Abstract
PURPOSE:
This study compares a web-based teleophthalmology assessment with a clinical slit lamp examination to screen for diabetic retinopathy (DR) and age-related macular degeneration (AMD) among diabetic patients in a rural East African district.
METHODS:
Six hundred and twelve eyes from 306 diabetic patients underwent both a clinical slit lamp examination and a teleretina (TR) assessment by an experienced ophthalmologist. Both assessments were compared for any DR and AMD using the early treatment diabetic retinopathy study and age-related eye disease study grading scales, respectively.
RESULTS:
Of the 612 TR assessment photos, 74 (12%) were deemed ungradable due to media opacities, poor patient cooperation, or unsatisfactory photographs. The ability to detect DR and AMD showed a fair agreement (kappa statistic 0.27 and 0.23, respectively) between the TR and clinical slit lamp examination. Relative to a clinical slit lamp evaluation, a positive TR diagnosis carried a 75.0% positive predictive value when diagnosing DR and a 27.3% positive predictive value when diagnosing AMD. A negative TR diagnosis carried a 97.2% negative predictive value for the diagnosis of DR and a 98.1% negative predictive value for the diagnosis of AMD.
CONCLUSION:
When comparing TR assessments to clinical slit lamp examinations to diagnose DR and AMD, there was a fair agreement. Although further validation is needed, the TR approach provides a promising method to diagnose DR and AMD, two major causes of ocular impairment worldwide.
Keywords: Age-related macular degeneration, diabetic retinopathy, slit lamp examination, teleretina, teleophthalmology
Introduction
Diabetic retinopathy (DR) and age-related macular degeneration (AMD) are the fifth and third most common causes of moderate-to-severe visual impairment worldwide.[1] As of 2010, there are an estimated 127 million cases of DR,[2] and as of 2014, 170 million cases of AMD.[3] These numbers are projected to rise to 191 million,[2] and 243 million,[3] respectively, by the year 2030.
The international diabetes foundation estimates that the number of Africans with diabetes will increase by 162% from 15.9 million in 2017 to 41.6 million in 2045.[4] With between 30.2% and 31.6% of African diabetics experiencing DR, there is projected to be an increase of approximately eight million African individuals experiencing DR between now and 2045.[5] Furthermore, Africa is the region with the highest percentage of undiagnosed diabetics in the world, with approximately 70% of people with diabetes unaware of their condition.[4] Consequently, the prevalence of severe DR in Africans has been reported to be 52% of DR cases, significantly higher than in individuals from other regions.[6] Similarly, the number of Africans with AMD is projected to increase by 105% from 19.0 million in 2018 to 39.1 million by 2040.[3]
Unfortunately, in the developing world, and particularly in Sub-Saharan Africa, there are problems with poor patient attendance in clinics, inadequate or even nonexistent referral systems to ophthalmic services, poor record-keeping, nonexistent systematic screening programs, and inadequate access to imaging and therapeutic technology.[5] These additional barriers add another degree of complexity when trying to treat AMD and DR in these regions.
Teleretina (TR) refers to the use of telecommunication and technology to evaluate and treat patients with retinal diseases at a distance. TR programs allow for a diagnosis to be made most commonly through the use of stereoscopic photography, examining for clinical signs of DR and AMD.[7] TR programs have been shown to be effective in reducing office visits,[8] as well as in increasing screening rates and access to recommended eye care in those with DR and AMD.[9,10] Early detection and treatment of retinal abnormalities is critical in preventing vision loss in those with DR and AMD,[11,12] and TR has been shown to be an effective early form of screening.[12,13] Despite this, more research is needed to evaluate the efficacy and reliability of TR on a global scale.[14]
The aim of this study was to compare the efficacy of a web-based TR assessment with an in-person clinical slit lamp examination to screen for DR and AMD among diabetic patients in a rural African district.
Materials and Methods
Ethics approval
Ethics approval was obtained from the Aga Khan University East Africa Research Committee as part of the Faculty of Health Sciences Medical College in Nairobi, Kenya. The investigation was carried out following the tenets of the Declaration of Helsinki.
Patient selection
The study was carried out in the Muranga District Hospital diabetes and ophthalmology clinics. A precision-based method was used to calculate the appropriate sample size. All patients above the age of 30 years who were capable of giving informed consent and who attended the Muranga Hospital Diabetic Clinic between August and October 2011 were included in the study. Individuals under the age of 30 years, with ocular anatomy (natural or traumatic) inhibiting adequate fundus photography, or with physical deformities that inhibited proper positioning for fundus photography and visual field examination were excluded. Patients were also excluded at the discretion of the nurses in cases with the potential for aggressive behavior or violence. Finally, blurry fundus photos that were not conducive to TR assessment were excluded from the study.
Patient prescreening
After obtaining informed consent, patients underwent both a general medical and an ocular history by a trained ophthalmic assistant (OA) using a predetermined form [Appendix]. The OAs were trained to take thorough histories before the study at the Aga Khan University Hospital in Nairobi. The initial ocular assessment conducted by the OAs included visual acuity, external ocular exam with a penlight, intraocular pressures with a Tonopen (Reichert Technologies, Depew, NY, USA), frequency doubling technology (FDT) visual field screening perimetry (screening C-20 program), and fundus photography [Appendix]. A photograph of the visual field test printout was digitally uploaded. Stereo images of fundus were acquired following pupil dilation with 1% tropicamide. All fundus photos were obtained with a Topcon 777 (Topcon Corp., Tokyo, Japan) three field fundus camera at 45°. Each 172 MB (24 bits/pixel) file was 16:1 compressed to a 1.1 MB (1.5 bits/pixel) JPEG image. The OAs uploaded the history form, the examination form, the color photographs, and the results of the visual field testing onto a secure patient data website operated by secure diagnostic imaging (SDI) based at the University of Alberta (www.teleophthalmology.com). The software to date has already been validated for DR and utilized for Teleretinal screening at the University of Alberta, Canada.[7,15] An internet modem from Safaricom Limited was used to access the website. Patients were subjected to both slit lamp (see protocol #1 below) and teleophthalmology examination (see protocol #2 below), and results were compared.[INLINE:1]
Protocol #1 slit lamp examination
Following prescreening, each patient was seen by a comprehensive ophthalmologist based in the district. The ophthalmologist reviewed the history form, the examination form, and the FDT printout. They subsequently completed a dilated fundus exam to diagnose and grade the DR and/or AMD using the predetermined form [Appendix]. Lens opacity was recorded based on the lens opacities classification system III classification.[16] During the slit lamp examination, a 90D lens was used to examine the optic nerve and macula to record any DR and/or AMD features. DR was graded based on the early treatment diabetic retinopathy study classification and AMD based on age-related eye disease study grading scale [Appendix].
Protocol #2 remote teleopthalmology investigation
After uploading the data onto the SDI website, a remote ophthalmologist reviewed the history form, the examination form, the color photographs, and the visual fields. The ophthalmologist viewed the fundus photos in three-dimensions and indicated any observed microaneurysms, venous beading, intraretinal microvascular abnormality, drusen size/area, pigmentary changes, geographic atrophy and/or macular edema. Figure 1 demonstrates a nurse taking fundus photographs and an ophthalmologist grading the teleophthalmology images. A positive DR and/or AMD diagnosis in the setting of TR analysis was based on a synthesis of history and retinal examination. For a summary of the criteria used to diagnose the DR and AMD, refer to the appendix.
Figure 1.

(a) Dr. Dan Kiage seen grading teleophthalmology images wearing LCD shutter goggles. The fundus image appears on one screen while the grading form appears on a separate screen. (b) Nurse Florence taking a fundus photograph in Muranga
Blinding protocol
To ensure a fair comparison, the district comprehensive ophthalmologist doing the in-person fundus exam and the ophthalmologist performing the remote grading were blinded from each other's findings. The information obtained from the clinical examination and the SDI program software was entered into Microsoft Excel and SPSS 11.0 (IBM Corp, New York, NY, USA) for analysis and comparison.
Statistical analysis
Predictive values were used to compare the results from the TR assessment and the clinical slit lamp examination. The kappa (κ) statistic was calculated using individual eyes as the unit of analysis to analyze the reproducibility of the measurements and diagnoses between the two assessments. To reduce the effects of intra-patient clustering introduced by using the results from both eyes for the slit lamp and TR protocols, the standard error was adjusted in the random-effects model using the bootstrapping method described by Landis and Koch.[17] For all other analyses, the exact agreement was used. Photographs deemed ungradable were excluded from the κ analysis. κ values >0.81 illustrate almost perfect strength of agreement, values between 0.61 and 0.80 illustrate substantial agreement, values between 0.41 and 0.60 illustrate moderate agreement, values between 0.21 and 0.40 illustrate fair agreement, and values between 0 and 0.20 illustrate slight agreement.[17]
Sample size
Given that the literature has reported rates of DR and AMD among African Diabetics to be 31%,[18] and 1.3%,[19] respectively, a sample size of at least 329 eyes was required (α = 0.05, d = 0.05).
Results
Study population analysis
The study included 314 patients and 628 eyes, thereby fulfilling the sample size requirement for this cross-sectional analysis. Of note, the mean age of included patients was 62 ± 11.6 years; the median number of years that patients had been diagnosed with diabetes mellitus was five. In addition, 60% of patients had a history of hypertension, and 5% had a history of blindness [Table 1]. All 314 patients completed the clinical examination, whereas eight patients did not complete the entire TR exam. Six individuals did not complete the TR portion of the examination and two were missing clinical data. Six hundred and twelve fundus photos were taken, 74 (12%) of which were deemed ungradable. Thirty-nine were ungradable due to media opacities: 22 cataracts, 10 corneal opacities, and 7 postcataract posterior capsular opacities [Appendi × 1], whereas the other 35 cases were ungradable due to poor pupil dilation, unsatisfactory photographs, or uncooperative patients. Ninety-four cases of DR and 13 cases of AMD were identified. Figure 2 demonstrates fundus photographs taken in this investigation and their associated retinal pathologies.
Table 1.
Patient population overview
| General characteristics | Specific Item | Value (percentage of total) |
|---|---|---|
| Age (years) | Mean±SD | 62±11.6 |
| SD | 11.6 | |
| Median | 63 | |
| Range | 33-91 | |
| Gender | Male | 124 (40) |
| Female | 187 (60) | |
| Missing information | 3 | |
| Duration of previous diabetes mellitus (years) | Median | 5 |
| Range | 0-44 | |
| Presence of comorbidities and risk factors | Hypertension | 188 (60) |
| Heart disease | 11 (4) | |
| Asthma | 15 (5) | |
| Other systemic diseases and complications | 20 (6) | |
| Alcohol consumption | 8 (3) | |
| Cigarette smoking | 2 (1) | |
| Past ocular history | Prior eye surgery | 18 (6) |
| Known glaucoma | 11 (4) | |
| Using glaucoma medication(s) | 17 (5) | |
| Family history of blindness | 15 (5) | |
| Mean intraocular pressure (mmHg) | 22±8.5 | |
| Total patient size | Total | 314 (100) |
SD: Standard deviation
Figure 2.
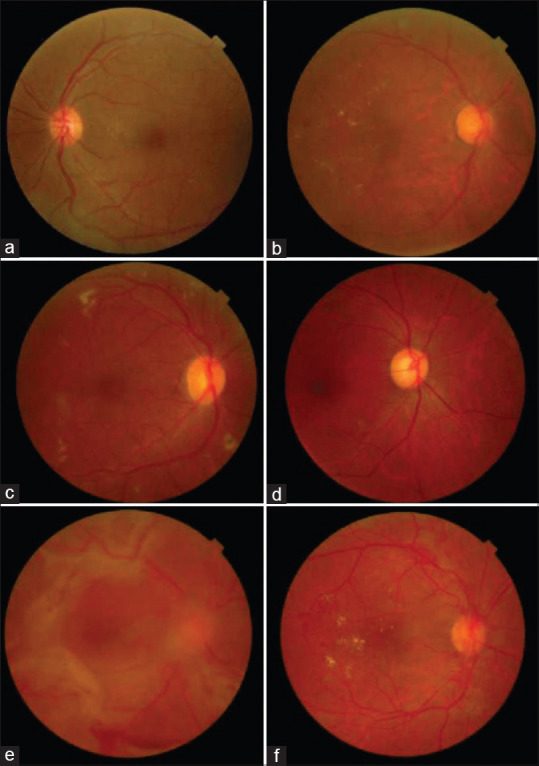
Fundus photographs demonstrating a variety of retinal pathologies. (a) Microaneurysms (ETDRS level 20) (b) CSME (ETDRS level 35D). (c) Moderate NPDR (ETDRS level 35F) (d) Moderate NPDR (ETDRS level 43B) e) PDR (ETDRS level 65C) f) PDR (ETDRS level 65B) ETDRS: Early treatment diabetic retinopathy study, CSME: Clinically significant macular edema, NPRDR: Non-proliferative diabetic retinopathy, PDR: Proliferative diabetic retinopathy
Comparison of the ability to diagnose diabetic retinopathy and age-related macular degeneration
The diagnosis of DR and AMD was based on a synthesis of history and retinal examination. When comparing the ability to diagnose these two conditions, the κ score of agreement between the TR analysis and the clinical slit lamp examination was 0.27 for DR (95% confidence interval [CI]: 0.21–0.33), and 0.23 for AMD (95% CI: 0.001–0.47).
Thereby these results indicate a fair agreement between the two methods for the diagnosis of DR and AMD [Table 2].
Table 2.
Comparison of the agreement in diagnosis between teleretina and clinical slit lamp examination in the analysis of both patient eyes
| Assessment | κ | 95% CI (κ) |
|---|---|---|
| Overall diabetic retinopathy diagnosis | 0.27 | 0.21-0.33 |
| Overall age-related macular degeneration diagnosis | 0.23 | 0.001-0.47 |
CI: Confidence interval
Relative to the clinical slit lamp examination, TR has a 76.6% (95% CI: 62.8–86.4) sensitivity, and a 96.9% (95% CI: 94.7–98.2) specificity for diagnosing DR and a 23.1% (95% CI: 5.0–53.8) sensitivity, and a 98.48% (95% CI: 97.0–99.3) specificity for diagnosing AMD; a diagnosis of DR using TR assessment had a 75.0% (95% CI: 61.2–85.1) positive predictive value and a 97.2% (95% CI: 95.0–98.4) negative predictive value. A diagnosis of AMD carried a 27.3% (95% CI: 10.1–55.6) positive predictive value and 98.1% (95% CI: 97.5–98.6) negative predictive value. The likelihood ratio of a positive diagnosis of DR with TR relative to clinical slit lamp examination was 25.1 (95% CI: 14.1–44.8), and the likelihood ratio of a negative diagnosis was 0.2 (95% CI: 0.2). For the AMD assessment, the likelihood ratio of a positive diagnosis was 15.1 (95% CI: 4.5–50.3) and the likelihood ratio of a negative diagnosis was 0.8 (95% CI: 0.6–1.1) [Table 3]. The prevalence of DR and AMD within our sample of African diabetics analyzed by clinical slit lamp examination was 10.7% and 2.4%, respectively; the prevalence of DR and AMD by TR assessment was 10.9% and 2.0%, respectively.
Table 3.
Ability of teleretina screening to diagnose diabetic retinopathy and age-related macular relative to the clinical slit lamp examination
| Characteristic | Diabetic retinopathy |
Age-related macular degeneration |
||
|---|---|---|---|---|
| Percentage | 95% confidence interval | Percentage | 95% confidence interval | |
| Sensitivity | 76.6 | 62.8-86.4 | 23.1 | 5.0-53.8 |
| Specificity | 96.9 | 94.7-98.2 | 98.5 | 97.0-99.3 |
| PPV | 75.0 | 61.2-85.1 | 27.3 | 10.1-55.6 |
| NPV | 97.2 | 95.0-98.4 | 98.1 | 97.5-98.6 |
| LR+ | 25.1 | 14.1-44.8 | 15.1 | 4.5-50.7 |
| LR− | 0.2 | 0.1-0.4 | 0.8 | 0.6-1.1 |
PPV: Positive predictive value, NPV: Negative predictive value, LR+: Likelihood ratio of the positive test, LR−: Likelihood ratio of negative test
Discussion
In this investigation, TR assessments yielded a fair agreement with clinical slit lamp examinations in their ability to diagnose DR and AMD. A positive diagnose via TR carried a 75.0% and 27.3% positive predictive value and a negative diagnosis carried a 97.2% and 98.1% negative predictive value for DR and AMD respectively relative to a clinical examination.
TR screening has shown much promise in both rural and in inner-city settings to deliver more efficient and effective healthcare to patients.[9,10,11,12,13,14,20,21] Irrespective of geographical barriers, patients have limited access to appropriate eye care, leading to a delay in diagnosis and subsequent treatment of retinal diseases.[5,22] TR assessments have shown to be effective in combatting this issue, highlighted by one study demonstrating a six-fold increase in the rate of retinal examinations through TR when compared to usual care.[20] Furthermore, previous research suggests, that with strategic workflow planning, TR screening can be fiscally advantageous from a health systems perspective by avoiding unnecessary physician services, and from a patient perspective by reducing travel costs and the loss of income secondary to traveling to retinal specialists.[23] This is exceptionally important when considering the immense financial constraints of treating ocular conditions in the developing world.[22] Another important factor to consider is that patients welcome the prospect of TR assessments. One study examining patient satisfaction with TR screening for DR found that close to 99% of patients were satisfied with a TR system and that 95% of patients would actually prefer a TR screening to a conventional clinical face-to-face examination.[24] Before TR approaches are fully adopted; however, they must prove to have a similar diagnostic ability to the current gold standards for evaluating retinal diseases. A recent literature review examining the feasibility of TR for DR and AMD found that as with the results of our investigation, the current literature tends to report a high specificity for TR screening for DR with specificities varying depending on the severity of the DR.[23] Another systematic review and meta-analysis found similar results and calculated the pooled specificity of TR evaluations to exceed 90% and the pooled sensitivity to exceed 80%.[25] There exists substantially less literature examining the role of TR evaluations for AMD. One hypothesized explanation for this trend is that until relatively recently, there was a lack of effective treatments for AMD.[23] The literature review examining the feasibility of TR for DR and AMD reported a large range of sensitivity and specificity values for the accuracy of AMD diagnosis through TR screening. The results varied greatly depending on the technique used and on the signs that each study was examining for, underscoring the need for further standardized investigations in the future.[23] As with our study, a concern expressed in this review were the frequency of ungradable digital images with the rates ranging from 0% to 14.9% of all fundus photographs.[23]
The results of this investigation are encouraging for the future implementation of a TR screening program in Sub-Saharan Africa. We were able to obtain digital fundus photographs of at-risk patients for DR and AMD from a rural African district at minimum cost and discomfort to the patient. These photographs were then reviewed by a remote ophthalmologist. The methodology and screening system utilized in this investigation can be used for future research to build off of in the pursuit of providing high-quality healthcare in lower-resource settings, as well as to improve efficiency and resource allocation in urban centers.
There are, however, several limitations must be considered when interpreting the results of this investigation. First, 74 (12%) of the fundus photographs were deemed ungradable due to media opacities, poor pupil dilation, unsatisfactory photographs, or uncooperative patients. Thus, while a TR approach may increase patient access to ophthalmic care, poor quality information may limit its effectiveness. In cases in which an appropriate clarity of photograph was achieved, factors such as differences in physician training or variations in the grading approach may have limited the level of agreement. Finally, there are inherent drawbacks to any telemedicine screening program that should also be considered. Notably, a potentially diminished physician-patient relationship due to a reduction in the level of face-to-face encounters and a reduction in patients' ability to ask questions.[26]
We suggest that future investigations involve a larger patient population to better confirm the agreement between the two assessment techniques. We also suggest that in order to reduce the effects of inter-observer variation and of variable training by the assessors, that the same ophthalmologist, with adequate time lapse to reduce the effects of recall bias, completes the slit lamp examination and grades the images virtually.
Conclusion
In conclusion, AMD and DR are major causes of visual impairment and blindness around the world. Given the ability to manage these conditions, and the detrimental consequences of progressing to the late stages of these diseases, much attention is given to the early detection and initiation of treatment in patients with a positive diagnosis. TR screening protocols have been proposed as a viable solution to improve patient access to specialist care, especially in rural areas where financial, structural, and cognitive barriers result in less than ideal healthcare delivery. Our investigation demonstrated a fair correlation between TR screening and traditional slit-lamp examination in the diagnosis of DR and AMD. Although further validation is needed, the TR approach provides a promising method to diagnose and treat DR and AMD.
Financial support and sponsorship
A grant for this study was obtained from the Aga Khan University Research council with a supplement from Pfizer Canada. The SDI software was provided at no charge by the directors of the company.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
A grant for this study was obtained from the Aga Khan University Research council with supplement from Pfizer Canada. The SDI software was provided at no charge by the directors of the company. Special thanks are given to Abshir Moalin, who was invaluable in the software set up and support throughout the study. We thank the Ophthalmic Assistants Florence Mutisya and Beatrice Nyaga who took the photographs, filled the history and examination forms, and uploaded all information to the website. We also thank Anne Kinyanjui, the Muranga District Hospital Ophthalmic Nurse, for booking patients and assisting in the pre-exam work up as well as Dr. Stephen Gichuhi, for his help with the statistical analysis. We appreciate all patients who participated in the study as well as the administration of Muranga District hospital for allowing us to use their facility. Finally, we thank Dr. Michael Gichangi of the Kenya Division of Ophthalmic Services, for facilitating the collaboration with the Muranga District Hospital Eye Clinic.
Appendix
Case definitions for diabetic retinopathy and age-related macular degeneration
| DR: ETDRS classification | AMD: AREDS grading scale |
|---|---|
| None CSME Mild NPDR Moderate NPDR Severe NPDR PDR Retinal laser completed |
Large drusen Pigmentary changes |
CSME: Clinically significant macular edema, NPDR: Nonproliferative diabetic retinopathy, PDR: Proliferative diabetic retinopathy, ETDRS: Early treatment diabetic retinopathy study, AREDS: Age-related eye disease study
Reasons for ungradable fundus photographs (n=74)
| Reason | Number of cases (percentage of total) |
|---|---|
| Media opacification | |
| Cataract | 22 (4) |
| Corneal opacity | 10 (2) |
| Intraocular lens posterior capsular opacity | 7 (1) |
| Nonopacification* | 35 (6) |
| Total | 74 (12) |
*Including: Dilation difficulty, poor quality photograph, uncooperative patient
History forms
| Date____________ Name: ________________________, Project No: __________, DOB: _____ Male/female: ______ | ||
| History | RE | LE |
|---|---|---|
| Known glaucoma | Yes/no | Yes/no |
| Current ocular meds | ||
| Eye surgery | Trab/cataract - IOL/aphakia Others | Trab/Cataract - IOL/aphakia Others |
| Family history of glaucoma | ||
| Systemic history | ||
| Systemic medication | ||
| Duration of diabetes | ||
| Hypertension | Yes/no | |
| Heart disease | Yes/no | |
| Asthma | Yes/no | |
| Alcohol consumption | Yes/no | |
| Cigarette smoking | Yes/no | |
Examination form
| Date____________ Name: ___________________________, Project No: __________, DOB:_____ Male/female: _____ | ||
| Exam | RE | LE |
|---|---|---|
| VA | SC/CC/PH | SC/CC/PH |
| IOP by tonopen | ||
| FDT visual fields | ||
| photography | ||
| Ophthalmologists examination | ||
| Cornea | ||
| Lens status | Cataract/IOL/aphakia | Cataract/IOL/aphakia |
| If Cataract - type and grade | ||
| Vertical CDR | ||
| Notch | Yes/no | Yes/no |
| PPA | Yes/no | Yes/no |
| Disc hemorrhage | Yes/no | Yes/no |
| Visual fields defect | Yes/no | Yes/no |
| Glaucoma | Category 0 Category 1 Category 2 Category 3 Glaucoma suspect |
Category 0 Category 1 Category 2 Category 3 Glaucoma suspect |
| Diabetic retinopathy | None CSME Mild NPDR Moderate NPDR Severe NPDR PDR Retinal Laser done |
None CSME Mild NPDR Moderate NPDR Severe NPDR PDR Retinal Laser done |
| AMD | Yes/no | Yes/no |
| Other fundus pathology (describe) | ||
CSME: Clinically significant macular edema, NPDR: Nonproliferative diabetic retinopathy, PDR: Proliferative diabetic retinopathy, ETDRS: Early treatment diabetic retinopathy study, AREDS: Age-related eye disease study
DR: ETDRS grading protocol. International clinical diabetic retinopathy disease severity scale
| Proposed disease severity level | Findings observable with dilated ophthalmoscopy |
|---|---|
| No apparent DR | No abnormalities |
| Mild nonproliferative DR | Micro aneurysms only |
| Moderate nonproliferative DR | More than “mild” but less than “severe” |
| Severe nonproliferative DR | Any of the following 20 or more intraretinal hemorrhages in 4 quadrants Definite venous beading in 2 or more quadrants Prominent IRMA in 1 or more quadrants and no neovascularization |
| Proliferative DR | 1 or more of the following Definite neovascularization Preretinal or vitreous hemorrhage |
IRMA: Intraretinal microvascular abnormalities, ETDRS: Early treatment diabetic retinopathy study
International clinical diabetic macular edema disease severity scale
| Proposed disease severity level | Findings on dilated ophthalmoscopy |
|---|---|
| DME absent | No retinal thickening or hard exudates present in the posterior pole |
| DME present | Some retinal thickening or hard exudates present in the posterior pole |
| If DME is present, it can be categorized as follows | |
| Proposed disease severity level | Findings observable on dilated ophthalmoscopy* |
| Mild DME | Some retinal thickening or hard exudates in posterior pole but distant from the center of the macula |
| Moderate DME | Retinal thickening or hard exudates approaching the center of the macula but not involving the center |
| Severe DME | Retinal thickening or hard exudates involving the center of the macula |
*Hard exudates are a sign of current or previous macular edema. DME is defined as retinal thickening, and this requires a 3-dimensional assessment that is best performed through a dilated pupil examination using slit-lamp biomicroscopy with an accessory lens and/or stereo fundus photography. DME: Diabetic macular edema
AMD: AREDS grading scale
Age-related eye disease study research group. Arch Ophthalmol 2005;123:1484-149
References
- 1.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990-2010: A systematic analysis. Lancet Glob Heal. 2013;1:e339–49. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428–31. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Heal. 2014;2:e106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas-2017 Atlas. Diabetes Atlas. 2017. [Last accessed on 2018 Jul 08]. p. 150. Available from: http://diabetesatlas.org/resources/2017-atlas.html .
- 5.Burgess PI, MacCormick IJ, Harding SP, Bastawrous A, Beare NA, Garner P. Epidemiology of diabetic retinopathy and maculopathy in Africa: A systematic review. Diabet Med. 2013;30:399–412. doi: 10.1111/j.1464-5491.2012.03756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalk WJ, Joannou J, Ntsepo S, Mahomed I, Mahanlal P, Becker PJ. Ethnic differences in the clinical and laboratory associations with retinopathy in adult onset diabetes: studies in patients of African, European and Indian origins. J Intern Med. 1997;241:31–7. doi: 10.1046/j.1365-2796.1997.70892000.x. [DOI] [PubMed] [Google Scholar]
- 7.Rudnisky CJ, Tennant MT, Weis E, Ting A, Hinz BJ, Greve MD. Web-based grading of compressed stereoscopic digital photography versus standard slide film photography for the diagnosis of diabetic retinopathy. Ophthalmology. 2007;114:1748–54. doi: 10.1016/j.ophtha.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Hanson C, Tennant MT, Rudnisky CJ. Optometric referrals to retina specialists: Evaluation and triage via teleophthalmology. Telemed e-Health. 2008;14:441–5. doi: 10.1089/tmj.2007.0068. [DOI] [PubMed] [Google Scholar]
- 9.Taylor CR, Merin LM, Salunga AM, Hepworth JT, Crutcher TD, O'Day DM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: The vine hill study. Diabetes Care. 2007;30:574–8. doi: 10.2337/dc06-1509. [DOI] [PubMed] [Google Scholar]
- 10.Le Tien V, Strého M, d'Athis P, Taillandier-Heriche E, Paillaud E, Mahiddine H, et al. Interobserver and intraobserver reliability of detecting age-related macular degeneration using a nonmydriatic digital camera. Am J Ophthalmol. 2008;146:520–6.e1. doi: 10.1016/j.ajo.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Keane PA, de Salvo G, Sim DA, Goverdhan S, Agrawal R, Tufail A. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin Ophthalmol. 2015;9:353–66. doi: 10.2147/OPTH.S59012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vashist P, Singh S, Gupta N, Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: an overview. Indian J Community Med. 2011;36:247–52. doi: 10.4103/0970-0218.91324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sreelatha OK, Ramesh SV. Teleophthalmology: Improving patient outcomes? Clin Ophthalmol. 2016;10:285–95. doi: 10.2147/OPTH.S80487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walton OB, Garoon RB, Weng CY, Gross J, Young AK, Camero KA, et al. Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol. 2016;134:204. doi: 10.1001/jamaophthalmol.2015.5083. [DOI] [PubMed] [Google Scholar]
- 15.Tennant MT, Greve MD, Rudnisky CJ, Hillson TR, Hinz BJ. Identification of diabetic retinopathy by stereoscopic digital imaging via teleophthalmology: A comparison to slide film. Can J Ophthalmol. 2001;36:187–96. doi: 10.1016/s0008-4182(01)80039-9. [DOI] [PubMed] [Google Scholar]
- 16.Chylack LT, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. Arch ophthalmol. 1993;111:831. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 18.Burgess PI, Msukwa G, Beare NA. Diabetic retinopathy in sub-saharan Africa: Meeting the challenges of an emerging epidemic. BMC Med. 2013;11:157. doi: 10.1186/1741-7015-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathenge W, Bastawrous A, Peto T, Leung I, Foster A, Kuper H. Prevalence of age-related macular degeneration in Nakuru, Kenya: A cross-sectional population-based study. In: Lewallen S, editor. PLoS Med. 10. p. e1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis RM, Fowler S, Bellis K, Pockl J, Al Pakalnis V, Woldorf A. Telemedicine improves eye examination rates in individuals with diabetes: A model for eye-care delivery in underserved communities. Diabetes Care. 2003;26:2476. doi: 10.2337/diacare.26.8.2476. [DOI] [PubMed] [Google Scholar]
- 21.Cavallerano J, Aiello LM. Emerging trends in ocular telemedicine: The diabetic retinopathy model. J Telemed Telecare. 2005;11:163–6. doi: 10.1258/1357633054068874. [DOI] [PubMed] [Google Scholar]
- 22.Sommer A, Taylor HR, Ravilla TD, West S, Lietman TM, Keenan JD, et al. Challenges of ophthalmic care in the developing world. JAMA Ophthalmol. 2014;132:640–4. doi: 10.1001/jamaophthalmol.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaziri K, Moshfeghi DM, Moshfeghi AA. Feasibility of telemedicine in detecting diabetic retinopathy and age-related macular degeneration. Semin Ophthalmol. 2015;30:81–95. doi: 10.3109/08820538.2013.825727. [DOI] [PubMed] [Google Scholar]
- 24.Boucher MC, Nguyen QT, Angioi K. Mass community screening for diabetic retinopathy using a nonmydriatic camera with telemedicine. Can J Ophthalmol J Can d'Ohtalmol. 2005;40:734–42. doi: 10.1016/S0008-4182(05)80091-2. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Wu H, Dong J, Jiang K, Lu X, Shi J. Telemedicine for detecting diabetic retinopathy: A systematic review and meta-analysis. Br J Ophthalmol. 2015;99:823–31. doi: 10.1136/bjophthalmol-2014-305631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabesan S, Allen D, Caldwell P, Loh PK, Mozer R, Komesaroff PA, et al. Practical aspects of telehealth: Doctor-patient relationship and communication. Intern Med J. 2014;44:101–3. doi: 10.1111/imj.12323. [DOI] [PubMed] [Google Scholar]


