Abstract
Background:
Frailty, originally characterized in community-dwelling older adults, is increasingly being studied and implemented for adult patients with end-stage kidney disease (ESKD) of all ages (>18 years). Frailty prevalence and manifestation are unclear in younger adults (18–64 years) with ESKD; differences likely exist based on whether the patients are treated with hemodialysis (HD) or Kidney Transplantation (KT).
Methods:
We leveraged three cohorts: 378 adults initiating hemodialysis (HD) (2008–2012), 4,304 adult kidney transplantation (KT) candidates (2009–2019), and 1,396 KT recipients (2008–2019). The frailty phenotype was measured within 6-months of dialysis initiation, at KT evaluation, and KT admission, respectively. Prevalence of frailty and its components was estimated by age (≥65vs.<65years). A Wald test for interactions was used to test whether risk factors for frailty differed by age.
Results:
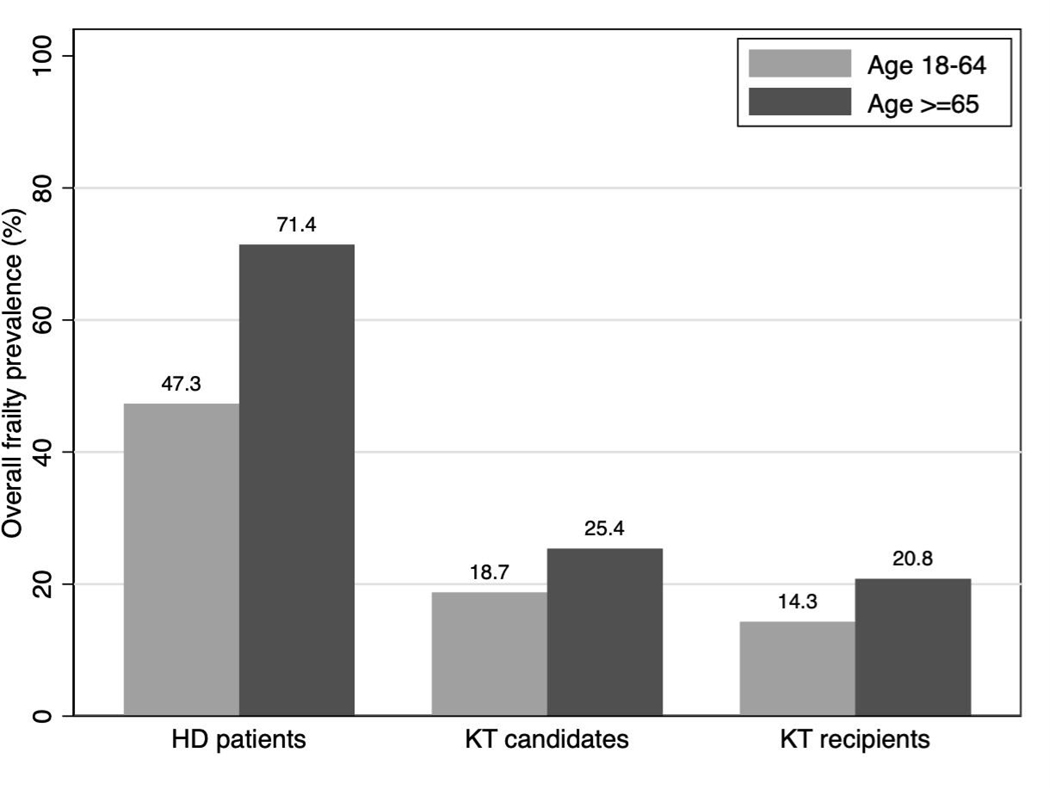
In all three cohorts, frailty prevalence was higher among older than younger adults (HD:71.4%vs.47.3%; candidates:25.4%vs.18.8%; recipients:20.8%vs.14.3%). In all cohorts, older patients were more likely to have slowness and weakness, but less likely to report exhaustion. Among candidates, older age (OR=1.79, 95%CI:1.47–2.17), non-Hispanic black race (OR=1.30, 95%CI:1.08–1.57), and dialysis type (HD vs. no dialysis: OR=2.06, 95%CI:1.61–2.64; peritoneal dialysis vs. no dialysis OR=1.78, 95%CI:1.28–2.48) were associated with frailty prevalence, but sex and Hispanic ethnicity were not. These associations did not differ by age (pinteractions>0.1). Similar results were observed for recipients and HD patients.
Conclusions:
Although frailty prevalence increases with age, younger patients have a high burden. Clinicians caring for this vulnerable population should recognize that younger patients may experience frailty, and screen all age groups.
Keywords: frailty, age, ESRD, dialysis, kidney transplantation
INTRODUCTION
Frailty has recently gained the attention of nephrologists, transplant surgeons, and renal healthcare professionals for its promise of identifying vulnerable older patients living with end-stage kidney disease (ESKD) [1–6]. Frailty, originally identified in community-dwelling older adults, is defined as a medical syndrome of multisystem dysregulation and is manifested as a compromised resistance to stressors that increases vulnerability to dependence and mortality [7]. The most commonly researched measure of frailty is the Physical Frailty Phenotype (PFP) [8], which was developed [9] and validated [10] among community-dwelling older adult populations. Importantly, this tool to measure frailty identifies those with diminished physiologic reserve even without disability and comorbidity [9, 11], which has great applicability to patients with ESKD.
Frailty, as measured by the PFP, is present in approximately 35%−48% among patients undergoing dialysis [12, 13] and is associated with adverse outcomes including falls [14], hospitalizations [15], cognitive impairment [12], and mortality [16–18]. Among kidney transplant (KT) candidates, frailty is associated with decreased access to KT [19], waitlist mortality [19–21], cognitive impairment [22], and poor health-related quality of life (HRQOL) [23]. KT recipients who are frail at the time of admission for transplantation, are more likely to experience depressive symptoms [24], poor HRQOL [25], longer length of stay [26], delirium [27], delayed graft function [28], reduction in mycophenolate mofetil (MMF) immunosuppression dose [29], early hospital readmission [30], and mortality [31, 32]. Importantly, the association between frailty and these adverse outcomes does not differ by whether the patients were older or younger, suggesting that frailty is equally as predictive for older and younger patients. While frailty research has primarily focused on risk stratification for adverse outcomes among ESKD patients of all ages, less is known about frailty among younger patients with ESKD, which may be common due to elevated levels of inflammation, lack of physical activity, and build-up of uremic toxins as a result of years on dialysis. It is likely that the prevalence of frailty and its components differ by age among patients undergoing dialysis, KT candidates, and KT recipients.
While the original studies of frailty focused on older community-dwelling adults, it is likely that frailty occurs in younger adult ESKD patients. Therefore, we estimated and compared the prevalence of frailty and each of its five components between younger and older adult patients with ESKD initiating hemodialysis (HD), being evaluated for KT, and undergoing KT. We also, examined the risk factors for frailty in each of these three cohorts.
MATERIALS AND METHODS
Study Design: Incident HD Patients
We leveraged an existing prospective cohort study of 378 patients initiating thrice weekly HD, the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study [33]. Briefly, participants were recruited from 27 dialysis units in Baltimore, Maryland, from November 2008 to August 2012. Inclusion criteria were: 1) ≥18 years of age and 2) ability to speak English. Exclusion criteria were: 1) institutionalized persons; 2) persons with a cancer diagnosis other than nonmelanoma skin cancer; 3) persons with a pacemaker or an automatic implantable cardioverter defibrillator; and 4) pregnant or nursing women [33].
All clinical and research activities being reported are consistent with the Declaration of Helsinki and the Declaration of Istanbul. The study protocol was approved by the Johns Hopkins University Institutional Review Board, MedStar Health Systems, and the medical director of each dialysis unit. Participants provided written informed consent.
Study Design: KT Candidates
We leveraged a two-center, prospective cohort study of 4,304 adult ESKD participants who were being evaluated for KT at the Johns Hopkins Hospital (1/2009–9/2019; n=4,010) and the University of Michigan Medical Center (6/2014–4/2016; n=294). All participants were enrolled at the time of KT evaluation. Inclusion criteria were: 1) ≥18 years of age and 2) ability to speak English. The Johns Hopkins Institutional Review Board and the University of Michigan Institutional Review Board approved the study, and all participants provided written informed consent.
Study Design: KT Recipients
We leveraged a two-center, prospective study of 1,396 adult KT recipient who received a transplant at the Johns Hopkins Hospital (12/2008–9/2019; n=1278) or the University of Michigan Medical center (6/2014–6/2017; n=118). All KT recipients were enrolled at admission for KT and inclusion criteria were the same as for KT candidates. The Johns Hopkins Institutional Review Board and the University of Michigan Institutional Review Board approved the study, and all participants provided written informed consent.
Frailty
Frailty was ascertained on non-dialysis days within 6 months of dialysis initiation for HD patients, at the time of evaluation for KT candidates, and at admission for KT for KT recipients. In all three cohorts, the PFP, as was defined by Fried et al [9] among community-dwelling older adults in the Cardiovascular Health Study, was used to measure frailty. The five PFP components include: shrinking (self-report of unintentional weight loss of 10 pounds in the past year, on the basis of dry weight), weakness (grip strength below an established cut-off on the basis of sex and BMI), exhaustion (self-report), low activity (kilocalories per week below an established cut-off), and slowed walking speed (walking time of 15 feet below an established cut-off by sex and height) [9]. Each of the five components was scored as 0 or 1, representing the absence or presence of that component, respectively. The aggregate frailty score was calculated as the sum of the component scores (range, 0–5), and frailty was defined as a score of 3 or greater.
Correlates of Frailty
Participant characteristics were ascertained within 6 months of dialysis initiation for HD patients, at the time of evaluation for KT candidates, and at admission for KT for KT recipients. All characteristics that were potential correlates of frailty were self-reported, measured, or abstracted from medical records (age, sex, race/ethnicity, education, household income, years on dialysis, type of dialysis for KT candidates and recipients, and smoking status). Additionally, we abstracted comorbidity information from patient electronic medical records to calculate the Charlson Comorbidity Index (CCI) adapted for ESKD [34, 35], and supplemented the data on comorbidities with participant self-report.
Descriptive Statistics by Age Group
We generated percentages for categorical characteristics, means and standard deviations (SD) for normally distributed continuous variables, and medians and interquartile ranges (IQR) for non-normally distributed continuous variables by age group. We estimated the prevalence frailty by age as a continuous variable using restricted cubic splines with three knots, which were placed at percentiles based on prior recommended approaches [36]. We tested whether the prevalence of frailty and the prevalence of the PFP components varied by older (≥65 years) compared to younger (18–64 years) HD patients, KT candidates, and KT recipients using logistic regression, and adjusted for sex, race/ethnicity, and dialysis type (among KT candidates and recipients). Additionally, we tested whether associations between age and frailty status differed by other correlates of frailty (sex, race, and ethnicity) among HD patients, KT candidates, and KT recipients by including an interaction between age and the three risk factors in separate models.
Statistical Analyses
We estimated adjusted prevalence of frailty by age using logistic regression via Stata version 15 (StataCorp, College Station, TX). Two-sided p-values < 0.05 were considered statistically significant.
RESULTS
Study Populations
Among the 378 patients initiating HD, the median age was 55 years (interquartile range [IQR]= 47–63), 42.1% were female, and 70.9% were black. In the cohort of patients initiating HD, 22.2% were older adults.
Among the 4,304 KT candidates, the median age was 56 years (IQR=45–65), 40.8% were female, and 48.0% were black. The median number of years on dialysis was 1.6 (IQR=0.6–4.4), and 63.3% of participants were undergoing HD, while 13.4% were undergoing peritoneal dialysis. In the cohort of KT candidates, 25.9% were older adults.
Among the 1,396 KT recipients, the median age was 54 years (IQR=43–64), 40.0% were female, and 40.1% were black. The median number of years on dialysis was 3.0 (IQR=1.2–5.5), and 69.8% of participants were undergoing HD and 18.3% were undergoing PD. In this cohort of KT recipients, 21.1% were older adults.
Prevalence of Frailty Among Younger and Older Patients Initiating HD
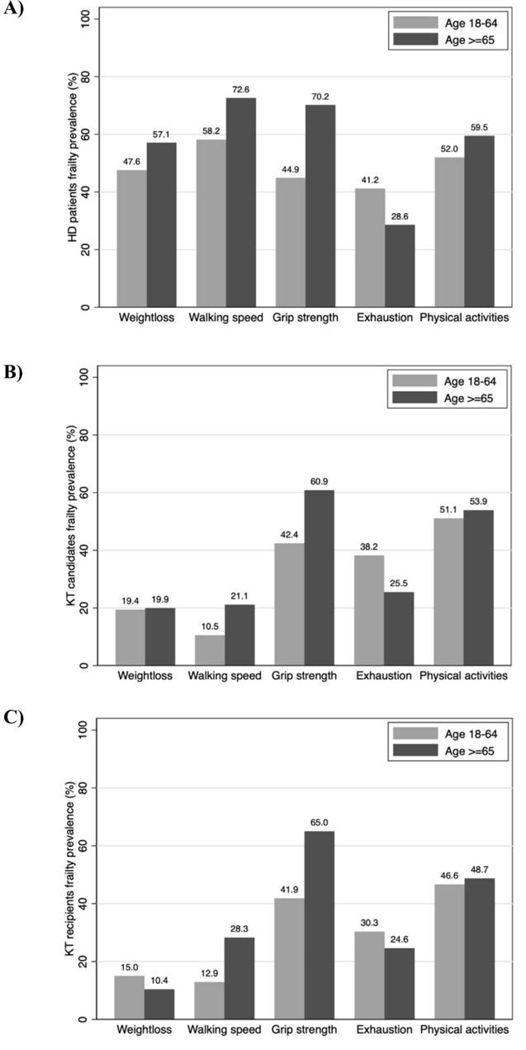
Among patients initiating HD, frailty prevalence was 71.4% for older patients and 47.3% for younger patients (Figure 1). Older patients were more likely to have unintentional weight loss (57.1% vs. 47.6%), slowness (72.6% vs. 58.2%), weakness (70.2% vs. 44.9%), and low physical activity (59.5% vs. 52.0%), but less likely to report exhaustion (28.6% vs. 41.2%) (Figure 2A).
Figure 1.
Overall frailty prevalence by age group among incident hemodialysis (HD) patients (n=378), kidney transplant (KT) candidates (n=4,304), and KT recipients (n=1,396).
Figure 2.
Prevalence of physical frailty phenotype components by age among A) incident hemodialysis (HD) patients (n=378), B) kidney transplant candidates (n=4,304), and C) kidney transplant recipients (n=1,396).
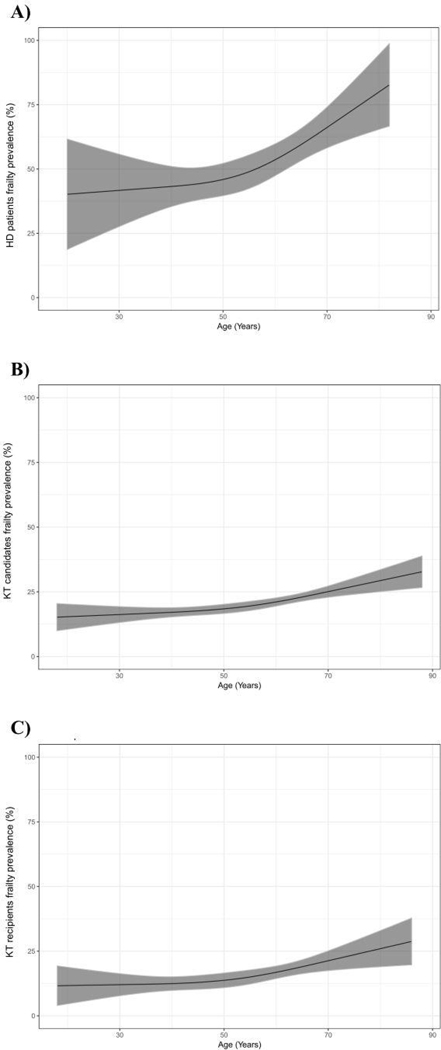
While the burden was high among younger ages, frailty prevalence increased with age (Figure 3A). Older age was associated with a 2.90-fold (95% CI: 1.69–5.11) increased odds of being frail, however there were no other factors that were associated with frailty prevalence among patients initiating HD (Table 2). Furthermore, the association between age and frailty did not differ by sex (p for interaction=0.23), race (p for interaction=0.98), or Hispanic ethnicity (p for interaction=0.95) (Table 2).
Figure 3. Prevalence of by age among A) incident hemodialysis (HD) patients (n=378), B) kidney transplant candidates (n=4,304), and C) kidney transplant recipients (n=1,396).
Recipient age was treated as a continuous variable at time of dialysis initiation for HD, evaluation for KT candidates, and admission for KT recipients. Restricted cubic splines were used. 95% confidence intervals are depicted as the grey colored region.
Table 2.
Correlates of frailty and interactions with older age among incident hemodialysis (HD) patients (n=378), kidney transplant (KT) candidates (n=4,304), and KT recipients (n=1,396).
| Risk factors for frailty | HD patients | p value (interaction) | KT Candidates | p value (interaction) | KT recipients | p value (interaction) | |
|---|---|---|---|---|---|---|---|
| Older age (≥65 years) | cOR (95% CI) | 2.79 (1.67–4.79) | 1.47 (1.25–1.74) | 1.57 (1.12–2.21) | |||
| aOR (95% CI)+ | 2.90 (1.69–5.11) | - | 1.79 (1.47–2.17) | - | 1.74 (1.16–2.62) | - | |
| Female sex | cOR (95% CI) | 1.06 (0.70–1.59) | 0.97 (0.83–1.14) | 1.04 (0.77–1.40) | |||
| aOR (95% CI) + | 0.96 (0.62–1.46) | 0.23 | 0.99 (0.83–1.19) | 0.19 | 1.24 (0.86–1.77) | 0.93 | |
| Non-Hispanic Black* | cOR (95% CI) | 0.88 (0.55–1.41) | 1.35 (1.15–1.59) | 1.32 (0.94–1.85) | |||
| aOR (95% CI) + | 1.15 (0.70–1.91) | 0.98 | 1.30 (1.08–1.57) | 0.26 | 1.28 (0.88–1.85) | 0.88 | |
| Hispanic* | cOR (95% CI) | 2.15 (0.43–15.27) | 0.85 (0.51–1.40) | 0.91 (0.35–2.38) | |||
| aOR (95% CI) + | 2.55 (0.49–19.10) | 0.95 | 0.99 (0.58–1.67) | 0.21 | 0.80 (0.27–2.35) | 0.24 | |
| Hemodialysis** | cOR (95% CI) | - | 2.12 (1.67–2.68) | 1.51 (0.84–2.72) | |||
| aOR (95% CI) + | - | - | 2.06 (1.61–2.64) | 0.51 | 1.45 (0.77–2.72) | 0.27 | |
| Peritoneal dialysis** | cOR (95% CI) | - | 1.68 (1.23–2.31) | 1.22 (0.61–2.43) | |||
| aOR (95% CI) + | - | - | 1.78 (1.28–2.48) | 0.13 | 1.22 (0.58–2.55) | 0.80 |
Abbreviations: cORs, Crude odds ratios; aORs, adjusted odds ratios.
The reference group is non-Hispanic White.
The reference group is no dialysis.
Adjusted model includes older age, sex, and race/ethnicity. The models among KT candidates and recipients also adjusted for dialysis type (no dialysis, hemodialysis, or peritoneal dialysis).
Prevalence of Frailty Among Younger and Older KT Candidates
Frailty prevalence was 25.2% for older candidates and 18.8% for younger candidates (Figure 1). Older candidates were more likely to have slowness (21.1% vs. 10.5%) and weakness (60.9% vs. 42.4%), but less likely to report exhaustion (25.5% vs. 38.2%); there were no differences in unintentional weight loss or low activity by age (Figure 2B).
Like patients initiating HD, there was a high burden of frailty across all ages, and prevalence increased with age (Figure 3B). Among KT candidates, older age (OR=1.79, 95% CI: 1.47–2.17), non-Hispanic black race (OR=1.30, 95% CI: 1.08–1.57), and dialysis type (HD vs. no dialysis: OR=2.06, 95% CI: 1.61–2.64; PD vs. no dialysis OR=1.78, 95% CI: 1.28–2.48) were all associated with frailty prevalence, but female sex (OR=0.97, 95% CI: 0.83–1.14) and Hispanic ethnicity (OR=0.85, 95% CI: 0.51–1.40) were not. Furthermore, the association between age and frailty did not differ by sex (p for interaction=0.19), race (p for interaction=0.26), ethnicity (p for interaction=0.21) or dialysis type (p for interaction of HD vs. other=0.51; p for interaction of PD vs. other=0.13).
Prevalence of Frailty Among Younger and Older KT Recipients
Among KT recipients, frailty prevalence was 20.8% for older recipients and 14.3% for younger recipients (Figure 1). Older recipients were more likely to have slowness (28.3% vs. 12.9%) and weakness (65.0% vs. 41.9%), similar levels of low physical activity (48.7% vs. 46.6%), but less likely to report exhaustion (24.6% vs. 30.3%) and unintentional weight loss (10.4% vs. 15.0%) (Figure 2C).
Among KT recipients, frailty prevalence was high across all age groups, and it increased with age (Figure 3C). Like patients initiating HD, only older age (OR=1.74, 95% CI: 1.47–2.17) was associated with frailty prevalence among KT recipients. Furthermore, the association between age and frailty did not differ by sex (p for interaction=0.93), race (p for interaction=0.88), ethnicity (p for interaction=0.24) or dialysis type (p for interaction of HD vs. other=0.27; p for interaction of PD vs. other=0.80).
DISCUSSION
Among each of the three cohorts of 378 patients initiating HD, 4,304 KT candidates, and 1,396 KT recipients, frailty was common. Additionally, frailty prevalence was higher among older compared to younger HD patients (71.4% vs. 47.3%), KT candidates (25.4% vs. 18.8%), and KT recipients (20.8% vs. 14.3%). In all three cohorts, older patients with ESKD were more likely to have slowness and weakness, but were less likely to report exhaustion. Among all three cohorts, older age was associated with frailty prevalence; non-Hispanic black race and dialysis type were also associated with frailty prevalence but only among KT candidates. Among all three cohorts of ESKD patients, older age was associated with frailty status similarly between men and women and between black and non-black patients; among KT candidates and recipients, it was additionally similar between HD and non-HD patients.
It is well recognized that frailty is a key predictor of adverse events while on dialysis [12, 37], on the waitlist [19, 20], or during/after KT [19, 26, 38]. Consequently, clinical applications of frailty assessments have emerged, such as for improving screening. Our findings reinforce the importance of such applications among patients with ESKD given the substantial burden of frailty across all stages—dialysis initiation, KT evaluation, and KT admission. Differences in prevalence across the ESKD care continuum may be attributable to the greater risk of mortality and reduced chance of KT among frail patients as suggested by prior studies [19]. Additionally, prior studies have demonstrated the dynamic nature of frailty among ESKD patients undergoing HD, on the waitlist, or recipients post-KT such that improvements in frailty scores can be observed over time [39–41]. This suggests that frailty in many cases is not an irreversible state of low physiological reserve, [42, 43] which provides an optimistic avenue for intervention.
This study also bolsters prior findings among community-dwelling older adults and patients with ESKD that frailty prevalence is associated with older age [44, 45]. It is not surprising that the highest burden of frailty was among older HD patients (71.4%). HD is recognized as a chronic stressor that can propel frailty individuals into a state of vulnerability at higher risk of adverse outcome [3, 12, 15, 16]. Together with the steady increase in the number of older adults aged 65 years and older initiating HD [46], it has become increasingly important to consider novel metrics of aging, such as frailty, to help with risk-stratification for long-term ESKD outcomes.
Despite the association with age, this study highlights that a substantial burden of frailty exists across all age groups; there is a high prevalence of frailty even among those who are not chronologically older, and irrespective of sex, race/ethnicity, and dialysis type. Unlike their healthy counterparts, younger ESKD patients present with years of cardiovascular disease burden and other comorbidities [21, 47], to the extent that their disease profiles often resemble those of older adults. Their unique medical history along with the physical and emotional burden of undergoing renal replacement therapy (dialysis or KT) can often catalyze the cycle of multisystem dysregulation and propel them into a frail state. This supports prior theories that frailty is a “biological” marker of deterioration that resembles “accelerated aging,” capturing underlying multisystem dysfunction that manifests as a vulnerability to stressors distinct from comorbidity and disability [11].
This study also builds upon prior findings by demonstrating that younger ESKD patients across all three cohorts (HD, KT candidates, and KT recipients) are more likely to report exhaustion compared to older ESKD patients, who are more likely to report slowness and weakness. However, these differences in reported exhaustion by age were less dramatic among KT recipients. This may simply suggest a difference in life circumstances. For example, younger adults remain in the work force, while older adults may enjoy the liberties of retirement. Notably, the exhaustion component of frailty is meant to capture low energy and feelings of weakness and fatigue [48]. However, it is a self-reported measure based on questions from the Centers for Epidemiologic Studies-Depression (CES-D) [9]. As a measure that is related but distinct from depression, it is possible that part of the differences observed across cohorts may be explained by differences in prevalence of undiagnosed or under-diagnosed depression [49]. Further studies are needed to confirm potential mechanisms.
This study is limited by the dialysis and transplant centers included; though results are likely reflective of the regions these centers represent, frailty prevalence is expected to vary by geographic location across the U.S., as is observed among community-dwelling older adults [44, 50]. Additionally, these data are cross-sectional in nature; these are three different cohorts representing distinct stages across the ESKD care continuum, and as such, results only apply to patients at a particular stage. Another potential limitation is frailty prevalence measured by the PFP could be partially biased due to misclassification; frailty components may measure frailty differentially across subgroups, such as by age, sex, and race [44, 51], when they should be invariant across extraneous factors. PFP criteria should be evaluated closely to determine if the current thresholds that were developed among community-dwelling older adults would also be appropriate for patients with ESKD. Nevertheless, this study has notable strengths. To date, this is the largest study of frailty prevalence among three different populations, including HD patients, KT candidates, and KT recipients.
In conclusion, although frailty prevalence is particularly high among older adults with ESKD, frailty burden exists across all age groups. Our study found that younger HD patients experienced a frailty burden that was two times higher than older KT candidates and recipients. Therefore, relying on age alone to screen for frailty can lead to a great number of vulnerable patients being overlooked for appropriate care. This reinforces the importance of frailty as a well-established tool with a biological basis that is not exclusively driven by chronological age. Additionally, the manifestation of frailty components differed by age; further research is needed to investigate whether this is a function of measurement error, or whether this a result of differing life circumstances or stages by age that may impact how frailty is manifested in patients. Findings emphasize that frailty screening should not be limited to older ESKD patients, but rather should be implemented across all age groups. Additionally, screening should take place earlier among ESKD patients initiating HD and at evaluation for KT to identify patients who would benefit most from pre-KT interventions like prehabilitation [52].
Table 1.
Demographic and health characteristics by age group for incident hemodialysis (HD) patients (n=378), kidney transplant (KT) candidates (n=4,304), and KT recipients (n=1,396).
| HD patients | KT candidates | KT recipients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Age 18–64 (N=294) | Age ≥65 (N=84) | p value | Age 18–64 (N=3,190) | Age ≥65 (N=1,114) | p value | Age 18–64 (N=1,102) | Age ≥65 (N=294) | p value |
| Female sex, % | 40.5 | 47.6 | 0.30 | 42.6 | 35.6 | <0.001 | 41.5 | 34.4 | 0.03 |
| Race/ethnicity, % | <0.001 | <0.001 | 0.004 | ||||||
| Non-Hispanic White | 19.4 | 46.4 | 44.8 | 60.2 | 53.6 | 65.6 | |||
| Non-Hispanic Black | 76.9 | 50.0 | 51.3 | 38.6 | 42.4 | 31.5 | |||
| Hispanic | 1.7 | 2.4 | 3.8 | 1.3 | 4.0 | 2.9 | |||
| Education, % | 0.26 | <0.001 | 0.001 | ||||||
| Grade school | 36.7 | 41.7 | 5.8 | 6.7 | 5.2 | 4.8 | |||
| High school | 44.6 | 35.7 | 40.0 | 33.6 | 35.9 | 32.4 | |||
| Two-year technical school | 2.0 | 2.4 | 8.4 | 5.7 | 8.8 | 6.4 | |||
| College | 11.6 | 14.3 | 31.8 | 27.2 | 32.1 | 26.0 | |||
| Graduate school | 4.8 | 6.0 | 13.8 | 26.4 | 18.0 | 30.4 | |||
| Other | 0 | 0 | 0.2 | 0.4 | 0.1 | 0.0 | |||
| Annual household income, % | 0.96 | <0.001 | 0.56 | ||||||
| < $35,000 | 48.6 | 50.0 | 11.7 | 8.8 | 7.4 | 5.8 | |||
| $35,000–74,999 | 16.7 | 15.5 | 13.9 | 17.9 | 12.7 | 10.2 | |||
| $75,000–99,999 | 4.1 | 2.4 | 4.9 | 5.3 | 5.3 | 5.1 | |||
| ≥$100,000 | 5.4 | 4.8 | 11.4 | 14.7 | 13.7 | 16.0 | |||
| Not reported | 20.1 | 22.6 | 58.1 | 53.3 | 61.0 | 62.9 | |||
| Years since dialysis initiation, median (IQR) | 0.28 (0.21–0.41) | 0.31 (0.24–0.45) | 0.09 | 1.7 (0.6, 4.9) | 1.5 (0.6, 3.5) | 0.04 | 3.2 (1.2, 6.0) | 2.4 (1.3, 3.9) | 0.04 |
| Type of dialysis | - | <0.001 | 0.01 | ||||||
| No dialysis | - | - | 21.6 | 28.4 | 10.5 | 17.1 | |||
| Hemodialysis | 100.0 | 100.0 | 64.2 | 60.8 | 70.1 | 68.5 | |||
| Peritoneal dialysis | - | - | 14.2 | 10.8 | 19.4 | 14.4 | |||
| Current smoker | 29.3 | 17.9 | 0.04 | 15.1 | 6.1 | <0.001 | 10.2 | 3.8 | 0.01 |
| Charlson comorbidities index (CCI), median (IQR) | 5.1 (3.0, 6) | 5.5 (4.8, 6.0) | <0.001 | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | <0.001 | 0.0 (0.0, 2.0) | 2.0 (0.0, 3.0) | <0.001 |
| Comorbidities, % | |||||||||
| Myocardial infarction | - | - | - | 9.4 | 14.9 | <0.001 | 4.7 | 9.9 | <0.001 |
| Peripheral vascular disease | 16.7 | 29.8 | 0.01 | 5.7 | 9.3 | 0.001 | 4.5 | 11.0 | <0.001 |
| Cerebral vascular disease | 19.7 | 31.0 | 0.04 | 7.5 | 7.3 | 0.01 | 3.4 | 4.2 | 0.08 |
| Dementia | 2.4 | 1.2 | 0.69 | 0.4 | 0.3 | 0.51 | 0.1 | 0.4 | 0.52 |
| Chronic lung disease | 10.9 | 4.8 | 0.09 | 3.0 | 5.4 | 0.01 | 5.0 | 3.9 | 0.11 |
| Rheumatological disease | 10.5 | 19.0 | 0.06 | 6.3 | 6.4 | 0.46 | 12.8 | 17.0 | 0.02 |
| Peptic ulcer disease | 6.5 | 17.9 | 0.004 | 2.5 | 3.7 | 0.02 | 3.6 | 5.0 | 0.52 |
| Diabetes | 55.4 | 65.5 | 0.11 | 40.6 | 50.7 | <0.001 | 27.1 | 44.7 | <0.001 |
| Diabetes with complications | 47.3 | 60.7 | 0.04 | 25.2 | 21.6 | 0.08 | 26.2 | 37.0 | 0.02 |
| Moderate/severe liver disease | 2.7 | 0.0 | - | 4.6 | 3.1 | 0.03 | 2.5 | 2.5 | 0.59 |
| Metastatic cancer | 0.0 | 0.0 | - | 0.9 | 2.1 | 0.03 | 0.3 | 0.7 | 0.34 |
| Lymphoma or leukemia | 3.7 | 23.8 | <0.001 | 0.9 | 1.5 | 0.22 | 0.7 | 0.7 | 0.96 |
| HIV | 12.9 | 0.0 | - | 4.6 | 0.7 | <0.001 | 2.9 | 0.7 | 0.03 |
| Congestive heart failure | 39.1 | 52.4 | 0.03 | 15.9 | 16.6 | 0.29 | 5.1 | 10.6 | 0.004 |
Acknowledgments
FUNDING SOURCES
Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Disease and the National Institute of Aging: grant numbers K01AG064040 (PI: Chu), P30AG021334 (PI: Bandeen-Roche), R01AG055781 (PI: McAdams-DeMarco), R01DK114074 (PI: McAdams-DeMarco), and K24DK101828 (PI: Segev).
Funders had no role in the study design, data collection, analysis, reporting, or decision to submit for publication.
Footnotes
STATEMENT OF ETHICS
All clinical and research activities being reported are consistent with the Declaration of Helsinki and the Declaration of Istanbul. The study protocol was approved by the Johns Hopkins University Institutional Review Board, MedStar Health Systems, and the medical director of each dialysis unit. Participants provided written informed consent.
DISCLOSURE STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and Cognitive Impairment, Frailty, and Adverse Health Outcomes in Older Patients Reaching ESRD-A Systematic Review. Clin J Am Soc Nephrol 2016;11(9):1624–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sy J, Johansen KL. The impact of frailty on outcomes in dialysis. Curr Opin Nephrol Hypertens 2017;26(6):537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen KL, Delgado C, Bao Y, et al. Frailty and dialysis initiation. Semin Dial 2013;26(6):690–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant 2019;19(4):984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdams-DeMarco MA, Van Pilsum Rasmussen SE, Chu NM, et al. Perceptions and Practices Regarding Frailty in Kidney Transplantation: Results of a National Survey. Transplantation 2020;104(2):349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Pilsum Rasmussen S, Konel J, Warsame F, et al. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol 2018;19(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14(6):392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–156 [DOI] [PubMed] [Google Scholar]
- 10.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 2006;61(3):262–266 [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2004;59(3):M255-M263 [DOI] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clin J Am Soc Nephrol 2015;10(12):2181–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Loon IN, Goto NA, Boereboom FTJ, et al. Frailty Screening Tools for Elderly Patients Incident to Dialysis. Clin J Am Soc Nephrol 2017;12(9):1480–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdams-Demarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol 2013;14(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013;61(6):896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao Y, Dalrymple L, Chertow GM, et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 2012;172(14):1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick J, Sozio S, Jaar B, et al. Frailty, body composition and the risk of mortality in incident hemodialysis patients. American Journal of Kidney Disease 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sy J, McCulloch CE, Johansen KL. Depressive symptoms, frailty, and mortality among dialysis patients. Hemodial Int 2019;23(2):239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haugen CE, Chu NM, Ying H, et al. Frailty and Access to Kidney Transplantation. Clinical Journal of the American Society of Nephrology 2019;14(4):576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients with End-Stage Renal Disease in a Prospective Cohort Study. Transplantation 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez Fernandez M, Martinez Miguel P, Ying H, et al. Comorbidity, Frailty, and Waitlist Mortality among Kidney Transplant Candidates of All Ages. Am J Nephrol 2019;49(2):103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu NM, Shi Z, Haugen CE, et al. Cognitive Function, Access to Kidney Transplantation, and Waitlist Mortality Among Kidney Transplant Candidates With or Without Diabetes. Am J Kidney Dis 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Frailty and Health-Related Quality of Life in End Stage Renal Disease Patients of All Ages. J Frailty Aging 2016;5(3):174–179 [PMC free article] [PubMed] [Google Scholar]
- 24.Konel JM, Warsame F, Ying H, et al. Depressive Symptoms, Frailty, and Adverse Outcomes among Kidney Transplant Recipients. Clin Transplant 2018:e13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg 2012;147(2):190–193 [DOI] [PubMed] [Google Scholar]
- 29.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, Mycophenolate Reduction, and Graft Loss in Kidney Transplant Recipients. Transplantation 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant 2013;13(8):2091–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant 2015;15(1):149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual Frailty Components and Mortality In Kidney Transplant Recipients. Transplantation 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parekh RS, Meoni LA, Jaar BG, et al. Rationale and design for the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. BMC Nephrology 2015;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. Journal of clinical epidemiology 1994;47(11):1245–1251 [DOI] [PubMed] [Google Scholar]
- 35.Hemmelgarn BR, Manns BJ, Quan H, et al. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 2003;42(1):125–132 [DOI] [PubMed] [Google Scholar]
- 36.Harrell FE Jr, Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer, Placed Published: 2001. [Google Scholar]
- 37.McAdams-DeMarco MA, Daubresse M, Bae S, et al. Dementia, Alzheimer’s Disease, and Mortality after Hemodialysis Initiation. Clinical Journal of the American Society of Nephrology 2018;13(9):1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu NM, Gross AL, Shaffer AA, et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. J Am Soc Nephrol 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu NM, Deng A, Ying H, et al. Dynamic Frailty Before Kidney Transplantation-Time of Measurement Matters. Transplantation 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansen KL, Dalrymple LS, Delgado C, et al. Factors Associated with Frailty and Its Trajectory among Patients on Hemodialysis. Clin J Am Soc Nephrol 2017;12(7):1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in Frailty After Kidney Transplantation. Journal of the American Geriatrics Society 2015;63(10):2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in Frailty After Kidney Transplantation. J Am Geriatr Soc 2015;63(10):2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAdams-DeMarco M A, Chu N M, Segev D L. Frailty and Long-Term Post-Kidney Transplant Outcomes, Placed Published: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci 2015;70(11):1427–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual Frailty Components and Mortality In Kidney Transplant Recipients. Transplantation 2016:in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.United States Renal Data System, 2016 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 47.Foley RN, Parfrey PS. Cardiovascular disease and mortality in ESRD. Journal of nephrology 1998;11(5):239–245 [PubMed] [Google Scholar]
- 48.Xue QL, Bandeen-Roche K, Varadhan R, et al. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci 2008;63(9):984–990 [DOI] [PubMed] [Google Scholar]
- 49.Konel JM, Warsame F, Ying H, et al. Depressive symptoms, frailty, and adverse outcomes among kidney transplant recipients. Clinical Transplantation 2018;32(10):e13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haugen CE, Thomas AG, Chu NM, et al. Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplant 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bandeen-Roche KJ, Usher T, Kasper JD. DISPARATE MEASUREMENT AS WELL AS STATUS? FRAILTY CRITERIA MANIFEST DIFFERENTLY IN BLACK AND WHITE AMERICANS. Innovation in Aging 2018;2(Suppl 1):603–603 [Google Scholar]
- 52.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clinical Transplantation 2019;33(1):e13450 [DOI] [PMC free article] [PubMed] [Google Scholar]