1. Introduction
Increasing evidence indicates that hypercoagulability plays a significant role in the pathophysiology of severe coronavirus disease 2019 (COVID-19), contributing to macro- and microvascular thromboses [1,2]. It is of practical relevance to identify adequate diagnostic and prophylactic approaches to recognize and limit these complications. We report D-dimer performance in venous thromboembolism (VTE) diagnosis and the comparison of intermediate-dose versus standard-of-care prophylactic anticoagulation in VTE-prevention among critically-ill COVID-19 patients.
2. Methods
We performed a retrospective study at Lausanne University Hospital (CHUV). We included patients aged ≥18 years admitted to ICU for severe COVID-19 with microbiologically confirmed SARS-CoV-2 infection, between 28 February and 26 April 2020. Patients' follow-up was updated as of 3 May 2020. The study was approved by the competent ethical board (CER-VD 2020-00815).
Until 6 April 2020, internal guidelines recommended for ICU-patients with COVID-19, in absence of contraindications, a standard-of-care prophylactic anticoagulation [enoxaparin 40 mg (60 mg for patients >120 kg) q.d. or unfractionated heparin 5′000 UI bid for those with creatinine clearance <30 ml/min]. Internal guidelines implemented intermediate-dose prophylactic anticoagulation [enoxaparin 40 mg bid (60 mg bid if >120 kg) or unfractionated heparin iv 200 UI/kg/24 h in case of impaired renal function] on 7 April 2020. D-dimers were measured irregularly prior to 29 March 2020, afterwards every other day.
Primary outcome was VTE [deep venous thrombosis (DVT) assessed by compression ultrasonography, and pulmonary embolism (PE) assessed by computer tomography (CT)]. Secondary outcomes were bleeding, classified as major and non-major according to ISTH-criteria [3] and arterial thromboembolism (ATE) [ischaemic stroke assessed by CT/magnetic resonance imaging, myocardial infarction (confirmed after cardiology review), and peripheral ATE (diagnosed by experienced angiologists)]. VTE/ATE-imaging was performed according to clinical judgement. VTE diagnosed until day 1 of hospitalisation was considered as prehospital-acquired; the others as hospital-acquired. VTE until 9 April 2020 were considered related to standard-of-care prophylactic anticoagulation.
The significance level was set at 0.05. Qualitative variables were compared using Fisher's exact and Chi-square tests, and continuous variables using Mann-Whitney test.
3. Results
From 28 February to 26 April 2020, 113 patients with a microbiologically confirmed SARS-CoV-2 infection were admitted to ICU. Thirteen (12%) were excluded: eight refused to sign the “CHUV general consent to research” and five were pauci-symptomatic for SARS-CoV-2 infection and admitted to ICU for other reasons; 100 patients were included (Supplementary Fig. 1). As of 3 May 2020, 64 patients had been discharged from ICU, eight remained in ICU, and 28 had died. Males represented 74% of our sample. Median age was 63.5 years (IQR 56–73; range 30–87). The population is detailed in Supplementary Table 1.
Forty-six patients had VTE-imaging because of symptoms (n = 23), increased D-dimers (n = 9) or both (n = 14). All but two patients with a marked D-dimer increase were investigated. Twenty-two patients (n = 8 clinical symptomatology; n = 5 increased D-dimers; n = 9 both) were diagnosed with VTE: 12 lobar/segmental PE, three subsegmental PE, three proximal DVT, two distal DVT, and two patients with VTE and ATE (one with proximal DVT and cerebrovascular stroke; one with proximal DVT and peripheral ATE). Six other patients developed ATE (two cerebrovascular strokes, one ST-elevation and one non-ST-elevation myocardial infarction; two peripheral ATE).
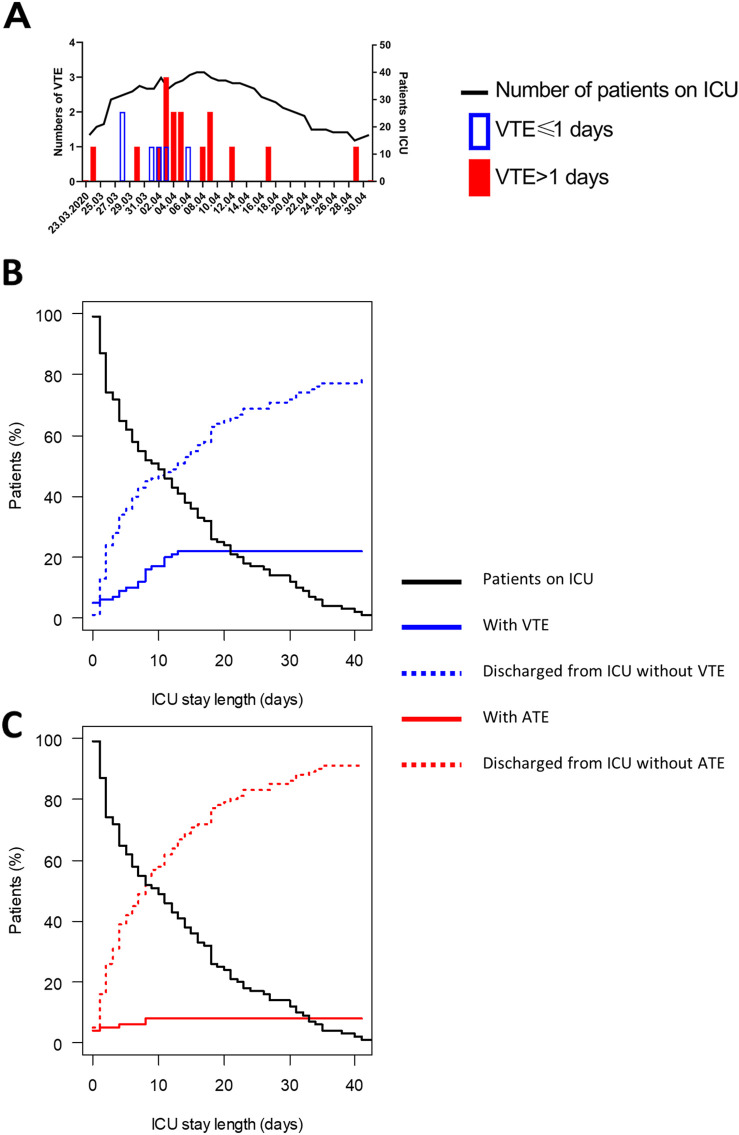
The incidence of hospital-acquired VTE decreased significantly after implementation of intermediate-dose prophylactic anticoagulation (Fig. 1A): thirteen vs. three hospital-acquired VTE, corresponding to a drop from 18.5 hospital-acquired VTE per 1′000 ICU-days during 702 patient-days to 4.9 during the following 608 patient-days (p-value 0.04).
Fig. 1.
Timing of V/ATE. Panel A: Number of patients in ICU and VTE diagnosed [open blue columns: VTE occurring before 2 days of hospitalisation (VTE ≤ 1 day); full red columns: VTE occurring starting at day 2 of hospitalisation (VTE > 1 day)]. Panel B: Cumulative proportion of VTE (continuous blue line) and evolution of the number of patients discharged from ICU without VTE (dotted blue line) and of patients still in the ICU (black line). Panel C: Cumulative proportion of ATE (continuous red line), evolution of the number of patients discharged from ICU without ATE (dotted red line) and of patients still in the ICU (black line).
A/VTE, arterial and venous thromboembolic events; ICU, intensive care unit. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 1B and C show the VTE-/ATE-cumulative proportions. The VTE-incidence was 5% at hospitalisation and 5% at ICU-admission, the ATE-incidence 2% and 4%. VTE-prevalence increased steadily during the first two weeks of hospitalisation/ICU-stay.
Four patients presented a major and 10 a non-major bleeding. The major bleedings occurred in patients receiving therapeutic anticoagulation for extracorporeal membran oxygenation (ECMO, n = 3) or no anticoagulation (n = 1, recent stroke). The non-major bleedings occurred under therapeutic anticoagulation (n = 5), prophylactic anticoagulation (n = 4) or no anticoagulation (n = 1).
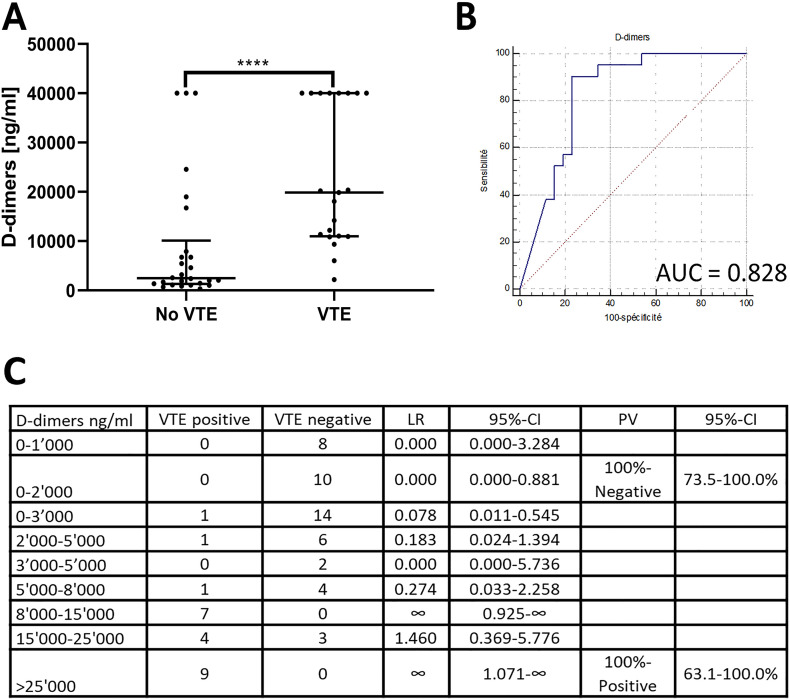
Considering the 45 patients (one excluded for suspected heparin-induced thrombocytopenia) with VTE-imaging (23 negative, 22 positive), maximal D-dimers before imaging were significantly higher in patients with VTE compared to those without (median 19′870 ng/ml vs. 2′475 ng/ml; p-value <0.0001; Fig. 2A) with an area under the ROC-curve of 0.828 (Fig. 2B). The optimal cut-off was >7′909 [sensitivity 91%, specificity 77%, positive predictive value (PPV) 76%, negative predictive value (NPV) 91%]. A NPV of 100% (95%-CI 74–100%) was obtained with a cut-off ≤2′065 ng/ml (sensitivity 100%, specificity 46%). Three patients without VTE had D-dimers >35′200 ng/ml. However, two had alternative causes for the increased D-dimers: the first underwent a 13-minute cardio-respiratory arrest a few hours before sampling and the other developed DIC under ECMO. The third patient had been treated with therapeutic anticoagulation, started just after the measurement of D-dimers >35′200 ng/ml because of clinical PE-suspicion without CT-scan. A CT-scan infirmed this suspicion three weeks later, so that PE at time of the high D-dimers value could not be excluded. After exclusion of these patients, the area under the ROC-curve increased to 0.911 and a PPV of 100% (95%-CI 63–100%) was obtained with a cut-off of >24′552 ng/ml (sensitivity 38%, specificity 100%). Likelihood ratios for D-dimer intervals are presented in Fig. 2C. Of practical utility, D-dimers were significantly higher in patients with VTE starting 3 days before diagnosis [median D-dimers 1′191 ng/ml in patients without VTE vs. 3′074 ng/ml in those with (p-value 0.021); Supplementary Fig. 2].
Fig. 2.
Diagnostic value of D-dimers in patients with VTE-imaging (n = 45). Maximal D-dimers until discharge for patients without VTE and maximal D-dimers until VTE-diagnostics were included. Panel A: Maximal D-dimers in patients with and without VTE. Comparisons between the groups were performed using Mann-Whitney test. Error bars represent median and interquartile range. Panel B represent the ROC-curve; Panel B: ROC-curve for maximal D-dimer values; Panel C: Likelihood ratios of D-dimers intervals for prediction of the VTE-occurrence based on patients with specific VTE-investigation. ****, p-value <0.0001; 95%-CI, 95%-confidence interval; AUC, area under the ROC-curve; LR, likelihood ratio; PV, predictive value; ROC-curve, receiver-operating characteristic curve; VTE, venous thromboembolic events.
Data for VTE and ATE in the whole population are presented in Supplementary Fig. 3, data for the mortality in Supplementary Fig. 4.
4. Discussion
In this retrospective study performed in COVID-19 patients admitted to ICU, we observed that serial D-dimer measurement could be helpful in identifying patients with symptomatic VTE (Fig. 2 and Supplementary Fig. 2). Additionally, our data indicates that an intermediate-dose prophylactic anticoagulation in critically-ill COVID-19 patients could safely reduce VTE-incidence.
VTE-incidence was 22%, similar to published data in COVID-19 patients [2,4] and ICU-patients with sepsis (37%) [5] but higher than in general ICU-populations (4.5–9.6%) [6,7] and non-COVID-19 ARDS-patients (3.4%) [2]. However, the observed VTE-incidence could be underestimated because VTE-imaging was performed according to clinical judgement. This is supported by an autopsy report that observed a VTE-prevalence of 58% [1]. ATE-incidence was 8% and similar to reported data [2]. Although VTE-incidence in COVID-19 approximates that of sepsis-patients [5], the pathophysiological mechanisms of hypercoagulability are different. In particular, hyperinflammation may lead to immunothrombosis, constituting a relevant cause of thromboembolic events in COVID-19 patients. This could limit the diagnostic potential of D-dimers.
A non-negligible VTE-percentage was present on admission, demonstrating hypercoagulability already in the pre-hospital phase. During hospitalisation, VTE-incidence increased steadily (Fig. 1A). Therefore, VTE must be suspected with a low threshold on admission and during the entire hospitalisation.
The rate of hospital-acquired VTE decreased from 18.5 to 4.9 per 1′000 ICU-days with intermediate-dose prophylactic anticoagulation. This is coherent with the high failure-rate observed in ARDS-patients under standard-of-care prophylactic anticoagulation [8] and supports adoption of a more intense anticoagulation in severe COVID-19 cases. Moreover, four bleedings occurred under prophylactic anticoagulation (three standard-of-care, 1 intermediate-dose) and all were minor, indicating that intermediate-dose prophylactic anticoagulation could be safe in this population.
D-dimers were significantly higher in patients with VTE compared to patients without, already three days before imaging, indicating a wide diagnostic window (Supplementary Fig. 2). D-dimers showed good diagnostic performances (Fig. 2A–C). According to analyses on patients with VTE-imaging (Fig. 2C), a cut-off of 2000 ng/ml had a 100%-NPV for VTE, which confirmed published results [4,9]. In presence of D-dimers ≥8′000 ng/ml, the VTE-likelihood ratio increased. A D-dimer cut-off of 25′000 ng/ml had a 100%-PPV. This could be used to take diagnostic decisions in emergency settings with limited resources or until imaging can be performed, in absence of increased bleeding risk.
Of note, two patients under ECMO without thromboembolic events developed D-dimers >35′200 ng/ml, despite intensive anticoagulation. High D-dimers in absence of VTE has been reported to reflect the coagulation activity and clot formation within the membrane oxygenator [10]. Our observation may indicate that clot formation in the oxygenator could be frequent in COVID-19 patients.
Our study has limitations. First, D-dimer analysis was performed during periods with standard-of-care and intermediate-dose prophylactic anticoagulation. Because only three VTE were diagnosed in the second phase, it was impossible to perform separate analyses. Second, this is a retrospective, mono-centric study limiting its generalisation. Third, VTE/ATE-imaging was performed according to clinical judgement and VTE-incidence is likely underestimated. However, since during the second period the awareness of the thromboembolic risk in COVID-19 was higher, the reduction of VTE-incidence observed is even more robust. Fourth, the SARS-CoV-2 epidemic was ongoing at time of data analysis and eight patients were still in ICU. Five, follow-up was shorter than three months, which is the standard for the absence of hospital-acquired VTE.
In conclusion, critically-ill COVID-19 patients are at high VTE-risk. Physicians should keep a high suspicion for thromboembolism during the whole hospitalisation. An intermediate-dose prophylactic anticoagulation appears to reduce their incidence. D-dimer cut-offs of 2′000 ng/ml and 8′000 ng/ml appear useful to identify patients with low, respectively high probability of having developed VTE. Our findings need to be confirmed in prospective studies.
Ethics approval and consent to participate
This investigation was conducted according to the guidelines of the competent ethical board [Commission cantonale (VD) d'éthique de la recherche sur l'être humain, CER-VD, protocol number CER-VD 2020-00815, approved on April 20, 2020]. Specific patient informed consent for the study was waived for this retrospective study, according to the Federal Act on Research involving Human Beings on Human Research (Human Research Act, HRA). However, our centre is using a general research consent for reuse of patient's data. Patients were excluded in case of documented refusal of this general research consent.
Funding
No external funding.
CRediT authorship contribution statement
MGZ designed the study, collected data, performed statistical analysis, analysed and interpreted data, and wrote the manuscript. OP designed the study, was in charge of patient care, collected data, participated in data validation, and revised the manuscript. FG collected data, and revised the manuscript. AS was in charge of patient care, collected data, and revised the manuscript. MM analysed and interpreted data, and revised the manuscript. LM analysed and interpreted data, and revised the manuscript. OH analysed and interpreted data, and revised the manuscript. PAB analysed and interpreted data, and revised the manuscript. MPO analysed and interpreted data, and revised the manuscript. LA designed the study, analysed and interpreted data, participated in data validation, and wrote the manuscript.
All authors read and approved the submitted version of the manuscript.
Declaration of competing interest
The authors do not have competing interest to declare in relation to this manuscript.
Acknowledgment
We thank Mrs. Amagoia Madina Berastegui (Data science & Research, CHUV) for excellent administrative support and Dr. Caroline Peltier for the help for Figs. 2 and Supplementary Fig. 4. We also thank all paramedics and physicians who took care of COVID-19 patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2020.08.027.
Appendix A. Supplementary data
Supplementary material
References
- 1.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020 doi: 10.7326/m20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 4.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan D., Casper T.C., Elliott C.G., Men S., Pendleton R.C., Kraiss L.W. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224–1230. doi: 10.1378/chest.15-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boddi M., Barbani F., Abbate R., Bonizzoli M., Batacchi S., Lucente E. Reduction in deep vein thrombosis incidence in intensive care after a clinician education program. J. Thromb. Haemost. 2010;8(1):121–128. doi: 10.1111/j.1538-7836.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C., Zhang Z., Mi J., Wang X., Zou Y., Chen X. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore) 2019;98(23) doi: 10.1097/MD.0000000000015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanify J.M., Dupree L.H., Johnson D.W., Ferreira J.A. Failure of chemical thromboprophylaxis in critically ill medical and surgical patients with sepsis. J. Crit. Care. 2017;37:206–210. doi: 10.1016/j.jcrc.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dornia C., Philipp A., Bauer S., Stroszczynski C., Schreyer A.G., Muller T. D-dimers are a predictor of clot volume inside membrane oxygenators during extracorporeal membrane oxygenation. Artif. Organs. 2015;39(9):782–787. doi: 10.1111/aor.12460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material