Abstract
The novel coronavirus (COVID-19) has become a global pandemic outbreak. Patients with COVID-19 are prone to progress to acute respiratory distress syndrome (ARDS), and even severe ARDS with ineffective mechanical ventilation, and an extremely high mortality. Extracorporeal membrane oxygenation (ECMO) provides effective respiratory support and saves time for the treatment of severe COVID-19. The present study reports that a 31-year-old pregnant female infected by COVID-19, who suffered from fever, dyspnea, and rapid ARDS. The patient's pulmonary function gradually recovered by combining early mechanical ventilation and ECMO, and finally, this patient was successfully weaned from ECMO and the ventilator. No fibrosis lesions were found in the chest CT, and the patient recovered very well after leaving from the hospital for one month.
Keywords: ECMO, ARDS, COVID-19, Prgnance, Mechanical ventilation
Introduction
COVID-19 has presently become a global pandemic that seriously endangers human health. Among the patients with COVID-19 in Wuhan, 31% of them progressed to ARDS, and some cases were even complicated by developing from severe ARDS refractory into mechanical ventilation (MV), with a high mortality rate.1
Due to the better efficacy achieved by ECMO during the H1N1 pandemic in 20092, this was successfully applied for the treatment of H7N9 and Middle East respiratory syndrome.3 , 4 However, recent studies have shown that the mortality rate of COVID-19 patients treated with ECMO remains high.5 , 6 At present, there is no case report on the successful treatment of COVID-19 pregnant women complicated with ARDS by ECMO. The first critically ill COVID-19 pregnant woman in China was successfully treated by combining early MV and ECMO. By reporting the present case, we aim to provide experience for the treatment of critically ill COVID-19 pregnant women.
Case report
A-31-year old pregnant woman with 35 weeks of gestation developed sore throat and cough after returning to Zhongshan City from Hubei Province on January 27, 2020, and went to the hospital on February 1 due to fever with dyspnea. The chest CT revealed an infection in the left lower lung (Fig. 1 , day 1), the white blood cell count was 6.8 × 109/L, the lymphocyte count was 0.14, and the oropharyngeal swabs tested by the CDC for 2019-nCoV was positive. Then the patient was sent to the ICU for treatment, 39 breaths per minute and SPO2 78% were performed under high-flow nasal cannula oxygen therapy (HFNC), and tracheal intubation, MV and emergency cesarean section were performed.
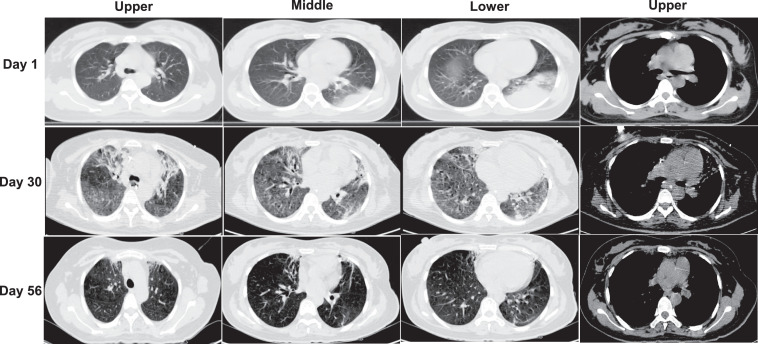
Fig. 1.
Chest computed tomography (CT) in COVID-19 patient.
Day 1. Chest CT images on day 1 of admission. Pulmonary consolidation was observed in the posterior basal segment of inferior lobe of left lung. The main pulmonary artery diameter was 29.8 mm.
Day 30. Chest CT images on day 30 of admission. Diffuse distributed ground-glass shadows in both lung fields were observed. The main pulmonary artery diameter was 35.5 mm.
Day 57. Chest CT images on day 57 of admission. The ground glass-like shadows in both lungs were completely absorbed, remaining mild bronchiectasis. The main pulmonary artery diameter was 27.8mm.
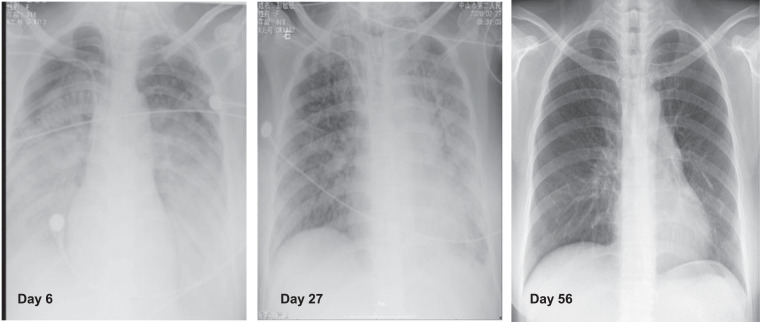
On the 6th day after admission, the chest X-ray revealed an increase in diffuse ground-glass opacity (Fig. 2 , day 6). Under the MV support of FiO2 100% and PEEP 16 cmH2O, the blood gas analysis revealed that pH was 7.15, PO2 was 62 mmHg, and PCO2 was 68 mmHg. Then, ECMO (Medtronic, USA, auxiliary flow 3.0 L/min) was performed. After one hour, the PO2/FiO2 returned to 85 mmHg and PCO2 decreased to 39 mmHg. Afterwards, “lung-protective ventilation” was performed under adequate sedation. After the patient's condition was stable, PEEP was gradually down regulated, and the fluid balance was negative. On the 27th day of admission, the chest X-ray revealed the reduced absorption of ground-glass opacities in both lungs (Fig. 2, day 27), and with the ECMO flow of 2.0 L/min, the blood gas analysis revealed that PO2/FiO2 was 370 mmHg and PCO2 was 36 mmHg, then the ECMO was successfully withdrawn.
Fig. 2.
Chest X-ray images of the COVID-19 patient.
Day 6. Chest X-rays on day 6 of admission. Diffuse high-density shadows with fuzzy patches and ground glass like changes in both lung fields were observed.
Day 27. Chest X-rays on day 27 of admission. Scattered high-density shadows with fuzzy patches and ground glass like changes in both lung fields were observed. Compared with the X-ray on day 6, the shadows diminished.
Day 56. Chest X-rays on day 56 of admission. The shadows with fuzzy patches and ground glass like changes in both lung fields disappeared.
On the 30th day of admission, the chest CT revealed diffuse ground-glass lesions with an inner diameter of the main pulmonary artery of 35.5 mm (Fig. 1, day 30). After pulmonary improvement, the ventilator parameters and dose of sedative drugs were gradually reduced. On the 38th day after admission, the patient was successfully weaned from the ventilator. On the 56th day after admission, the chest CT revealed no fibrosis lesions. Furthermore, the inner diameter of main pulmonary artery was 27.8 mm (Fig. 1, day 56). The patient was in good health at the one-month follow-up after discharge.
Discussion
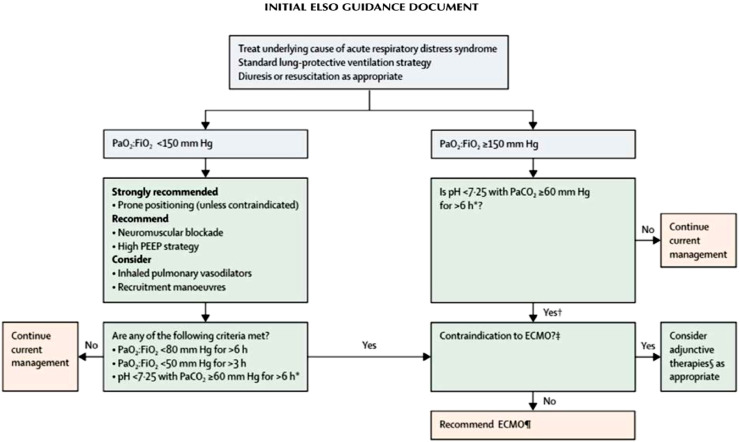
ECMO is a method of life support that maintains cardiopulmonary function. In ARDS management, ECMO enhances the gas exchange and mitigates ventilator-associated lung injury.2 Therefore, among COVID-19 patients complicated with ARDS who become unresponsive to conventional treatment, the use of ECMO, which temporarily replaces pulmonary function, can represent a life-saving alternative, providing more time to resolve the underlying cause. Fig. 3 presents the decision-making algorithm for ECMO consideration endorsed by the ELSO guidelines. 7Although the ECMO is presently widely used for the treatment of severe COVID-19, the role of ECMO for the rescue therapy of respiratory failure in critically ill COVID-19 patients remains unclear.
Fig. 3.
Algorithm for management of acute respiratory distress syndrome.
*With respiratory rate increased to 35 breaths per minute and mechanical ventilation settings adjusted to keep a plateau airway pressure of 32 cm of water. †Consider neuromuscular blockade. ‡There are no absolute contraindications that are agreed upon except end-stage respiratory failure when lung transplantation will not be considered; exclusion used in the EOLIA trial1 can be taken as a conservative approach to contraindications to ECMO. ∫ Eg neuromuscular blockade, high PEEP strategy, inhaled pulmonary vasodilators, recruitment maneuvers, high-frequency oscillatory ventilation. ¶Recommend early ECMO as per EOLIA trial criteria; salvage ECMO, which involves deferral of ECMO initiation until further decompensation (as in the crossovers to ECMO in the EOLIA control group), is not supported by the evidence but might be preferable to not initiating ECMO at all in such patients. PEEP, positive end-expiratory pressure; PaO2: HO2, ratio of partial pressure of oxygen in arterial blood to the fractional concentration of oxygen in inspired air; ECMO, extracorporeal membrane oxygenation; PaCO2, partial pressure of carbon dioxide in arterial blood.
The data obtained from Wuhan revealed that the mortality rate of severe COVID-19 treated with ECMO was 94.6%.5 Li et al. reported 16 ICU patients, which included eight ECMO patients in Shanghai. Among these eight patients, three patients were weaned from ECMO, four patients died, and one patient was still on ECMO.8 In summary, the use of ECMO is associated with the high mortality of patients with ARDS caused by COVID-19 in China. Studies have shown that the main cause of death of COVID-19 patients treated with ECMO in China was bleeding and infection.5
After analyzing the data of COVID-19 patients treated with ECMO who died, it could be observed that these patients had shortness of breath after admission, underwent MV after HFNC, and had non-invasive ventilator failure.1 , 9 The studies revealed that the high transpulmonary pressure caused by shortness of breath and continuous high-concentration oxygen therapy would aggravate the lung injury.10 When the MV failed, even when the patient received ECMO, it was found that the patient's pulmonary function slowly recovered and was prone to complications such as bleeding and infection5, which seriously affected the patient's prognosis. Therefore, the cause for the high mortality might be correlated to the delayed MV. The pregnant woman underwent MV and terminated pregnancy as early as possible after HFNC failure on the day of admission, avoiding lung injury caused by delayed mechanical ventilation.
On the 6th day after admission, the patient had a poor oxygenation index, with CO2 retention. Due to the postural change, the patient's blood pressure decreased. Hence, the prone position ventilation was not performed. Then, the ECMO was performed. After the ECMO, the hypoxemia was improved, and the CO2 retention was corrected and downregulated the plateau pressure, reducing the risk of ventilator-associated lung injury. A study revealed that spontaneous breathing during ECMO increased the transpulmonary pressure in early ARDS.11 In addition, the quick downregulation of PEEP increased the mortality12, coupled with more maternal blood volume, when compared to normal subjects. Therefore, the patient was given “lung-protective ventilation” under adequate sedation, the PEEP was slowly downregulated, the fluid balance was negative, and the patient's pulmonary function gradually improved.
In addition, the autopsy pathology revealed that there was damage to the alveoli and pulmonary vessels in critically ill COVID-19 patients13, and the patient's chest CT revealed that the main pulmonary artery was widened after withdrawing the ECMO, suggesting that critically ill COVID-19 patients are prone to pulmonary hypertension, which could increase the ECMO pipeline recirculation during ECMO. Hence, attention should be given in preventing Acute Cor Pulmonale. The patient's chest CT before leaving the hospital revealed no fibrotic lesions, and the diameter of the main pulmonary artery returned to normal, suggesting that the pulmonary lesions caused by the critical COVID-19 might be reversible.
Therefore, critically ill COVID-19 pregnant women need to terminate pregnancy as early as possible. Furthermore, when early MV is not effective, ECMO needs to be performed as soon as possible. Moreover, some COVID-19 pregnant patients complicated with ARDS can still benefit from the treatment of early MV and ECMO.
Contributors
YY conceived the study. YY designed and supervised the overall study. LH and WW collected specimens. LH, WW, ML, BL and KG collected clinical data with the assistance of YY. HL, ML and KG collected pathological images. YY, LH, WW, ML, BL, JL and KG analyzed and interpreted the data. YY and LH formulated the treatment regimen and analyzed the X-ray images. LH, ML, JL and KG made the tables and figures and wrote the manuscript. YY, WW and ML critically revised the manuscript.
Declaration of Competing Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “First successful treatment of a COVID-19 pregnant woman with severe ARDS by combination of early Mechanical Ventilation and ECMO”
Acknowledgements
We thank the patient; the nurses and clinical staff who provided care for the patient; staff at the administrative departments.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hrtlng.2020.08.015.
Appendix. Supplementary materials
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies A., Jones D., Ziegenfuss M. Extracorporeal membrane oxygenation for 2009; influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 3.Alshahrani M.S., Sindi A., Alshamsi F. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensiv Care. 2018;8(1):3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L., Zhang W., Yang Yi. Application of extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome induced by avian influenza A (H7N9) viral pneumonia: national data from the Chinese multicentre collaboration. BMC Infect Dis. 2018;18(1):23. doi: 10.1186/s12879-017-2903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry B.M., Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs, J.P., Stammers, A.H., St Louis, J., et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in COVID-19: experience with 32 patients. ASAIO J. 2020;66(7):722–730. Apr 17. [DOI] [PMC free article] [PubMed]
- 7.Bartlett R.H., Ogino M.T., Brodie D. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66(5):472–474. doi: 10.1097/MAT.0000000000001173. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., Guo Z., Li B. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J. 2020;66(5):475–481. doi: 10.1097/MAT.0000000000001172. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauri T., Yoshida T., Bellani G. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensiv Care Med. 2016;42(9):1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 11.Mauri T., Langer T., Zanella A. Extremely high transpulmonary pressure in a spontaneously breathing patient with early severe ARDS on ECMO. Intensiv Care Med. 2016;42(12):2101–2103. doi: 10.1007/s00134-016-4470-9. [DOI] [PubMed] [Google Scholar]
- 12.Chmidt M.P, Arcadipane T., Combes A. Mechanical ventilation management during extracorporealmembrane oxygenation for acute respiratory distress syndrome. An international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200(8):1002–1012. doi: 10.1164/rccm.201806-1094OC. 10 15. [DOI] [PubMed] [Google Scholar]
- 13.Lou W., Yu H., Gou J. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) Med Pharmacol. 2020 Preprints2020020407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.