Abstract
With the development of the COVID-19 epidemic, there is an urgent need to establish a system for determining the effectiveness and neutralizing activity of vaccine candidates in biosafety level 2 (BSL-2) facilities. Previously, researchers had developed a pseudotyped virus system for SARS-CoV and MERS-CoV, based on HIV-1 core, bearing virus spike protein. During the development of a pseudotyped SARS-CoV-2 system, a eukaryotic expression plasmid expressing SARS-CoV-2 spike (S) protein was constructed and then co-transfected with HIV-1 based plasmid which containing the firefly luciferase reporter gene, into HEK293T cells to prepare the pseudotyped SARS-CoV-2 virus (ppSARS-2). We have successfully established the pseudotyped SARS-CoV-2 system for neutralization and entry inhibition assays. Huh7.5 cell line was found to be the most susceptible to our pseudotyped virus model. Different levels of neutralizing antibodies were detected in convalescent serum samples of COVID-19 patients using ppSARS-2. The recombinant, soluble, angiotensin-converting enzyme 2 protein was found to inhibit the entry of ppSARS-2 in Huh7.5 cells effectively. Furthermore, the neutralization results for ppSARS-2 were consistent with those of live SARS-CoV-2 and determined using the serum samples from convalescent patients. In conclusion, we have developed an easily accessible and reliable tool for studying the neutralizing efficiency of antibodies against SARS-CoV-2 and the entry process of the virus in a BSL-2 laboratory.
Keywords: Pseudotyped, Pseudovirus, SARS-CoV-2, COVID-19, Neutralization assay, Viral entry assay
Highlights.
Scientific question
To establish a pseudotyped SARS-CoV-2 system for determining the effectiveness and neutralizing activity of vaccine candidates in biosafety level 2 (BSL-2) facilities.
Evidence before this study
Coronavirus disease 2019 (COVID-19) has become a global pandemic. Currently, SARS-CoV-2 live virus-associated experiments need to be handled in biosafety level 3 (BSL-3) facilities. Previously, researchers had successfully established an HIV based pseudotyped virus system for studies on MERS-CoV and Ebola virus. Using the pseudotyped virus system, viral entry associated research, e.g. neutralization assays and in vitro pharmacodynamics, can thus be carried out in the BSL-2 facilities.
New findings
In this study, we have developed a pseudotyped SARS-CoV-2 system that efficiently operates in a BSL-2 facility. With transfection of two plasmids into HEK293T cells, we have developed an HIV-1 core-based pseudotyped virus consisting of SARS-CoV-2 spike protein and found Huh7.5 cell line suitable for analysis of our pseudotyped SARS-CoV-2 system. We use the Convalescent serum from 11 COVID-19 patients to compare the results of SARS-CoV-2 live-virus microneutralization and the pseudotyped SARS-CoV-2 system and notice a significant correlation between the results obtained by the two methods.
Significance of the study
The pseudotyped SARS-CoV-2 system, developed in this study, seems highly reliable for conducting the SARS-CoV-2 viral entry associated research in a BSL-2 facility. The system is suitable for high-throughput analysis and R&D of vaccines and drugs.
Alt-text: Unlabelled Box
1. Introduction
The causative agent of the unprecedented global pandemic of coronavirus disease 2019 (COVID-19) is a novel beta-coronavirus [[1], [2], [3]], named as SARS-CoV-2 (also called as COVID-19 virus in China) [4]. The control of the COVID-19 pandemic is a great challenge because SARS-CoV-2 is a highly contagious virus, and COVID-19 has diverse clinical manifestations (such as asymptomatic infection, common cold, and pneumonia) [5]. Therefore, there is an urgent need to develop vaccines or therapeutics against COVID-19. However, currently, SARS-CoV-2 live virus-associated experiments can only be conducted in a biosafety level 3 (BSL-3) facility, which limits the development of SARS-CoV-2 vaccines and drugs to several scientific teams and local departments of disease control and prevention. Hence, a reliable, rapid, and convenient neutralization assay, that can be handled in a BSL-2 laboratory is essential for screening and evaluation of antibodies and therapeutic agents against the SARS-CoV-2 infection.
Previous studies have suggested that the glycosylated spike (S) protein is the major surface protein responsible for receptor binding and entry of the virus into the host cell [[6], [7], [8]], and that both SARS-CoV-2 and SARS-CoV have the same main receptor, which is angiotensin-converting enzyme 2 (ACE2) [9,10]. Pseudotyped viruses based on the HIV-1 backbone consist of an envelope protein of a heterologous virus and reporter genes, which make them reliable and safe models for assessment of neutralization efficiency and entry inhibition [[11], [12], [13]].
Here, a pseudotyped model with the SARS-CoV-2 infection property has been developed, based on optimized S expression plasmid and the HIV-1 packaging system incorporating luciferase reporter [14,15]. Cell lines sensitive to this pseudotyped model were identified. The application and authenticity of the system were verified by the neutralization assay based on live SARS-CoV-2.
2. Materials and Methods
2.1. Cells and serum
HEK293T, Vero, Huh7, and Huh7.5 cells were purchased from ATCC (US), and Vero E6 cells were provided by Beijing Sinovac Biotech Co. (China) All cells were cultured under the same conditions throughout the study. DMEM (Hyclone, US) containing 10% fetal bovine serum (FBS; GEMINI Co., China) was used to culture the cells in a 5% CO2 incubator at 37 °C.
2.2. Construction and identification of S expressing plasmid
The gene coding for the S protein of SARS-CoV-2 (GISAID, No. EPI_ISL_402119) was synthesized (Genescript Co., China) using a mammalian-optimized codon. It was then inserted into the eukaryotic expression vector, pcDNA3.1 (+), via Hind III and Xba I digestion, and named as pcDNA3.1-nCoV S. The expression plasmid was transfected into HEK293T cells with jetPRIME (Polyplus, France) transfection reagent for 48 h. The expression of S protein was identified by indirect immunofluorescence assay. After transfection, the cells were resuspended and fixed on a microporous glass plate in 4% paraformaldehyde for 1 h. Later, the cells were permeabilized and blocked using 10% goat serum in 0.2% TritonX-100 PBS. Next, the cells were incubated with primary antibodies at 37 °C for 2 h. Mouse anti-SARS-CoV-2 S pAb and convalescent serum from COVID-19 patients, diluted at 1:200 and 1:50, respectively, were used as primary antibodies. After the incubation period, the cells were washed with PBST 10 times and were incubated with goat anti-mouse IgG FITC or anti-human IgG FITC in 0.5% Evans blue PBS at RT for 1 h. The cells were then washed with PBST, and mounted on cover slips using mounting buffer. Fluorescence was observed using OLYMPUS IX50FL microscope and the fluorescent images were captured using the DP70 digital camera system.
2.3. Preparation and identification of pseudotyped SARS-CoV-2 virus
Two micrograms of pcDNA3.1-SARS-CoV-2 S expression plasmid (or mock plasmid pcDNA3.1) and four micrograms of HIV-1 pseudotype packaging plasmid, pNL4–3.Luc.R-E-, containing the Fluc reporter gene, were diluted into Opti-MEM and mixed with 20 μL X-tremeGENE HP transfection reagent (Roche, Germany) for 15 min before the plasmids were transfected into the HEK293T cells. The culture medium was refreshed after 6 h. The transfected cells were cultured for 48 h, then supernatant was harvested and frozen at −70 °C.
SARS-CoV-2 pseudotyped virus and mock virus were ultracentrifuged at 24,000 ×g using 1 mL of 20% sucrose as a cushion. Then, the medium was discarded, and the precipitated pseudoviruses were suspended in 200 μL of SDS-PAGE loading buffer. The samples were heated for 10 min at 95 °C and then subjected to SDS-PAGE for immunoblotting, as previously described [16]. SARS-CoV-2 S protein was identified using the mouse anti-SARS-CoV-2 S pAb (Sino biological, China), while HIV p24, used as reference, was identified using the rabbit anti-p24 polyclonal Ab (Sino biological).
2.4. Sensitive cell screening test for pseudotyped SARS-CoV-2 infection
Vero, Vero E6, Huh7, Huh7.5, and BHK21 cells were seeded into 96-well plates one day before infection. When infected, the cells were approximately 60%–80% confluent. Following infection, the culture medium was removed, and 50 μL of serum-free DMEM medium was added. The cells were stabilized for 30 min, following which 50 μL of pseudotype virus culture suspension was added to the cells. The culture medium was replaced after 12–16 h with fresh DMEM supplemented with 2% FBS, and the culture was continued for 24 h. Bright-Glo Luciferase Assay System (Promega Co., US) was used to detect Fluc expression in the cells, and the titer of pseudotype virus in different cell lines was obtained. Thus, sensitive cell lines were identified.
2.5. Pseudotyped virus-based neutralization assay
The sensitive cells were grown to 60%–80% confluency for the experiment. The serum was subjected to quadruplicate serial diluted (from 1:100 to 1:6,400) in DMEM with a total volume of 50 μL. The diluted serum was mixed with equal volume of pseudovirus particles, and the mixture was incubated at 37 °C for 1 h. Subsequently, the mixture was added to Vero cells in 96-well plates in quadruplicates. The Bright-Glo Luciferase Assay System (Promega) was used to detect the expression of Fluc in cells, and the appearance was recorded as relative light units (RLU). After 48 h, the Bright-Glo luciferase assay substrate was added to each well, and the luminescence was measured with GLOMAX luminometer, and the neutralization efficiency was then calculated. The inhibition rate of serum antibody in pseudotyped virus-based neutralization test (ppNT) was expressed as the inhibition percentage and was calculated using the following formula: Neutralization potency (percentage) = (RLU without serum - RLU with serum)/RLU without serum*100%. The median neutralization dose (ND50) was calculated by Reed-Munch method.
2.6. Soluble ACE2 entry inhibition assay
Prof. Wenhui Li has generously provided the soluble human ACE2 protein. The experiment was conducted as previously described [7,17]. In brief, recombinant soluble human ACE2 protein was serially diluted in DMEM and mixed with pseudotyped viruses at room temperature. After 30 min of incubation, the mixture was added to Huh7.5 cells cultured in a 96-well plate to 60%–80% confluency. After 48 h of 5% CO2 incubation at 37 °C, the infectious titer was measured using the Bright-Glo firefly luciferase kit (Promega). The inhibition ratio was calculated as follows: Inhibition ratio = (Blank RLU-treatment RLU)/Blank RLU *100%. The median inhibitory concentration (IC50) was calculated by nonlinear regression.
2.7. Live SARS-CoV-2-based neutralization (inhibition) assay
The experiment was conducted in a BSL-3 laboratory, as previously reported [18]. Briefly, serum and live SARS-CoV-2 (C-Tan-nCoV Wuhan strain 01) were diluted using 2% FBS-DMEM, and the mixtures were incubated at 37 °C for 1 h, following which they were added to the seeded Vero cells. After incubation at 37 °C for 48 h, CPE was observed, and 100 μL of the culture supernatant was harvested for nucleic acid extraction and real-time fluorescence RT-PCR reaction. Median tissue culture infective dose (TCID50) of the virus in the sample was calculated according to the CT value of the sample and standard curve. The neutralization potency (or inhibition rate) was calculated as follows: Inhibition ratio = (TCID50 without serum - TCID50 with serum)/ TCID50 without serum *100%. The median neutralization dose (ND50) was calculated by Reed-Munch method.
2.8. Statistical analysis
The neutralization percentage curve was analyzed using nonlinear regression function of GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA, USA). One-way ANOVA was used for the comparison of multiple groups of data. The correlation between results was analyzed using linear regression function of using GraphPad Prism 7.
3. Results
3.1. Identification of SARS-CoV-2 S protein expression and SARS-CoV-2 pseudotyped virus
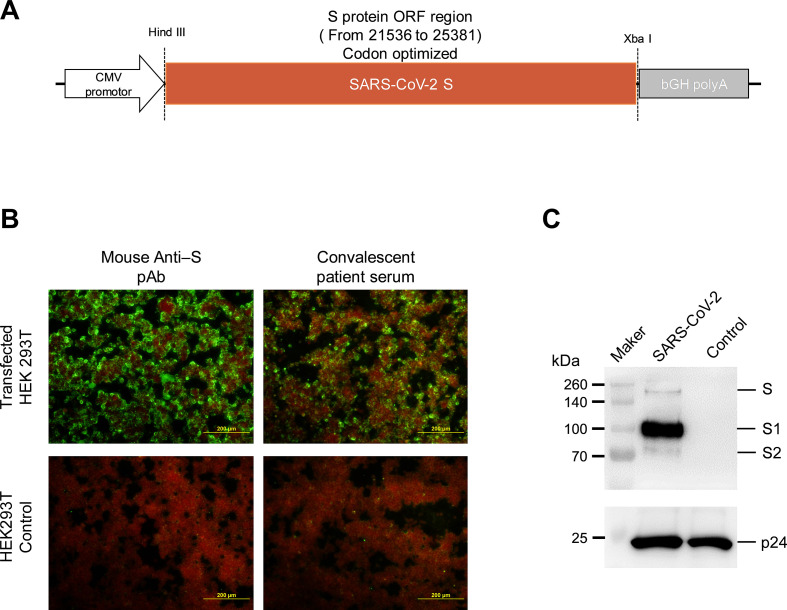
The construction of SARS-CoV-2 S protein expression plasmid (pcDNA3.1- SARS-CoV-2 S) is shown in Fig. 1A. The expression of S protein in HEK293T cells was analyzed via the immunofluorescence assay (Fig. 1B), in which the cells were stained by the serum of COVID-19 convalescent patients, as it exhibited a robust expression of SARS-CoV-2 S protein.
Fig. 1.
Identification of SARS-CoV-2 S protein expression and SARS-CoV-2 pseudotyped virus.
(A) Construction and identification of S expressing plasmid. SARS-CoV-2 S protein gene was inserted in the pCDNA3.1 vector. (B) Immunofluorescence assay for S protein expression in pcDNA3.1-SARS-CoV-2 S plasmid. The expression was determined using mouse pAb against SARS-CoV-2 S protein and convalescent serum samples from COVID-19 patients. (C) Identification of S protein expression in SARS-CoV-2 pseudotyped virus by immunoblot assay. Bands corresponding to SARS-CoV-2 S and HIV-1 p24 proteins were detected at the same sample line in the gel.
Pseudotyped virus bearing SARS-CoV-2 S protein was identified by western blotting using antibodies against SARS-CoV-2 S and HIV-1 p24. Dedicated bands of full-length S, S1, and S2 proteins were observed in concentrated pseudotyped virus cultural supernatant. HIV p24 can be detected at the same sample line (Fig. 1C), indicating the HIV core bearing SARS-CoV-2 spike protein pseudotyped virus were successful packaged.
3.2. Screening of sensitive cell lines for pseudotyped SARS-CoV-2 virus infection
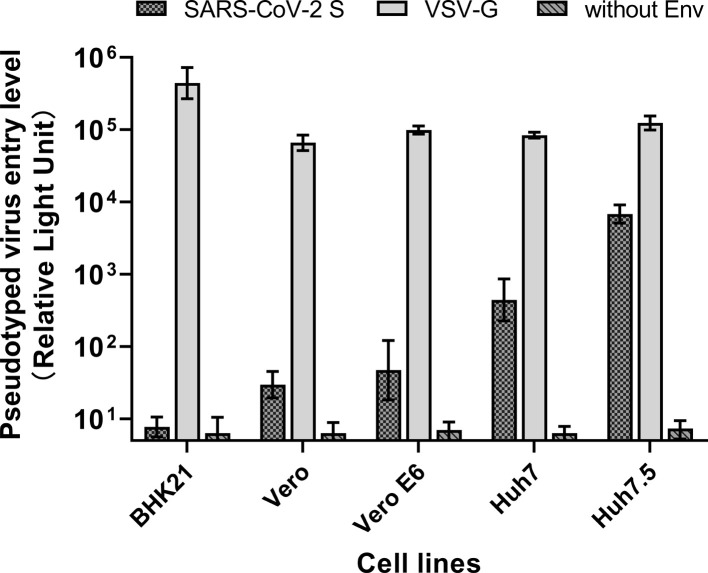
We analyzed several cell lines to screen for a cell line that is suitable for infecting with the SARS-CoV-2 pseudotyped virus (Fig. 2 ). Among them, the Huh7.5 cell line exhibited the highest reporter gene expression after transduction with pseudotyped SARS-CoV-2 virus, while other cell lines (Vero, Vero E6, BHK21) were substantially less permissive to SARS-CoV-2 pseudotyped virus entry.
Fig. 2.
Screening of sensitive cell lines for pseudotyped SARS-CoV-2 virus infection.
(A) Vero, Vero E6, Huh7, and Huh7.5 cells were analyzed to screen the cell line most sensitive to infection by the pseudotyped SARS-CoV-2 system. BHK21 cells were used as the negative control in this system, due to lack of SARS-CoV-2 receptor in the cells.
3.3. Application of pseudotyped SARS-CoV-2 virus for neutralization or entry inhibition assay
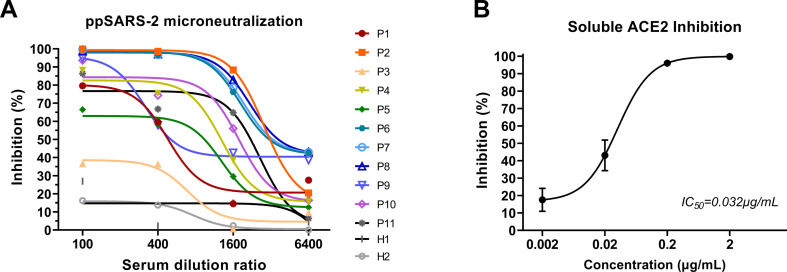
The pseudotyped SARS-CoV-2 system was employed to test the neutralizing activities of several convalescent serum samples from COVID-19 patients (Fig. 3A). The neutralizing titer of serially diluted serum samples of convalescent patients can be determined using the pseudotyped SARS-CoV-2 virus. Various levels of neutralizing antibodies were detected in the serum samples from COVID-19 convalescent patients, which exhibited 50% neutralization (ND50) against ppSARS-2 from less than 1:50 dilution to more than 1:2,048 dilution.
Fig. 3.
Neutralization or entry inhibition assay based on pseudotyped SARS-CoV-2 virus.
(A) Neutralization assay based on SARS-CoV-2 pseudotyped-virus system. The serum samples of 11 convalescent patients (P1-P11) was tested. The inhibition ratio decreased gradually with dilution. Ten out of 11 samples neutralized over 50% pseudotyped-virus at 1:100 dilution.
(B) Soluble ACE2 entry inhibition assay based on SARS-CoV-2 pseudotyped-virus system. Soluble ACE2 were serial diluted ten times. Results were obtained from three technical replicates, and the medium inhibitory concentration is calculated by nonlinear regression equation.
Since hACE2 has been reported as the SARS-CoV-2 entry receptor, we have utilized soluble hACE2 as a binding competitor to inhibit the entry of pseudotyped SARS-CoV-2 [9]. ppSARS-CoV-2 entry was blocked using soluble ACE2, as indicated by the significant reduction in reporter gene expression level (Fig. 3B). The IC50 of soluble hACE2 was identified as 0.032 μg/mL.
3.4. Correlation analysis between pseudotyped SARS-CoV-2 system and live-SARS-CoV-2 assays
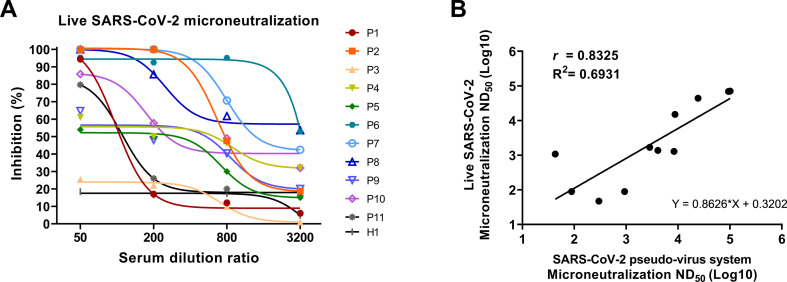
To verify the true reliability of the system, serum samples of the SARS-CoV-2 convalescent patients were analyzed by microneutralization using live SARS-CoV-2 virus (Fig. 4A). Results revealed the similar neutralizing potency between the pseudotyped-SARS-CoV-2 system and live-SARS-CoV-2.
Fig. 4.
Live SARS-CoV-2 virus microneutralization and correlation between SARS-CoV-2 pseudo-virus system and live SARS-CoV-2 virus assay.
(A) Live-SARS-CoV-2 microneutralization. Serum samples of the same 11 COVID-19 convalescent patients (P1-P11) were used to perform live SARS-CoV-2 virus microneutralization. Ten out of 11 samples neutralized over 50% live-virus at 1:50 dilution.
(B) Correlation between two methods was evaluated by ND50. A significant correlation was observed between the results of the two methods (r = 0.8325). Data is analyzed by Pearson's correlation comparison.
We then compared the results of the analysis conducted using the ppNT and the live virus (Fig. 4B) and found that the results from the ppNT were strongly correlated with those obtained using live virus (R2 = 0.6931 and p < 0.005).
4. Discussion
A reliable, rapid, and convenient neutralization assay is essential for screening and evaluation of antibodies and therapeutic agents against SARS-CoV-2 infection. The pseudotyped SARS-CoV-2 system developed here contains firefly luciferase reporter gene, which is suitable for high-throughput analysis and easy to handle in a BSL-2 facility. Various levels of neutralizing antibodies were detected in serum samples of COVID-19 convalescent patients, and soluble ACE2 protein was found to effectively inhibit the entry of ppSARS-2 in Huh7.5 cells. Moreover, we have verified the correlation between the results from using a pseudotyped platform and live SARS-CoV-2. Our data support that the pseudotyped SARS-CoV-2 platform could be employed for R&D of vaccines and drugs as well as for a deeper understanding of the transmission and infection of SARS-CoV-2.
SARS-CoV-2 has been successfully isolated and cultured in Vero, Huh7, and Calu-3 cell lines [19]. We have analyzed several cell lines to screen the most susceptible one to SARS-CoV-2 pseudotyped virus infection. Huh7.5 cell line exhibits the highest infectious potency as compared to Vero and Huh7 cell lines. We speculated that the reason for the excellent performance in the Huh7.5 cell line might be related to the defect of the interferon system and the expression of the new coronavirus-related receptor, ACE2 [20]. Besides, we have validated that ppSARS-CoV-2 entry in vitro can be effectively blocked using a novel, soluble ACE2, whose IC50 was determined as 0.032 μg/mL, indicating that soluble ACE2 can be a promising alternative strategy for prevention and treatment of COVID-19 [21,22].
Recently, two research groups have also reported the development of pseudotyped SARS-CoV-2 based on the VSV platform bearing the full-length S or C-terminal 18 amino-acids truncated S [22,23]. VSV-based pseudotyped SARS-CoV-2 shows a broader range of target cell lines. However, the former platform, analyzed using the Huh7 cell line and Fluc reporter, was not validated by live SARS-CoV-2 assays, whereas the latter platform, analyzed using BHK21-hACE2 cell line and GFP reporter, was not suitable for high-throughput quantitative analysis. In general, VSV-based pseudotyped virus is not as practicable as an HIV-1-based pseudotyped system, which can be effectively packaged via co-transfection of two or three plasmids [14,15].
Collectively, the pseudotyped SARS-CoV-2 virus system, established in this study, has good authenticity and reliability. Overcoming the limitation of requirement of a BSL-3 lab, the pseudotyped SARS-CoV-2 virus system can be widely used for large-scale serological screening for epidemiological investigation on SARS-CoV-2. It can also be used to evaluate the neutralization activity of vaccines and therapeutic antibodies, as well as provide technical support for optimized drug development for inhibition of viral entry.
Acknowledgments
Acknowledgements
The following grants support this work: The National Key Research and Development Program of China (No. 2016YFD0500301, No. 2020YFC0842100) and the National Major Project for Control and Prevention of Infectious Disease in China (No. 2018ZX10101002). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of China CDC, funding sources, and other organizations.
Conflict of interest statement
The authors declare that there are no conflicts of interest. Given their roles as Editorial Board Member, Wenjie Tan had no involvement in the peer-review of this article and had no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to the editor Di Qu.
Author contributions
Ren Yang: Conceptualization, Data curation, Writing - original draft. Baoying Huang: Data curation, Methodology. Ruhan A: Project administration. Wenhui Li: Resources. Wenling Wang: Resources. Yao Deng: Methodology, Supervision. Wenjie Tan: Conceptualization, Editing - original draft, Validation.
References
- 1.Tan W., Zhao X., Ma X., Wang W., Niu P., Xu W., Gao G.F., Wu G. A novel coronavirus genome identified in a cluster of pneumonia cases-Wuhan, China 2019–2020. China CDC Weekly. 2020;2:61–62. doi: 10.46234/ccdcw2020.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;10224:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;8:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;4:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, Lancet. 2020;10223:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;7461:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;8:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;2:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;2:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B beta-coronaviruses. Nat. Microbiol. 2020;4:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutner R.H., Zhang X.Y., Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 12.Steffen I., Simmons G. Pseudotyping viral vectors with emerging virus envelope proteins. Curr. Gene Ther. 2016;1:47–55. doi: 10.2174/1566523216666160119093948. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q., Nie J., Huang W., Jiao Y., Li L., Zhang T., Zhao J., Wu H., Wang Y. Development and optimization of a sensitive pseudovirus-based assay for HIV-1 neutralizing antibodies detection using A3R5 cells. Hum. Vaccin. Immunother. 2018;1:199–208. doi: 10.1080/21645515.2017.1373922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;8:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju B., Zhang Q., Ge X., Wang R., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Ge J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Wang X., Zhang Z., Zhang L. 2020. Potent Human Neutralizing Antibodies Elicited by SARS-CoV-2 Infection, bioRxiv. [DOI] [PubMed] [Google Scholar]
- 16.Zhao G., Du L., Ma C., Li Y., Li L., Poon V.K., Wang L., Yu F., Zheng B.J., Jiang S., Zhou Y. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol. J. 2013;10:266. doi: 10.1186/1743-422X-10-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado D.P.C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.004. 905-913.e7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman C.M., Frieman M.B. Growth and quantification of MERS-CoV infection. Curr. Protoc. Microbiol. 2015;37 doi: 10.1002/9780471729259.mc15e02s37. , 15E.2.1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J., Li C., Liu X., Chiu M.C., Zhao X., Wang D., Wei Y., Lee A., Zhang A.J., Chu H., Cai J.P., Yip C.C., Chan I.H., Wong K.K., Tsang O.T., Chan K.H., Chan J.F., K.K. To, Chen H., Yuen K.Y. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020;26:1077–1083. doi: 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- 20.Blight K.J., McKeating J.A., Rice C.M. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 2002;24:13001–13014. doi: 10.1128/jvi.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei C., Qian K., Li T., Zhang S., Fu W., Ding M., Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020;1:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., Lu Q., Li X., Sun Q., Liu J., Fan C., Huang W., Xu M., Wang Y. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;1:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong H., Wu Y., Cao Ji., Yang R., Ma J., Qiao X., Yao X., Zhang B., Zhang Y., Hou W., Shi Y., Xu J., Zhang L., Wang S., Fu B., Yang T., Ge S., Zhang J., Yuan Q., Huang B., Li Z., Zhang T. Robust neutralization assay based on SARS-CoV-2 S-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressed BHK21 cells. bioRxiv. 2020 doi: 10.1101/2020.04.08.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]