Abstract
Background
There is an increasing interest in safely delivering high dose of inhaled nitric oxide (NO) as an antimicrobial and antiviral therapeutics for spontaneously breathing patients. A novel NO delivery system is described.
Methods
We developed a gas delivery system that utilizes standard respiratory circuit connectors, a reservoir bag, and a scavenging chamber containing calcium hydroxide. The performance of the system was tested using a mechanical lung, assessing the NO concentration delivered at varying inspiratory flows. Safety was assessed in vitro and in vivo by measuring nitrogen dioxide (NO2) levels in the delivered NO gas. Lastly, we measured the inspired and expired NO and NO2 of this system in 5 healthy subjects during a 15-min administration of high dose NO (160 parts-per-million, ppm) using our delivery system.
Results
The system demonstrated stable delivery of prescribed NO levels at various inspiratory flow rates (0–50 L/min). The reservoir bag and a high flow of entering air minimized the oscillation of NO concentrations during inspiration on average 4.6 ppm for each 10 L/min increment in lung inspiratory flow.
The calcium hydroxide scavenger reduced the inhaled NO2 concentration on average 0.9 ppm (95% CI -1.58, −0.22; p = .01). We performed 49 NO administrations of 160 ppm in 5 subjects. The average concentration of inspired NO was 164.810.74 ppm, with inspired NO2 levels of 0.70.13 ppm. The subjects did not experience any adverse events; transcutaneous methemoglobin concentrations increased from 1.050.58 to 2.260.47%.
Conclusions
The system we developed to administer high-dose NO for inhalation is easy to build, reliable, was well tolerated in healthy subjects.
Keywords: Nitric oxide, Nitrogen dioxide, Delivery system
Highlights
-
•
We conceived and tested a NO delivery system for spontaneously breathing subjects.
-
•
A scavenger containing calcium hydroxide reduces the inspired NO2 concentration.
-
•
A reservoir bag reduces variations of NO concentration during breathing.
-
•
In five healthy subjects breathing 164.810.74 ppm of NO, inspired NO2 was 0.70.13 ppm.
-
•
In a healthy subject breathing 153 ppm of NO, the exhaled NO2 was 0.03 ppm.
1. Introduction
Nitric oxide (NO) is a therapeutic gas approved by the US Food and Drug Administration (FDA) in 1999 for the treatment of “term and near-term (>34 weeks) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension where it improves oxygenation and reduces the need for extracorporeal membrane oxygenation” [1]. In addition to its pulmonary vasodilator effect, NO produces broad antimicrobial activity on bacteria [2] and viruses such as SARS CoV [3,4], the virus responsible for the SARS epidemic in 2003. Based on the established anti-viral effects, several clinical trials are now testing the efficacy of NO inhalation on patients infected by SARS CoV-2, in the midst of the ongoing pandemic [[5], [6], [7]].
In spontaneously breathing patients, NO gas is traditionally delivered through a mechanical ventilator or through a high flow nasal cannula (HFNC) system [8]. Nitric oxide is blended with medical air and oxygen in the ventilator and may be delivered to the patient through a snug fitting facemask [8]. Despite this approach's ability to administer a high concentration of NO gas (>100 parts per million [ppm]), widespread adoption is challenged by the lack of a safe delivery system. In addition, in the phase of the SARS-CoV-2 pandemic, delivering NO via HFNC or ventilator-driven respiratory systems potentially aerosolizes droplets with virus, which raises further concerns for safety.
Two major patient safety aspects of administering high-dose NO are the generation of nitrogen dioxide (NO2) and methemoglobin (MetHb). Nitrogen dioxide is formed by the reaction between NO and oxygen, and when combined with water in the airways, NO2 forms nitric acid, leading to a caustic burn of the airways. When inhaling NO, methemoglobin is generated by oxidation of the iron contained in circulating hemoglobin. Methemoglobin cannot bind oxygen, so levels must be closely monitored in all subjects receiving NO (particularly high doses). Methemoglobin levels of 10%, or less, are well tolerated in a healthy subject [9]. After cessation of NO treatment, intracellular methemoglobin reductase rapidly reduces RBC MetHb levels.
An in-hospital system that is simple, inexpensive, and capable of delivering a constant and predictable concentration of NO over time, while minimizing NO2, without generating aerosolized particles is needed to allow use of high-dose NO outside the intensive care unit (ICU).
In this study, we designed and developed a breathing system capable of delivery high concentrations of NO. We evaluated performance and safety of the device both in vitro and in healthy adults by accurately sampling and measuring NO and NO2 concentrations in the inhaled and exhaled breath.
2. Materials and METHODS
2.1. System design
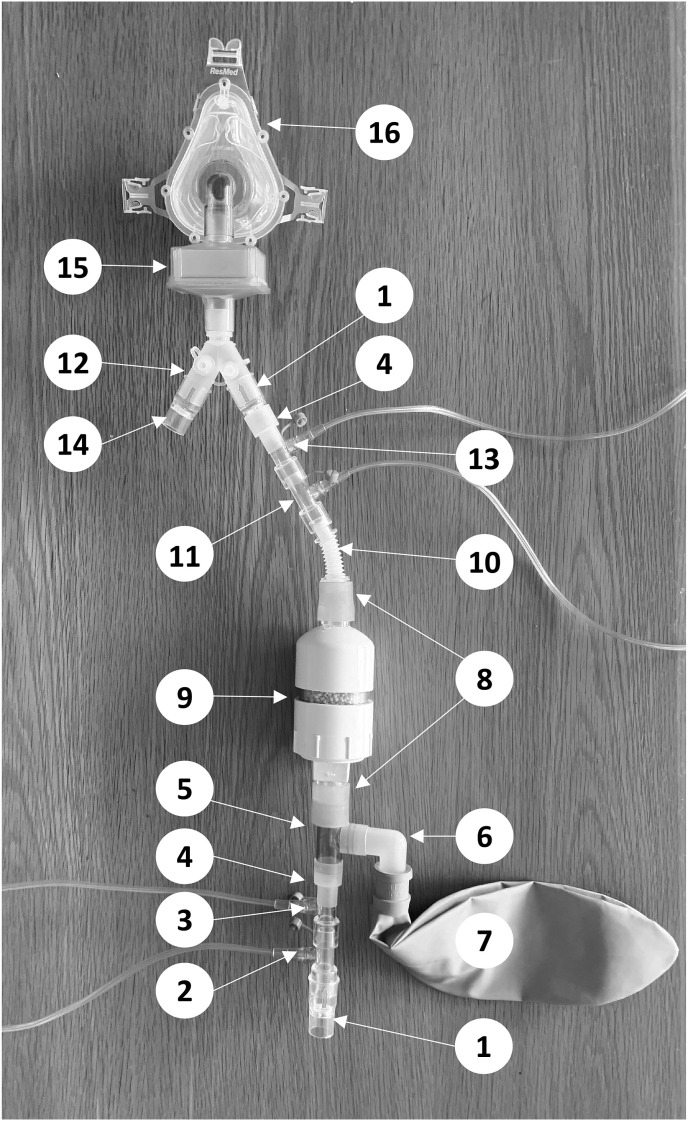
The system incorporates standard respiratory circuit connectors (Fig. 1 ). The distal portion of the inspiratory limb begins with a one-way valve (Hudson RCI, Wayne, PA, USA) and two gas inlet connectors (Hudson RCI, Wayne, PA, USA), which inject medical air and NO gas, respectively. A T-connector joins a 3 L bag to the inspiratory limb. The bag serves as an NO reservoir to stabilize the NO concentration throughout the inspiratory phase. A scavenger (internal diameter = 60 mm, internal length = 53 mm, volume = 150 mL) containing 100 g of calcium hydroxide (Spherasorb™, Intersurgical Ltd, Berkshire, UK) absorbs the NO2 generated in the gas mixture [10]. A flexible connector was inserted to accommodate patient movement and positioning. Two gas inlet connectors act as oxygen inlet and NO/NO2 sampling line, respectively. A second inspiratory one-way valve was placed after the set reservoir/scavenger to avoid additional gas mixing due to expired backflow. This system was created for the treatment of subjects with coronavirus disease 2019 (COVID-19), and a high-efficiency particulate air (HEPA) filter was connected between the Y-piece and the patient interface (full face mask or mouthpiece) to remove any aerosolized virus.
Fig. 1.
System for delivering inhaled nitric oxide (NO) during spontaneous breathing. 1. Inspiratory one-way valve; 2. Gas connector for medical air; 3. Gas connector for NO; 4. Two-step adapter; 5. T Adaptor; 6. Elbow Adaptor; 7. Reservoir bag (3 L); 8. Silicone adapter; 9. NO2 Scavenger; 10. Flex Connector; 11. Gas connector for O2; 12. Y-piece; 13. Gas sample port for NO and NO2 analyzers; 14. Expiratory one-way valve; 15. HEPA filter; 16. Snug fitting full face mask.
Active humidification was not added, and relative humidity ratio was not tested, as the device, here described, has been built for delivering intermittent, short periods of high dose nitric oxide [11].
2.2. Experimental assessments
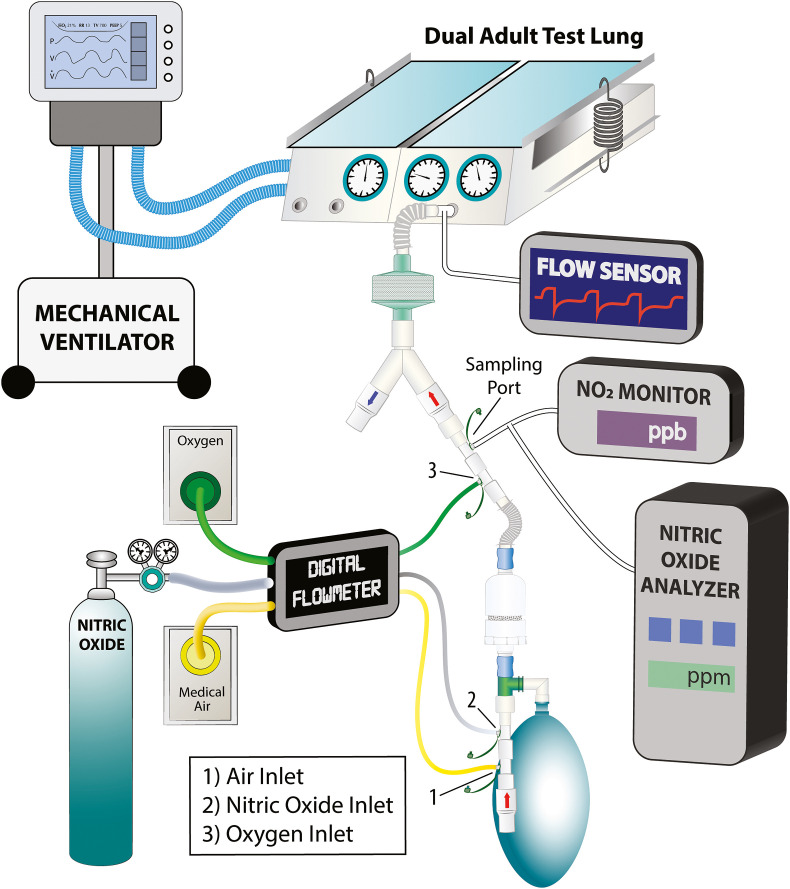
The NO delivery system performance was tested using a bench testing lung (Dual Adult Test Lung, Michigan Instruments, Michigan, USA) (Fig. 2 ) and a mechanical ventilator to simulate an inspiratory effort (Hamilton G5, Hamilton Medical AG, Bonaduz, Switzerland). The ventilator was connected to the right lung, which acts as the “diaphragm” to lift the left lung by a coupling clip. An inspiratory sinusoid flow waveform was produced by the ventilator during a volume-controlled ventilation mode. The iNO system was connected to the left lung (the “breathing” lung), which was set with a compliance of 0.05 L/cmH2O. No airway resistor was added. A digital flowmeter (Mallinckrodt Puritan-Bennett PTS 2000) was used to measure the air, O2, and NO gas flow rates. The inspired oxygen fraction was assessed with an oxygen analyzer (MiniOX® 1, Ohio Medical Corporation®, 1111 Lakeside Drive, Gurnee, IL 60031 USA).
Fig. 2.
Experimental setup. The mechanical ventilator connected to the right lung acts as a diaphragm of the left lung. The left lung is connected with the tested delivery system. During the experiment we continuously monitor the inspiratory/expiratory flow after the Y and FiO2, NO and NO2 concentration on the inspiratory limb of the circuit.
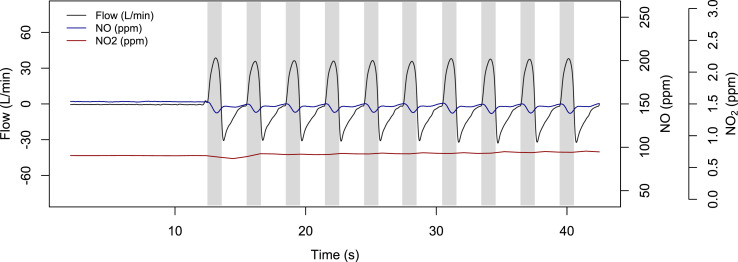
The NO concentration was measured by an NO analyzer (Sievers 280i Nitric Oxide Analyzer, GE Analytical Instruments, Boulder, CO) connected to the inspiratory limb via a sampling line proximal to the Y-connector. NO2 levels were simultaneously evaluated by the Cavity Attenuated Phase Shift (CAPS) NO2 monitor (Aerodyne Research Inc, Billerica, MA) using the same sampling port and line. NO and NO2 concentrations were measured during the inspiratory phase (Fig. 3 ). Additionally, we measured NO and NO2 concentration using an electrochemical gas sensor from a commercially available NO delivery system (iNOmax DSIR® Plus, Mallinckrodt Pharmaceuticals, Bedminster, NJ, USA).
Fig. 3.
NO and NO2 signals synchronized with the airway flow during a bench test with the artificial lung. In all the subsequent analyses, we used the average concentration during the inspiratory time (grey/shadowed area) for the NO and NO2 concentration.
The NO tank was provided at either 857 ppm (150 A, Airgas, Radnor Township, Pennsylvania, content = 4089 L at STP) or 800 ppm (Noxivent, size AQ aluminum cylinders Praxair Shimersville Road Bethlehem Pennsylvania, content = 2239 L at STP). The duration of a 2239 L tank at STP ranged between 3.1 and 37.3 h when NO was delivered at 50 ppm or 250 ppm, respectively. One should note, however, that the delivery system we described here is independent from the tank of NO employed. By introducing a standard gas connector, an operator could use our delivery system with any desirable NO source.
2.3. Performance tests
2.3.1. Reservoir effect
To reduce the fluctuations of delivered NO, we examined the efficacy of adding a reservoir bag to stabilize the concentration of the inspired NO. The experimental setup involved a respiratory rate (RR) of 15 breaths/min; tidal volume (VT) of 0.25, 0.5, 0.75 and 1 L; an inspiratory time of 1 s, and a sinusoidal flow wave. During this test the calcium hydroxide scavenger was incorporated into the system. The average inspiratory flow required to archive the set VT was used as an independent variable in the analysis. Nitric oxide concentration was measured over 2 min during the inspiratory phase of each set of tidal volume, with and without the reservoir bag. We used three NO concentrations: 50, 150 and 250 ppm. FiO2 was set at 0.21.
Similarly, we evaluated the inspired concentration of NO2, using the same experimental settings, with or without the 3 L reservoir bag.
2.3.2. Air flow effect
We examined the effect of various levels of air flow on NO concentration during ventilation. We measured the inspiratory NO concentrations at 5, 10, and 15 L/min of air flow. At every level of air flow, before starting ventilation, we set the NO gas flow to achieve a static concentration of 180 ppm NO in the inspiratory limb of the circuit.
Ventilator settings included a respiratory rate of 20 bpm, a tidal volume of 0.5 L, an inspiratory time of 1 s and sinusoidal flow wave.
2.3.3. NO and NO2 assessment in vitro
We tested the performance of the system to find the NO and oxygen flow required to obtain the desired concentration of inspired NO. Mechanical ventilation was set with a tidal volume 0.5 L, a respiratory rate of 20 bpm, an inspiratory time of 1 s and a sinusoidal flow wave. We tested 3 different target NO concentrations: 50, 150, and 250 ppm at different FiO2: 0.21, 0.30, and 0.40. NO2 levels were also measured.
2.4. Safety assessment
2.4.1. Scavenger effect
To assess the efficacy of the calcium hydroxide scavenger in reducing the inspiratory levels of NO2, we used the same mechanical ventilator settings as above, a range of target NO concentrations (50, 150, and 250 ppm), two different levels of FiO2 (0.21 and 0.40) and measured NO2 levels in the inspiratory limb with and without the scavenger.
2.4.2. NO2 assessment with healthy subjects
We administered high-dose NO with our system to healthy adult subjects as part of a randomized controlled trial [7] conducted in our center (NCT04312243). Each administration lasted for 15 min. FiO2, NO and NO2 concentrations were monitored in the inspiratory limb of the circuit. Peripheral oxygen saturation (SpO2) and methemoglobin (MetHb) were continuously and non-invasively monitored with a pulse co-oximeter (Masimo rainbow SET, Irvine, CA 92618) [12,13].
2.4.3. Exhaled NO2 measurement
Additionally, we evaluated the exhaled concentration of NO2 in one healthy subject. We administered 150 ppm NO using the previously described system. To monitor the expiratory NO2 concentration we placed the sampling line between the mouthpiece and the HEPA filter (Fig. 1). Since the continuous gas flow from the inspiratory limb of the circuit can interfere with the measure, a 3-way stopcock (2100 series, Hans Rudolph INC. Shawnee, KS, USA) was positioned before the Y: its closure at the beginning of exhalation stopped the washout effect of fresh gas coming from the inhalation arm of the circuit, allowing a sampling of exhaled gas only.
2.5. Statistical analysis
We used linear mixed models to analyze the effect of the reservoir and the scavenger on the system's performance. Statistical significance was assumed at a two-tailed P value < .05. The statistical analyses were performed in R (R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). Variables were expressed as mean and standard deviation (SD).
3. Results
3.1. System inspiratory and expiratory resistance
The total inspiratory resistance, from the inspiratory one-way valve to the HEPA filter, was on average 8.6 cmH2O/L/s. The total expiratory resistance, measured from the HEPA filter to expiratory one-way valve, was on average 7.2 cmH2O/L/s.
3.2. Reservoir effect
For NO concentrations of 50, 150, and 250 ppm, the reservoir bag decreased the inspiratory NO fluctuations, respectively, by 0.79 ppm (95% CI -1.7, 0.2; p = .09), 5.2 ppm (95% CI -7.6, −2.6; p = .004), and 7.8 ppm (95% CI -14.3, −1.3; p = .02) for each 10 L/min increment in the average lung inspiratory flow (Fig. 4 ).
Fig. 4.
Changes in inspiratory NO (ppm) during incremental inspiratory flow, with and without the reservoir bag. Nitric oxide concentrations were 50 ppm (Panel A), 150 ppm (Panel B) and 250 ppm (Panel C).
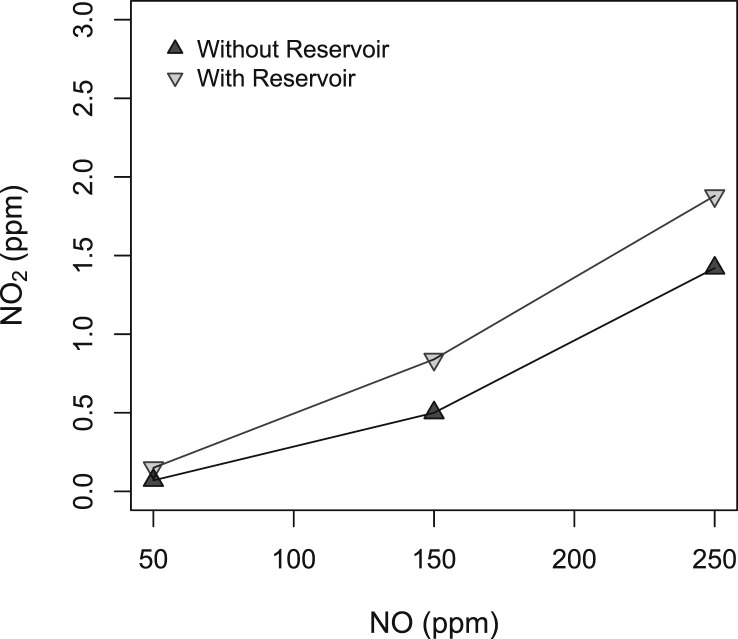
The accumulation of NO and O2 in the reservoir bag produced an increase of inspiratory NO2 on average of 0.29 ppm (95% CI 0.14, 0.43; p < .001) (Fig. 5 ). The NO2 levels were kept below 2 ppm in all NO concentrations with the reservoir bag. These data suggest that adding a reservoir bag (3 L) efficiently reduces the fluctuations of delivered NO and keeps NO2 concentrations below the recommended level [14].
Fig. 5.
Inspiratory NO2 concentrations (ppm) at increasing NO concentrations (ppm) with and without the reservoir bag. FiO2 was 0.21 in each step.
3.3. Air flow effect
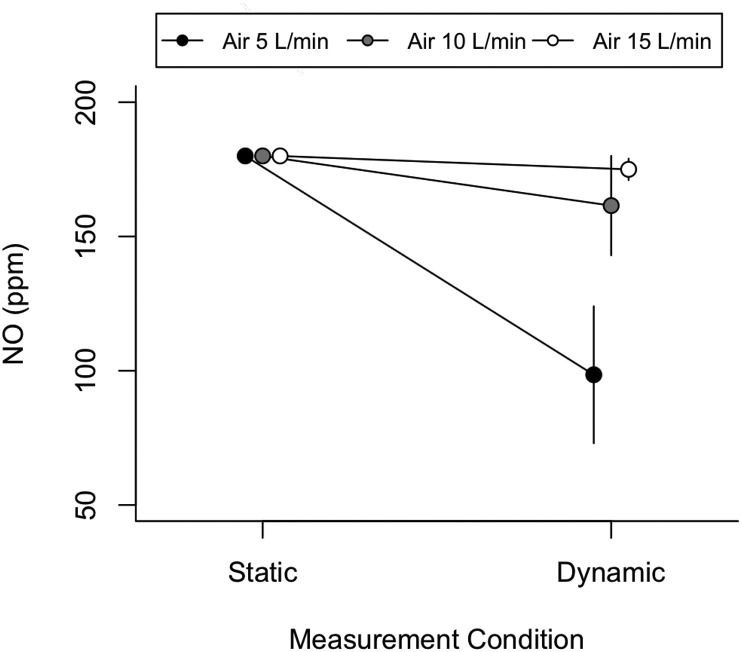
At a low air flow of 5 L/min, we measured a marked reduction of NO concentration in the inspiratory limb of the circuit: from 180 ppm to 96 ppm with 51 ppm of variation during the inspiratory phase. At 15 L/min, the concentration during ventilation was stable and decreasing of only about 8 ppm from 180 ppm to 169 ppm (Fig. 6 ). These findings indicate that maintaining air flow at 15 L/min reduces the effect of ventilation on the variation of the NO concentration in the respiratory limb.
Fig. 6.
Nitric oxide concentration (ppm) in static and dynamic conditions (during mechanical ventilation) at different air flows: 5 L/min (black dots), 10 L/min (grey dots) and 15 L/min (white dots). The dots represent the average inspiratory concentration, and error bars represent the intra-tidal swing of NO concentration during the inspiration phase.
3.4. Scavenger effect
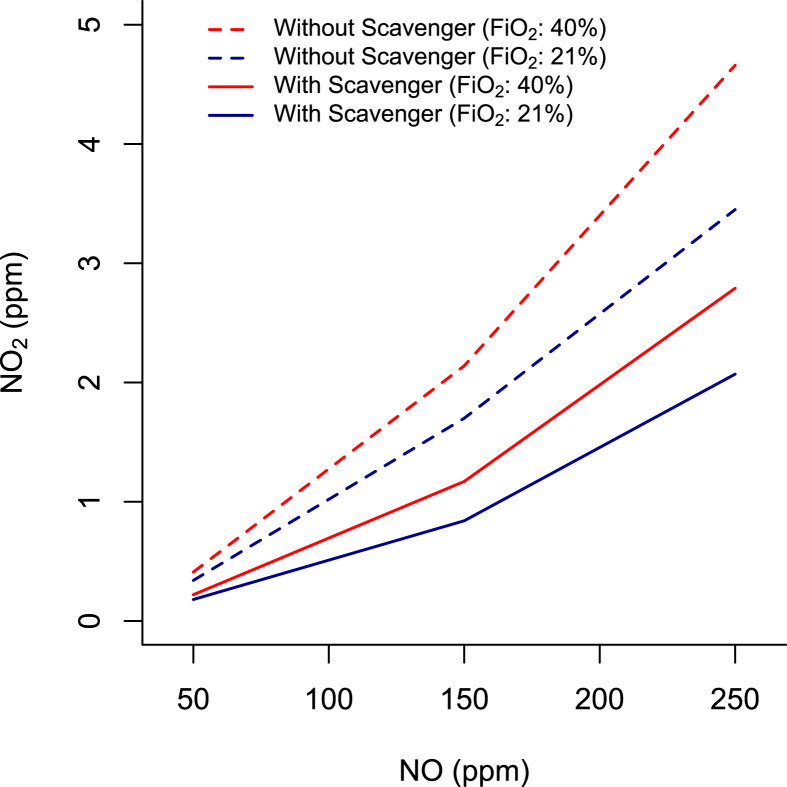
The scavenger positioned in the inspiratory limb of the circuit reduced the inhaled NO2 concentration to an average of 0.9 ppm (95% CI -1.58, −0.22; p = .01) (Fig. 7 ). At 150 ppm of inhaled NO, the NO2 concentration was maintained below 1.2 ppm with FiO2 from 0.21 to 0.40. Our data suggest that the scavenger can efficiently reduce NO2 in the circuit for NO delivery.
Fig. 7.
NO2 concentration with increasing NO concentrations (50 ppm, 150 ppm, 200 ppm) at 2 levels of FiO2 (0.21 and 0.4) with (continuous line) or without (dotted line) the calcium hydroxide scavenger.
3.5. NO flow for different NO and FiO2 target
The delivered NO concentrations varied depending on the flows of NO and O2. Three concentrations of NO (50, 145, and 245 ppm) targeted for delivery were tested. First, to obtain a concentration of 50 ppm NO (48.5 ppm measured with the chemiluminescence method, 44 ppm using the electrochemical gas phase sensor), the flows of NO ranged from 1.0 to 1.4 L/min for FiO2 levels of 0.21 and 0.41, respectively, using the 800 ppm NO/N2 tank. Using the 857 ppm tank, NO flows were set from 0.9 to 1.2 L/min for FiO2 levels of 0.21 and 0.41, respectively. Second, to obtain an NO concentration of 145 ppm (143 ppm measured with the chemiluminescence method, 130 ppm using the electrochemical gas phase sensor), the required NO flow was at 3.5 and 5.8 L/min of O2 for FiO2 levels of 0.21 and 0.41, respectively, using the 800 ppm tank. Using the 857 ppm tank, NO flows was at 3.1 L/min, and 4.5 L/min for FiO2 levels of 0.21 and 0.41, respectively. Finally, to obtain an NO concentration of 245 ppm, the required NO flow was at 7.0 and 11.9 L/min for FiO2 levels of 0.21 and 0.41, respectively, using the 800 ppm tank. Using the 857 ppm tank, NO flow was at 6.2 and 10.1 L/min for FiO2 levels of 0.21 and 0.41, respectively.
NO2 concentration was measured by the most accurate Cavity Attenuated Phase Shift (CAPS) NO2 monitor and by a hospital commonly used electrochemical gas phase sensors. In-vitro studies were performed by using 800 ppm of NO/N2 tanks. Same studies, then, were repeated with 857 ppm NO/N2 tanks. When NO tank was set to deliver 50 ppm of NO, NO2 concentration in the inspiratory limb of the circuit by CAPS monitoring was 0.13 at FiO2 of 0.21, 0.18 at FiO2 0.3 and 0.18 at FiO2 0.41. When NO flow was increased to reach 250 ppm of NO, NO2 was 1.57 at FiO2 0.21, 2.35 at FiO2 0.3 and 2.61 at FiO2 0.41.
By electrochemical methodology, at 50 ppm of NO, NO2 concentration measured 0.1 at FiO2 0.21, 0.3 at FiO2 0.3 and 0.3 at FiO2 0.41. At 250 ppm of NO, NO2 concentration measured 2.37 at FiO2 0.21, 3.17 at FiO2 0.3 and 3.61 at FiO2 0.41.
Similar NO2 values were found when 857 ppm NO/N2 tanks were used (see Table 1 ). These data provide a reference for adjusting desired NO delivery in our designed system.
Table 1.
Nitric oxide and oxygen flow (in liters/minute) were set to obtain a desired NO inspiratory concentration (50, 150, and 250 ppm) at a desired FiO2. We set medical air flow at 15 Liters/min in every setting. We used two different tanks with different NO concentrations (800 and 857 ppm).
|
NO/N2tank concentration = 800 ppm | ||||
|---|---|---|---|---|
| NO (ppm) | NO2 (ppm) | NO flow (L/min) | O2 flow (L/min) | |
| FiO2 = 0.21 | 49 | 0.13 | 1 | 0.36 |
| 145.5 | 0.72 | 3.46 | 1.11 | |
| 249.5 | 1.57 | 6.99 | 2.10 | |
| FiO2 = 0.30 | 48.5 | 0.18 | 1.2 | 3.50 |
| 140 | 0.97 | 4.25 | 4.82 | |
| 250 | 2.35 | 9.10 | 7.30 | |
| FiO2 = 0.41 | 48 | 0.18 | 1.40 | 7.12 |
| 145 | 1.17 | 5.08 | 10.20 | |
| 241.5 |
2.61 |
11.91 |
17.40 |
|
| NO/N2 tank concentration = 857 ppm | ||||
| NO (ppm) |
NO2 (ppm) |
NO flow (L/min) |
O2 flow (L/min) |
|
| FiO2 = 0.21 | 47.50 | 0.24 | 0.92 | 0.58 |
| 143.50 | 0.77 | 3.07 | 1.16 | |
| 237.50 | 1.98 | 6.18 | 2.37 | |
| FiO2 = 0.30 | 49 | 0.26 | 1.05 | 3.46 |
| 143 | 1.24 | 3.84 | 5.40 | |
| 253 | 2.79 | 7.66 | 7.22 | |
| FiO2 = 0.42 | 40 | 0.29 | 1.22 | 8.48 |
| 142 | 1.40 | 4.48 | 10.74 | |
| 236 | 3.11 | 10.05 | 15.70 | |
3.6. Clinical NO and NO2 assessment with healthy subjects
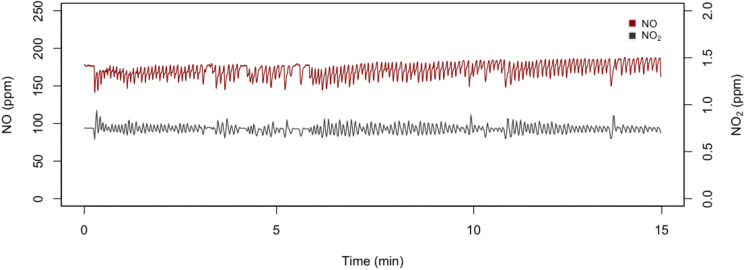
We administered NO to 5 adult health care subjects: 2 males and 3 females. Median age was 32. The subjects had no history of cardiovascular or lung disease. The total number of NO administrations was 48. The average concentration of inspired NO was 164.8 10.74 ppm with NO2 levels of 0.7 0.13 ppm; these levels remained stable throughout the administration (Fig. 8 ). We administer oxygen to keep FiO2 0.21 (see Table 1). During 15 min of administration of gaseous NO, methemoglobin levels increased from a baseline value of 1.05 0.58% to 2.26 0.47%. The subjects did not experience any discomfort during the procedure. No adverse events were reported. Despite the small number of administrations, these results indicate that breathing high concentration of NO for short period of time using our newly developed NO breathing system is feasible and well tolerated without adverse events.
Fig. 8.
NO and NO2 concentrations during 15 min of NO gas inhalation using a mechanical lung. Target NO dose = 160 ppm.
3.7. Exhaled NO2 measurement
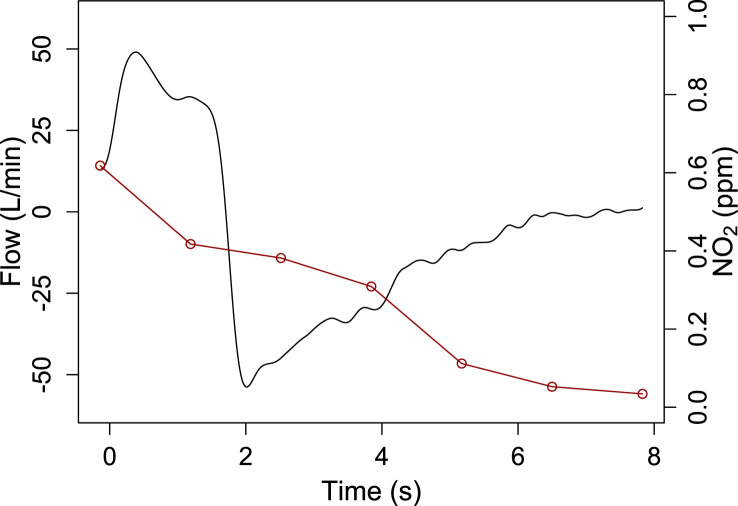
The average inspired NO and NO2 concentration were 153 ppm and 0.51 ppm, respectively, using an FiO2 of 0.205. At the end of exhalation NO2 concentration decreased to 0.03 ppm (see Fig. 9 ). Exhaled FiO2 was 0.195.
Fig. 9.
Exhaled nitrogen dioxide concentration (red line) during a single breath in a healthy subject. The black line represents flow.
4. Discussion
We built a device to reliably administer high concentration of NO in spontaneously breathing subjects without the need for a mechanical ventilator. The use of a 3-Liter reservoir and high medical air flow allow this system to maintain a stable concentration of NO throughout a wide range of tidal volumes (from 0.25 to 1 L) independently form the source of gaseous nitric oxide. This system administers a concentration of NO up to 250 ppm at a range of FiO2 from 0.21 to 0.4. Using this system, NO2 levels are maintained at the prescribed low levels [14].
Over the last few years, there has been increased interest in administering high-dose NO to spontaneously breathing patients outside the ICU setting and, possibly outside the hospital environment. Previous in vitro and in vivo evidence support the use of high-dose of NO as an antimicrobial [2,15]. Over the past two years, at Massachusetts General Hospital (Boston, MA), we have treated a teenage patient with 46-intermittent inhalation of 160 ppm NO for antibiotic resistant Burkholderia multivorans lung infection in the setting of cystic fibrosis. No adverse events or delivery system failures were observed, respiratory symptoms of the patient improved and the antibiotic pattern of the Burkholderia multivorans changed to allow common antibiotic coverage (i.e., Bactrim and Levofloxacin) [16]. Additionally, inhaled NO therapy has also shown to be a potent anti-inflammatory agent, reducing lung thrombosis after lung transplant [17].
In the setting of the current COVID-19 pandemic, we are examining whether the administration of high-dose NO in spontaneously breathing COVID-19 patients leads to a reduced rate of hospital admission (NCT04338828) and respiratory failure requiring intubation and mechanical ventilation (NCT04305457). Furthermore, we recently published a case series of 6 COVID 19 positive pregnant patients that received high dose (160–200 ppm) nitric oxide using our delivery system [18].
To avoid overwhelming COVID-19 systemic inflammation, we designed a way to deliver NO treatment early. Therapy with NO inhalation starts in the emergency department or upon hospital admission to the general care wards. One could envision treatments with NO gas with a similar prototype of NO delivery system for home use [19].
The system we designed and evaluated in this study allows for the administration of NO outside the ICU setting without a mechanical ventilator. If evidence continues to mount that high-dose inhaled NO is an effective anti-inflammatory, anti-thrombotic and anti-microbial agent, the system described can be used to treat or prophylactically treat many patients without the need for a dedicated mechanical ventilator. It has potential applications on the front lines, in an emergency room or rural clinic setting, or in low-resource settings, in the face of a pandemic due to a susceptible respiratory pathogen. The system is reproducible, inexpensive, reliable, and easy to build and maintain.
There are two important limitations of this system. First, this system does not continuously measure NO and NO2 during administration without the use of external gas analyzers. However, once calibrated, using consistent gas flows and concentrations, and fresh calcium hydroxide, the NO and NO2 levels showed to remain consistent in repeated laboratory tests. Second, to set medical air, NO, and oxygen flows, we used a high precision digital flowmeter. These flowmeters are not in widespread clinical use. The flowmeters commonly used in the clinical setting cannot reach this level of resolution, which may introduce some unexpected variability in the predicted NO and NO2 levels.
In conclusion, we built an NO delivery system that provides an alternative to a ventilator-based system [8] to give high dose NO to spontaneously breathing patients. Despite some limitations, this system can efficient to allow administering high concentrations of NO via a comfortable fitting mask.
Funding information
S.G, C.C.A.M., G.L, R.P, R.C. have nothing to declare.
LB receives salary support from K23 HL128882/NHLBI NIH as principal investigator for his work on hemolysis and nitric oxide. LB receives technologies and devices from iNO Therapeutics LLC, Praxair Inc, Masimo Corp. LB receives a grant from iNO Therapeutics LLC. This study was supported by the Reginald Jenney Endowment at Harvard Medical School to LB, by grants from NHLBI B-BIC/NCAI (#U54HL119145) to W.M.Z, an NHLBI grant (#R21HL130956) to B.Y. This study was also supported by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (MGH). W.M.Z. and B.Y. received patents at MGH on the electric generation of nitric oxide (NO). W.M.Z. is on the scientific advisory board of Third Pole Inc, which has licensed patents on electric NO generation from MGH.
CRediT authorship contribution statement
Stefano Gianni: Formal analysis, Writing - review & editing, Writing - original draft. Caio C.A. Morais: Writing - original draft, Formal analysis, Writing - review & editing. Grant Larson: Writing - original draft, Writing - review & editing. Riccardo Pinciroli: Writing - original draft. Ryan Carroll: Writing - original draft, Writing - review & editing. Binglan Yu: Writing - original draft, Writing - review & editing. Warren M. Zapol: Writing - original draft, Writing - review & editing. Lorenzo Berra: Writing - original draft, Writing - review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.niox.2020.08.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.20845_INOmax_Approv.pdf. https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20845_INOmax_Approv.pdf [cited 2020 May 25]
- 2.McMullin B.B., Chittock D.R., Roscoe D.L., Garcha H., Wang L., Miller C.C. The antimicrobial effect of nitric oxide on the bacteria that cause nosocomial pneumonia in mechanically ventilated patients in the intensive care unit. Respir. Care. 2005;50(11):6. [PubMed] [Google Scholar]
- 3.Akerström S., Gunalan V., Keng C.T., Tan Y.-J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395(1):1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist A., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79(3):1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei C, Su B, Dong H, et al. Title: Protocol of a Randomized Controlled Trial Testing Inhaled Nitric Oxide in Mechanically Ventilated Patients with Severe Acute Respiratory Syndrome in COVID-19 (SARS-CoV-2).:16..
- 6.Berra L., Lei C., Su B. 2020. Protocol for a Randomized Controlled Trial Testing Inhaled Nitric Oxide Therapy in Spontaneously Breathing Patients with COVID-19 [Internet]. Intensive Care and Critical Care Medicine.http://medrxiv.org/lookup/doi/10.1101/2020.03.10.20033522 [cited 2020 May 5] [Google Scholar]
- 7.Gianni S, Fakhr BS, Morais CCA, et al. Nitric Oxide Gas Inhalation to Prevent COVID-2019 in Healthcare Providers. :14.
- 8.Marrazzo F., Spina S., Zadek F. Protocol of a randomised controlled trial in cardiac surgical patients with endothelial dysfunction aimed to prevent postoperative acute kidney injury by administering nitric oxide gas. BMJ Open. 2019;9(7) doi: 10.1136/bmjopen-2018-026848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehman H.U. Methemoglobinemia. West J Med. 2001;175(3):193–196. doi: 10.1136/ewjm.175.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickett J.A., Mahmood N., Latimer R.D., Powroznyk A., Ghosh S., Oduro A. Effective absorption of nitrogen dioxide with soda lime. Br. J. Anaesth. 1995;74(1):107–108. doi: 10.1093/bja/74.1.107. [DOI] [PubMed] [Google Scholar]
- 11.Davidson A.C., Banham S., Elliott M. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(Suppl 2) doi: 10.1136/thoraxjnl-2015-208209. ii1–35. [DOI] [PubMed] [Google Scholar]
- 12.Feiner J.R., Bickler P.E. Improved accuracy of methemoglobin detection by pulse CO-oximetry during hypoxia. Anesth. Analg. 2010;111(5):1160–1167. doi: 10.1213/ANE.0b013e3181f46da8. [DOI] [PubMed] [Google Scholar]
- 13.Barker S.J., Curry J., Redford D., Morgan S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: a human volunteer study. Anesthesiology. 2006;105(5):892–897. doi: 10.1097/00000542-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 14.1988 OSHA PEL project - nitrogen dioxide | NIOSH | CDC [internet] 2020. https://www.cdc.gov/niosh/pel88/10102-44.html [cited 2020 May 30]
- 15.Miller C.C., Hergott C.A., Rohan M., Arsenault-Mehta K., Döring G., Mehta S. Inhaled nitric oxide decreases the bacterial load in a rat model of Pseudomonas aeruginosa pneumonia. J. Cyst. Fibros. 2013;12(6):817–820. doi: 10.1016/j.jcf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Bartley B.C.R. High-dose inhaled nitric oxide as adjunct therapy in cystic fibrosis targeting Burkholderia multivorans. Case Reports in Pediatrics. 2020 doi: 10.1155/2020/1536714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno I., Mir A., Vicente R. Analysis of interleukin-6 and interleukin-8 in lung transplantation: correlation with nitric oxide administration. Transplant. Proc. 2008;40(9):3082–3084. doi: 10.1016/j.transproceed.2008.08.124. [DOI] [PubMed] [Google Scholar]
- 18.Safaee Fakhr B., Wiegand S.B., Pinciroli R., Gianni S. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19) Obstet. Gynecol. 2020:136. doi: 10.1097/AOG.0000000000004128. Online ahed of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez R.A., Berra L., Gladwin M.T. Home NO therapy for COVID-19. Am. J. Respir. Crit. Care Med. 2020;202(1):16–20. doi: 10.1164/rccm.202005-1906ED. rccm.202005-1906ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.