Abstract
Background
Lianhuaqingwen (LH) has been proven effective for influenza. However, the promotion of LH for the treatment of patients with COVID-19 remains controversial. Therefore, our study aimed to assess the efficacy and safety of Lianhuaqingwen (LH) in treating patients with COVID-19 by a systematic review and meta-analysis.
Methods
We conducted the literature search using six electronic databases from December 1, 2019, to June 2, 2020. Cochrane Risk of Bias tool was used to assess the quality of randomized controlled trials. Newcastle-Ottawa Scale was used to assess the quality of case control studies. Agency for Healthcare Research and Quality checklist was used to assess the quality of case series. All analyses were conducted by RevMan 5.3. For outcomes that could not be meta-analyzed were performed a descriptive analysis.
Results
Eight studies with 924 patients were included. Three studies were RCTs, three were case control studies, and two were case series. The quality of the included studies was poor. Compared with patients treated by conventional treatment, patients treated by LH combined with conventional treatment have a higher overall effective rate (RR = 1.16, 95%CIs: 1.04∼1.30, P = 0.01) and CT recovery rate (RR=1.21, 95%CIs: 1.02∼1.43, P = 0.03). Patients of LH groups have a lower incidence of diarrhea (5.6% vs.13.4%), and have statistically significant (P = 0.026). But the rate of abnormal liver function in the combined medication group is higher than that in the single LH group.
Conclusion
LH combined with conventional treatment seems to be more effective for patients with mild or ordinary COVID-19.
Keywords: COVID-19, Lianhuaqingwen, Herbal medicine, Systematic review, Meta-analysis
1. Introduction
Initial and most clinical manifestations of patients with coronavirus disease 2019 (COVID-19) include fever, fatigue, and dry cough.1 Patients with severe disease may exhibit pulmonary inflammation and infiltration, as well as systemic inflammatory cytokine storms, even death.2 Besides, some patients were undetected relatively mild infections.3 However, these are no specific antiviral treatment for COVID-19, currently. Supportive cares, including symptomatic controls and prevention of complications are remaining the most critical and cornerstone therapeutic regimens.4
Traditional Chinese medicine (TCM) has been proven to be effective in patients with influenza.5, 6 In the prevention and treatment of COVID-19, TCM have been widely used, especially in treating cases of mild symptoms.7 The combination of traditional Chinese and Western medicine is seemed to more effective than Western medicine alone for the management of Covid-19.8 Variety of TCM have been endorsed by the National Health Commission for the treatment of COVID-19. Lianhuaqingwen (LH) is one of them, which is used to treat COVID-19 with a solid theoretical foundation in the field of TCM.9 Experimental research also showed that LH exerted broad-spectrum effects on a series of influenza viruses by inhibiting viral propagation and regulating immune function.10
As for as we know, only one systematic review evaluated the efficacy and safety of LH combined with western medicine treatment COVID-19.11 But four studies with poor quality were included and the statistical method through single rate in their study.11 Therefore, it is necessary to provide a higher level of evidence to assess the efficacy and safety of LH treatment of patients with COVID-19. Our study aim is to evaluate the efficacy and safety of LH for treating patients with COVID-19 by a systematic review and meta-analysis.
2. Methods
2.1. Study registration
Our study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement12 and the synthesis without meta-analysis (SWiM) in systematic reviews.13 We prospectively registered this study protocol to the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020176332).
2.2. Eligibility criteria
Randomized controlled trials (RCTs), nonrandomized controlled trials, case-control trials, cohort studies, or cross-sectional studies meet the following criteria were included: (1) patients with COVID-19 were confirmed by a laboratory test; (2) patients treated with LH or LH combines with other drugs; (3) reported data on efficacy or safety of LH on COVID-19; (4) included a sample size of larger than 10; (5) published in Chinese or English language.
We excluded the following studies: (1) studies included suspected cases; (2) studies did not focus on LH; (3) case reports, reviews, animal studies, letters, comments, abstracts, and editorials.
2.3. Search strategy
We conducted a comprehensive literature search using in the China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), Wanfang database, PubMed, Embase, and Web of Science (WoS) from December 1, 2019, to June 2, 2020. The following search terms were used: “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “coronavirus disease-19”, “COVID-19”, “novel corona virus”, “new coronavirus”, “2019 novel coronavirus”, “2019-nCoV”, “novel coronavirus”, “nCoV-2019”, “novel coronavirus pneumonia”, “coronavirus disease 2019”, “Lianhua Qingwen”, and “Lianhuaqingwen”. The detailed search strategy of PubMed is presented in Supplement 1. We also manually searched the reference lists of eligible studies and relevant systematic reviews to identify additional potentially eligible studies.
2.4. Study selection
We used Endnote X8 (Thomson Reuters (Scientific) LLC Philadelphia, PA, US) software to manage the identified records and remove duplicates. Two reviewers (ML and YG) independently screened the titles and abstracts to determine studies that should be further evaluated. Then, two reviewers (ML and YG) reviewed the full-texts of potential studies to determine the final eligibility. Any disagreements were solved by consulting a senior reviewer (JHT). Considering multiple studies with overlapping patients, the study with a larger sample size was included.
2.5. Quality assessment
Two reviewers (YY and KLY) independently assessed the quality for each included study, and a third reviewer (JHT) was consulted in case of disagreement. The quality of included studies was evaluated by three tools. Cochrane Risk of Bias (RoB) tool was been used to assess the quality of randomized controlled trials. Newcastle-Ottawa Scale (NOS) was been used to assess the quality of case control studies. Agency for Healthcare Research and Quality (AHRQ) checklist was been used to assess the quality of case series studies.
2.6. Data extraction
A pre-designed data extraction form was used to extract information from including studies. This form included the following information: authors, publication year, country of the first and corresponding author, journal name, publication language, study setting, study period, study design, sample size, age and sex of patients, existing comorbidities, interventions, doses of LH, duration of treatment, follow-up, and outcomes of interest. Two reviewers (ML and YG) independently conducted data extraction and disagreements were resolved by consulting a senior reviewer (JHZ).
2.7. Statistical analysis
We conducted pairwise meta-analyses using the Mantel–Haenszel method with the random-effects model for RCTs to estimate the overall effect size between LH and control group. The pooled risk ratio (RR) with 95% confidence intervals (CIs) was used for the dichotomous variables. Heterogeneity between trials was evaluated using I² statistics. The values of 25%, 50%, and 75% for the I² were indicated of low, moderate, and high statistical heterogeneity, respectively. We planned to explore the publication bias using funnel plots if the number of included studies exceeded nine.14 All analyses were conducted by RevMan 5.3. P value < 0.05 is considered statistically significant.
For outcomes that could not be meta-analyzed, we performed a descriptive analysis. In this process, we followed the SWiM guideline, which was used in systematic reviews to examine the quantitative effects of interventions for which meta-analysis of effect estimates is not possible, or not appropriate.13
3. Results
3.1. Study selection
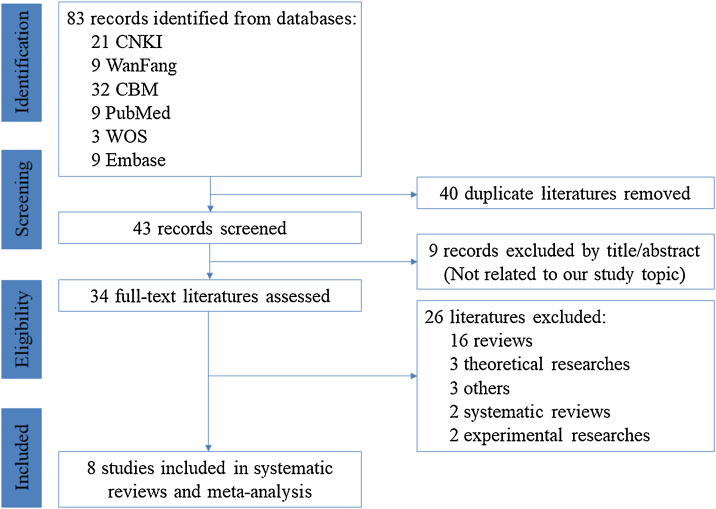
The literature selection process is described in detail in Fig. 1. We identified 83 records from the six electronic databases in the initial search. After removing 40 duplications, screening of the remaining 43 titles/abstracts yielded 34 potentially eligible literatures. By screening the full-text, 26 literatures were excluded because they were not in the field of interest and eight studies were included.15, 16, 17, 18, 19, 20, 21, 22
Fig. 1.
PRISMA flow diagram of study selection process (CNKI = China National Knowledge Infrastructure, CBM = Chinese Biomedical Literature Database, WOS = Web of Science, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
3.2. Characteristics of the included studies
Table 1 present detailed characteristics of the included studies. All studies were published in 2020 and performed in China. The disease stages of patients with COVID-19 were mild or ordinary. Included clinical trials enrolled patients from January 1, 2020 to March 06, 2020. Only one study was published in English.17 Three trials are RCTs,17, 21, 22 three are retrospective case control studies15, 18, 20 and two are retrospective case series.16, 19 The total sample size was 924 (506 males), and sample size per study ranged from 32 to 295. The mean age of the patients was between 44.1- and 60.1-year old.
Table 1.
Characteristics of included studies.
| Study | Study type | COVID-19 stage | Age(M ± SD) | Treatment |
Manufacturer (LH) | Dose (LH)/Durations | Main outcomes | Basic treatment | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention/control | Intervention | Control | |||||||
| Yu 21 | RCT | Mild and Ordinary | 48.3 ± 9.6 47.3 ± 9.7 | (A) LHG+(B) (n = 147) | (B) Arbidol Dispersible Tablets + Moxifloxacin tablets + Ambroxol tablets (n = 148) | BY | 6 g/times, tid n.r. | ①②③④⑤⑥⑦ | None |
| Hu 17 | RCT | Mild and Ordinary | 50.4 ± 15.2 51.8 ± 14.8 | (A) LHC+(B) (n = 142) | (B) Arbidol Dispersible Tablets (n = 142) | SY | No 14days | ①②③④⑤⑥⑦ | Oxygen therapy + Antiviral medications + Symptomatic therapies |
| Wang 22 | RCT | Ordinary | n.r. n.r. | (A) LHC + Lopinavir (Ritonavir)+(B) (n = 30) | (B) LHC + Interferon-alpha (n = 30) | n.r. | 1.4 g/times, tid 7∼10 days | ① | None |
| Chen 15 | Case control | Ordinary | 55.5 ± 12.3 55.8 ± 11.6 | (A) LHG+(B) (n = 51) | (B) Antiviral medications + Antimicrobial medication(n = 51) | SY | 6 g/times, tid 7 days | ②③④⑤ | Nutritional support therapy + Symptomatic treatment |
| Yao 20 | Case control | Ordinary | 57.1 ± 14.0 62.4 ± 12.3 | LHG+(B) (n = 21) | Conventional therapy (n = 21) | n.r. | 6 g/times, tid n.r. | ④⑤ | No |
| Liu 18 | Case control | Mild and Ordinary | n.r. n.r | LHC + Arbidol Dispersible Tablets (n = 14) | LHC(n = 18) | SY | 1.4 g/times, tid | ②⑤⑥⑦ | Oxygen therapy + Symptomatic therapies |
| Wang 19 | Case series | Mild and Ordinary | 44.1 ± 12.6 NA | LHG + Conventional therapy(n = 55) | None | BY | 6 g/times, tid n.r. | ④⑤⑥ | No |
| Chen 16 | Case series | Ordinary | 60.1 ± 17.0 NA | LHG + Conventional therapy (n = 54) | None | BY | 6 g/times, tid (8.0 ± 4.1) days | ④⑤⑦ | Human immunoglobulin + Ganciclovir injection + Levofloxacin injection + Methylprednisolone sodium succinate |
RCT = Randomized Controlled Trial; M = Mean; SD = Standard Deviation; LHG = Lianhua QingWen Granule; LHC = Lianhua QingWen Capsule; No = Not Provide; NA = No Apply; nr = Not Reported; BY: Beijing Yiling Pharmaceutical Co., Ltd; SY: Shijiazhuang Yiling Pharmaceutical Co., Ltd; ①Overall effective rate; ②CT recovery rate; ③Aggravation rate; ④Symptom recovery rate; ⑤Symptom recovery time; ⑥Laboratory index; ⑦Safety.
Four studies provided the durations of LH treatment patients with COVID-19. The shortest was 7 days and the longest was 14 days. Four studies provided information of patients’ comorbidities (Supplement 2). The details of the vital signs of including patients were shown in Supplement 3. The details the diagnostic criteria of included study were listed in Supplement 4.
3.3. Quality of including studies
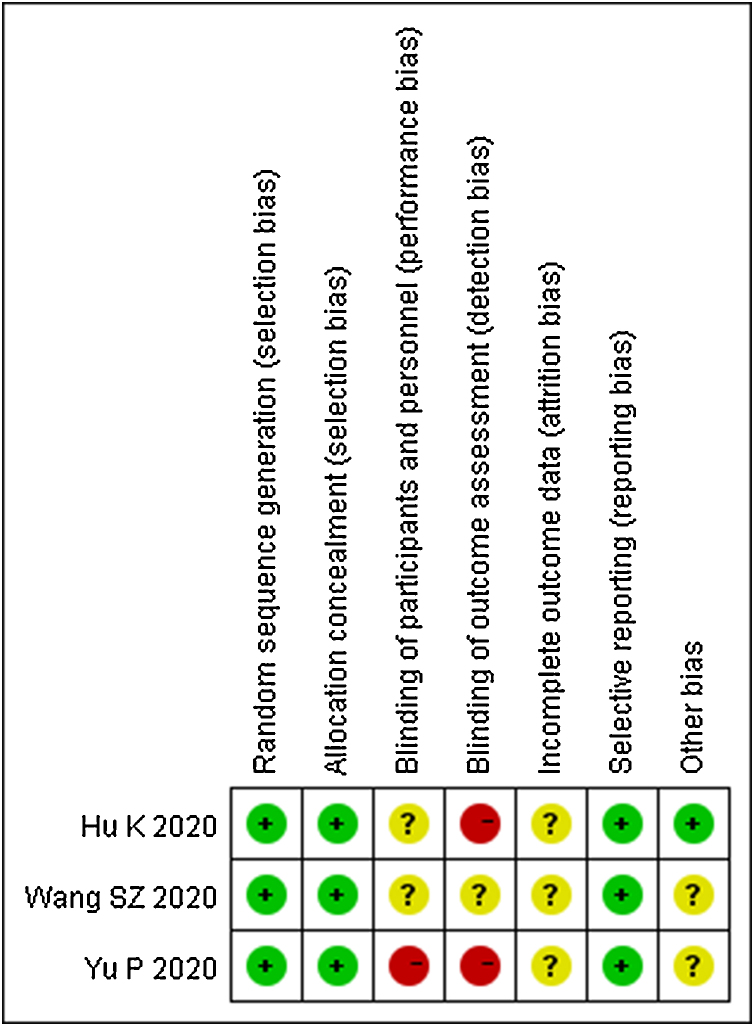
The quality of the three included RCTs is shown in Fig. 2. The quality of three case control studies was assessed by NOS. The study published by Yao KT et al. scored 4,20 Cheng DZ et al.’s15 scored 6 and Liu LL et al.’s18 scored 5. The quality of two case series which published by Wang FC et al.19 and Cheng DZ et al.16 were all fair quality.
Fig. 2.
The risk of bias of including randomized controlled trials.
3.4. Outcomes
3.4.1. RCT
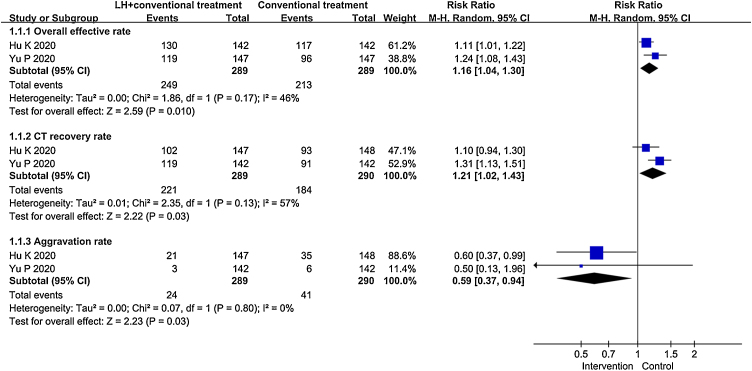
Two RCTs reported the comparison of overall effective rate, CT recovery rate and aggravation rate between LH combined with conventional treatment and conventional treatment.17, 21 Fig. 3 is the statistical analysis results. Compared with patients treated by conventional treatment, patients treated by LH combined with conventional treatment have a higher overall effective rate (RR = 1.16, 95%CIs: 1.04∼1.30, P = 0.01) and CT recovery rate (RR=1.21, 95%CIs: 1.02∼1.43, P = 0.03). The aggravation rate of patients treated by conventional treatment was higher than patients treated by LH combined with conventional treatment (RR=0.59, 95%CIs: 0.37∼0.94), and the difference was statistically significant (P = 0.03). Another RCT conducted by Wang SZ et al. showed that LH combined with lopinavir/ritonavir was more effective than LH alone for patients with COVID-19.22
Fig. 3.
Forest plot for meta-analysis of LH treat patients with COVID-19.
In addition, Hu K et al. also found that patients treated by LH combined with conventional treatment had shorter time to recovery of fever, fatigue and coughing.17 About the safety of LH, Hu K et al. provided the data of adverse event in both group of patients.17 They found that patients whom treated by LH combined with conventional treatment had a statistically significant low incidence of diarrhea (5.6%﹤13.4%, P = 0.026). And both groups of patients appeared abnormal liver function, renal dysfunction, headache, nausea, vomiting and loss of appetite. But the rate of these adverse events between the two groups did not have statistically significant difference. An important finding of Yu P et al. in RCT is that LH combined with conventional treatment changed the expression of inflammatory factor (e.g. WBC, LYM, CRP, PCT) more than the conventional treatment.21
3.4.2. Case studies
Chen DZ et al. and Yao KT et al. showed that LH combined with conventional treatment can significantly improve the symptoms of fever, cough, sputum and shortness of breath in patients with COVID-19 by retrospective case control study.15, 20 In addition, Chen DZ et al. also revealed that LH can improve the clinical symptoms of fatigue, chest tightness, loss of appetite of patients with COVID-19.15 And Liu LL et al. found that compared with treatment by LH alone, LH combined with abidole was more effective.18 However, the rate of abnormal liver function in the combined medication group was higher than that in the single medication group, and should be of concern.18 In addition, Chen DZ et al. and Wang FC et al. showed that LH combined with conventional treatment had a significant effect in treating patients with COVID-19 by retrospective case cerise.16, 19 And the effective rate of treatment for 7 days was significantly higher than that of treatment for 5 days.16 Wang FC et al. also showed that the longer the course of treatment, the easier it is for laboratory test indicators to return to normal.19
4. Discussion
4.1. Summary of the main results
We conducted an extensive systematic review of the efficacy and safety of LH treatment patients with COVID-19 across a broad range of condition. We included eight clinical trials with 924 patients. And the disease stages of all patients were mild or ordinary. Our study found that LH combined with conventional treatment (e.g. oxygen therapy, antiviral, antimicrobial) may improve the clinical efficacy of patients with COVID-19. There are different results regarding the safety of LH. A study found that it may reduce the occurrence of some adverse events, such as diarrhea.17 But another study found that the rate of abnormal liver function in the combined medication group is higher than that in the single LH group.18
4.2. Overall completeness and applicability of the evidence
LH is a manufactured product of the TCM formula marketed in Global. Both in vitro experiments and clinical trials proved that it is effective for COVID-19.17, 23 Clinical investigations showed that the most common clinical symptoms of COVID-19 are fever, fatigue, and cough.24, 25 And some studies indicated that LH can affect the relevant cytokines and ameliorating lung injury associated with inflammatory cell infiltration.26 Therefore, in addition to improving clinical symptoms, LH may also improve relevant laboratory and physical detection indicators. Thus, LH may be used not only for mild patients with fever, fatigue, and cough, but also for severe patients with lung injury. And more studies are needed to prove more potential therapeutic effects for patients with COVID-19.
4.3. Potential biases in the review process
In our study, we systematically evaluated the efficacy and safety of LH treatment of patient with COVID-19. The results of this study have certain reference value for the treatment of patients with COVID-19. Moreover, the in-depth study of LH also has a guiding role. However, our study also has some limitations. Firstly, all the studies included were from China, and the sample size was small, so the current findings may not fully reflect the global situation and should be interpreted with caution. Secondly, although the basic characteristics of the patients included in the study did not differ significantly, but some factors were not evaluated and may affect the accuracy of the results. Thirdly, the patient overlap was still possible between two studies that published by Chen DZ.15, 16 Fourth, there was a lack of data for subgroup analysis. Moreover, the data for meta-analysis was only contained two original studies, so the results needed to be interpreted carefully. As more data become available, it is necessary to update this systematic review and performed more comprehensive analyses to answer questions to guide clinical practice.
4.4. Agreements and disagreements with other studies or reviews
Cai LL et al.’s study showed that LH was more effective than other Chinese medicinal and western drugs in alleviating flu-like symptoms when treating influenza by a systematic review.27 Lu ZJ et al. showed that LH combined with conventional Western medicine in the treatment of pneumonia could improve the clinical treatment efficiency, shorten the time of fever, cough, rale disappearance and imaging recovery, improve CRP index and accelerate the recovery of pneumonia patients.28 So far, there is only one systematic review on LH treatment of patients with COVID-19.11 However, it only included four studies, and the analysis method has big flaws.11 The conclusions drawn are not convincing and cannot guide the clinical application of LH. Our study has strict acceptance criteria and reasonable evidence summary methods. The results showed that for patients with COVID-19, LH combined with conventional treatment was significantly better than conventional treatment alone. Therefore, our study will provide a good guidance for LH in the treatment of patients with COVID-19.
4.5. Implication for practice
Like the treatment of influenza, LH plays an irreplaceable role in the treatment of patient with COVID-19.29 However, like other TCM, it needs to be used in combination with western medicines, and the effect of single use is general.30 We all know the control of SARS-CoV-2 still presents multiple challenges in the short term, more potent antiviral drugs are urgent to be developed.31 Therefore, before the development of special antiviral drugs, integrated Chinese and Western medicine treatment will be the most effective treatment for patients with COVID-19.
4.6. Implication for research
Worldwide, the TCM market is increasing by 10∼20% annually.32 But TCM cannot be more widely accepted, and other important factors are safety and consistent quality. LH is a proprietary Chinese medicine, and our study found that there are different results regarding the safety of LH. Therefore, when LH combines with other drugs, more attention should be paid to safety. More clinical trials are needed to confirm the safety of LH combines with other drugs.
4.7. Conclusion
Our study indicated that LH may improve the clinical symptoms of patients with COVID-19, such as fever, fatigue and muscle ache. When LH combines with other drugs to treat patients with COVID-19, the safety is uncertain. As more data become available, it is necessary to update this study and performed more comprehensive analyses to answer questions to guide clinical practice.
Acknowledgments
The authors thank all investigators and supporters involved in this study.
Author contributions
Conceptualization: ML, YG, JHT, and JHZ. Methodology: ML, YG, YY, and KLY. Software: YG and ML. Formal Analysis: ML and YG. Writing – Original Draft: ML and JHT. Writing – Review & Editing: ML, YG, JHT, and JHZ.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was supported by the Emergency Research Project of Key Laboratory of Evidence-based Medicine and Knowledge Translation of Gansu Province (Grant No. GSEBMKT-2020YJ01).
Ethical statement
This research did not require an ethical approval as it does not involve any human or animal experiment.
Data availability
The data will be made available upon request.
Footnotes
Supplementary material related to this article can be found in the online version, at doi:https://doi.org/10.1016/j.imr.2020.100644.
Contributor Information
Jinhui Tian, Email: tianjh@lzu.edu.cn.
Junhua Zhang, Email: zjhtcm@foxmail.com.
Supplementary material
The following are Supplementary material to this article:
References
- 1.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England). 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye G., Pan Z., Pan Y., Deng Q., Chen L., Li J. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. The Journal of infection. 2020;80:e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng W.Y., Jiang Z.W., Wang W.C., Liu X.H., Chen H., Ling Z.X. Clinical characteristics of 32 asymptomatic patients infected with novel coronavirus. Medical Journal of Wuhan University. 2020:1–5. [Google Scholar]
- 4.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nature reviews Drug discovery. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., Manheimer E., Shi Y., Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. Journal of alternative and complementary medicine (New York, NY). 2004;10:1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 6.Duan Z.P., Jia Z.H., Zhang J., Liu S., Chen Y., Liang L.C. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chinese medical journal. 2011;124:2925–2933. [PubMed] [Google Scholar]
- 7.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacological research. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M., Gao Y., Yuan Y., Yang K.L., Shi S.Z., Zhang J.H. Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacological research. 2020;158:104896. doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H.R., Chang L.P., Wei C., Jia Z.H. Theoretical Research Basis and Clinical Efficacy of Lianhua Qingwen in Treating Novel Coronavious Pneumonica. World Chinese Medicine. 2020;15:332–336. [Google Scholar]
- 10.Pan W.Q., Li R.F., Hou Y.L., Huang J.C., Ma Q.H., Shi Y.X. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacological research. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi G.D., Qi W., Jiang Q., Shen K.Q., Zhang X., Zhang L. The Efficacy of Lianhua Qingwen Combined with Western Medicine Scheme on COVID-19 General Type Patients : a Systematic Review. Clinical Journal of Traditional Chinese Medicine. 2020:1–9. [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell M., McKenzie J.E., Sowden A., Katikireddi S.V., Brennan S.E., Ellis S. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ (Clinical research ed). 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D.Z., Wang W.J., Li Y., Wu X.D., Zhou B., Song Q.Y. 51 cases of COVID-19 patients with Chinese medicine Lianhuaqingwen curative effect analysis: a multi-center retrospective study. Tianjin Journal of Tradition Chinese Medicine. 2020;37:509–516. [Google Scholar]
- 16.Cheng D.Z., Li Y. Clinical Effectiveness and Case Analysis in 54 NCP Patients Treated with Lanhuaqingwen Granules. World Chinese Medicine. 2020;15:150–154. [Google Scholar]
- 17.Hu K., Guan W.J., Bi Y., Zhang W., Li L.J., Zhang B.L. Efficacy and Safety of Lianhuaqingwen Capsules, a repurposed Chinese Herb, in Patients with Coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. international journal of phytotherapy and phytopharmacology. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L.L., Yuan L.F., Feng Y., Sun D., Liu W.S., Wang Y.J. Clinical study on combined scheme of Lianhuaqingwen Capsules and abidole in the treatment for coronavirus disease 2019. Guangdong Medical Journal. 2020:1–4. [Google Scholar]
- 19.Wang F.C., Shen B.X., He C.Y., Zhao W.C., Nie S.L. Clinical Efficacy and Mechanism of Lianhua Qingwen Granule on COVID-19 Based on Network Pharmacology Research. Pharmacology and Clinics of Chinese Materia Medica. 2020;36:93–101. [Google Scholar]
- 20.Yao K.T., Liu M.Y., Li X., Huang J.H., Cai H.B. Retrospective Clinical Analysis on Treatment of Coronavirus Disease 2019 with Traditional Chinese Medicine Lianhua Qingwen. Chinese Journal of Experimental Traditional Medical Formulae. 2020;26:8–12. [Google Scholar]
- 21.Yu P., Li Y.Z., Wan S.B., Wang Y. Observation of Therapeutic Effect of Lianhuaqingwen Granule Combined with Abidor on Mild COVID-19. Chinese Pharmaceutical Journal. 2020:1–9. [Google Scholar]
- 22.Wang S.Z., Wang H.J., Chen H.M., Yue Y., Bu F.J., Zhang X.M. Lianhua Qingwen capsule and interferon-α combined with lopinavir /ritonavir for the treatment of 30 COVID-19 patients. J Bengbu Med Coll. 2020;45:154–155. [Google Scholar]
- 23.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nature reviews Drug discovery. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 24.Liu X.H., Lu S.H., Chen J., Lu X., Yang Z.G., Charles S. Clinical characteristics of foreign-imported COVID-19 cases in Shanghai, China. Emerging microbes & infections. 2020;9:1230–1232. doi: 10.1080/22221751.2020.1766383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International journal of antimicrobial agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y., Zeng L., Li R., Chen Q.Y., Zhou B.X., Chen O.L. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC complementary and alternative medicine. 2017;17:130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai L.L., Jiang H.L., Fang T., Zhong Y.Q., Zhou W., Mao B. Effectiveness and Safety of Lianhuaqingwen Capsule for Influenza: A Systematic Review. Chin J Evid-based Med. 2012;12:1396–1403. [Google Scholar]
- 28.Lu Z.J., Wu L.Y., Mou Y.Y., Duan H.M., Chen R.C., Xiao R., et al. Meta-analysis and systematic review of efficacy and safety of Lianhua Qingwen in adjuvant treatment of adult pneumonia China. Journal of Chinese Materia Medica. 1–11. [DOI] [PubMed]
- 29.Niu Q.Q., Chen Y., Liu Y., Mao S.Z., Wang H., Zheng W.K. Efficacy and safety of Lianhua Qingwen capsule for influenza: a systematic review. ZhongGuo ZhongYao ZaZhi. 2017;42:1474–1481. doi: 10.19540/j.cnki.cjcmm.2017.0044. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M.M., Liu X.M., He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World journal of gastroenterology. 2004;10:3500–3505. doi: 10.3748/wjg.v10.i23.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) Coronavirus. American journal of respiratory and critical care medicine. 2020;201:p7–p8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 32.Liu S.H., Chuang W.C., Lam W., Jiang Z., Cheng Y.C. Safety surveillance of traditional Chinese medicine: current and future. Drug safety. 2015;38:117–128. doi: 10.1007/s40264-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available upon request.