOI] [PubMed] [Google Scholar]
- [116].Mihic A, Cui Z, Wu J, Vlacic G, Miyagi Y, Li SH, et al. A Conductive Polymer Hydrogel Supports Cell Electrical Signaling and Improves Cardiac Function After Implantation into Myocardial Infarct. Circulation 2015;132:772–84. [DOI] [PubMed] [Google Scholar]
- [117].Ismail YA, Martínez JG, Al Harrasi AS, Kim SJ, Otero TF. Sensing characteristics of a conducting polymer/hydrogel hybrid microfiber artificial muscle. Sens Actuators B 2011;160:1180–90. [Google Scholar]
- [118].Jeong B, Gutowska A. Lessons from nature: stimuli-responsive polymers and their biomedical applications. Trends Biotechnol 2002;20:305–11. [DOI] [PubMed] [Google Scholar]
- [119].Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J Biomed Mater Res A 2011;99:376–85. [DOI] [PubMed] [Google Scholar]
- [120].Mawad D, Artzy-Schnirman A, Tonkin J, Ramos J, Inal S, Mahat MM, et al. Electroconductive Hydrogel Based on Functional Poly(Ethylenedioxy Thiophene). Chem Mater 2016;28:6080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Naficy S, Razal JM, Spinks GM, Wallace GG, Whitten PG. Electrically Conductive, Tough Hydrogels with pH Sensitivity. Chem Mater 2012;24:3425–33. [Google Scholar]
- [122].Hassarati RT, Dueck WF, Tasche C, Carter PM, Poole-Warren LA, Green RA. Improving cochlear implant properties through conductive hydrogel coatings. IEEE Trans Neural Syst Rehabil Eng 2014;22:411–8. [DOI] [PubMed] [Google Scholar]
- [123].Wu Q, Wei J, Xu B, Liu X, Wang H, Wang W, et al. A robust, highly stretchable supramolecular polymer conductive hydrogel with self-healability and thermo-processability. Sci Rep 2017;7:41566/1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Aouada FA, Guilherme MR, Campese GM, Girotto EM, Rubira AF, Muniz EC. Electrochemical and mechanical properties of hydrogels based on conductive poly(3,4-ethylene dioxythiophene)/poly(styrenesulfonate) and PAAm. Polym Test 2006;25:158–65. [Google Scholar]
- [125].Zhang X, Chang D, Liu J, Luo Y. Conducting polymer aerogels from supercritical CO2 drying PEDOT-PSS hydrogels. J Mater Chem 2010;20:5080–5. [Google Scholar]
- [126].Larsson KC, Kjall P, Richter-Dahlfors A. Organic bioelectronics for electronic-to-chemical translation in modulation of neuronal signaling and machine-to-brain interfacing. Biochim Biophys Acta 2013;1830:4334–44. [DOI] [PubMed] [Google Scholar]
- [127].Yoon SG, Chang ST. Microfluidic capacitive sensors with ionic liquid electrodes and CNT/PDMS nanocomposites for simultaneous sensing of pressure and temperature. J Mater Chem C 2017;5:1910–9. [Google Scholar]
- [128].Zhou T, Gao X, Dong B, Sun N, Zheng L. Poly(ionic liquid) hydrogels exhibiting superior mechanical and electrochemical properties as flexible electrolytes. J Mater Chem A 2016;4:1112–8. [Google Scholar]
- [129].Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem 2001;3:156–64. [Google Scholar]
- [130].Chanie Y, Díaz I, Pérez E. Kinetics and mechanisms of adsorption/desorption of the ionic liquid 1-buthyl-3-methylimidazolium bromide into mordenite. J Chem Technol Biotechnol 2016;91:705–10. [Google Scholar]
- [131].Lei Z, Chen B, Koo YM, MacFarlane DR. Introduction: Ionic Liquids. Chem Rev 2017;117:6633–5. [DOI] [PubMed] [Google Scholar]
- [132].Gallagher S, Florea L, Fraser KJ, Diamond D. Swelling and shrinking properties of thermo-responsive polymeric ionic liquid hydrogels with embedded linear pNIPAAM. Int J Mol Sci 2014;15:5337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Egorova KS, Gordeev EG, Ananikov VP. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem Rev 2017;117:7132–89. [DOI] [PubMed] [Google Scholar]
- [134].Robinson SS, O’Brien KW, Zhao H, Peele BN, Larson CM, Mac Murray BC, et al. Integrated soft sensors and elastomeric actuators for tactile machines with kinesthetic sense. Extreme Mech Lett 2015;5:47–53. [Google Scholar]
- [135].Liang X, Qu B, Li J, Xiao H, He B, Qian L. Preparation of cellulose-based conductive hydrogels with ionic liquid. React Funct Polym 2015;86:1–6. [Google Scholar]
- [136].Raney JR, Lewis JA. Printing mesoscale architectures. MRS Bull 2015;40:943–50. [Google Scholar]
- [137].Agarwala S, Lee JM, Ng WL, Layani M, Yeong WY, Magdassi S. A novel 3D bioprinted flexible and biocompatible hydrogel bioelectronic platform. Biosens Bioelectron 2018;102:365–71. [DOI] [PubMed] [Google Scholar]
- [138].Leberfinger AN, Ravnic DJ, Dhawan A, Ozbolat IT. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl Med 2017;6:1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Coburn JC, Grant GT. FDA Regulatory Pathways and Technical Considerations for the 3D Printing of Medical Models and Devices. In: Rybicki FJ, Grant GT, editors. 3D Printing in Medicine Cham: Springer, 2017. p. 97–111. [Google Scholar]
- [140].Liu Y, He K, Chen G, Leow WR, Chen X. Nature-Inspired Structural Materials for Flexible Electronic Devices. Chem Rev 2017;117:12893–941. [DOI] [PubMed] [Google Scholar]
- [141].Tai Y, Mulle M, Aguilar Ventura I, Lubineau G. A highly sensitive, low-cost, wearable pressure sensor based on conductive hydrogel spheres. Nanoscale 2015;7:14766–73. [DOI] [PubMed] [Google Scholar]
- [142].Wei S, Qu G, Luo G, Huang Y, Zhang H, Zhou X, et al. Scalable and Automated Fabrication of Conductive Tough-Hydrogel Microfibers with Ultrastretchability, 3D Printability, and Stress Sensitivity. ACS Appl Mater Interfaces 2018;10:11204–12. [DOI] [PubMed] [Google Scholar]
- [143].Tian K, Bae J, Bakarich SE, Yang C, Gately RD, Spinks GM, et al. 3D Printing of Transparent and Conductive Heterogeneous Hydrogel-Elastomer Systems. Adv Mater 2017;29:1604827/1–8. [DOI] [PubMed] [Google Scholar]
- [144].Guo SZ, Qiu K, Meng F, Park SH, McAlpine MC. 3D Printed Stretchable Tactile Sensors. Adv Mater 2017;29:1701218/1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Li L, Pan L, Ma Z, Yan K, Cheng W, Shi Y, et al. All Inkjet-Printed Amperometric Multiplexed Biosensors Based on Nanostructured Conductive Hydrogel Electrodes. Nano Lett 2018;18:3322–7. [DOI] [PubMed] [Google Scholar]
- [146].Darabi MA, Khosrozadeh A, Mbeleck R, Liu Y, Chang Q, Jiang J, et al. Erratum: Skin-Inspired Multifunctional Autonomic-Intrinsic Conductive Self-Healing Hydrogels with Pressure Sensitivity, Stretchability, and 3D Printability. Adv Mater 2018;30:1705922/1–2. [DOI] [PubMed] [Google Scholar]
- [147].Odent J, Wallin TJ, Pan W, Kruemplestaedter K, Shepherd RF, Giannelis EP. Highly Elastic, Transparent, and Conductive 3D-Printed Ionic Composite Hydrogels. Adv Funct Mater 2017;27:1–10. [Google Scholar]
- [148].Liu X, Lu Y, Iseri E, Shi Y, Kuzum D. A Compact Closed-Loop Optogenetics System Based on Artifact-Free Transparent Graphene Electrodes. Front Neurosci 2018;12:132/1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wu GH, Hsu SH. Review: Polymeric-Based 3D Printing for Tissue Engineering. J Med Biol Eng 2015;35:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Abidian MR, Daneshvar ED, Egeland BM, Kipke DR, Cederna PS, Urbanchek MG. Hybrid conducting polymer-hydrogel conduits for axonal growth and neural tissue engineering. Adv Healthc Mater 2012;1:762–7. [DOI] [PubMed] [Google Scholar]
- [151].Lee SJ, Zhu W, Nowicki M, Lee G, Heo DN, Kim J, et al. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J Neural Eng 2018;15:016018/1–12. [DOI] [PubMed] [Google Scholar]
- [152].Zhu K, Shin SR, van Kempen T, Li YC, Ponraj V, Nasajpour A, et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv Funct Mater 2017;27:1605352/1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jung IY, Kim JS, Choi BR, Lee K, Lee H. Hydrogel Based Biosensors for In Vitro Diagnostics of Biochemicals, Proteins, and Genes. Adv Healthc Mater 2017;6:1601465/1–19. [DOI] [PubMed] [Google Scholar]
- [154].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nature Mater 2017;16:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kresse H, Schonherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol 2001;189:266–74. [DOI] [PubMed] [Google Scholar]
- [156].Scott JE. Extracellular matrix, supramolecular organisation and shape. J Anat 1995;187 ( Pt 2):259–69. [PMC free article] [PubMed] [Google Scholar]
- [157].Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng 2005;11:101–9. [DOI] [PubMed] [Google Scholar]
- [158].Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–62. [DOI] [PubMed] [Google Scholar]
- [159].Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 2003;60:107–14. [DOI] [PubMed] [Google Scholar]
- [160].Huang Z-M, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compo Sci Technol 2003;63:2223–53. [Google Scholar]
- [161].Darrell HR, Iksoo C. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996;7:216/1–7. [Google Scholar]
- [162].Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer 2008;49:5603–21. [Google Scholar]
- [163].Boudriot U, Dersch R, Greiner A, Wendorff JH. Electrospinning approaches toward scaffold engineering--a brief overview. Artif Organs 2006;30:785–92. [DOI] [PubMed] [Google Scholar]
- [164].Xie J, MacEwan MR, Schwartz AG, Xia Y. Electrospun nanofibers for neural tissue engineering. Nanoscale 2010;2:35–44. [DOI] [PubMed] [Google Scholar]
- [165].Jun I, Jeong S, Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials 2009;30:2038–47. [DOI] [PubMed] [Google Scholar]
- [166].Bendrea AD, Cianga L, Cianga I. Review paper: progress in the field of conducting polymers for tissue engineering applications. J Biomater Appl 2011;26:3–84. [DOI] [PubMed] [Google Scholar]
- [167].Chen MC, Sun YC, Chen YH. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater 2013;9:556272. [DOI] [PubMed] [Google Scholar]
- [168].Nisbet DR, Forsythe JS, Shen W, Finkelstein DI, Horne MK. Review paper: a review of the cellular response on electrospun nanofibers for tissue engineering. J Biomater Appl 2009;24:7–29. [DOI] [PubMed] [Google Scholar]
- [169].Li M, Guo Y, Wei Y, MacDiarmid AG, Lelkes PI. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006;27:2705–15. [DOI] [PubMed] [Google Scholar]
- [170].Malki M, Fleischer S, Shapira A, Dvir T. Gold Nanorod-Based Engineered Cardiac Patch for Suture-Free Engraftment by Near IR. Nano Lett 2018;18:4069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Liu Y, Lu J, Xu G, Wei J, Zhang Z, Li X. Tuning the conductivity and inner structure of electrospun fibers to promote cardiomyocyte elongation and synchronous beating. Mater Sci Eng C 2016;69:865–74. [DOI] [PubMed] [Google Scholar]
- [172].McKenzie M, Betts D, Suh A, Bui K, Kim LD, Cho H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015;20:20397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Norouzi M, Nazari B, Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today 2016;21:1835–49. [DOI] [PubMed] [Google Scholar]
- [174].Naderi-Meshkin H, Andreas K, Matin MM, Sittinger M, Bidkhori HR, Ahmadiankia N, et al. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int 2014;38:72–84. [DOI] [PubMed] [Google Scholar]
- [175].Chung SE, Park W, Shin S, Lee SA, Kwon S. Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nat Mater 2008;7:581–7. [DOI] [PubMed] [Google Scholar]
- [176].Tasoglu S, Yu CH, Liaudanskaya V, Guven S, Migliaresi C, Demirci U. Magnetic Levitational Assembly for Living Material Fabrication. Adv Healthc Mater 2015;4:1469–76, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Fabiilli ML, Wilson CG, Padilla F, Martin-Saavedra FM, Fowlkes JB, Franceschi RT. Acoustic droplet-hydrogel composites for spatial and temporal control of growth factor delivery and scaffold stiffness. Acta Biomater 2013;9:7399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Albrecht DR, Underhill GH, Mendelson A, Bhatia SN. Multiphase electropatterning of cells and biomaterials. Lab Chip 2007;7:702–9. [DOI] [PubMed] [Google Scholar]
- [179].Kim D, Kim J, Ko Y, Shim K, Kim JH, You J. A Facile Approach for Constructing Conductive Polymer Patterns for Application in Electrochromic Devices and Flexible Microelectrodes. ACS Appl Mater Interfaces 2016;8:33175–82. [DOI] [PubMed] [Google Scholar]
- [180].Wu Y, Chen YX, Yan J, Quinn D, Dong P, Sawyer SW, et al. Fabrication of conductive gelatin methacrylate-polyaniline hydrogels. Acta Biomater 2016;33:122–30. [DOI] [PubMed] [Google Scholar]
- [181].Navaei A, Moore N, Sullivan RT, Truong D, Migrino RQ, Nikkhah M. Electrically conductive hydrogel-based micro-topographies for the development of organized cardiac tissues. RSC Adv 2017;7:3302–12. [Google Scholar]
- [182].Lee JM, Moon JY, Kim TH, Lee SW, Ahrberg CD, Chung BG. Conductive hydrogel/nanowire micropattern-based sensor for neural stem cell differentiation. Sens Actuators B 2018;258:1042–50. [Google Scholar]
- [183].Tee BC, Wang C, Allen R, Bao Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat Nanotechnol 2012;7:825–32. [DOI] [PubMed] [Google Scholar]
- [184].Han L, Lu X, Wang M, Gan D, Deng W, Wang K, et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017;13:1601916/1–9. [DOI] [PubMed] [Google Scholar]
- [185].Srinivasan S, Babu SS, Praveen VK, Ajayaghosh A. Carbon nanotube triggered self-assembly of oligo(p-phenylene vinylene)s to stable hybrid pi-gels. Angew Chem Int Ed 2008;47:5746–9. [DOI] [PubMed] [Google Scholar]
- [186].Okesola BO, Suravaram SK, Parkin A, Smith DK. Selective Extraction and In Situ Reduction of Precious Metal Salts from Model Waste To Generate Hybrid Gels with Embedded Electrocatalytic Nanoparticles. Angew Chem Int Ed 2016;55:183–7. [DOI] [PubMed] [Google Scholar]
- [187].Chakraborty P, Bairi P, Mondal S, Nandi AK. Co-assembled conductive hydrogel of N-fluorenylmethoxycarbonyl phenylalanine with polyaniline. J Phys Chem B 2014;118:13969–80. [DOI] [PubMed] [Google Scholar]
- [188].Seidlits SK, Lee JY, Schmidt CE. Nanostructured scaffolds for neural applications. Nanomedicine 2008;3:183–99. [DOI] [PubMed] [Google Scholar]
- [189].Xu Y, Sheng K, Li C, Shi G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010;4:4324–30. [DOI] [PubMed] [Google Scholar]
- [190].Kopecek J, Yang J. Smart self-assembled hybrid hydrogel biomaterials. Angew Chem Int Ed 2012;51:7396–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Pugliese R, Marchini A, Saracino GAA, Zuckermann RN, Gelain F. Cross-linked self-assembling peptide scaffolds. Nano Res 2018;11:586–602. [Google Scholar]
- [192].Thompson CB, Korley LTJ. Harnessing Supramolecular and Peptidic Self-Assembly for the Construction of Reinforced Polymeric Tissue Scaffolds. Bioconjug Chem 2017;28:1325–39. [DOI] [PubMed] [Google Scholar]
- [193].Cheng QY, Han BH. Supramolecular hydrogel based on graphene oxides for controlled release system. J Nanosci Nanotechno 2013;13:755–60. [DOI] [PubMed] [Google Scholar]
- [194].Sun Y, Wu Q, Shi G. Supercapacitors based on self-assembled graphene organogel. Phys Chem Chem Phys 2011;13:17249–54. [DOI] [PubMed] [Google Scholar]
- [195].Cai G, Wang J, Qian K, Chen J, Li S, Lee PS. Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection. Adv Sci 2017;4:1600190/1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Haraguchi K, Uyama K, Tanimoto H. Self-healing in nanocomposite hydrogels. Macromol Rapid Commun 2011;32:1253–8. [DOI] [PubMed] [Google Scholar]
- [197].Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids Surf B 2000;18:197–219. [DOI] [PubMed] [Google Scholar]
- [198].Gerard M, Chaubey A, Malhotra BD. Application of conducting polymers to biosensors. Biosens Bioelectron 2002;17:345–59. [DOI] [PubMed] [Google Scholar]
- [199].Li L, Shi Y, Pan L, Shi Y, Yu G. Rational design and applications of conducting polymer hydrogels as electrochemical biosensors. J Mater Chem B 2015;3:2920–30. [DOI] [PubMed] [Google Scholar]
- [200].Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater Sci Eng R Rep 2015;93:1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Peppas NA, Van Blarcom DS. Hydrogel-based biosensors and sensing devices for drug delivery. J Control Release 2016;240:142–50. [DOI] [PubMed] [Google Scholar]
- [202].Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Prog Polym Sci 2007;32:876–921. [Google Scholar]
- [203].Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S. Applications of conducting polymers and their issues in biomedical engineering. J R Soc Interface 2010;7 Suppl 5:S559–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Wang H, Han H, Ma Z. Conductive hydrogel composed of 1,3,5-benzenetricarboxylic acid and Fe(3+) used as enhanced electrochemical immunosensing substrate for tumor biomarker. Bioelectrochemistry 2017;114:48–53. [DOI] [PubMed] [Google Scholar]
- [205].Gao G, Fang D, Yu Y, Wu L, Wang Y, Zhi J. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 2017;167:208–16. [DOI] [PubMed] [Google Scholar]
- [206].Bayer CL, Peppas NA. Advances in recognitive, conductive and responsive delivery systems. J Control Release 2008;132:216–21. [DOI] [PubMed] [Google Scholar]
- [207].Shi Y, Ma C, Peng L, Yu G. Conductive “Smart” Hybrid Hydrogels with PNIPAM and Nanostructured Conductive Polymers. Adv Funct Mater 2015;25:1219–25. [Google Scholar]
- [208].Merino S, Martin C, Kostarelos K, Prato M, Vazquez E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015;9:4686–97. [DOI] [PubMed] [Google Scholar]
- [209].Heo DN, Song SJ, Kim HJ, Lee YJ, Ko WK, Lee SJ, et al. Multifunctional hydrogel coatings on the surface of neural cuff electrode for improving electrode-nerve tissue interfaces. Acta Biomater 2016;39:25–33. [DOI] [PubMed] [Google Scholar]
- [210].Indermun S, Choonara YE, Kumar P, Du Toit LC, Modi G, Luttge R, et al. Patient-controlled analgesia: therapeutic interventions using transdermal electro-activated and electro-modulated drug delivery. J Pharm Sci 2014;103:353–66. [DOI] [PubMed] [Google Scholar]
- [211].Mario Cheong GL, Lim KS, Jakubowicz A, Martens PJ, Poole-Warren LA, Green RA. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta Biomater 2014;10:1216–26. [DOI] [PubMed] [Google Scholar]
- [212].Goding J, Gilmour A, Martens P, Poole-Warren L, Green R. Interpenetrating Conducting Hydrogel Materials for Neural Interfacing Electrodes. Adv Healthc Mater 2017;6:1601177/1–13. [DOI] [PubMed] [Google Scholar]
- [213].Paradee N, Sirivat A. Electrically controlled release of benzoic acid from poly(3,4-ethylenedioxythiophene)/alginate matrix: effect of conductive poly(3,4-ethylenedioxythiophene) morphology. J Phys Chem B 2014;118:9263–71. [DOI] [PubMed] [Google Scholar]
- [214].Pairatwachapun S, Paradee N, Sirivat A. Controlled release of acetylsalicylic acid from polythiophene/carrageenan hydrogel via electrical stimulation. Carbohydr Polym 2016;137:214–21. [DOI] [PubMed] [Google Scholar]
- [215].Sanz R, Calpena AC, Mallandrich M, Clares B. Enhancing topical analgesic administration: review and prospect for transdermal and transbuccal drug delivery systems. Curr Pharm Des 2015;21:2867–82. [DOI] [PubMed] [Google Scholar]
- [216].Green JJ, Elisseeff JH. Mimicking biological functionality with polymers for biomedical applications. Nature 2016;540:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Liu X, Gilmore KJ, Moulton SE, Wallace GG. Electrical stimulation promotes nerve cell differentiation on polypyrrole/poly (2-methoxy-5 aniline sulfonic acid) composites. J Neural Eng 2009;6:065002/1–10. [DOI] [PubMed] [Google Scholar]
- [218].Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng Part A 2009;15:3605–19. [DOI] [PubMed] [Google Scholar]
- [219].Sirivisoot S, Pareta R, Harrison BS. Protocol and cell responses in three-dimensional conductive collagen gel scaffolds with conductive polymer nanofibres for tissue regeneration. Interface Focus 2014;4:20130050/1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Liu X, Miller AL 2nd, Park S, Waletzki BE, Zhou Z, Terzic A, et al. Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl Mater Interfaces 2017;9:14677–90. [DOI] [PubMed] [Google Scholar]
- [221].Weng B, Liu X, Shepherd R, Wallace GG. Inkjet printed polypyrrole/collagen scaffold: A combination of spatial control and electrical stimulation of PC12 cells. Synth Met 2012;162:1375–80. [Google Scholar]
- [222].Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials 2001;22:1055–64. [DOI] [PubMed] [Google Scholar]
- [223].Yow S-Z, Lim TH, Yim EKF, Lim CT, Leong KW. A 3D Electroactive Polypyrrole-Collagen Fibrous Scaffold for Tissue Engineering. Polymers 2011;3:527–44. [Google Scholar]
- [224].Yang S, Jang L, Kim S, Yang J, Yang K, Cho SW, et al. Polypyrrole/Alginate Hybrid Hydrogels: Electrically Conductive and Soft Biomaterials for Human Mesenchymal Stem Cell Culture and Potential Neural Tissue Engineering Applications. Macromol Biosci 2016;16:1653–61. [DOI] [PubMed] [Google Scholar]
- [225].Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci USA 2010;107:565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Zhou J, Chen J, Sun H, Qiu X, Mou Y, Liu Z, et al. Engineering the heart: evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci Rep 2014;4:3733/1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Laiva AL, Venugopal JR, Navaneethan B, Karuppuswamy P, Ramakrishna S. Biomimetic approaches for cell implantation to the restoration of infarcted myocardium. Nanomedicine 2015;10:2907–30. [DOI] [PubMed] [Google Scholar]
- [228].Dong R, Zhao X, Guo B, Ma PX. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl Mater Interfaces 2016;8:17138–50. [DOI] [PubMed] [Google Scholar]
- [229].Gelmi A, Cieslar-Pobuda A, de Muinck E, Los M, Rafat M, Jager EW. Direct Mechanical Stimulation of Stem Cells: A Beating Electromechanically Active Scaffold for Cardiac Tissue Engineering. Adv Healthc Mater 2016;5:1471–80. [DOI] [PubMed] [Google Scholar]
- [230].Liu L, Li P, Zhou G, Wang M, Jia X, Liu M, et al. Increased proliferation and differentiation of pre-osteoblasts MC3T3-E1 cells on nanostructured polypyrrole membrane under combined electrical and mechanical stimulation. J Biomed Nanotechnol 2013;9:1532–9. [DOI] [PubMed] [Google Scholar]
- [231].Wang Y, Rouabhia M, Zhang Z. Pulsed electrical stimulation benefits wound healing by activating skin fibroblasts through the TGFbeta1/ERK/NF-kappaB axis. Biochim Biophys Acta 2016;1860:1551–9. [DOI] [PubMed] [Google Scholar]
- [232].Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017;122:34–47. [DOI] [PubMed] [Google Scholar]
- [233].Kaur G, Adhikari R, Cass P, Bown M, Gunatillake P. Electrically conductive polymers and composites for biomedical applications. RSC Adv 2015;5:37553–67. [Google Scholar]
- [234].Sarwar MS, Dobashi Y, Preston C, Wyss JK, Mirabbasi S, Madden JD. Bend, stretch, and touch: Locating a finger on an actively deformed transparent sensor array. Sci Adv 2017;3:e1602200/18. [DOI] [PMC free article] [PubMed] [Google Scholar]