Abstract
Background
COVID-19 is a novel viral disease. Severe courses may present as ARDS. Several publications report a high incidence of coagulation abnormalities in these patients. We aimed to compare coagulation and inflammation parameters in patients with ARDS due to SARS-CoV-2 infection versus patients with ARDS due to other causes.
Methods
This retrospective study included intubated patients admitted with the diagnosis of ARDS to the ICU at Munich university hospital. 22 patients had confirmed SARS-CoV2-infection (COVID-19 group), 14 patients had bacterial or other viral pneumonia (control group). Demographic, clinical parameters and laboratory tests including coagulation parameters and thromboelastometry were analysed.
Results
No differences were found in gender ratios, BMI, Horovitz quotients and haemoglobin values. The median SOFA score, serum lactate levels, renal function parameters (creatinine, urea) and all inflammation markers (IL-6, PCT, CRP) were lower in the COVID-19 group (all: p < 0.05).
INR (p < 0.001) and antithrombin (p < 0.001) were higher in COVID-19 patients. D-dimer levels (p = 0.004) and consecutively the DIC score (p = 0.003) were lower in this group.
In ExTEM®, Time-to-Twenty (TT20) was shorter in the COVID-19 group (p = 0.047), these patients also had higher FibTEM® MCF (p = 0.005). Further, these patients presented with elevated antigen and activity levels of von-Willebrand-Factor (VWF).
Conclusion
COVID-19 patients presented with higher coagulatory potential (shortened global clotting tests, increased viscoelastic and VWF parameters), while DIC scores were lower. An intensified anticoagulation regimen based on an individual risk assessment is advisable to avoid thromboembolic complications.
Abbreviations: aPTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; BMI, body mass index; CFT, clot formation time; COVID-19, coronavirus disease 19; CRP, C-reactive protein; CT, clotting time; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; FEU, fibrinogen equivalent units; GGT, gamma-glutamyl-transferase; GOT, glutamate-oxaloacetate-transaminase; GPT, glutamate-pyruvate-transaminase; ICU, intensive care unit; IL-6, interleukin 6; INR, international normalized ratio; MCF, maximum clot firmness; ML, maximum lysis; PCT, procalcitonin; SOFA, sequential organ failure assessment; TT20, Time-to-Twenty; VWF, von-Willebrand-Factor
Keywords: Anaesthesia and Intensive Care (4), Acute respiratory distress syndrome (ARDS) (4.01), Intensive Care (4.24), SARS-CoV-2
Highlights
-
•
COVID-19 patients presented with higher coagulatory potential than non-COVID-ARDS.
-
•
DIC scores were lower in COVID-19 ARDS patients.
-
•
An intensified anticoagulation regimen might be advisable.
-
•
Use of platelet inhibitors may be considered.
1. Background
COVID-19 is a novel viral disease in humans that is caused by infection with SARS-CoV-2 and presents mainly as a respiratory tract infection. Severe courses of the disease may present as acute respiratory distress syndrome (ARDS) or even develop lethal multi organ failure [1]. Several publications report about a frequent incidence of thromboembolic events and coagulation abnormalities in this patient collective [[2], [3], [4]]. As deep vein thrombosis and pulmonary embolism are not only frequent complications in critically ill patients but also contribute to increased rates of morbidity and mortality, coagulation management is key in critical care. In this retrospective observational study, we compared coagulation and inflammatory parameters of patients with ARDS due to infection with SARS-CoV-2 versus patients with ARDS due to other causes.
2. Methods
This study complied with the edicts of the 1975 Declaration of Helsinki and was approved by the institutional review board of the university of Munich (20-345). This retrospective study included intubated patients who were admitted with the diagnosis of ARDS to the intensive care units at Munich university hospital between March 4th and April 4th 2020 (COVID-19 group), and were compared to non-COVID-19 ARDS patients admitted to our institution between January 1st 2019 and March 31st 2020 (control group).
The COVID-19 group consisted of 22 consecutive patients who had confirmed SARS-CoV2 infection and underwent extended haemostasis monitoring within 48 h after intensive care unit (ICU) admission. The diagnosis of COVID-19 was confirmed by detection of SARS-CoV-2 N-gene-1 RNA by RT-PCR in endotracheal secretions and serum [5]. There was no evidence for superinfection by another pathogen in any of the COVID-19 patients.
The control group consisted of 14 ARDS patients who had either bacterial or viral pneumonia. Pathogens in this group comprised influenza A virus (n = 3), herpes simplex virus (n = 2), Streptococcus pneumoniae (n = 2), Legionella pneumophila (n = 1), Klebsiella pneumoniae (n = 1), Mycoplasma pneumoniae (n = 1), Pseudomonas aeruginosa (n = 1), Stenotrophomonas maltophilia (n = 1) and Pneumocystis jirovecii (n = 1). In one patient, Candida albicans was found; in two patients, no pathogen was detected.
Pathogens were identified by real time PCR in case of viral infections and microbiological culture of endotracheal secretions or bronchoalveolar lavage samples in case of bacterial infection. All patients admitted to the ICU after February 1st 2020 were tested several times for presence SARS-CoV-2; all tests were negative.
In this group, extended coagulation testing was performed within 48 h after ICU admission as a standard of care for patients who were deemed potential candidates for treatment by extracorporeal membrane oxygenation (ECMO). All patients received prophylactic anticoagulation according to the institutional standard for critically ill patients with continuous infusion of heparin with individual aPTT targets defined by the treating physician according to their thromboembolic and bleeding risk.
Clinical and laboratory data at ICU admission were retrieved from the hospital's electronic patient data records. This included age, SOFA score at ICU admission, Horovitz oxygenation index, infectious pathogens, full blood count, liver function tests, renal parameters, inflammatory and coagulation parameters.
Extensive coagulation monitoring included not only prothrombin time and international normalized ratio (INR), activated partial thromboplastin time (aPTT) and D-dimers, but also measurements of fibrinogen (Clauss method), antithrombin, protein C and protein S levels, von-Willebrand-Factor (VWF) antigen and activity as well as thromboelastometric tests (ROTEM®). These extended coagulation tests were gradually added to the testing routine, as coagulopathy and an increased risk of thromboembolisms were reported in COVID-19 patients [2,3]. Thus, only 11 out of 22 COVID-19 patients received a ROTEM® diagnostic.
Thromboelastometry was performed using a ROTEM® delta analyser (Tem Innovations, Munich, Germany). Two thromboelastometric tests were performed: ExTEM®, using a tissue factor containing activator and therefore representing the extrinsic pathway and a FibTEM® test containing cytochalasin D - a platelet inhibitor - thus clot firmness represents fibrin-polymerization and contribution of fibrinogen to clot formation [6].
In EXTEM®, clotting time (CT), clot formation time (CFT), maximum clot firmness (MCF), maximum lysis (ML) and in FibTEM® maximum clot firmness were measured. In addition, in EXTEM®, the time from the initial clot formation up to a clot amplitude of 20 mm (Time-to-Twenty (TT20)) was analysed.
All laboratory tests were performed by the LMU Munich Institute for Laboratory Medicine, according to institutional standards. aPTT, INR and fibrinogen levels (Clauss method) were measured by optical coagulometry (Dade Actin® FSL Activated PTT reagent, Thromborel®S Reagent, Dade Thrombin Reagenz, Siemens Healthineers, Germany). D-dimer levels were determined using a latex agglutination method (Innovance D-dimer, Siemens Healthineers, Germany). Results are provided in fibrinogen equivalent units (FEU). VWF antigen and activity were measured by turbidimetry using vWF-Ag reagent and Innovance vWF Ac (Siemens Healthineers, Germany) respectively.
The Disseminated Intravascular Coagulation (DIC) score was calculated according to the British Society for Haematology guidelines for the diagnosis and management of disseminated intravascular coagulation [7]. It is based on platelet count, fibrinogen levels, D-dimer levels, and prothrombin time.
Data analysis was performed as a complete case analysis in Python 3.5 using the following libraries:
-
-
Pandas: 0.24.2
-
-
Scipy 1.3.0
-
-
Numpy: 1.18.1
-
-
Seaborn: 0.9.0
-
-
Matplotlib: 3.0.3
The Shapiro-Wilk test (scipy library) was used to test for normal distribution. As only four out of 46 parameters showed non-significant p-values and the number of patients in each group was less than 30, we assumed that none of the outcome distributions were approximately normally distributed [8]. Hence, the use of non-parametric tests was deemed appropriate.
The median and the interquartile range (25th, 75th percentiles) were calculated with the numpy library and the pandas library, respectively. The Mann-Whitney-U test (scipy library) was used to test for differences in distributions between groups. Fisher's Exact test (scipy library) was used for analysis of contingency tables. The Chi-Square goodness-of-fit test (scipy library) was used for analysis of frequencies between groups. A p-value of less than 0.05 was considered to be statistically significant.
3. Results
36 Patients were included in the study. 22 patients formed the COVID-19 group (COVID-19), 3 of them were female and 19 male. The comparative group (control) comprised 14 patients, 5 of them female, 9 male. Thus, the odds ratios for female patients to catch the SARS-CoV-2 virus/infection were 3.52 times less than for male patients, although the p-value was not significant.
Patients in the COVID-19 group were significantly older than patients in the comparative group (63.5 vs. 49.0 years, p = 0.005). The mean body mass index (BMI) was 27.0 and 25.5 in the COVID-19 and the control group respectively; patients in both groups tended to be slightly obese - with no statistically significant difference (p = 0.34).
No significant differences were found in the occurrences of comorbidities (diabetes, obesity, renal or liver failure, cardiac diseases or coagulation disorders). In the COVID-19 group, significantly more patients presented with the diagnosis of arterial hypertension resulting in an odds ratio for hypertension of 11.9 (p = 0.013), showing that patients presenting with hypertension were seven times as likely to be in COVID-19 group than in the non COVID-19 group.
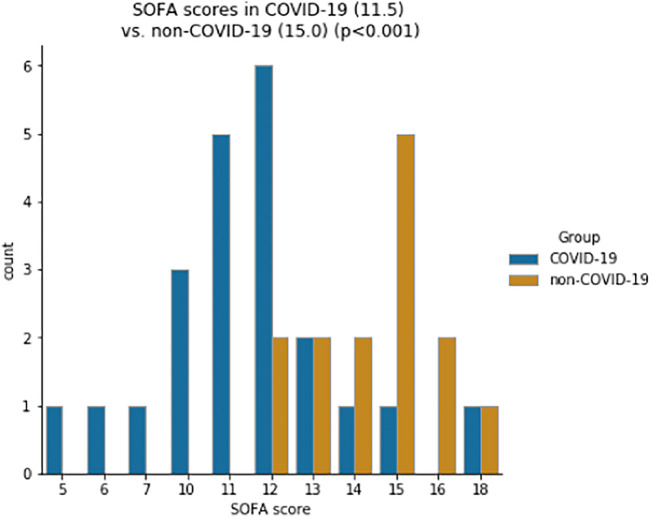
In the COVID-19 group, the median SOFA score was 11.5 which was significantly lower than the median of 15.0 in the control group (p < 0.001; Fig. 1 ).
Fig. 1.
Distribution of SOFA score for COVID-19 and control group (median).
There were no significant differences in the Horovitz oxygenation index (160.5 vs. 85.5, p = 0.115) nor in bilirubin (0.6 vs. 0.8 mg/dl, p = 0.172), gamma-glutamyl-transferase (GGT) (86 vs. 90 U/l, p = 0.487) or glutamate-oxaloacetate-transaminase (GOT) (54 vs. 68 U/l, p = 0.243) levels. However, glutamate-pyruvate-transaminase (GPT) was lower in the control group (47 vs. 31 U/l, p = 0.017). While in the COVID-19 group all patients had serum lactate levels below 3.0 mmol/l with a median of 1.1 mmol/l, lactate levels were significantly higher (median 2.1 mmol/l; p = 0.014).
Renal function parameters such as creatinine and urea levels were significantly lower in the COVID-19 group (creatinine: 1.1 vs 1.4 mg/dl, p = 0.047; urea: 30 vs. 58 mg/dl, p = 0.020, Fig. 2 ). All demographic and laboratory values are displayed in Table 1 .
Fig. 2.
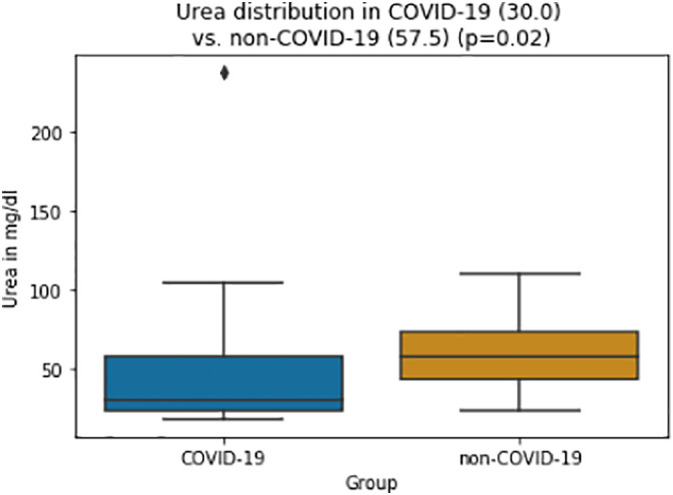
Box-and-whisker plots of Urea values for COVID-19 and control group (median, whiskers (1.5 IQR) and outliers).
Table 1.
Descriptive statistics of demographic and laboratory parameters (median and IQR).
| Normal range | COVID-19 (n = 22) | Non-COVID-19 (n = 14) | |
|---|---|---|---|
| Age [years]⁎ | 64 (52, 70) | 49 (36, 57) | |
| Male/female | 19/3 | 9/5 | |
| BMI [kg/m2] | 18.5–24.9 | 27 (24, 31) | 26 (22, 32) |
| Horovitz index | 161 (119, 190) | 86 (76, 171) | |
| Haemoglobin [g/dl]⁎⁎ | 13.1 (11.8, 13.3) | 9.3 (8.3, 12.6) | |
| Female | 11.5–15.4 | 11.1 (10.3, 12.4) | 9.3 (8.7, 9.7) |
| Male | 13.5–17.5 | 13.1 (12.0, 13.3) | 10.2 (7.8, 13.7) |
| Haematocrit⁎⁎ | 0.356 (0.334, 0.377) | 0.301 (0.268, 0.326) | |
| Female | 0.346–0.453 | 0.348 (0.290, 0,363) | 0.298 (0.285, 0.304) |
| Male | 0.396–0.506 | 0.361 (0.337, 0.376) | 0.308 (0.257, 0.434) |
| SOFA score⁎ | 11.5 (10.3, 12.0) | 15.0 (13.3, 15.0) | |
| Leukocytes [G/l] | 3.9–10.4 | 11.9 (7.3, 15.3) | 16.0 (9.6, 24.6) |
| Bilirubin [mg/dl] | ≤1.2 | 0.6 (0.4, 0.9) | 0.8 (0.6, 1.1) |
| GOT [U/l] | ≤49 | 54 (42, 74) | 68 (45, 104) |
| GPT [U/l]⁎ | ≤49 | 47 (33, 87) | 31 (25, 36) |
| GGT [U/l] | ≤59 | 86 (35, 149) | 90 (38, 131) |
| Lactate [mmol/l]⁎ | ≤2.4 | 1.1 (1.0, 1.4) | 2.1 (1.1, 7.1) |
| Creatinine [mg/dl]⁎ | 0.5–1.2 | 1.1 (0.9, 1.4) | 1.4 (1.1, 2.6) |
| Urea [mg/dl]⁎ | 17–49 | 30 (23, 58) | 58 (44, 74) |
| CRP [mg/dl]⁎ | ≤0.5 | 15.6 (10.3, 18.8) | 27.4 (16.0, 32.8) |
| PCT [ng/dl]⁎ | ≤0.1 | 0.4 (0.1, 0.9) | 3.5 (1.0, 86.9) |
| IL-6 [pg/ml]⁎ | ≤5.9 | 147 (70, 431) | 2710 (271, 45202) |
BMI body mass index, SOFA score sequential organ failure assessment score, GOT glutamate-oxaloacetate-transaminase, GPT glutamate-pyruvate-transaminase, GGT gamma-glutamyl-transferase, CRP C-reactive protein, PCT procalcitonin.
p < 0.05.
No differences between genders.
After adjustment for sex, both haemoglobin and haematocrit values showed no significant differences between the groups (see Table 1). Likewise, leukocyte and platelet count did not differ significantly between the groups (leukocytes: 11.9 vs. 16.0 G/l, p = 0.079; platelets: 227 vs. 175 G/l, p = 0.223).
All inflammation markers were significantly lower in the COVID-19 group: while the median interleukin-6 (IL-6) level of the COVID-19 group was 147 pg/ml with a maximum at 6594 pg/ml, IL-6 levels in the control group were significantly higher with a median of 2710 pg/ml and maximum levels as high as in the hundred thousand (p = 0.003). Similarly, procalcitonin (PCT) levels as well as C-reactive protein (CRP) levels were significantly higher in the non-COVID-19 group (PCT: 0.4 vs. 3.5 ng/dl, p < 0.001; CRP: 15.6 vs. 27.4 mg/dl, p = 0.037).
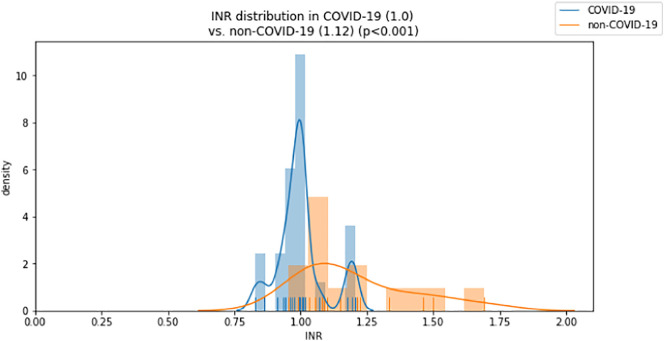
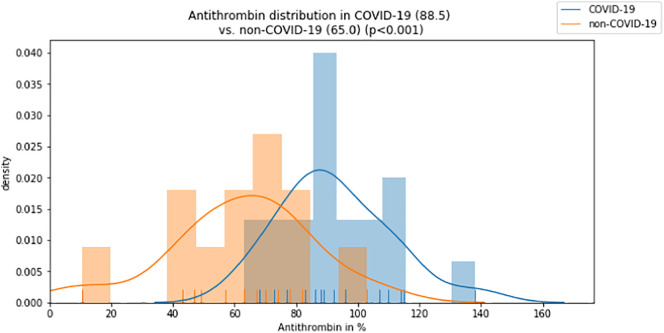
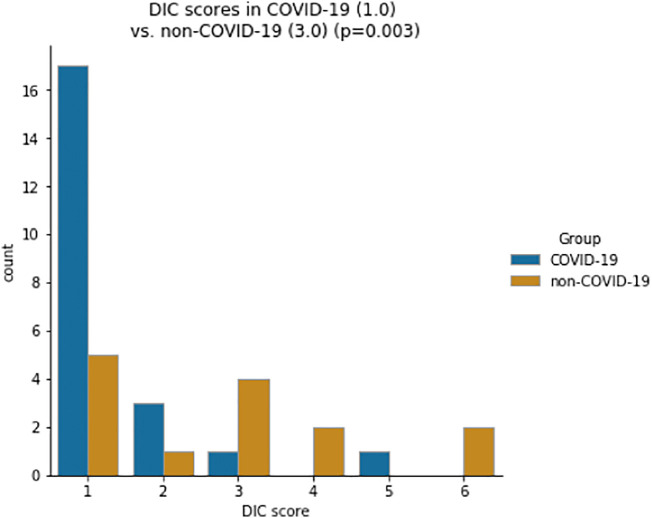
While activated partial thromboplastin time (aPTT) and fibrinogen levels did not differ significantly between groups (aPTT: 29 vs. 29 s, p = 0.274; fibrinogen: 709 vs. 598 mg/dl, p = 0.058), INR was lower and antithrombin was significantly higher in the COVID-19 group compared to the non-COVID-19 group (INR: 1.0 vs. 1.1, p < 0.001; antithrombin: 89 vs. 65%, p < 0.001; Fig. 3, Fig. 4 ). D-dimer levels and the DIC score were significantly lower in the COVID-19 group (D-dimer 2.4 vs. 11.3 μg/ml, p = 0.004; DIC score 1 vs. 3, p = 0.003; Fig. 5, Fig. 6 ).
Fig. 3.
Distribution of INR values for COVID-19 and control group (median).
Fig. 4.
Distribution of Antithrombin values for COVID-19 and control group (median).
Fig. 5.
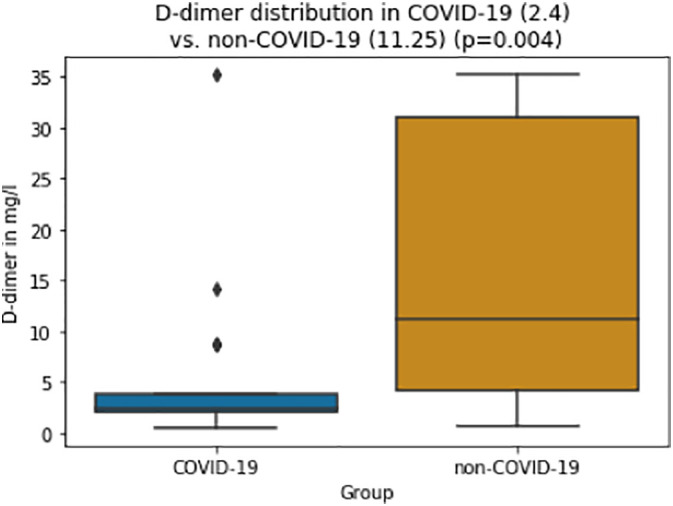
Box-and-whisker plots of D-dimer values for COVID-19 and control group (median, whiskers (1.5 IQR) and outliers).
Fig. 6.
Distribution of DIC scores for COVID-19 and control group (median).
While ExTEM® clotting time (CT) and clot formation time (CFT) tended to be shorter in the COVID-19 group and thus were not statistically significant (CT: 62 vs. 70 s, p = 0.094; CFT: 93 vs. 84 s; p = 0.301), TT20 was significantly shorter in the COVID-19 group (143 vs. 155, p = 0.047).
Neither maximum clot firmness (MCF) nor maximum lysis (ML) in ExTEM® differed between groups (MCF: 65 vs. 66 mm; p = 0.456; ML: 6.5 vs. 5.0%; p = 0.151).
In FibTEM®, median MCF of the COVID-19 group was 7 mm higher compared to the non-COVID-19 group (29 vs. 22 mm; p = 0.005). In addition, nine of eleven COVID-19 patients had readings above the normal range of 9–25 mm.
The readings of VWF activity and antigen as well as protein C and S levels were only available for seven COVID-19 patients only but none of the control patients. Median VWF activity and antigen levels were 225% and 284%, respectively. While protein C levels were 104% in median, the median protein S level was 74%.
All coagulation parameters are displayed in Table 2 .
Table 2.
Descriptive statistics of standard coagulation parameters, thromboelastometry, platelet function analysis, and DIC score (median and IQR).
| Normal range | COVID-19 | Non-COVID-19 | |
|---|---|---|---|
| Platelets [G/l] | 146–391 | 227 (175, 324) | 175 (113, 347) |
| VWF activity [%] | 48–148 | 226 (204, 312) | NA |
| VWF antigen [%] | 53–154 | 300 (249, 371) | NA |
| aPTT [sec] | 22–34 | 29 (26, 32) | 29 (26, 42) |
| INR⁎ | 0.8–1.2 | 1.00 (0.96, 1.01) | 1.12 (1.06, 1.31) |
| Fibrinogen [mg/dl] | 210–400 | 709 (530, 786) | 598 (502, 645) |
| Antithrombin [%]⁎ | 75–130 | 89 (82, 104) | 65 (49, 75) |
| D-dimer [mg/l]⁎ | ≤0.5 | 2.4 (2.0, 3.9) | 11.3 (4.1, 31.0) |
| EXTEM® CT [sec] | 38–79 | 62 (56, 68) | 70 (58, 78) |
| EXTEM® CFT [sec] | 34–159 | 93 (55, 97) | 84 (80, 113) |
| EXTEM® Time to twenty⁎ | 143 (119, 151) | 155 (140, 177) | |
| EXTEM® MCF [mm] | 50–72 | 65 (63, 70) | 66 (53, 72) |
| EXTEM® ML [%] | ≤15 | 6.5 (4.5, 9.0) | 5.0 (2.3, 7.0) |
| FIBTEM® MCF [mm]⁎ | 9–25 | 29 (24, 34) | 22 (18, 24) |
| DIC score⁎ | 1 (1, 1) | 3 (1, 4) |
NA not available, VWF von-Willebrand-Factor, aPTT activated partial thromboplastin time, INR international normalized ratio, D-dimer in fibrinogen equivalent units (FEU), CT clotting time, CFT clot formation time, MCF maximal clot firmness, ML maximum lysis, DIC Disseminated Intravascular Coagulation.
p < 0.05.
4. Discussion
As COVID-19 is not only a pandemic disease but also new in its appearance, there is little knowledge about the characteristics of the disease and the best suitable treatment. As there are several reports about thromboembolic complications and coagulation alterations leading to a worse outcome in COVID-19 patients, coagulation management and the understanding of underlying haemostasis alterations are of key importance [2,4,9].
This retrospective study assessed the coagulation profiles at ICU admission of critically ill SARS-CoV-2 positive ARDS patients in comparison to patients suffering from ARDS due to other pathogens. There was almost no significant difference regarding pulmonary and liver functions. Only GPT differed significantly between both groups but was still just above the normal range.
Patients in the COVID-19 group presented with significantly lower international normalized ratios and shorter ExTEM® TT20 values. Furthermore, fibrinogen plasma concentrations in the COVID-19 group were above normal range and elevated compared to the control group. Though no statistical significance was found here, this trend is reflected in a significantly higher MCF in FibTEM® (p = 0.005). D-dimer levels and DIC-score values of the COVID-19 group were significantly lower than in the control group (Fig. 5, Fig. 6).
In this cohort, COVID-19 patients had a median age of 64 years with quite a narrow interquartile range from 52 to 70 years. Most of the patients were male (odds = 19/3), which is in line with findings from other publications delineating COVID-19 patients [10].
In contrast, patients in the control group were younger (median of 49 years) and had a higher proportion of female patients (odds = 5/9).
IL-6, a plasma cytokine marker of inflammation, was markedly lower in the COVID-19 group.
However, as IL-6 tends to remain at lower levels in viral infections, this effect might be explained by the higher proportion of bacterial infections in the control group [11].
Similarly, CRP and PCT were higher in the control group. These acute phase proteins can be stimulated by both viral or bacterial infections, but typically reach higher values in the latter [11,12]. IL-18 and ferritin levels are described as markers more sensitive to viral infections but were not measured in our patients.
Patients in the control group had a significantly higher SOFA score at admission, which can be explained to a large extent by an impaired renal function - as represented by increased serum creatinine and urea levels. In addition, the presence of a septic shock, which can be suspected due to increased lactate levels, would result in higher values in the SOFA score.
However, lung function, platelet count and liver function did not show any clinically relevant differences between groups.
In this study, the majority of platelet counts in the COVID-19 group was within or even below the normal range. This finding is supported by the reports of Chen et al. and Guan et al. [10,13]. Conversely, Yin et al. described a higher platelet count in patients with severe pneumonia induced by SARS-CoV-2 than in those induced by non-SARS-CoV2 [2].
Though activation of thrombocytes is well described during any infection and systemic inflammation [14], COVID-19 patients clinically exhibit an increased risk for cardiovascular events: both Guo et al. as well as Chen et al. reported on myocardial injury, manifestation of cardiovascular disease and myocardial infarction in COVID-19 patients [15,16].
Elevated VWF activity and antigen levels are factors contributing to an enhanced platelet aggregation. Both levels were markedly elevated in the COVID-19 patients reaching values up to 490% of the normal range. VWF is a major determinant of platelet adhesion after vessel injury and consequently clot formation. High VWF antigen levels are an independent risk factor for ischaemic stroke as well as myocardial infarction [17]. Hence, the application of platelet inhibitors might be considered in order to avoid cardiovascular events.
However, elevated VWF levels do not seem to be a COVID-19 specific phenomenon. As VWF levels have not been routinely measured in our unit in the past, we cannot provide any VWF levels for the control group. Still, Ware et al. as well as Bajaj and Tricomi already described elevated VWF levels ranging from 350% to 425% in ARDS patients [18,19].
In our study population, COVID-19 patients also showed elevated plasmatic coagulation parameters compared to non-COVID-19 ARDS patients. 12 out of 22 COVID-19 patients had shortened prothrombin times, with two patients having international normalized ratios below the normal range.
This is in line with the findings from Han et al. [20] and also reflected in the thromboelastometry results: TT20 in ExTEM® - which correlates to prothrombin time or INR - was significantly shorter in the COVID-19 group. However, recent information suggest that COVID-19 can affect liver cells as well. Of note to our knowledge information is missing on whether COVID-19 can cause viral hepatitis [21]. Irrespective of this, COVID-19 cases with compromised liver function have been reported [13], whereas in our cohort only GPT showed a clinically irrelevant but statistically significant difference.
Furthermore, COVID-19 patients in our study revealed markedly elevated fibrinogen levels and concordantly supra-normal maximum clot firmness levels in FibTEM®. While in 7 out of 11 COVID-19 patients, FibTEM® MCF values were above the normal range, FibTEM® MCF values in the remaining group were within normal range. In the control group, only one patient showed elevated FibTEM® MCF values, and two patients even showed values below the normal range.
Reports about thromboembolic events including fatal pulmonary embolism underline the clinical relevance of these findings [3]. Tang et al. demonstrated that anticoagulant therapy can improve outcome of COVID-19 patients with elevated D-dimer levels and positive DIC-scores [22].
Several publications report a correlation of elevated D-dimer levels and a poor outcome [9,23]. However, in our cohort, D-dimer levels of COVID-19 patients were comparably low and DIC-score values were not suggesting ongoing DIC.
As our patients therefore presented with a high procoagulant potential - especially when compared to non-COVID-19 ARDS patients, these results not only underline the recommendations of a strict and consequent thrombosis prophylaxis but also might support references that advise aiming for a high prophylactic dosing. While many authors recommend the use of low-molecular-weight-heparin and an intermediate or half-therapeutic dosing for COVID-19 patients, a therapeutic anticoagulation regimen might be an option in selected intubated and critically-ill patients [4,24,25]. Given the still scarce evidence regarding COVID-19 patients, dosing considerations and the choice of the anticoagulant are probably best subject to a patient individual risk assessment including thrombosis and haemorrhage risk as well as considering liver and renal functions [25].
Although our results extend the current state of knowledge regarding coagulation in COVID-19 patients, this study faces some limitations. Due to a small sample size only non-parametric tests were applied, which have less power and lack the ability to detect small effects. The smaller the true-effect size, the larger the study needs to be to distinguish between real effects and random variations [26]. However, smaller effect sizes might indicate less clinical significance. Our findings are based on an effect size detectable by a small sample size and thus, show a clinical significance.
Another limitation is the retrospective nature of our study. As there was no systematic screening for thromboembolic events during the ICU stay, there were no data available about clinical complications in these patients. Unfortunately, as some of the patients were admitted from other departments or even other hospitals for evaluation of ECMO therapy, we could not retrieve any data about the pre-ICU course. Here, possible confounders might be hidden as a higher SOFA score in the control group might indicate. Still, the Horovitz oxygenation indices imply similar pulmonary functions in both groups.
Since most COVID-19 patients are still in the ICU and mechanically ventilated, no outcome parameters are available as of this date.
In summary, COVID-19 patients presented with a higher procoagulatory potential but lower DIC score values. Based on our findings of an increased plasmatic coagulation and in line with other recent publications [4,22], we may suggest an anticoagulation treatment with high prophylactic doses. Additionally, against the background of a high platelet aggregation risk, the use of antiplatelet therapy may be considered in patients with increased cardiovascular risk factors.
Further studies are needed to investigate the aetiology of coagulation disturbances and to evaluate the effectiveness and risks of such an anticoagulation therapy in COVID-19 patients.
CRediT authorship contribution statement
DJH, MZ, MS contributed to the study conception and design. Data collection and analysis were performed by DJH, JL, ABP. Data interpretation was performed by DJH, ABP, MZ, MB, MS, SS, LCH, and BZ. The first draft of the manuscript was written by DJH and ABP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
This study compiled with the edicts of the 1975 Declaration of Helsinki and was approved by the institutional review board of the university of Munich (20-345).
Consent to participate
Consent was waived by review board of the university of Munich.
Consent for publication
All authors read and approved the final version of the manuscript and consented to submission to Thrombosis Research.
Availability of data and material
All anonymous data available upon request from corresponding author.
Code availability
Custom code available upon request from corresponding author.
Funding
Not applicable/no funding.
Declaration of competing interest
All authors declare that they have no competing interests.
Acknowledgements
We thank Kornelia Lesser-Wetzold and the team of the Institute of Laboratory Medicine as well as all personnel on the ICU for their ongoing support.
References
- 1.Zhang J., Yang S., Xu Y., Liu J., Guo J., Tian S. Epidemiological and clinical characteristics of COVID-19 infection outside Wuhan, China: a multicenter study. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3546040. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3546040 [DOI] [Google Scholar]
- 2.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Max von Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie. Mikrobiologische Diagnostik und Krankenhaushygiene. http://www.mvp.uni-muenchen.de/fileadmin/diagnostik/Teaserbilder/2020-02-14-_Leistungsverzeichnis.pdf [cited 15 Apr 2020]. Available.
- 6.https://www.rotem.de/produkte/rotem-delta/reagenzien/ Website. [cited 12 Apr 2020]. Available.
- 7.Levi M., Toh C.H., Thachil J., Watson H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 8.Krithikadatta J. Normal distribution. J. Conserv. Dent. 2014;17:96–97. doi: 10.4103/0972-0707.124171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slaats J., Ten Oever J., van de Veerdonk F.L., Netea M.G. IL-1β/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gendrel D., Raymond J., Coste J., Moulin F., Lorrot M., Guérin S. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr. Infect. Dis. J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinosoglou K., Alexopoulos D. Use of antiplatelet agents in sepsis: a glimpse into the future. Thromb. Res. 2014;133:131–138. doi: 10.1016/j.thromres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P. Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 17.Andersson H.M., Siegerink B., Luken B.M., Crawley J.T.B., Algra A., Lane D.A. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555–1560. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 18.Ware L.B., Eisner M.D., Thompson B.T., Parsons P.E., Matthay M.A. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am. J. Respir. Crit. Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj M.S., Tricomi S.M. Plasma levels of the three endothelial-specific proteins von Willebrand factor, tissue factor pathway inhibitor, and thrombomodulin do not predict the development of acute respiratory distress syndrome. Intensive Care Med. 1999:1259–1266. doi: 10.1007/s001340051054. [DOI] [PubMed] [Google Scholar]
- 20.Han H., Yang L., Liu R., Liu F., Wu K.-L., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arachchillage D.R., Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluge S., Janssens U., Welte T., Weber-Carstens S., Marx G., Karagiannidis C. Empfehlungen zur intensivmedizinischen Therapie von Patienten mit COVID-19*. InFo Hämatologie Onkologie. 2020:17–19. doi: 10.1007/s15004-020-8072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackshaw A. Small studies: strengths and limitations. Eur. Respir. J. 2008:1141–1143. doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All anonymous data available upon request from corresponding author.