Abstract
With the advent of combined antiretroviral therapies (cART), the face of HIV infection has changed dramatically from a disease with almost certain mortality from serious comorbidities, to a manageable chronic condition with an extended lifespan. In this paper we present the more recent investigations into the epidemiology, microbiology, and pathogenesis of periodontal diseases in patients with HIV, and the effects of cART on the incidence and progression of these diseases both in adults and perinatally infected children. In addition, comparisons and potential interactions between the HIV-associated microbiome, host responses and pathogenesis in the oral cavity with the gastrointestinal tract and other areas of the body are presented. In addition, the effects HIV and cART on comorbidities such as hyposalivation, dementia, and osteoporosis on periodontal disease progression are discussed.
Over the past 40 years the prognosis of human immunodeficiency virus (HIV) disease has changed dramatically. What was once a disease with a high mortality rate preceded by dramatic declines in immune function accompanied with variety of life altering morbidities, has become a manageable chronic condition. This change in the prognosis of the disease is due in large part to the advent and continued improvements in combination antiretroviral therapy (cART). However, despite the many successful outcomes of cART, new challenges associated with its possible detrimental side-effects have arisen. Furthermore, additional complications inherent to the aging of the population living with HIV infection also need to be addressed. Globally in 2017, there were approximately 36.9 million people living with HIV at the end of 2017 with 1.8 million people becoming newly infected (http://www.who.int/news-room/fact-sheets/detail/hiv-aids). However in developed countries such as the United States, the demographics of HIV infected individuals have shifted with more than half the population with HIV being currently over the age of 50 years.1 For the dental practitioner treating these individuals, there is an increasing need to address the common age related dental diseases such as periodontal disease. Furthermore, these older individuals may present with other co-morbidities associated with HIV infection, the antiretroviral treatment, or a combination of both, that could contribute to a higher incidence and severity of periodontal disease.
In addition to the challenge inherent to a population of aging HIV patients, there remain challenges on the opposite end of the demographic spectrum with children and adolescents who were infected either at birth or early childhood due to maternal exposure. These HIV infected children/adolescents, most of whom became infected perinatally prior to routine HIV-testing of pregnant women in the US before the early 2000s, may also develop HIV and ART related co-morbidities including periodontal disease as they enter adulthood.
In addition to these changing epidemiologic trends in HIV infection in the United States and in other developed and developing countries, there remain many unanswered questions for dental research: These include: 1. What are the underlying mechanisms and relationships between HIV and cART and their effects on the oral microbiome and immune milieu in the development of oral and systemic pathology?; 2. What new insights can be gained in periodontal disease progression from recent laboratory and clinical studies in HIV-infected patients which compare and contrast the microbiology, immunology, and inflammatory host response between connected areas of the body such as the oral cavity and periodontium to the gut and blood circulation?
In this paper, we will review the current scientific literature pertaining to changes in the epidemiology and microbiology of periodontal disease in populations with HIV disease in the era of cART. In addition, there has been a more recent focus on the interactions between periodontal diseases and systemic conditions and co-morbidities associated with HIV infection. These more recent investigations have brought together research and clinical disciplines that have focused on the mouth and periodontal disease with disciplines that have focused on other areas of the body such as the GI tract, brain, and vagina. These interactions and comparisons between oral and systemic health are explored in this review.
RECENT TRENDS IN THE EPIDEMIOLOGY OF PERIODONTAL DISEASE IN POPULATIONS WITH HIV DISEASE
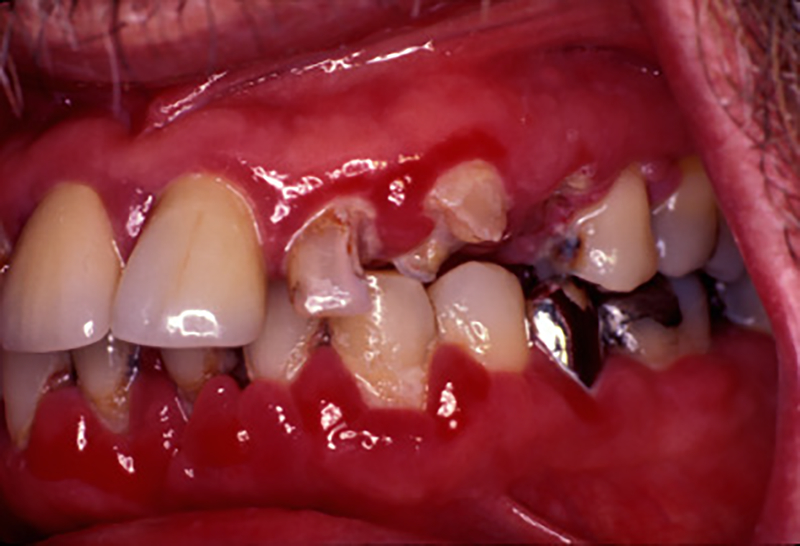
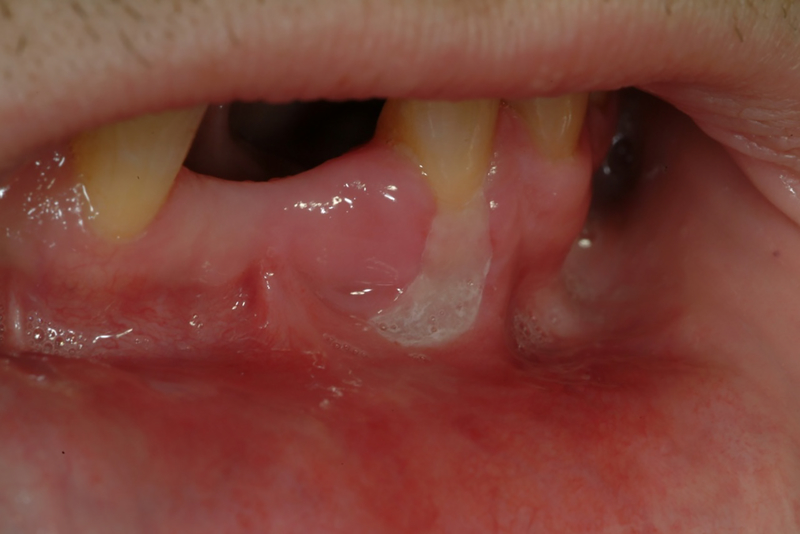
Before the advent of cART, atypical lesions involving the periodontal tissues were observed including linear gingival erythema (Figure 1) and a range of necrotizing periodontal diseases either restricted to the gingiva per se (e.g. necrotizing ulcerative gingivitis), or extending further into the periodontium to involve the soft tissue attachment and alveolar bone (e.g necrotizing ulcerative periodontitis (Figure 2)) 2. Similar lesions involving the adjacent hard and soft tissues of the mandible, maxilla, hard palate and buccal vestibule (necrotizing stomatitis) were also described (Figure 3). It has been hypothesized that necrotizing ulcerative gingivitis, necrotizing ulcerative periodontitis, and necrotizing stomatitis each represent a different stage of the same disease3–5 and may reflect the overall systemic progression of HIV infection to more severe collapse of the immune system and progression to full blown AIDS 6–9. However, with the advent of cART a marked decline in the frequency of destructive periodontal disease in HIV patients has been reported. The frequency of these conditions continue to be higher in developing countries due to a lack of access to the most effective cART regimen and to dental hygiene and care 10–12.
Figure 1.
Severe linear gingival erythema (white arrows) (from a previous Periodontology 2000. Will get permission from Wiley)
Figure 2.
Necrotizing ulcerative periodontitis involving maxillary left lateral incisor, canine, and first premolar
Figure 3.
Necrotizing stomatitis or ulcerative necrotizing ulcerative stomatitis on buccal aspect of mandibular left canine (need copyright permission; J Oral Pathol Med (2009) 38: 481–488)
Nevertheless, with the increase in the average age of the general HIV population in the United States, other developed countries, and some developing countries, attention has now turned to two areas of periodontal research: First is the incidence, severity and management of the more common chronic periodontal diseases in the aging adult population, where age is a major risk indicator as seen in non-HIV infected individuals13. Second is the incidence and progression of periodontal disease in the population of children and adolescents who acquired the HIV perinatally.
Epidemiological studies on the most common form of periodontal disease in the older population, previously termed “chronic periodontitis”, have and continue to show a range of possible associations between HIV and the incidence and severity of periodontal diseases. Before the advent of ART, some studies reported a greater attachment loss in HIV patients with preexisting chronic periodontal disease when compared to non-infected patients which correlated with declining CD4 counts 14–18, 19 and a greater extent of gingival recession with or without greater alveolar bone loss 20, 21.
In the cART era, studies have reported reductions in oral candidiasis and hairy leukoplakia 9,22–27, and a decrease the prevalence of periodontal diseases in HIV adults 28. In addition, the cART era studies that compare the incidence and severity of periodontal disease between HIV infected patients receiving cART and non-HIV patients have shown no significant differences between these two groups21,22,29,30. Furthermore, in a large longitudinal study conducted from 1995 to 2002 on a female cohort, no significant differences were found in baseline mean clinical attachment levels and probing depths or progression of attachment loss and pocket depths between HIV positive and HIV negative women 31.
One area of continued investigation is the finding that while there may be no increase in clinical attachment loss in HIV infected vs non infected patients with chronic periodontitis, there has been a reported increase in gingival recession in HIV patients20. One possible explanation is that the HIV patient may share some local microbial or destructive inflammatory characteristics with the necrotic periodontal lesions seen more commonly in the pre-cART era 32,33. Furthermore, it is still possible the characteristics of the local microbiome, virome and mycobiome of the chronic periodontitis lesion in the aging HIV patient may share some of the same patterns as seen in the destructive periodontal lesions in the pre-cART era. Recent finding of these possible unique microbial signatures persistent in the cART era as well as their implications for the pathogenesis of periodontal disease will be discussed in the next section.
In the cART era, as in the pre cART era correlations between CD4 and/or HIV viral load with periodontal attachment loss or pocket depth have been inconclusive with some studies reporting no major differences in other periodontal parameters between HIV infected and non-infected patients 34 or in tooth loss patterns 35 particularly for patients with CD4 counts ≥ 500 36. By contrast patients under cART who may have either developed a resistance to cART or lack of compliance to therapy and who experienced a 10-fold increase in viral load did show a marginal increase in tooth loss 31 while another study reported that patients under cART but with CD4 count <200 cells/mm3 were at greater risk for periodontal disease 7. These observations point to the need for earlier initiation, continuation, and patient compliance to cART as this approach may decrease the risk of marked immunosuppression in HIV which may in turn reduce the incidence, severity and progression of periodontal disease.
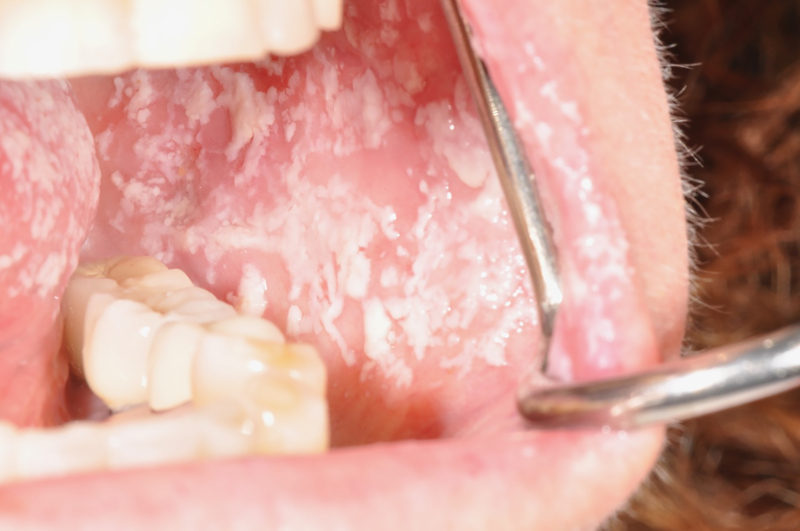
As discussed previously, while one focus of periodontal research in HIV has shifted to the aging population undergoing cART a second focus on periodontal disease has shifted towards studies on children 37. While some of these studies have focused on the less common prepubertal aggressive forms of periodontitis, other studies have focused on the more common periodontal changes in gingival health per se in children and adolescents that would normally precede manifestations of more advanced periodontal disease. These clinical changes include gingivitis and early indications of periodontitis, including increased pocket depths and the subtle clinical signs of attachment loss. Early studies of children in the USA in the pre cART era using clinical parameters of bleeding on probing, increases in probing depths and or loss of clinical attachment, reported a range of the incidence of gingivitis and early periodontitis from 55% to 94% 38. A similar wide range of gingivitis using a range of diagnostic criteria including bleeding on probing and visual clinical inflammation was reported in other countries. For example there have been reported rates of gingivitis at 49% in Romania 39, 13.5 to 17.5% in Brazil 40,41 2.2% in Thailand 42,43 and 10.8% in India 44. More recently in the cART era, several large scale cross sectional and prospective epidemiological studies have been conducted to examine the effects of these treatments on the incidence and severity of periodontal diseases in children and adolescents. One large multicenter prospective cohort study of 2767 HIV infected children at sites in the United States and Puerto Rico reported a much lower incidence of herpes zoster and oral candidiasis (Figure 4) in children on cART when compared to those in the pre-cART era 45.
Figure 4.
Pseudomembranous candidiasis, buccal mucosa and lateral tongue
One advantage of studying periodontal diseases in perinatally exposed children as they progress through childhood, adolescence, and adulthood is that comparisons in disease progression can be made between HIV infected children and other children in the similar environment and socioeconomic who are not HIV infected. Such studies are much less affected by the potential multiple environmental and behavioral differences that often make it difficult to determine the effects of HIV infection itself on the incidence and progression as well as the underlying microbiological, immunological, inflammatory, genetic, and other considerations for the progression of periodontal disease. Recently a multicenter study was undertaken in the United States to assess the effects of HIV on periodontal diseases and other oral diseases and conditions by comparing children and adolescents perinatally exposed and infected with HIV (PHIV) with perinatally HIV exposed but uninfected (PHEU) children (PHACS-AMP Oral Health Substudy). The majority of these study participants shared similar socioeconomic status. In this baseline AMP Oral Health Substudy, the prevalence of periodontal disease did not differ between PHIV and PHEU with 32% of this youth population having mild/moderate periodontitis as defined by the modified CDC classification system. However, using this same classification system, this prevalence was higher than the 22% reported in the Nutrition Examination Survey (NHANES) survey of an older general population of 30–34 year-old subjects 46,47. Among PHIV youth, younger age of first exposure to cART was associated with fewer number of teeth with multiple sites of bleeding on probing.48 A current follow-up study on this HIV infected and HIV exposed uninfected cohort is currently underway to assess and compare the periodontal heath of these cohorts as they progress to young adulthood.
CURRENT AND NEW INVESTIGATIONS INTO THE MICROBIOLOGY AND THE HOST RESPONSE IN PERIODONTITIS IN THE HIV PATIENT
In the pre cART era, the vast majority of microbiological studies on periodontal diseases in HIV and non-HIV infected patients focused on the bacterial profiles or microbiome of both the atypical necrotic periodontal lesions and linear gingival erythema as well as more common acute and chronic periodontal diseases 49,50. The atypical lesions of the pre cART era were characterized by a diffuse invasion from viruses, fungi, and other opportunistic organisms into the gingival tissue and underlying periodontal support from both the tooth surface and soft tissue surface, with a reduction of cells of the local innate and acquired immune system 51–61. This is in contrast to chronic periodontal diseases in HIV uninfected individuals where inflammatory cells are localized primarily to the connective tissue regions under the junctional and sulcular epithelium adjacent to the supra and subgingival biofilm on the tooth surface 50.
The advent of cART has provided the opportunity to make comparisons between HIV- infected patients and non-infected patients, and within HIV patients before and after cART for the more common forms of periodontal diseases. In addition, and equally important, newer avenues of research have turned their attention to not only the microbiome, but also other viruses (the virome) and fungi (the mycobiome) associated with HIV and the interactions between all of these three populations of the microbiome with each other. In addition, there are new investigations and insights into the interactions of this complex microbiome with immunological and inflammatory responses and with the effectiveness of cART. In this section, these trends in changes in the microbiology of HIV, in the periodontium and in other oral and systemic sites, will be presented for each of these microbiome, virome and mycobiome populations with newly discovered interactions and clinical implications.
The Microbiome in HIV in the cART Era
In the pre cART era, with the greater prevalence of the less common HIV associated lesions and conditions of the periodontium such as linear gingival erythema, necrotizing ulcerative gingivitis and necrotizing ulcerative periodontitis, as well as conventional periodontal diseases such as chronic periodontitis, each of these conditions may have presented alone or in combination of these lesions and conditions. Earlier studies demonstrated that the bacterial microbiome profile of each of these conditions was characterized by species normally associated with periodontal diseases as well as unique opportunistic flora that may present in the more destructive and/or necrotic lesions. For example, the similarities in the microbiome between linear gingival erythema and necrotizing ulcerative periodontitis in HIV patients implied that there was a continuum between these lesions 62–67.
For conventional chronic periodontitis most studies demonstrated that the microbiome was similar between HIV positive and HIV negative patients, with the exception of several opportunistic microorganisms. In addition certain combinations of suspected periodontal pathogens are more prevalent in the HIV positive patient 68. In particular the higher detection rates of a variety of treponemes (spirochetes) in HIV patients may have clinical significance as they have an important role in the pathogenesis of chronic periodontal diseases and necrotizing periodontal diseases 69,70. In the cART era, different microbiome studies have reported both similarities and differences between HIV patients with cART and non-infected HIV patients. For example, some studies have demonstrated no differences in microbiota between HIV infected cART patients and non HIV infected patients 71 while other studies have shown marked differences72–76. For example, in patients on cART, Enterococcus faecalis and Fusobacterium nucleatum were observed in higher numbers inversely related to lower CD4 counts 77,78. More recent microbial studies in the cART era mirror the previous findings for the pre-cART era in that in general there are no major differences in the periodontal microflora in chronic periodontitis between HIV positive and negative patients, particularly for the classic periodontal pathogens 79. However, in the cART era there continue to be reports of atypical/opportunistic microbial species isolated from the periodontal pockets of HIV patients 80. For example, some recent reports have demonstrated that in HIV patients, opportunistic pathogens such as Helicobacter pylori, E. faecalis and Pseudomonas aeruginosa 81 are still detected in higher frequency in the subgingival biofilm.
For longitudinal studies of the periodontal microbiome in the cART era, several recent studies have taken advantage of the opportunity to examine changes in the microbiome in the individual patient before and after cART. Some of these studies have found differences in the microbiome with cART before and after treatment 74,75. For example, in the Women’s Interagency HIV study which specifically examined species with pathogenic potential in periodontitis, cART use increased the risk of recovering pathogenic bacteria such as Fusobacterium species, enteric gram-negative rods, Peptostreptococcus micros, Campylobacter species, Eubacterium species, and Tannerella forsythia 72. In addition, several studies have shown that cART may reduce the counts of commensal bacteria which have a protective effect on the colonization of pathogenic species 72,74,75.
The oral microbiome in children with HIV is an area that has been the focus of several recent studies. Children infected with HIV from their mother perinatally present a unique opportunity to study the development of the oral microbiome. Most of these earlier studies on infants were performed in developing countries in Africa, with some recent studies performed in the United States 82–84. From the time of birth to their later development, children acquire their initial oral microbiome from their mother, and their early environment plays a major role in the development and maturation of the oral microbiome under the influence of HIV and cART. In addition, there is an opportunity to compare the development of this oral microbiome with those children/youth under similar beneficial or detrimental environmental conditions 85. For example, in the recent PHACS-AMP cross sectional multicenter study on HIV and oral health in both PHIV and PHEU youth, no significant differences in the oral microbiome were noted with some exceptions, such as lower levels of Corynebacterium and Streptococcus mutans among PHIV youth. As Corynebacterium is considered one of the health-associated taxa in plaque, this may be one possible contributing factor in the higher caries prevalence in HIV infected youth observed in one study 86. In addition, follow-up studies of these types of cohorts may yield new insights into changes in the microbiome associated with changes in periodontal health.
There is considerable evidence that alterations in the oral microbiome as a result of cART may have impact on a range of local and systemic diseases and conditions. The pathogenic properties of this altered microbiome can produce products that stimulate the local inflammatory and immune response and release of inflammatory cytokines and chemokines, which contribute to the initiation and progression of periodontal diseases 87. In addition, with this emerging evidence that cART may alter the composition of the oral microbiome, two other important interrelationships between the effectiveness of cART and the oral microbiome have been proposed. The first is determining if there are differences in the microbial diversity in saliva from persistent high HIV viral load and low CD4 to patients with HIV patients normal CD4 count and minimal to no viral load as another approach to assess the effectiveness of cART 75. The second are the recent observations that alterations of the microbiome in the vagina have the potential to metabolize cART drugs 88 and thereby reduce their clinical effectiveness. The possible effects of the oral microbiome in metabolizing cART drugs has yet to be explored. The implications of each of these proposed interrelationships merits further investigation.
The Virome in the cART Era
In the HIV infected patient the role of other oral non-HIV viruses in the initiation and progression of both the necrotic periodontal conditions more commonly reported in the pre cART era, as well as more common chronic periodontal diseases that are frequently detected in the cART era has been extensively studied. In HIV infected patients, a full range of viruses including cytomegalovirus 89, herpes zoster 90 and human papilloma virus 91 have been isolated from both necrotic periodontitis as well as chronic periodontitis. In particular for chronic periodontitis, viruses in the herpesvirus family which include cytomegalovirus, Epstein-Barr virus, and herpes simplex virus 1 and 2 have also been detected in periodontal pockets of HIV infected patients, and are found in higher numbers when compared to HIV non-infected patients92,93. More recent research on Epstein-Barr virus have shown that they are present in both chronic and aggressive forms of periodontal diseases94,95, and that they, along with other herpesviruses, are shed into the oral cavity at a greater rate with more severe immunosuppression 96.
Several mechanisms for the direct and indirect role of these viruses in the initiation and pathogenesis of periodontal diseases have been proposed. These include the role of these viruses in promoting the overgrowth of periodontal pathogens and opportunistic infections by suppressing both innate and acquired immunity to pathogenic bacteria, and by directly or indirectly promoting the production and release of a range of inflammatory mediators that result in destruction of the periodontal support.97 Insights into the interactions between herpesviruses and the microbiome and host response and implications for periodontal disease progression are presented in more detail in the paper by Chen et al. (add to the reference list) in this volume.
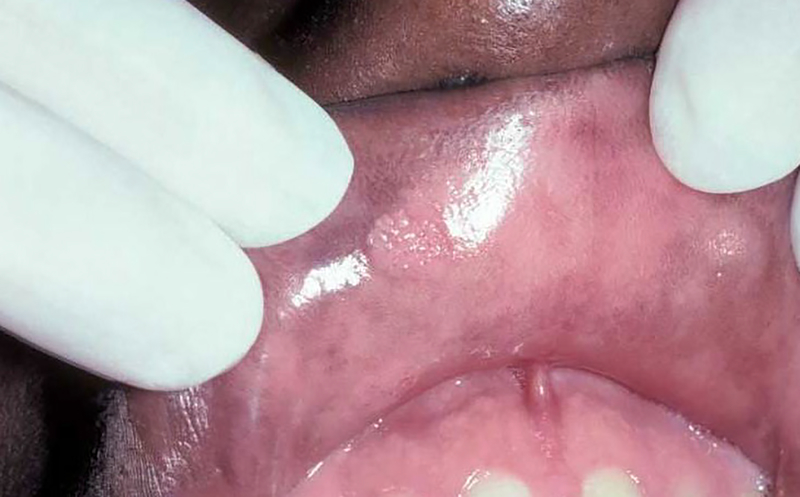
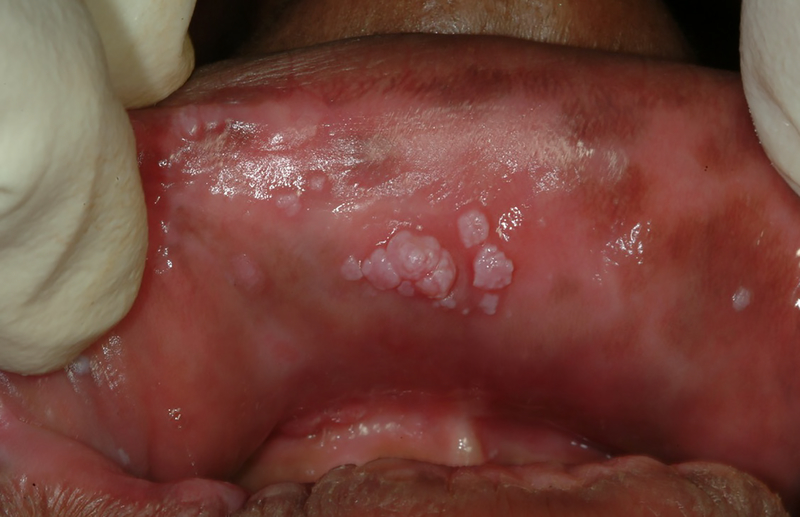
With the advent of cART it would appear that detection levels of these non-HIV viruses would be lower than in the pre-cART level. While these declining trends have been reported for many classes of viral species, detection of oral human papilloma virus and their clinical manifestations in the oral cavity (Figures 5 and 6) have remained essentially the same 98. Control of human papilloma virus presents a unique challenge to the health practitioner as there is a strong association of the presence of human papilloma virus with oral pharyngeal carcinomas99. In several studies conducted in the cART era, the detection rates of oral human papilloma virus has been ranging from 14 to 37% of HIV-infected persons compared to less than 10% of non-HIV infected persons 100–103. In addition, indirect evidence for the association of cART and persistence of human papilloma virus has been demonstrated in the recent PHACS (Pediatric HIV and AIDS Cohort study), where human papilloma virus infection was associated with lower nadir CD4 levels 104. Therefore, as in studies of the bacterial microbiome, the direct or indirect effects of cART in altering the viral microbiome may have both beneficial and detrimental effects.
Figure 5.
Single human papilloma virus wart on upper right labial mucosa
Figure 6.
Multiple clustered human papilloma virus warts on upper labial mucosa
The Mycobiome in the cART Era
Oral candiasis is a common clinical manifestation of immuosuppression due to HIV infection (Figure 4). In addition, the role of candida and possible other yeasts in the pathology of the atypical periodontal lesions as well as in more common periodontal conditions has received considerable attention since the beginning of HIV research. Candida species have been detected in high numbers in the plaque biofilm of HIV positive patients 16,33,69,105–108. As with viruses, candida and other yeasts may also play a role in the progression of these necrotic lesions into the supporting alveolar bone. In the pre-cART era, the invasion of candida into the periodontal soft tissues appeared to be directly connected with the occurrence of atypical periodontal lesions including linear gingival erythema, and necrotizing ulcerative gingivitis and necrotizing ulcerative periodontitis 33,108,109. As with periodontal bacterial pathogens and viruses, the presence of candida in the periodontal pocket and/or invasion of candida into the periodontal tissues may trigger a destructive inflammatory response 108,110,111 leading to tissue necrosis seen more commonly in the pre-cART era. In addition, this candida induced inflammatory response may promote loss of clinical attachment and resorption of alveolar bone in chronic or aggressive periodontal diseases in the HIV positive patient. This destructive inflammatory effect induced by candida and other yeasts could include the release of potentially destructive enzymes to periodontal tissue from adjacent “primed” neutrophils attempting to neutralize this candida invasion 111. In addition to a “primed” neutrophil response, priming of other inflammatory responses to HIV may play a role in the pathogenesis of periodontal disease8. These include excessive production of interferon-gamma87,112, prostaglandins113, matrix metalloproteinase-9, tissue inhibitors of metalloproteinases-1, and other metalloproteinases57,58, interleukin-1β, interleukin-6 114, transforming growth factor-beta 115, and interleukin-2 and interleukin-18 87. As in HIV uninfected patients, several of these inflammatory cytokines are found in higher concentrations in deeper vs. shallower pockets 53–55,116. This elevated inflammatory cytokine response in HIV infected patients may be in response in part to the HIV infection per se, and/or opportunistic infections such as candida. This primed inflammatory response may in turn play a role in the pathogenesis of chronic periodontitis in HIV subjects 112. These patterns of tissue destruction in the HIV infected patient would occur both within the gingival crevice and within the oral gingival epithelium.
In the past, the focus of the effects of candida on periodontal disease have centered on these destructive inflammatory effects. However, more recent research has also indicated that candida itself may have protective effects against HIV. This recent novel finding was proposed in studies that observed that increased vaginal candidiasis is associated with reduced levels of HIV particles 117. In contrast to the role of periodontal bacterial pathogens in promoting the adherence, invasion and activation of HIV particles, candida may exhibit these protective effects against HIV through the stimulation of the production of anti-HIV chemokines including RANTES and Interferon alpha beta, by sequestering HIV particles, and by inhibiting the binding of HIV to target cells. 118. Therefore, the balance between the protective and destructive effects of candida in HIV infection and implications for periodontal and oral health merit further investigation.
MICROBIOLOGY AND PATHOGENESIS OF HIV IN THE ORAL CAVITY AND GUT: SIMILARITIES, DIFFERENCES AND INTERACTIONS
In the non-HIV infected patient with periodontal disease, there is now a large body of evidence demonstrating the ability of oral bacteria and their toxic by-products to translocate across the mucous membrane resulting in systemic diseases. Infective endocarditis is the most well-known of these, specifically after dental procedures. Other systemic diseases associated with periodontal disease are diabetes, rheumatoid arthritis and premature delivery which are likely due to the toxic by-products and bacterial translocation from the oral cavity, which then induce chronic systemic inflammation. The presence of an HIV infection adds a third component that can play into these oral and systemic interactions. When considering the interactions between HIV and the oral and gut microbiome, there are three areas of investigation that have received considerable attention. The first area of investigation centers on the ability of HIV to attach and invade the surface epithelium and underlying tissues of the oral cavity and gut. The second area of investigation centers on the effects of HIV on altering the microbiome of the oral cavity and gut and promoting the translocation of other bacteria, fungi, and viruses into the bloodstream to other sites of the body. The third area of investigation centers on the role of the microbiome of the oral cavity and gut in activating latent HIV reservoirs. For each of these three areas of investigation, the gut and oral cavity share some similarities as well as some important differences which are discussed in this section.
When assessing the potential of HIV to attach to the surface of the oral cavity or gut and invade deeper tissues, one important question arises: Why is the gut more susceptible to attachment and invasion of HIV, while the mouth in general is more resistant to HIV invasion? This difference may be due in part to differences in the type and magnitude of host defenses in these two areas of the oral/gastrointestinal tract. In addition, in health, the oral mucosa normally expresses low levels of CCR5 which may account for the low binding affinity of the HIV virus to the oral epithelium 119. While studies on periodontal diseases have shown that there is an increase in surface HIV primary receptors (glycoprotein [gp]120) and co-receptors (such as CCR5) in gingival epithelium which would promote HIV attachment and invasion of target cells, this is offset by a significant local increase in antiviral alpha and beta defensins of the innate immune system 119,120. These observations may help explain why there is a lack of direct evidence of invasion of HIV in the oral cavity in periodontally healthy and periodontally diseased patients 119.
For the actual translocation of bacteria, fungi, and non-HIV viruses into the bloodstream, both the oral cavity in general, the inflamed periodontal pocket in particular, and the lining of the gut mucosa are potential sites for bacteria to cross a damaged or compromised protective epithelial barrier and enter the blood stream where they may have detrimental effects on other organ systems and other locations in the body. This process of bacterial translocation has been proposed as a central mechanism for the possible periodontal-systemic connection in the field of periodontal medicine. As previously discussed, while the role of HIV itself in promoting this translocation through the inflamed periodontal pocket has not been investigated, there is a considerable body of evidence from research on the gut that HIV itself may affect the composition of the gut microbiome, which in turn would affect the permeability of the protective gut mucosa, thereby leading to translocation of gut bacteria into the bloodstream 121,122. Furthermore, in patients with cART there is residual chronic inflammation as well as residual elevation of opportunistic bacteria, and decreases in beneficial bacteria both in the gut and in the oral cavity 76,123. This persistent inflammation and presence of a more pathological microbiome can also lead to dysfunction of the mucosal barrier and translocation of the microbiome. The resulting persistent local and systemic inflammation and bacterial translocation can in turn result in the co-morbidities associated with HIV infection in patients with cART. It is well established that when the gingival epithelial barrier is damaged or when there are inflammatory changes in the underlying periodontal tissues, bacterial translocation can occur leading to systemic bacteremia124 In particular, HIV protease inhibitors used as part of the cART regimen have been of interest because of their known mechanism of action and undesired side effects. For example Danaher et al125showed that certain protease inhibitors can block oral epithelial cell DNA synthesis, which causes a reduction in healing and potential microbial shifts and destruction of the mucosal barrier, thereby enhancing translocation.
One other mechanism that has been shown to promote the translocation of bacteria through the gut lining but as of yet not in the oral cavity, is the effect of HIV on depletion of TH-17cells 126,127. In the oral cavity, the presence of high numbers of TH17 cells are associated with higher levels of secretion of the pro inflammatory cytokine interleukin-17. However, as with the prostaglandins, at lower concentrations this cytokine is also required to maintain mucosal integrity in the gut 128. Thus, depletion of interleukin-17 may therefore further promote bacterial translocation 129. Furthermore, this relationship between the gut microbiota and HIV infection has been shown to be reciprocal in that alterations in the gut microbiota can in turn promote the attachment of HIV to the gut mucosa as well as to cells of the immune system which are the primary target of the HIV130,131.
Perhaps no other area of research in the connections between periodontal disease, systemic disease, and HIV has received as much attention as the role of the periodontal microbiome in the invasion of the HIV virus and activation of latent viruses. A similar phenomenon may occur in the gut as changes in the microbiota may enhance the attachment and invasion of HIV 130,131 In particular, the role of one keystone periodontal pathogen, Porphyromonas gingivalis and its interactions with HIV has been extensively investigated in vitro. Studies have shown that P. gingivalis can first promote invasion of immune cells through a variety of mechanisms. One of the these mechanisms is the interaction of P. gingivalis gingipains at the HIV gp120 domain 132 which can then promote internalization of the HIV into a variety of cells 133. In addition, P. gingivalis has been shown in vitro to increase the expression of the CCR5 receptor for HIV on oral epithelial cells 134,135 and may facilitate the transfer of HIV from oral epithelial cells to oral dendritic cells and other oral antigen presenting cells 134. However, these effects of P. gingivalis in the promotion of HIV infection in vivo have yet to be determined.
With the advent of cART, while effective reductions in viral load and reconstitution of some of the immune system is a beneficial outcome, there is also a persistent residual level of systemic immune activation and inflammation that can potentially reactivate any latent pools of HIV particles. Products of bacteria in periodontal disease may both directly promote this reactivation through direct actions on cells harboring latent HIV reservoirs, or indirectly through the local and systemic effects of bacterial translocation. Direct effects of periodontal bacteria on HIV activation include evidence that the periodontal pathogens P. gingivalis, F. nucleatum, and Treponema denticola can enhance reactivation of HIV in infected monocytes and macrophages by binding to Toll-like receptors 2 and 9 on these cells 94,136–139 and by directly activating the promoter locus of HIV in these cells 140–144. Similar effects in increased HIV gene transcription and activation have been observed on cultured HIV infected macrophages and dendritic cells 142,143,145. Lipopolysaccaride from periodontal pathogenic bacteria can also induce HIV reactivation by a similar action on the promoter locus of HIV.146
While periodontal disease severity may decrease after cART, systemic inflammatory markers may remain 147 leading to persistent immune activation148 and barrier dysfunction of gut mucosal tract148,149,150. This persistent immune activation seen in some HIV patients receiving cART can be enhanced by the presence of chronic translocation of bacteria and release of inflammatory mediators from inflamed periodontal tissues 148. There is now a considerable body of evidence that several of these inflammatory mediators derived directly from inflamed periodontal tissues or from extraoral inflammatory responses to translocated bacteria can in turn activate latent reservoirs of HIV. For example, common elevated inflammatory mediators from periodontal diseases including interleukin-6, interleukin-8 and granulocyte-macrophage colony stimulating factor have been shown to activate HIV in monocytes, macrophages, dendritic cells, and T-cells 87,137,138,151,152.
OTHER CONSIDERATION IN THE INCIDENCE AND PROGRESSOIN OF PERIODONTAL DISEASES AND OTHER ORAL CONDITIONS IN THE cART ERA
While the principal focus of the effects of cART on the incidence and progression of periodontal diseases in HIV patients has been on the interactions between alterations in the microbiota and the host response, there are several other considerations that may also play a direct or indirect role for the HIV patient on cART that may also contribute to periodontal diseases and caries. These include the effects of cART on salivary flow, reduced cognitive function, effects of HIV infection and cART on bone mineralization and systemic diseases, and conditions that may adversely affect periodontal health such as diabetes. Each of these conditions or diseases share some common characteristics that increase the HIV patient’s susceptibility to periodontal diseases and are discussed below. These characteristics include an altered immune response and impaired host response to other harmful microbiota, an enhanced destructive inflammatory response, and/or impairment of periodontal tissue integrity leading to greater susceptibility to breakdown or resorption. In the following section, some examples the implications of HIV on some of these other factors and their potential role in periodontal diseases progression as well as in other dental diseases will be presented.
Effects of salivary hypofunction on periodontal disease
Reduced salivary flow, with or without salivary gland enlargement, is one of the known complications of HIV infection 153,154. Several studies have observed reduced salivary flow rate with HIV infection 155 particularly at advanced stages of the disease. In the cART era, reduced salivary flow and xerostomia is a common side effects of some cART regimens156,157. These reduced salivary flow conditions have been clearly shown to be associated with an increase in dental caries, and may also have an adverse effect on periodontal diseases. While several studies have demonstrated a strong association of cART with an increase in dental caries and periodontal diseases, the role of hyposalivation in cART treatment in periodontal disease progression merits further investigation 158,159.
Associations and Connections between HIV, cART and Dementia
As with non HIV infected patients, it is expected that the aging population of HIV infected patients receiving cART will experience reductions in cognitive function ranging from mild cognitive impairment to severe dementia 160. Such impairments of cognitive function may have a major adverse effect on both periodontal health and caries through the reduced ability to perform routine oral hygiene. With the HIV infected patient this would be a particular problem as reduced cognitive function appears to be more severe and at earlier ages161. Recent research on these patients, has provided new insights to the interplay between HIV infection, cART therapy, periodontal diseases, and cognitive function. There is evidence that bacteria themselves, and bacterial products from inflamed periodontal tissues can enter the brain 162 resulting in a local inflammatory reaction and impairment of neural function. While the integrity of the protective blood brain barrier is maintained through the structural integrity of the cell to cell junctions of the barrier lining, there is evidence that both HIV and bacteria from the periodontal tissues can damage this barrier, and thereby allow the entry of these bacterial into the brain 163.
Effects of osteoporosis on the initiation, progression and severity of periodontal diseases
It is now well established that patients with reduced bone mineralization, particularly in the more severe forms of osteoporosis are more prone to periodontal breakdown. As populations with HIV live longer, complications associated with aging such as osteoporosis become more important considerations for the patient’s overall health and health of the periodontium. In addition, both HIV infection and cART may have direct or indirect roles in the earlier initiation and greater severity of osteoporosis. For example, osteoporosis has been reported to be more prevalent in HIV patients including those under cART 164–167. Furthermore, in a population of HIV infected younger men, the levels of bone mineralization were reported to be lower than their HIV uninfected counterparts 168,169.
One possible underlying cause for a decrease in bone mineralization in HIV infected patients is the persistent chronic systemic immune activation observed even in those ‘successfully” treated with cART. The elevation of inflammatory cytokines associated with systemic immune activation such as transforming growth factor-alpha, interleukin-6, interleukin-17, nuclear factor-kappa B and interleukin-1 beta151,170 directly or indirectly leads to both systemic bone loss and localized alveolar bone loss. In the younger HIV infected patient, poor nutrition, and a lower adherence to the cART regimen may also contribute to the observed lower levels of bone mineralization and osteoporosis 171.
CONCLUSIONS
Over the past two decades the face of HIV infection has changed dramatically from a disease with almost certain mortality due to direct effects of the HIV and serious co-morbidities, to a manageable chronic condition with an extended lifespan through newer therapies (cART). However, many clinical and research challenges remain in addressing the nature of this disease and the interactions between different sites of HIV infection and areas of the body where HIV exerts a direct and/or indirect effect. In addition, new issues in the diagnosis, treatment and pathogenesis of an aging population infected with HIV at different stages of their lifetime will arise. This article has presented some of the most important of these epidemiological and research trends. One common theme in this presentation is the increasing importance of interactions between clinicians and researchers in the fields of periodontal diseases, other oral diseases, as well as in other systemic conditions. New and continued collaborations between these different dental, medical and basic science disciplines should yield new insights into the interactions between periodontal diseases and systemic conditions, not only for the HIV infected patient, but for non HIV infected patients with periodontal diseases and associated local and systemic diseases and conditions.
Acknowledgments
Funding information
US National Institutes of Health, Grant/Award Number U01-HD052102
REFERENCES
- 1.Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med. 2007;23(3):567–583, vii. [DOI] [PubMed] [Google Scholar]
- 2.ECC. EC-Clearinghouse on Oral Problems related to HIV infection and WHO collaborating center on Oral manifestations of the immunodeficiency virus Classification and diagnosis criteria for oral lesion in HIV infection.. J Oral Pathol Med 1993;22:289–291. [PubMed] [Google Scholar]
- 3.Williams CA, Winkler JR, Grassi M, Murray PA. HIV-associated periodontitis complicated by necrotizing stomatitis. Oral Surg Oral Med Oral Pathol. 1990;69(3):351–355. [DOI] [PubMed] [Google Scholar]
- 4.Patton LL, McKaig R. Rapid progression of bone loss in HIV-associated necrotizing ulcerative stomatitis. J Periodontol. 1998;69(6):710–716. [DOI] [PubMed] [Google Scholar]
- 5.Robinson PG, Sheiham A, Challacombe SJ, Wren MW, Zakrzewska JM. Gingival ulceration in HIV infection. A case series and case control study. J Clin Periodontol. 1998;25(3):260–267. [DOI] [PubMed] [Google Scholar]
- 6.Vernon LT, Babineau DC, Demko CA, et al. A prospective cohort study of periodontal disease measures and cardiovascular disease markers in HIV-infected adults. AIDS Res Hum Retroviruses. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernon LT, Demko CA, Whalen CC, et al. Characterizing traditionally defined periodontal disease in HIV+ adults. Community Dent Oral Epidemiol. 2009;37(5):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umadevi M, Adeyemi O, Patel M, Reichart PA, Robinson PG. (B2) Periodontal diseases and other bacterial infections. Adv Dent Res. 2006;19(1):139–145. [DOI] [PubMed] [Google Scholar]
- 9.Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ, Jr. Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(3):299–304. [DOI] [PubMed] [Google Scholar]
- 10.Wandera MN, Engebretsen IM, Rwenyonyi CM, Tumwine J, Astrom AN, Group P-ES. Periodontal status, tooth loss and self-reported periodontal problems effects on oral impacts on daily performances, OIDP, in pregnant women in Uganda: a cross-sectional study. Health Qual Life Outcomes. 2009;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood NH, Blignaut E, Lemmer J, Meyerov R, Feller L. Necrotizing periodontal diseases in a semirural district of South Africa. AIDS Res Treat. 2011;2011:638584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phiri R, Feller L, Blignaut E. The severity, extent and recurrence of necrotizing periodontal disease in relation to HIV status and CD4+ T cell count. J Int Acad Periodontol. 2010;12(4):98–103. [PubMed] [Google Scholar]
- 13.Eke PI, Wei L, Thornton-Evans GO, et al. Risk Indicators for Periodontitis in US Adults: NHANES 2009 to 2012. J Periodontol. 2016;87(10):1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr C, Lopez MR, Rua-Dobles A. Periodontal changes by HIV serostatus in a cohort of homosexual and bisexual men. J Clin Periodontol. 1992;19(10):794–801. [DOI] [PubMed] [Google Scholar]
- 15.Robinson PG, Sheiham A, Challacombe SJ, Zakrzewska JM. Periodontal health and HIV infection. Oral Dis. 1997;3 Suppl 1:S149–152. [DOI] [PubMed] [Google Scholar]
- 16.Lucht E, Heimdahl A, Nord CE. Periodontal disease in HIV-infected patients in relation to lymphocyte subsets and specific micro-organisms. J Clin Periodontol. 1991;18(4):252–256. [DOI] [PubMed] [Google Scholar]
- 17.Yeung SC, Stewart GJ, Cooper DA, Sindhusake D. Progression of periodontal disease in HIV seropositive patients. J Periodontol. 1993;64(7):651–657. [DOI] [PubMed] [Google Scholar]
- 18.Choromanska M, Waszkiel D. Periodontal status and treatment needs in HIV-infected patients. Adv Med Sci. 2006;51 Suppl 1:110–113. [PubMed] [Google Scholar]
- 19.Ndiaye CF, Critchlow CW, Leggott PJ, et al. Periodontal status of HIV-1 and HIV-2 seropositive and HIV seronegative female commercial sex workers in Senegal. J Periodontol. 1997;68(9):827–831. [DOI] [PubMed] [Google Scholar]
- 20.McKaig RG, Thomas JC, Patton LL, Strauss RP, Slade GD, Beck JD. Prevalence of HIV-associated periodontitis and chronic periodontitis in a southeastern US study group. J Public Health Dent. 1998;58(4):294–300. [DOI] [PubMed] [Google Scholar]
- 21.Aichelmann-Reidy ME, Wrigley DL, Gunsolley JC. HIV infection and bone loss due to periodontal disease. J Periodontol. 2010;81(6):877–884. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Amador V, Esquivel-Pedraza L, Sierra-Madero J, Anaya-Saavedra G, Gonzalez-Ramirez I, Ponce-de-Leon S. The changing clinical spectrum of human immunodeficiency virus (HIV)-related oral lesions in 1,000 consecutive patients: A 12-year study in a referral center in Mexico. Medicine (Baltimore). 2003;82(1):39–50. [DOI] [PubMed] [Google Scholar]
- 23.Arribas JR, Hernandez-Albujar S, Gonzalez-Garcia JJ, et al. Impact of protease inhibitor therapy on HIV-related oropharyngeal candidiasis. AIDS. 2000;14(8):979–985. [DOI] [PubMed] [Google Scholar]
- 24.Greenspan D, Gange SJ, Phelan JA, et al. Incidence of oral lesions in HIV-1-infected women: reduction with HAART. J Dent Res. 2004;83(2):145–150. [DOI] [PubMed] [Google Scholar]
- 25.Nicolatou-Galitis O, Velegraki A, Paikos S, et al. Effect of PI-HAART on the prevalence of oral lesions in HIV-1 infected patients. A Greek study. Oral Dis. 2004;10(3):145–150. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson TA, Greenspan D, Greenspan JS. Oral lesions of HIV disease and HAART in industrialized countries. Adv Dent Res. 2006;19(1):57–62. [DOI] [PubMed] [Google Scholar]
- 27.Tappuni AR, Fleming GJ. The effect of antiretroviral therapy on the prevalence of oral manifestations in HIV-infected patients: a UK study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(6):623–628. [DOI] [PubMed] [Google Scholar]
- 28.Kroidl A, Schaeben A, Oette M, Wettstein M, Herfordt A, Haussinger D. Prevalence of oral lesions and periodontal diseases in HIV-infected patients on antiretroviral therapy. Eur J Med Res. 2005;10(10):448–453. [PubMed] [Google Scholar]
- 29.Fricke U, Geurtsen W, Staufenbiel I, Rahman A. Periodontal status of HIV-infected patients undergoing antiretroviral therapy compared to HIV-therapy naive patients: a case control study. Eur J Med Res. 2012;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goncalves LS, Goncalves BM, Fontes TV. Periodontal disease in HIV-infected adults in the HAART era: Clinical, immunological, and microbiological aspects. Arch Oral Biol. 2013;58(10):1385–1396. [DOI] [PubMed] [Google Scholar]
- 31.Alves M, Mulligan R, Passaro D, et al. Longitudinal evaluation of loss of attachment in HIV-infected women compared to HIV-uninfected women. J Periodontol. 2006;77(5):773–779. [DOI] [PubMed] [Google Scholar]
- 32.Odden K, Schenck K, Hurlen B. High numbers of T cells in gingiva from patients with human immunodeficiency virus (HIV) infection. J Oral Pathol Med. 1995;24(9):413–419. [DOI] [PubMed] [Google Scholar]
- 33.Odden K, Schenck K, Koppang H, Hurlen B. Candidal infection of the gingiva in HIV-infected persons. J Oral Pathol Med. 1994;23(4):178–183. [DOI] [PubMed] [Google Scholar]
- 34.Brito A, Escalona LA, Correnti M, Perrone M, Bravo IM, Tovar V. Periodontal conditions and distribution of Prevotella intermedia, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans in HIV-infected patients undergoing anti-retroviral therapy and in an HIV-seronegative group of the Venezuelan population. Acta Odontol Latinoam. 2008;21(1):89–96. [PubMed] [Google Scholar]
- 35.Engeland CG, Jang P, Alves M, Marucha PT, Califano J. HIV infection and tooth loss. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(3):321–326. [DOI] [PubMed] [Google Scholar]
- 36.Vastardis SA, Yukna RA, Fidel PL Jr, Leigh JE, Mercante DE. Periodontal disease in HIV-positive individuals: association of periodontal indices with stages of HIV disease . J Periodontol. 2003;74(9):1336–1341. [DOI] [PubMed] [Google Scholar]
- 37.Yengopal V, Bhayat A, Coogan M. Pediatric oral HIV research in the developing world. Adv Dent Res. 2011;23(1):61–66. [DOI] [PubMed] [Google Scholar]
- 38.Howell RB, Jandinski JJ, Palumbo P, Shey Z, Houpt MI. Oral soft tissue manifestations and CD4 lymphocyte counts in HIV-infected children. Pediatr Dent. 1996;18(2):117–120. [PubMed] [Google Scholar]
- 39.Vaseliu N, Carter AB, Kline NE, et al. Longitudinal study of the prevalence and prognostic implications of oral manifestations in romanian children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 2005;24(12):1067–1071. [DOI] [PubMed] [Google Scholar]
- 40.Santos LC, Castro GF, de Souza IP, Oliveira RH. Oral manifestations related to immunosuppression degree in HIV-positive children. Braz Dent J. 2001;12(2):135–138. [PubMed] [Google Scholar]
- 41.Magalhaes MG, Bueno DF, Serra E, Goncalves R. Oral manifestations of HIV positive children. J Clin Pediatr Dent. 2001;25(2):103–106. [DOI] [PubMed] [Google Scholar]
- 42.Khongkunthian P, Grote M, Isaratanan W, Piyaworawong S, Reichart PA. Oral manifestations in 45 HIV-positive children from Northern Thailand. J Oral Pathol Med. 2001;30(9):549–552. [DOI] [PubMed] [Google Scholar]
- 43.Reichart PA, Khongkhunthian P, Bendick C. Oral manifestations in HIV-infected individuals from Thailand and Cambodia. Med Microbiol Immunol. 2003;192(3):157–160. [DOI] [PubMed] [Google Scholar]
- 44.Ranganathan K, Geethalakshmi E, Krishna Mohan Rao U, Vidya KM, Kumarasamy N, Solomon S. Orofacial and systemic manifestations in 212 paediatric HIV patients from Chennai, South India. Int J Paediatr Dent. 2010;20(4):276–282. [DOI] [PubMed] [Google Scholar]
- 45.Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296(3):292–300. [DOI] [PubMed] [Google Scholar]
- 46.Moscicki AB, Yao TJ, Ryder MI, et al. The Burden of Oral Disease among Perinatally HIV-Infected and HIV-Exposed Uninfected Youth. PLoS One. 2016;11(6):e0156459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryder MI, Yao TJ, Russell JS, Moscicki AB, Shiboski CH, Pediatric HIVACS. Prevalence of periodontal diseases in a multicenter cohort of perinatally HIV-infected and HIV-exposed and uninfected youth. J Clin Periodontol. 2017;44(1):2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiboski CH, Yao TJ, Russell JS, et al. The association between oral disease and type of antiretroviral therapy among perinatally HIV-infected youth. AIDS. 2018;32(17):2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryder MI, Nittayananta W, Coogan M, Greenspan D, Greenspan JS. Periodontal disease in HIV/AIDS. Periodontol 2000. 2012;60(1):78–97. [DOI] [PubMed] [Google Scholar]
- 50.Ryder MI. An update on HIV and periodontal disease. J Periodontol. 2002;73(9):1071–1078. [DOI] [PubMed] [Google Scholar]
- 51.Myint MM, Steinsvoll S, Odden K, Dobloug J, Schenck K. Salivary IgA responses to bacteria in dental plaque as related to periodontal and HIV infection status. Eur J Oral Sci. 1997;105(6):562–570. [DOI] [PubMed] [Google Scholar]
- 52.Steinsvoll S, Myint M, Odden K, Berild D, Schenck K. Reduced serum IgG reactivities with bacteria from dental plaque in HIV-infected persons with periodontitis. J Clin Periodontol. 1997;24(11):823–829. [DOI] [PubMed] [Google Scholar]
- 53.Grbic JT, Lamster IB, Mitchell-Lewis D. Inflammatory and immune mediators in crevicular fluid from HIV-infected injecting drug users. J Periodontol. 1997;68(3):249–255. [DOI] [PubMed] [Google Scholar]
- 54.Lamster IB, Grbic JT, Bucklan RS, Mitchell-Lewis D, Reynolds HS, Zambon JJ. Epidemiology and diagnosis of HIV-associated periodontal diseases. Oral Dis. 1997;3 Suppl 1:S141–148. [DOI] [PubMed] [Google Scholar]
- 55.Baqui AA, Meiller TF, Jabra-Rizk MA, Zhang M, Kelley JI, Falkler WA Jr Enhanced interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in gingival crevicular fluid from periodontal pockets of patients infected with human immunodeficiency virus 1. Oral Microbiol Immunol. 2000;15(2):67–73. [DOI] [PubMed] [Google Scholar]
- 56.Mellanen L, Ingman T, Lahdevirta J, et al. Matrix metalloproteinases-1, −3 and −8 and myeloperoxidase in saliva of patients with human immunodeficiency virus infection. Oral Dis. 1996;2(4):263–271. [DOI] [PubMed] [Google Scholar]
- 57.Mellanen L, Salo T, Ingman T, Konttinen YT, Lahdevirta J, Sorsa T. 72-kDa and 92-kDa gelatinases in saliva of patients with human immunodeficiency virus infection. Acta Odontol Scand. 1998;56(3):135–142. [DOI] [PubMed] [Google Scholar]
- 58.Alpagot T, Suzara V, Bhattacharyya M. The associations between gingival crevice fluid matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and periodontitis in human immunodeficiency virus-positive patients. J Periodontal Res. 2006;41(6):491–497. [DOI] [PubMed] [Google Scholar]
- 59.Myint M, Odden K, Schreurs O, Halstensen TS, Schenck K. The gingival plasma cell infiltrate in HIV-positive patients with periodontitis is disorganized. J Clin Periodontol. 1999;26(6):358–365. [DOI] [PubMed] [Google Scholar]
- 60.Myint M, Yuan ZN, Schenck K. Reduced numbers of Langerhans cells and increased HLA-DR expression in keratinocytes in the oral gingival epithelium of HIV-infected patients with periodontitis. J Clin Periodontol. 2000;27(7):513–519. [DOI] [PubMed] [Google Scholar]
- 61.Myint M, Steinsvoll S, Yuan ZN, Johne B, Helgeland K, Schenck K. Highly increased numbers of leukocytes in inflamed gingiva from patients with HIV infection. AIDS. 2002;16(2):235–243. [DOI] [PubMed] [Google Scholar]
- 62.Murray PA, Winkler JR, Peros WJ, French CK, Lippke JA. DNA probe detection of periodontal pathogens in HIV-associated periodontal lesions. Oral Microbiol Immunol. 1991;6(1):34–40. [DOI] [PubMed] [Google Scholar]
- 63.Moore LV, Moore WE, Riley C, Brooks CN, Burmeister JA, Smibert RM. Periodontal microflora of HIV positive subjects with gingivitis or adult periodontitis. J Periodontol. 1993;64(1):48–56. [DOI] [PubMed] [Google Scholar]
- 64.Murray PA, Grassi M, Winkler JR. The microbiology of HIV-associated periodontal lesions. J Clin Periodontol. 1989;16(10):636–642. [DOI] [PubMed] [Google Scholar]
- 65.Rams TE, Andriolo M Jr, Feik D, Abel SN, McGivern TM, Slots J Microbiological study of HIV-related periodontitis. J Periodontol. 1991;62(1):74–81. [DOI] [PubMed] [Google Scholar]
- 66.Zambon JJ. Rapid diagnosis of periodontal infections: findings in AIDS patients. Immunol Invest. 1997;26(1–2):55–65. [DOI] [PubMed] [Google Scholar]
- 67.Zambon JJ, Reynolds HS, Genco RJ. Studies of the subgingival microflora in patients with acquired immunodeficiency syndrome. J Periodontol. 1990;61(11):699–704. [DOI] [PubMed] [Google Scholar]
- 68.Patel M, Coogan M, Galpin JS. Periodontal pathogens in subgingival plaque of HIV-positive subjects with chronic periodontitis. Oral Microbiol Immunol. 2003;18(3):199–201. [DOI] [PubMed] [Google Scholar]
- 69.Cobb CM, Ferguson BL, Keselyak NT, Holt LA, MacNeill SR, Rapley JW. A TEM/SEM study of the microbial plaque overlying the necrotic gingival papillae of HIV-seropositive, necrotizing ulcerative periodontitis. J Periodontal Res. 2003;38(2):147–155. [DOI] [PubMed] [Google Scholar]
- 70.Salama C, Finch D, Bottone EJ. Fusospirochetosis causing necrotic oral ulcers in patients with HIV infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(3):321–323. [DOI] [PubMed] [Google Scholar]
- 71.Goldberg BE, Mongodin EF, Jones CE, et al. The oral bacterial communities of children with well-controlled HIV Infection and without HIV Infection. PLoS One. 2015;10(7):e0131615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Navazesh M, Mulligan R, Pogoda J, et al. The effect of HAART on salivary microbiota in the Women’s Interagency HIV Study (WIHS). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(6):701–708. [DOI] [PubMed] [Google Scholar]
- 73.Dang AT, Cotton S, Sankaran-Walters S, et al. Evidence of an increased pathogenic footprint in the lingual microbiome of untreated HIV infected patients. BMC Microbiol. 2012;12:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moyes DL, Saxena D, John MD, Malamud D. The gut and oral microbiome in HIV disease: a workshop report. Oral Dis. 2016;22 Suppl 1:166–170. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Saxena D, Chen Z, et al. HIV infection and microbial diversity in saliva. J Clin Microbiol. 2014;52(5):1400–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kistler JO, Arirachakaran P, Poovorawan Y, Dahlen G, Wade WG. The oral microbiome in human immunodeficiency virus (HIV)-positive individuals. J Med Microbiol. 2015;64(9):1094–1101. [DOI] [PubMed] [Google Scholar]
- 77.Goncalves Lde S, Ferreira SM, Silva A Jr, et al. Association of T CD4 lymphocyte levels and subgingival microbiota of chronic periodontitis in HIV-infected Brazilians under HAART. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(2):196–203. [DOI] [PubMed] [Google Scholar]
- 78.Paster BJ, Russell MK, Alpagot T, et al. Bacterial diversity in necrotizing ulcerative periodontitis in HIV-positive subjects. Ann Periodontol. 2002;7(1):8–16. [DOI] [PubMed] [Google Scholar]
- 79.Aas JA, Barbuto SM, Alpagot T, Olsen I, Dewhirst FE, Paster BJ. Subgingival plaque microbiota in HIV positive patients. J Clin Periodontol. 2007;34(3):189–195. [DOI] [PubMed] [Google Scholar]
- 80.Mataftsi M, Skoura L, Sakellari D. HIV infection and periodontal diseases: an overview of the post-HAART era. Oral Dis. 2011;17(1):13–25. [DOI] [PubMed] [Google Scholar]
- 81.de Souza Goncalves L, Souto R, Colombo AP. Detection of Helicobacter pylori, Enterococcus faecalis, and Pseudomonas aeruginosa in the subgingival biofilm of HIV-infected subjects undergoing HAART with chronic periodontitis. Eur J Clin Microbiol Infect Dis. 2009;28(11):1335–1342. [DOI] [PubMed] [Google Scholar]
- 82.Smith C, Jalbert E, de Almeida V, et al. Altered natural killer cell function in HIV-exposed uninfected infants. Front Immunol. 2017;8:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mussi-Pinhata MM, Motta F, Freimanis-Hance L, et al. Lower respiratory tract infections among human immunodeficiency virus-exposed, uninfected infants. Int J Infect Dis. 2010;14 Suppl 3:e176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kidzeru EB, Hesseling AC, Passmore JA, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS. 2014;28(10):1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans C, Humphrey JH, Ntozini R, Prendergast AJ. HIV-exposed uninfected infants in Zimbabwe: Insights into health outcomes in the pre-antiretroviral therapy era. Front Immunol. 2016;7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Starr JR, Huang Y, Lee KH, et al. Oral microbiota in youth with perinatally acquired HIV infection. Microbiome. 2018;6(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falasca K, Vecchiet F, Ucciferri C, et al. Periodontitis and cytokine patterns in HIV positive patients. Eur J Med Res. 2008;13(4):163–168. [PubMed] [Google Scholar]
- 88.Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356(6341):938–945. [DOI] [PubMed] [Google Scholar]
- 89.Glick M, Cleveland DB, Salkin LM, Alfaro-Miranda M, Fielding AF. Intraoral cytomegalovirus lesion and HIV-associated periodontitis in a patient with acquired immunodeficiency syndrome. Oral Surg Oral Med Oral Pathol. 1991;72(6):716–720. [DOI] [PubMed] [Google Scholar]
- 90.Moskow BS, Hernandez G. Aggressive periodontal destruction and herpes zoster in a suspected AIDS patient. J Parodontol. 1991;10(4):359–369. [PubMed] [Google Scholar]
- 91.Madinier I, Monteil RA. Human papillomaviruses in oral epithelial lesions. Comparative study between histopathology and immunohistochemistry in routine diagnosis. J Biol Buccale. 1987;15(2):105–110. [PubMed] [Google Scholar]
- 92.Mardirossian A, Contreras A, Navazesh M, Nowzari H, Slots J. Herpesviruses 6, 7 and 8 in HIV- and non-HIV-associated periodontitis. J Periodontal Res. 2000;35(5):278–284. [DOI] [PubMed] [Google Scholar]
- 93.Contreras A, Mardirossian A, Slots J. Herpesviruses in HIV-periodontitis. J Clin Periodontol. 2001;28(1):96–102. [DOI] [PubMed] [Google Scholar]
- 94.Slots J Periodontal herpesviruses: prevalence, pathogenicity, systemic risk. Periodontol 2000. 2015;69(1):28–45. [DOI] [PubMed] [Google Scholar]
- 95.Zhu C, Li F, Wong MC, Feng XP, Lu HX, Xu W. Association between herpesviruses and chronic periodontitis: A meta-analysis based on case-control studies. PLoS One. 2015;10(12):e0144319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dittmer DP, Tamburro K, Chen H, et al. Oral shedding of herpesviruses in HIV-infected patients with varying degrees of immune status. AIDS. 2017;31(15):2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slots J, Contreras A. Herpesviruses: a unifying causative factor in periodontitis? Oral Microbiol Immunol. 2000;15(5):277–280. [DOI] [PubMed] [Google Scholar]
- 98.Shiboski CH, Lee A, Chen H, et al. Human papillomavirus infection in the oral cavity of HIV patients is not reduced by initiating antiretroviral therapy. AIDS. 2016;30(10):1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stein AP, Saha S, Kraninger JL, et al. Prevalence of human papillomavirus in oropharyngeal cancer: A systematic review. Cancer J. 2015;21(3):138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189(4):686–698. [DOI] [PubMed] [Google Scholar]
- 101.Cameron JE, Mercante D, O’Brien M, et al. The impact of highly active antiretroviral therapy and immunodeficiency on human papillomavirus infection of the oral cavity of human immunodeficiency virus-seropositive adults. Sex Transm Dis. 2005;32(11):703–709. [DOI] [PubMed] [Google Scholar]
- 102.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beachler DC, Sugar EA, Margolick JB, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol. 2015;181(1):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moscicki AB, Farhat S, Yao TJ, et al. Oral human papillomavirus in youth from the Pediatric HIV/AIDS Cohort Study. Sex Transm Dis. 2016;43(8):498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holmstrup P Non-plaque-induced gingival lesions. Ann Periodontol. 1999;4(1):20–31. [DOI] [PubMed] [Google Scholar]
- 106.Grbic JT, Mitchell-Lewis DA, Fine JB, et al. The relationship of candidiasis to linear gingival erythema in HIV-infected homosexual men and parenteral drug users. J Periodontol. 1995;66(1):30–37. [DOI] [PubMed] [Google Scholar]
- 107.Chattin BR, Ishihara K, Okuda K, Hirai Y, Ishikawa T. Specific microbial colonizations in the periodontal sites of HIV-infected subjects. Microbiol Immunol. 1999;43(9):847–852. [DOI] [PubMed] [Google Scholar]
- 108.Gomez RS, da Costa JE, Loyola AM, de Araujo NS, de Araujo VC. Immunohistochemical study of linear gingival erythema from HIV-positive patients. J Periodontal Res. 1995;30(5):355–359. [DOI] [PubMed] [Google Scholar]
- 109.Portela MB, Souza IP, Abreu CM, et al. Effect of serine-type protease of Candida spp. isolated from linear gingival erythema of HIV-positive children: critical factors in the colonization. J Oral Pathol Med. 2010;39(10):753–760. [DOI] [PubMed] [Google Scholar]
- 110.Glick M, Muzyka BC, Lurie D, Salkin LM. Oral manifestations associated with HIV-related disease as markers for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. 1994;77(4):344–349. [DOI] [PubMed] [Google Scholar]
- 111.Ryder MI, Winkler JR, Weinreb RN. Elevated phagocytosis, oxidative burst, and F-actin formation in PMNs from individuals with intraoral manifestations of HIV infection. J Acquir Immune Defic Syndr. 1988;1(4):346–353. [PubMed] [Google Scholar]
- 112.Alpagot T, Font K, Lee A. Longitudinal evaluation of GCF IFN-gamma levels and periodontal status in HIV+ patients. J Clin Periodontol. 2003;30(11):944–948. [DOI] [PubMed] [Google Scholar]
- 113.Alpagot T, Remien J, Bhattacharyya M, Konopka K, Lundergan W, Duzgunes N. Longitudinal evaluation of prostaglandin E2 (PGE2) and periodontal status in HIV+ patients. Arch Oral Biol. 2007;52(11):1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74(3):391–401. [DOI] [PubMed] [Google Scholar]
- 115.Alpagot T, Konopka K, Bhattacharyya M, Gebremedhin S, Duzgunes N. The association between gingival crevicular fluid TGF-beta1 levels and periodontal status in HIV-1(+) patients. J Periodontol. 2008;79(1):123–130. [DOI] [PubMed] [Google Scholar]
- 116.Lamster IB, Grbic JT, Mitchell-Lewis DA, Begg MD, Mitchell A. New concepts regarding the pathogenesis of periodontal disease in HIV infection. Ann Periodontol. 1998;3(1):62–75. [DOI] [PubMed] [Google Scholar]
- 117.Ohmit SE, Sobel JD, Schuman P, et al. Longitudinal study of mucosal Candida species colonization and candidiasis among human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188(1):118–127. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez Rodrigues C, Remes Lenicov F, Jancic C, et al. Candida albicans delays HIV-1 replication in macrophages. PLoS One. 2013;8(8):e72814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cutler CW, Jotwani R. Oral mucosal expression of HIV-1 receptors, co-receptors, and alpha-defensins: tableau of resistance or susceptibility to HIV infection? Adv Dent Res. 2006;19(1):49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jotwani R, Muthukuru M, Cutler CW. Increase in HIV receptors/co-receptors/alpha-defensins in inflamed human gingiva. J Dent Res. 2004;83(5):371–377. [DOI] [PubMed] [Google Scholar]
- 121.Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7(4):983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nowak P, Troseid M, Avershina E, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS. 2015;29(18):2409–2418. [DOI] [PubMed] [Google Scholar]
- 123.Saxena D, Li Y, Devota A, et al. Modulation of the orodigestive tract microbiome in HIV-infected patients. Oral Dis. 2016;22 Suppl 1:73–78. [DOI] [PubMed] [Google Scholar]
- 124.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33(6):401–407. [DOI] [PubMed] [Google Scholar]
- 125.Danaher RJ, Wang C, Roland AT, Kaetzel CS, Greenberg RN, Miller CS. HIV protease inhibitors block oral epithelial cell DNA synthesis. Arch Oral Biol. 2010;55(2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chege D, Sheth PM, Kain T, et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS. 2011;25(6):741–749. [DOI] [PubMed] [Google Scholar]
- 128.Horliana AC, Chambrone L, Foz AM, et al. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. 2014;9(5):e98271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dandekar S, George MD, Baumler AJ. Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS. 2010;5(2):173–178. [DOI] [PubMed] [Google Scholar]
- 130.Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol. 2015;36:22–30. [DOI] [PubMed] [Google Scholar]
- 131.Dillon SM, Frank DN, Wilson CC. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS. 2016;30(18):2737–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie H, Belogortseva NI, Wu J, Lai WH, Chen CH. Inhibition of human immunodeficiency virus type 1 entry by a binding domain of Porphyromonas gingivalis gingipain. Antimicrob Agents Chemother. 2006;50(9):3070–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mantri CK, Chen C, Dong X, Goodwin JS, Xie H. Porphyromonas gingivalis-mediated Epithelial Cell Entry of HIV-1. J Dent Res. 2014;93(8):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Giacaman RA, Asrani AC, Gebhard KH, et al. Porphyromonas gingivalis induces CCR5-dependent transfer of infectious HIV-1 from oral keratinocytes to permissive cells. Retrovirology. 2008;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giacaman RA, Nobbs AH, Ross KF, Herzberg MC. Porphyromonas gingivalis selectively up-regulates the HIV-1 coreceptor CCR5 in oral keratinocytes. J Immunol. 2007;179(4):2542–2550. [DOI] [PubMed] [Google Scholar]
- 136.Das B, Dobrowolski C, Shahir AM, et al. Short chain fatty acids potently induce latent HIV-1 in T-cells by activating P-TEFb and multiple histone modifications. Virology. 2015;474:65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gonzalez OA, Li M, Ebersole JL, Huang CB. HIV-1 reactivation induced by the periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves Toll-like receptor 2 [corrected] and 9 activation in monocytes/macrophages. Clin Vaccine Immunol. 2010;17(9):1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Imai K, Victoriano AF, Ochiai K, Okamoto T. Microbial interaction of periodontopathic bacterium Porphyromonas gingivalis and HIV-possible causal link of periodontal diseases to AIDS progression. Curr HIV Res. 2012;10(3):238–244. [DOI] [PubMed] [Google Scholar]
- 139.Kantor B, Ma H, Webster-Cyriaque J, Monahan PE, Kafri T. Epigenetic activation of unintegrated HIV-1 genomes by gut-associated short chain fatty acids and its implications for HIV infection. Proc Natl Acad Sci U S A. 2009;106(44):18786–18791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gonzalez OA, Ebersole JL, Huang CB. Supernatants from oral epithelial cells and gingival fibroblasts modulate human immunodeficiency virus type 1 promoter activation induced by periodontopathogens in monocytes/macrophages. Mol Oral Microbiol. 2010;25(2):136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gonzalez OA, Li M, Ebersole JL, Huang CB. HIV-1 reactivation induced by periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves TLR2 and TLR9 activation in monocytes/macrophages. Clin Vaccine Immunol. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang CB, Alimova YV, Ebersole JL. HIV-1 reactivation in HIV-latently infected dendritic cells by oral microorganisms and LPS. Cell Immunol. 2011;268(2):105–111. [DOI] [PubMed] [Google Scholar]
- 143.Huang CB, Alimova YV, Strange S, Ebersole JL. Polybacterial challenge enhances HIV reactivation in latently infected macrophages and dendritic cells. Immunology. 2011;132(3):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009;182(6):3688–3695. [DOI] [PubMed] [Google Scholar]
- 145.Huang CB, Emerson KA, Gonzalez OA, Ebersole JL. Oral bacteria induce a differential activation of human immunodeficiency virus-1 promoter in T cells, macrophages and dendritic cells. Oral Microbiol Immunol. 2009;24(5):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gonzalez OA, Ebersole JL, Huang CB. Oral infectious diseases: a potential risk factor for HIV virus recrudescence? Oral Dis. 2009;15(5):313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Valentine J, Saladyanant T, Ramsey K, et al. Impact of periodontal intervention on local inflammation, periodontitis, and HIV outcomes. Oral Dis. 2016;22 Suppl 1:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. [DOI] [PubMed] [Google Scholar]
- 149.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200(8):1212–1215. [DOI] [PubMed] [Google Scholar]
- 150.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–1543. [DOI] [PubMed] [Google Scholar]
- 151.Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202(5):723–733. [DOI] [PubMed] [Google Scholar]
- 152.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9(2):139–147. [DOI] [PubMed] [Google Scholar]
- 153.Navazesh M, Mulligan R, Komaroff E, Redford M, Greenspan D, Phelan J. The prevalence of xerostomia and salivary gland hypofunction in a cohort of HIV-positive and at-risk women. J Dent Res. 2000;79(7):1502–1507. [DOI] [PubMed] [Google Scholar]
- 154.Navazesh M, Mulligan R, Barron Y, et al. A 4-year longitudinal evaluation of xerostomia and salivary gland hypofunction in the Women’s Interagency HIV Study participants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(6):693–698. [DOI] [PubMed] [Google Scholar]
- 155.Nittayananta W, Talungchit S, Jaruratanasirikul S, et al. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J Oral Pathol Med. 2010;39(5):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Diz Dios P, Scully C. Antiretroviral therapy: effects on orofacial health and health care. Oral Dis. 2014;20(2):136–145. [DOI] [PubMed] [Google Scholar]
- 157.Navazesh M, Mulligan R, Karim R, et al. Effect of HAART on salivary gland function in the Women’s Interagency HIV Study (WIHS). Oral Dis. 2009;15(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cavasin Filho JC, Giovani EM. Xerostomy, dental caries and periodontal disease in HIV+ patients. Braz J Infect Dis. 2009;13(1):13–17. [DOI] [PubMed] [Google Scholar]
- 159.Nittayananta W, Chanowanna N, Jealae S, Nauntofte B, Stoltze K. Hyposalivation, xerostomia and oral health status of HIV-infected subjects in Thailand before HAART era. J Oral Pathol Med. 2010;39(1):28–34. [DOI] [PubMed] [Google Scholar]
- 160.Farhadian S, Patel P, Spudich S. Neurological Complications of HIV Infection. Curr Infect Dis Rep. 2017;19(12):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Smith R, Chernoff M, Williams PL, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31(6):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol. 2002;17(2):113–118. [DOI] [PubMed] [Google Scholar]
- 163.Groeger SE, Meyle J. Epithelial barrier and oral bacterial infection. Periodontol 2000. 2015;69(1):46–67. [DOI] [PubMed] [Google Scholar]
- 164.Caputo BV, Traversa-Caputo GC, Costa C, Giovani EM. Evaluation of bone alterations in the jaws of HIV-infected menopausal women. Braz Oral Res. 2013;27(3):231–237. [DOI] [PubMed] [Google Scholar]
- 165.Yin MT, Zhang CA, McMahon DJ, et al. Higher rates of bone loss in postmenopausal HIV-infected women: a longitudinal study. J Clin Endocrinol Metab. 2012;97(2):554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Beck JM, Schloss PD, Venkataraman A, et al. Multicenter comparison of lung and oral microbiomes of HIV-infected and HIV-uninfected Individuals. Am J Respir Crit Care Med. 2015;192(11):1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Noguera-Julian M, Guillen Y, Peterson J, et al. Oral microbiome in HIV-associated periodontitis. Medicine (Baltimore). 2017;96(12):e5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS. 2014;28(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Negredo E, Domingo P, Ferrer E, et al. Peak bone mass in young HIV-infected patients compared with healthy controls. J Acquir Immune Defic Syndr. 2014;65(2):207–212. [DOI] [PubMed] [Google Scholar]
- 170.Nittayananta W, Amornthatree K, Kemapunmanus M, Talungchit S, Sriplung H. Expression of oral cytokines in HIV-infected subjects with long-term use of antiretroviral therapy. Oral Dis. 2014;20(3):e57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Warriner AH, Mugavero M, Overton ET. Bone alterations associated with HIV. Curr HIV/AIDS Rep. 2014;11(3):233–240. [DOI] [PubMed] [Google Scholar]