Graphical abstract

Keywords: COVID-19, Pandemic, Severe acute respiratory syndrome coronavirus 2, Antibodies, Plasma therapy, Immunotherapy
Abstract
COVID-19, the disease induced by the recently emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has imposed an unpredictable burden on the world. Drug repurposing has been employed to rapidly find a cure; but despite great efforts, no drug or vaccine is presently available for treating or prevention of COVID-19. Apart from antivirals, immunotherapeutic strategies are suggested considering the role of the immune response as the host defense against the virus, and the fact that SARS-CoV-2 suppresses interferon induction as an immune evasion strategy. Active immunization through vaccines, interferon administration, passive immunotherapy by convalescent plasma or synthesized monoclonal and polyclonal antibodies, as well as immunomodulatory drugs, are different immunotherapeutic approaches that will be mentioned in this review. The focus would be on passive immunotherapeutic interventions.
Interferons might be helpful in some stages. Vaccine development has been followed with unprecedented speed. Some of these vaccines have been advanced to human clinical trials. Convalescent plasma therapy is already practiced in many countries to help save the lives of severely ill patients. Different antibodies that target various steps of SARS-CoV-2 pathogenesis or the associated immune responses are also proposed.
For treating the cytokine storm induced at a late stage of the disease in some patients, immune modulation through JAK inhibitors, corticosteroids, and some other cognate classes are evaluated.
Given the changing pattern of cytokine induction and immune responses throughout the COVID-19 disease course, different adapted approaches are needed to help patients. Gaining more knowledge about the detailed pathogenesis of SARS-CoV-2, its interplay with the immune system, and viral-mediated responses are crucial to identify efficient preventive and therapeutic approaches. A systemic approach seems essential in this regard.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a newly emerged betacoronavirus, is responsible for coronavirus disease 2019 (COVID-19), which was first reported in Wuhan, China in December 2019. SARS-CoV-2, the third fatal virus of its group, is an enveloped positive-sense, single-stranded RNA virus [1]. While the other viruses of this family induce only mild cold symptoms, Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), the two other virulent betacoronaviruses, have been presented with higher fatality rates than SARS-CoV-2. On the other hand, SARS-CoV-2 has shown a higher transmission rate and therefore a wider spread around the globe [2].
The burden of coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been very huge on health, economy, and many other aspects of life. It has already claimed more than 750,000 lives (as of 15 August 2020 according to WHO COVID-19 dashboard at https://covid19.who.int/). The urgency of this threat has prompted scientists in many countries to seek solutions through drug repurposing and repositioning of the previously approved drugs, while the fast-tracking of vaccine and drug development are seriously followed. However, some of the repurposed candidate drugs have already failed in some clinical trials [3].
Some antiviral drugs, developed for other similar viruses, are suggested, which may inhibit the cell entry or replication of the virus [2]. On the other hand, supporting the immune system’s potential to function properly and fight with the virus is another viable strategy. Normalization of the dysregulated immune responses or even their suppression at the final stages of the disease may also be required [4].
Although the role of the immune system in overcoming COVID-19 is indisputable, and many therapeutic and preventive approaches implicate modulation of the immune system activity, there are still many questions to be answered in this regard. For instance, the pattern of cytokine secretion during the disease course has been the subject of vast investigations. In this review, the pathogenesis of SARS-CoV-2 and its interplay with the immune system are briefly stated. A classification of possible immune-based approaches to combat COVID-19 is presented with a focus on convalescent plasma therapy, antibodies, and immunomodulators.
2. Pathogenesis of SARS-CoV-2 in brief
SARS-CoV-2 enters the lungs through the nasopharyngeal mucosal membrane and infect alveolar macrophages and type I and II epithelial cells in the lungs [5]. The most prominent way of viral entry was shown to be through the attachment of S protein and angiotensin-converting enzyme 2 (ACE2) receptors, which may be enhanced through some proteases such as a serine protease called TMPRSS2 (transmembrane protease, serine 2) or Cathepsin L/B (CTSL/B) [6]. Another serine exopeptidase receptor, called dipeptidyl peptidase 4 (DPP4) or cluster of differentiation 26 (CD26), was also shown to provide additional interactions with SARS-CoV-2 spike beside the ACE2 receptor [7]. It was revealed that the virus can also enter the cell through clathrin-dependent and -independent endocytosis pathways. For instance, SARS-CoV-2 may attack lymphocytes through the JAK-STAT pathway [8].
COVID-19 patients manifest mild to severe symptoms, including fever, non-productive cough, dyspnea, malaise, fatigue, lymphopenia, and pneumonia 2–14 days after the viral attack. Moreover, laboratory results including leucopenia, elevated C-reactive protein (CRP), and higher erythrocyte sedimentation rate (ESR) are detected. A wide range of other clinical manifestations has been observed in COVID-19 involving different organs, namely heart, eyes, nose, brain, pancreas, kidney, and bladder. As reported, 7–14 days upon the manifestation of the initial symptoms of the disease, the virus may cause a second attack and an aggravation of the disease symptoms in which severe pneumonia, ground-glass opacity, acute cardiac injury, and RNAaemia are observed [9]. Moreover, patients who succumbed to COVID-19 represented a higher level of neutrophils, D-dimer, blood urea nitrogen (BUN), and creatinine than the survivors [10], [11].
3. Immunotherapeutic approaches against SARS-CoV-2 based on its immunopathology
Viral antigens are presented to T cells and B cells via major histocompatibility complex (MHC) on antigen-presenting cells (APCs), thus innate and adaptive immunities are activated. Innate immunity response is initiated by interferon secretion from the infected cells in viral infections for signaling to other cells and making them ready for the battle [4]. SARS-CoV-2 is found to antagonize the induction of type I interferons (interferon-alpha and -beta) [12], [13] thereby evading the innate immune system defense [14]. Moreover, it was shown that the immune system decision between Th1 response (cellular response) or Th2 response (humoral response), which is affected by the cytokine pattern, determines the viral infection control. In fact, it was reported that some infections were well controlled by a Th1 response, and this response was observed in 20 recovered COVID-19 patients [15]. SARS-CoV-2 was found to suppress antigen presentation through the downregulation of MHC class I and II molecules, which may lead to the impediment of T cell-mediated immune defense [16].
In the second attack phase of the disease (usually one or two weeks after the presentation of the first symptoms), the level of lymphocytes drops dramatically, and the cytokine storm occurs. Cytokine storm is an uncontrolled release of inflammatory cytokines, including IFN-α, IFN-γ, GM-CSF, G-CSF, IL-1ß, IL-6, IL-12, IL-18, IL-33, TNF-α, TGF-ß, and chemokines, particularly CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10. The cytokine level reduces as the patient recovers. Cytokine storm has been recognized as the main cause of acute respiratory distress syndrome (ARDS), which causes lung injury and multiple organ failure. Taken together, the immune system is highly impaired in critically ill COVID-19 patients.
Given the role of the immune system in host defense, immunomodulation could be regarded as an important strategy to curtail COVID-19 considering the patient’s immune system condition at various phases of the disease. Such immunomodulatory interventions can be achieved using vaccines, interferons, convalescent plasma, anti-inflammatory agents, antibodies, and other classes of immunomodulators, which are described in the following.
4. Interferons
The suppression of interferon I-mediated immune responses by SARS-CoV-2 is already confirmed [12], [13]. Although interferon was shown to fight against the virus and is suggested to treat the disease [17], some contradictory data demonstrated that interferon may enhance ACE2 expression and thus viral entry [18]. On the other hand, positive results were found by using interferons type I, including interferon-beta-1a in several clinical trials [19]. The difference in the route of administration, either subcutaneous (s.c.) and intravenous (i.v.), was proposed as a reason for the diverse effects reported about interferon beta-1a in some studies [20]. The outcomes of the investigations on interferon therapy in COVID-19 were presented in some other publications [17], [19], [20] and as a systematic review [21]. Interferon-beta is already being examined in a combination protocol in the international clinical trial launched by WHO, called the “Solidarity” trial, in the partner countries [3].
5. Active immunization using vaccines
Vaccines are believed as the ultimate protection for saving the public from the novel virus. The lack of previous exposure of the human immune system to SARS-CoV-2 [22] is regarded as the major contributor to its high risk. Hence, active immunization through vaccines could prepare the body to resist against this infection. Very soon after finding the virus genetic sequence, vaccine development was started and followed with unprecedented speed by several research groups and pharmaceutical companies. Huge investments are dedicated by several public and private bodies to advance this project [2].
As of 13 August, 29 candidate vaccines have entered the clinical phase, eight of which are already in phase 2 or 3. In addition, 138 candidate vaccines are in preclinical development. The six pioneer vaccines have already entered in clinical trial phase 3, including ChAdOx1-S, developed by the University of Oxford in collaboration with AstraZeneca Pharmaceutical Company, an inactivated vaccine from Sinovac, two RNA-based vaccines, one by Moderna and the other through a collaboration of BioNTech, Fosun Pharma, and Pfizer; two other inactivated vaccines and one with an adenovirus vector are also under clinical trial in China (accessed on https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
Various platforms are employed in these investigational vaccines, including inactivated, killed or weakened pathogen, non-replicating viral vector, RNA, DNA, VLP (virus-like particle), and protein subunit structures. There also some replicating viral vector-based vaccines under development. Each of these platforms has its own advantage and limitations. For instance, while DNA and RNA vaccines are intensively studied mainly because of their rapid development, easy production, and safety, their large-scale production might need more time to set up, due to novelty and lack of previous experience in their commercial production. Additionally, more than one dose of these vaccine types is required for the immunization because of their short half-life [23]. This topic is reviewed in details elsewhere [23], [24], [25].
All in all, vaccine development is a time-consuming process. Moreover, the induction of memory in the immune system is still under question. Therefore, despite preliminary promising results, the efficiency of the investigational vaccines should be confirmed in large clinical trials.
6. Passive immunotherapy
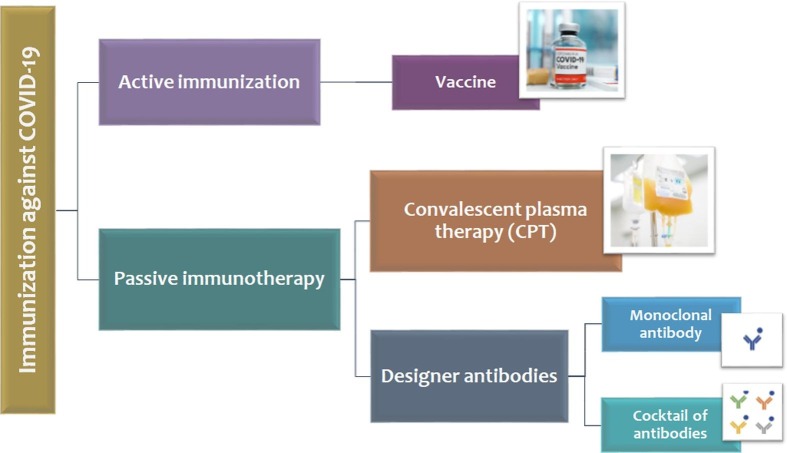
Considering the lack of an approved drug or vaccine against SARS-CoV-2, taking advantage of a helpful alternative intervention is an urgent need [26], [27], [28]. Passive immunotherapy via neutralizing antibodies has been considered as a possible strategy for defeating COVID-19 apart from anti-viral therapeutics and vaccines (Fig. 1 ). Antibodies can be either isolated from a convalescent patient or produced in a lab [29]. These two approaches would be discussed in more detail in the following.
Fig. 1.
Immunization approaches against COVID-19. Active immunization is provided through vaccines, which are still under development for COVID-19. Passive immunization can be performed via natural antibodies using convalescent plasma therapy (CPT) or antibodies that are manufactured. In CPT, neutralizing antibodies derived from a hyperimmune patient would be administered to a COVID-19 patient through plasma transfusion. This approach is already being used and investigated in many countries with acceptable levels of success. On the other hands, different polyclonal or monoclonal antibodies could be produced via using hybridoma cell-lines, animals, or cell-free protein synthesis, which may be administered in patients as a monoclonal antibody or a cocktail of antibodies.
6.1. Convalescent plasma therapy (CPT)
Neutralizing antibodies derived from a hyperimmune patient through plasma transfusion is the most prevalent and accessible empirical approach used to treat several viral infections previously. This method, so-called convalescent plasma therapy (CPT), is also considered as a viable therapy for COVID-19 [30], [31], [32].
It has been used to reduce the hospital stay and the mortality rate of critically ill patients with severe acute respiratory syndromes [32], [33] and was found beneficial in some previous epidemics of infectious diseases [34]. The use of this approach was suggested for the first time during the outbreak of Spanish influenza (pandemic of 1918–1920) [33]. Subsequently, plasma transfusion was recommended as a safe and effective way for the prevention or treatment of the Ebola virus in 2014 and also several other severe viral infections, including MERS, SARS-CoV, and avian influenza A [35], [36]. In fact, neutralizing antibodies in the convalescent plasma (CP) could suppress the viremia through binding to the external antigens of the viruses and blocking their entry into the host cells [34], [37]. The effectiveness of CPT could vary according to the type of microorganism, it’s pathogenesis, and treatment protocols, such as timing, dosing, and volume of administration [33].
According to the previous evidence for plasma therapy of other coronaviruses, such as SARS-CoV and MERS, the early transfusion of CP can probably be more effective and improves the survival rate of critical COVD-19 patients at the early disease stage. It could be explained by the fact that in most viral infections, viremia rises in the first week of the disease [37], [38]. It should be noted that CPT may not be useful for mild or end-stage patients. Indeed, CPT is not able to significantly reduce the mortality rate among end-stage patients because of their disease severity. On the other hand, mild patients can be self-recovered, and CPT would not be required [39].
The titer of SARS-CoV-2 neutralizing antibodies in the CP could be another important factor to increase the treatment efficacy. Although, the antibodies level in the donor plasma before transfusion is not determined, some studies indicated that the specific IgG increases about three weeks after symptom onset and peaks at week 12. Therefore, the CP from donors who are at week 12 after the initiation of the symptoms is predicted to be more efficient [27], [40].
Generally, patients with primary and secondary antibody deficiencies, need intravenous immunoglobulin (IVIG) treatment as the standard replacement therapy [41]; and historically, administration of IVIG has been one of the important treatments in immunodeficient patients [42]. These patients are considered as a high-risk group, which can encounter several severe complications if infected with SARS-CoV-2 virus [43]. Therefore, an effective treatment is required to help such patients survive. CP extracted from the SARS-COV-2 survivors may be a promising approach for the protection of COVID-19 patients with antibody deficiency before the development of an effective vaccine [44].
However, the data about the potency of CPT in COVID-19 patients with primary and secondary humoral immunodeficiency is limited and has not been fully established, there are some case studies that have reported its proper effectiveness. For example, Clark et al. reported a 76-year-old COVID-19 case with lymphoma who was treated with a combination of bendamustine and rituximab that could lead to the impairment of humoral and cellular responses. After the failure of different treatment protocols against SARS-COV-2, the administration of hyperimmune plasma resulted in a rapid recovery in this patient [45]. In another report, an immunosuppressed COVID-19 patient with myeloid malignancy, disseminated tuberculosis, and kidney disease, was successfully treated after transfusion of CP and tocilizumab [46]. Furthermore, Mira et al. reported a primary antibody immunodeficient (x-linked agammagloblulinemia) male patient with COVID-19, whose health condition was improved following CP administration [47].
Presently, several clinical trials investigating the usage of CPT in COVID-19 are ongoing (as recorded in https://clinicaltrials.gov/ ), a number of which are summarized in Table 1 . The trials are selected according to the recruitment status of the study (recruitment or completed state), age (18 years and older), and severity of symptoms in participants (admitted to the hospital or ICU with severe symptoms). Several other publications have discussed the usage of CPT in COVID-19 [32], [33], [35], thus more details would not be addressed here.
Table 1.
Summary of some clinical trials on CPT)recorded in https://clinicaltrials.gov/ at 6 June 2020).
| No | Status | Estimated participants | Volume of administration | Outcome measurement | Time of transfusion | Study phase | Identifier |
|---|---|---|---|---|---|---|---|
| 1 | Recruiting | 100 | 1 unit single dose | Changes in respiratory status after CP* transfusion/ Length of ICU and hospital stay/ Development of plasma transfusion reactions and immune complex disorders at days 1, 3, 7, and 28/ Change in anti CoV-2 IgM and IgG levels at days 1, 3, 7, and 28 | Within 21 days of symptoms onset | Early phase I | NCT04412486 |
| 2 | Recruiting | 60 | 1–2 units on days 0, 3, 6 (Based on the availability of plasma) | Feasibility of administering CP to patients in the ICU who intubated and mechanically ventilated/ Overall survival of patients in the ICU receiving at least once dose of CP | – | Early phase I | NCT04353206 |
| 3 | Recruiting | 100 | 500 ml IV single dose | Mortality/ Requirement for and duration of mechanical ventilation/ Adverse events | – | Early phase I | NCT04355897 |
| 4 | Recruiting | 90 | 1–2 units on days 0, 2, 4, 6, and 8 (based on plasma availability) | Ventilation free days/ Mortality/ Duration of hospital and ICU stay | – | I | NCT04411602 |
| 5 | Recruiting | 20 | 250 ml on days 0 and 1 | Adverse events/ Heart Failure, Pulmonary Edema, Allergic Reaction during CP transfusion or after it/ Viral load of SARS-CoV-2 | – | I | NCT04333355 |
| 6 | Recruiting | 80 | 2 units single dose | Adverse events/Severity of symptoms/ Clinical status assessment/ Time to discharge/ Oxygen-free days/ Days of non-invasive ventilation/ Duration of hospitalization/ Mortality/ Changes in WBC with differential/ Changes in hemoglobin, platelets, creatinine, glucose, bilirubin, ALT, AST, PT measurement | – | I | NCT04397757 |
| 7 | Recruiting | 10 | 100 ml on day 0, 3, and 6 | Change of INR, CRP, Oxygenation Index (OI) and Chest X-ray compared to pre and post transfusion/ Severe adverse events | – | I | NCT04407208 |
| 8 | Recruiting | 50 | 200 ml CP daily until SARS-CoV-2 is no longer detectable in the blood up to a maximum of 7 infusions | Duration of ventilation or oxygen therapy/ Adverse events related to CP/Dose of plasma needed to clear viremia/ SARS-CoV-2 RNA detection by PCR in blood or serum/Duration of symptoms/ Inflammatory parameters/ Antibody response to SARS-CoV-2 | – | I-II | NCT04384497 |
| 9 | Recruiting | 131 | 1–2 units (200–400) ml single IV infusion | Overall mortality and length of admission | Within 21 days of symptoms onset | II | NCT04354831 |
| 10 | Recruiting | 10 | 200 ml single dose | Overall survival/ Adverse events/ Lung injury | – | II | NCT04357106 |
| 11 | Completed | 29 | 200 to 600 ml according to the patient requirement | Proportion of patients remaining free of mechanical ventilation/ Mortality/Duration of hospital and ICU stay/ Improvement of respiratory status/ Requirements of Vasopressor | II | NCT04346446 | |
| 12 | Recruiting | 120 | 2 units of 200–220 ml. In the absence of acute adverse events in the first 3 patients, an additional 2 units will be transfused 1 day after first 2 units: a total of 4 units / patient. | Severe adverse events/ Overall survival/ Time to discharge/ Oxygen-free days | Within 10 days of symptoms onset | II | NCT04345991 |
| 13 | Recruiting | 100 | 1–2 units (200–600 ml) | Number and type of adverse events/ Duration of hospital and ICU stay and intubation/Survival rate/ Changes in complete blood count, CRP*, fibrinogen, PT*, PTT* and BMP* in patients after receiving CP at day 0 and 7 (or the day of hospital discharge) | – | II | NCT04389710 |
| 14 | Recruiting | 30 | 200 ml single dose over 3 h | Improvement in respiratory disease/ Radiographic improvement/ Tolerability of CP/ Length of stay in hospital or ICU/ Duration of ventilation | – | II | NCT04385199 |
| 15 | Recruiting | 30 | 200 ml day 1 and 2 only if worsening of respiratory function or persistence of COVID symptoms for greater than 7 days after enrolment | Percentage and duration of mechanical Ventilation/ Hospitalization longer than 14 days or death during hospitalization/ Duration of fever/ length of ICU stay and admission/ Re-admission rate/ Length of viral clearance | Within 7 days of symptoms onset | II | NCT04375098 |
| 16 | Recruiting | 40 | 10–15 ml/kg at least once and if possible, daily for up to five sessions | Duration of mechanical Ventilation/ Mortality/ Adverse events/ Length of stay in ICU/ Days to clinical recovery | II | NCT04347681 | |
| 17 | Recruiting | 126 | 200 ml single dose | Need of invasive mechanical ventilation/ Mortality rates/ Time to virologic cure/ Adverse events/ Length of stay in hospital | – | II | NCT04393727 |
| 18 | Recruiting | 200 | – | Mortality rate: Significant reduction (P < 0.05) in mortality | – | II-III | NCT04385043 |
| Lymphocytes: Significant increase (P < 0.05) in lymphocyte levels after 7 and 14 days after treatment | |||||||
| Antibody levels and clinical improvement Significant correlation (P < 0.05) between hyperimmune plasma antibody levels and clinical improvement time | |||||||
| Inflammatory cytokines Significant reduction (P < 0.05) of plasma levels of IL-6 and TNF-alpha 7 and 14 days after treatment initiation | |||||||
| 19 | Recruiting | 426 | 300 ml single IV infusion | Overall mortality/ Duration of symptoms/ Length of stay in ICU and hospital/ Duration of oxygen therapy/ Duration of SARS-CoV-2 shedding from airways (airway samples will be taken on day 1–3-5–7-10–14, and at discharge)/Safety of plasma therapy | – | II-III | NCT04342182 |
| 20 | Recruiting | 500 | Plasma will be administered at a rate of 500 ml/h | Mortality/ Hospitalization status/ Oxygen and ventilator free days/ Vasopressor-free days/ Hospital and ICU free days/ Adverse events | – | III | NCT04362176 |
*CP = convalescent plasma.
*CRP = C-reactive protein.
*PT = prothrombin time.
*PTT = partial thromboplastin time.
*BMP = Basic Metabolic Panel. Tests include measures of glucose, calcium, sodium, potassium, bicarbonate, chloride, blood urea nitrogen, and creatinine.
All in all, CPT has demonstrated acceptable safety, and it currently seems a promising choice for treating severe COVID-19 patients besides other therapeutic strategies [48].
6.1.1. Advantage and disadvantages of CPT
The previous experiences and efficacy in other similar diseases, as well as its feasibility, are the important advantages of CPT. Moreover, according to different reports, CPT is well-tolerated by receivers [35], but as all treatment approaches, this method may have some minor adverse effects as well. Generally, the most common adverse effects of CPT is related to transfusion events, such as chills, fever, rash, allergic reactions, circulatory overload, and hemolysis [40], [49].
Additionally, several other problems are attributed to the mentioned approach, including lack of plasma donors, risk of cross-contamination, inconsistency from batch to batch, non-scalability, and the possibility of host reaction [50]. These pitfalls behoove pharmaceutical companies to manufacture polyclonal or monoclonal antibodies.
6.2. Antibodies
6.2.1. Targets of monoclonal antibodies against SARS-CoV-2
Different steps involved in the pathogenesis of SARS-CoV-2 can be targeted via antibodies. Our data in regards to the designing of an antibody against SARS-CoV-2 highly rely on the previous studies concerning SARS-CoV, the virus that is very similar to SARS-CoV-2. It has been shown that SARS-CoV-2 shares 89.1% and 50% nucleotide sequence identity with SARS-CoV and MERS-CoV, respectively [51], [52]. Hence, in this review, only SARS-CoV-related data about the pathogenesis and the design of monoclonal antibody are included.
Monoclonal antibodies designed against SARS-CoV-2 can be categorized in three main groups based on their target: 1) antibodies that inhibit the virus attachment and entry by either targeting the virus structure or host receptors, 2) antibodies that interfere with the virus replication and transcription, 3) antibodies that hinder various steps of the immune system response. The schematic targets of monoclonal antibodies against SARS-CoV-2 are depicted in Fig. 2 .
Fig. 2.
Potential targets to curtail COVID-19. 26 potential targets for COVID-19 are depicted. 1) receptor binding domain (RBD) located in S1 protein, it is considered as the first and the main target for neutralizing antibodies, 2) S2 protein, consists of HR1 and HR2 domains, SARS-CoV neutralizing antibodies, including CR3022 and S309 potently cross-neutralize SARS-CoV-2 through binding with S2 protein, 3) viroporin, ion channel proteins, hypothesized to be targeted by dewetting monoclonal antibodies, 4) nucleoprotein, target of neutralizing antibodies, 5) envelope, target of neutralizing antibodies, 6) ACE2, angiotensin converting enzyme receptor 2, a receptor found on the cells of respiratory system, gastrointestinal tract, and endothelium, strongly binds with the virus spike, some monoclonal antibodies are designed to compete with the virus in attachment to the ACE2 receptor, 7) TMPRSS2, it is responsible for spike protein cleavage and viral entry, targeted by TMPRSS2 inhibitors including nafamostat and camostat mesylate 8) vimentin, cytoskeleton protein that is important in the formation of SARS-CoV-2-ACE2 complex, 9) viral RNA, 10) cysteine-like protease (3clPro), one of the important viral proteases and targets of antiviral drugs, 11) Papain-like proteases (PLpro), another important viral protease and target of antiviral drugs 12) Bruton tyrosine kinase (BTK), BTK inhibitors, including acalabrutinib, modulate cytokine storm in COVID-19 patients, 13) AP2-associated protein kinase 1 (AAK1), regulates viral endocytosis and is the target of immunomodulators, 14) signal transducer and activator of transcription proteins/Janusassociated kinase (STAT/JAK), modulates viral entry and cytokine storm, JAK inhibitors, including baricitinib are repurposed for SARS-CoV-2 inhibition, 15) dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN), mediates viral entry and is the target of human monoclonal antibody, 16) IL-6, one of the most important cytokines that activates downstream inflammatory process and causes acute respiratory distress syndrome, inhibited by different monoclonal antibodies, including Tocilizumab, 17) granulocyte–macrophage colony-stimulating factor (GM-CSF), causes positive feedback in inflammatory mediators and acute respiratory distress syndrome, target of monoclonal antibodies, 18) TNF, inflammatory mediator and cause of acute respiratory distress syndrome, target of monoclonal antibodies and TNF blockers, including etanercept, 19) IL-17, responsible for aggravation of cytokine storm and pulmonary edema and target of secukinumab, 20) IL-1, responsible for aggravation of cytokine storm and pulmonary edema, target of canakinumab, 21) nicotinamide phosphoribosyltransferase (NAMPT), upregulated by physical stress and causes an increase in the number of TLR4 and lung inflammation, target of monoclonal antibodies, 22) calcineurin, calcineurin inhibitors, including tacrolimus, block T-cell activation, 23) mTOR, mTOR inhibitors including sirolimus, inhibit memory B-cell activation and the antibody-dependent enhancement mechanism, 24) CTLA-4, immune check point and negative regulator of T-cell, target of monoclonal antibodies, 25) PD-1, immune check point and negative regulator of T-cell, target of monoclonal antibodies, 26) intercellular adhesion molecule 3 (ICAM-3), mediates the viral entry.
The first and most common targets are spike (S) proteins, which are located on the virus surface, generating its specific ‘crown’ shape [53]. SARS-CoV-2 starts its pathogenesis through the attachment of receptor-binding domain (RBD), located in the S1 subunit of the S protein, with ACE2. Thus, S proteins are considered as the most antigenic part of the virus with the main responsibility for the host immune responses [54]. It has been widely suggested that the previously-experimented SARS-CoV RBD neutralizing antibodies can be repurposed for SARS-CoV-2 [1], [55], [56]. The cross-neutralization capacity of antibodies relies on the conservation of the particular residues that are essential for the formation of specific bonds between RBD and the antibody, between the two types of viruses. For instance, an analysis was performed to determine which of the previously-proposed monoclonal antibodies against SARS-CoV can also cross-neutralize SARS-CoV-2. In the mentioned study, the residues for the formation of a salt bridge and electrostatic interaction between the m396 antibody and RBD were conserved between SARS-CoV and SARS-CoV-2. By contrast, antibodies, including R80 and F26G19 failed to interact with SARS-CoV-2 RBD in a similar way they did with SARS-CoV RBD due to the differences in their residues [57]. Surprisingly, it was shown that F26G19 neutralized SARS-CoV-2 more potently than SARS-CoV through other interactions [51]. Studies including cryoelectron microscopy of SARS-CoV-2 spike and analysis of the protein–protein interactions of SARS-CoV-2 and ACE2 receptor with energy-based methods revealed the amino acids 319–591 of SARS-CoV-2 RBD as important residues; the latter study also introduced the linear and conformational epitopes within this region as antibody targets [54], [58].
Though the RBD has been considered as the main target of interest, some neutralizing or blocking antibodies have been shown to recognize other epitopes, including domains in S1 subunit, S-ectodomain, HR1 and HR2 domains in the S2 subunit, nucleoprotein (NP), or envelope (E) protein [59], [60], [61]. For example, CR3022 cross-neutralized SARS-CoV-2 more strongly than other neutralizing antibodies against SARS-CoV, while it did not compete with ACE2 for binding to SARS-CoV-2. This observation indicates that CR3022 neutralizes SARS-CoV-2 through binding epitopes other than RBD [57]. Another study showed that the HR2 domain with an identity of 93% is highly conserved between SARS-CoV and SARS-CoV- 2, and thus neutralizing antibodies that target the HR2 domain, including 2B2, 1A9, 4B12, and 1G10 potently cross-neutralized SARS-CoV-2 [62]. Similarly, the S309 monoclonal antibody, retrieved from convalescent SARS-CoV patients, potently neutralized SARS-CoV-2 through a highly conserved domain distinct from RBD and did not interfere with the binding of S protein with the ACE2 receptor [63]. Moreover, in contrast to the above-mentioned importance of RBD in designing monoclonal antibodies, some studies have revealed the antibody-dependent enhancement of viral entry when the monoclonal antibody targets the RBD. It was shown that the binding of monoclonal antibody to the RBD triggers conformational changes that are similar to the alterations made following the binding of viral receptors to the viral RBD; hence, the binding mediates the virus entry to the cell via viral receptor-dependent pathways. However, as stated in the mentioned study, this mechanism depends on the monoclonal antibody dosage, expression of particular viral receptors (e.g. Fc receptors), and particular features of the monoclonal antibody [64]. Although ACE2 has been introduced as the main receptor of SARS-CoV, it may not be sufficient for the interaction between the cell and the virus. Other cellular factors, such as vimentin, a cytoskeleton protein, were revealed to be important in the formation of the ACE2-SARS-CoV complex [65]. Therefore, surface vimentin was recognized as a potential target for SARS-CoV. DS-SIGN/CD209 is also a transmembrane adhesion molecule, which is mainly expressed on interstitial dendritic cells and lung alveolar macrophages. It was shown that DS-SIGN also mediates the entry of SARS-CoV. A humanized monoclonal antibody was produced to interfere with the interaction of DS-SIGN and intercellular adhesion molecule 3 (ICAM-3), and thus inhibit SARS-CoV entry to the cell [66]. Similar proteins may be identified in regards to SARS-CoV-2.
In addition to inhibiting the virus entry, antibodies can intrude into the biological activities of the virus thereby preventing its replication. Fully human antibodies, which are capable of traversing across the cell membrane of infected cells and preventing virus replication, were made against several kinds of viruses, including influenza, hepatitis C virus, and Ebola [67]. Papain-like proteases (PLpro), cysteine-like protease (3CLpro), and other non-structural proteins (nsps) can be suggested as targets of interests that hinder SARS-CoV-2 replication [1], [68].
Besides structural parts of the virus, various steps associated with the innate and the adaptive immune responses have been proposed as the most important targets of interest. For instance, the significant rise in the level of chemokines and cytokines, including IL-1β, IFN-γ, IP-10, and MCP-1, which is called the cytokine storm, can be inhibited by antibodies [69]. The preliminary studies of critically ill COVID-19 patients have shown that IL-6 may cause severe inflammatory responses that lead to acute respiratory distress syndrome [70]. Herein, tocilizumab, an IL-6 inhibitor monoclonal antibody, has gained significant attention. In a 21-patient clinical study recruited in China, tocilizumab resulted in the reduction of oxygen need in 75% of patients, lung lesion opacity absorption in 90.5% of patients, and correction of lymphocyte and C-reactive protein levels [70]. The significance of tocilizumab can be better explained since a noticeable number of 46 clinical trials regarding its use against SARS-CoV-2 related pneumonia and respiratory tract infections were recorded in NIH until May 27, 2020. An increase in the level of some of these pro-inflammatory cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF) results in a positive feedback in the number of other inflammatory mediators, including IL6, IL-23, and TNF. GM-CSF along with IL6 and IL23 also induce Th1/Th17 differentiation and the polarization of macrophages to M1 phenotypes, which in turn boost the inflammatory responses [71]. Th17 immune response also can aggravate the cytokine storm by raising the level of IL17, GM-CSF, IL21, and IL22 [72]. High levels of GM-CSF and Th17 cells were observed in the plasma of severe- to critically ill COVID-19 patients [73], [74]. Herein, harnessing the upregulation of GM-CSF can prevent a cascade of inflammatory responses, which result in acute respiratory syndrome. Since monoclonal antibodies targeting one inflammatory mediator, may fail to control the whole cytokine storm and prevent the lung injury induced by acute respiratory distress syndrome, a newer approach to prevent lung injury was proposed. It was shown that physical stress such as excessive mechanical stress caused by ventilators upregulates the expression of a gene, called nicotinamide phosphoribosyltransferase (NAMPT). As the bioavailability of NAMPT increases, Toll-like receptor 4 (TLR4), which is responsible for lung inflammation, gets activated [75], [76]. Herein, neutralization of circulating NAMPT by monoclonal antibodies can be another viable approach in preventing the lung injury caused by SARS-CoV-2.
In addition to the role of inflammatory mediators, understanding the adaptive immune responses helps us to repurpose or invent immunomodulatory antibodies to defeat SARS-CoV-2. For example, CD4+ and CD8+ T-cells are expected to promote the proliferation of neutralizing antibodies and the destruction of infected cells, respectively. However, lymphocytopenia is identified to play a role in the pathogenesis of severely-ill COVID-19 patients [77], which can be prevented or restored by regulating lymphocyte proliferation and apoptosis [78]. Herein, some studies have shown that sepsis may occur secondary to inflammatory responses. The immune imbalance, which occurs in sepsis, maybe as a result of T-cell depletion. PD-1 and CTLA-4 receptors are immune checkpoints that are expressed on the surface of T-cells and play a role as the negative regulator of T-cell function. Therefore, inhibiting the immune checkpoints by monoclonal antibodies is also an intriguing approach in defeating SARS-CoV-2 [79]. Furthermore, CD4+ cells express a receptor called c-chemokine receptor 5 (CCR5), which was established as a way of HIV entry to the cell [80]. This receptor could also be a potential target for SARS-CoV-2.
The level of different immunoglobulins is raised in response to SARS-CoV-2. Even though an increase in the level of immunoglobulins is attributed to pose neutralizing effect on SARS-CoV-2, a rise in anti-S IgG is associated with lung failure [81], [82]. Complement systems also play a role in conferring both humoral and natural immunity; however, they have been also attributed to the refractory inflammatory diseases. Herein, C5a and C5a receptor, the members of the complement system, have been successfully targeted in different clinical trials of inflammatory diseases and thus can be recognized as possible targets in new diseases such as COVID-19 [83]. Additionally, TLR3, CD16, immunoreceptor tyrosin-based activation motif (ITAM), G-CSF, monocyte chemoattractant protein 1 (MCP1), TNFα, IL4, and IL10 are the other members of the immune system for which anti-SARS-CoV monoclonal antibodies were patented. These monoclonal antibodies could be reevaluated for SARS-CoV-2 [84].
Besides the main strategies described above for designing monoclonal antibodies against SARS-CoV-2, some more novel approaches with a completely different mechanism were suggested. One of these strategies is using dewetting monoclonal antibodies. Dewetting transition is a process in which hydrophobic pores of the ion channels inhibit water transmission and thus impair the cellular performance. This phenomenon can be deployed to block viruses, bacteria, and autoimmune activities. Dewetting monoclonal antibodies are antibodies with a lipophilic fragment that target the transmembrane receptors and hinder the physiological water flow inside the channel. Such antibodies were produced against the influenza virus [85], [86]. Recently, viroporins were identified in SARS-CoV-2, the ion channel proteins that are generated by the virus E protein and are responsible for different parts of the virus life cycle, including virus entry, assembly, release, and the whole pathogenesis cycle [87]. As suggested, dewetting monoclonal antibodies could be developed against SARS-CoV-2 viroporins and administered through the nose. These antibodies can deactivate the virus by targeting viroporins even before the virus will be able to bind to the host cells [88].
6.2.2. Design and production of monoclonal antibodies against SARS-CoV-2
Generally speaking, the antibodies could be usually produced at large-scale, either as monoclonal or polyclonal antibodies, using hybridoma cell-lines, animals, or cell-free protein synthesis [89]. Monoclonal antibodies can be produced via several technologies, including the production of high-affinity human antibodies in immunized transgenic mice (e.g. XenoMouse® or HuMAB® mice), various phage-display systems such as generating antibodies from immunoglobulin cDNA libraries in bacteria or mammalian cells, and obtaining memory B cells of convalescent patients that are immortalized by EBV transformation. All of these techniques were previously recruited to produce monoclonal antibodies against SARS-CoV [90]. The production of monoclonal antibodies against SARS-CoV-2 is in its incipient phase. Currently, our data about SARS-CoV-2 antibodies greatly comes from the studies in which the antibodies derived from the plasma of convalescent patients were analyzed. A recent study of the convalescent patients’ antibodies demonstrated that anti-SARS-CoV-2 antibodies were versatile among the convalescent patients and each patient represented a unique pattern of antibody biodistribution. These results explain why it may be difficult to design specific anti-SARS-CoV-2 antibodies [91]. Therefore, the introduced antibodies in this study were mainly tested against SARS-CoV or approved for other immune inflammatory diseases such as rheumatoid arthritis, cancers, and other viral infections. An extra method for designing antibodies against SARS-CoV-2 can be based on the antibody-antigen computational simulation [51]. For instance, an online docking server using the CoDockPP engine is constructed to predict the docking modes between antibodies or other peptides. This server is freely available at http://ncov.schanglab.org.cn. Identified structural parts of SARS-CoV-2 and the homologous parts of other coronaviruses were gathered to produce the mentioned docking server [92].
6.2.3. Examples of monoclonal antibodies
The antibodies retrieved from the previous studies on SARS-CoV or computational studies concerning SARS-CoV-2, which mainly target the virus structure or host receptors are shown in Table 2 . Despite a great homology between SARS-CoV and SARS-CoV-2, the cross-reactivity of SARS-CoV antibodies against SARS-CoV-2 is still under debate. For instance, some highly-conserved regions are found in SARS-CoV-2, which are absent in SARS-CoV. The c-terminal of SARS-CoV-2 RBD highly differs from that of SARS-CoV. Moreover, an extra furin cleavage site was found between S1 and S2 subunits in SARS-CoV-2, which is absent in SARS-CoV. These differences may not affect the ability of both viruses in interacting with the ACE2 receptor but explain the different levels of affinity among neutralizing antibodies with the two viruses [51], [57], [93], [94]. Moreover, a recent study revealed that the antibodies targeting RBD of the coronavirus family are virus species-specific, while those that target viral parts outside RBD are capable of cross recognition [91]. In an antibody epitope computational analysis, it was revealed that 85.3% of antibody epitopes in the SARS-CoV-2 spike were novel in comparison with those in SARS-CoV [95]. Therefore, antibodies suggested in Table 2 ought to be reevaluated to be used against SARS-CoV-2. Taken together, among the introduced monoclonal antibodies in these study, F26G19, CR3022, and 47D11 were shown to cross-neutralize SARS-CoV and SARS-CoV-2.
Table 2.
Monoclonal antibodies identified based on the previously studied SARS-CoV antibodies or computational studies.
| Antibody | Mechanism of action | Identification method | Ref |
|---|---|---|---|
| 80R | Competes with ACE2 for association with S1 domain | Screening phage display library | [96] |
| S3.1 | Prevents the cytopathic effect of virus and viral entry by recognizing spike | Analysis of immune SARS-CoV patients’ serum and in vivo study in mice | [60] |
| A group containing 20 neutralizing antibodies, including S101.1, S102.1, S103.3, S104.1, S105.2, S106.1, S107.4, S108.1, S109.2, S132.9, S128.5, S127.6, S124.4, S159.1, S160.1, S215.13, S216.9, S217.2, S218.6, S219.2 | Neutralize spike by binding to residues 318–510 | ||
| A group containing five neutralizing antibodies, including S18.1, S20.1, S21.1, S23.4, S24.1 | Neutralize nucleoprotein | ||
| S5.1 | Neutralizes envelope protein | ||
| S13.1 | Not defined | ||
| CR3014 | Blocks S1 domain | Screening phage display library | [97] |
| CR3022 | Blocks S1 domain, neutralizes mutated SARS-CoV escape from CR3014, induces synergistic effect and dose reduction in combination with CR3014 | Screening phage display library | [98] |
| Higher affinity to SARS-CoV-2 S protein than SARS-CoV | Antibody-antigen docking simulation | [51] | |
| Neutralizes SARS-CoV and SARS-CoV-2 S protein by binding epitopes other than RBD | Cross neutralization determined by ELISA and BLI | [57] | |
| m396 | Competes with ACE2 for association with S1 domain | Screening phage display library | [99] |
| Neutralizes SARS-CoV resistant against 80R and S3.1 antibody | |||
| Neutralizes all zoonotic SARS-CoV except bat-originated ones | |||
| S230.15 | Competes with ACE2 for association with S1 domain | ||
| S230 | Mimics receptor attachment and promotes conformational rearrangement of S protein | Cryoelectron microscopy study of S protein in combination with antibody | [100] |
| B1 | Neutralizes S2 epitope | Screening phage display library | [101] |
| A group containing 27 human monoclonal antibodies | Neutralizes S1 domain by binding to residues 318–510 within RBD or 12–261 located at the upstream of RBD; antibodies targeted RBDs were the most reactive ones | Screening human monoclonal antibodies produced in XenoMouse® against SARS-CoV S protein | [61] |
| A group containing 57 human monoclonal antibodies | Neutralizes S2 domain | ||
| 201 | Neutralizes S1 domain by binding to residues 490–510; provides complete protection against SARS-CoV infection in murine model | Screening human monoclonal antibodies produced in HuMAB mice® against SARS-CoV S protein | [102] |
| 68 | Neutralizes S1 domain by binding to residues 130–150; provides complete protection against SARS-CoV infection in murine model | ||
| A group containing nine human monoclonal antibodies, including 1F8, 4A4, 1D12, 2A12, 5C3, 2B12, 6H2, 6C9, and 4F9 | Neutralize HR1 domain | Screening human monoclonal antibodies produced in XenoMouse® against SARS-CoV S protein | [59] |
| A group containing 13 human monoclonal antibodies, including 5G8, 5B10, 3A11, 5E9, 6H1, 1E10, 3H11, 5B9, 5D7, 2D2, 3E10, 5G9, and 2D6 | Neutralize HR2 domain | ||
| A group containing 17 human monoclonal antibodies, including 1F1, 3F1, 4E11, 6C5, 4G10, 3F9, 6D8, 2C6, 2G11, 1D11, 4E6, 1C1, 2B9, 2E11, 1G12, 6H6, and 1D5 | Neutralize S-ectodomain domain | ||
| F26G19 | Antibody that binds to SARS-CoV RBD and blocks the contact of virus with ACE2 receptors | Studying x-ray crystal structure of Fab of mouse monoclonal antibody in complex with SARS-CoV RBD | [103] |
| Higher affinity to SARS-CoV-2 S protein than SARS-CoV | Antibody-antigen docking simulation | [51] | |
| F26G15 | Neutralizes nucleoprotein | Screening murine monoclonal antibodies by enzyme immunoassays | [104] |
| F26G1, F26G6, F26G8, F26G18, F26G19 | Neutralize spike | ||
| A group containing 8 antibodies, including five mutated forms of the antibody with the PDB ID of 2GHW and three mutated forms of the antibody with the PDB ID of 6NB6 | Neutralize spike protein | Analysis of 1933 antibody against SARS-CoV-2 via machine learning, neutralization was identified based on neutralizing scaffold of 80R antibody | [105] |
| 1C6, 1H1, 6B9, 4B12, 1G10 | Interfere with the HR1 and HR2 interaction and inhibit the membrane fusion and virus entry | Monoclonal antibodies generated in immunized mice against S fragment were analyzed by immunoassays | [106] |
| 2B2,2G2, 1A9 | Occupy the upstream of HR2 domain and cause steric hindrance | ||
| 256 | Neutralizes virus by enhancing binding of S protein to the surface of target cell | Identified in scFv libraries | [107] |
| 4D4 | Binds to the N-terminal of RBD and inhibits post binding steps | Screening human monoclonal antibodies produced in XenoMouse® against SARS-CoV S protein | [108] |
| 47D11 | Cross-neutralizes S1 subunit of SARS-CoV and SARS-CoV-2 | Derived from immunized transgenic H2L2 mice and cross-reactivity identified by ELISA | [109] |
| 1A9, 2B2, 4B12, 1G10 | Neutralize HR2 domain | Murine monoclonal antibodies generated using S protein fragment and neutralization capacity identified by immunoassay | [62] |
| Dewetting monoclonal antibodies | Dewetting viroporin of SARS-CoV-2 | Hypothesized based on dewetting transition phenomenon | [88] |
| P2C-1F11, P2B-2F6, P2C-1A3 | Block RBD | Antibodies derived from convalescent patients and tested via immune assays | [91] |
| S309, S306 | Neutralize S protein through glycan containing epitope distinct from RBD, does not compete with receptor attachment | Identified from memory B-cell of SARS-CoV patients | [63] |
| Induce NK-mediated antibody-dependent cell cytotoxicity | |||
| B38 and H4 | Have synergistic action in binding with RBD and neutralizing the virus, their synergistic action avoids immune escape | Isolated from SARS-CoV-2 convalescent patients | [110] |
Further information in this study concerning monoclonal antibody data against SARS-CoV-2 was retrieved from clinical trial data recorded in clinicaltrials.gov or biotechnology and pharmaceutical companies’ websites, which are summarized in Table 3, Table 4 , respectively. Monoclonal antibodies, which are proposed to date against SARS-CoV-2, mainly target immune responses rather than the virus structure. Various inclusion and exclusion criteria were considered in clinical trials as it is stated in clinicaltrials.gov. Most of the clinical trials were indicated for severe to critically COVID-19 patients with progressed pneumonia. However, one clinical trial related to tocilizumab, being held in the University of Chicago (NCT04331795), aims to prevent clinical decompensation in the hospitalized non-critically ill patients. Some of the trials aim to assess the efficacy of monoclonal antibodies alone, while others are administered in combination with other medications. For example, in one study in Spain (NCT04332094), tocilizumab is administered with hydroxychloroquine and azithromycin. Moreover, studies evaluate the efficacy of a medication alone or in comparison with other treatment options, including a study in China (NCT04306705) in which tocilizumab is compared with continuous renal replacement therapy. In some clinical trials, such as the one related to thymosin, lymphocytopenia was an eligibility criterion along with severe pneumonia. Different primary and secondary endpoints were considered in clinical trials, among them a reduction in fever and decreased need to oxygen can be mentioned. Pharmaceutical companies have proposed various kinds of monoclonal antibodies against SARS-CoV-2, details of which are explained in Table 4. Many of the proposed antibodies target immune system responses, including GM-CSF, IL-1β, IL-17, C5a, and NAMPT, while some others, developed by Eli Lilly and AbCellera, Vir Biotechnology, Adaptive, Amgen, and Harbour Biomed, are designed to neutralize SARS-CoV-2 structure. However, the mechanisms and specificities of the latter antibodies have not been elaborated to date and thus should be followed in companies’ websites.
Table 3.
Clinical trials of monoclonal antibodies against SARS-CoV-2 recorded in clinicaltrials.gov.
| Antibody name | Mechanism | Sponsor | Clinical trial identifier | Start-end | Participants number | Study location | Study protocol |
|---|---|---|---|---|---|---|---|
| Sarilumab (Kevzara®) (REGN88) (SAR153191) | Anti-IL-6 receptor | Regeneron & Sanofi | NCT04315298 | Mar 2020-Mar 2021 | 400 | Global (63 study locations) | Single IV low & high dose |
| Lisa Barrett Nova Scotia Health Authority Dalhousie University | NCT04321993 | Mar 2020-Feb 2021 | 1000 | – | 200 mg single SQ dose | ||
| Tocilizumab (TCZ) (Roactemra®) (Actemra®) | Anti-IL-6 receptor | Fundació Institut de Recerca de l'Hospital de la Santa Creu i Sant Pau Instituto de Salud Carlos III | NCT04332094 | Apr 2020-Sep 2020 | 276 | Spain | 162 mg q12 h SQ (one day) + hydroxychloroquine + Azithromycin |
| National Cancer Institute, Naples | NCT04317092 | Mar 2020-Dec 2020 | 330 | Global (27 study locations) | 8 mg/kg (max 800 mg per dose) q 12 h | ||
| University of Chicago | NCT04331795 | Apr 2020-Jul 2020 | 50 | USA | Single low dose (starting with 80 mg) or high dose (starting with 200 mg), repeat dose if needed | ||
| University Hospital Inselspital, Berne Roche Pharma AG | NCT04335071 | Apr 2020-Oct 2020 | 100 | Switzerland | 8 mg/kg (max 800 mg per dose), IV infusion; repeat dose if needed | ||
| Hoffmann-La Roche | NCT04320615 | Apr2020-Aug 2021 | 330 | – | |||
| University of L'Aquila | NCT04332913 | Apr 2020- Dec 2020 | 30 | – | – | ||
| Tongji Hospital | NCT04306705 | Feb 2020-May 2020 | 120 | China | 8 mg/kg IV infusion | ||
| Peking University First Hospital | NCT04310228 | Mar 2020-May 2020 | 150 | China | 8 mg/kg (max 800 mg per dose) + favipiravir | ||
| MedSIR | NCT04335305 | Mar 2020-May 2020 | 24 | – | Single dose of 8 mg/kg (max 800 mg per dose), IV infusion + pembrolizumab | ||
| Centre Leon Berard | NCT04333914 | Apr 2020-Jun 2020 | 273 | France | Single dose 400 mg IV + nivolumab + chloroquine | ||
| University Hospital, Ghent | NCT04330638 | Apr 2020-Sep 2020 | 342 | Belgium | Single dose of 8 mg/kg (max 800 mg per dose), IV infusion | ||
| Marius Henriksen Lars Erik Kristensen | NCT04322773 | Mar 2020-Jun 2021 | 200 | Denmark | Single dose 400 mg IV | ||
| Università Politecnica delle Marche | NCT04315480 | Mar 2020-Apr 2020 | 30 | Italy | Single IV dose of 8 mg/kg | ||
| Assistance Publique - Hôpitaux de Paris | NCT04331808 | Mar 2020-Mar 2021 | 240 | – | IV dose of 8 mg/kg, repeat dose if needed | ||
| Siltuximab (Sylvant®) | Anti-IL-6 | University Hospital, Ghent Belgium Health Care Knowledge Centre | NCT04330638 | Apr 2020-Sep 2020 | 342 | Belgium | Single dose of 11 mg/kg IV infusion + anakinra + tocilizumab |
| Judit Pich Martínez | NCT04329650 | Apr 2020-May 2020 | 100 | Spain | Single dose of 11 mg/kg IV infusion | ||
| A.O. Ospedale Papa Giovanni XXIII | NCT04322188 | Mar 2020-May 2020 | 50 | Italy | – | ||
| Bevacizumab (An ke da) | VEGF inhibitor | Qilu Hospital of Shandong University | NCT04305106 | Mar 2020-Jun 2020 | 140 | China | 7.5 mg/kg IV |
| Qilu Hospital of Shandong University Renmin Hospital of Wuhan University Moriggia-Pelascini Gravedona Hospital | NCT04275414 | Feb 2020-Apr 2020 | 20 | China | 500 mg IV | ||
| Emapalumab (Gamifant®) | Anti-IFNγ | Swedish Orphan Biovitrum | NCT04324021 | Mar 2020-Jul 2020 | 54 | Italy | IV infusion every three days for a total of five infusions. Day 1: 6 mg/kg. Days 4, 7, 10 and 13: 3 mg/kg + anakinra |
| Thymosin | Anti-PD-1 | Southeast University, China | NCT04268537 | Feb 2020-Apr 2020 | 120 | China | 1.6 mg SQ, qd (for five days) |
| Pembrolizumab (MK-3475) (Keytruda®) | MedSIR | NCT04335305 | Mar 2020-May 2020 | 24 | – | Single dose of 200 mg IV infusion + tocilizumab | |
| Nivolumab | Centre Leon Berard | NCT04333914 | Apr 2020-Jun 2020 | 273 | France | Single dose of 0.3 mg/kg IV infusion + tocilizumab + chloroquine | |
| Eculizumab (Soliris®) | Distal complement inhibitor, preventing formation of the membrane attack complex | Hudson Medical | NCT04288713 | – | – | – | – |
| Meplazumab | Anti -CD147 | Tang-Du Hospital | NCT04275245 | Feb 2020-Dec 2020 | 20 | China | 10 mg IV infusion, every two days |
Table 4.
Antibodies candidate against SARS-CoV-2 under investigation by pharmaceutical and biotechnology companies.
| Antibody | Mechanism | Company | Stage of study/identification method | Ref |
|---|---|---|---|---|
| Gimsilumab | Anti GM-CSF monoclonal antibody | Roviant Sciences | In clinical stage for inflammation and rheumatic disease Prioritized in clinical trial for SARS-CoV-2 | [111] |
| TJM2 | I-MAB Biopharma | Phase 1 clinical trial completed and revealed favorable safety | [112] | |
| Lenzilumab | Humanigen Inc. | Currently in clinical stage for leukemia and lymphoma | [113] | |
| Canakinumab (Ilaris®) | IL-1β inhibitor | Novartis | In clinical stage for several inflammatory diseases including arthritis, periodic fever and lung cancer; | [114] |
| Repurposed by Novartis for COVID-19 | ||||
| Secukinumab (Cosentyx®) | IL-17 inhibitor | Novartis | In clinical stage for several autoimmune diseases including psoriasis; repurpose by Novartis for COVID-19 | [69] |
| Not mentioned | Binds to highly-conserved epitopes within SARS-CoV and SARS-CoV-2 | Vir biotechnology with WuXi biologics and Biogen | Enters clinical trial within 3–5 months Aimed to confer short- and long-term immunity and use as prophylaxis |
[115] |
| Not mentioned | Block virus RBD interaction with ACE2 | Distributed Bio | Computational studies; Thousands of fully human high affinity monoclonal antibodies engineered by Tumbler and SuperHuman 2.0 technology |
[116] |
| Not mentioned | Anti SARS-CoV-2 | Eli Lilly and AbCellera | Experimental stage; screened functional antibodies of recovered COVID-19 patients | [117] |
| TZLS-501 | Fully human monoclonal antibody targeting the receptor of IL-6, it binds to both membrane-bound and soluble forms of IL-6R, and rapidly depletes the circulating levels of IL-6 in blood. | Tiziana Life Sciences and Novimmune | Preclinical stage | [118] |
| ALT-100 | Neutralize circulating NAMPT | Aqualung Therapeutics Corp. | Preclinical stage | [119] |
| Not mentioned | Fully human monoclonal antibody | Harbour Biomed; Mount Sinai Health System | Experimental stage | [120] |
| Produced with the technology of H2L2 Harbour mice® | ||||
| Pritumumab | Fully human IgG antibody targeting vimentin | Nascent Biotech Inc. | Received FDA approve for several carcinoma | [121] |
| Research began for COVID-19 | ||||
| Leronlimab (PRO140) | Antagonizes CCR5 on T-cells and prevents viral entry | CytoDyn | A 10-patient clinical study against COVID-19 | [122] |
| Initially developed against HIV; in clinical trial for HIV and breast cancer | ||||
| BDB-1 | Anti C5a | Beijing Defengrei Biotechnology | Beijing Defengrei Biotechnology passes the phase II of clinical trial | [123] |
| IFX-1 | InflaRx | InflaRx received approval for starting the clinical trial in Netherlands | ||
| Not mentioned | Fully human neutralizing antibodies targeting SARS-CoV-2 | Adaptive and Amgen | Screening B cell receptors of patients recovered from COVID-19 to find neutralizing antibodies | [124] |
| VIR-7831/VIR-7832 | Neutralize highly conserved epitope in s protein | VIR biotechnology and GSK | Designed based on S309 (isolated from SARS-Cov patients) | [125] |
| Induce NK-mediated antibody-dependent cell cytotoxicity | ||||
| SAB | Anti SARS-CoV-2 fully human poly clonal antibodies | SAB Biotherapeutics | Antibodies produced in genetically engineered cattle will enter clinical trial by early summer | [126] |
| SAB-301 against MERS passes phase 1 of clinical trial and entered phase II/III | ||||
| – | Target multiple viral S epitope | ImmunoPrecise | Using B cell Select® and Deep Display® technology | [127] |
| COVID-HIG and COVID-EIG | Hyperimmune polyclonal antibody derived from human plasma or immunized horse | Emergent BioSolutions | Enter clinical trial within 4–5 months | [128] |
| rCIG | Recombinant anti SARS-CoV-2 hyperimmune gammaglobulin, polyclonal antibodies | GigaGen | Preclinical stage | [129] |
| Aimed for COVID19 hospitalized patients and prophylaxis in high risk individuals | ||||
| Antibody cocktail including REGN3048-3051 | Fully human multivalent antibodies against the spike protein isolated from genetically modified mice or recovered COVID-19 patients | Regeneron | Phase 1 clinical trial for MERS completed last year | [130] |
| Clinical trial for SARS-CoV-2 starts by early summer |
6.2.4. Polyclonal antibodies against SARS-CoV-2
For effective passive immunotherapy, several epitopes ought to be targeted rather than one epitope. Moreover, the design of monoclonal antibodies and their testing at a clinical stage is a long pathway followed by the fact that the massive production of monoclonal antibodies might be costly, time-consuming, and labor-intensive; factors that cannot be underestimated in the time of a pandemic outbreak and an urgent need to effective therapeutics. Herein, the administration of a cocktail of monoclonal antibodies or multivalent antibodies for the neutralization of SARS-CoV-2 also seems more rational [130]. For example, Regeneron Pharmaceuticals proposed the use of a cocktail of the neutralizing antibodies derived from a genetically engineered mouse to fight with SARS-CoV-2. Three other pharmaceutical companies, including GigaGen, Emergent BioSolution, and SAB Biotherapeutics also have developed polyclonal antibodies against SARS-CoV-2, called rCIG, COVID-EIG/HIG, and SAB, respectively (Table 4). Polyclonal antibodies produced by immunized animals or a particular cell line technology are expected to mimic convalescent plasma therapy with a higher potency than their plasma-derived equivalents as well as better clinical outcomes. Furthermore, the risk of contamination and host reactions will be reduced compared with their plasma equivalents; dosing and kinetics also would be more predictable and scalable. Moreover, targeting more than one epitope can cause synergistic effects in neutralization and would limit the formation of escape-mutants. For instance, a recent study revealed that a cocktail of antibody noticeably enhanced SARS-CoV-2 neutralization compared with the use of one monoclonal antibody [63].
6.3. Immunomodulators
Several classes of immunomodulators including tyrosine kinase inhibitors, mTOR inhibitors, calcineurin inhibitors, antimetabolites, TNF blockers, metal-based agents, and other anti-inflammatory agents might be of value in the treatment of COVID-19. Janus-associated kinase (JAK) inhibitors are of high interest among the mentioned immunomodulators.
6.3.1. JAK inhibitors
Although ACE2 receptors have been recognized as the main receptors for the entry of SARS-CoV-2, it was shown that SARS-CoV-2 also attacks the cells that do not have ACE2 receptors, including lymphocytes. It is proposed that SARS-CoV-2 may also enter the cell through clathrin-mediated endocytosis, thus it can be defeated through inhibiting endocytosis. Members of numb-associated kinase (NAK) family including AP2-associated protein kinase 1 (AAK1) and Janus-associated kinase (JAK) are two of the main regulators of endocytosis, which can be inhibited and suggested as a possible target for controlling different viral infections such as SARS-CoV-2 infection [131]. JAK inhibitors include ruxolitinib, baricitinib, fedratinib, upadacitinib, tofacitinib, and filgotinib, which are mainly used for the treatment of myelofibrosis or other inflammatory diseases, including rheumatoid arthritis, ulcerative colitis, and psoriasis. Myelofibrosis is a blood cancer in which chronic leukemia is observed. Lymphocytopenia notably occurs in COVID-19 patients as well and is considered as one of the major markers of the disease. Hence, it seems logical to repurpose JAK inhibitors in COVID-19 patients, since the disease mimics the symptoms that occur in myelofibrosis. Moreover, JAK inhibitors with their high anti-inflammatory properties can curtail the cytokine storm that happens in COVID-19 patients. It is of special notice that the use of JAK inhibitors for the prevention of SARS-CoV-2 may be a double-edged sword. For instance, although JAK inhibitors are considered as relatively safe therapeutics for SARS-CoV-2, they may inhibit inflammatory mediators, such as INF-α, which play a vital role in the immune responses and thwarting SARS-CoV-2 [132]. In fact, broad immunosuppression either by JAK inhibitors or other immunosuppressants may delay the virus clearance and perpetuate the illness. The possible benefits of immunosuppressants should be assessed versus their potential to impair the immune system and cause bacterial infections as well [133].
JAK inhibitors are classified as JAK1, 2, or 3 inhibitors, and their inhibition is either exclusive (JAK2 inhibitor) or non-exclusive (JAK1/3, JAK1/2 inhibitor). Several in vitro/ in vivo or computational studies concerning various viral infections or SARS-CoV-2 were performed to assess the safety and efficacy of different JAK inhibitors and compare the advantage of one JAK inhibitor over another member of the family. For instance, JAK2 inhibitors, such as fedratinib, function more precisely in the control of cytokine storm. JAK2 inhibitors block the Th17 differentiation thereby reducing the level of IL-17, IL22, and GM-CSF, which are responsible for the aggravation of cytokine storm and pulmonary edema. Moreover, JAK2 inhibitors have a marginal effect on IL21, which is responsible for B cell function, and do not disrupt innate immune response, since the inhibition caused by JAK2 inhibitors is transient and reversible [72]. Another member of this family, baricitinib, is of special interest due to its advantages over other JAK inhibitors and has been highly studied. Baricitinib inhibits another regulator of endocytosis, called cyclin G associated kinase, through which it can defeat the viral infection [134]. Baricitinib has been also suggested as the best choice among other JAK inhibitors due to its acceptable profile of side effects, the possibility of once-daily dosing, higher potency, and advantageous pharmacokinetics. The inhibitory doses of baricitinib were well-tolerated by patients with inflammatory diseases in comparison with other kinase inhibitors [135], [136]. Moreover, baricitinib represents low protein binding and minimal interaction with drug transporters or metabolic enzymes; thus, it is preferred over other JAK inhibitors for administration along with an anti-viral regimen [136]. In contrast to the above-mentioned benefits of baricitinib, some clinical studies suggested that baricitinib may not be an ideal option for the treatment of COVID-19 due to the possibility of causing lymphocytopenia, neutropenia, viral reactivation, and enhancement of coinfection [137]. Other JAK inhibitors, including upadacitinib and filgotinib, were also shown to impair interferon-mediated antiviral responses and perpetuate SARS-CoV-2 infection. Herein, the incidence of secondary viral infections, including herpes zoster was observed, which was shown to be more prevalent in immunocompromised patients. Hence, JAK inhibitors, particularly baricitinib, should be administered with meticulous consideration, especially in susceptible and immunocompromised patients [138]. Next, ruxolitinib was listed as one of the top hit compounds in an advanced bioinformatics analysis of available medications for SARS-CoV-2. The mentioned study aimed to identify compounds that counteract the expression of SARS-CoV-2-related genes [139]. To date, only three JAK inhibitors have entered clinical studies on COVID-19 patients, including baricitinib, ruxolitinib, and tofacitinib, among which many of the studies are related to baricitinib and ruxolitinib (Table 5 ).
Table 5.
Clinical studies on immunomodulators in COVID-19.
| Immunomodulator | Mechanism | Sponsor | Clinical trial identifier | Start-end | Participant number | Study location | Administration |
|---|---|---|---|---|---|---|---|
| Baricitinib (Olumiant®) | JAK inhibitor | University of Colorado | NCT04340232 | Apr 2020-Aug 2020 | 80 | USA | 2 mg PO |
| Hospital Universitario de Fuenlabrada | NCT04346147 | Apr 2020-Aug 2020 | 165 | Spain | 4 mg PO, QD + 200 mg BID Hydroxychloroquine | ||
| Hospital of Prato | NCT04320277 | Mar 2020-Apr 2020 | 60 | Italy | 4 mg PO, QD (2 weeks) + ritonavir 600 mg BID | ||
| Thomas Benfield | NCT04345289 | Apr 2020-Jun 2021 | 1500 | Denmark | 4 mg PO, QD (one week) | ||
| Lisa Barrett Nova Scotia Health Authority Dalhousie University | NCT04321993 | Apr 2020-Feb 2021 | 1000 | Canada | 2 mg PO, QD (10 days) | ||
| Tofacitinib | JAK 1/3 inhibitor | Università Politecnica delle Marche | NCT04332042 | Apr 2020-Jun 2020 | 50 | Italy | 5 mg, BID (2 weeks) |
| Ruxolitinib (Jakafi®, Jakavi®) | JAK 1/2 inhibitor | University of Colorado, Denver | NCT04348071 | Apr 2020-Aug 2020 | 80 | USA | 10 mg, BID (2 weeks) |
| Grupo Cooperativo de Hemopatías Malignas | NCT04334044 | Apr 2020-Jun 2020 | 20 | Mexico | 10 mg, BID (until observation of changes in pneumonia) | ||
| University Health Network, Toronto | NCT04331665 | Apr 2020-Oct 2020 | 64 | Canada | 10 mg, BID (two weeks) followed by 5 mg, BID (2 days) and 5 mg, QD (one day) | ||
| Prof. Dr. med. Andreas Hochhaus | NCT04338958 | May 2020-Jan 2021 | 200 | Germany | 10 mg, BID (7 days) | ||
| Fundación de investigación HM Apices Soluciones S.L. | NCT04348695 | Apr 2020-May 2020 | 94 | Spain | 10 mg, BID (seven days) + 40 mg simvastatin QD (2 weeks) | ||
| Acalabrutinib | BTK inhibitor | AstraZeneca Acerta Pharma B.V. | NCT04346199 | Dec 2020-Jan 2020 | 428 | – | PO |
| Tacrolimus (Advagraf®, Modigraf®) | Calcineurin inhibitor | Hospital Universitari de Bellvitge Institut d'Investigació Biomèdica de Bellvitge | NCT04341038 | Apr 2020-Jun 2020 | 84 | Spain | Necessary dose to obtain the blood level of 8–10 ng/ml + 120 mg methylprednisolone QD (3 consecutive days) |
| Sirolimus (rapamycin) (rapamune®) | mTOR inhibitor | University of Cincinnati | NCT04341675 | Apr 2020-Jul 2020 | 30 | USA | 6 mg loading dose + 2 mg, QD (2 weeks) |
| Thalidomide (fanyingting®) | Anti-inflammatory, TNF-α inhibitor | Wenzhou Medical University | NCT04273529 | Feb 2020-May 2020 | 100 | – | 100 mg, PO, QN, (2 weeks) |
| CD24Fc | Anti-inflammatory | OncoImmune, Inc. | NCT04317040 | Apr 2020-May 2021 | 230 | USA | 480 mg, IV infusion, single dose |
| Fingolimod | Sphingosine-1-phosphate receptor regulators | Hospital of Fujian Medical University | NCT04280588 | Feb 2020-Jul 2020 | 30 | China | 0.5 mg, PO, QD (3 days) |
6.3.2. Other tyrosine kinase inhibitors
Bruton tyrosine kinase (BTK) inhibitors are another group of tyrosine kinase inhibitors, which has been repurposed to modulate the cytokine storm ensuing COVID-19 infection. BTK signaling leads to B cell proliferation and activation of cytokine pathway. BTK inhibitors are mainly approved for the treatment of an aggressive form of B cell lymphoma, called mantle cell lymphoma. This group includes acalabrutinib, zanubrutinib, tirabrutinib, and ibrutinib, among which ibrutinib was shown to have less efficacy and more toxicity [140]. AstraZeneca® incorporation has designed a clinical trial to assess the efficacy of one of these BTK inhibitors, called acalabrutinib, to alleviate the cytokine storm of SARS-CoV-2 infection (Table 5). In addition to BTK inhibitors, other kinase inhibitors, including erlotinib and sunitinib were also shown to interfere in viral entry through inhibiting JAK and AAK1. However, they are not classified as JAK inhibitors and are not preferred over JAK inhibitors due to less efficacy and higher toxicity [135], [136]. Sorafenib was also hypothesized to be repurposed in COVID-19 based on a drug-gene interaction analysis [141].
6.3.3. mTOR inhibitors
Another mechanism of immunosuppression, which has been proposed, is related to the use of mTOR inhibitors. The cytokine storm in COVID-19 patients is attributed to a mechanism, called antibody-dependent enhancement, in some systematic reviews [142]. This phenomenon happens when the virus triggers the production of cross-reactive antibodies by memory B cells. These cross-reactive antibodies enhance virus delivery to the macrophages and thus contribute to the massive replication of the virus without being captured by the immune system. mTOR inhibitors were found to inhibit the activation of memory B cell and prevent the antibody-dependent enhancement mechanism. mTOR inhibitors also were shown to inhibit the replication of MERS-CoV in the in vitro studies [142]. Some mTOR inhibitors, including rapamycin or sirolimus, were hypothesized to be repurposed in COVID-19 clinical studies. Sirolimus was shown to inhibit viral replication and release in patients with severe pneumonia and acute respiratory failure [143]. The computational analysis of protein–protein interactions and gene-enrichment network also suggested that sirolimus can be repurposed for SASR-CoV-2 [144]. Moreover, a clinical study of sirolimus on COVID-19 patients has been recently started (Table 5).
6.3.4. Antimetabolites
Papain-like protease (PLpro) is a viral protease responsible for coronavirus genome replication with deubiquitinating activity [145]. PLpro has been mainly the target of viral inhibitor class of medications. However, antimetabolites were also shown to be effective on PLpro due to their pharmacological action. For instance, 6-mercaptopurine (6MP) and 6-thioguanine (6TG) were shown to be the specific inhibitors of SARS-CoV PLpro [146]. Mycophenolate mofetil is another immunosuppressant that was shown to target PLpro in SARS-CoV and MERS-CoV in both in vitro and in vivo studies. However, further clinical studies are needed to assess its efficacy against SARS-CoV-2 [147]. There is no definitive evidence about the efficacy of other antimetabolites including methotrexate in COVID-19 patients [147].
6.3.5. Calcineurin inhibitors
Calcineurin inhibitors such as tacrolimus might be effective against SARS-CoV-2, since they inhibit calcineurin, thereby blocking T cell activation. Tacrolimus, which is mainly used in organ transplant, was shown to be effective against MERS-CoV in a renal transplant patient compared with a similar patient who did not receive tacrolimus as a part of the transplant regimen [148]. Tacrolimus was also found to be effective against SARS-CoV in a study on cell lines. However, further studies undoubtedly are required to assess its efficacy against SARS-CoV-2 [149]. There is no definitive evidence about the efficacy of other calcineurin inhibitors including cyclosporine [147].
6.3.6. Metal-based agents
Metal-based agents with different metal centers, including gold, ruthenium, and bismuth were suggested to be used in COVID-19 patients [150]. The gold compound, called auranofin (Ridaura®) is an FDA approved compound, which was initially proposed for rheumatoid arthritis. The exact mechanism of auranofin is still unclear; however, it is classified as an immunomodulatory and anti-inflammatory agent. In recent years, auranofin has gained attention in viral infections, including HIV. In the case of HIV, it was revealed to be more effective than hydroxychloroquine in control of viral production, latency, and viral reactivation [151]. It was also hypothesized that auranofin can interfere with IL-6 signaling by inhibiting JAK1 and STAT3 pathways [152]. It was shown that a low micromolar concentration of auranofin strongly inhibited SARS-CoV-2 viral replication and reduced the viral-induced cytokine expression in human cells [153].
6.3.7. TNF-α blockers and other anti-inflammatories
TNF-α was shown to be associated with SARS-CoV pulmonary injury; therefore, TNF-α blockers, which are mainly used in the treatment of autoimmune and inflammatory diseases, such as rheumatoid arthritis, ankylosing spondylitis, and psoriasis, can be suggested as a potential target for SARS-CoV-2 [154]. Besides the monoclonal antibodies that modulate the TNF-α responses, etanercept was suggested as another immunomodulator for COVID-19 patients [147].
Lenalidomide and thalidomide, which are not specifically classified as TNF-α blockers, were hypothesized to be repurposed based on a drug-gene interaction analysis [141]. Thalidomide has anti-inflammatory, anti-fibrotic, and immunoregulatory effects, which proved to be safe and effective in the treatment of lung injuries with different etiologies, including H1NI-induced lung injury [155]. It has also entered a clinical trial of COVID-19, as shown in Table 5.
CD24Fc, which is a fusion protein constituted of human CD24 attached to the human IgG Fc region, is another biological immunomodulator. CD24Fc was demonstrated to successfully ameliorate cytokine responses in viral infections and reduce the graft versus host disease [156], [157]. Hence, CD24Fc could be effective against the SARS-CoV-2 cytokine storm and the associated pneumonia. CD24Fc has entered a clinical trial by OncImmune® incorporation (Table 5).
6.3.8. Corticosteroids
The rationale for using corticosteroids in COVID-19 patients relies on their inhibitory effects on the inflammatory factors. Corticosteroids inhibit a massive proportion of cytokines, chemokines, inflammatory enzymes, and receptors that are overexpressed in response to the viral infections [158]. Results regarding the beneficial effects of corticosteroids against coronavirus are controversial. A meta-analysis including 5270 patients from 15 studies revealed that the use of corticosteroids resulted in higher mortality rate, the longer length of stay, a higher rate of bacterial infection, hypokalemia, and hypercalcemia [159]. Furthermore, Russell and colleagues in a comment published in The Lancet did not advocate the use of corticosteroids in COVID-19-induced lung injury or shock, except in the clinical trial settings [160]. By contrast, a retrospective analysis of 401 patients with severe SARS revealed that corticosteroids led to reduced mortality rate and shortened hospital stay [161]. Taken together, corticosteroids are indicated for critically ill COVID-19 patients, and its use in mild to moderate patients is highly disregarded. For instance, the use of corticosteroids is recommended in mechanically-ventilated COVID-19 patients with respiratory failure (with ARDS), while is inappropriate for COVID-19 patients without ARDS [162]. In fact, administering corticosteroids in the onset of disease was shown to deter the immune system and increase the viral load, thereby inducing additional complications, such as diabetes and vascular necrosis [163], [164]. The timing and dosage of corticosteroids are of special importance in the outcomes of treatment in critically ill patients [165]. For instance, in patients with high inflammatory responses including severe deterioration of oxygenation indicators and rapid imaging progress, a short term (3–5 days) of corticosteroids doses equivalent to 1–2 mg/kg/day methylprednisolone is recommended [166]. Moreover, a recent study revealed that low doses of corticosteroids (e.g. 25–150 mg/day methylprednisolone) did not delay viral clearance and did not increase the mortality rate compared with the control group [167].
Recently dexamethasone was introduced as the first medicine presented with a survival benefit in treating critically ill COVID-19 patients, and WHO welcomes preliminary results regarding its use [168], [169]. According to a large, multi-center and randomized clinical trial called RECOVERY (Randomized Evaluation of COVid-19 thERapY) trial, the use of 6 mg/day dexamethasone in COVID-19 patients on mechanical ventilation or oxygen supplementation was associated with reduced mortality rate compared with those who received an standard treatment. In the patients who did not need respiratory support, no improvement was observed [170], [171]. This well-known steroid is believed to support the suppression of hyperactive inflammatory responses, so-called cytokine storm [168]. Interestingly, an extra mechanism in defeating SARS-CoV-2 by dexamethasone was suggested. A computational study revealed that dexamethasone tightly binds to 3C-like protease (the main protease) in SARS-CoV-2 as remdesivir does; however, dexamethasone surprisingly forms a better contact with the enzyme active site than remdesivir [172].
In addition to the importance of dose and timing, other considerations concerning the use of corticosteroids ought to be made according to a recent correspondence consensually made by experts in The Lancet, including: 1) the pros and cons of corticosteroids should be carefully evaluated prior to administration; 2) prudent consideration should be made for the further use of corticosteroids in patients who are on the regular use of corticosteroids for chronic diseases [173].
7. Conclusion
Modulation of the immune system is obviously a major strategy in the control and treatment of COVID-19, which should be adjusted according to different stages of the disease. It can be done through vaccines, interferons, antibodies (either as convalescent plasma therapy or monoclonal and polyclonal antibodies), or potential therapeutic immunomodulators.
At the pre-disease or disease prevention stage, active immunization by vaccines could be an optimal approach. However, vaccine development is a time-consuming and complicated process. Despite enormous efforts in finding vaccines against SARS-CoV-2, there is still a fairly long way to the market availability of a proper vaccine.
At the early stages of the disease, stimulation of the immune system through passive immunotherapy is regarded as a therapeutic objective to support the patient’s immune system and compensate for its suppression by SARS-CoV-2. CPT, as a viable emergency immunotherapy, is already being investigated in many countries with positive results. Yet, some limitations of CPT suggest the employment of manufactured monoclonal or polyclonal antibodies. Given the specificity of antibodies, using a cocktail of monoclonal antibodies, which can target SARS-CoV-2 in several locations, is proposed to prevent viral entry into the host cells more thoroughly. The higher speed of antibody development process versus vaccines and small molecules are regarded as the benefits of antibody therapy for the management of COVID-19. However, the probability of adverse events caused by antibody administration such as disease aggravation through antibody-dependent enhancement (ADE) and the intensification of inflammatory responses and worsening of the disease should be taken into serious consideration. Designing novel antibodies with strong viral neutralization capability and less proinflammatory function could be a new research avenue.
In critically ill COVID-19 patients, at the later stages of the disease, dysregulation of the immune system may occur, which may lead to a cytokine storm. In such conditions, immunosuppression by JAK inhibitors, mTOR inhibitors, TNF-α blockers, corticosteroids, or other immunosuppressant and anti-inflammatory drugs could be potentially the life-saving strategy. Currently, the efficacy and adverse effects of different drugs of these categories are under evaluation in clinical trials to completely evaluate their risk and benefits and avoid the possibility of unwanted immune suppression in favor of the virus replication and proliferation in the body. Recently, the survival benefit of low dose dexamethasone was reported in hospitalized patients.
Despite the similarities of SARS-CoV-2 with SARS and MERS, some differences such as higher transmission rate and longer incubation period indicate its varied interaction with the immune system. Given that most of the present data are interpreted from SARS and MERS studies, more specific data about SARS-CoV-2 immunopathogenicity and viral-mediated responses are demanded. Finally, taking into account the complexity of the immune system activities and their complicated interconnections, a systemic approach could be a key element in gaining a more complete picture of this disease and the virus pathogenicity. Such insight is urgently warranted to plan for proper interventional therapies with predictable and favorable outcomes.
Acknowledgement
This study was supported by Grant No. 99-01-36-23491 from the Research Council of Shiraz University of Medical Sciences, Shiraz, Iran.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106924.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Dhama K., et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines & Immunotherapeutics. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negahdaripour M. The battle against COVID-19: where do we stand now? Iranian J. Med. Sci. 2020;45(2):81–82. doi: 10.30476/ijms.2020.46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negahdaripour M. The rise and fall in therapeutic candidates for COVID-19. Iranian J. Med. Sci. 2020;45(4):231–232. doi: 10.30476/ijms.2020.46689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahabinezhad F., et al. Therapeutic approaches for COVID-19 based on the dynamics of interferon-mediated immune responses. Preprints. 2020 doi: 10.20944/preprints202003.0206.v2. 2020030206. [DOI] [Google Scholar]
- 5.Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20(7):397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muus C., et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. BioRxiv. 2020 doi: 10.1101/2020.04.19.049254. 2020.04.19.049254. [DOI] [Google Scholar]
- 7.Strollo R., Pozzilli P. DPP4 inhibition: Preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3330. e3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? The Lancet. 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X., et al. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;2:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L., et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection——a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020:1–14. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuen C.K., et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020;9(1):1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Y. Konno, et al., SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant. bioRxiv, 2020: 2020.05.11.088179. [DOI] [PMC free article] [PubMed]
- 14.Keam S., et al. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grifoni A., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.015. 1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paces J., et al. COVID-19 and the immune system. Physiol. Res. 2020;69(3) doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallard E., et al. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104791. 104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziegler C., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is enriched in specific cell subsets across tissues. Cell. 2020;5 doi: 10.1016/j.cell.2020.04.035. 1016-1035.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dastan F., et al. Subcutaneous administration of interferon beta-1a for COVID-19: A non-controlled prospective trial. Int. Immunopharmacol. 2020;85 doi: 10.1016/j.intimp.2020.106688. 106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalkanen J., Hollmén M., Jalkanen S. Interferon beta-1a for COVID-19: critical importance of the administration route. Crit. Care. 2020;24(1):335. doi: 10.1186/s13054-020-03048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu C., et al. Evaluation of safety, efficacy, tolerability, and treatment-related outcomes of type I interferons for human coronaviruses (HCoVs) infection in clinical practice: An updated critical systematic review and meta-analysis. Int. Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106740. 106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negahdaripour M. A world of changes: the inheritance of COVID-19. Iranian J. Med. Sci. 2020;45(3):155–156. doi: 10.30476/ijms.2020.46530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lurie N., et al. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 24.Belete T.M. A review on promising vaccine development progress for COVID-19 disease. Vacunas. 2020 doi: 10.1016/j.vacun.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koirala A., et al. Vaccines for COVID-19: The current state of play. Paediatr. Respir. Rev. 2020 doi: 10.1016/j.prrv.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan K., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B., et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020;158(1):e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta N., et al. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am. J. Emergency Med. 2020;38(7):1488–1493. doi: 10.1016/j.ajem.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q., et al. Clinical trial analysis of 2019-nCoV therapy registered in China. J. Med. Virol. 2020;92:540–545. doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arabi Y., et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4(1):1–8. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan K.-H., et al. Cross-reactive antibodies in convalescent SARS patients' sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J. Infect. 2013;67(2):130–140. doi: 10.1016/j.jinf.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiberghien P., et al. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how. Vox Sang. 2020 doi: 10.1111/vox.12926. [DOI] [PubMed] [Google Scholar]
- 33.Brown B.L., McCullough J. TreaTment for emerging viruses: Convalescent plasma and COVID-19. Transfus. Apheres. Sci. 2020 doi: 10.1016/j.transci.2020.102790. 102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marando M., Tamburello A. Immunoglobulins or convalescent plasma to tackle COVID-19: buying time to save lives–current situation and perspectives. Swiss Med. Wkly. 2020;150(1718) doi: 10.4414/smw.2020.20264. [DOI] [PubMed] [Google Scholar]
- 35.Rajendran K., et al. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J. Med. Virol. 2020;2020:1–9. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen C., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L., et al. Convalescent plasma as a potential therapy for COVID-19. Lancet. Infect. Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar G.V., Jeyanthi V., Ramakrishnan S. A short review on antibody therapy for COVID-19. New Microbes New Infect. 2020 doi: 10.1016/j.nmni.2020.100682. 100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng Q.-L., et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J. Infect. Dis. 2020;1(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Q., He Y. Challenges of convalescent plasma therapy on COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104358. 104358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan V., et al. Prospective audit of adverse reactions occurring in 459 primary antibody-deficient patients receiving intravenous immunoglobulin. Clin. Exp. Immunol. 2003;133(2):247–251. doi: 10.1046/j.1365-2249.2003.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas M., et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102554. 102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J., et al. SARS-CoV-2 infection in immunocompromised patients: humoral versus cell-mediated immunity. J. ImmunoTher. Cancer. 2020;8(2) doi: 10.1136/jitc-2020-000862. e000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammarström L., et al. Development of passive immunity against SARS-CoV-2 for management of immunodeficient patients—a perspective. J. Allergy Clin. Immunol. 2020;146(1):58–60. doi: 10.1016/j.jaci.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark E., et al. Convalescent plasma for persisting Covid-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br. J. Haematol. 2020;190:e154–e156. doi: 10.1111/bjh.16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Çınar O.E., et al. Convalescent (immune) plasma treatment in a myelodysplastic covid-19 patient with disseminated tuberculosis. Transfus. Apheres. Sci. 2020;102821 doi: 10.1016/j.transci.2020.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mira E., et al. Rapid recovery of a SARS-CoV-2 infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J. Allergy Clin. Immunology Practice. 2020 doi: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn J.Y., et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020;35(14) doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langhi D.M., Santis G., Bordin J.O. COVID-19 convalescent plasma transfusion. Hematol., Transfus. Cell Ther. 2020 doi: 10.1016/j.htct.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanmugaraj B., et al. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 51.Park T., et al. Spike protein binding prediction with neutralizing antibodies of SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.02.22.951178. [DOI] [Google Scholar]
- 52.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tay M.Z., et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wrapp D., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng L.-P., et al. Cross-neutralization of SARS coronavirus-specific antibodies against bat SARS-like coronaviruses. Sci. China Life Sci. 2017;60(12):1399–1402. doi: 10.1007/s11427-017-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian X., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D'Annessa I., Marchetti F., Colombo G. Differential antibody recognition by novel SARS-CoV-2 and SARS-CoV spike protein receptor binding domains: mechanistic insights and implications for the design of diagnostics and therapeutics. bioRxiv. 2020 doi: 10.1101/2020.03.13.990267. [DOI] [Google Scholar]
- 59.Elshabrawy H.A., et al. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traggiai E., et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coughlin M., et al. Generation and characterization of human monoclonal neutralizing antibodies with distinct binding and sequence features against SARS coronavirus using XenoMouse®. Virology. 2007;361(1):93–102. doi: 10.1016/j.virol.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Z., et al. Monoclonal antibodies for the S2 subunit of spike of SARS-CoV cross-react with the newly-emerged SARS-CoV-2. Euro Surveill. 2020;25(28) doi: 10.2807/1560-7917.ES.2020.25.28.2000291. 16 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto D., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020:1–10. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 64.Wan Y., et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2020;94(5) doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu Y.-T.-C., et al. Surface vimentin is critical for the cell entry of SARS-CoV. J. Biomed. Sci. 2016;23(1):14. doi: 10.1186/s12929-016-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Z.-Y., et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seesuay W., et al. Human transbodies that interfere with the functions of Ebola virus VP35 protein in genome replication and transcription and innate immune antagonism. Emerg. Microbes Infect. 2018;7(1):1–15. doi: 10.1038/s41426-018-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anand K., et al. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 69.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020;16(10):1718–1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Y., et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv. 2020 doi: 10.1101/2020.02.12.945576. [DOI] [Google Scholar]
- 72.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor fedratinib. J. Microbiol. Immunol. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J. Am. Med. Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 74.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camp S.M., et al. Unique toll-like receptor 4 activation by NAMPT/PBEF induces NFκB signaling and inflammatory lung injury. Sci. Rep. 2015;5:13135. doi: 10.1038/srep13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oita R.C., et al. Novel mechanism for nicotinamide phosphoribosyltransferase inhibition of TNF-α–mediated apoptosis in human lung endothelial cells. Am. J. Respir. Cell Mol. Biol. 2018;59(1):36–44. doi: 10.1165/rcmb.2017-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bermejo-Martin J.F., et al. Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection: Lymphopenia in severe COVID-19 infection. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Future Med. 2020;12(5) doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Platt E.J., et al. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998;72(4):2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woo P.C., et al. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin. Diagn. Lab. Immunol. 2004;11(4):665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu L., et al. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horiuchi T., Tsukamoto H. Complement-targeted therapy: development of C5-and C5a-targeted inhibition. Inflamm. Regen. 2016;36(1):11. doi: 10.1186/s41232-016-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu C., et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Homeyer N., et al. Interpreting thermodynamic profiles of aminoadamantane compounds inhibiting the M2 proton channel of influenza A by free energy calculations. J. Chem. Inf. Model. 2016;56(1):110–126. doi: 10.1021/acs.jcim.5b00467. [DOI] [PubMed] [Google Scholar]
- 86.Cho A., Wrammert J. Implications of broadly neutralizing antibodies in the development of a universal influenza vaccine. Curr. Opin. Virol. 2016;17:110–115. doi: 10.1016/j.coviro.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tozzi A. Three options against SARS-CoV-2: pediatric sera, mithridatization, dewetting antibodies. Preprint. 2020 doi: 10.13140/RG.2.2.19452.69767. [DOI] [Google Scholar]
- 89.Stech M., Kubick S. Cell-free synthesis meets antibody production: a review. Antibodies. 2015;4(1):12–33. [Google Scholar]
- 90.Coughlin M.M., Prabhakar B.S. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev. Med. Virol. 2012;22(1):2–17. doi: 10.1002/rmv.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ju B., et al. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.03.21.990770. [DOI] [PubMed] [Google Scholar]
- 92.R. Kong, et al., COVID-19 Docking Server: An interactive server for docking small molecules, peptides and antibodies against potential targets of COVID-19. arXiv preprint arXiv:2003.00163, 2020. [DOI] [PMC free article] [PubMed]
- 93.Walls A.C., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.058. 281-292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie L., et al. SARS-CoV-2 and SARS-CoV spike-RBD structure and receptor binding comparison and potential implications on neutralizing antibody and vaccine development. bioRxiv. 2020 doi: 10.1101/2020.02.16.951723. [DOI] [Google Scholar]
- 95.Zheng M., Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell. Mol. Immunol. 2020:1–3. doi: 10.1038/s41423-020-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sui J., et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. PNAS. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van den Brink E.N., et al. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79(3):1635–1644. doi: 10.1128/JVI.79.3.1635-1644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ter Meulen J., et al. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3(7) doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Z., et al. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. PNAS. 2007;104(29):12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walls A.C., et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(5) doi: 10.1016/j.cell.2018.12.028. 1026–1039. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duan J., et al. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem. Biophys. Res. Commun. 2005;333(1):186–193. doi: 10.1016/j.bbrc.2005.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greenough T.C., et al. Development and characterization of a severe acute respiratory syndrome—associated coronavirus—neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J. Infect. Dis. 2005;191(4):507–514. doi: 10.1086/427242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pak J.E., et al. Structural insights into immune recognition of the severe acute respiratory syndrome coronavirus S protein receptor binding domain. J. Mol. Biol. 2009;388(4):815–823. doi: 10.1016/j.jmb.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berry J.D., et al. Development and characterisation of neutralising monoclonal antibody to the SARS-coronavirus. J. Virol. Methods. 2004;120(1):87–96. doi: 10.1016/j.jviromet.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Magar R., Yadav P., Farimani A.B. Potential neutralizing antibodies discovered for novel corona virus using machine learning. arXiv. 2020 doi: 10.1038/s41598-021-84637-4. arXiv:2003.08447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rao S.K., et al. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 2004;279(19):20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 107.Sui J., et al. Broadening of neutralization activity to directly block a dominant antibody-driven SARS-coronavirus evolution pathway. PLoS Pathog. 2008;4(11) doi: 10.1371/journal.ppat.1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lonberg N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005;23(9):1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 109.Wang C., et al. A human monoclonal 1 antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu Y., et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roivant Announces Development of Anti-GM-CSF Monoclonal Antibody to Prevent and Treat Acute Respiratory Distress Syndrome (ARDS) in Patients with COVID-19. Available from: https://www.biospace.com/article/releases/roivant-announces-development-of-anti-gm-csf-monoclonal-antibody-to-prevent-and-treat-acute-respiratory-distress-syndrome-ards-in-patients-with-covid-19/.
- 112.I-Mab Biopharma Announces Development of TJM2 to Treat Cytokine Release Syndrome Associated with Severe and Critically-Ill Patients with Coronavirus Disease (COVID-19). Available from: http://ir.i-mabbiopharma.com/news-releases/news-release-details/i-mab-biopharma-announces-development-tjm2-treat-cytokine?from=timeline&isappinstalled=0.
- 113.Humanigen Partners With CTI, A Leading Contract Research Organization, For Planned Phase III Study For Lenzilumab For Coronavirus Treatment. 30 March 2020]; Available from: https://www.humanigen.com/press/Humanigen-Partners-With-CTI%2C-A-Leading-Contract-Research-Organization%2C-For-Planned-Phase-III-Study-For-Lenzilumab-For-Coronavirus-Treatment.
- 114.COVID-19 Novartis response.
- 115.Vir Biotechnology Proceeding with Two Clinical Development Candidates for COVID-19. [25 March 2020]; Available from: https://investors.vir.bio/news-releases/news-release-details/vir-biotechnology-proceeding-two-clinical-development-candidates.
- 116.Distributed Bio optimizes anti-SARS protective antibodies to block the novel coronavirus. [31 March 2020]; Available from: https://www.distributedbio.com/covid19.
- 117.AbCellera and Lilly to Co-develop Antibody Therapies for the Treatment of COVID-19. [12 March 2020]; Available from: https://www.abcellera.com/news/2020-03-abcellera-and-lilly-codevelopment.
- 118.Tiziana Life Sciences plc To Expedite Development of its Fully Human Anti-Interleukin-6-Receptor Monoclonal Antibody, a Potential Treatment of Certain Patients Infected with Coronavirus COVID-19. [11 March 2020]; Available from: https://www.tizianalifesciences.com/news-item?s=2020-03-11-tiziana-life-sciences-plc-to-expedite-development-of-its-fully-human-anti-interleukin-6-receptor-monoclonal-antibody-a-potential-treatment-of-certain-patients-infected-with-coronavirus-covid-19.
- 119.Aqualung Therapeutics Advances Its Investigational Monoclonal Antibody into IND-Enabling Studies of Acute Respiratory Distress Syndrome (ARDS) And Ventilator-Induced Lung Injury (VILI). [18 March 2020]; Available from: https://www.accesswire.com/581410/Aqualung-Therapeutics-Advances-Its-Investigational-Monoclonal-Antibody-into-IND-Enabling-Studies-of-Acute-Respiratory-Distress-Syndrome-ARDS-And-Ventilator-Induced-Lung-Injury-VILI.
- 120.Mount Sinai and Harbour BioMed Collaborate to Advance Novel Biotherapies for the Treatment of Cancer and Coronavirus COVID-19. [6 March 2020]; Available from: http://www.harbourbiomed.com/en/newsContent.html#135.
- 121.Nascent biotech investigation drug product, pritumumab, to be studied as a potential therapeutic agent against (coronavirus) COVID-19. [3 March 2020]; Available from: https://apnews.com/6ff39d8992ddd98d4422acc9b5e4b9c2.
- 122.Three Additional Patients with Severe COVID-19 Treated with Leronlimab in New York Medical Center Bringing the Total to 10 Patients. [30 March 2020]; Available from: https://www.cytodyn.com/newsroom/press-releases/detail/401/three-additional-patients-with-severe-covid-19-treated-with.
- 123.InflaRx Doses First Patient in Multicenter Randomized Clinical Trial in Severe Progressed COVID-19 Pneumonia in Europe upon Receipt of Initial Positive Human Data with InflaRx’s anti-C5a Technology. [31 March 2020]; Available from: https://www.inflarx.de/Home/Investors/Press-Releases/03-2020-InflaRx-Doses-First-Patient-in-Multicenter-Randomized-Clinical-Trial-in-Severe-Progressed-COVID-19-Pneumonia-in-Europe-upon-Receipt-of-Initial-Positive-Human-Data-with-InflaRx-s-anti-C5a-Technology.html.
- 124.Amgen And Adaptive Biotechnologies Announce Strategic Partnership To Develop A Therapeutic To Prevent Or Treat COVID-19. Available from: https://investors.adaptivebiotech.com/news-releases/news-release-details/amgen-and-adaptive-biotechnologies-announce-strategic.
- 125.Identification and Characterization of a Potential Therapeutic COVID-19 Antibody by Vir Biotechnology Published in Nature [May 18, 2020]; Available from: https://investors.vir.bio/node/6981/pdf.
- 126.SAB Biotherapeutics: rapidly responding to COVID-19. Available from: https://sabbiotherapeutics.com/covid-19/.
- 127.ImmunoPrecise Provides Updates on B Cell Select™ and Deep Display™ Programs for SARS-CoV-2 Antibody Discovery. [April 17,2020]; Available from: https://www.immunoprecise.com/immunoprecise-provides-updates-on-b-cell-select-and-deep-display-programs-for-sars-cov-2-antibody-discovery/.
- 128.Emergent BioSolutions Initiates Development of Plasma-Derived Product Candidates for the Treatment and Prevention of Coronavirus Disease. [11 March 2020]; Available from: https://investors.emergentbiosolutions.com/node/19256/pdf.
- 129.GigaGen Initiates Development of Recombinant Polyclonal Antibody Therapy for COVID-19. [30 March 2020]; Available from: https://www.globenewswire.com/news-release/2020/03/30/2008438/0/en/GigaGen-Initiates-Development-of-Recombinant-Polyclonal-Antibody-Therapy-for-COVID-19.html.
- 130.NuGenerex. Ii-Key-nCOV Peptide Vaccine.Overview. [19 March 2020]; Available from: https://storage.googleapis.com/wzukusers/user-26831283/documents/5e73891471930g8BqVxh/Ii-Key-nCOV%20Peptide%20Vaccine.Overview.3%2019%2020.pdf.
- 131.Bekerman E., et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Invest. 2017;127(4):1338–1352. doi: 10.1172/JCI89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang W., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020 doi: 10.1016/j.clim.2020.108393. 108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? The Lancet. 2020;395(10230) doi: 10.1016/S0140-6736(20)30691-7. P1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sorrell F.J., et al. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24(3):401–411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pu S.Y., et al. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antiviral Res. 2018;155:67–75. doi: 10.1016/j.antiviral.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stebbing J., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Praveen D., Chowdary P.R., Aanandhi M.V. Baricitinib-a januase kinase inhibitor-not an ideal option for management of covid 19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Favalli E.G., et al. Baricitinib for COVID-19: a suitable treatment? Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim J., et al. Advanced bioinformatics rapidly identifies existing therapeutics for patients with coronavirus disease-2019 (COVID-19) J. Translational Med. 2020;18:257. doi: 10.1186/s12967-020-02430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Owen C., et al. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr. Oncol. 2019;26(2) doi: 10.3747/co.26.4345. e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar S. COVID-19: A drug repurposing and biomarker identification by using comprehensive gene-disease associations through protein-protein interaction network analysis. Preprints. 2020 doi: 10.20944/preprints202003.0440.v1. 2020030440. [DOI] [Google Scholar]
- 142.Y.F. Zheng, S.A. Liu, Prevent COVID-19 Severity by Repurposing mTOR Inhibitors. 2020.Preprints. doi:10.20944/preprints202004.0060.v1.
- 143.Wang C.-H., et al. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit. Care Med. 2014;42(2):313–321. doi: 10.1097/CCM.0b013e3182a2727d. [DOI] [PubMed] [Google Scholar]
- 144.Zhou Y., et al. Network-based drug repurposing for human coronavirus. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barretto N., et al. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 2005;79(24):15189–15198. doi: 10.1128/JVI.79.24.15189-15198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen X., Chou C.-Y., Chang G.-G. Thiopurine analogue inhibitors of severe acute respiratory syndrome-coronavirus papain-like protease, a deubiquitinating and deISGylating enzyme. Antiviral Chem. Chemother. 2009;19(4):151–156. doi: 10.1177/095632020901900402. [DOI] [PubMed] [Google Scholar]
- 147.Russell B., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.AlGhamdi M., et al. MERS CoV infection in two renal transplant recipients: case report. Am. J. Transplant. 2015;15(4):1101–1104. doi: 10.1111/ajt.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carbajo-Lozoya J., et al. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marzo T., Messori L. A role for metal-based drugs in fighting Covid-19 infection? the case of auranofin. ACS Med. Chem. Lett. 2020;11(6):1067–1068. doi: 10.1021/acsmedchemlett.0c00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Savarino A., Shytaj I.L. Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology. 2015;12(1):51. doi: 10.1186/s12977-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim N.H., et al. Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology. 2007;122(4):607–614. doi: 10.1111/j.1365-2567.2007.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rothan H.A., et al. The FDA-approved gold drug Auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology. 2020;547:7–11. doi: 10.1016/j.virol.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tobinick E. TNF-[alpha] inhibition for potential therapeutic modulation of SARS coronavirus infection. Curr. Med. Res. Opin. 2004;20(1):39. doi: 10.1185/030079903125002757. [DOI] [PubMed] [Google Scholar]
- 155.Zhu H., et al. Anti-inflammatory effect of thalidomide on H1N1 influenza virus-induced pulmonary injury in mice. Inflammation. 2014;37(6):2091–2098. doi: 10.1007/s10753-014-9943-9. [DOI] [PubMed] [Google Scholar]
- 156.Chen G.-Y., et al. CD24 and Siglec-10 selectively repress tissue damage–induced immune responses. Science. 2009;323(5922):1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tian R.-R., et al. CD24 and Fc fusion protein protects SIVmac239-infected Chinese rhesus macaque against progression to AIDS. Antiviral Res. 2018;157:9–17. doi: 10.1016/j.antiviral.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 158.Cruz-Topete D., Cidlowski J.A. One hormone, two actions: anti-and pro-inflammatory effects of glucocorticoids. NeuroImmunoModulation. 2015;22(1–2):20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang Z., et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J. Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen R.C., et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.W. Alhazzani, et al., Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive Care Med., 2020, 1–34. [DOI] [PMC free article] [PubMed]
- 163.Xiao J., et al. Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi. 2004;43(3):179–182. [PubMed] [Google Scholar]
- 164.Li Y., et al. Factors of avascular necrosis of femoral head and osteoporosis in SARS patients' convalescence. Zhonghua Yi Xue Za Zhi. 2004;84:1348. [PubMed] [Google Scholar]
- 165.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qin Y., et al. Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial. Chin. Med. J. 2020;133(9):1080–1086. doi: 10.1097/CM9.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fang X., et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-1. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 169.WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients. [cited 2020 29 Jun]; Available from: https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients.
- 170.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. [cited 2020 29 Jun]; Available from: https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 171.Horby P., et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19. Preliminary Report. medRxiv. 2020 doi: 10.1101/2020.06.22.20137273. [DOI] [Google Scholar]
- 172.S.U. Khan, T.T. Htar, Deciphering the binding mechanism of Dexamethasone against SARS-CoV-2 Main Protease: Computational molecular modelling approach. ChemRxiv. 2020. Preprint. doi:10.26434/chemrxiv.12517535.v1.
- 173.Shang L., et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.