Abstract
Testing for SARS-CoV-2 has attracted a tremendous amount of attention as a tool to manage the ongoing COVID-19 pandemic. Although diagnostic laboratory testing is used ubiquitously by physicians and encountered regularly by individuals receiving medical care, several aspects of test interpretation are incompletely understood by medical communities and the general population, creating a significant challenge in minimizing the damage caused by disease spread through informed decision making and proper testing utilization. Here, general principles of test interpretation are reviewed and applied to specific examples, such as whether asymptomatic individuals should be tested, what it means to test positive (or negative), and how to interpret tests for “immunity passports.” Unexpectedly, the answers seem to run contrary to many of the popular narratives about testing as a tool for managing COVID-19. Although testing is an important and essential part of managing diseases such as COVID-19, improper utilization can have unintended negative consequences.
Stites and Wilen discuss the interpretation of COVID-19 testing, highlighting probabilistic interpretation with timely examples. As COVID-19 testing is rapidly changing and as information on test performance is still being acquired, this perspective highlights an approach that can adapt to new information and to new testing environments.
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic that is caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has presented a massive public health crisis.1 Issues such as quarantines and stay-at-home orders, critical equipment shortages, healthcare provider shortages, vaccines, experimental therapeutics, and the community use of masks are but some of the issues that have become major topics of discussion. In addition, laboratory diagnostic testing has received a tremendous amount of attention. This article focuses on the statistical interpretation of laboratory diagnostic tests for COVID-19, but these concepts are broadly applicable to other diagnostics for other diseases.
There are 2 major classes of laboratory diagnostic tests used for SARS-CoV-2.2, 3, 4, 5, 6 The first major class detects fragments of the virus—either the viral RNA genome or viral proteins. These tests are more useful for diagnosing a current (or very recent) infection, although they appear less useful in the first days of infection.7 Viral RNA is most commonly detected with a quantitative real-time reverse transcriptase-PCR (RT-PCR), or more commonly, a qRT-PCR, qPCR, or RT-PCR test. Although these tests are technically “quantitative” in that they provide a measure for the abundance of viral genetic material detected, they are more commonly used to report a “qualitative” assessment of whether the virus is present (at an abundance above a threshold chosen to minimize false positives). Nucleic acid amplification tests (NAAT), which measure viral RNA but do not use conventional PCR, are also in service.6 In contrast, viral antigen tests rely on immunoassays to measure specific viral proteins.5 , 6 Viral antigen tests are less analytically sensitive than NAATs (which can detect as few as 10 viral RNA fragments in a sample), but may be faster to perform and more economical.
The second major class of tests measure proteins associated with the immune response to the virus. Serological tests detect the presence of antibodies against SARS-CoV-2 within an individual who has been exposed to the virus. There are multiple isotypes of antibodies, and the developed tests most commonly measure immunoglobulin M (IgM) and/or IgG.6 As the antibody response requires time to develop, these tests are less useful for the diagnosis of an acute, active infection. However, serological tests may be advantageous for documenting a previous infection, such as would be useful in epidemiological studies or for monitoring the proportion of healthcare workers in an organization who may have been infected with SARS-CoV-2.
That testing can be valuable, of course, is not a contentious claim. Testing is routinely discussed and communicated to the public by government leaders, business leaders, medical experts, and members of the media. Laboratory diagnostic testing is a ubiquitous part of medicine, and almost everyone has experience with diagnostic testing as a patient. This sense of familiarity with testing and a recognition of the need for information that can be provided by testing, however, does not equate to a finer understanding of how testing is used. There are several nuances to laboratory testing that are not well appreciated by the general healthcare provider; for example, multiple studies have found that physicians struggle with the ability to properly interpret test results.8, 9, 10
A major source of the confusion is how to interpret and apply results from imperfect tests. A perfect test for a disease is easy to understand and interpret; the test would only be positive if the disease were present, and it would only be negative if it were absent. However, diagnostic tests are not perfect, as all tests have false positives and false negatives. As a consequence, test results cannot state definitively whether a disease (or virus) is or is not present. That does not mean that the test is not useful—it simply means that test results must be evaluated probabilistically on the basis of test performance characteristics, patient data, and disease prevalence. Based on these types of information, the “positive predictive value” of a test can be estimated. The positive predictive value is a probability that the person who tested positive actually has the disease. Similarly, “negative predictive values” can be calculated based on similar information to qualify the chance that someone who tested negative truly does not have the disease.
Two key metrics that characterize the test are needed to interpret the results of imperfect tests: the diagnostic sensitivity and diagnostic specificity (or commonly, “sensitivity” and “specificity”).11 At present, there is limited information about these values for widely used SARS-CoV-2 tests.5 , 12 , 13 To properly interpret an imperfect test, it is also necessary to consider the estimated likelihood that the individual being tested has the condition. This prior probability is a fairly subjective estimate that a healthcare provider would make on the basis of medical history, presentation, and factors such as known prevalence of the disease in the community. This value is never exact, but consideration of specific values helps provide estimates for how much the test results should influence decision making.
To facilitate the understanding of diagnostic laboratory testing with an emphasis on the COVID-19 pandemic, each of these three aspects is discussed here in more detail. Illustrative examples that highlight specific and salient aspects are presented. Examples are ordered based upon the statistical concepts being discussed, and the examples alternate between RT-PCR and serological tests, as appropriate, for the clinical situation. We both highlight and stress that information for many of the values used in test interpretation is limited.5 , 12 , 13 Thus, the challenge of interpreting imperfect tests is further compounded by increased uncertainty in the critical information needed to interpret tests. In addition, there has been rapid development of new tests, and many have made their way into the clinical arena without the standard preclinical validation processes required by the US Food and Drug Administration (FDA).4 , 5 , 14 Thus, clinicians will likely be faced with interpreting imperfect tests that have received suboptimal validation through the near future. This perspective was therefore written to both highlight uncertainties and communicate the process of using information so that practitioners can responsibly adapt their practices as tests continue to evolve.
Test Sensitivity
The first major property needed to properly interpret a test result is sensitivity, which refers to the proportion of patients with the disease that the test correctly classifies as positive (Figure 1 ). Although the analytical sensitivity11 of RT-PCR can be quite good (i.e., it is capable of detecting very low quantities of viral RNA), the diagnostic sensitivity for a single test in clinical practice is believed to be much less good. This is an important point that is surprising to those who are familiar with RT-PCR as a highly analytically sensitive test in both research and clinical settings. There have been many reports of frequent false negatives for RT-PCR testing of SARS-CoV-2,15, 16, 17 and reports suggest the sensitivity of RT-PCR in clinical practice could be in the range of 50%–70%.16 , 18 This is a rapidly changing area in which there is more uncertainty than would be encountered in more established areas of medicine. In one recent commentary, a laboratory director confided that he does not know what the clinical sensitivity of his tests is as a consequence of the rapid pace of COVID-19 diagnostics.19 Many reviews estimate a value of 70% for RT-PCR diagnostic sensitivity,6 , 14 , 20 which is approximately the same as previous RT-PCR tests for the original SARS-CoV.21 For our examples, we estimate sensitivity at 80% to account for the widespread perception that tests are performing better than described in the literature. The use of this value does not change conclusions largely from an estimate of 70%, whereas use of a lower estimate could cause the reader to wrongly discount the examples as worst-case scenarios.
Figure 1.
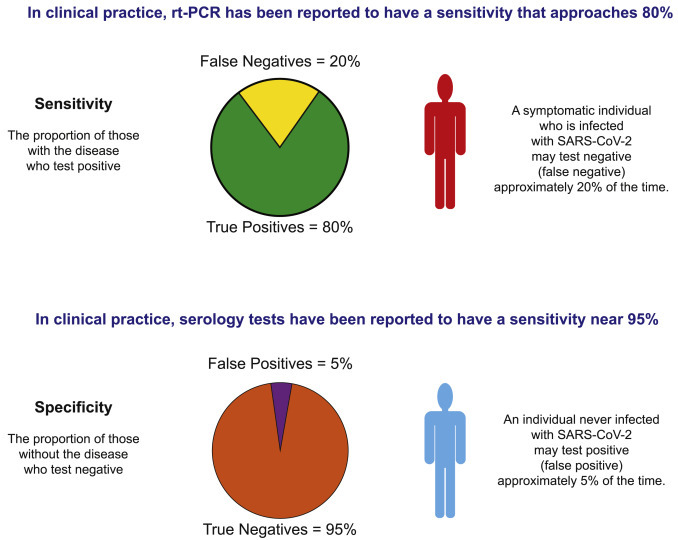
Current Sensitivity and Specificity of SARS-CoV-2 Tests Allow for Problems If Overinterpreted
The sensitivity of RT-PCR is reported to be only ∼80% in practice. Thus, someone infected with SARS-CoV-2 may test negative. Thus, negative tests should not be overinterpreted for individuals who are likely to be positive by other indications. The specificity of serology tests may be 95%, which still suggests a 5% false positive rate. Thus, the testing of a population such as a workplace may falsely suggest 5% of the non-infected population has been infected.
There are many reasons why an infection may not be detected by RT-PCR, ranging from whether the nasopharyngeal swab was performed properly, to the abundance of SARS-CoV-2 in the tested anatomical location, to when in the course of an infection the sample is obtained.3 , 7 , 22, 23, 24 False negatives can also occur due to clinical laboratory errors involving steps such as sample preparation, machine and/or operator error, and reporting errors.24 , 25 For example, during the early days of COVID-19 in the United States, a labeling error resulted in a patient receiving a false-negative test result, which in turn led to the patient being discharged from a hospital.26 Viral evolution could also result in reduced sensitivity.27 In addition, the timing of the test relative to the course of the infection is an important variable, as the tests have their lowest sensitivity both early and late in the infection.7
This relatively high rate of false negatives is problematic for several reasons. RT-PCR is less effective early in the course of a SARS-CoV-2 infection,7 which limits the potential value of RT-PCR for screening asymptomatic individuals before they exhibit robust viral replication. For individuals with symptoms and/or known exposures, false negatives may cause the individual to believe that they are not infected and that they can return to work and other activities.13 It is therefore important for there to be a clear understanding that a negative result needs to be interpreted as “no virus detected” rather than “not infected with the virus.”
The reported sensitivities for serological tests are typically higher (85%–99%).28 However, timing is also a very important variable due to the timing of the immune response to infection that produces IgM and then IgG antibodies.3 For this reason, serology is not as useful in the early stages of an infection in which the virus is present but a significant antibody response has not yet been mounted.3 Multiple studies that compare serological assays have reported poor diagnostic sensitivity if they are used early in infection.28 , 29
It is important to note that reported sensitivities for serological tests may not apply to all of the clinical scenarios encountered. For example, individuals with mild SARS-CoV2 infection have lower antibody titers.4 If the sensitivity reported for a serological assay was obtained by studying samples from hospitalized individuals with advanced COVID-19, the value reported may not reflect the sensitivity for detecting mild cases.
Example Demonstrating How Sensitivity Affects Clinical Test Interpretation
Consider an individual who was exposed to multiple coworkers who developed COVID-19. Several days later, the individual develops symptoms that are consistent with a mild case of COVID-19. Let us assume that the individual works in an essential industry, and that working through a “common cold” would be routine in most years. Due to the number of infected employees and the resulting shortage of workers in this essential industry, there is pressure on the employee to return to work if the illness is not due to SARS-CoV-2.
The employee is referred for RT-PCR for SARS-CoV-2 testing by a healthcare provider to determine whether the employee is currently infected. The test result is negative. Should the employee be classified as SARS-CoV-2-free and be cleared to return to work? As mentioned above, the sensitivity of RT-PCR is ∼70%.16 , 18 , 21 That is, if this patient is truly positive and infectious, the test may still come back as negative 30% of the time (Figure 1). A negative test does not mean the patient does not have the disease.30 The decision about the individual and whether he or she should return to work needs to involve this likely possibility. A better estimate of the likelihood that the patient has SARS-CoV-2 is possible with additional considerations, and this example is revisited after those topics are discussed.
Example Demonstrating Relevance of Sensitivity to the Concerns of Re-infection with SARS-CoV-2
There have been reports that appear to describe individuals who cleared their SARS-CoV-2 infection and then became infected again.31, 32, 33 The evidence is typically one or more negative tests after the patient’s illness has improved, followed by one or more negative tests. With false negatives common by RT-PCR, the possibility that there was one or more false-negative tests should be considered. With a 70% sensitivity, the chance of 2 false-negative tests in a row is ∼10%, the chance of 3 in a row is ∼3%, and for 4, 1%. With hundreds of thousands of patients with confirmed SARS-CoV-2 infections, it should be anticipated that there will be many patients who have a series of false-negative tests followed by a true-positive test. For example, one study considered 70 COVID-19 patients and found that just over 20% had a positive RT-PCR test after 2 consecutive negative tests.34
Test Specificity
Test specificity is the second major property that needs to be considered to evaluate a test. Specificity is the proportion of patients who do not have the disease who test negative (Figure 1B). Like sensitivity, this is measured under clinical conditions and reflects the full spectrum of challenges that may complicate measurement. RT-PCR tests for SARS-CoV-2 are expected to be very specific (∼99%),35 , 36 while serology tests for SARS-CoV-2 generally have reported specificities in the range of 85%–100%.28 , 37 Although these assays can be very specific, false positives are possible. For example, contamination of a sample with SARS-CoV-2 nucleic acids could cause a false-positive RT-PCR test, and false positives in serological testing may come from cross-reactivity to other coronaviruses.4 One recent FDA Alert communicated a 3% false-positive rate for a commercial RT-PCR system designed to detect SARS-CoV-2. False-positive results can also have negative consequences. For example, a false-positive test could result in a patient who has been admitted to the hospital with non-COVID-19 being placed in a room or unit containing other individuals who tested positive for the disease, rendering that individual with a false-positive result at high risk of a nosocomial COVID-19 infection.
Example Demonstrating the Limitation of Test Specificity in Screening
For this hypothetical example, we consider an essential industry workplace with hundreds of employees. To date, there has been little to no SARS-CoV-2 in the community surrounding the workplace. The workplace engages a local clinical laboratory to implement periodic, large-scale SARS-CoV-2 serological testing to monitor the possible spread of SARS-CoV-2. The company performs serology testing on its 500 employees with a very good test that is 95% specific (Figure 1). If nobody was previously exposed to SARS-CoV-2, the test should still report ∼5% of the employees, or 25 people, are “positive,” with all being false positives.
Example Demonstrating the Importance of Specificity in Prevalence Studies
Seroprevalence studies in communities can provide important information, such as how far the disease has spread. A better understanding of how many total people have been infected can allow better estimates for the lethality of the virus. Data that appear to address these issues can have a profound impact on rapidly evolving public health policies. In addition, public perception of the risks of the disease are likely to contribute to compliance with both suggested and mandated measures to limit the spread of the virus. For all of these reasons, it is important that reported data concerning the spread and lethality of the disease be accurate.
Concerns have been raised about the limited specificity of many commercially available serology tests and the possible consequences of their use in seroprevalence studies.38 Seroprevalence studies with low-specificity tests could lead to an overestimate of the number of people who have previously been infected, which would in turn wrongly suggest that the disease is less lethal and that we are closer to herd immunity. It is therefore important that the studies use properly validated serological reagents and statistically account for the estimated false-positive rate. One recent study reported an ∼3% seroprevalence for SARS-CoV-2. The study used a serology test with a very high specificity reported by the manufacturer (99.8%); thus, the number of expected false positives would be much less than that detected by the study.39 The authors performed additional statistical analyses to account for possible likely ranges of test sensitivity and specificity. The authors of this seroprevalence study also performed an additional, small, in-house validation test with samples from patients with known SARS-CoV-2 and from pre-COVID-19 samples. It is illustrative to note that although the manufacturers reported a sensitivity of 91.8% and a specificity of 99.5%, the authors’ validation found a sensitivity of 82.8% and a specificity of 99.5%; the difference in sensitivity highlights that manufacturer-reported values may not always be indicative of test performance within end-user laboratories.39
Pre-test Probability That the Disease Is Present
Diagnostic tests provide important and actionable information, even though a test result should not be treated as absolutely certain. This is because test results can be interpreted probabilistically to provide a likelihood that the person tested does or does not have a disease. The sensitivity and specificity of the test are not enough to calculate the likelihood. An estimated probability that the individual being tested has the condition, or the pre-test probability, is also required.
One way that the pretest probability can be estimated is based upon the overall prevalence rates in the relevant population. For example, if a patient arrives from New York City, where antibodies to SARS-CoV-2 have recently been reported to be present in 15%–20% of the population,40 a reasonable estimate for prevalence would be 15%–20% if that individual receives serological testing. If an emergency department finds that ∼5% of individuals currently presenting with influenza symptoms are found to have a SARS-CoV-2 infection,41 this may also be a good estimate for the pre-test probability.
The estimated pre-test probability can also be improved by including additional information about the individual being tested. The quantity and quality of symptoms; the patient history, including travel and close contacts; and results from imaging studies and/or other laboratory tests could lead a physician to adjust the pre-test probability. If the physician has been treating and diagnosing many patients with COVID-19, the estimate may be very good. If the physician has never before treated a patient with COVID-19, the estimate may be less good.
Example Comparing High and Low Pre-test Probability of Patients Receiving the Same Test
Here, we consider two different members of the community (Figure 2 ). The first person presents to the emergency department with high fever, hypoxia, and a lower respiratory cough, and whose spouse recently had a severe case of SARS-CoV-2 that was first managed at home but then progressed to require hospitalization. A chest X-ray finds ground-glass opacities.42 RT-PCR testing is ordered in an attempt to detect a current infection. This person has a high pre-test probability for having SARS-CoV-2. We would estimate the likelihood that he or she has SARS-CoV-2 at 90%.
Figure 2.
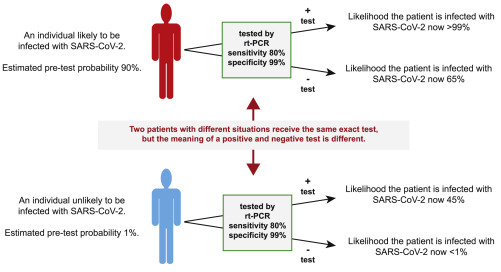
Test Result Interpretation Is Largely Affected by Whether Other Factors Suggest the Tested Individual Is Infected with SARS-CoV-2
If the same exact RT-PCR test is given to 2 different individuals—one whose presentation is consistent with SARS-CoV-2 infection and one whose presentation is not—then the probability that a positive test indicates an infection with SARS-CoV-2 can vary greatly. For example, the individual whose presentation suggests a 90% chance of infection is >99% likely to have an infection if he or she tests positive and 65% likely to have an infection if he or she tests negative. The individual at low risk remains more likely not to have an infection if he or she tests positive, and the absolute reduction in the chance that he or she has a SARS-CoV-2 infection has reduced by <1%.
The second individual presents to a drive-through RT-PCR testing site on referral from his concierge physician. This individual has heard that it is possible to be infected with SARS-CoV-2, yet be asymptomatic. This individual has also heard that anyone who wants a test can receive a test, and this person wants a test. When the individual’s physician took the patient’s history, it is found that this individual lives alone, does not have any infected contacts, and has been strictly sheltering in place. In addition, the individual lives in an area that has been minimally affected by COVID-19, with a recent study measuring a seroprevalence of <2%, with most of those individuals being essential workers or nursing home residents. We would estimate the likelihood that this individual has SARS-CoV-2 as 1%, which is more likely an overestimate, considering the history and presentation. The concierge physician provides a prescription for this individual to receive SARS-CoV-2 testing nevertheless.
A nasopharyngeal swab is obtained from both patients for RT-PCR testing that has a sensitivity of 80% and a specificity of 99%. The test is negative for the first person but positive for the second. For the first individual, we estimated the pretest probability at 90%. If the test result is positive, the probability that the patient has SARS-CoV-2 increases to >99%. (This is the positive predictive value of the test for that patient.) If the test comes back negative, the probability that the patient has SARS-CoV-2 decreases, but the patient still has a nearly 65% chance of having SARS-CoV-2; i.e., it is still more likely that the patient is infected and received a negative test than the patient is truly negative and received a negative test.
In contrast, we consider the patient who simply wants a test. If the patient receives a positive test, then the chance that the patient actually has SARS-CoV-2 increases to only 45% i.e., it is still more likely that the individual is negative for the disease despite the positive test. If the test comes back negative, then there is now a <0.3% chance that the individual has SARS-CoV-2. Although the individual may feel comforted to see a negative test, going from 1% to 0.3% is a very small change, and it should be difficult for any physician to justify recommending these patients for testing when testing is limited.
It is important to note that the interpretation of a positive test was different for the two individuals. In other words, the positive predictive value depends both upon the characteristics of the test and the characteristics of the patient that suggest the presence or absence of infection. An online interactive calculator that provides rounded estimates is included with a recent article on COVID-19 testing, and this calculator can be useful for those who want to explore how alternative values of the pre-test probability, sensitivity, and specificity would change the likelihood of a positive (and negative) diagnosis.20
Example Applying All of These Concepts to the Concept of “Immunity Passports”
The issue of whether those who have had COVID-19 can later be re-infected is an important issue. There have been discussions about various forms of immunity passports that document those who have had previous infections and may no longer be at risk from this illness, such that these individuals could return to more normal interpersonal interactions, such as those before the COVID-19 pandemic, without fear of disease.43 , 44 However, this concept depends on those who have cleared an infection to have immunity and to be non-infectious. Although some immunity seems likely, the quality and duration of immunity remains uncertain at the present time. In addition, the concept of immunity passports raises many important ethical issues that practicing bioethicists have investigated.43, 44, 45
We consider a workplace that wants to implement immunity passports on the basis of serological tests that may identify a previous infection with SARS-CoV-2 (Figure 3 ). One employee is 25 years old and the other is 65 years old. Neither had previous known exposure to an individual ill with COVID-19, and both are assumed to have a pre-test probability of 5% on the basis of serology rates within their community. The commercial serology test offered to the workplace has 95% sensitivity and 95% specificity, which is quite good for many applications. The 25-year-old tests negative and the 65-year-old tests positive. The 65-year-old individual is given the immunity passport, and the 25-year-old is not. Does this make sense?
Figure 3.
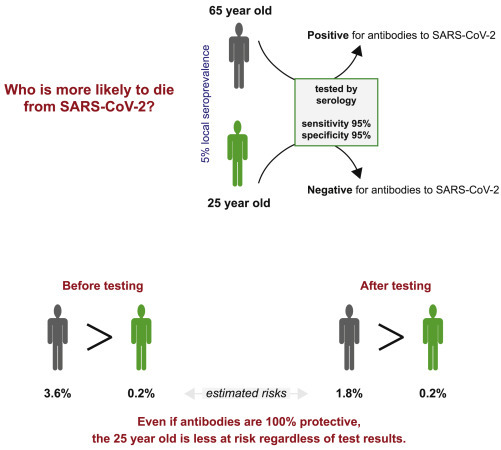
Immunity Passport Decisions Need to Consider More Factors Than the Presence of Antibodies
In a hypothetical example, a 65-year-old and a 25-year-old are tested for antibodies to SARS-CoV-2. They have no known exposure, the local seroprevalence is 5%, and they receive a serology test with sensitivity and specificity of 95%. The 65-year-old tests positive, which has a 50% probability of being a true positive. The 25-year-old tests negative, which has a >99% chance of being a true negative. Even if we assume the 65-year-old has immunity to SARS-CoV-2 when antibodies are present (an overly aggressive assumption), the 25-year-old is still at less risk of death for a SARS-CoV-2 infection. The estimated risk of death for individuals 60–70 years old is 3.6% and 0.2% for individuals 20–30 years old. Thus, if we assume that the risk of the 65-year-old is zero when antibodies are present and 3.6% when they are not, then the overall risk, considering test results, is a 1.8% risk of death, which is nearly 10× the risk faced by the 25-year-old. Therefore, although test results are valuable sources of information, other factors need to be considered.
A probabilistic assessment of the test results for the above conditions gives the 65-year-old employee a 50% chance of truly having antibodies to SARS-CoV-2, and the 25-year-old is almost certain not to have any such antibodies. The 65-year-old is thus more likely to have some immunity to SARS-CoV-2. However, it is also imperative to consider that age is a major risk factor for death in those who are infected with SARS-CoV-2. The estimated case fatality rate for the 65-year-old employee is ∼10 times higher than it is for the 25-year-old, with reports of a 3.6% case fatality rate for those aged 60–70 years and a 0.2% rate for those aged 20–25 years.46 Even if we could optimistically assume that there would be absolutely no chance that the 65-year-old could die from SARS-CoV-2 when the positive test result is true (an overly optimistic assumption), we must assume that the risk of death remains ∼3.6% for the times that the test was a false positive (50% of the time for the current example, even though the test was 95% specific). Thus, we should estimate a case fatality rate of ∼1.8% for the 65-year-old, with a 50% chance of immunity. The 25-year-old with no exposure to SARS-CoV-2 would still be at less risk for death, despite not having any antibodies to the virus. This example highlights that after a test is properly interpreted from a probabilistic perspective, the clinician must also consider other factors when managing a patient.
Discussion
Dialogues about testing have focused more upon the importance of testing and the ongoing testing shortages than upon test interpretation and how test performance impacts utility. These issues have been mentioned in passing within the academic medical literature as issues to be aware of, but detailed discussions have been relatively limited.14 , 20 This is problematic, as there is abundant evidence that physicians struggle with proper probabilistic test interpretation.8, 9, 10 As government, business, and institutional leaders turn to physicians and healthcare providers for advice, it seems likely that a reasonable fraction may be giving incorrect advice based on these systemic weaknesses. The consequences of policies and practices that are based upon the improper use of laboratory testing are potentially fatal during a pandemic.
Many communities are limiting tests to those who are most in need. This is often presented as a problem. It is not necessarily a problem; it is actually consistent with best practices in laboratory medicine. One of the examples above highlighted the limited value of testing for those without a good indication for testing. Positive results are likely to be false positives; negative results do not provide much additional information, and the patient is not at less risk from a future infection. The idea that anyone who wants a test can undergo a test is complicated because the results of a test are not absolute and what they actually mean depends upon the history of exposures and symptoms of the person being tested and the community within which he or she lives. This issue does not appear to have been as prominently discussed in ongoing COVID-19 public dialogs.
Not only are government and organizational leaders faced with an urgent need to consider aspects of medicine that they have rarely considered in such detail but also many scientists and engineers have been mobilized to lend their expertise to the ongoing crisis. The limitations of testing are often surprising to them. Although there are many differences between best practices in a clinical laboratory and in a research laboratory, the lack of absolute certainty with any one test should not be a surprise. Scientists do not publish the results from a single iteration of a single experiment. Rather, they expect tests to be reproducible and therefore perform experiments multiple times to demonstrate that the results were not a single outlier. In addition, they support their conclusion with multiple lines of new experimental evidence and historical evidence from the literature that, taken together, support their finding. This is akin to a physician considering laboratory results on the basis of the patient history, physical examination, and any other imaging or laboratory test results.
With the reported suboptimal sensitivities of RT-PCR tests in practice and the reported poor performance of some serology tests that are flooding the market after emergency use authorizations reduced many of the regulatory burdens in an effort to expedite the development and delivery of tests,5 , 47 , 48 probabilistic test interpretation is going to play an important role in test interpretation. In addition, as disease burdens shift through communities and the list of symptoms and syndromes associated with COVID-19 grow,49 , 50 assessments of pre-test probability will need to continually be adapted to current information. This is in contrast to traditional medical conditions, in which physicians may have familiarity with test results developed over years and in which the concepts of probabilistic test interpretation have been replaced by years of experience that influence judgment and interpretation.
Acknowledgments
This work was supported by NIH K22CA216318 (E.C.S.), NIH K08AI128043 (C.B.W.), and NIH R01AI148467 (C.B.W.).
Author Contributions
E.C.S. and C.B.W. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020 doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Cheng M.P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M., Dittrich S., Yansouni C.P. Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020;172:726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 4.Torres R., Rinder H.M. Double-Edged Spike-Are SARS-CoV-2 Serologic Tests Safe Right Now? Lab. Med. 2020;51:236–238. doi: 10.1093/labmed/lmaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020;58:e00512-20. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissleder R., Lee H., Ko J., Pittet M.J. COVID-19 diagnostics in context. Sci. Transl. Med. 2020;12:eabc1931. doi: 10.1126/scitranslmed.abc1931. [DOI] [PubMed] [Google Scholar]
- 7.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bramwell R., West H., Salmon P. Health professionals’ and service users’ interpretation of screening test results: experimental study. BMJ. 2006;333:284. doi: 10.1136/bmj.38884.663102.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steurer J., Fischer J.E., Bachmann L.M., Koller M., ter Riet G. Communicating accuracy of tests to general practitioners: a controlled study. BMJ. 2002;324:824–826. doi: 10.1136/bmj.324.7341.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffrage U., Gigerenzer G. Using natural frequencies to improve diagnostic inferences. Acad. Med. 1998;73:538–540. doi: 10.1097/00001888-199805000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Saah A.J., Hoover D.R. “Sensitivity” and “specificity” reconsidered: the meaning of these terms in analytical and diagnostic settings. Ann. Intern. Med. 1997;126:91–94. doi: 10.7326/0003-4819-126-1-199701010-00026. [DOI] [PubMed] [Google Scholar]
- 12.Mallapaty S. Will antibody tests for the coronavirus really change everything? Nature. 2020;580:571–572. doi: 10.1038/d41586-020-01115-z. [DOI] [PubMed] [Google Scholar]
- 13.West C.P., Montori V.M., Sampathkumar P. COVID-19 Testing: The Threat of False-Negative Results. Mayo Clin. Proc. 2020;95:1127–1129. doi: 10.1016/j.mayocp.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woloshin S., Patel N., Kesselheim A.S. False Negative Tests for SARS-CoV-2 Infection - Challenges and Implications. N. Engl. J. Med. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 15.Xu H., Yan L., Qiu C., Jiao B., Chen Y., Tan X., Chen Z., Ai L., Xiao Y., Luo A., Li S. Analysis and Prediction of False Negative Results for SARS-CoV-2 Detection with Pharyngeal Swab Specimen in COVID-19 Patients: A Retrospective Study. medRxiv. 2020 doi: 10.1101/2020.03.26.20043042. [DOI] [Google Scholar]
- 16.Li Y., Yao L., Li J., Chen L., Song Y., Cai Z., Yang C. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J. Med. Virol. 2020;92:903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly J.C., Dombrowksi M., O’neil-Callahan M., Kernberg A.S., Frolova A.I., Stout M.J. False-Negative COVID-19 Testing: Considerations in Obstetrical Care. Am. J. Obstet. Gynecol. MFM. 2020:100130. doi: 10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Kang H., Liu X., Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J. Med. Virol. 2020;92:538–539. doi: 10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titus K. The laboratory tests of pandemic summer. CAP Today. 2020 https://www.captodayonline.com/the-laboratory-tests-of-pandemic-summer/ [Google Scholar]
- 20.Watson J., Whiting P.F., Brush J.E. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 21.Drosten C., Chiu L.L., Panning M., Leong H.N., Preiser W., Tam J.S., Günther S., Kramme S., Emmerich P., Ng W.L., et al. Evaluation of advanced reverse transcription-PCR assays and an alternative PCR target region for detection of severe acute respiratory syndrome-associated coronavirus. J. Clin. Microbiol. 2004;42:2043–2047. doi: 10.1128/JCM.42.5.2043-2047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijaykumar P., Geng B., Muenker M.C., Moore A.J., Vogels C.B.F., et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv. 2020 doi: 10.1101/2020.04.16.20067835. [DOI] [Google Scholar]
- 23.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh B.A., Bailey T.C., Lyons P.G., Anderson N.W. The Brief Case: “Not Positive” or “Not Sure”-COVID-19-Negative Results in a Symptomatic Patient. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.01195-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin. Chim. Acta. 2009;404:16–23. doi: 10.1016/j.cca.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Bogel-Burroughs N. Labeling Error to Blame for Hospital’s Release of Coronavirus Patient. The New York Times, February 11, 2020. 2020 https://www.nytimes.com/2020/02/11/us/san-diego-coronavirus-patient.html [Google Scholar]
- 27.Osório N.S., Correia-Neves M. Implication of SARS-CoV-2 evolution in the sensitivity of RT-qPCR diagnostic assays. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang M.S., Hock K.G., Logsdon N.M., Hayes J.E., Gronowski A.M., Anderson N.W., Farnsworth C.W. Clinical Performance of Two SARS-CoV-2 Serologic Assays. Clin. Chem. 2020;66:1055–1062. doi: 10.1093/clinchem/hvaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitman J.D., Hiatt J., Mowery C.T., Shy B.R., Yu R., Yamamoto T.N., Rathore U., Goldgof G.M., Whitty C., Woo J.M., et al. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv. 2020 doi: 10.1101/2020.04.25.20074856. [DOI] [Google Scholar]
- 30.Bullis S.S.M., Crothers J.W., Wayne S., Hale A.J. A Cautionary Tale of False-Negative Nasopharyngeal COVID-19 Testing. IDCases. 2020;20:e00791. doi: 10.1016/j.idcr.2020.e00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D., Xu W., Lei Z., Huang Z., Liu J., Gao Z., Peng L. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int. J. Infect. Dis. 2020;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng J., Wang M., Zhang G., Lu E. Seven discharged patients turning positive again for SARS-CoV-2 on quantitative RT-PCR. Am. J. Infect. Control. 2020;48:725–726. doi: 10.1016/j.ajic.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao A.T., Tong Y.X., Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J. Med. Virol. 2020 doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalla A.K., Casto A.M., Huang M.W., Perchetti G.A., Sampoleo R., Shrestha L., Wei Y., Zhu H., Jerome K.R., Greninger A.L. Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer-Probe Sets and One Assay Kit. J. Clin. Microbiol. 2020;58:e00557-20. doi: 10.1128/JCM.00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X., Wang L., Sakthivel S.K., Whitaker B., Murray J., Kamili S., Lynch B., Malapati L., Burke S.A., Harcourt J., et al. US CDC Real-Time Reverse Transcription PCR Panel for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 2020;26:1654–1665. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C.Y., Fomsgaard A., Krogfelt K.A., Jørgensen C.S. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv. 2020 doi: 10.1101/2020.04.09.20056325. [DOI] [Google Scholar]
- 38.Oxford C. How (Not) to Do an Antibody Survey for SARS-CoV-2. The Scientist, April 28, 2020. 2020 https://www.the-scientist.com/news-opinion/how-not-to-do-an-antibody-survey-for-sars-cov-2-67488 [Google Scholar]
- 39.Bendavid E., Mulaney B., Sood N., Shah S., Ling E., Bromley-Dulfano R., Lai C., Weissberg Z., Saavedra-Walker R., Tedrow J., et al. COVID-19 Antibody Seroprevalence in Santa Clara County, California. medRxiv. 2020 doi: 10.1101/2020.04.14.20062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman J.D., Rothfeld M. 1 in 5 New Yorkers May Have Had Covid-19, Antibody Tests Suggest. The New York Times, April 23, 2020. 2020 https://www.nytimes.com/2020/04/23/nyregion/coronavirus-antibodies-test-ny.html [Google Scholar]
- 41.Spellberg B., Haddix M., Lee R., Butler-Wu S., Holtom P., Yee H., Gounder P. Community Prevalence of SARS-CoV-2 Among Patients With Influenzalike Illnesses Presenting to a Los Angeles Medical Center in March 2020. JAMA. 2020;323:1966–1967. doi: 10.1001/jama.2020.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persad G., Emanuel E.J. The Ethics of COVID-19 Immunity-Based Licenses. (“Immunity Passports”) JAMA. 2020 doi: 10.1001/jama.2020.8102. [DOI] [PubMed] [Google Scholar]
- 44.Hall M.A., Studdert D.M. Privileges and Immunity Certification During the COVID-19 Pandemic. JAMA. 2020;323:2243–2244. doi: 10.1001/jama.2020.7712. [DOI] [PubMed] [Google Scholar]
- 45.Kofler N., Baylis F. Ten reasons why immunity passports are a bad idea. Nature. 2020;581:379–381. doi: 10.1038/d41586-020-01451-0. [DOI] [PubMed] [Google Scholar]
- 46.Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 47.Petherick A. Developing antibody tests for SARS-CoV-2. Lancet. 2020;395:1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon D. Why the Accuracy of SARS-CoV-2 Antibody Tests Varies So Much. The Scientist, May 5, 2020. 2020 https://www.the-scientist.com/news-opinion/why-the-accuracy-of-sars-cov-2-antibody-tests-varies-so-much-67513 [Google Scholar]
- 49.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C., Herman P., Manley G.T., Lyon D.M., Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30293-0. S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


