Abstract
Lung injury is characterized by inflammatory processes demonstrated as loss of function of the pulmonary capillary endothelial and alveolar epithelial cells. Autophagy is an intracellular digestion system that work as an inducible adaptive response to lung injury which is a resultant of exposure to various stress agents like hypoxia, ischemia-reperfusion and xenobiotics which may be manifested as acute lung injury (ALI), acute respiratory distress syndrome (ARDS), chronic lung injury (CLI), bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD), asthma, ventilator-induced lung injury (VILI), ventilator-associated lung injury (VALI), pulmonary fibrosis (PF), cystic fibrosis (CF) and radiation-induced lung injury (RILI). Numerous regulators like LC3B-II, Beclin 1, p62, HIF1/BNIP3 and mTOR play pivotal role in autophagy induction during lung injury possibly for progression/inhibition of the disease state. The present review focuses on the critical autophagic mediators and their potential cross talk with the lung injury pathophysiology thereby bringing to limelight the possible therapeutic interventions.
Keywords: Lung injury, Autophagy, Regulators, Therapeutics
Graphical abstract
1. Introduction
Autophagy is an evolutionary cellular program that serves for the breakdown of cytoplasmic components within lysosomes [[1], [2], [3]]. In 1960's, electron microscopic studies first identified autophagy in mammalian cells, but the molecular pathways were not understood until the discovery of autophagy genes (ATGs) in yeast by performing genetic screening [4]. It is a cytoprotective rather than a self-destructive process [5]. It is extensively accepted as a main regulator of innate and adaptive immune mechanisms, the change in which completely impact the pathogenesis of disease and the processes that are influenced by autophagy includes the regulation of inflammation, antigen presentation and bacterial clearance [6]. Moreover autophagy aids in the maintenance of fundamental organelle populations such as mitochondria, which is necessary for cellular bioenergetics and homeostasis [7]. Homeostasis of the cell is been accomplished by maintaining the biosynthesis and turnover.
There are two broader protein degrading systems in eukaryotic cells: first is the ubiquitin-proteasome system, responsible for the selective breakdown of most short-lived proteins and second is the lysosomal system [8,9]. The primary organelle called lysosome in eukaryotes is known for degradation through its acid hydrolases. In unfavourable nutrient deprivation condition, autophagy arbitrates a regulated phagocytosis via lysosomes [10]. Autophagy is mediated by autophagosome that is thought to be an on selective degradation system as it engulfs some of the cytoplasmic contents. The ubiquitin–proteasome system concedes only ubiquitinated proteins for degradation and it marks a remarkable contrast to autophagosome process [11].
Autophagosomes, a double-membered vesicle that engulfs durable proteins, impaired organelles, intracellular pathogenic organisms and transports it to the lysosomes that are fused to form autolysosome and the inner vesicle along with its cargo, is been degraded. At the time of starvation, the remaining macromolecules are again recycled to the cytosol for reuse [12]. The precise mechanism in cargo recognition is uncertain but this process involves ubiquitination [13]. Autophagic process is separated into distinct steps, which includes induction, recognition, selection of cargo, formation of vesicle, then occurs the fusion of autophagosome-vacuole followed by the degradation of the cargo and release into the cytosol. Various Atg proteins are indulged in this process and it consists of the central autophagic machinery [10].
Autophagy encompasses different process by which cells deliver cytoplasmic substrates for lysosomal degradation. They are macroautophagy, chaperone-mediated autophagy (CMA) and microautophagy [14]. All the three processes of autophagy are morphologically distinct [15]. In microautophagy, the lysosomal membrane invaginations or protrusions are needed to seize cargo [16]. Once the cargo is captured, the uptake directly happens at the limiting membrane of the lysosome that includes intact organelles. CMA uses chaperones to sequester cargo proteins that have a pentapeptide motif. These proteins are unfolded and translocated directly across the lysosomal membrane via LAMP2A receptor [17,18]. Macroautophagy involves requisition of cargo, vesicle formation and its subsequent transport to the lysosome [12].
In recent years, deletion of the autophagy related genes (Atg) in various model organisms has proved that autophagy plays decisive role in adaptive responses to stress, cellular differentiation and development [1]. An oncogenic event may establish by the partial minimization in the autophagic capacity. Atg6/Beclin 1, one of the phylogenetically premeditated autophagy genes, is often subdued at one locus in human cancers. Studies in mice have shown that Beclin 1 is a haplo insufficient tumor suppressor [19]. Autophagic programmed cell death was primarily depicted in actively developing tissues [18]. Several conjugation systems comprising of the Atg genes are available that take part in autophagasomal elongation [20]. One such system is the LC3 (microtubule associated protein 1 light chain 3) and Atg8 conjugation system [21]. LC-3 is the mammalian conjugative protein ortholog of yeast protein Atg8 [22]. The lipid derivative phosphatidylethanolamine binds to LC-3 to form LC3-II, an important molecular marker of autophagy. LC3-II remains on the mature autophagosome until it fuses with the lysosomes (Fig. 1 ) [23]. The Beclin 1 complex gives rise to an incipient autophagosome membrane and it assemble around cargo in a vesicle that combines with a lysosome, forming autolysosome that is degraded by acid hydrolases present in the lysosomes [24].
Fig. 1.
Mechanism of autophagy. ULK1/PI3K/mTOR signalling pathway binds to endoplasmic reticulum and activates DCEDP1 that initiates Beclin 1 and Atg5/12 conjugation system which forms isolation membrane. This is followed by the sequestration process that leads to autophagosomal formation. The autophagosome fuses with lysosomes to form autolyososome that leads to degradation.
2. Lung injury: clinical implications
The primary organ of gas exchange is the pair of lungs. They mediate inspiration of oxygen and elimination of mono and dioxides of carbon. Lung also serves as an attractive target for the entry of the pathogens. Regulation of the pulmonary functions is mediated by intricate cells of endothelial and epithelial lining, dendritic cells, alveolar macrophages and fibroblasts [25]. All these pulmonary cells are highly heterogeneous in nature. They together in association, respond to the lung injury by provoking inflammatory and immune responses [26]. Airway epithelial cells express pattern recognition receptors (PRRs) along with Toll like receptors [27], C-type lectins [28,29], RIG-I [30] and inflammasome components [31,32] which are involved in the innate response against microbes. Microbial cell wall constituents like lipopolysaccharide are sensed by PRRs and induce an inflammatory response. Alveolar epithelium comprises type I and type II alveolar cells. Apart from these the mucus layer and the physical barrier made by the epithelium contribute to the first line of defense [33]. Lung injury is described as any damage to the associated tissues and compartments of the pulmonary system. The concept of lung injury influenced by the microenvironment can be demonstrated via series of changes in cell deformability and manifestation of intercellular adhesive molecules [34]. The major causes of lung injury are atelectasis, alveolar instability, volutrauma, barotrauma, infections and oxygen toxicity [35]. The pathogenicity of acute and chronic lung injury is correlated with the release of proteases, free radicals and growth regulatory proteins by the alveolar macrophages [36]. Neutrophil takes care of the extreme ranges of lungs searching for pathogens thereby eliminating via phagocytosis. They undergo trans-endothelial and trans-epithelial migration [37]. Primary host defense mechanism of lungs include the immunity conferred by surfactant proteins (SP) namely SP-A and SP-D. They link both innate and adaptive immunity by regulating the responses of innate immune cells, antigen presenting cells (APCs) and T-cells. They can act as potential biomarkers of lung injury [38]. The implication of autophagy over lung injury is tortuous, as its function varies highly with the cell types specific to the pulmonary disorders [39]. It provides both defensive and injurious outcomes of lung injury [40].
2.1. Lung injury – types and associated pathologies
2.1.1. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
Acute lung injury and acute respiratory distress syndrome are common, grievous diseases among critically ill-patients and are evidential sources of mortality and morbidity. They are expressed along with hypercapnia and hypoxemia. ARDS is outlined as the most serious form of ALI [41]. ALI is characterized by inflammation of lung surfaces resulting in the disruption of alveolar-capillary membrane followed by transmigration of neutrophils (significant event in the procession of ALI and ARDS) and outbreak of cytotoxic mediators. Immune response mediated by several components like T-cells, macrophages, natural killer (NK) cells, chemokines and pro-inflammatory cytokines also has a predominant role in the progression of the acute injury and results in immune reconstitution inflammatory syndrome (IRIS). Immune cells involved in the immune response like macrophages and monocytes are thought to be involved in the pathophysiology of IRIS [42,43]. Elevated levels of CD14+ CD16− monocyte population and an resulting increase in the pro-inflammatory cytokines IL-6, TNF-α and C- reactive protein (CRP) are also observed in tuberculosis (TB)-IRIS patients [44]. Lung endothelial biomarkers (like VWF) and epithelial biomarkers (like SP-D) are the diagnostic targets of ALI. Sepsis, pancreatitis, pneumonia, trauma, transfusions, aspiration and inhalation of toxic gases are some of the clinical factors of ALI and ARDS [45,46]. Histological patterns of ALI and ARDS have the chances of demonstrating diffuse alveolar damage, alveolar haemorrhage, eosinophilic pneumonia and acute fibrinous [47]. Pneumonia associated with ARDS may also result from pathogens like fungal, viral, bacterial, and parasitic. Most common pathogen strains include Streptococcus pneumoniae, respiratory viruses, Staphylococcus aureus, fungal pathogens like Pneumocystis jirovecii and Aspergillus fumigatus, Legionella pneumophila and enteric gram-negative organisms [48]. Among bacteria the virulence capability of Pseudomonas aeruginosa is one of the prime determinant of the severity of the lung injury [49]. Disease progress takes place in three degrees namely acute phase (exudative), subacute phase (proliferative) and chronic phase (fibrotic) [50]. Radiological features during the acute phase signify two-sided patchy ground-glass densities relating to interstitial edema and hyaline membranes. The important constituent of innate immune system, the pattern recognition receptors (PRRs) are affiliated with the advancement of ALI and ARDS. The ligands of PRRs include pathogen-associated molecular patterns (PAMPs) which induce inflammatory signalling events and the damage-associated molecular patterns (DAMPs) which induce neutrophil-mediated tissue damage. Increased incidences of mitochondrial DAMPs result in high mortality rates [51]. A common type of ALI is the Transfusion-related acute lung injury (TRALI). TRALI is defined as adult respiratory distress syndrome occurring with transfusions. It is manifested by pulmonary insufficiency, severe hypoxia and dyspnea. It is often reported as the neutrophil (PMN)-mediated syndrome [52]. A particular study has reported that the potential risk factors for ALI and ARDS may be associated with larger tidal volume (VT) and higher airway pressure (Paw) during one lung ventilation (OLV) in post-pneumonectomy ALI/ARDS patients and also in patients who developed ALI/ARDS [53]. Long-term effects after acute lung injury are related to neurological impairments namely neuromuscular dysfunction, neurocognitive dysfunction and neuropsychological dysfunction. Some of the minor impacts include heterotopic ossification, stiae and frozen joints [54].
2.1.2. Chronic lung injury (CLI) and bronchopulmonary dysplasia (BPD)
Chronic lung injury is characterized by a condition called pleuroparenchymal fibroelastosis (PPFE) in which a rapid multiplication of subpleural, intestinal elastic fibres majorly in the upper lobes predominates along with other clinicopathological conditions [55]. Following chronic lung injury, cells undergoing apoptosis is cleared by a process called efferocytosis. It is a type of phagocytosis confined only to the cells that undergo apoptosis to maintain the cell-turnover in pulmonary airways, carried out mainly by the macrophages while immature dendritic cells, mesenchymal cells and epithelial cells can also perform efferocytosis [56]. Recently SARS-CoV-2 manifested as severe respiratory infection resulted in number of deaths worldwide. Common risk factors associated with death in SARS-CoV-2 patients identified are hypertension, diabetes, cardiovascular disease or chronic lung disease [57]. The most prevailing chronic lung injury of infants is the bronchopulmonary dysplasia. BPD occurs when preterm infants suffering from various respiratory syndromes like meconium aspiration syndrome and neonatal pneumonia, are subjected to treatment with supplemental oxygen and extensive mechanical ventilators. It is proven that the common risk factors of BPD increases with a fall in birth weight, prematurity and gestational period [58,59]. Apart from the above other perinatal risk factors with which BPD is associated with are intrauterine growth restrictions [60], race or ethnicity [61], chorioamnionitis [62], and genetic risk factors [63]. It is characterized by saccular formation and elastic fibre aggregation of distal air spaces, inflation and edema. Barotrauma and pulmonary oxygen toxicity are pathologies of BPD [64,65]. BPD is multifactorial in nature. Studies declare that surviving patients of BPD have established pulmonary dysfunction incidence. Adults with BPD history are strictly prohibited to cigarette smoking [66]. Another form of BPD called the New BPD when surfactants are used as treatment. New BPD is depicted by pulmonary hypertension and abnormalities in vasculo-pulmonary development. Glucocorticoids and TGF-beta are the efficient modulators that initiate BPD injury [67]. BPD infants have significantly lower levels of vascular endothelial growth factor and platelet endothelial cell adhesion molecules [68].
2.1.3. Chronic obstructive pulmonary disease (COPD) and asthma
COPD is a general illness worldwide. It is defined as the complete irreversible state of airflow limitation, characterized by weak inflammatory response of the lungs, emphysema, chronic obstructive bronchiolitis, fibrosis, blocking and narrowing of airways, deprivation of lung parenchyma and elasticity. Immune cells like T-lymphocytes with CD8+ dominance, B-lymphocytes, macrophages and neutrophils are the regulators of COPD [69]. Acute provocation of COPD is defined as the continuous deterioration from steady state facilitating an alteration in typical medication for rudimentary COPD [70]. Systemic inflammation responses are a result of leukotriene B4, TNF-alpha, interleukin IL-8 and proteases. A decrease in the ratio of CD4+ to CD8+ is a typical feature of pulmonary inflammatory responses. Additional impacts of COPD are cardiovascular diseases, nervous effects and osteoskeletal effects [71]. Smoking is an important cause of systemic oxidative stresses, immoderate inflammatory responses and emphysema as manifested as COPD implications. COPD being an hetergoenous disease, patients with exacerbations are found to be associated with bacterial infections like Moraxella catarrhalis, Streptococcus pneumonia and Haemophilus influenza. Apart from bacteria, other microbes like virus and fungi also comtribute to the lung microbiota and pathogenesis of lung microbiota through initiation of chronic inflammation. It has been found that bacteria and viruses, fungi can promote local and systemic inflammation that may contribute to the pathogenesis of COPD [72]. Specific biomarkers of COPD observed are C-reactive protein (CRP), IL-6, IL-10 and CCL18/PARC [73]. Skeletal muscle loss takes place in COPD. Wasting of the cell mass is evidenced to be the result of TNF-α participation in the pathogenesis of COPD. Muscle Glutamate reduction is linked with lactic acidosis in COPD patients ending in muscle wastage [74]. Similar to COPD, asthma is characterized by pulmonary obstruction and similar immune responses. Only variation from COPD is that the airway obstruction in asthma is reversible and it does not affect lung parenchyma. Dendritic cells are the modulators of TH2 cells playing an important immune response of asthma. Severe asthma is equal to the effects of COPD, illustrated by the increase in neutrophils, tumor necrosis factor (TNF), CXCL8 and decreased reception to corticosteroids. While reversible COPD is potentially to have subsequent asthma and COPD [75].
2.1.4. Ventilator-induced lung injury (VILI) and ventilator-associated lung injury (VALI)
The prevailing lung injury cases with ARDS when given ventilator assistance mechanically in a clinical set-up may develop additional lung injuries ending up in ventilator-associated lung injury (VALI). Similarly, in experimental models lung injury can be provoked by external application of injurious ventilation procedures contributing to ventilator-induced lung injury (VILI). Thus VALI can adversely aggravate the health of the ARDS patients. At low lung volumes of ventilation, atelectrauma occurs. At high lung volumes of ventilation, barotrauma and pulmonary edema occur. Biomarkers studied via experimental models of VALI include several pro-inflammatory molecules like TNF-α, IL-1β, IL-8 and anti-inflammatory molecules like IL-10, IL-6 and sTNFR1 respectively [[76], [77], [78]]. Positive end-respiratory pressure (PEEP) and tidal volume applied have direct influence on VALI and are to be studied specifically during ALI and edema. It is reported that PEEP when provokes over-inflation, the extent of edema also increases. Mechanism of surfactant inactivation is seen in alveolar micro-vessels with an increase in fluid filtration. Further, greater the lung volume, higher is the transmural pressure in them [79]. A retrospective cohort study revealed that height and gender of the patients should be taken into considerations along with establishing limitations to large tidal volumes, before setting up a ventilator [80]. Experiments explain that decrease in the peak pulmonary arterial pressure or respiratory frequency can lessen the grimness effects of VALI [81]. It is proven that hypercapnic acidosis affords protection to VILI [82]. Addressable implications of VALI are barotrauma, volutrauma, atelectrauma, biotrauma and oxy-toxic effects. Cellular pathology includes physical disruption of cells and tissues and activation of cytotoxic responses. Leucocytes are raised to likely interact with the endothelium as the increasing intra-alveolar pressure fastens the transit time of them [83]. Inferences for current medical practices involve lung-protective ventilation. Survival of patients can be encouraged by prone positioning. Future clinical practices involve precisioned ventilation-individualized tidal volumes using driving pressure, individualized PEEP and extracorporeal strategies [84].
2.1.5. Pulmonary fibrosis (PF) and cystic fibrosis (CF)
Idiopathicpulmonary fibrosis (IPF) is a degenerative interstitial fibrosing disease prevalent worldwide. It is also termed as cryptogenic fibrosing alveolitis. A typical honeycomb-like structure of asymmetric airspaces covered by dense fibrosis is observed. IPF is followed by interstitial pneumonia which is featured by deficit inflammation and with absence of homogeneous participation of lung tissues [85]. Studies have been made since ages which revealed several inherited forms of pulmonary fibrosis. Mutations in various genes were associated to PF namely SFTPC, SFTPA2, TERT, TERC and MUC5B. Incidence with rheumatoid arthritis and scleroderma are more likely to form PF. There is a chance of clearance of alveolar basement membrane and occurrence of hyperplastic epithelial cells [86]. IPF ensures migration of fibroblasts into the fibrin-rich exudates thus a chemoattractant activity is created in the airspaces after lung injury. This process is proven to be regulated by lysophosphatidic acid [87]. Experiment proves that the pro-inflammatory cytokines IL-1β directly regulates the initiation of acute and chronic inflammation making it a valuable target of IPF [88]. Cystic fibrosis is characterized as a most common autosomal recessive genetic disorder caused by the mutation in CFTR gene (transmembrane protein gene-cystic fibrosis transmembrane conductance receptor) [89]. Pathology of the disease involves bronchiolitis, obstruction of pathways, endobronchiolar infection, impaired ciliary actions, atelectasis finally leading to secondary alveolar injury. Bacterial infections of S. aureus, P. aeruginosa and H. influenzae causes CF. It is the pulmonary macrophages and polymorphonuclear neutrophils that forms the defense against infections. While lymphocyte-mediated mucosal injury is observed in CF patients [90]. CF patients have increased flow of TNF-α, IL-8 and IL-1β [91]. Submucosal glands of the lungs are the crucial hosts of CFTR genes as the number expressed are high. Hence CF contributes to epithelial airway lining abnormalities with respect to the changes taking place in the submucosal glands respectively. The macromolecular secretions of the submucosal glands have changes in their composition, viscosity and greatly impacts on the mucociliary clearance [92].
2.1.6. Radiation-induced lung injury (RILI)
Several complications of the lung malignancies require treatments involving Radiation therapy (RT). Extensive reports claim that RT may lead to a state of RILI. The three-dimensional dosimetric predictors can be emphasized for the risk of symptomatic RILI [93]. The alveolar-capillary subunit forms the most radiosensitive complex of the lungs. Thus the RILI is also called as the diffuse alveolar damage. Radiation exposure provokes the production of reactive oxygen species creating high toxicity levels in the lung parenchyma. This may ultimately lead to lung fibrosis which develops one to six months after RT, accompanied by dyspnea [94]. In addition to fibrosis, RILI also develops radiation-induced pneumonitis gradually after months to years. Almost all the patients undergoing RT have the chance of developing fibrosis. Radiation-induced pneumonitis is characterized by cough, occasional fever, non-specific symptoms of dyspnea and chest pain with or without deformities in pulmonary functional tests. While radiation-induced pulmonary fibrosis is characterized by cough, differential levels of dyspnea, chest pain or symptomless and stable scarring of the lung tissues when detected radiographically [95]. It has been stated that the incidence and occurrence of RILI is directly influenced by dose and volume determinants of RT [96]. Several studies provide information on RILI that result in provoking inflammatory responses. A biphasic manifestation of cytokines is observed in the lung tissues once exposed to RT [97]. One such study carried out to assess the cytokine production in C3H/HeN irradiated mice, revealed a spatial change in the expression of pro-inflammatory cytokines among various cellular compartments of the lungs. Further, it was noted that the bronchoalveolar lavage cells responded immediately while the interstitial cells contributed only in the later stages during pneumonitis [98]. Profiling of cytokines namely the interleukins, interferons, monocyte chemotactic protein1, tumor necrosis factors, macrophage inflammatory proteins and granulocyte colony-stimulating factors can be performed to analyse the developmental risks of symptomatic radiation-induced lung injury [99]. In vitro studies experiments state that the application of melatonin and carnosine compounds reduced the reactive oxygen species and inflammatory cytokines produced following RILI [100,101].
2.2. Regulators of autophagy in lung injury
Numerous regulators of autophagy play a vital part in the development of lung injury. With regard to autophagy the following are the important regulators (Fig. 2 ). The mechanism by which these autophagy regulators act is shown in the Table 1 .
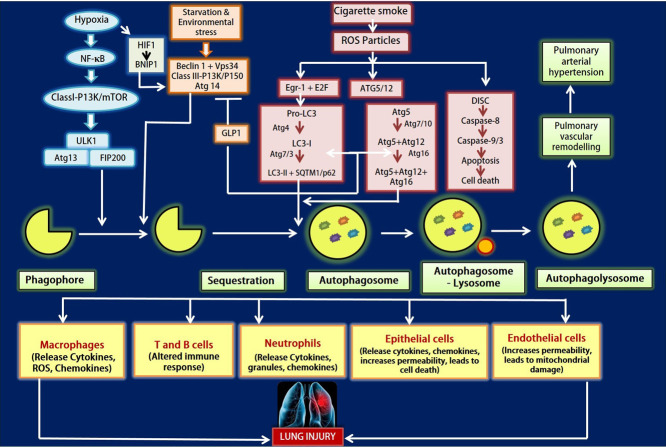
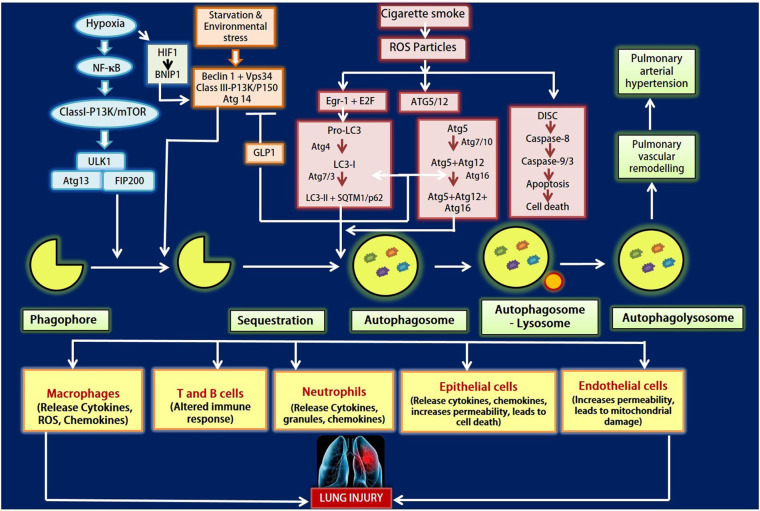
Fig. 2.
Figure depicts the autophagy regulators in regard to lung injury. Various conditions like hypoxia, starvation, environmental stress and cigarette smoke leads to autophagy that in turn causes lung injury. Activation of Class I-PI3K/mTOR pathway takes place under hypoxic and starvation conditions. While environmental stress results in provoking Beclin 1/Vps34 pathway that induces the sequestration of the phagophore formation. Cigarette smoke induces ROS accumulation which in turn causes EGR-1 & E2F signalling to activate LC3-II along with SQTM1/p62 and ATG5/12 conjugation system. The ROS generated may also leads to apoptosis. The autophagosome fuses with lysosomes and forms autophagolysosome that causes pulmonary arterial hypertension and lung injury.
Table 1.
Regulators of autophagy in lung injury and their mechanism.
| Regulators | Mechanism | Reference No. |
|---|---|---|
| LC3B-II | Elongation of autophagosome and its maturation requires Atg8/MAP1LC3 protein. LC3-I combines with phosphatidylethanolamine forming LC3-II, essential for autophagosome formation. | [174] |
| Beclin 1 | The initiation of the isolation membrane that forms autophagosome after the sequestration process is regulated by Beclin 1 and PI3K. | [119] |
| p62 | p62 along with SQSTM1 is the receptor for polyubiquitinated substrates. It helps in the transportation of cargo into the autophagosome by binding with LC3-II for degradation. | [139] |
| HIF1/BNIP3 | During hypoxic conditions, HIF1 induces BNIP3 that in turn brings about cell survival through autophagy and provoke cell death by apoptosis. | [155] |
| mTOR | The mammalian homologue Atg13 is phosphorylated by mTOR that binds ULK1 proteins. FIP200 phosphorylates ULK and initiates the isolation membrane formation in autophagy. | [175] |
2.2.1. LC3B-II
The microtubule-associated protein 1 light chain 3 (LC3) is the principle autophagic protein expressed on the double-membraned autophagosome. Nearly eight homologues of LC3 proteins are studied in mammals. The amino acid composition of these homologues, classifies them into two subfamilies - first one comprising of LC3A (2 splicing variants), LC3B and LC3C taking part in autophagosomal membrane elongation while the second one comprising of GABARAP, GABARAPL1, GABARAPL2 and GABARAPL3 taking part in the maturation of autophagosome. Generally, LC3B occurs in cytosol as LC3B-I by the proteolytic cleavage of c-terminal of LC3B, which then unites with phosphatidylethanolamine to form LC3B-II to assemble on the autophagosomal membrane. Conjugation of phosphatidylethanolamine with LC3B-II can be revoked by the activity of Atg4. Thus it is clear that occurrence of LC3B-II is crucial in the process of autophagy [102]. LC3B-II levels are analysed through immunoblotting. While limitations are degradation of LC3B-II by autophagy itself and the non-indication of autophagic flux at distinct time points. This can be overcome by comparison studies with LC3B-I and using lysosomal protease inhibitors [103]. Anti-LC3B antibodies can be applied to detect autophagy in various cell types. Application of anti-LC3B to glioblastoma tissues demonstrated a positive detection of LC3B levels both in vitro and in vivo, suggesting a latent monitoring system of LC3B [104]. Several studies state that hyperoxic conditions can result in ALI and ARDS. This further triggers the morphological biomarker of autophagy, LC3B-II to accumulate thereby, determining the fate of cell clearance. Experiments performed in hyperoxia-induced human bronchial epithelial cells and cultured epithelial cells (Beas-2B) clearly stated that the expression of LC3B-II was high, mediated by the apoptotic regulators [105]. Similar experiment carried out in the hyperoxia-induced lung injury in C57BL/6 mice inferred the involvement of apoptotic pathways in the activation of LC3B-II. Interaction of LC3B-II with Fas proteins was observed marking the importance of LC3B-II in the management of ALI [106]. Pulmonary hypertension is a main cause of COPD manifestation in lungs that affects vascular architecture. When chronic hyperoxia was induced in the lung tissue extracts of patients with pulmonary hypertension, the upregulation of LC3B prevailed indicating the regulatory role of LC3B in vascular cell proliferation and mediating adaptive cellular responses [107]. Smoking results in various implications of lung injury in due course. A multimeric protein complex comprising of LC3B-Caveolin1-Fas occurs under basal state. Extensive studies reveal that smoking triggers LC3B to initiate the dissociation of caveolin1 from Fas protein, thus facilitating apoptotic pathways accordingly [108]. Emphysema, a destructive expression of COPD is worsened by cigarette smoking. In vitro studies in lung tissues of mice, on exposure to cigarette smoking ensured the driving role of LC3B in regulating apoptotic mechanisms and finally developing emphysema respectively. All these experiments prove the comprehensive bridge between autophagy and apoptosis, highly regulated by the expression LC3B [109]. LC3B-I and LC3B-II bind to Microtubule associated protein 1B (MAP1B), wherein over-expression of MAP1B decreases the levels of LC3B-II. Protein kinase C is known to cease autophagy by interfering with autophagosomal formation. Both in vitro and in vivo studies confirmed that the LC3B phosphorylation by protein kinase C takes place consistently [110]. In lungs, the emergence of autophagy either as a protective role or maladaptive response due to sepsis was studied in a cecal ligation and puncture (CLP) - induced septic mice. It was ensured autophagy to be a protective response. Yet, an over-expression of LC3B-II in the later stages of sepsis leads to ALI, describing a maladaptive role [111]. Lung injury can be provoked by ischemia/reperfusion, in which autophagy is stated as the safeguarding mechanism by moderately maintaining the level of LC3B-II. Also, the ischemia/reperfusion-induced lung injury is positively governed by the ERK1/2 signalling pathway that regulates the cellular expression of LC3B-II respectively [112]. Nanoparticles of Zinc Oxide, on exposure to lungs may induce ALI. Further, zinc oxide nanoparticles resulted in the raise of autophagosomal structures followed by the accumulation of LC3B-II proteins. Thus ZnO nanoparticles-induced ALI is autophagy dependent. 3-methyladenine, a classical autophagy inhibitor reduced the manifestation of LC3B-II and lowered the release of zinc particles thereby, stopping ZnO nanoparticles-related toxicity of lungs [113]. Similarly, the artificially synthesized polyamidoamine dendrimers (PAMAM) used as an effective drug-delivery system, may sometimes result in PAMAM nanoparticles-induced ALI. The levels of LC3B-II biomarkers were high, indicating the autophagic responses [114].
2.2.2. BECLIN 1
Beclin 1 was first identified by Beth Levine as BECN1/ATG6 in chromosome 17q21 in the year 1999 and it is the major autophagy regulating gene [115]. It is a coiled-coil protein of molecular weight ~60 kDa, comprising of 450 amino acids and acts together with Bcl-2, an anti-apoptotic protein [116,117]. Beclin 1 is an indispensable autophagy-promoting gene that is homologue to the mammalian yeast Atg6 gene which regulates cell survival of different types and is involved in the constitution of autophagosomes [118]. The initiation of the organization of autophagosomes is regulated by class III phsophoinositide 3-Kinase (PI3K) and autophagy related gene Beclin 1 [119]. Beclin 1 has got a novel Bcl-2 homology region-3 (BH3) domain. The BH3 domain in Beclin1 can bind to Bcl family proteins that initiate apoptotic signalling and prevents the Beclin 1-mediated autophagy by removing Beclin 1 from hVps34 [120]. Either phosphorylation or ubiquitination of Beclin 1 or Bcl-2 can disunite Bcl-2 from Beclin 1 and increase the activity of Vps34 kinase which brings about increase in function [121]. During autophagy, autophagy-associated protein Beclin1 binds to LC3-I that adapts to its membrane-bound form (LC3-II) and it cooperates with the ubiquitin-binding protein p62/sequestosome 1 (SQSTM1) [122]. The first organ that fails during sepsis is lungs. The familiar complications of sepsis are ALI and ARDS. ALI activated various autophagy related proteins like LC3-II, Beclin 1 and lysosome-related protein 2 (LAMP2) and Rab7 expressions in sepsis-induced ALI [123]. To evaluate the function of autophagy in severe sepsis, an experiment is carried out using endotoxemia that frequently uses septic shock and CLP which is a clinical polymicrobial sepsis model. Here, BECN1+/− mice was susceptible to CLP-induced sepsis [124]. In cystic fibrosis transmembrane conductance regulator (CFTR), autophagy by Beclin 1 overexpression, cystamine or antioxidants and the restoration of Beclin 1 recovers the localization of Beclin 1 to endoplasmic reticulum and regresses the CF airway phenotype both in vitro and in vivo in Scnn1b-transgenic and cftr F508del homozygous mice and also in human CF nasal biopsies [125]. In LPS-induced ALI, there are three distinct complexes of Beclin-1-Vps34 have been identified, the first complex contains Beclin-1, Vps34, Vps15 and Atg14L, second complex contains Beclin-1, Vps34, Vps15 and ultraviolet irradiation resistance-associated gene (UVARAG) and the final complex contains Beclin-1, Vps34, Vps15, UVRAG and Rubicon. Among these complex, the one that contains Atg14L is concerned in the formation of autophagosome while others are in the autophagosome and endosome maturation. Beclin 1 forms the bridge in the recruitment of inducers and suppressors of autophagy and simultaneously behaves as a key modulator in autophagosome formation [126]. Mesenchymal stem cells (MSCs) increases the translation level of Beclin 1 but not its transcription rate. MSCs might alleviate LPS-ALI via downregulation of miR-142a-5p that permits pulmonary epithelial cells (PECs) to proceed with Beclin 1-mediated cell autophagy [127]. In lung disease, the bacterial studies demonstrated that the penetration of Acinetobacter baumannii activated the ubiquitin-mediated autophagic response that depends on the septins SEPT2 and SEPT9 and it is due to the Beclin 1-dependent AMPK/ERK/mTOR pathway [128].
Beclin 1 increases G1-phase cells via mitogenic signalling and also decrease the cell cycle progression that leads to inhibition of tumor growth [129]. Beclin 1 is the primary target for miR-17-5p in the lung cancer cells (A549-T24 and H596-TxR) which shows resistant to Paclitaxel. The expression of miR-17-5p was confined with the increased regulation of Beclin 1 and protected the cells from cell death [130]. The expression of Beclin1 is negatively regulated by miRNA through the mimic and antagomir by modulating the activity of autophagy and suggests the new role of miRNA in cellular processes [131]. In A549 non-small cell lung cancer (NSCLC) cells, the higher expression of Beclin 1 promotes programmed cell death and markedly decreased invasion and increases the caspase-3 & -9 activity and upregulates the manifestation of esophageal cancer-related gene 4 (ECRG4) [132]. There was a significant decrease in the levels of Beclin 1 in NSCLC tissues in comparison with the nearby normal tissues and is negatively related with tumor recurrence rate. There occurs subsequent development of cancers (for eg: lymphoma, liver and lung cancer) and inhibition of autophagy in mice with Beclin 1 knockdown [133]. In NSCLC, the use of chloroquine or Beclin 1 knockdown could enhance the apoptosis/programmed cell death that is been activated by the JAK2/STAT3 inhibitor [134]. In hepatocellular carcinoma, the increased and decreased levels of Bcl-XL and Beclin 1 respectively, results in high frequency of malignancy and poor prognosis [135]. High mobility group box 1 (HMGB1) protein encourages the core complex formation of Beclin-1-PI3K-III by enhancing mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK1/2) pathway in Lung adenocarcinoma (LAD) cells [136]. In CFTR, autophagy prevention by reactive oxygen species (ROS)-TG-2-meditaed aggresome requisition of Beclin 1 is driven by defective CFTR. Therefore by rescuing Beclin 1 from endoplasmic reticulum and then the autophagy clears the formed aggresomes that ameliorates CF lung inflammation and enhances CFTR trafficking both in vitro and in vivo [137]. The clinical lung samples collected from COPD patients described significantly increased level of autophagy and it is been observed that the autophagic proteins such as LC3B, Beclin 1, ATG7 and ATG5 were upregulated and then the formation of autophagosome was observed using electron microscopy [138].
2.2.3. p62
p62 is a tyrosine kinase p561ck binding autophagy adaptor protein that is destined for autophagic turnover by regulating the protein aggregates formation and facilitated the degradation of cargo protein through the process of autophagy. It is now well understood that it plays a crucial role as adaptor, then binds to the ubiquitinylated aggregates of protein and transport it to autophagosomes [139]. p62/SQTSM1 are capable of interacting with polyubiquitinated proteins and Atg8/LC3 are essential for autophagosome formation [140]. p62 protein migrates to the place of autophagosome and it binds to LC3 and ubiquitin-degraded protein [141]. It is because of this, p62 is thought to be the receptor for ubiquitin-degraded substrates that which forms autophagosome [142]. This requires LC3B that combines with p62 with the help of p62-LC3-interacting region (the LIR domain) and it serves as the major component in the breakdown of cytoplasmic contents. It is also observed that p62 along with LC3B prevents apoptosis by eliminating proapoptotic protein tBID [143]. p62 plays a major role as scaffold proteins for several transduction mechanism by interacting with various other signalling proteins (for eg: RIP, TRAF6, ERK, aPKC and caspase-8) [144]. p62 binds with NF-κB, Wnt signalling, apoptosis and it has a major role in survival and decease signalling pathways. When p62 accumulation occurs it brings about dysregulated initiation of these processes and it is well understood that inclusion bodies are one of the component of p62. The higher accretion of p62 brings about persistent initiation of nuclear factor erythroid 2-related factor 2 (Nrf2) [145]. The involvement of p62 in disease-related inclusion formation is been observed by N-terminal Phox and Bemlp (PB1) domain which exhibits self-aggregation and the C-terminal ubiquitin-associated domain that binds to ubiquitinated protein [146]. In autophagy, the amount of p62 is been modulated by autophagy which in turn suppress autophagy through mTORC1 activation [147]. In smoke-induced epithelial cells, it is expected to initiate Nrf2 signalling and SQTSM1/p62 expression by the release of higher levels of free radicals and these activation leads to the mTORC1 exhilaration and suppress autophagy. It is observed that higher the expression of SQTSM1/p62 inhibits the cigarette smoke extract (CSE)-induced autophagy in lung epithelial cells [148]. The COPD-emphysema pathogenesis mechanism is been initiated by the CS exposure that brings proteostasis dysfunction results in the aggregation of ubiquitin-degraded proteins and impairs autophagic marker p62 [149]. In CFTR, during defective autophagic conditions p62 will get accumulated and then it controls the development and removal of intracellular aggregates. The knockdown of p62 in defective CF epithelial cells results in the decrease of aggresome accumulation but it also plays major role in CFTR development and trafficking. By silencing the p62 gene, it mediates two major effects of defective autophagy in CF airways and involved in CF pathogenesis [137]. Experiment in CF shows that CFTRF508del maturation and trafficking that deregulates intracellular chaperone system by reducing the p62 levels [125]. In normal cells, it is been suggested that Nrf2 is involved with p62 for the activation of tumorigenesis because p62 degrades the Keap1/Nrf2 binding and brings about the stabilization of Nrf2. In NSCLC, p62 accumulation did not bring about the stabilization of p62 [150]. As like nrf2, Twist1 is an important downstream effector of p62 that helps in the regulation of cell division and translocation [151]. In lung cancer, the polysorbitol-mediated transporter (PSMT)/siRNA p62 complexes activated autophagy that silences p62 and leads to the downregulation of tumorigenesis and decrease in angiogenesis [152].
2.2.4. HIF1/BNIP3
Hypoxic conditions upregulates the transcription of Bcl-2 nineteen-kilo Dalton interacting protein (BNIP3) by the induction through Hypoxia-inducing factor 1 (HIF1). HIF1 comprises of two subunits, HIF1-α and HIF1-β. HIF1-α shows unique response to oxygen and expressed during hypoxic conditions while HIF1-β, the aryl hydrocarbon receptor-nuclear translocator, is expressed during normoxic conditions respectively. BNIP3 is a unique member that comes under BH3-only Bcl-2 family. It consist two major domains namely transmembrane domain (TM) and PEST domain. HIF1 stabilization acts to express BNIP3 genes and it are known to be a highly regulated process as BNIP3 overexpression may lead to cell death. Cellular stresses developed on exposure to toxic substances like nitric oxide, arsenic trioxide, cyanide triggers HIF1-regulated BNIP3 expression respectively. BNIP3 possesses the ability to provoke cell death via necrosis, autophagy and apoptosis [153,154]. BNIP3 functions dually by either enhancing cell survival mechanism through autophagy or cell death by apoptosis. Expression of BNIP3 causes it to moves into the mitochondria where it generates reactive oxygen species and causes mitophagy through autophagy. BNIP3 further interacts with the autophagosomal-expressing protein LC3-II and Dynamin-related protein 1 (DRP1) to promote mitochondrial-mediated autophagy. It also said that BNIP3 binds with Bcl-2 which activates Beclin 1 from the inactive complex, to undergo autophagy. While interaction of BNIP3 with OPA1 (mitochondrial fusion protein-optic atrophy1) to promote cell death. Phosphorylation of C-terminus regions of BNIP3 provides an integral mechanism of regulating both pro-death and pro-survival proteins. Thus phosphorylation of BNIP3 results in halting cell death while autophagy pursues, providing a regulation process [155]. Several studies proved that hypoxia induced pressure is overcome by mitochondrial autophagy. It acts as a protection mechanism from over-production of free radicals and is therefore an adaptive metabolic maintenance. All these survival mechanisms are the impacts of the expression of BNIP3 via HIF1 respectively [156]. Another member of the BH3-only Bcl-2 family that shows around 56% sequence homology with BNIP3 is the NIX or BNIP3L protein which is also expressed during hypoxic conditions that may or may not mediate mitophagy [157. NIX is known to play as a canonical BH3-only protein; moreover an unproven hypothesis arises with the functional overlapping between NIX and BNIP3 during hypoxic responses [158 158]. LPS-induced acute lung injury reaps HIF1 expression that worsens epithelial cell injury by addressing the stimulation of BNIP3 transcription and pro-inflammatory cytokines. A study revealed that the suppression of HIF1 in lung tissues of trauma/hemorrhagic shock-induced injury emerged to be a protective mechanism [159. PLAGL2 is a pleomorphic adenoma gene and its expression was studied in COPD patients. It was studied that PLAGL2 upregulated HIF1/BNIP3 expression. It was also noted that PLAGL2 have many downstream genes like the surfactant proteins SP-C and respond accordingly [160]. Lung ischemia-reperfusion injury (LIRI) is a manifestation occurring during ALI conditions during which the rates of apoptosis and autophagy increase. It is proved that dexmedetomidine upregulates HIF1 and downregulates BNIP3 during ischemia-reperfusion injury (IRI) of lungs in a dose-dependent way thereby, partly suppressing autophagy. Thus dexmedetomidine can be opted as a potential applicant in LIRI cases [161]. During ALI, the extracellular signalling molecule adenosine is identified to protect lungs via its receptors namely ADORA1, ADORA2A, ADORA2B and ADORA3. Among them, ADORA2B works to protect lungs from acute inflammation responses. ADORA2B signalling is regulated by the HIF1 activational pathways [162].
2.2.5. mTOR
Mammalian target of rapamycin (serine/threonine kinase) (mTOR)/PI3K/Akt is important signalling pathway that regulates macroautophagy/autophagy, metabolism, proliferation and survival [163]. In normal mouse or human cells, deletion of phosphatase and PTEN-the homologue of tensin activates mTOR or constitutive expression of serine/threonine kinase-Akt initiate senescence. Few drugs are necessary for the prolonged survival in some species, one such drug is rapamycin that inhibit the activity of mTOR [164]. In pulmonary epithelium, mTOR is activated by LPS and the suppressed autophagy brings about LPS-induced inflammatory responses [165]. In alveolar epithelial cells (AECs), insufficient autophagy and aberrant mTOR signalling pathway activation are concerned with lung pathogenesis. Continuous repetition of injury in AECs and repair are the critical function in pulmonary fibrosis [163]. In lung cells of mouse, autophagic cell death is activated by carboxylic acid- carbon nanotubes (COOH-CNT) through the Akt-TSC2-mTOR pathway and induced ALI [166]. During Traumatic brain injury (TBI)-induced ALI, the ERK1/2/mTOR/Stat3 signalling pathway important in autophagy-mediated protection [167]. In COPD patients, there occurs an alteration between AMPK and mTOR pathway was observed. ULK1 kinase, mTORC1 and AMPK activity are important for the regulation of the initiation of autophagy [168]. Experiment in human monocytic U937 cells showed that the CSE activated PI3K/Akt that shortly induced mTORC1/S6K pathway and decreased corticosteroid sensitivity [169]. In A549 cells, ATP depletion and AMPK phosphorylation by iron oxide nanoparticles induces autophagy by suppressing mTOR [170]. mTOR signalling is induced by the lower doses of ROS and the higher accumulation of ROS activated by LPS leads to the suppression of mTOR signalling pathway [166]. The activity and expression of mTOR in lungs is decreased by cigarette smoke that induces cell death and immediate inflammation in airway epithelial cells and in lung epithelium, selective disruption of mTOR leads to emphysema in COPD patients [171]. In hyperoxia-exposed developing lungs, the inhibition of RPTOR, a major element of mTORC1 induces elevated levels of autophagy, lowers apoptotic cell death and improved lung architecture [172]. In lung cancer cells, the signalling pathway PI3K/AKT/mTOR is involved in the ginsenoside Rg3 antitumor effects [173.
3. Discussion
Autophagy is a hallmark cell survival phenomenon which proceeds with the clearance of organellar and macromolecular contents of the cell [1,3]. Noted as the basic modulator of immune responses, autophagy may also influence the disease pathogenicity and inflammatory responses [6]. Homeostatic maintenance of the turn-over of crucial cellular organelles like mitochondria is achieved through autophagy [7]. The mechanism of autophagy characterized as self-eating phenomena, is carried out primarily by the lysosomal complexes that contains the acid hydrolases [10]. Various cargos of autophagy are engulfed by the autophagosomal structures for transportation to the lysosomes [12]. The mechanism of autophagy includes recognition and selection of cargo, formation of autophagosome and its fusion with lysosome, release of cargo for degradation and expelling the degraded particles into the cytosol [10]. The mechanism of delivering the contents for degradation into the lysosomes classifies autophagy into three major types namely, macroautophagy, chaperone-mediated autophagy and microautophagy [14]. The combination of autophagy related genes known as Atg genes perform the integral role in uptaking the core mechanism of autophagy. The Atg-LC3 conjugation system helps in the organization of autophagosome [1,24].
The heterogeneous pulmonary system comprises of the epithelial lining, endothelial cells, alveolar macrophages, fibroblasts etc., to carry out the basic and immunological functions of the system [25,26]. Lung injury is defined as the state of deformities or damages of the lung tissues due to barotrauma, valotrauma, infections and toxicity. Injurious state of lungs addresses the immunological responses like release of proteases, free radicals and other regulatory proteins by the alveolar macrophaghes, neutrophil-mediated transmigration and phagocytosis, and host defense conferred by the surfactant proteins [34,38]. The governance of autophagy over pulmonary system varies with the type of lung injury and can be either deleterious or protective, ultimately providing a survival phenomenon [39,40]. The major lung injury types are listed as: acute lung injury (ALI), acute respiratory distress syndrome (ARDS), chronic lung injury (CLI), bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD), ventilator-induced lung injury (VILI), fibrosis and radiation-induced lung injury (RILI).
The most common types of lung injury, the ALI and ARDS are the prominent sources of relative incidence and increased death rate across the world. They are characterized by the damage of alveolar-capillary membrane, neutrophil transmigration and cytotoxic mediated responses. Transfusion-related ALI is a type of adult ARDS that causes serious hypoxia and dyspnea [41,46,52]. CLI is characterized by pleuroparenchymal fibroelastosis and efferocytosis. BPD is a type of the manifestation of CLI in infants and is multifactorial in occurrence [55,66]. Other familiar type of lung injury is COPD which is characterized by weakened immune responses, airway constriction and emphysema. Tobacco smoking is the main cause of COPD. Asthma varies from COPD from the fact that it does not target the parenchymous cells and the condition of airway obstruction can be reversed [69,75].
VILI is provoked when the ARDS patients undergo the assistance of ventilators. Low lung volumes may cause atelectrauma while higher lung volumes may have the chances of developing barotrauma and pulmonary edema respectively [76,78]. Idiopathic pulmonary fibrosis called as the cryptogenic fibrosing alveolitis, prevails with the development of dense masses of fibrosis and interstitial pneumonia. The genetic disorder occurring with the mutation of CFTR gene results in cystic fibrosis, which leads to secondary alveolar injury and lymphosal-mediated lung mucosal injury [85,89,90]. Radiation therapy may sometimes lead to the state of RILI that impacts highly on the alveolar-capillary system, developing radiation-induced pneumonitis and radiation-induced fibrosis [93,95].
Autophagic regulators in the cases of lung injury are LC3B-II, Beclin 1, p62, HIF1/BNIP3 and mTOR. LC3B-II is the basic biomarker studied in autophagy since its recruitment is important for the formation of an autophagosome. LC3B-I combines with phosphatidylethanolamine to form LC3B-II [81]. ALI and ARDS conditions express LC3B-II to ensure cell clearance. Hyperoxia-mediated lung injury encompasses the interaction between LC3B-II and apoptotic proteins [105,107]. The LC3B-Caveolin1-Fas protein complex is important in regulating apoptosis and autophagy. Smoking-induced emphysema and pulmonary injuries manifest a disturbance in the multimeric protein complex to dissociate, resulting in apoptosis [108,109]. Ischemia/reperfusion-induced lung injury showcases a positive administration of the ERK1/2 cell signalling pathway that governs the expression of LC3B-II. Nanoparticle-mediated lung toxicity accomplishes an increased rate of LC3B-II manifestation [112,114].
Beclin 1 is a major protein that regulates autophagy by assisting in the formation of autophagasome along with PI3K. Beclin 1 co-ordinates with LC3B and p62 sequestosome during the time of autophagy. It interacts with Bcl-2 family proteins that mediate apoptosis while its regulation by Vps34 kinase corresponds to autophagy [118,122]. Several inducers and inhibitors of autophagy are recruited by the regulation of Beclin 1 genes [105]. Conditions like ALI, sepsis, COPD and cystic fibrosis encounter an over-expression of Beclin 1 genes [123,125,129,138]. Beclin-1 overexpression was found to be associated with increased activity of caspase-3 and caspase-9 and expression of Esophageal Cancer-Related Gene 4 (ECRG4) which is involved in decreased invasion in A549 lung adenocarcinoma cells. This highlights the importance of Beclin-1 as a target for gene therapy for treatment of lung cancer [136].
p62 is regarded as the key adaptor protein of autophagy. It helps to transport the ubiquitinylated protein aggregates and brings into the autophagosome for degradation, mediated by its interaction with LC3B, also preventing the apoptotic process [139,141]. SQTSM/p62 over-expression suppresses smoking-induced autophagy. Interactions with Nrf2 occurs during smoking, thereby accumulating more of free radicals and leading to the death of epithelial cells in airway lining [148]. Excessive cigarette smoking in the cases of COPD-emphysema manifestations may impair p62. In defective CFTR implications, p62 silencing may promote CFTR pathogenesis disturbing autophagy [137,149].
BNIP3, a unique Bcl-2 family member is activated by HIF1 when the pulmonary system encounters hypoxic and toxic conditions. BNIP3 mediates mitophagy in mitochondria. It interacts with LC3B-II, Beclin 1, DRP1 and OPA1 regulatory genes [154,156]. Stimulation of BNIP3 by HIF1 may deteriorate lung epithelial injury in ALI. PLAGL2 increases the expression of HIF1/BNIP3 under COPD state [160]. Dexmedetomidine possess protective effect in ischemia-reperfusion lung injury mediated by autophagy markers. Detailed investigation has reported that the effect is due to inhibition of autophagy and apoptosis. Levels of HIF-1α proteins are found to be upregulated in dexmedetomidine preconditioning group. Further the study also demonstrated that BNIP3 levels are overexpressed following injury and that preconditioning treatment with dexmedetomidine effectively attenuated ischemia-reperfusion lung injury by downregulation of BNIP3. Since BNIP3 is one of the target genes of HIF1-α in the present study it was observed that HIF-1α levels were upregulated however BNIP3 levels were downregulated which demonstrated inconsistency. Hence more studies focussing on different aspects are needed for proper understanding of the detailed mechanism [162].
mTOR is a key regulator and it's signalling pathway is significant for autophagy and other cellular mechanisms [163164]. Balancing the activities of mTORC1, ULK1 kinase and AMPK is very important for initiating autophagy [169]. The LPS-induced lung injury activates mTOR likely to highlight the inflammatory responses and inadequate autophagy [166]. The autophagic protection is insecure by the ERK1/2/mTOR/Stat3 signalling in the adverse condition of traumatic brain injury-induced ALI [163]. Over-accumulation of free radicals may affect mTOR pathway. Smoking leads to selective disruption of mTOR favoring emphysema [172].
4. Conclusion
Lung injury is recognized as a considerable cause of substantial morbidity and mortality worldwide. Although a profound knowledge on the implications of the various types of lung injuries are being addressed, the involvement of autophagy and their corresponding regulators along with its potential link in lung injury induced by various causes is to be dealt extensively for development of therapeutics targeting novel factors. Only a more advancement and in-depth progressive studies, allow a better characterization of the molecular understanding of the chief autophagic regulation mechanisms in the pathophysiology of the lung injury cases, which will ensure in developing novel therapeutic strategies and possible targets for drug discoveries.
5. Future perspectives
Currently several novel therapeutics and clinical interventions are being investigated and mechanisms deciphered to understand the possible involvement of autophagy-associated diseases which also includes apoptosis inhibitors/activators. Autophagy being a double edged sword, potential research is at present focussing on understanding the upregulation/downregulation of autophagy mediators which could therapeutically benefit a number of diseases conditions including lung injury. Possible understanding of the differences between pathological and ohysiological autophagy may help researchers in designing therapeutic target against pathological autophagy without hampering its physiological effects.
Declaration of competing interest
The authors have no competing interest to declare.
Acknowledgement
The authors acknowledge financial support from Department of Health Research (Grant No:12014/11/2018-HR/E-office:3151264 to RRR). The authors are grateful to Dr. D. Brindha, Principal, PSG College of Arts & Science, Coimbatore for providing valuable support.
References
- 1.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N., Yoshimori T., Levine B. Methods in mammalian research. Cell. 2010;140(3):313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuervo A.M. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14(2):70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Klionsky D.J., Cregg J.M., Dunn W.A., et al. A unified nomenclature for yeast autophagy-related genes. Dev. Cell. 2003;5(4):539–545. doi: 10.1016/s1534-5807(03)00296-x. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G., Levine B. Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008;9(12):1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine B., Mizushima N., Virgin H.M. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–325. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67(1):425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 9.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 10.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43 doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 12.Yorimitsu T., Klionsky D.J. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaid S., Brandts C.H., Serve H., et al. Ubiquitination and selective autophagy. Cell Death Differ. 2013;20(1):21–30. doi: 10.1038/cdd.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubinsztein D.C., Marino G., Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z., Klionsky D.J. Mammalian autophagy: core molecular mechanism and signalling regulation. Curr. Opin. Cell Biol. 2010;22(2):124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mijaljica D., Prescott M., Devenish R.J. Microautophagy in mammalian cells: revisiting a 40-year old conundrum. Autophagy. 2011;7(7):673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 17.Massey A., Kiffin R., Cuervo A.M. Pathophysiology of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 2004;36(12):2420–2434. doi: 10.1016/j.biocel.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima N., Levine B., Cuervo A.M., et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine B., Yuan J. Autophagy in cell death: an innocent convict? J. Clin. Invest. 2005;115(10):2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryter S.W., Koo M., Choi A.M.K. Molecular regulation of autophagy and its implication for metabolic diseases. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17(4):329–337. doi: 10.1097/MCO.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi A.M.K., Ryter S.W., Levine B. Autophagy in human health and disease. N. Engl. J. Med. 2013;368(7):651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 22.Tanida I., Ueno T., Kominami E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004;36(12):2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sou Y., Tanida I., Komatsu M., et al. Phosphatidylserine in addition to phosphatidylethanolamine is an invitro target of the mammalian Atg8 modifiers, LC3, GABARAP and GATE-16. J. Biol. Chem. 2006;281(6):3017–3024. doi: 10.1074/jbc.M505888200. [DOI] [PubMed] [Google Scholar]
- 24.Rabinowitz J.D., White E. Autophagy and metabolism. Science. 2011;330(6009):1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryter S.W., Choi A.M.K. Autophagy in the lung. Proceed. Am. Thorac. Soc. 2010;7(1):13–21. doi: 10.1513/pats.200909-101JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens T., Phan S., Frid M.G., et al. Lung vascular cell heterogeneity:endothelium, smooth muscle, and fibroblasts. Proceed. Am. Thorac. Soc. 2008;5(7):783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer A.K., Muehmer M., Mages J., et al. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J. Immunol. (Baltimore Md.: 1950) 2007;178(5):3134–3142. doi: 10.4049/jimmunol.178.5.3134. [DOI] [PubMed] [Google Scholar]
- 28.Gavino A.C.P., Chung J., Sato K., et al. Identification and expression profiling of a human C-type lectin, structurally homologous to mouse dectin-2. Exp. Dermatol. 2005;14(4):281–288. doi: 10.1111/j.0906-6705.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- 29.heyl K.A., Klassert T.E., Heinrich A., et al. Dectin-1 is expressed in human lung and mediates the proinfla mmatory immune response to nontypeable Haemophilus influenzae. mBio. 2014;5(5) doi: 10.1128/mBio.01492-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P., Jamaluddin M., Li K., et al. Retinoic acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 2007;81(3):1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirota J.A., Hirota S.A., Warner S.M., et al. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. J. Allergy Clin. Immunol. 2012;129(4):1116–1125. doi: 10.1016/j.jaci.2011.11.033. e6. [DOI] [PubMed] [Google Scholar]
- 32.kummaer J.A., Broekhuizen R., Everett H., et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007;55(5):443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 33.Weitnauer M., Mijošek V., Dalpke A.H. Control of local immunity by airway epithelial cells. Mucosal Immunol. 2016;9(2):287–298. doi: 10.1038/mi.2015.126. [DOI] [PubMed] [Google Scholar]
- 34.Donelly S.C., Haslett C. Cellular mechanisms of acute lung injury:implications for future treatment in the adult respiratory distress syndrome. Thorax. 1992;47(4):260–263. doi: 10.1136/thx.47.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark R.H., Gertsmann D.R., Jobe A.H., et al. Lung injury in neonates-causes, strategies for prevention and long-term consequences. J. Pediatr. 2001;139(4):478–486. doi: 10.1067/mpd.2001.118201. [DOI] [PubMed] [Google Scholar]
- 36.Oberdorster G., Ferin J., Gelein R., et al. Role of alveolar macrophage in lung injury-studies with ultrafine particles. Environ. Health Perspect. 1992;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zemans R.L., Colgan S.P., Downey G.P. Transepithelial migration of neutrophils- mechanisms and implications for acute lung injury. Am. J. Resp. Cell Mol. 2009;40(5):519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastva A.M., Wright J.R., Williams K.L. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proceed. Am. Thorac. Soc. 2007;4(3):252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel A.S., Morse D., Choi A.M.K. Regulation and functional significance of autophagy in respiratory cell biology and disease. Am. J. Resp. Cell Mol. 2013;48(1):1–9. doi: 10.1165/rcmb.2012-0282TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizumura K., Cloonan S.M., Haspel J.A., et al. The emerging importance of autophagy in pulmonary diseases. Chest. 2012;142(5):1289–1299. doi: 10.1378/chest.12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ragallar M., Richter T. Acute lung injury and acute respiratory distress syndrome. J. emerg. Trauma Shock. 2010;3(1):43–51. doi: 10.4103/0974-2700.58663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran H.T., Van den Bergh R., Loembé M.M., et al. Modulation of the complement system in monocytes contributes to tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2013;27(11):1725–1734. doi: 10.1097/QAD.0b013e328361648b. [DOI] [PubMed] [Google Scholar]
- 43.Tran H.T., Van den Bergh R., Vu T.N., et al. The role of monocytes in the development of tuberculosis-associated immune reconstitution inflammatory syndrome. Immunobiology. 2014;219(1):37–44. doi: 10.1016/j.imbio.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Gopal R., Rapaka R.R., Kolls J.K. Immune reconstitution inflammatory syndrome associated with pulmonary pathogens. Eur. Respir. Rev. 2017;26 doi: 10.1183/16000617.0042-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson E.R., Matthay M.A. Acute lung injury-epidemiology, pathogenesis and treatment. J. Aerosol Med. Pulm. D. 2010;23(4):243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grommes J., Soehnlein O. Contribution of neutrophils to acute lung injury, Mol. Med. 2011;17(3):293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beasley M.B. The pathologist's approach to acute lung injury. Arch. Pathol. Lab. Med. 2010;134(5):719–727. doi: 10.5858/134.5.719. [DOI] [PubMed] [Google Scholar]
- 48.Kao K.C., Chiu L.C., Hung C.Y., et al. Coinfection and mortality in pneumonia-related acute respiratory distress syndrome patients with bronchoalveolar lavage: a prospective observational study. Shock (Augusta, Ga.) 2017;47(5):615. doi: 10.1097/SHK.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moskowitz S.M., Wiener-Kronish J.P. Mechanisms of bacterial virulence in pulmonary infections. Curr. Opin. Crit. Care. 2010;16(8):12. doi: 10.1097/MCC.0b013e3283354710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackay A., Al-Haddad M. Acute lung injury and acute respiratory distress syndrome. BJA CEPD Reviews. 2010;9(5):152–156. [Google Scholar]
- 51.Butt Y., Kurdowska A., Allen T.C. Acute lung injury-a clinical and molecular review. Arch. Pathol. Lab. Med. 2010;140(4):345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 52.Silliman C.C., Boshkov L.K., Mehdizadehkashi Z., et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors, blood. Am. J. Hematol. 2003;101(2):454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 53.Jeon K., Yoon J.W., Suh G.Y., et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth. Intensive Care. 2009;37(1):14–19. doi: 10.1177/0310057X0903700110. [DOI] [PubMed] [Google Scholar]
- 54.Rubenfeld G.D., Herridge M.S. Epidemiology and outcomes of acute lung injury. Chest. 2007;131(2):554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 55.Rosenbaum J.N., Butt Y.M., Johnson K.A., et al. Pleuroparenchymal fibroelastosis-a pattern of chronic lung injury. Hum. Pathol. 2015;46(1):137–146. doi: 10.1016/j.humpath.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Grabiec A.M., Hussell T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin. Immunopathol. 2016;38(4):409–423. doi: 10.1007/s00281-016-0555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. Bmj. 2020:368. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 58.Oh W., Poindexter B.R., Perritt R., et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J. Pediatr. 2005;147(6):786–790. doi: 10.1016/j.jpeds.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 59.Thebaud B., Goss K.N., Laughon M., et al. Bronchopulmonary dysplasia. Nature reviews. Disease Primers. 2019;5(1):78. doi: 10.1038/s41572-019-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bose C., Van Marter L.J., Laughon M., et al. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124(3):e450–e458. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemons J.A., Bauer C.R., Oh W., et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development neonatal research network, January 1995 through December 1996. Pediatrics. 2001;107(1):e1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 62.Hartling L., Liang Y., Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2012;97(1):F8–F17. doi: 10.1136/adc.2010.210187. [DOI] [PubMed] [Google Scholar]
- 63.Lavoie P.M., Pham C., Jang K.L. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the National Institutes of Health. Pediatrics. 2008;122(3):479–485. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eber E., Zach M.S. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy) Thorax. 2001;56(4):317–323. doi: 10.1136/thorax.56.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albertine K.H., Jones G.P., Starcher B.C., et al. Chronic lung injury in preterm lambs-disordered respiratory tract development. Am. J. Respir. Crit. Care Med. 1999;159(3):945–958. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 66.Northway W.H., Moss R.B., Carlisle K.B., et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N. Engl. J. Med. 1990;323(26):1793–1799. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 67.Jobe A.H., Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 68.Bhandari A., Bhandari V. Pathogenesis, pathology and pathophysiology of pulmonary sequelae of bronchopulmonary dysplasia in premature infants. Front. Biosci. 2003;8(5):e370–e380. doi: 10.2741/1060. [DOI] [PubMed] [Google Scholar]
- 69.Barnes P.J., Shapiro S.D., Pauwels R.A. Chronic obstructive pulmonary disease-molecular and cellular mechanisms. Eur. Respir. J. 2003;22(4):672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 70.Hogg J.C., Timens W. The pathology of chronic obstructive pulmonary disease. Annu. Re. Pathol: Mechanisms of Disease. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 71.Agusti A.G.N., Noguera A., Sauleda J., et al. Systemic effects of chronic obstructive pulmonary disease. Eur. Respir. J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 72.Gates K., Martinez F. The human microbiome in the lung: are infections contributing to lung health and disease? Chronic Obstructive Pulmonary Diseases. 2016;3(1):466. doi: 10.15326/jcopdf.3.1.2015.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim V., Rogers T.J., Criner G.J. New concepts in the pathoiology of chronic obstructive pulmonary disease. Proceed. Am. Thorac. Soc. 2008;5(4):478–485. doi: 10.1513/pats.200802-014ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wouters E.F.M. Chronic obstructive pulmonary disease-5 systemic effects of COPD. Thorax. 2002;57(12):1067. doi: 10.1136/thorax.57.12.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008;8(3):183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 76.Frank J.A., Parsons P.E., Matthay M.A. Pathogenic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest. 2006;130(6):1906–1914. doi: 10.1378/chest.130.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dreyfuss D., Saumon G. Ventilator-induced lung injury-lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 78.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 79.Richard J.D., Dreyfuss D., Saumon G. Ventilator-induced lung injury. Eur. Respir. J. 2003;33(42 suppl):2s–9s. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 80.Gahlot L., Milbrandt E.B., Snyder J. Higher initial tidal volumes associated with the subsequent development of acute lung injury in dose-response relationship. Crit. Care. 2005;9(6):E25. [Google Scholar]
- 81.Hotchkiss J.R., Blanch L., Murias G. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2000;161(2):463–468. doi: 10.1164/ajrccm.161.2.9811008. [DOI] [PubMed] [Google Scholar]
- 82.A.F. Broccard, J.R. Hotchkiss, C. Vannay, et al., Protective effects of hypercapnic acidosis on ventilator-induced lung injury, Am. J. Respir. Crit. Care Med. 164 (5) (201) 802–806. [DOI] [PubMed]
- 83.Pinhu L., Whitehead T., Evans T., et al. Ventilator-associated lung injury. Lancet. 2003;361(9354):332–340. doi: 10.1016/S0140-6736(03)12329-X. [DOI] [PubMed] [Google Scholar]
- 84.Curley G.F., Laffey J.G., Zhang H., et al. Biotrauma and ventilator-induced lung injury-clinical implications. Chest. 2016;150(5):1109–1117. doi: 10.1016/j.chest.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Wolters P.J., Collard H.R., Jones K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol:mechanisms of disease. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noble P.W., Barkauskas C.E., Jiang D. Pulmonary fibrosis-patterns and perpetrators. J. Clin. Invest. 2012;122(8):2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tager A.M., LaCamera P., Shea B.S., et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 2008;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 88.Kolb M., Margetts P.J., Anthony D.C., et al. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Invest. 2001;107(12):1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheppard M.N., Nicholson A.G. The pathology of cystic fibrosis. Curr. Diagn. Pathol. 2002;8(1):50–59. [Google Scholar]
- 90.Elborn J.S., Shale D.J. Cystic fibrosis. 2. Lung injury in cystic fibrosis. Thorax, 1990. 1990;45(12):970. doi: 10.1136/thx.45.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonfield T.L., Panuska J.R., Konstan M.W., et al. Inflammatory cytokines in cyctic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995;152(6):2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 92.Gibson R.L., Burns J.L., Ramsey B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 93.Marks L.B., Yu X., Vujaskovic Z., et al. Radiation-induced lung injury. Semin. Radiat. Oncol. 2003;13(3):333–345. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 94.Ghafoori P., Marks L.B., Vujaskovic Z., et al. Radiation-induced lung injury-assessment, management, and prevention. Lung Cancer. 2008;22(1) [PubMed] [Google Scholar]
- 95.Madani I., Ruyck K.D., Goeminne H., et al. Predicting risk of radiation-induced lung injury. J. Thorac. Oncol. 2007;2(9):864–874. doi: 10.1097/JTO.0b013e318145b2c6. [DOI] [PubMed] [Google Scholar]
- 96.Marks L.B. Dosimetric predictors of radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2002;54(2):313–316. doi: 10.1016/s0360-3016(02)02928-0. [DOI] [PubMed] [Google Scholar]
- 97.Rube C.E., Wilfert F., Palm J., et al. Irradiation induces a biphasic expression of pro-inflammatory cytokines in the lung. Strahlenther. Onkol. 2004;180(7):442–448. doi: 10.1007/s00066-004-1265-7. [DOI] [PubMed] [Google Scholar]
- 98.Hong J., Jung S., Tsao Thomas C.Y., et al. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int. J. Radiat. Biol. 2003;79(3):159–167. doi: 10.1080/0955300031000076894. [DOI] [PubMed] [Google Scholar]
- 99.Hart J.P., Broadwater G., Rabbani Z., et al. Cytokine profiling for prediction of symptomatic radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2005;63(5):1448–1454. doi: 10.1016/j.ijrobp.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 100.Jang S.S., Kim H.G., Lee J.S., et al. Melatonin reduces X-ray radiation-induced lung injury in mice by modulating oxidative stress and cytokine expression. Int. J. Radiat. Oncol. 2013;89(2):97–105. doi: 10.3109/09553002.2013.734943. [DOI] [PubMed] [Google Scholar]
- 101.Guney Y., Turkcu U.O., Hicsonmez A., et al. Carnosine may reduce lung injury caused by radiation therapy. Med. Hypotheses. 2006;66(5):957–959. doi: 10.1016/j.mehy.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 102.Lai S., Devenish R.J. LC3-associated phagocytosis (LAP)-connections with host autophagy. Cells. 2012;1(3):396–408. doi: 10.3390/cells1030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mizushima N., Yoshimori T. How ito interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 104.Aoki H., Kondo Y., Aldape K., et al. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy. 2008;4(4):467–475. doi: 10.4161/auto.5668. [DOI] [PubMed] [Google Scholar]
- 105.Jin Y., Tanaka A., Choi A.M.K., et al. Autophagic proteins-new facets of the oxygen paradox. Autophagy. 2012;8(3):426–428. doi: 10.4161/auto.19258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanaka A., Jin Y., Lee S., et al. Hyperoxia-induced LC3B interacts with the Fas apoptotic pathway in epithelial cell death. Am. J. Respir. Cell Mol. 2012;46(4):507–514. doi: 10.1165/rcmb.2009-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S., Smith A., Guo L. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011;183(5):649–658. doi: 10.1164/rccm.201005-0746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryter S.W., Lam H.C., Chen Z., et al. Deadly triplex- smole, autophagy and apoptosis. Autophagy. 2011;7(4):436–437. doi: 10.4161/auto.7.4.14501. [DOI] [PubMed] [Google Scholar]
- 109.Chen Z., Lam H.C., Jin Y., et al. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl. Acad. Sci. 2010;107(44):18880–18885. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang H., Cheng D., Liu W., et al. Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem. Biophys. Res. Commun. 2010;395(4):471–476. doi: 10.1016/j.bbrc.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stevan L., Shyng-Shiou Y., Chin H., et al. Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann. Surg. 2013;257(2):352–363. doi: 10.1097/SLA.0b013e318269d0e2. [DOI] [PubMed] [Google Scholar]
- 112.Zhang D., Li C., Zhou J., et al. Autophagy protects against ischemia/reperfusion-induced lung injury through alleviating blood-air barrier damage. J. Heart Lung Transpl. 2015;34(5):746–755. doi: 10.1016/j.healun.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 113.Jiang X., Tang Q., Zhang J., et al. Autophagy-dependent release of zinc ions is critical for acute lung injury triggered by zinc oxide nanoparticles. Nanotoxicology. 2018;12(9):1068–1091. doi: 10.1080/17435390.2018.1513094. [DOI] [PubMed] [Google Scholar]
- 114.Li C., Liu H., Sun Y., et al. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signalling pathway. J. Mol. Cell Biol. 2009;1(1):37–45. doi: 10.1093/jmcb/mjp002. [DOI] [PubMed] [Google Scholar]
- 115.Levine B., Klionsky D.J. Development of self-digestion: molecular mechanism and biological functions of autophagy. Dev. Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 116.Liang X.H., Kleeman L.K., Jiang H.H., et al. Protection against fatal sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998;72(11):8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Z., Chen B., Wu Y., et al. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010;10(98) doi: 10.1186/1471-2407-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pickford F., Masliah E., Britschgi M., et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Invest. 2008;118(6):2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kondo Y., Kanzawa T., Sawaya R., et al. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer. 2005;5(9):726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 120.Maiuri M.C., Toumelin G., Criollo A., et al. Functional and physical interaction between Bcl-XL and a BH3-like domain in Beclin-1. EMBO J. 2007;26(10):2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abrahamsen H., Stenmark H., Platta H.W. Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuing class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett. 2012;586(11):1584–1591. doi: 10.1016/j.febslet.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 122.Wang X., Du Z., Li L., et al. Beclin 1 and p62 expression in non-small cell lung cancer: relation with malignant behaviours and clinical outcome. Int. J. Clin. Exp. Pathol. 2015;8(9):10644–10652. [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao H., Chen H., Xiaoyin M., et al. Autophagy activation improves lung injury and inflammation in sepsis. Inflammation. 2019;42(2):426–439. doi: 10.1007/s10753-018-00952-5. [DOI] [PubMed] [Google Scholar]
- 124.Mizumura K., Cloonan S., Choi M.E., et al. Autophagy: friend or foe in lung disease? Ann. Am. Thorac. Soc. 2016;13(Supplement 1):S40–S47. doi: 10.1513/AnnalsATS.201507-450MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luciani A., Rachela V., Esposito S., et al. Defective CFTR induces aaggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 2010;12(9):863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y., Zhang J. Saturated hydrogen saline ameliorates lipopolysaccharide-induced acute lung injury by reducing excessive autophagy. Exp. Ther. Med. 2017;13(6):2609–2615. doi: 10.3892/etm.2017.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Z., You Z. Mesenchymal stem cells alleviate LPS-induced acute lung injury in mice by MiR-142a-5p controlled pulmonary endothelial cell autophagy. Cell. Physiol. Biochem. 2016;38:258–266. doi: 10.1159/000438627. [DOI] [PubMed] [Google Scholar]
- 128.Liao S., Sun P., Gu Y., et al. Autophagy and pulmonary disease. Ther. Adv. Respir. Dis. 2019;13:1–13. doi: 10.1177/1753466619890538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koneri K., Goi T., Hirono Y., et al. Beclin 1 gene inhibits tumor groth in colon cancer cell lines. Anticancer Res. 2007;27:1453–1457. [PubMed] [Google Scholar]
- 130.Chatterjee A., Chattopaghyay D., Chakrabarti G. miR-17-5p Downregulation contributes to paclitaxel resistance of lung cancer cells through altering Beclin 1 expression. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu H., Wu H., Liu X., et al. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2009;5(6):816–823. doi: 10.4161/auto.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W., Fan H., Li X., et al. Beclin 1 promotes apoptosis and decreases invasion by upregulating the expression of ECRG4 in A549 human lung adenocarcinoma cells, Mol. Med. Rep. 2016;14:355–360. doi: 10.3892/mmr.2016.5219. [DOI] [PubMed] [Google Scholar]
- 133.Zhou W., Yue C., Deng J., et al. Autophagic positive Beclin 1 serves as an independent positive prognostic biomarker for non-small cell lung cancer. PLoS One. 2013;8:e80338. doi: 10.1371/journal.pone.0080338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim S.Y., Song X., Zhang L., et al. Role of BCL-XL/Beclin 1 in interplay between apoptosis and autophagy in oxaplatin and bortezomib-induced cell death. Biochem. Pharmacol. 2014;88:178–188. doi: 10.1016/j.bcp.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dalby K.N., Tekedereli I., Lopez-Berestein G., et al. Targgeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pan B., Chen D., Huang J., et al. HMGB1-mediated autophagy promotes docetaxel resistance in human lung adenocarcinoma. Mol. Cancer. 2014;13(1):165. doi: 10.1186/1476-4598-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luciani A., Villella V.R., Esposito S., et al. Cystic fibrosis: a disorder with defective autophagy. Autophagy. 2011;7(1):104–106. doi: 10.4161/auto.7.1.13987. [DOI] [PubMed] [Google Scholar]
- 138.Hussain S.N.A., Sandri M. Role of autophagy in COPD skeletal muscle dysfunction. J. Appl. Physiol. 2013;114(9):1273–1281. doi: 10.1152/japplphysiol.00893.2012. [DOI] [PubMed] [Google Scholar]
- 139.Jiang T., Harder B., Vega M., et al. p62 links autophagy and Nrf2 signaling. Free Rad. Biol. Med. 2015;88 (:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fujii S., Hara H., Araya J., et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology. 2012;1(5):630–641. doi: 10.4161/onci.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bjorkoy G., Lamark T., Brech A., et al. p62/SQSTM1 forms aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. Cell Biol. 2005;171(4):603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johansen T., Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liang X., Wei S., Lee S., et al. p62 sequestosome 1/light chain 3b complex confers cytoprotection on lung epithelial cells after hyperoxia. Am. J. Respir. Cell Mol. 2013;48(4):489–496. doi: 10.1165/rcmb.2012-0017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moscat J., Diaz-Meco M.T., Albert A., et al. Cell signalling and function organized by PBI domain interactions. Mol. Cell. 2006;23:631–640. doi: 10.1016/j.molcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 145.Komatsu M. Potential role of p62 in tumor development. Autophagy. 2011;7(9):1088–1090. doi: 10.4161/auto.7.9.16474. [DOI] [PubMed] [Google Scholar]
- 146.Ichimura Y., Komatsu M. Selective degradation of p62 by autophagy. Semin. Immunopathol. 2010;32(4):431–436. doi: 10.1007/s00281-010-0220-1. [DOI] [PubMed] [Google Scholar]
- 147.Komatsu M., Kageyama S., Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol. Res. 2012;66(6):457–462. doi: 10.1016/j.phrs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 148.Sukkar M.B., Harris J. Potential impact of oxidative stress induced growth inhibitor 1 (OSGIN1) on airway epithelial cell autophagy in chronic onstructive pulmonary disease (COPD) J. Thorac. Dis. 2017;9(12):4825. doi: 10.21037/jtd.2017.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vij N., Chandramani-Shivalingappa P., Westphal C.V., et al. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol:Cell Physiol. 2018;314(1):C73–C87. doi: 10.1152/ajpcell.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Inoue D., Suzuki T., Mitsuishi Y., et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103(4):760–766. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qiang L., Zhao B., Ming M., et al. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc. Natl. Acad. Sci. 2014;111(25):9241–9246. doi: 10.1073/pnas.1322913111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152.Islam M.A., Shin J., Yun C., et al. The effect of RNAi silencing of p62 using an osmotic polysorbitol transporter on autophagy and tumorigenesis in lungs of K-rasLA1 mice. Biomaterials. 2014;35(5):1584–1596. doi: 10.1016/j.biomaterials.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 153.Burton T.R., Gibson S.B. The role of Bcl-2 family member BNIP3 in cell death and disease-NIPping at the heels of cell death. Cell Death Differ. 2009;16(4):515–523. doi: 10.1038/cdd.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haddad J.J. Science review: redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury:role for hypoxia-inducible factor-1α. Crit. Care. 2002;7(1):1–8. doi: 10.1186/cc1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu K.E., Frazier W.A. Phosphorylation of the BNIP3 C-terminus inhibits mitochondrial damage and cell death without blocking autophagy. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang H., Bosch-Marce M., Shimoda L.A., et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283(16):10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 157.Mazure N.M., Pouyssegur J. Hypoxia-induced autophagy - cell death or cell survival? Curr. Opin. Cell Boil. 2010;22(2):177–180. doi: 10.1016/j.ceb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 158.Tracy K., Macleod K.F. Regulation of mitochondrial integrity, autophagy and cell survival by BNIP3. Autophagy. 2007;3(6):616–619. doi: 10.4161/auto.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jiang H., Huang Y., Xu H., et al. Inhibition of hypoxia inducible factor-1alpha ameliorates lung injury induced by trauma and hemorrhagic shock in rats. Acta Pharmacol. Sin. 2012;33(5):635–643. doi: 10.1038/aps.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang Y., Yang M.W., Guo Y., et al. PLAGL2 expression-induced lung epithelium damages at bronchiolar alveolar duct junction in emphysema - bNip3- and SP-C-associated cell death/injury activity. Am. J. Physiol: Lung Cell Mol. Physiol. 2009;297(3):L455–L466. doi: 10.1152/ajplung.00144.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang W., Zhang J. Dexmedetomidine preconditioning protects lung injury induced by ischemia-reperfusion through inhibition of autophagy. Exp. Ther. Med. 2017;14(2):973–980. doi: 10.3892/etm.2017.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eckle T., Kewley E.M., Brodsky K.S., et al. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J. Immunol. 2014;192(3):1249–1256. doi: 10.4049/jimmunol.1100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gui Y., Wang L., Tian X., et al. mTOR overactivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Houssaini A., Breau M., Kebe S.A.K., et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI insight. 2018;3(3) doi: 10.1172/jci.insight.93203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu Y., Lou J., Mao Y., et al. Activation of mTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy. 2016;12(12):2286–2299. doi: 10.1080/15548627.2016.1230584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu H., Zhang Y., Zhang Y., et al. A functionalized single-walled carbon nanotube-induced autophagic cell death in human lung cells through Akt-TSC2-mTOR signalling. Cell Death Dis. 2011;2:e159. doi: 10.1038/cddis.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu X., Zhi T., Chao H., et al. ERK1/mTOR/Stat3 pathway-mediated autophagy alleviates traumatic brain injury-induced acute lung injury. BBA-Mol. Basis Dis. 2018;1864:1663–1674. doi: 10.1016/j.bbadis.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 168.Guo Y., Gosker H.R., Schols A., et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188(11):1313–1320. doi: 10.1164/rccm.201304-0732OC. [DOI] [PubMed] [Google Scholar]
- 169.Mitani A., Ito K., Vuppusetty C., et al. Inhibition of mTOR restores corticosteroid sensitivity in chronic obstructive pulmonary disease. Am. J. Crit. Care Med. 2015;193:143–153. doi: 10.1164/rccm.201503-0593OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Khan M.I., Mohammad A., Patil G., et al. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33(5):1477–1488. doi: 10.1016/j.biomaterials.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 171.Wang Y., Liu J., Zhou J., et al. mTOR suppresses cigarette smoke-induced epithelial cell death and airway inflammation in chronic obstructive pulmonary disease. J. Immunol. 2018;200(8):2571–2580. doi: 10.4049/jimmunol.1701681. [DOI] [PubMed] [Google Scholar]
- 172.Sureshbabu A., Syed M., Das P., et al. Inhibition of regulatory-associated protein of mechanistic target of rapamycin prevents hyperoxia-induced lung injury by enhancing autophagy and reducing apoptosis in neonatal mice. Am. J. Respir. Cell Mol. 2016;55(5):722–735. doi: 10.1165/rcmb.2015-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang J., Li S., Wang L., et al. Ginsenoside Rg3 attenuates lipopolysaccharide-induced acute lung injury via MerTK-dependent activation of the PI3K/AKT/mTOR pathway. Front. Pharmacol. 2018;9:850. doi: 10.3389/fphar.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee Y., Lee J. Role of the mammalian ATG8/LC3 family in autophagy: differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Rep. 2016;49(8):424. doi: 10.5483/BMBRep.2016.49.8.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jung C.H., Jun C.B., Ro S., et al. ULK-Atg13FIP200 complexes mediate mTOR signalling to the autophagy machinery. Mol. Biol. Cell. 2009;20(7):1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]