Abstract
Background
Coronavirus disease 2019 (COVID-19) is currently spreading worldwide. This study examined whether serum Krebs von den Lungen-6 (KL-6) level is a useful biomarker for evaluating the severity of COVID-19.
Methods
We retrospectively examined patients diagnosed with COVID-19 at the Japanese Red Cross Medical Center between February 1, 2020, and May 15, 2020. Patients were divided into four categories based on clinical and radiological findings: mild, moderate, severe, and critical. Patients who presented with a mild or moderate illness and patients who started with or worsened to a severe or critical illness were classified as the non-severe and severe groups, respectively. The two groups were compared for patient characteristics, including serum KL-6 levels. Receiver operating characteristic curves were used to define the optimum cut-off value of serum KL-6 level to evaluate COVID-19 severity.
Results
A total of 54 patients were enrolled, including 33 in the non-severe group and 21 in the severe group, of which four died. Compared with those in the non-severe group, more patients in the severe group were significantly older and had comorbidities. Serum KL-6 levels were significantly higher in the severe group than in the non-severe group both at diagnosis (median, 338 U/mL) and at peak levels within one week after diagnosis (median, 781 U/mL) (both p < 0.001). Serum KL-6 value at peak level (371 U/mL) was used as the optimal cut-off to evaluate disease severity (sensitivity, 85.7%; specificity, 96.6%).
Conclusions
Serum KL-6 levels were significantly elevated in severe COVID-19 and is useful for evaluating its severity.
Keywords: Biomarker, Coronavirus disease 2019, Krebs von den Lungen-6, Pneumonia, Viral infection
List of Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; NIH, National Institutes of Health; PCR, polymerase chain reaction; ROC, receiver operating characteristic; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; sIL2-R, soluble interleukin-2-receptor; SpO2, saturation of percutaneous oxygen; WBC, white blood cell; WHO, World Health Organization
1. Introduction
Coronavirus disease 2019 (COVID-19), an infectious disease caused by a new type of coronavirus, which emerged at the end of 2019, and has spread worldwide [1]. In Japan, there have been more than 16,000 cases of infections and 800 deaths toward the end of May 2020 [2]. The World Health Organization (WHO) declared COVID-19 as a pandemic on January 30, 2020. Despite many infection prevention strategies and countermeasures undertaken in each country, the pandemic has not been controlled so far.
The common symptoms of COVID-19 are fever, cough, and malaise, which ranges from mild to severe. According to a meta-analysis from China, abnormalities in chest computed tomography (CT) were observed in 96.6% of COVID-19 cases, and the percentage of severe cases was 18.1% [3]. Severe illness is more likely to occur in elderly people and in patients with comorbidities, such as diabetes or chronic cardiovascular disease [4]. Some studies have shown that serum markers, such as ferritin, soluble interleukin-2-receptor (sIL2-R), and lactate dehydrogenase (LDH), are elevated in severe COVID-19 patients and are useful for predicting disease progression [[5], [6], [7], [8], [9]]. However, all of these were small-scale studies. Furthermore, an optimal biomarker to assess COVID-19 severity has not been established. Therefore, further development of biomarkers is urgently required to identify the severity and to predict the progression of COVID-19.
In severe cases of COVID-19 pneumonia, ground-glass opacity and consolidation spreads entirely over the bilateral lungs, resulting in acute respiratory distress syndrome (ARDS) [10]. ARDS causes diffuse alveolar damage characterized by injury and detachment of the alveolar epithelial cells, hyaline membrane formation, and neutrophil infiltration [11]. Krebs von den Lungen-6 (KL-6), a glycoprotein secreted by type II alveolar pneumocytes and bronchiolar epithelial cells, is a useful biomarker for alveolar epithelial proliferation and injury [12]. Serum KL-6 levels are elevated in patients with various respiratory diseases, such as ARDS [13], interstitial lung diseases (ILDs), idiopathic pulmonary fibrosis, hypersensitivity pneumonitis, and collagen vascular disease–associated interstitial pneumonitis [12]. It is plausible that serum KL-6 levels are elevated in severe COVID-19 pneumonia with ARDS, but this has not yet been investigated.
We conducted a retrospective observational study in COVID-19 patients. The purpose of this study was to investigate whether serum KL-6 level is a useful biomarker to evaluate the severity of COVID-19.
2. Patients and methods
2.1. Eligibility criteria
We retrospectively assessed all consecutive adult patients diagnosed with COVID-19 at the Japanese Red Cross Medical Center, Tokyo, Japan, between February 1, 2020, and May 15, 2020. COVID-19 diagnosis was confirmed by polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from sputum or nasopharyngeal swab; patients with positive results were enrolled. SARS-CoV-2 RNA was detected using TaqMan One-Step RT-PCR Kits (QIAGEN, Co., Ltd, Hilden, Germany). Patients with other pathogens, such as bacteria, fungi, and other respiratory viruses, were excluded. A total of 54 patients were eventually enrolled.
2.2. Clinical and radiological analyses
Age, sex, race, body mass index, smoking history, comorbidities, regular medications, body temperature at diagnosis, saturation of percutaneous oxygen (SpO2), laboratory data, treatment received, use of mechanical ventilation and extracorporeal membrane oxygenation (ECMO), and outcome were collected from electronic medical records. We also extracted information on comorbidities, such as diabetes, cardiovascular disease, hypertension, hemodialysis, cancer, chronic obstructive pulmonary disease, and ILD. Laboratory data included white blood cell (WBC) count, lymphocyte count, eosinophil count, LDH, C-reactive protein (CRP), ferritin, sIL2-R, KL-6, and D-dimer at diagnosis, and peak levels of LDH, ferritin, sIL2-R, KL-6, and D-dimer within one week after diagnosis. Serum levels of KL-6 were measured using the Nanopia KL-6 Reagent kit (Sekisui Medical Co., Ltd, Tokyo, Japan). Clinical outcomes were monitored up to May 31, 2020. We classified COVID-19 patients into four categories according to the National Institutes of Health (NIH) classification criteria: 1) mild illness, patients who had any of the various signs and symptoms of COVID-19 without shortness of breath, dyspnea, or abnormal chest imaging; 2) moderate illness, patients who had evidence of lower respiratory disease on clinical assessment or imaging and SpO2 ≥ 94% on room air at sea level; 3) severe illness, patients who had a respiratory rate of >30 breaths per minute, SpO2 < 94% on room air at sea level, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen < 300 mmHg, or lung infiltrates > 50%; and 4) critical illness, patients who had respiratory failure, septic shock, and/or multiple organ dysfunction [14]. The WHO interim guidelines was used to define ARDS [15]. The number of days between symptom onset and the worst severity during clinical course was recorded, as was the number of days between diagnosis and the worst severity.
Two skilled pulmonologists (N.A. and M.I.) evaluated the chest CT images and combined their individual results. Chest high–resolution CT (HRCT) scan was performed at the time of diagnosis, eight days after diagnosis, and when the respiratory condition changed. HRCT images were reconstructed with 1.0–2.0 mm collimation and 10–20 mm slice intervals.
2.3. Statistical analysis
Results for continuous variables are shown as median with interquartile ranges. Patients who presented with a mild or moderate illness and patients whose condition deteriorated to a severe or critical illness were classified as the non-severe and the severe groups, respectively. To compare the two groups, a Fisher's exact test was used for categorical variables and a Mann–Whitney U test was used for continuous variables. Receiver operating characteristic (ROC) curve analyses were used to assess the serum biomarker levels for evaluating COVID-19 severity and obtain the optimum cut-off values. The Youden index was used to determine the optimal cut-off values. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). We used a modified version of the R commander, which has been designed to add statistical functions frequently used in biostatistics. A two-tailed p-value < 0.05 was considered statistically significant.
2.4. Ethics statement
This study was approved by the Ethical Committee for Clinical Studies, Japanese Red Cross Medical Center (No. 1112; April 15, 2020). As this was a retrospective analysis, informed consent was not obtained individually, but an opt-out method based on clinical practice guidelines in Japan was used.
3. Results
3.1. Patient characteristics
A total of 54 patients were enrolled, with 33 in the non-severe group (four mild, 29 moderate) and 21 in the severe group (11 severe, 10 critical) (Table 1 ). The median time from onset of symptoms or diagnosis to the worst severity was eight days and one day, respectively. The median age of patients was 46 years and 38 (70.4%) patients were men. Fifty-one patients (94.4%) were Japanese. There was no difference in the median time from diagnosis to the worst severity between the non-severe and severe groups. However, COVID-19 severity worsened in nine patients after three or more days in the severe group, whereas in the non-severe group, the disease severity worsened from mild to moderate in only two patients after two days. Compared with those in the non-severe group, patients in the severe group were significantly older and more patients had comorbidities, such as diabetes and hypertension. Although many drugs were administrated to patients in the severe group, hydroxychloroquine, favipiravir, steroid, and tocilizumab were used significantly more in this group than in the non-severe group. Of the five patients who received steroids, three died. All ten patients who were classified as having critical illness developed ARDS. All of them required mechanical ventilation, and two were treated with ECMO; however, four patients died. Laboratory findings showed significantly lower lymphocytes and eosinophil counts in the severe group than in the non-severe group (Table 2 ). CRP level was significantly higher in the severe group than in the non-severe group. LDH, ferritin, sIL2-R, and D-dimer levels were significantly higher in the severe group than in the non-severe group both at diagnosis and at peak levels (within one week after diagnosis). Similarly, serum KL-6 levels were significantly higher in the severe group than in the non-severe group both at diagnosis (median, 338 U/mL) and at peak levels within one week after diagnosis (median, 781 U/mL) (both p < 0.001). Furthermore, the increase in serum KL-6 level (Δ KL-6) was significantly greater in the severe group (median, 404 U/mL) than in the non-severe group (median, 0 U/mL) (p < 0.001).
Table 1.
COVID-19 patient characteristics and comparisons between the non-severe and the severe groups.
| All patients (n = 54) | Non-severe group (n = 33) (Mild: n = 4; Moderate: n = 29) |
Severe group (n = 21) (Severe: n = 11; Critical: n = 10) |
p-value | |
|---|---|---|---|---|
| Number of days between symptom onset and the worst severity | 8 (4.3–10.8) | 8 (4–11) | 8 (5–10) | 0.55 |
| Number of days between diagnosis and the worst severity | 1 (1–1.8) | 1 (1–1) | 1 (1–4) | <0.001 |
| Age, years | 46 (34–66) | 40 (33–50) | 64 (56–78) | <0.001 |
| Men, n (%) | 38 (70.4) | 23 (69.7) | 15 (71.4) | 1 |
| Race | 0.27 | |||
| Japanese, n (%) | 51 (94.4) | 30 (90.9) | 21 (100) | |
| Caucasian, n (%) | 3 (5.6) | 3 (9.1) | 0 (0) | |
| Body mass index, kg/m2 | 23.0 (20.7–25.7) | 22.1 (20.5–24.2) | 24.4 (21.7–27.0) | 0.095 |
| Smoking history | 0.78 | |||
| Smoker, n (%) | 29 (53.7) | 17 (51.5) | 12 (57.1) | |
| Non-smoker, n (%) | 25 (46.3) | 16 (48.5) | 9 (42.9) | |
| Comorbidities, n | 0 (0–1) | 0 (0–0) | 1 (0–2) | <0.001 |
| Diabetes, n (%) | 11 (20.4) | 1 (3.0) | 10 (47.6) | <0.001 |
| Cardiovascular disease, n (%) | 5 (9.3) | 1 (3.0) | 4 (19.0) | 0.069 |
| Hypertension, n (%) | 11 (20.4) | 1 (3.0) | 10 (47.6) | <0.001 |
| Hemodialysis, n (%) | 6 (11.1) | 2 (6.1) | 4 (19.0) | 0.19 |
| Cancer, n (%) | 2 (3.7) | 1 (3.0) | 1 (4.8) | 1 |
| COPD, n (%) | 2 (3.7) | 2 (6.1) | 0 (0) | 0.52 |
| ILD, n (%) | 2 (3.7) | 2 (6.1) | 0 (0) | 0.52 |
| Regular medications | ||||
| ACEI and/or ARB, n (%) | 5 (9.3) | 1 (3.0) | 4 (19.0) | 0.069 |
| Anti-cancer agent, n (%) | 1 (1.9) | 1 (3.0) | 0 (0) | 1 |
| Body temperature, °Celsius | 37.6 (36.7–38.6) | 37.6 (36.8–38.5) | 37.6 (36.5–38.7) | 0.49 |
| Treatment | ||||
| No antiviral drugs | 24 (44.4) | 22 (66.7) | 2 (9.5) | <0.001 |
| Lopinavir-Ritonavir, n (%) | 2 (3.7) | 1 (3.0) | 1 (4.8) | 1 |
| Hydroxychloroquine, n (%) | 21 (38.9) | 7 (21.2) | 14 (66.7) | 0.001 |
| Favipiravir, n (%) | 26 (48.1) | 8 (24.2) | 18 (85.7) | <0.001 |
| Steroid, n (%) | 5 (9.3) | 0 (0) | 5 (23.8) | 0.006 |
| Tocilizumab, n (%) | 12 (22.2) | 0 (0) | 12 (57.1) | <0.001 |
| Mechanical ventilation, n (%) | 10 (18.5) | 0 (0) | 10 (47.6) | <0.001 |
| ECMO, n (%) | 2 (3.7) | 0 (0) | 2 (9.5) | 0.15 |
| Death, n (%) | 4 (7.4) | 0 (0) | 4 (19.0) | 0.019 |
Continuous variables are shown as medians (interquartile ranges).
COVID-19: Coronavirus disease 2019, COPD: chronic obstructive pulmonary disease, ILD: interstitial lung disease, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin II receptor blocker, ECMO: extracorporeal membrane oxygenation.
Fisher's exact tests were performed on categorical variables and Mann–Whitney U tests were used to compare continuous variables between the non-severe and the severe groups.
Table 2.
Laboratory findings of COVID-19 patients and comparisons between the non-severe and the severe groups.
| All patients (n = 54) | Non-severe group (n = 33) (Mild: n = 4; Moderate: n = 29) |
Severe group (n = 21) (Severe: n = 11; Critical: n = 10) |
p-value | |
|---|---|---|---|---|
| At diagnosis | ||||
| WBC,/μL | 5420 (4173–6730) | 5140 (3940–6570) | 5740 (4210–8290) | 0.25 |
| Lymphocyte,/μL | 1125 (703–1393) | 1280 (890–1460) | 820 (520–1190) | <0.001 |
| Eosinophil,/μL | 10 (0–50) | 20 (0–60) | 0 (0–20) | 0.007 |
| LDH, U/L | 258 (190–355) | 208 (169–275) | 356 (293–480) | <0.001 |
| CRP, mg/dL | 3.5 (1.1–7.4) | 1.9 (0.7–4.0) | 8.8 (4.8–13.6) | <0.001 |
| Ferritin, ng/mL | 369 (190–771) | 305 (157–438) | 819 (476–1318) | <0.001 |
| sIL2-R, U/mL | 693 (506–1123) | 616 (459–734) | 1152 (715–1773) | <0.001 |
| KL-6, U/mL | 229 (184–336) | 223 (166–255) | 338 (303–529) | <0.001 |
| D-dimer, μg/mL | 0.9 (0.1–2.2) | 0.9 (0–1.1) | 2.7 (0.6–7.6) | 0.004 |
| Peak levels within one week after diagnosis | ||||
| LDH, U/L | 307 (219–449) | 243 (173–313) | 479 (356–700) | <0.001 |
| Ferritin, ng/mL | 517 (321–1324) | 344 (167–533) | 1326 (785–2147) | <0.001 |
| sIL2-R, U/mL | 886 (583–1232) | 664 (500–869) | 1431 (1126–1963) | <0.001 |
| KL-6, U/mL | 283 (222–540) | 234 (194–282) | 781 (429–1435) | <0.001 |
| D-dimer, μg/mL | 1.9 (0.9–8.2) | 1.6 (0.6–1.7) | 10 (4.9–21.2) | <0.001 |
| Differences between serum biomarker levels at diagnosis and peak levels within one week after diagnosis | ||||
| Δ LDH, U/L | 6.5 (0–73) | 0 (0–28) | 70 (0–169) | 0.001 |
| Δ Ferritin, ng/mL | 33 (0–209) | 18 (0–58) | 152 (0–686) | 0.011 |
| Δ sIL2-R, U/mL | 20 (0–257) | 0 (0–40) | 243 (0–438) | <0.001 |
| Δ KL-6, U/mL | 29 (0–110) | 0 (0–28) | 404 (89–634) | <0.001 |
| Δ D-dimer, μg/mL | 0.7 (0.5–3.8) | 0.6 (0.5–0.7) | 4.2 (0.5–14.3) | 0.003 |
Data are shown as medians (interquartile ranges).
COVID-19: Coronavirus disease 2019, WBC: white blood cell, LDH: lactate dehydrogenase, CRP: C-reactive protein, sIL2R: soluble interleukin-2-receptor, KL-6: Krebs von den Lungen-6.
Mann–Whitney U tests were used to compare the non-severe and the severe group.
3.2. Predictive value of serum biomarker levels for evaluating disease severity
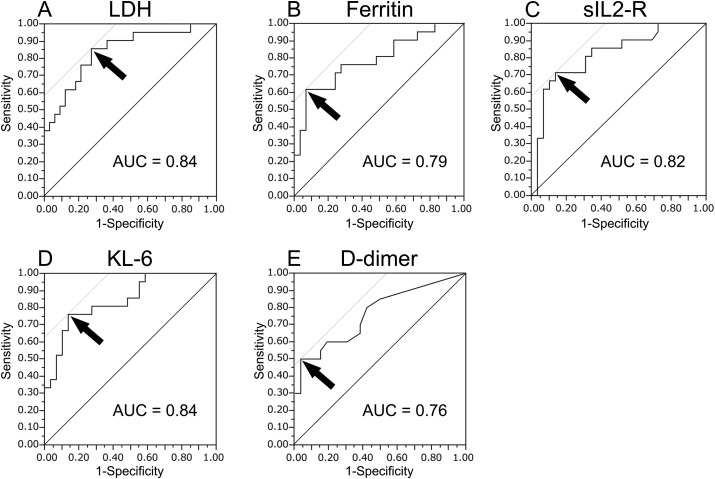
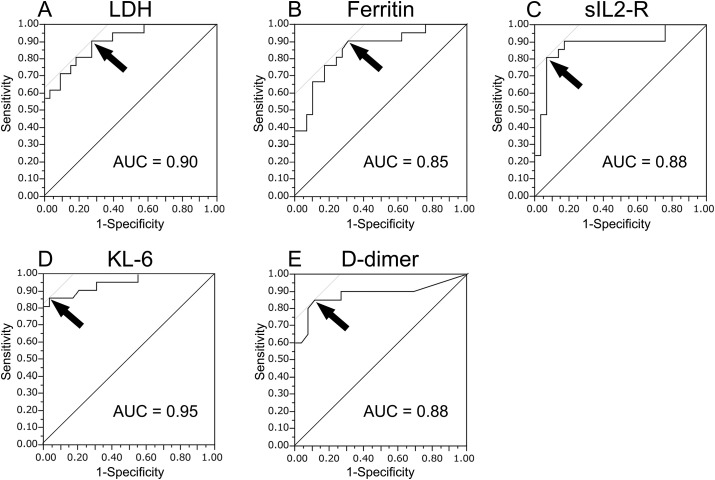
ROC curve analyses were performed using the serum biomarker levels at diagnosis (Fig. 1 ) and at their peak levels within one week after diagnosis (Fig. 2 ) to obtain the optimal cut-off values to assess the severity of COVID-19. The optimal cut-off values of LDH, ferritin, sIL2-R, KL-6, and D-dimer levels at diagnosis were 265 U/L, 770 ng/mL, 929 U/mL, 303 U/mL, and 2.7 μg/mL, respectively. At the respective cut-off values, serum KL-6 level had a sensitivity of 76.2% and a specificity of 86.2% for evaluating disease severity (area under the curve [AUC] = 0.84). The optimal cut-off values of LDH, ferritin, sIL2-R, KL-6, and D-dimer at their peak levels within one week after diagnosis were 305 U/L, 533 ng/mL, 1093 U/mL, 371 U/mL, and 3.8 μg/mL, respectively. At the respective cut-off values, serum KL-6 level had a sensitivity of 85.7% and a specificity of 96.6% for evaluating disease severity (AUC = 0.95).
Fig. 1.
Receiver operating characteristic curve analyses of serum biomarker levels at diagnosis. The curves show the power of LDH (A), ferritin (B), sIL2-R (C), KL-6 (D), and D-dimer (E) levels for evaluating COVID-19 severity, respectively. The arrows indicate the cut-off values. The optimal cut-off values of LDH, ferritin, sIL2-R, KL-6, and D-dimer levels at diagnosis were 265 U/L, 770 ng/mL, 929 U/mL, 303 U/mL, and 2.7 μg/mL, respectively. At the cut-off values, serum KL-6 level had a sensitivity of 76.2% and a specificity of 86.2% in evaluating disease severity. LDH: lactate dehydrogenase, sIL2-R: soluble interleukin-2-receptor, KL-6: Krebs von den Lungen-6, AUC: area under the curve.
Fig. 2.
Receiver operating characteristic curve analyses of serum biomarker at the peak levels within one week after diagnosis. The curves show the power of LDH (A), ferritin (B), sIL2-R (C), KL-6 (D), and D-dimer (E) levels for evaluating COVID-19 severity, respectively. The arrows indicate the cut-off values. The optimal cut-off values of LDH, ferritin, sIL2-R, KL-6, and D-dimer at the peak levels within one week after diagnosis were 305 U/L, 533 ng/mL, 1093 U/mL, 371 U/mL, and 3.8 μg/mL, respectively. At the cut-off value, serum KL-6 level had a sensitivity of 85.7% and a specificity of 96.6% in evaluating disease severity. LDH: lactate dehydrogenase, sIL2-R: soluble interleukin-2-receptor, KL-6: Krebs von den Lungen-6, AUC: area under the curve.
4. Discussion
We investigated the relationship between serum KL-6 levels and COVID-19 severity. Since the mortality rate of patients with severe and critical COVID-19 is high, assessing severity is important. To our knowledge, no previous study has discussed the usefulness of serum KL-6 levels for assessing severity of COVID-19. Our study found that serum KL-6 was a useful biomarker for severity of COVID-19.
In our study, 21 of the 54 enrolled patients were stratified to the severe group either on admission or during their treatment. Their characteristics, such as old age, presence of many comorbidities, and abnormal laboratory findings, were consistent with those reported by previous studies [[5], [6], [7], [8], [9],16]. Additionally, according to previous meta-analyses, ARDS develops in 14.8%–32.8% of COVID-19 patients and the all-cause mortality is 28.8%–52.4% in COVID-19 patients with ARDS [3,[17], [18], [19]]. In our study, 10 patients developed ARDS and four died; this prognosis was similar to that reported in previous studies. Therapeutic agents, such as hydroxychloroquine, favipiravir, and tocilizumab, were used more frequently for patients in the severe group than for those in the non-severe group. In the early stages of the COVID-19 pandemic, these drugs were expected to be effective, but none of them have been proven effective to date. In contrast, methylprednisolone has been used for ARDS and its safety and efficacy has been reported [20]. In our study, steroids were administered to five COVID-19 patients with ARDS, but three of them died. The efficacy of steroids cannot be discussed because of the small number of cases. Recently, a retrospective cohort study revealed that steroid administration significantly reduced the risk of death in COVID-19 patients with ARDS (hazard ratio, 0.38) [18]. In addition, the United Kingdom reported that dexamethasone reduced mortality in patients with severe COVID-19 [21]. The American Thoracic Society's guidelines on COVID-19 makes no recommendation either for or against treatment with systemic corticosteroids owing to the risk of increased viral replication and prolonged viral shedding [22]. Further studies are thus needed on effective treatments for severe COVID-19.
There are several reports on how to classify the severity of COVID-19 and some of them classify patients into mild, moderate, severe, and critical illness [14,23], but no criteria have yet been adopted as a standard classification. In the present study, we used the NIH criteria, which is concise and globally prevalent. Similarly, there are no established standards for identifying the risk factors for COVID-19 progression. Ji et al. proposed a risk factor scoring system called the CALL score, based on age, comorbidities, lymphocyte counts, and serum LDH levels [9] and Gong et al. developed a prognostic nomogram [16]. Consistent with these reports, in our study, patients in the severe group had significantly higher LDH and CRP levels and lower lymphocyte counts than those in the non-severe group. While these models may help to predict disease progression, there is a high risk of overfitting because of the small sample size in these studies. Furthermore, because of worldwide variation in factors, such as the rate of spread of infection; the number of hospital beds, including intensive care unit beds; and the number of available health care workers, the mortality rates differ greatly even among patients with the same severity and risk of progression.
It may be difficult to create a global severity classification and an algorithm to predict progression. However, since it is so important to accurately evaluate severity, we investigated the usefulness of serum KL-6, which is known as a biomarker of lung injury. High serum levels of KL-6 correlate with the presence and severity of ILDs and ARDS [[24], [25], [26], [27]], indicating the extent of damaged alveolar epithelium and alveolar capillary permeability [12]. Our results showed that serum KL-6 levels both at diagnosis and at peak levels were significantly higher in patients in the severe group than those in the non-severe group, with a high sensitivity and specificity. Of the AUC values of serum biomarkers, those of serum KL-6 levels were the highest both at diagnosis and at peak levels within one week after diagnosis, indicating that it was particularly useful for assessing disease severity. It is necessary to closely monitor patients with high serum KL-6 levels and provide aggressive treatments. Additionally, since serum KL-6 level is a useful prognostic marker for patients with ILDs and ARDS [13,27,28], it may also be useful for predicting the prognosis of COVID-19 patients. Although the sensitivity and specificity were slightly lower, the evaluation of serum KL-6 levels at diagnosis than at the peak level was considered more important for assessing prognosis and considering therapeutic interventions.
In our study, the peak serum KL-6 levels occurred within one week after diagnosis in patients in the severe group were remarkably increased (median, 781 U/mL). However, the cut-off value of serum KL-6 level for evaluating COVID-19 severity was slightly lower than the threshold value for distinguishing healthy people from those with ILD or evaluating the activity of other ILDs [12,29]. This is probably because the maximum diameter of KL-6 is large (200 nm or more) and it takes some time for serum KL-6 levels to increase after disruption of alveolar capillary permeability [12]. In severe COVID-19 patients, serum KL-6 levels could be further elevated even after one week of diagnosis. Therefore, repeated assessment of serum biomarkers, including KL-6 and respiratory status, is important.
This study had several limitations. First, it was a single-center retrospective study with a small number of patients. In addition, there were correlations and collinearities in the serum biomarkers, which were candidates for prognostic factors. As a result, we could not perform multivariate analyses and assess the precise relationship between the serum KL-6 levels and COVID-19 severity. Second, we used various therapeutic agents after diagnosis of COVID-19, some at a significantly different frequency in the severe and non-severe groups. Although no drug has been established to be effective against COVID-19, it is possible that the drugs we used had an impact on disease progression.
5. Conclusions
In conclusion, this study demonstrated that serum KL-6 levels were high in severe COVID-19 patients and useful for evaluating disease severity. Patients with high serum KL-6 levels have high disease severity and require close monitoring and aggressive treatments.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japanese Ministry of Health, Labour and Welfare . 2020. About coronavirus Disease 2019 (COVID-19)https://www.mhlw.go.jp/english/ accessed. [Google Scholar]
- 3.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92:612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate coronavirus Disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y., Zhang Z., Tian J., Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann Cardiothorac Surg. 2020;9:428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 8.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheum. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji D., Zhang D., Xu J., Chen Z., Yang T., Zhao P. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;9 doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan F., Ye T., Sun P., Gui S., Liang B., Li L. Time course of lung changes at chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro C.Y. ARDS and diffuse alveolar damage: a pathologist's perspective. Semin Thorac Cardiovasc Surg. 2006;18:13–19. doi: 10.1053/j.semtcvs.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa N., Hattori N., Yokoyama A., Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50:3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Ishizaka A., Matsuda T., Albertine K.H., Koh H., Tasaka S., Hasegawa N. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health . 2020. Management of persons with COVID-19.https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ accessed. [Google Scholar]
- 15.World Health Organization . 2020. Clinical management of severe acute respiratory infection when COVID-19 is suspected: interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected accessed. [Google Scholar]
- 16.Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L. A tool to early predict severe corona virus Disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71:833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X., Du R., Wang R., Cao T.Z., Guan L.L., Yang C.Q. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032. S0012–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Meduri, Golden E., Freire A.X., Taylor E., Zaman M., Carson S.J. Affiliations expand. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 21.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19 — preliminary report. N Engl J Med. 2020;NEJMoa2021436 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Thoracic Society . 2020. COVID-19: interim guidance on management pending empirical evidence from an American Thoracic Society-led International Task Force.https://www.thoracic.org/covid/covid-19-guidance.pdf accessed. [Google Scholar]
- 23.General Office of National Health Committee, Office of State Administration of Traditional Chinese Medicine . 2020. 2019–nCoV. Notice on the issuance of a programme for the diagnosis and treatment of novel coronavirus pneumonia.http://yzs.satcm.gov.cn/zhengcewenjian/2020-02-19/13221.html trial sixth edition. accessed. [Google Scholar]
- 24.Yanaba K., Hasegawa M., Hamaguchi Y., Fujimoto M., Takehara K., Sato S. Longitudinal analysis of serum KL-6 levels in patients with systemic sclerosis: association with the activity of pulmonary fibrosis. Clin Exp Rheumatol. 2003;21:429–436. [PubMed] [Google Scholar]
- 25.Bandoh S., Fujita J., Ohtsuki Y., Ueda Y., Hojo S., Tokuda M. Sequential changes of KL-6 in sera of patients with interstitial pneumonia associated with polymyositis/dermatomyositis. Ann Rheum Dis. 2000;59:257–262. doi: 10.1136/ard.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukaya S., Oshima H., Kato K., Komatsu Y., Matsumura H., Ishii K. KL-6 as a novel marker for activities of interstitial pneumonia in connective tissue diseases. Rheumatol Int. 2000;19:223–225. doi: 10.1007/s002960000064. [DOI] [PubMed] [Google Scholar]
- 27.Sato H., Callister M.E., Mumby S., Quinlan G.J., Welsh K.I., duBois R.M. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur Respir J. 2004;23:142–145. doi: 10.1183/09031936.03.00070303. [DOI] [PubMed] [Google Scholar]
- 28.Satoh H., Kurishima K., Ishikawa H., Ohtsuka M. Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med. 2006;260:429–434. doi: 10.1111/j.1365-2796.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y., Luo Q., Han Q., Huang J., Ou Y., Chen M. Sequential changes of serum KL-6 predict the progression of interstitial lung disease. J Thorac Dis. 2018;10:4705–4714. doi: 10.21037/jtd.2018.07.76. [DOI] [PMC free article] [PubMed] [Google Scholar]