Abstract
Stroke is among the commonest causes of adult disability worldwide, and its disease burden is shifting towards that of a long-term condition. Therefore, the development of approaches to enhance recovery and augment neural repair after stroke will be critical. Recovery after stroke involves complex interrelated systems of neural repair. There are changes in both structure (at the molecular, cellular, and tissue levels) and function (in terms of excitability, cortical maps, and networks) that occur spontaneously within the brain. Several approaches to augment neural repair through enhancing these changes are under study. These include identifying novel drug targets, implementing rehabilitation strategies, and developing new neurotechnologies. Each of these approaches has its own array of different proposed mechanisms. Current investigation has emphasized both cellular and circuit-based targets in both gray and white matter, including axon sprouting, dendritic branching, neurogenesis, axon preservation, remyelination, blood brain barrier integrity, blockade of extracellular inhibitory signals, alteration of excitability, and promotion of new brain cortical maps and networks. Herein, we review for clinicians recovery after stroke, basic elements of spontaneous neural repair, and ongoing work to augment neural repair. Future study requires alignment of basic, translational, and clinical research. The field continues to grow while becoming more clearly defined. As thrombolysis changed stroke care in the 1990s and thrombectomy in the 2010s, the augmentation of neural repair and recovery after stroke may revolutionize care for these patients in the coming decade.
Keywords: stroke, recovery, neural repair, translation
Introduction
Stroke is among the commonest causes of adult disability worldwide (GBD 2016 Stroke Collaborators, 2019; Murray & Lopez, 2013; Regenhardt, Biseko et al., 2019). Furthermore, there has been an epidemiological shift of its disease burden towards that of a long-term condition, suggesting that the number of patients with stroke will continue to rise (Crichton, Bray, McKevitt, Rudd, & Wolfe, 2016). Therefore, the development of approaches to enhance recovery and augment neural repair after stroke will be critical. Herein, we provide a narrative review for clinicians broadly summarizing recovery after stroke, basic elements of neural repair, and ongoing work to augment repair. To devise novel interventions, an understanding of the spontaneous repair processes that occur in the brain after stroke is essential. Ongoing work builds on mounting data to identify strategies to improve the brain’s capacity to repair itself, through pharmacological, rehabilitative, and neurotechnological means.
Many factors make the clinical translation of therapies a challenging task. Stroke is a heterogeneous disease. Repair and recovery are highly dependent upon the brain regions involved (Minnerup et al., 2018) and the time point after stroke through a biphasic progression as damaged brain transitions from injury into repair (E. H. Lo, 2008). Interventions that target repair are unique compared to acute reperfusion and neuroprotection strategies. Those that target repair have a different timeline (weeks to months versus hours to days) and different target (augmenting the function of surviving tissue rather than saving dying tissue) (Cramer, 2018). Unlike acute therapies to “save” brain, repair-focused therapies emphasize repairing, replacing, and “re-wiring” damaged brain (i.e. plasticity) (Corbett, Nguemeni, & Gomez-Smith, 2014; Cramer et al., 2011; Regenhardt et al., 2017; Regenhardt et al., 2018). In the weeks to months after stroke, spontaneous changes are seen in areas surrounding the infarct and those with network connections to the infarct (Cramer & Chopp, 2000; Hermann & Chopp, 2012; Nudo, 2011; Overman & Carmichael, 2014). These changes are interrelated and occur at both the structural (molecular, cellular, and tissue) and functional levels (excitability, cortical maps, and networks).
At the level of the individual patient, recovery after stroke can be variable and difficult to predict (Ward, 2017). While both reflect functional improvement, a distinction should be made between compensation and true recovery (J. W. Krakauer, Carmichael, Corbett, & Wittenberg, 2012). Compensation is the use of alternative strategies to accomplish a task or goal, such as the use of the unaffected limb. In contrast, true recovery occurs through neural repair, when brain regions regain function or when undamaged brain regions are recruited to generate commands to the same muscles that were used before injury. Complicating our understanding of recovery, many early trials failed to measure true recovery directly and instead measured global outcomes.
Different symptoms after stroke, including motor weakness, aphasia, neglect, and apraxia, spontaneously recover at different rates (Cassidy, Lewis, & Gray, 1998; Desmond, Moroney, Sano, & Stern, 1996; Hier, Mondlock, & Caplan, 1983; Lazar et al., 2010; Nijboer, Kollen, & Kwakkel, 2013; Pedersen, Jorgensen, Nakayama, Raaschou, & Olsen, 1995; Sunderland, Tinson, & Bradley, 1994; Wade, Parker, & Langton Hewer, 1986). The most data exist for motor recovery as it is amenable to more objective measurement (Duncan, Goldstein, Matchar, Divine, & Feussner, 1992). The dominant predictive factor is the initial severity of impairment (Coupar, Pollock, Rowe, Weir, & Langhorne, 2012), where milder deficits recover faster and achieve higher levels of function (Cramer, 2008). One classic study showed that arm function achieved maximum recovery in 95% of patients within 9 weeks (Nakayama, Jorgensen, Raaschou, & Olsen, 1994). More recently, the proportional recovery rule has been described for upper limb motor impairment: at 3 months, patients with mild to moderate initial impairments achieve approximately 70% of their maximum potential for recovery (Byblow, Stinear, Barber, Petoe, & Ackerley, 2015; J. W. Krakauer & Marshall, 2015; Prabhakaran et al., 2008; Winters, van Wegen, Daffertshofer, & Kwakkel, 2015; Zarahn et al., 2011). This “rule” has come under controversy recently (Hawe, Scott, & Dukelow, 2018; Hope et al., 2019; Senesh & Reinkensmeyer, 2019). Nevertheless, two themes hold for spontaneous recovery after stroke. First, most recovery occurs in the first weeks after stroke and involves stereotyped biological processes (as described below). Second, there is a substantial amount of individual variation in recovery after stroke that likely involves personal, genetic, and environmental factors. Ongoing research aims to identify other prognostic biomarkers, such as corticospinal tract lesion extent and prediction tools based on clinical variables; there has been progress predicting ultimate limb motor impairment, walking, and swallowing (Stinear, Smith, & Byblow, 2019).
Changes in structure: Repair at the molecular, cellular, and tissue levels
After stroke, there are spontaneous changes in both structure and function in the brain (Table 1, Figure 1). Repair refers to these collective changes if they lead to functional recovery (Krakauer,J.W., Carmichael,S.T., 2017). The distinction is important because it emphasizes that reorganization described in histological studies should not be considered repair unless the changes have functional consequences. The basic elements of structural changes were first described in animal models and involve both gray and white matter (Carmichael, 2016; Murphy & Corbett, 2009; Wahl & Schwab, 2014; Wieloch & Nikolich, 2006). Subsequently, humans have been found to have similar changes (Jin et al., 2006; Sanin, Heess, Kretzschmar, & Schuller, 2013). These changes occur in areas connected to the infarcted area, including peri-infarct, ipsilateral brain, contralateral brain, and spinal cord (Benowitz & Carmichael, 2010; Murphy & Corbett, 2009; Overman & Carmichael, 2014; Starkey & Schwab, 2014). In preclinical models, the temporal and spatial ordering of these events is governed by alterations in gene expression. Cytokines, chemokines, growth factors, and other molecules, including SDF-1, Ang-1, Ang II, CCL2, LIF, TNFalpha, TGFbeta, NGF, GDF10, BDNF, and FGF-2, are secreted from activated astrocytes, microglia, and endothelial cells to initiate and govern these processes (Berry et al., 2005; Davis et al., 2019; Kahle & Bix, 2013; Klassen et al., 2003; Krakauer,J.W., Carmichael,S.T., 2017; Ohab & Carmichael, 2008; Regenhardt et al., 2013; Regenhardt, Bennion, & Sumners, 2014). Details of these pathways are beyond the scope of this review; however, we summarize these terms and others with references (Table 2). Interestingly, while post stroke brain resembles developing brain in many ways at the cellular and tissue levels, the regenerative and developmental transcriptomes are different (Cramer & Chopp, 2000; Li et al., 2010).
Table 1.
Hierarchical structural and functional changes in spontaneous neural repair after stroke. Neural repair is defined by these collective processes if they lead to functional recovery. A hierarchical framework can be helpful to understand these changes. Structural changes occur from the transcriptome (molecular program) level to the cell and tissue level. Functional changes occur from the excitability level to the cortical map and plasticity levels. The levels are often bidirectionally inter-related, whereby changes at one level induce changes at another. Further, structural changes can induce functional changes and vice versa.
| Structural changes |
|---|
| Transcriptome: Molecular program of repair through gene expression |
| Mediators and pathways: Transcription factors, cytokines, chemokines, growth factors, and other molecules. Examples include: |
| Ang-1, BDNF, CCL2, CCR5, CREB, CSP, DLK, Ephrins, EPO, FGF-2, GAP43, G-CSF, GDF10, LIF, MAG, MCT-1, |
| MOBP, NGF, NMNAT1, NogoA, SDF-1, Semaphorins, STAT3, TGFbeta, TLR3/TLR4, TNFalpha, VEGF |
| Cells: Neurons, astrocytes, oligodendrocytes, endothelial cells, pericytes, microglia, infiltrating inflammatory cells |
| Tissue: Gray versus white matter, infarct versus peri-infarct versus distant brain/cord, specific regions and nuclei |
| Changes result in axonal sprouting, dendritic branching, neurogenesis, axon preservation, remyelination, glial scar |
| Functional changes |
| Excitability: Acute excitotoxicity versus subacute pathologic inhibition |
| Enhance by: Increasing glutamate-AMPA, further decreasing phasic GABA (synaptic), decreasing tonic GABA (extrasynaptic) |
|
Brain cortical maps and networks: Through macro-level changes and plasticity Enhance by: Altering signaling to augment repair processes |
Figure 1. Structural and functional changes in spontaneous neural repair after stroke.
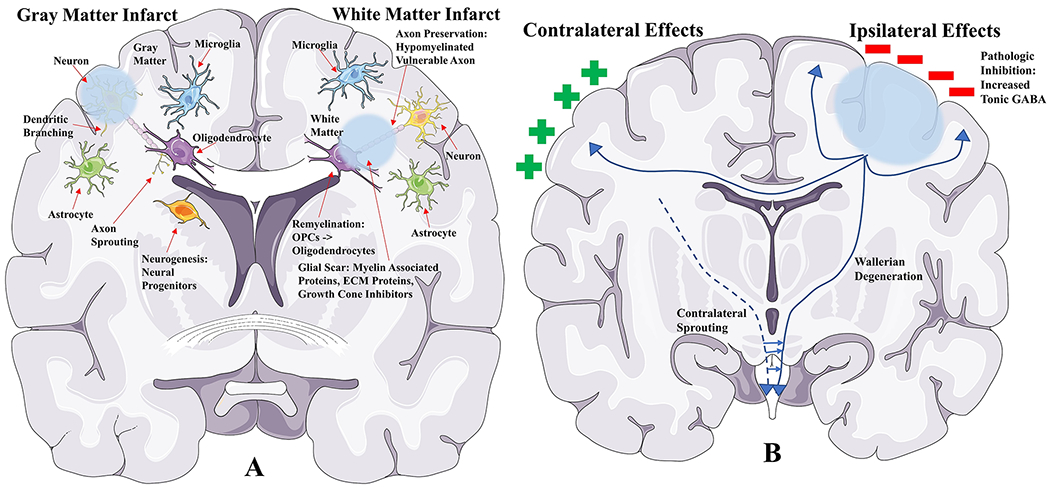
A. Structural changes hypothesized to play a role in neural repair after gray matter and white matter stroke. Several processes are represented at the histologic level, including axon sprouting, dendritic branching, neurogenesis, axon preservation, remyelination, and glial scarring.
B. Functional changes hypothesized to play a role in neural repair through integrated mechanisms involving the whole brain. There are changes in excitability, brain cortical maps, and plasticity.
Table 2.
Abbreviations and terms.
| Abbreviation | Term | References |
|---|---|---|
| AMPA | Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid | Clarkson et al., 2011; Carmichael, 2012 |
| Ang II | Angiotensin II | Regenhardt, Bennion, & Sumners, 2014 |
| Ang-1 | Angiopoietin-1 | Ohab & Carmichael, 2008 |
| BDNF | Brain-derived neurotrophic factor | Schabitz et al., 2004; Clarkson et al., 2015 |
| cAMP | Cyclic adenosine monophosphate | Xu, Zhang, & O’Donnell, 2011 |
| CCL2, MCP-1 | Chemokine ligand 2, Monocyte chemoattractant protein-1 | Kahle & Bix, 2013 |
| CCR5 | C-C chemokine receptor type 5 | Joy, Zhou, Cai, Silva, & Carmichael, 2015; Joy et al., 2019 |
| cGMP | Cyclic guanosine monophosphate | Xu, Zhang, & O’Donnell, 2011 |
| CREB | cAMP response element-binding protein | Clarkson, Parker, Nilsson, Walker, & Gowing, 2015 |
| CSPs | Chondroitin sulfate proteoglycans | Alia et al., 2016 |
| DLK | Dual leucine zipper kinase | Hinman, 2014 |
| EPO | Erythropoietin | Tsai et al., 2006; L. Wang, Zhang, Wang, Zhang, & Chopp, 2004 |
| FGF-2 | Fibroblast growth factor-2 | Berry et al., 2005; Kawamata et al., 1997 |
| GABA | Gamma aminobutyric acid | Clarkson, Huang, Macisaac, Mody, & Carmichael, 2010 |
| GAP43 | Growth associated protein 43 | Overman et al., 2012 |
| G-CSF | Granulocyte-colony stimulating factor | Schneider et al., 2007 |
| GDF10 | Growth differentiation factor 10 | Li et al., 2015 |
| LIF | Leukemia inhibitory factor | Davis et al., 2019 |
| MAG | Myelin-associated glycoprotein | Cramer et al., 2013 |
| MCT-1 | Monocarboxylate transporter-1 | Hinman, 2014; Rinholm et al., 2011 |
| MOBP | Myelin-associated oligodendrocytic basic protein | Ward, 2017 |
| NGF | Nerve growth factor | Krakauer,J.W., Carmichael,S.T., 2017 |
| NgR1 | Nogo receptor 1 | Takase, Kurihara, Yokoyama, & Takei, 2017; Sozmen et al., 2016 |
| NMNAT1 | Nicotinamide mononucleotide adenylyltransferase 1 | Hinman, 2014; Hinman et al., 2015 |
| NogoA | Neurite outgrowth inhibitor A | Lindau et al., 2014 |
| OPC | Oligodendrocyte progenitor cell | Shindo et al., 2016; Xiao & Hinman, 2016 |
| PDE3 | Phosphodiesterase-3 | Krakauer,J.W., Carmichael,S.T., 2017 |
| PDE4 | Phosphodiesterase-4 | MacDonald et al., 2007 |
| PDE5 | Phosphodiesterase-5 | Krakauer,J.W., Carmichael,S.T., 2017 |
| SDF-1 | Stromal cell-derived factor-1 | Kahle & Bix, 2013 |
| STAT3 | Signal transducer and activator of transcription 3 | Hinman, 2014 |
| TGFbeta | Transforming growth factor beta | Krakauer,J.W., Carmichael,S.T., 2017 |
| TLR4 | Toll-like receptor 4 | Hayakawa et al., 2016; Hayakawa et al., 2016 |
| TNFalpha | Tumor necrosis factor alpha | Klassen et al., 2003 |
| VEGF | Vascular endothelial growth factor | Z. G. Zhang et al., 2000 |
After infarction of gray matter, changes in axonal connections are initiated in the first week after stroke and persist for at least one month in the mouse (Carmichael, Wei, Rovainen, & Woolsey, 2001; Overman et al., 2012). There is evidence for axonal sprouting in humans as GAP43, highly involved in this process, is upregulated in the peri-infarct. Remaining neurons in the peri-infarct can form robust new connections (Krakauer,J.W., Carmichael,S.T., 2017; Overman et al., 2012). These processes have been found distances away from the infarct in the mouse (Li et al., 2015; Overman et al., 2012) and nonhuman primate (Dancause et al., 2005). Furthermore, axonal sprouting is not simply a function of proximity, but of functionally linked brain regions (Li et al., 2015; Overman et al., 2012). There is an early induction transcriptome for axonal sprouting in the first week post stroke and a later maintenance transcriptome at 3 weeks (Li et al., 2010). Repair in gray matter likely also involves dendritic branching. Initially, there is a loss of dendritic spines in the peri-infarct during the first week after stroke in preclinical models (Brown, Wong, & Murphy, 2008; Mostany et al., 2010), reflecting a loss of synaptic connections. Subsequently, dendritic spines recover to premorbid levels and can even increase compared to controls. Branches remodel with retraction and growth that is maximal two weeks after infarct (Brown, Boyd, & Murphy, 2010).
Neurogenesis, gliogenesis, and angiogenesis may also pay a role in repair. These processes involve the generation of new neurons, glia, and blood vessels, respectively. While there remains limited evidence for these processes in primates (Imayoshi et al., 2008; Lee & Thuret, 2018), progenitors from the subventricular zone (SVZ) can proliferate, migrate to the peri-infarct cortex, and mature into new neurons in response to ischemia in rodent models (Ekdahl, Kokaia, & Lindvall, 2009; Kernie & Parent, 2010; Ohab & Carmichael, 2008). Depending on the distance of the stroke from the SVZ , there is variable neuroblast migration with distant cortical sites having lower numbers (Ohab & Carmichael, 2008). Experimentally augmenting neurogenesis is associated with improved recovery (Leker et al., 2007; Ohab, Fleming, Blesch, & Carmichael, 2006; L. Wang, Zhang, Wang, Zhang, & Chopp, 2004), while inhibiting neurogenesis impairs recovery (Garcia, Doan, Imura, Bush, & Sofroniew, 2004; Imayoshi et al., 2008). Recently, SVZ-derived neurons have been shown to synaptically integrate into peri-infarct cortex, in a mouse model, in a manner that is activity-dependent and sensitive to modulation (Liang et al., 2019).
Angiogenesis is also hypothesized to play a role in repair after stroke (Arai, Jin, Navaratna, & Lo, 2009). It has been shown to be triggered by experimental ischemia in the brain (Fan & Yang, 2007) and occurs primarily in the peri-infarct, although it also occurs in other regions including the contralesional cortex (Ergul, Alhusban, & Fagan, 2012; Liu et al., 2014). Endothelial cell proliferation may occur as early as 12 hours after ischemia and persist for several weeks in mice (Hayashi, Noshita, Sugawara, & Chan, 2003; Marti et al., 2000). Growth factors and chemokines are secreted (discussed subsequently), leading to new microvessels with pericytes and smooth muscle cells. Human autopsy studies confirmed angiogenesis occurs after stroke and the extent of which correlates with survival (Krupinski, Kaluza, Kumar, Kumar, & Wang, 1994).
In addition to gray matter, white matter also undergoes repair and has been under-studied as a target (Das, Regenhardt, Feske, & Gurol, 2019; Regenhardt, Das et al., 2019). Twenty-five percent of strokes involve exclusively white matter (Regenhardt, Das, Lo, & Caplan, 2018). Furthermore, white matter is involved to some degree in most other strokes, and its injury accounts for the second leading cause of dementia (Sozmen et al., 2016). When white matter undergoes ischemia, there is primary injury to axons involving calcium signaling and proteolysis. In the subacute period after stroke, a second progressive phase of injury occurs affecting axons that survive the initial infarct. Degeneration of axons occurs through contact with glia and altered energy metabolism in preclinical models (Hinman, 2014). A third degenerative/regenerative phase may further contribute involving anterograde Wallerian degeneration and retrograde signals to the soma that may influence axonal sprouting and reconnection (Hinman, 2014).
Injury to myelin also occurs in stroke, leaving axons dysfunctional and vulnerable. However, endogenous repair mechanisms are initiated in the mouse involving oligodendrocyte precursor cells (OPCs) and remyelination (Sozmen, Kolekar, Havton, & Carmichael, 2009). Much of our understanding of OPC function comes from models of multiple sclerosis (MS), showing that there are cues for resident OPCs to proliferate, migrate, and differentiate into myelinating oligodendrocytes (Franklin, 2002; Shindo et al., 2016; Sozmen & Carmichael, 2014). In the last several years, models have been developed, including cell, tissue, and whole animal platforms, to study white matter recovery after stroke, (Arai & Lo, 2009; Regenhardt et al., 2018).
Changes in function: Repair in terms of excitability, brain cortical maps, and networks
In addition to structural changes, there are also changes in neural function including excitability and changes in brain networks (Carmichael, 2012). Acutely, surviving brain becomes excitable relative to baseline. Indeed, blocking this excitotoxicity is a target for neuroprotection. In the mouse, there is a three-day period with excess excitation (Clarkson, Huang, Macisaac, Mody, & Carmichael, 2010); glutamate is excitotoxic and contributes to cell death, while GABA can counteract this toxicity (Lai, Zhang, & Wang, 2014). After this three-day period in mouse models (unclear timeline in humans), this is reversed and there is excess inhibition in the recovery phase, through an altered GABA vs glutamate balance when reactive astrocytes downregulate their GABA uptake systems (Carmichael, 2012). Interestingly, interventions that reduce the pathologic inhibitory tone in this phase can cause downstream beneficial changes in structure (J. L. Chen et al., 2011). It has been proposed that changes in excitability regulate growth factors and cytokines perhaps through epigenetic mechanisms (Felling & Song, 2015). Furthermore, preclinical models have shown reducing inhibition can lead to expanded and less-specific receptive fields (Alia et al., 2016; Winship & Murphy, 2008), enhanced long term potentiation (Hagemann, Redecker, Neumann-Haefelin, Freund, & Witte, 1998), and remapping of sensorimotor functions to surviving cortex in both hemispheres (Que et al., 1999; Takatsuru et al., 2009).
Experimental methods to reduce inhibition include increasing glutamate-AMPA signaling, further decreasing phasic GABA (synaptic) signaling, and decreasing tonic GABA (extrasynaptic) signaling (Murphy & Corbett, 2009). Increasing glutamate-AMPA signaling has been associated with improved recovery in a mouse model probably through downstream induction of BDNF (Clarkson et al., 2011). In addition, phasic GABA signaling has been shown to be spontaneously reduced in the first few weeks after stroke (Neumann-Haefelin, Hagemann, & Witte, 1995). This reduction can increase the likelihood of long-term potentiation (Hagemann et al., 1998). GABAA receptors are downregulated (Que et al., 1999; Schiene et al., 1996) and the density of inhibitory interneurons is reduced after stroke (Alia et al., 2016; Zeiler et al., 2013). In contrast, tonic GABA signaling has been shown to be increased after stroke in the mouse, through extrasynaptic GABA A receptor signaling (Clarkson et al., 2010; Lake et al., 2015). This increased tonic inhibitory signaling persists for one month post stroke (Carmichael, 2012). When this is reversed with a GABA A receptor inverse agonist, motor outcomes were improved in rodents (Clarkson et al., 2010; Neumann-Haefelin et al., 1995). Interactions between excitatory pyramidal neurons and inhibitory interneurons are complex, and this complexity likely increases after stroke (Clarkson, 2012). Strategies to reduce inhibition in human patients are being explored (discussed subsequently).
After stroke, there are also changes in cortical map representations and brain networks (Cramer, Moore, Finklestein, & Rosen, 2000; Ward & Cohen, 2004; Weiller, Ramsay, Wise, Friston, & Frackowiak, 1993). In non-human primate studies, infarcts that lead to a deficit in hand use are accompanied by a reduction in the cortical representation of the digit adjacent to the lesion (Nudo & Milliken, 1996). In humans, there are important network interactions after a single unilateral supratentorial stroke, where multiple brain areas are affected both in ipsilateral (Burke Quinlan et al., 2015; Carter et al., 2010; Grefkes et al., 2008; Grefkes & Fink, 2011; Sharma, Baron, & Rowe, 2009) and contralateral regions (Murase, Duque, Mazzocchio, & Cohen, 2004). There is increasing evidence that the contralateral hemisphere may play an adaptive role, especially in the subacute phase after stroke (Dodd, Nair, & Prabhakaran, 2017), and larger strokes employ more contralateral hemisphere (Heiss & Thiel, 2006; Netz, Lammers, & Homberg, 1997; Turton, Wroe, Trepte, Fraser, & Lemon, 1996). However, there is significant inter-patient variability, mechanistic details have yet to be established, and debate continues about a role for inhibiting contralateral hyperexcitability (Buetefisch, 2015; Dodd et al., 2017). Several approaches to augment repair, through enhancing these changes in structure and function, are under study. These include identifying novel drug targets, implementing rehabilitation strategies, and developing new neurotechnologies (Table 3). Each of these approaches has its own array of different proposed mechanisms.
Table 3.
Potential targets to augment neural repair after stroke.
| Target | Example |
|---|---|
| Gray matter | |
| Axonal sprouting | Inosine, PDE inhibitors, Growth factors, Stem cells |
| Dendritic branching | CCR5 blockade, Growth factors, Stem cells |
| Neurogenesis | Cerebrolysin, Growth factors, Stem cells |
| Angiogenesis | Growth factors, Stem cells |
| White matter | |
| Axon preservation | MCT-1 augmentation, NMNAT1/DLK/STAT3 manipulation |
| Remyelination | OPC proliferation/differentiation, Nogo blockade |
| Blood brain barrier | Endothelial cell metabolism, Pericyte proliferation |
| Microglia | TLR4 inhibition, Manipulating activation state |
| Glial scar | |
| Myelin-associated proteins | Nogo, MAG, MOBP blockade |
| Extracellular matrix proteins | Chondroitin sulfate proteoglycan inhibition |
| Growth cone inhibitors | Semaphorin, Eprhin blockade |
| Excitability | |
| Alter inhibition pharmacologically | SSRIs, CREB induction, GABA antagonism, Zolpidem, Amphetamines, L-dopa |
| Alter inhibition neurotechnologically | Transcranial magnetic stimulation, transcranial direct current stimulation, Deep brain stimulation, Vagal nerve stimulation |
| Brain cortical maps and networks | |
|
Macro level changes that depend may on changes in structure/excitability |
Rehabilitation: physical, occupational, speech Activity-based therapies, Sensory stimulation, Robot-based therapies Cognitive strategies, Functional MRI, Virtual reality Brain computer interfaces, C7 nerve root transfer |
Gray matter targets: Axon sprouting, dendritic branching, and neurogenesis
For gray matter stroke, several targets are under study. One promising agent, inosine, has been shown to increase axon growth and improve recovery in preclinical models. It promotes axonal sprouting, including into the corticospinal tract (Zai et al., 2009) by stimulating axonal growth programs (P. Chen, Goldberg, Kolb, Lanser, & Benowitz, 2002; Zai et al., 2009). Interestingly, inosine also augments the effects of anti-NogoA antibody (discussed subsequently) (Zai et al., 2011). Phosphodiesterase inhibitors may also have a role by blocking the degradation of cAMP and cGMP. cAMP stimulates cAMP response element-binding protein (CREB), which in turn regulates BDNF and axonal outgrowth (Clarkson, Parker, Nilsson, Walker, & Gowing, 2015; Xu, Zhang, & O’Donnell, 2011). PDE4 inhibitors have been shown to enhance motor performance when paired with rehabilitation in rats (MacDonald et al., 2007). In addition, the PDE5 inhibitor, Sildenafil, and PDE3 inhibitor, Cilostazol, are FDA approved agents that are under study (Krakauer,J.W., Carmichael,S.T., 2017). CCR5 blockade is another potential target in the translational pipeline. Involved in inflammatory processes post stoke, its blockade in a mouse model prevented the initial loss of dendritic spines and promoted recovery (Joy, Zhou, Cai, Silva, & Carmichael, 2015). A cohort of stroke patients with a loss of function mutation for CCR5 showed greater recovery of neurological function (Joy et al., 2019). Another agent under investigation is cerebrolysin, a proprietary mixture of amino acids and peptides from pig brain (Harrer, Melnizky, & Wagner, 1972). It may stimulate neurogenesis through specific effects on neural progenitor cells involving the sonic hedgehog pathway (L. Zhang et al., 2013). In the CARS clinical trial, 21 days of treatment in addition to rehabilitation, starting 24-72 hours after stroke onset, showed promise (Muresanu et al., 2016). However, this was a small, exploratory study that has yet to be replicated.
As discussed above, growth factors are highly involved in repair and may serve as therapeutic targets (Finklestein et al., 1990; Lanfranconi et al., 2011). Examples include FGF-2 (Berry et al., 2005; Kawamata et al., 1997), BDNF (Schabitz et al., 2004), VEGF (Z. G. Zhang et al., 2000), EPO (Tsai et al., 2006; L. Wang, Zhang, Wang, Zhang, & Chopp, 2004), G-CSF (Schneider et al., 2007), and GDF10 (Li et al., 2015). EPO shows some promise as it was shown in the BETAS study to be safe in humans (Cramer et al., 2010). Unfortunately, the REGENESIS study was terminated early likely given significant differences in rehabilitation practice and lack of statistical power (Cramer, Hill, & REGENESIS-LED Investigators, 2014). The AXIS study showed G-CSF was safe in humans (Schabitz et al., 2010), however AXIS 2 showed no benefit compared to placebo (Ringelstein et al., 2013). GDF10 is currently under study given it is highly upregulated in the regenerative transcriptome in peri-infarct neurons, including in humans, and has been shown to promote functionally useful axonal sprouting (Li et al., 2015). A primary concern for many growth factors is off target effects, prompting exploration of approaches for local delivery (Krakauer,J.W., Carmichael,S.T., 2017).
Stem cells offer another potential option to promote repair (Azad, Veeravagu, & Steinberg, 2016). Cell therapies may stimulate endogenous repair processes , possibly replace damaged tissue, or reduce secondary injury events (Krakauer,J.W., Carmichael,S.T., 2017). Preclinical studies and phase I clinical trials show promise (Kalladka et al., 2016; Steinberg et al., 2016). In addition to stimulating endogenous cells, there are two main classes of exogenous stem cells: embryonic and induced pluripotent cells as well as adult-derived cells. Embryonic and induced pluripotent stem cells can be differentiated into neural stem cells that can become neurons and glia. While some preclinical studies suggest they can differentiate into neurons and integrate into circuitry (Kelly et al., 2004; Park, Teng, & Snyder, 2002; Tornero et al., 2013), they more likely exert benefit through stimulating endogenous processes rather than replacing damaged brain since most transplanted cells die (Barker, Gotz, & Parmar, 2018; Lemmens & Steinberg, 2013; J. Zhang & Chopp, 2013).
In contrast, adult-derived stem cells, usually from bone marrow or adipose, do not form neurons or glia in vivo. Most commonly, these are mesenchymal stem cells, which can produce growth factors and extracellular matrix proteins. While they are often trapped in the lungs, liver, and spleen (Boltze et al., 2015; Hicks & Jolkkonen, 2009), they exert effects on the immune system that may mediate repair in preclinical models (Boltze et al., 2015; Eckert et al., 2013). A meta-analysis of preclinical mesenchymal stem cell transplantation found large effect sizes, consistent across route of administration (Vu, Xie, Eckert, Zhao, & Cramer, 2014). Clinical trials suggest they are safe, and there are trends for improvement (Kalladka et al., 2016; Steinberg et al., 2016). One study showed that post-mitotic human neuron-like cells derived from human testicular germ cell tumor could be feasibly implanted into humans (Kondziolka et al., 2000; Kondziolka et al., 2005). The more recent MASTERS trial showed that allogenic bone marrow-derived cells modulate the immune system, but do not likely enter the brain (Hess et al., 2017). Many questions about stem cells remain (Savitz, Cramer, Wechsler, & STEPS 3 Consortium, 2014): When to administer? Which cells and how many? Autologous vs allogenic? What route of administration: intravenous, intra-arterial, or surgical (i.e. Intraparenchymal)? Are scaffolding gels beneficial? For these reasons, further data are needed to determine efficacy and safety; patients seeking these therapies should be referred to academic centers conducting randomized controlled trials.
White matter targets: Axon preservation, remyelination, and blood brain barrier
In addition to gray matter targets, therapies to prevent axonal degeneration, aid remyelination, and stabilize the blood-brain barrier (BBB) have been examined after white matter stroke (Xiao & Hinman, 2016). A mouse model demonstrated that the volume of white matter infarcts enlarged over the first month (Sozmen et al., 2009). Studies in humans have also demonstrated that white matter infarcts can grow over time by serial imaging (Gouw et al., 2008). While the clinical significance of this slow stroke growth is unknown, it may prove a viable therapeutic target. Another mouse model of chronic white matter ischemia has demonstrated that injured axons show elongation of nodes and paranodes (Reimer et al., 2011), which slows conduction velocity (Babbs & Shi, 2013). The peri-infarct was examined in human white matter strokes, and axons up to 150% of the infarct diameter away showed disorganization of nodes and paranodes, despite maintaining intact structure of neurofilaments (Hinman, Lee, Tung, Vinters, & Carmichael, 2015).
There are three phases of axon injury and repair after stroke: primary injury, secondary progressive injury, and tertiary degeneration/regeneration (Hinman, 2014). In the secondary phase, axons swell and form retraction bulbs in preclinical models (Taguohi et al., 1989; Q. Zhang et al., 2012). These dying axons release signals that cause reactive astrocytes and microglia to respond within days to weeks (Block, Dihne, & Loos, 2005; J. Zhang, Zhang, Xing, Liang, & Zeng, 2012). Furthermore, when axons are disconnected from energy from the soma, MCT-1 from oligodendrocytes may serve as an alternative source through the transfer of lactate. Augmentation of the MCT-1 pathway may have therapeutic potential (Hinman, 2014; Rinholm et al., 2011). In the tertiary degeneration/regeneration phase, distal portions of axons undergo anterograde Wallerian degeneration mediated by NMNAT1 and retrograde signals to the cortical soma may involve degeneration/regeneration mediated by DLK and STAT3 (Hinman, 2014; Hinman et al., 2015). Manipulating these pathways to limit degeneration and promote regeneration may also prove fruitful.
Another proposed target in white matter is remyelination. It has been hypothesized that the aforementioned slow white matter stroke growth may occur secondary to eventual loss of vulnerable hypomyelinated peri-infarct axons in the mouse. Indeed, myelin loss was found to exceed axon damage in this model (Sozmen et al., 2009). Perhaps the disorganization of nodes and paranodes in human peri-infarct reflects a loss of normal cell-cell adhesion and signaling between axons and oligodendrocytes (Hinman et al., 2015). Remyelination has long been the Holy Grail for many neurologic diseases like MS. The obvious difference comparing MS to stroke is that the latter involves death of all cell types. Also, given there is likely a critical period after stroke, therapies may be high dose and short duration, minimizing off target effects. OPCs are delicate and highly susceptible to ischemia, have only a limited capacity to differentiate, and require proximity to the axons that they may remyelinate. Methods to increase OPC proliferation and differentiation, including the use of exogenous stem cells, are being explored (Shindo et al., 2016; Xiao & Hinman, 2016).
Other theoretical targets may exist in both white and gray matter. There may be approaches to manipulate microglia to promote a repair-oriented inflammatory milieu. In several mouse models of white matter stroke, microglia/macrophages were found near injured axons at day one, and increased until day seven while OPCs decreased (Hayakawa, Wang, & Lo, 2016; Hayakawa et al., 2016; Sozmen et al., 2009). Microglia have the potential to be detrimental in stroke by producing pro-inflammatory cytokines and reactive oxygen/nitrogen species (Hanisch & Kettenmann, 2007; Raivich & Banati, 2004; Regenhardt et al., 2014; Rosenzweig & Carmichael, 2015; J. Wang & Dore, 2007), impairing glutamate uptake (Arai & Lo, 2009; Takahashi, Giuliani, Power, Imai, & Yong, 2003), triggering excitotoxic oligodendrocyte death (Arai & Lo, 2009; Takahashi et al., 2003), and ingesting OPCs (Hughes, Kang, Fukaya, & Bergles, 2013). However, microglia can also produce anti-inflammatory cytokines and growth factors (Turrin & Rivest, 2006; J. Wang & Dore, 2007), secrete agents to promote oligodendrogenesis (Butovsky, Landa et al., 2006; Butovsky, Ziv et al., 2006; Mason et al., 2000; Mason, Suzuki, Chaplin, & Matsushima, 2001; Miron et al., 2013; Tanaka, Murakami, Bando, & Yoshida, 2013), and support remyelination through the phagocytosis of myelin debris and apoptotic cells (Olah et al., 2012; Turrin & Rivest, 2006; J. Wang & Dore, 2007). By inhibiting TLR4 signaling in microglia/macrophages, phagocytosis of OPCs was decreased and myelination was improved at day 14 after stroke in a mouse model (Hayakawa et al., 2016; Hayakawa et al., 2016). Another target may be the BBB, made up of tight junctions, multicellular interactions, and transcellular transport systems. It is hypothesized that failure of endothelial cells and pericytes to maintain this system underlies white matter injury. It may be possible to restore metabolic function to endothelial cells and promote pericyte proliferation with agents that do not have to cross the BBB (Xiao & Hinman, 2016).
Glial scar and blocking extracellular inhibitory signals
Another approach to augmenting repair is the modulation of glial scarring, relevant in both gray and white matter. After the acute phase, astrocytes and OPCs synergize with the inflammatory response and play a key role in the formation of glial scar. Scar is hypothesized to be beneficial acutely by sealing off the area of injury and closing the BBB. In preclinical models, reactive astrogliosis can be beneficial in certain circumstances (Hayakawa, Pham, Katusic, Arai, & Lo, 2012; Sofroniew, 2009), and without reactive astrocytes there is increased lesion size and inflammation acutely (Sofroniew, 2012). Chronically, however, several cell surface and extracellular matrix proteins have been shown to inhibit axon growth and impair the ability of oligodendrocytes to myelinate axons (Giger et al., 2008).
There are several inhibitory signals that may affect repair after stroke including myelin-associated proteins, extracellular matrix proteins, and growth cone inhibitors. Of the myelin-associated proteins, NogoA, MAG, and MOBP have undergone the most study(Cramer et al., 2013). In one model, rats administered anti-NogoA antibodies recovered motor control after sensorimotor cortex ablation with corticospinal tract sprouting across the midline into the denervated spinal hemicord, leading to a somatotopic anatomical and functional side switch(Lindau et al., 2014). Similar findings were seen with Nogo receptor 1 (NgR1) blockade in a mouse model of middle cerebral artery occlusion (Takase, Kurihara, Yokoyama, Kawahara, & Takei, 2017). Recently, NgR1 blockade was shown to overcome remyelination failure after white matter stroke to stimulate functional recovery in mice (Sozmen et al., 2016). Safety in humans has already been demonstrated (Meininger et al., 2014; Wahl & Schwab, 2014). Chondroitin sulfate proteoglycans (CSPs) are an example of extracellular matrix proteins. These mediate the inhibitory properties of perineuronal nets, which are responsible for synapse stabilization. In doing this, CSPs block axon growth. In a preclinical model, cortical infarcts lead to a reduced density of perineuronal nets in the peri-infarct, possibly allowing increased plasticity (Alia et al., 2016). Chondroitinase ABC can inactivate CSPs and perineuronal nets, thereby allowing additional plasticity (Pizzorusso et al., 2002) and restoring motor function (Gherardini, Gennaro, & Pizzorusso, 2015). Growth cone inhibitors, such as semaphorins and ephrins, inhibit the axonal growth cone, which is an extension of a regenerating neurite seeking its synaptic target. In the peri-infarct, ephrin-A5 is induced in astrocytes and has been shown to inhibit axonal sprouting. Blocking this pathway has been shown to promote recovery in a mouse model (Overman et al., 2012).
Altering excitability
Another potential mechanism to augment neural repair is through the alteration of excitability and neurotransmitters. As discussed above, animal models have demonstrated this proof of principle through experiments that decrease pathologic inhibitory tone. Since the 1980s, several FDA-approved drugs have been studied in humans. One of the first agents studied to augment recovery after stroke were amphetamines. They were shown to increase noradrenergic function and aid recovery after cortex ablation in a rat model when paired with rehabilitative therapy (Feeney, Gonzalez, & Law, 1982). Unfortunately, amphetamines failed to augment repair in clinical trials, possibly given lack of pairing with intense rehab, small heterogenous studies, and concerns for adverse effects (Goldstein, 2009; Martinsson, Hardemark, & Wahlgren, 2003). The STARS trial showed no benefit of amphetamine twice weekly for 5 weeks when patients were enrolled 5-10 days post stroke (Gladstone et al., 2006).
SSRIs have been the subject of recent attention, with the most data for fluoxetine. Several small trials showed positive effects on motor recovery (Mikami et al., 2014; Robinson et al., 2008). In the FLAME trial (Chollet et al., 2011), patients were enrolled within 10 days of stroke and treated for 3 months showing reduced motor impairment (Fugl-Meyer) and disability (modified Rankin). However, the larger FOCUS trial (FOCUS Trial Collaboration, 2019) did not replicate the reduced disability at 6 months, but the more sensitive impairment endpoint was not examined and the patients enrolled were heterogeneous with all levels of initial impairment. Preclinical models propose several possible mechanisms. It is hypothesized that treatment reinstates a critical period of plasticity in rats by decreasing inhibition through reducing extracellular GABA (Maya Vetencourt et al., 2008). Another study showed fluoxetine prolonged but did not reinstate this critical period in mice through a reduction of inhibitory interneuron expression in intact cortex (K. L. Ng et al., 2015). In human primary motor cortex slices, fluoxetine reduced the inhibitory tone through suppression of layer II-III monosynaptic excitatory connections from pyramidal neurons to inhibitory interneurons (Komlosi et al., 2012). Interestingly, SSRIs have been shown to activate CREB (Mostert, Koch, Heerings, Heersema, & De Keyser, 2008). In addition to regulating BDNF and axonal outgrowth (Clarkson et al., 2015; Xu et al., 2011), CREB activates genes that enhance excitability (Dong et al., 2006; Han et al., 2006), long term potentiation (Wu, Zhou, & Xiong, 2007), cortical re-mapping, and recovery in animal models (Caracciolo et al., 2018), exemplifying the interplay between structural and functional changes. Indeed, other pharmacological means of inducing CREB may be possible (Caracciolo et al., 2018; Krakauer,J.W., Carmichael,S.T., 2017).
Zolpidem has also been examined for potential effects after stroke. Indeed, it showed improved recovery in a mouse model (Hiu et al., 2016), and there are case reports of stroke patients with improved language, cognitive, and motor function (Cohen, Chaaban, & Habert, 2004; Hall et al., 2010). Zolpidem is believed to work through reducing tonic GABAA inhibition at high levels. In addition, there is an ongoing trial using an alpha 5 GABA antagonist (RESTORE Collaborators, 2018). L-dopa has also shown some promise in recovery, though perhaps through reward-based mechanisms. One small randomized, placebo-controlled trial of 53 patients enrolled within 6 months of stroke showed that it resulted in mild improvements in motor function (Scheidtmann, Fries, Muller, & Koenig, 2001). However, other trials including DARS, have failed to replicate this (Bhakta et al., 2014; Ford et al., 2019; Lokk, Salman Roghani, & Delbari, 2011). In addition to agents that may decrease inhibition, there are also agents that may worsen it. While this topic is not commonly discussed on neurology units and wards, careful attention should be paid to agents that could be maladaptive for recovery. For example, diazepam and haloperidol have been shown to have negative effects on recovery (Dombovy, 2004; Feeney et al., 1982; Hovda & Feeney, 1985).
In addition to pharmacologic means, transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are under study to stimulate pathologically inhibited cortex. TMS involves the placement of a stimulator on the scalp that issues magnetic pulses to induce action potentials in cortical neurons. A Cochrane review was inconclusive, but a more recent meta-analysis of arm motor recovery showed promising results (Hao, Wang, Zeng, & Liu, 2013; Hsu, Cheng, Liao, Lee, & Lin, 2012). High frequencies (5-20 Hz) are used to increase cortical excitability (Rossi, Hallett, Rossini, Pascual-Leone, & Safety of TMS Consensus Group, 2009). Mounting evidence suggests high frequency to ipsilateral motor cortex may improve hand function (Khedr, Ahmed, Fathy, & Rothwell, 2005; Y. H. Kim et al., 2006). Low frequencies (1 Hz), in contrast, are used to decrease cortical excitability with the aim to treat distant cortical regions (Dayan & Cohen, 2011; Liew, Santarnecchi, Buch, & Cohen, 2014; Naeser et al., 2005). Small studies have shown low frequency over the contralesional motor cortex may improve hand function after stroke (Fregni et al., 2006; Kobayashi, Hutchinson, Theoret, Schlaug, & Pascual-Leone, 2004; Nowak et al., 2008; Takeuchi, Chuma, Matsuo, Watanabe, & Ikoma, 2005). Another noninvasive approach to alter excitability utilizes tDCS. With this modality, DC current is supplied to the cortex via a scalp electrode. In contrast to TMS, tDCS does not cause action potentials but does alter resting membrane potentials (Hummel et al., 2005; Webster, Celnik, & Cohen, 2006). Early small studies suggest benefits in stroke recovery (Hummel et al., 2005; Hummel & Cohen, 2005). Through anodal stimulation, excitability can be increased; through cathodal stimulation, it can be decreased. A recent study showed enthusiasm for anodal tDCS to augment post-stroke swallowing function (Suntrup-Krueger et al., 2018). Interestingly, tDCS has been shown to induce CREB (Podda et al., 2016). Other approaches to stimulation are being explored, including work by Sabel et al. with transorbital stimulation (Fedorov et al., 2010; Sabel, Hamid, Borrmann, Speck, & Antal, 2019).
More invasive modalities of stimulation, including deep brain stimulation (DBS), epidural electrical stimulation, and vagal nerve stimulation, have been explored to a lesser extent. DBS has been well-established as a safe and effective treatment for movement disorders such as Parkinson’s disease. For stroke patients, DBS has been used to treat a variety of maladaptive consequences of stroke including neuropathic pain and dystonia. Only a few case reports have used DBS to enhance motor recovery (Elias, Namasivayam, & Lozano, 2018). A trial evaluating epidural stimulation combined with rehabilitation did not show an effect (Levy et al., 2016). Vagal nerve stimulation (VNS) has also been explored. Preclinical studies show that VNS enhances structural plasticity in motor networks (Meyers et al., 2018). A recent clinical trial of VNS paired with rehabilitation demonstrated safety and feasibility in humans (Dawson et al., 2016).
Promoting new brain cortical maps and networks
Macro-level changes in cortical maps and networks involve underlying changes in excitability and structure. Interestingly, they don’t always correlate temporally with behavioral recovery, leading some to question their role in repair (Krakauer,J.W., Carmichael,S.T., 2017). Translational approaches using rehabilitation and other neurotechnologies, many of which are incompletely understood, are believed to work through these mechanisms. The neurobiology of rehabilitation, including physical, occupational, and speech therapy, is only now becoming elucidated (L. Wang, Conner, Nagahara, & Tuszynski, 2016). The goals of rehabilitation are to enhance recovery, aid in adaptation to loss of neurologic function, and to continue preventative efforts to reduce recurrent stroke (Caplan, 2016). Several studies have shown that interdisciplinary stroke rehabilitation improves functional outcomes. As there is great heterogeneity in stroke symptoms, studies examining the effects of rehabilitation have measured different endpoints, including hemiplegic gait (Colborne, Olney, & Griffin, 1993; da Cunha et al., 2002; M. F. Ng, Tong, & Li, 2008), upper extremity function (Nakayama et al., 1994), aphasia (Shewan & Kertesz, 1984), and spatial neglect (Halligan & Marshall, 1994). A large meta-analysis collating studies from 1960-1990 including >3700 patients with hemiparesis showed that the average patient who took part in a rehabilitation program had better functional performance than 65% of patients in control groups (Ottenbacher & Jannell, 1993). There were greater effects of early treatment, and young patients did better than old.
Indeed, there is growing evidence suggesting that greater amount, duration, and intensity of activity based therapy improves outcomes in humans (Bhogal, Teasell, & Speechley, 2003; Cauraugh, Naik, Lodha, Coombes, & Summers, 2011; Galvin, Murphy, Cusack, & Stokes, 2008; Galvin, Cusack, O’Grady, Murphy, & Stokes, 2011; Kwakkel, Wagenaar, Twisk, Lankhorst, & Koetsier, 1999; Kwakkel et al., 2004; Legg, Langhorne, & Outpatient Service Trialists, 2004; Van Peppen et al., 2004). Another meta-analysis showed that the amount of therapy had positive influence on outcomes (Lohse, Lang, & Boyd, 2014). However, many patients don’t receive maximal therapy in clinical practice (Bernhardt, Dewey, Thrift, & Donnan, 2004; Bernhardt, Chan, Nicola, & Collier, 2007; Kimberley, Samargia, Moore, Shakya, & Lang, 2010; Lang et al., 2009; Wade, Skilbeck, Hewer, & Wood, 1984). Interestingly, well controlled animal studies show that the number of forelimb reaches must exceed a certain threshold before an effect on recovery is observed (MacLellan et al., 2011). Parenthetically, the number of repetitive movements in animals needed to induce plasticity is orders of magnitude greater than those observed in humans in current clinical practice. There is also evidence to support a critical time period of rehabilitation therapy. One rat model demonstrated that motor training of the forelimb starting 30 days post stroke had little improvement compared to those that underwent earlier training (Biernaskie, Chernenko, & Corbett, 2004). While not well defined in humans (Zeiler, 2019), animal models point to a time-limited period of heightened plasticity after stroke (Zeiler et al., 2016). Extensive reach training in a rodent model was not able to promote recovery when started late at 7 days. However, in the same model, when a second stroke was induced two days later, recovery was substantially improved (Zeiler et al., 2016). Two strokes are not likely better than one, but this adds to evidence that additional ischemia may re-open a window for repair.
Several studies support the use of intense training that focuses on reducing impairment in the first weeks to take advantage of biological repair (J. W. Krakauer et al., 2012). However, as with pharmacologic interventions, there is the theoretical risk of rehabilitative efforts having detrimental effects if they are too early or too intense, as demonstrated in the AVERT trial (AVERT Trial Collaboration group, 2015). Indeed, certain behaviors can have detrimental effects. The compensatory use of the contralesional forelimb may impair recovery of the affected limb (Allred, Maldonado, Hsu And, & Jones, 2005), possibly through aberrant synaptogenesis in the peri-stroke cortex (S. Y. Kim et al., 2015). As behaviors, drugs, and their combination may take advantage of post stroke plasticity in both positive and negative ways, trials should be conducted with caution.
In addition to traditional physical therapy, several advanced therapies are gaining traction in clinical practice and are the focus of ongoing research. These include activity based therapies, sensory stimulation, telerehabilitation, and robot-based therapies (Cramer et al., 2019; Johansson, Lindgren, Widner, Wiklund, & Johansson, 1993; Lin, Finklestein, & Cramer, 2018). Constraint-induced movement therapy (CIMT) is an example of an activity based therapy that involves overcoming learned disuse of the stroke-affected limb by preventing use of the unaffected limb and forcing use of the affected (Taub, Uswatte, Mark, & Morris, 2006). The phase 3 EXCITE study showed that improvements after CIMT persist for at least 1 year (Wolf et al., 2006). Sensory stimulation is another therapy with a growing body of literature. Repetitive sensorimotor training involves rocking movements performed by the affected arm; one study showed improvement that persisted at 5-year follow-up (Feys et al., 2004). Passive arm movement prior to active motor therapy may be superior to active motor therapy alone, possibly by balancing cortical excitability (Stinear, Barber, Coxon, Fleming, & Byblow, 2008). Neglect is also improved by many different sensory inputs (musical, optokinetic, neck proprioceptive, vestibular, and somatosensory) (Kerkhoff, 2003; Teasell & Kalra, 2004).
With advancing technology, telerehabilitation offers promise to increase access to therapy; it was recently shown noninferior to traditional in-clinic therapy (Cramer et al., 2019). Furthermore, robot-based therapies are becoming available though they are not often implemented clinically. Robots have been developed as tools to enhance post-stroke recovery (Hogan & Krebs, 2011; Krebs & Hogan, 2012), with numerous different robotic systems (Veerbeek, Langbroek-Amersfoort, van Wegen, Meskers, & Kwakkel, 2017; Volpe et al., 2009). A multicenter study showed that robot-assisted therapy was superior to usual physical therapy in reducing both upper extremity impairment and improving quality of life. The trial did not show benefits when compared to dose-matched intensive physical therapy (A. C. Lo et al., 2010), however high dose therapy involves thousands of repetitions and is thus not feasible routinely without the use of robotics. Furthermore, robots offer novel methods to measure effects of therapy and approaches can be personalized.
There are also several cognitive and behavioral strategies that may be implemented that synergize with motor rehabilitation. Some examples include cognitive rehearsal (Page, Levine, & Leonard, 2007), observing others perform a task (Celnik, Webster, Glasser, & Cohen, 2008), mirror visual feedback to overcome hemi-inattention (Ramachandran & Altschuler, 2009), real time neural feedback with fMRI (Berman, Horovitz, Venkataraman, & Hallett, 2012; Sitaram et al., 2012; Sulzer et al., 2013), and virtual reality (Fasotti & van Kessel, 2013). While there have been promising small pilot trials of virtual and augmented reality for stroke patients, larger trials and meta-analyses have not shown it to be more effective than conventional rehabilitation (Laver et al., 2017).
Brain-computer interfaces (BCIs) may afford both assistive and rehabilitation benefits after stroke. BCIs translate central nervous system signals into command signals for a technological device. For stroke rehabilitation, the underlying hypothesis is that by training patients to produce more normal brain activity patterns through feedback reinforcement, neuroplasticity mechanisms become engaged to allow for restoration of native neurologic function. A 2013 study showed that patients who underwent EEG-BCI training with an upper extremity orthosis showed significantly improved motor function. Perhaps most exciting, these improvements were correlated with adaptive changes in neural activity patterns on fMRI (Ramos-Murguialday et al., 2013). Another recent study demonstrated at-home use of an EEG-driven exoskeleton to open and close the affected hand from signals in the contralesional hemisphere resulted in significant motor improvements (Bundy et al., 2017). While not involving BCIs, another approach to bypass injured brain networks is through modification of peripheral nerve anatomy. Transfer of the C7 nerve root from the nonparalyzed side to the paralyzed side (28% had strokes) improved arm function compared to rehabilitation alone (Zheng et al., 2018).
Conclusion
In conclusion, the biology of recovery after stroke involves complex interrelated systems of neural repair. After a stroke, there are spontaneous changes in both structure and function within the brain. The future is optimistic, and several approaches to augment neural repair through pharmacological and neurotechnological means are under study. These include strategies that target both gray and white matter, including axon sprouting, dendritic branching, neurogenesis, axon preservation, remyelination, blood brain barrier integrity, blockade of extracellular inhibitory signals, alteration of excitability, and promotion of new brain cortical maps and networks.
Despite these many targets and promising preclinical data, progress has been more modest translating these interventions to augment neural repair in clinical trials. One fundamental hurdle to overcome lies in clinical trial design and appreciating the heterogeneity of stroke injury and repair; the right patient that stands to benefit must be selected for the right intervention at the right time point after stroke and the right outcome must be measured to determine efficacy. A second fundamental hurdle lies in our understanding the differences between preclinical models and human patients; the model brain’s structure (such as less white matter in rodent brains) and function (such as different timing of changes in excitability) must be considered when translating findings into clinical trials. When evaluating interventions, there are principles that should be considered in any study, be it basic, translational, or clinical (Table 4). The Stroke Recovery and Rehabilitation Roundtable (SRRR) established new standards for stroke recovery and neural repair research: better understanding the natural history of recovery, increasing neurobiological data in human subjects, characterizing different stroke recovery phenotypes, training new researchers, developing a network of clinical centers, and promoting collaborative dialogue (Bernhardt et al., 2017). The SRRR also discussed strategies to enhance alignment between pre-clinical studies of neural repair and clinical trials of recovery (Corbett et al., 2017).
Table 4.
Recommendations for approaches to future basic, translational, and clinical studies (Bernhardt et al., 2017; Corbett et al., 2017; Kwakkel et al., 2017).
| Overall Goals | Trial Considerations |
|---|---|
| Understand natural history of recovery Collect neurobiological data in humans Characterize stroke recovery phenotypes Organize network of clinical centers Align preclinical and clinical studies: Appropriate animal models, tissue and behavioral outcomes Train new neural repair and stroke recovery researchers |
Use standard definitions of recovery Include patient characteristics: Demographics, medical history, location/size of stroke, responders vs nonresponders Include time points post stroke: Enroll within 7 days and include outcomes at least 3 months post stroke Include time points post stroke: NIHSS (stroke severity), FM (function and structure), ARAT (upper limb), EQ-5D (quality of life), mRS (global disability) |
Importantly, the SRRR also suggested an approach to future clinical trials for rehabilitation and repair (Kwakkel et al., 2017). Future trials should incorporate standard definitions of recovery, patient characteristics (demographic, medical history, location/size of stroke, responders vs nonresponders), time points post stroke (enroll within 7 days and contain outcomes at least 3 months post stroke), and measured outcomes (NIH stroke scale for stroke severity, Fugl-Meyer for function and structure, Action Research Arm Test for upper limb, EQ-5D for quality of life, modified Rankin scale for global disability) (Kwakkel et al., 2017). Given stroke recovery is modality specific, we further recommend that trials employ modality-specific endpoints, in addition to global ones (Cramer, Koroshetz, & Finklestein, 2007). Having standardized approaches to trial design will facilitate multi-center trials, pooling of multiple studies, and aid meta-analyses. Momentum continues to grow with the accumulating understanding of the biology of neural repair. As thrombolysis changed stroke care in the 1990s and thrombectomy in the 2010s, the augmentation of neural repair for recovery after stroke may revolutionize care for these patients in the coming decade.
Acknowledgements
Seth P Finklestein, MD and Leigh R Hochberg, MD, PhD, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, provided comments to help guide this work. RWR is supported by R25 NS065743. EHL is supported by P01 NS055104, R01 NS099620, R01 AG055559, and R01 NS093415. DJL is supported by R25 NS065743. All grants are from the National Institutes of Health.
References
- Alia C, Spalletti C, Lai S, Panarese A, Micera S, & Caleo M (2016). Reducing GABAA-mediated inhibition improves forelimb motor function after focal cortical stroke in mice. Scientific Reports, 6, 37823. doi: 10.1038/srep37823 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RP, Maldonado MA, Hsu And JE, & Jones TA (2005). Training the “less-affected” forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restorative Neurology and Neuroscience, 23(5–6), 297–302. [PubMed] [Google Scholar]
- Arai K, Jin G, Navaratna D, & Lo EH (2009). Brain angiogenesis in developmental and pathological processes: Neurovascular injury and angiogenic recovery after stroke. The FEBS Journal, 276(17), 4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, & Lo EH (2009). Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Experimental & Translational Stroke Medicine, 1, 10.1186/2040-7378-1-6 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVERT Trial Collaboration group. (2015). Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet (London, England), 386(9988), 46–55. doi:S0140–6736(15)60690–0 [pii] [DOI] [PubMed] [Google Scholar]
- Azad TD, Veeravagu A, & Steinberg GK (2016). Neurorestoration after stroke. Neurosurgical Focus, 40(5), E2. doi: 10.3171/2016.2.FOCUS15637 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs CF, & Shi R (2013). Subtle paranodal injury slows impulse conduction in a mathematical model of myelinated axons. PloS One, 8(7), e67767. doi: 10.1371/journal.pone.0067767 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Gotz M, & Parmar M (2018). New approaches for brain repair-from rescue to reprogramming. Nature, 557(7705), 329–334. doi: 10.1038/s41586-018-0087-1 [doi] [DOI] [PubMed] [Google Scholar]
- Benowitz LI, & Carmichael ST (2010). Promoting axonal rewiring to improve outcome after stroke. Neurobiology of Disease, 37(2), 259–266. doi: 10.1016/j.nbd.2009.11.009 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BD, Horovitz SG, Venkataraman G, & Hallett M (2012). Self-modulation of primary motor cortex activity with motor and motor imagery tasks using real-time fMRI-based neurofeedback. NeuroImage, 59(2), 917–925. doi: 10.1016/j.neuroimage.2011.07.035 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt J, Chan J, Nicola I, & Collier JM (2007). Little therapy, little physical activity: Rehabilitation within the first 14 days of organized stroke unit care. Journal of Rehabilitation Medicine, 39(1), 43–48. doi: 10.2340/16501977-0013 [doi] [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Dewey H, Thrift A, & Donnan G (2004). Inactive and alone: Physical activity within the first 14 days of acute stroke unit care. Stroke, 35(4), 1005–1009. doi: 10.1161/01.STR.0000120727.40792.40 [doi] [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, … Cramer SC (2017). Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. International Journal of Stroke : Official Journal of the International Stroke Society, 12(5), 444–450. doi: 10.1177/1747493017711816 [doi] [DOI] [PubMed] [Google Scholar]
- Berry D, Ren J, Kwan CP, Sietsma DK, Sasisekharan R, & Finklestein SP (2005). Dimeric fibroblast growth factor-2 enhances functional recovery after focal cerebral ischemia. Restorative Neurology and Neuroscience, 23(3–4), 251–256. [PubMed] [Google Scholar]
- Bhakta BB, Hartley S, Holloway I, Couzens JA, Ford GA, Meads D, … Farrin AJ (2014). The DARS (dopamine augmented rehabilitation in stroke) trial: Protocol for a randomised controlled trial of co-careldopa treatment in addition to routine NHS occupational and physical therapy after stroke. Trials, 15, 10.1186/1745-6215-15-316 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal SK, Teasell R, & Speechley M (2003). Intensity of aphasia therapy, impact on recovery. Stroke, 34(4), 987–993. doi: 10.1161/01.STR.0000062343.64383.D0 [doi] [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Chernenko G, & Corbett D (2004). Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 24(5), 1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block F, Dihne M, & Loos M (2005). Inflammation in areas of remote changes following focal brain lesion. Progress in Neurobiology, 75(5), 342–365. doi:S0301-0082-(05)00032-8 [pii] [DOI] [PubMed] [Google Scholar]
- Boltze J, Arnold A, Walczak P, Jolkkonen J, Cui L, & Wagner DC (2015). The dark side of the force - constraints and complications of cell therapies for stroke. Frontiers in Neurology, 6, 155. doi: 10.3389/fneur.2015.00155 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Boyd JD, & Murphy TH (2010). Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 30(4), 783–791. doi: 10.1038/jcbfm.2009.241 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Wong C, & Murphy TH (2008). Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke, 39(4), 1286–1291. doi: 10.1161/STROKEAHA.107.498238 [doi] [DOI] [PubMed] [Google Scholar]
- Buetefisch CM (2015). Role of the contralesional hemisphere in post-stroke recovery of upper extremity motor function. Frontiers in Neurology, 6, 214. doi: 10.3389/fneur.2015.00214 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy DT, Souders L, Baranyai K, Leonard L, Schalk G, Coker R, … Leuthardt EC (2017). Contralesional brain-computer interface control of a powered exoskeleton for motor recovery in chronic stroke survivors. Stroke, 48(7), 1908–1915. doi: 10.1161/STROKEAHA.116.016304 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, … Cramer SC (2015). Neural function, injury, and stroke subtype predict treatment gains after stroke. Annals of Neurology, 77(1), 132–145. doi: 10.1002/ana.24309 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, … Schwartz M (2006). Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. The Journal of Clinical Investigation, 116(4), 905–915. doi: 10.1172/JCI26836 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, … Schwartz M (2006). Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Molecular and Cellular Neurosciences, 31(1), 149–160. doi:S1044–7431(05)00248–4 [pii] [DOI] [PubMed] [Google Scholar]
- Byblow WD, Stinear CM, Barber PA, Petoe MA, & Ackerley SJ (2015). Proportional recovery after stroke depends on corticomotor integrity. Annals of Neurology, 78(6), 848–859. doi: 10.1002/ana.24472 [doi] [DOI] [PubMed] [Google Scholar]
- Caplan LR (2016). Caplan’s stroke: A clinical approach (5th ed.). Cambridge, New York: Cambridge University Press. [Google Scholar]
- Caracciolo L, Marosi M, Mazzitelli J, Latifi S, Sano Y, Galvan L, … Carmichael ST (2018). CREB controls cortical circuit plasticity and functional recovery after stroke. Nature Communications, 9(1), 10.1038/s41467-018-04445-9 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST (2012). Brain excitability in stroke: The yin and yang of stroke progression. Archives of Neurology, 69(2), 161–167. doi: 10.1001/archneurol.2011.1175 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST (2016). Emergent properties of neural repair: Elemental biology to therapeutic concepts. Annals of Neurology, 79(6), 895–906. doi: 10.1002/ana.24653 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Wei L, Rovainen CM, & Woolsey TA (2001). New patterns of intracortical projections after focal cortical stroke. Neurobiology of Disease, 8(5), 910–922. doi: 10.1006/nbdi.2001.0425 [doi] [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, … Corbetta M (2010). Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of Neurology, 67(3), 365–375. doi: 10.1002/ana.21905 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy TP, Lewis S, & Gray CS (1998). Recovery from visuospatial neglect in stroke patients. Journal of Neurology, Neurosurgery, and Psychiatry, 64(4), 555–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauraugh JH, Naik SK, Lodha N, Coombes SA, & Summers JJ (2011). Long-term rehabilitation for chronic stroke arm movements: A randomized controlled trial. Clinical Rehabilitation, 25(12), 1086–1096. doi: 10.1177/0269215511410580 [doi] [DOI] [PubMed] [Google Scholar]
- Celnik P, Webster B, Glasser DM, & Cohen LG (2008). Effects of action observation on physical training after stroke. Stroke, 39(6), 1814–1820. doi: 10.1161/STROKEAHA.107.508184 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, & Nedivi E (2011). Structural basis for the role of inhibition in facilitating adult brain plasticity. Nature Neuroscience, 14(5), 587–594. doi: 10.1038/nn.2799 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, & Benowitz LI (2002). Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proceedings of the National Academy of Sciences of the United States of America, 99(13), 9031–9036. doi: 10.1073/pnas.132076299 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, … Loubinoux I (2011). Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): A randomised placebo-controlled trial. The Lancet.Neurology, 10(2), 123–130. doi: 10.1016/S1474-4422(10)70314-8 [doi] [DOI] [PubMed] [Google Scholar]
- Clarkson AN (2012). Perisynaptic GABA receptors the overzealous protector. Advances in Pharmacological Sciences, 2012, 708428. doi: 10.1155/2012/708428 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, & Carmichael ST (2010). Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature, 468(7321), 305–309. doi: 10.1038/nature09511 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, & Carmichael ST (2011). AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(10), 3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Parker K, Nilsson M, Walker FR, & Gowing EK (2015). Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 35(8), 1272–1279. doi: 10.1038/jcbfm.2015.33 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Chaaban B, & Habert MO (2004). Transient improvement of aphasia with zolpidem. The New England Journal of Medicine, 350(9), 949–950. doi: 10.1056/NEJM200402263500922 [doi] [DOI] [PubMed] [Google Scholar]
- Colborne GR, Olney SJ, & Griffin MP (1993). Feedback of ankle joint angle and soleus electromyography in the rehabilitation of hemiplegic gait. Archives of Physical Medicine and Rehabilitation, 74(10), 1100–1106. doi:0003–9993(93)90069-M [pii] [DOI] [PubMed] [Google Scholar]
- Corbett D, Carmichael ST, Murphy TH, Jones TA, Schwab ME, Jolkkonen J, … Joy MT (2017). Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable translational working group. International Journal of Stroke : Official Journal of the International Stroke Society, 12(5), 462–471. doi: 10.1177/1747493017711814 [doi] [DOI] [PubMed] [Google Scholar]
- Corbett D, Nguemeni C, & Gomez-Smith M (2014). How can you mend a broken brain? neurorestorative approaches to stroke recovery. Cerebrovascular Diseases (Basel, Switzerland), 38(4), 233–239. doi: 10.1159/000368887 [doi] [DOI] [PubMed] [Google Scholar]
- Coupar F, Pollock A, Rowe P, Weir C, & Langhorne P (2012). Predictors of upper limb recovery after stroke: A systematic review and meta-analysis. Clinical Rehabilitation, 26(4), 291–313. doi: 10.1177/0269215511420305 [doi] [DOI] [PubMed] [Google Scholar]
- Cramer SC (2008). Repairing the human brain after stroke: I. mechanisms of spontaneous recovery. Annals of Neurology, 63(3), 272–287. doi: 10.1002/ana.21393 [doi] [DOI] [PubMed] [Google Scholar]
- Cramer SC (2018). Treatments to promote neural repair after stroke. Journal of Stroke, 20(1), 57–70. doi: 10.5853/jos.2017.02796 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Abila B, Scott NE, Simeoni M, Enney LA, & MAG111539 Study Investigators. (2013). Safety, pharmacokinetics, and pharmacodynamics of escalating repeat doses of GSK249320 in patients with stroke. Stroke; a Journal of Cerebral Circulation, 44(5), 1337–1342. doi: 10.1161/STROKEAHA.111.674366 [doi] [DOI] [PubMed] [Google Scholar]
- Cramer SC, & Chopp M (2000). Recovery recapitulates ontogeny. Trends in Neurosciences, 23(6), 265–271. doi:S0166–2236(00)01562–9 [pii] [DOI] [PubMed] [Google Scholar]
- Cramer SC, Dodakian L, Le V, See J, Augsburger R, McKenzie A, … National Institutes of Health StrokeNet Telerehab Investigators. (2019). Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: A randomized clinical trial. JAMA Neurology, doi: 10.1001/jamaneurol.2019.1604 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Fitzpatrick C, Warren M, Hill MD, Brown D, Whitaker L, … Plon L (2010). The beta-hCG+erythropoietin in acute stroke (BETAS) study: A 3-center, single-dose, open-label, noncontrolled, phase IIa safety trial. Stroke, 41(5), 927–931. doi: 10.1161/STROKEAHA.109.574343 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Hill MD, & REGENESIS-LED Investigators. (2014). Human choriogonadotropin and epoetin alfa in acute ischemic stroke patients (REGENESIS-LED trial). International Journal of Stroke : Official Journal of the International Stroke Society, 9(3), 321–327. doi: 10.1111/ijs.12260 [doi] [DOI] [PubMed] [Google Scholar]
- Cramer SC, Koroshetz WJ, & Finklestein SP (2007). The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke, 38(4), 1393–1395. doi:01.STR.0000260087.67462.80 [pii] [DOI] [PubMed] [Google Scholar]
- Cramer SC, Moore CI, Finklestein SP, & Rosen BR (2000). A pilot study of somatotopic mapping after cortical infarct. Stroke, 31(3), 668–671. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, … Vinogradov S (2011). Harnessing neuroplasticity for clinical applications. Brain : A Journal of Neurology, 134(Pt 6), 1591–1609. doi: 10.1093/brain/awr039 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton SL, Bray BD, McKevitt C, Rudd AG, & Wolfe CD (2016). Patient outcomes up to 15 years after stroke: Survival, disability, quality of life, cognition and mental health. Journal of Neurology, Neurosurgery, and Psychiatry, 87(10), 1091–1098. doi: 10.1136/jnnp-2016-313361 [doi] [DOI] [PubMed] [Google Scholar]
- da Cunha IT Jr, Lim PA, Qureshy H, Henson H, Monga T, & Protas EJ (2002). Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: A randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation, 83(9), 1258–1265. doi:S0003–9993(02)00217–4 [pii] [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, … Nudo RJ (2005). Extensive cortical rewiring after brain injury. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 25(44), 10167–10179. doi:25/44/10167 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AS, Regenhardt RW, Feske SK, & Gurol ME (2019). Treatment approaches to lacunar stroke. Journal of Stroke and Cerebrovascular Diseases : The Official Journal of National Stroke Association, 28(8), 2055–2078. doi:S1052–3057(19)30224–1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Collier LA, Goodwin S, Lukins DE, Powell DK, & Pennypacker KR (2019). Efficacy of leukemia inhibitory factor as a therapeutic for permanent large vessel stroke differs among aged male and female rats. Brain Research, 1707, 62–73. doi:S0006–8993(18)30575–4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, … Engineer N (2016). Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke, 47(1), 143–150. doi: 10.1161/STROKEAHA.115.010477 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, & Cohen LG (2011). Neuroplasticity subserving motor skill learning. Neuron, 72(3), 443–454. doi: 10.1016/j.neuron.2011.10.008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Sano M, & Stern Y (1996). Recovery of cognitive function after stroke. Stroke, 27(10), 1798–1803. [DOI] [PubMed] [Google Scholar]
- Dodd KC, Nair VA, & Prabhakaran V (2017). Role of the contralesional vs. ipsilesional hemisphere in stroke recovery. Frontiers in Human Neuroscience, 11, 469. doi: 10.3389/fnhum.2017.00469 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombovy ML (2004). Understanding stroke recovery and rehabilitation: Current and emerging approaches. Current Neurology and Neuroscience Reports, 4(1), 31–35. [DOI] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, & Malenka RC (2006). CREB modulates excitability of nucleus accumbens neurons. Nature Neuroscience, 9(4), 475–477. doi:nn1661 [pii] [DOI] [PubMed] [Google Scholar]
- Duncan PW, Goldstein LB, Matchar D, Divine GW, & Feussner J (1992). Measurement of motor recovery after stroke. outcome assessment and sample size requirements. Stroke, 23(8), 1084–1089. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Vu Q, Xie K, Yu J, Liao W, Cramer SC, & Zhao W (2013). Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 33(9), 1322–1334. doi: 10.1038/jcbfm.2013.91 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, & Lindvall O (2009). Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience, 158(3), 1021–1029. doi: 10.1016/j.neuroscience.2008.06.052 [doi] [DOI] [PubMed] [Google Scholar]
- Elias GJB, Namasivayam AA, & Lozano AM (2018). Deep brain stimulation for stroke: Current uses and future directions. Brain Stimulation, 11(1), 3–28. doi:S1935–861X(17)30936–1 [pii] [DOI] [PubMed] [Google Scholar]
- Ergul A, Alhusban A, & Fagan SC (2012). Angiogenesis: A harmonized target for recovery after stroke. Stroke, 43(8), 2270–2274. doi: 10.1161/STROKEAHA.111.642710 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, & Yang GY (2007). Therapeutic angiogenesis for brain ischemia: A brief review. Journal of Neuroimmune Pharmacology : The Official Journal of the Society on NeuroImmune Pharmacology, 2(3), 284–289. doi: 10.1007/s11481-007-9073-3 [doi] [DOI] [PubMed] [Google Scholar]
- Fasotti L, & van Kessel M (2013). Novel insights in the rehabilitation of neglect. Frontiers in Human Neuroscience, 7, 780. doi: 10.3389/fnhum.2013.00780 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov A, Chibisova Y, Szymaszek A, Alexandrov M, Gall C, & Sabel BA (2010). Non-invasive alternating current stimulation induces recovery from stroke. Restorative Neurology and Neuroscience, 28(6), 825–833. doi: 10.3233/RNN-2010-0580 [doi] [DOI] [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, & Law WA (1982). Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science (New York, N.Y.), 217(4562), 855–857. [DOI] [PubMed] [Google Scholar]
- Felling RJ, & Song H (2015). Epigenetic mechanisms of neuroplasticity and the implications for stroke recovery. Experimental Neurology, 268, 37–45. doi: 10.1016/j.expneurol.2014.09.017 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys H, De Weerdt W, Verbeke G, Steck GC, Capiau C, Kiekens C, … Cras P (2004). Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: A 5-year follow-up study of a randomized trial. Stroke, 35(4), 924–929. doi: 10.1161/01.STR.0000121645.44752.f7 [doi] [DOI] [PubMed] [Google Scholar]
- Finklestein SP, Caday CG, Kano M, Berlove DJ, Hsu CY, Moskowitz M, & Klagsbrun M (1990). Growth factor expression after stroke. Stroke, 21(11 Suppl), III122–4. [PubMed] [Google Scholar]
- FOCUS Trial Collaboration. (2019). Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): A pragmatic, double-blind, randomised, controlled trial. Lancet (London, England), 393(10168), 265–274. doi:S0140–6736(18)32823-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford GA, Bhakta BB, Cozens A, Hartley S, Holloway I, Meads D, … Farrin AJ (2019). Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): A randomised, double-blind, placebo-controlled trial. The Lancet.Neurology, 18(6), 530–538. doi:S1474–4422(19)30147–4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ (2002). Remyelination of the demyelinated CNS: The case for and against transplantation of central, peripheral and olfactory glia. Brain Research Bulletin, 57(6), 827–832. doi:S0361923001007651 [pii] [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, … Pascual-Leone A (2006). A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain, 122(1–2), 197–209. doi:S0304–3959(06)00114-X [pii] [DOI] [PubMed] [Google Scholar]
- Galvin R, Cusack T, O’Grady E, Murphy TB, & Stokes E (2011). Family-mediated exercise intervention (FAME): Evaluation of a novel form of exercise delivery after stroke. Stroke, 42(3), 681–686. doi: 10.1161/STROKEAHA.110.594689 [doi] [DOI] [PubMed] [Google Scholar]
- Galvin R, Murphy B, Cusack T, & Stokes E (2008). The impact of increased duration of exercise therapy on functional recovery following stroke--what is the evidence? Topics in Stroke Rehabilitation, 15(4), 365–377. doi: 10.1310/tsr1504-365 [doi] [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, & Sofroniew MV (2004). GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nature Neuroscience, 7(11), 1233–1241. doi:nn1340 [pii] [DOI] [PubMed] [Google Scholar]
- GBD 2016 Stroke Collaborators. (2019). Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the global burden of disease study 2016. The Lancet.Neurology, 18(5), 439–458. doi:S1474–4422(19)30034–1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini L, Gennaro M, & Pizzorusso T (2015). Perilesional treatment with chondroitinase ABC and motor training promote functional recovery after stroke in rats. Cerebral Cortex (New York, N.Y.: 1991), 25(1), 202–212. doi: 10.1093/cercor/bht217 [doi] [DOI] [PubMed] [Google Scholar]
- Giger RJ, Venkatesh K, Chivatakarn O, Raiker SJ, Robak L, Hofer T, … Rader C (2008). Mechanisms of CNS myelin inhibition: Evidence for distinct and neuronal cell type specific receptor systems. Restorative Neurology and Neuroscience, 26(2–3), 97–115. [PMC free article] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Armesto A, McIlroy WE, Staines WR, Graham SJ, … Subacute Therapy with Amphetamine and Rehabilitation for Stroke Study Investigators. (2006). Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: A randomized, double-blind, placebo-controlled trial. Stroke, 37(1), 179–185. doi:01.STR.0000195169.42447.78 [pii] [DOI] [PubMed] [Google Scholar]
- Goldstein LB (2009). Amphetamine trials and tribulations. Stroke, 40(3 Suppl), S133–5. doi: 10.1161/STROKEAHA.108.533703 [doi] [DOI] [PubMed] [Google Scholar]
- Gouw AA, van der Flier WM, Pantoni L, Inzitari D, Erkinjuntti T, Wahlund LO, … LADIS study group. (2008). On the etiology of incident brain lacunes: Longitudinal observations from the LADIS study. Stroke; a Journal of Cerebral Circulation, 39(11), 3083–3085. doi: 10.1161/STROKEAHA.108.521807 [doi] [DOI] [PubMed] [Google Scholar]
- Grefkes C, & Fink GR (2011). Reorganization of cerebral networks after stroke: New insights from neuroimaging with connectivity approaches. Brain : A Journal of Neurology, 134(Pt 5), 1264–1276. doi: 10.1093/brain/awr033 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, & Fink GR (2008). Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Annals of Neurology, 63(2), 236–246. doi: 10.1002/ana.21228 [doi] [DOI] [PubMed] [Google Scholar]
- Hagemann G, Redecker C, Neumann-Haefelin T, Freund HJ, & Witte OW (1998). Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Annals of Neurology, 44(2), 255–258. doi: 10.1002/ana.410440217 [doi] [DOI] [PubMed] [Google Scholar]
- Hall SD, Yamawaki N, Fisher AE, Clauss RP, Woodhall GL, & Stanford IM (2010). GABA(A) alpha-1 subunit mediated desynchronization of elevated low frequency oscillations alleviates specific dysfunction in stroke--a case report. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 121(4), 549–555. doi: 10.1016/j.clinph.2009.11.084 [doi] [DOI] [PubMed] [Google Scholar]
- Halligan PW, & Marshall JC (1994). Spatial neglect: Position papers on theory and practice Lawrence Erlbaum Associates, Publishers. [Google Scholar]
- Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, … Nestler EJ (2006). Role of cAMP response element-binding protein in the rat locus ceruleus: Regulation of neuronal activity and opiate withdrawal behaviors. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 26(17), 4624–4629. doi:26/17/4624 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, & Kettenmann H (2007). Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience, 10(11), 1387–1394. doi:nn1997 [pii] [DOI] [PubMed] [Google Scholar]
- Hao Z, Wang D, Zeng Y, & Liu M (2013). Repetitive transcranial magnetic stimulation for improving function after stroke. The Cochrane Database of Systematic Reviews, (5):CD008862 doi(5), CD008862. doi: 10.1002/14651858.CD008862.pub2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer G, Melnizky U, & Wagner F (1972). Mode of action and therapeutic activity of a brain hydrolysate. [Wirkweise und therapeutische Wirksamkeit eines Gehirnhydrolysates] Arzneimittel-Forschung, 22(5), 900–904. [PubMed] [Google Scholar]
- Hawe RL, Scott SH, & Dukelow SP (2018). Taking proportional out of stroke recovery. Stroke, , STROKEAHA118023006. doi: 10.1161/STROKEAHA.118.023006 [doi] [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Katusic ZS, Arai K, & Lo EH (2012). Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proceedings of the National Academy of Sciences of the United States of America, 109(19), 7505–7510. doi: 10.1073/pnas.1121146109 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Seo JH, Miyamoto N, Maki T, Terasaki Y, … Lo EH (2016). CD200 restrains macrophage attack on oligodendrocyte precursors via toll-like receptor 4 downregulation. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 36(4), 781–793. doi: 10.1177/0271678X15606148 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Wang X, & Lo EH (2016). CD200 increases alternatively activated macrophages through cAMP-response element binding protein - C/EBP-beta signaling. Journal of Neurochemistry, 136(5), 900–906. doi: 10.1111/jnc.13492 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, & Chan PH (2003). Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 23(2), 166–180. doi: 10.1097/01.WCB.0000041283.53351.CB [doi] [DOI] [PubMed] [Google Scholar]
- Heiss WD, & Thiel A (2006). A proposed regional hierarchy in recovery of post-stroke aphasia. Brain and Language, 98(1), 118–123. doi:S0093–934X(06)00048–4 [pii] [DOI] [PubMed] [Google Scholar]
- Hermann DM, & Chopp M (2012). Promoting brain remodelling and plasticity for stroke recovery: Therapeutic promise and potential pitfalls of clinical translation. The Lancet.Neurology, 11(4), 369–380. doi: 10.1016/S1474-4422(12)70039-X [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, … Mays RW (2017). Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet.Neurology, 16(5), 360–368. doi:S1474–4422(17)30046–7 [pii] [DOI] [PubMed] [Google Scholar]
- Hicks A, & Jolkkonen J (2009). Challenges and possibilities of intravascular cell therapy in stroke. Acta Neurobiologiae Experimentalis, 69(1), 1–11. doi:6901 [pii] [DOI] [PubMed] [Google Scholar]
- Hier DB, Mondlock J, & Caplan LR (1983). Recovery of behavioral abnormalities after right hemisphere stroke. Neurology, 33(3), 345–350. [DOI] [PubMed] [Google Scholar]
- Hinman JD (2014). The back and forth of axonal injury and repair after stroke. Current Opinion in Neurology, 27(6), 615–623. doi: 10.1097/WCO.0000000000000149 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JD, Lee MD, Tung S, Vinters HV, & Carmichael ST (2015). Molecular disorganization of axons adjacent to human lacunar infarcts. Brain : A Journal of Neurology, 138(Pt 3), 736–745. doi: 10.1093/brain/awu398 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiu T, Farzampour Z, Paz JT, Wang EH, Badgely C, Olson A, … Steinberg GK (2016). Enhanced phasic GABA inhibition during the repair phase of stroke: A novel therapeutic target. Brain : A Journal of Neurology, 139(Pt 2), 468–480. doi: 10.1093/brain/awv360 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan N, & Krebs HI (2011). Physically interactive robotic technology for neuromotor rehabilitation. Progress in Brain Research, 192, 59–68. doi: 10.1016/B978-0-444-53355-5.00004-X [doi] [DOI] [PubMed] [Google Scholar]
- Hope TMH, Friston K, Price CJ, Leff AP, Rotshtein P, & Bowman H (2019). Recovery after stroke: Not so proportional after all? Brain : A Journal of Neurology, 142(1), 15–22. doi: 10.1093/brain/awy302 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovda DA, & Feeney DM (1985). Haloperidol blocks amphetamine induced recovery of binocular depth perception after bilateral visual cortex ablation in cat. Proceedings of the Western Pharmacology Society, 28, 209–211. [PubMed] [Google Scholar]
- Hsu WY, Cheng CH, Liao KK, Lee IH, & Lin YY (2012). Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: A meta-analysis. Stroke, 43(7), 1849–1857. doi: 10.1161/STROKEAHA.111.649756 [doi] [DOI] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, & Bergles DE (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neuroscience, 16(6), 668–676. doi: 10.1038/nn.3390 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, & Cohen LG (2005). Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain : A Journal of Neurology, 128(Pt 3), 490–499. doi:awh369 [pii] [DOI] [PubMed] [Google Scholar]
- Hummel F, & Cohen LG (2005). Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabilitation and Neural Repair, 19(1), 14–19. doi:19/1/14 [pii] [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, … Kageyama R (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nature Neuroscience, 11(10), 1153–1161. doi: 10.1038/nn.2185 [doi] [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, … Greenberg DA (2006). Evidence for stroke-induced neurogenesis in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 103(35), 13198–13202. doi:0603512103 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K, Lindgren I, Widner H, Wiklund I, & Johansson BB (1993). Can sensory stimulation improve the functional outcome in stroke patients? Neurology, 43(11), 2189–2192. [DOI] [PubMed] [Google Scholar]
- Joy MT, Ben Assayag E, Shabashov-Stone D, Liraz-Zaltsman S, Mazzitelli J, Arenas M, … Carmichael ST (2019). CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell, 176(5), 1143–1157.e13. doi:S0092–8674(19)30107–2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy MT, Zhou M, Cai Y, Silva AJ, & Carmichael ST (2015). The role of C-C chemokine receptor 5 in neural repair after stroke [Abstract]. Program no. 403.29/H22, Neuroscience Meeting Planner [Google Scholar]
- Kahle MP, & Bix GJ (2013). Neuronal restoration following ischemic stroke: Influences, barriers, and therapeutic potential. Neurorehabilitation and Neural Repair, 27(5), 469–478. doi: 10.1177/1545968312474119 [doi] [DOI] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, … Muir KW (2016). Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet (London, England), 388(10046), 787–796. doi: 10.1016/S0140-6736(16)30513-X [doi] [DOI] [PubMed] [Google Scholar]
- Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, & Finklestein SP (1997). Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proceedings of the National Academy of Sciences of the United States of America, 94(15), 8179–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, … Steinberg GK (2004). Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America, 101(32), 11839–11844. doi: 10.1073/pnas.0404474101 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff G (2003). Modulation and rehabilitation of spatial neglect by sensory stimulation. Progress in Brain Research, 142, 257–271. doi:S0079–6123(03)42018–9 [pii] [DOI] [PubMed] [Google Scholar]
- Kernie SG, & Parent JM (2010). Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiology of Disease, 37(2), 267–274. doi: 10.1016/j.nbd.2009.11.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, & Rothwell JC (2005). Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology, 65(3), 466–468. doi:65/3/466 [pii] [DOI] [PubMed] [Google Scholar]
- Kim SY, Allred RP, Adkins DL, Tennant KA, Donlan NA, Kleim JA, & Jones TA (2015). Experience with the “good” limb induces aberrant synaptic plasticity in the perilesion cortex after stroke. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 35(22), 8604–8610. doi: 10.1523/JNEUROSCI.0829-15.2015 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, … Hallett M (2006). Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke, 37(6), 1471–1476. doi:01.STR.0000221233.55497.51 [pii] [DOI] [PubMed] [Google Scholar]
- Kimberley TJ, Samargia S, Moore LG, Shakya JK, & Lang CE (2010). Comparison of amounts and types of practice during rehabilitation for traumatic brain injury and stroke. Journal of Rehabilitation Research and Development, 47(9), 851–862. [DOI] [PubMed] [Google Scholar]
- Klassen HJ, Imfeld KL, Kirov II, Tai L, Gage FH, Young MJ, & Berman MA (2003). Expression of cytokines by multipotent neural progenitor cells. Cytokine, 22(3–4), 101–106. doi:S1043466603001200 [pii] [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, Schlaug G, & Pascual-Leone A (2004). Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology, 62(1), 91–98. [DOI] [PubMed] [Google Scholar]
- Komlosi G, Molnar G, Rozsa M, Olah S, Barzo P, & Tamas G (2012). Fluoxetine (prozac) and serotonin act on excitatory synaptic transmission to suppress single layer 2/3 pyramidal neuron-triggered cell assemblies in the human prefrontal cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(46), 16369–16378. doi: 10.1523/JNEUROSCI.2618-12.2012 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, … Teraoka J (2005). Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. Journal of Neurosurgery, 103(1), 38–45. doi: 10.3171/jns.2005.103.1.0038 [doi] [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, … Bynum L (2000). Transplantation of cultured human neuronal cells for patients with stroke. Neurology, 55(4), 565–569. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST (2017). Broken movement: The neurobiology of motor recovery after stroke (1st ed.). Cambridge, MA: The MIT Press. [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, & Wittenberg GF (2012). Getting neurorehabilitation right: What can be learned from animal models? Neurorehabilitation and Neural Repair, 26(8), 923–931. doi: 10.1177/1545968312440745 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, & Marshall RS (2015). The proportional recovery rule for stroke revisited. Annals of Neurology, 78(6), 845–847. doi: 10.1002/ana.24537 [doi] [DOI] [PubMed] [Google Scholar]
- Krebs HI, & Hogan N (2012). Robotic therapy: The tipping point. American Journal of Physical Medicine & Rehabilitation, 91(11 Suppl 3), S290–7. doi: 10.1097/PHM.0b013e31826bcd80 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, & Wang JM (1994). Role of angiogenesis in patients with cerebral ischemic stroke. Stroke, 25(9), 1794–1798. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, … Bernhardt J (2017). Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. International Journal of Stroke : Official Journal of the International Stroke Society, 12(5), 451–461. doi: 10.1177/1747493017711813 [doi] [DOI] [PubMed] [Google Scholar]
- Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, … Langhorne P (2004). Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke, 35(11), 2529–2539. doi:01.STR.0000143153.76460.7d [pii] [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, & Koetsier JC (1999). Intensity of leg and arm training after primary middle-cerebral-artery stroke: A randomised trial. Lancet (London, England), 354(9174), 191–196. doi:S0140–6736(98)09477-X [pii] [DOI] [PubMed] [Google Scholar]
- Lai TW, Zhang S, & Wang YT (2014). Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Progress in Neurobiology, 115, 157–188. doi: 10.1016/j.pneurobio.2013.11.006 [doi] [DOI] [PubMed] [Google Scholar]
- Lake EM, Chaudhuri J, Thomason L, Janik R, Ganguly M, Brown M, … Stefanovic B (2015). The effects of delayed reduction of tonic inhibition on ischemic lesion and sensorimotor function. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 35(10), 1601–1609. doi: 10.1038/jcbfm.2015.86 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, … Bersano A (2011). Growth factors in ischemic stroke. Journal of Cellular and Molecular Medicine, 15(8), 1645–1687. doi: 10.1111/j.1582-4934.2009.00987.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, … Scheets PL (2009). Observation of amounts of movement practice provided during stroke rehabilitation. Archives of Physical Medicine and Rehabilitation, 90(10), 1692–1698. doi: 10.1016/j.apmr.2009.04.005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver KE, Lange B, George S, Deutsch JE, Saposnik G, & Crotty M (2017). Virtual reality for stroke rehabilitation. The Cochrane Database of Systematic Reviews, 11, CD008349. doi: 10.1002/14651858.CD008349.pub4 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar RM, Minzer B, Antoniello D, Festa JR, Krakauer JW, & Marshall RS (2010). Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke, 41(7), 1485–1488. doi: 10.1161/STROKEAHA.109.577338 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, & Thuret S (2018). Adult human hippocampal neurogenesis: Controversy and evidence. Trends in Molecular Medicine, 24(6), 521–522. doi:S1471–4914(18)30079–0 [pii] [DOI] [PubMed] [Google Scholar]
- Legg L, Langhorne P, & Outpatient Service Trialists. (2004). Rehabilitation therapy services for stroke patients living at home: Systematic review of randomised trials. Lancet (London, England), 363(9406), 352–356. doi: 10.1016/S0140-6736(04)15434-2 [doi] [DOI] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, & McKay RD (2007). Long-lasting regeneration after ischemia in the cerebral cortex. Stroke, 38(1), 153–161. doi:01.STR.0000252156.65953.a9 [pii] [DOI] [PubMed] [Google Scholar]
- Lemmens R, & Steinberg GK (2013). Stem cell therapy for acute cerebral injury: What do we know and what will the future bring? Current Opinion in Neurology, 26(6), 617–625. doi: 10.1097/WCO.0000000000000023 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, … Venkatesan L (2016). Epidural electrical stimulation for stroke rehabilitation: Results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabilitation and Neural Repair, 30(2), 107–119. doi: 10.1177/1545968315575613 [doi] [DOI] [PubMed] [Google Scholar]
- Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, … Carmichael ST (2015). GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nature Neuroscience, 18(12), 1737–1745. doi: 10.1038/nn.4146 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, … Carmichael ST (2010). An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nature Neuroscience, 13(12), 1496–1504. doi: 10.1038/nn.2674 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Zhao H, Gleichman A, Machnicki M, Telang S, Tang S, … Carmichael ST (2019). Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke. Proceedings of the National Academy of Sciences of the United States of America, 116(27), 13621–13630. doi: 10.1073/pnas.1811825116 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew SL, Santarnecchi E, Buch ER, & Cohen LG (2014). Non-invasive brain stimulation in neurorehabilitation: Local and distant effects for motor recovery. Frontiers in Human Neuroscience, 8, 378. doi: 10.3389/fnhum.2014.00378 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DJ, Finklestein SP, & Cramer SC (2018). New directions in treatments targeting stroke recovery. Stroke, 49(12), 3107–3114. doi: 10.1161/STROKEAHA.118.021359 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau NT, Banninger BJ, Gullo M, Good NA, Bachmann LC, Starkey ML, & Schwab ME (2014). Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-nogo-A therapy. Brain : A Journal of Neurology, 137(Pt 3), 739–756. doi: 10.1093/brain/awt336 [doi] [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, & Yang GY (2014). Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Progress in Neurobiology, 115, 138–156. doi: 10.1016/j.pneurobio.2013.11.004 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, … Peduzzi P (2010). Robot-assisted therapy for long-term upper-limb impairment after stroke. The New England Journal of Medicine, 362(19), 1772–1783. doi: 10.1056/NEJMoa0911341 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH (2008). A new penumbra: Transitioning from injury into repair after stroke. Nature Medicine, 14(5), 497–500. doi: 10.1038/nm1735 [doi] [DOI] [PubMed] [Google Scholar]
- Lohse KR, Lang CE, & Boyd LA (2014). Is more better? using metadata to explore dose-response relationships in stroke rehabilitation. Stroke, 45(7), 2053–2058. doi: 10.1161/STROKEAHA.114.004695 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokk J, Salman Roghani R, & Delbari A (2011). Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke--a randomized, double-blind, placebo-controlled trial. Acta Neurologica Scandinavica, 123(4), 266–273. doi: 10.1111/j.1600-0404.2010.01395.x [doi] [DOI] [PubMed] [Google Scholar]
- MacDonald E, Van der Lee H, Pocock D, Cole C, Thomas N, VandenBerg PM, … Kleim JA(2007). A novel phosphodiesterase type 4 inhibitor, HT-0712, enhances rehabilitation-dependent motor recovery and cortical reorganization after focal cortical ischemia. Neurorehabilitation and Neural Repair, 21(6), 486–496. doi:1545968307305521 [pii] [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Keough MB, Granter-Button S, Chernenko GA, Butt S, & Corbett D (2011). A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehabilitation and Neural Repair, 25(8), 740–748. doi: 10.1177/1545968311407517 [doi] [DOI] [PubMed] [Google Scholar]
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, & Risau W (2000). Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. The American Journal of Pathology, 156(3), 965–976. doi:S0002–9440(10)64964–4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson L, Hardemark HG, & Wahlgren NG (2003). Amphetamines for improving stroke recovery: A systematic cochrane review. Stroke, 34(11), 2766. doi: 10.1161/01.STR.0000098003.50143.17 [doi] [DOI] [PubMed] [Google Scholar]
- Mason JL, Jones JJ, Taniike M, Morell P, Suzuki K, & Matsushima GK (2000). Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. Journal of Neuroscience Research, 61(3), 251–262. doi: 10.1002/1097-4547(20000801)61:33.0.CO;2-W [pii] [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, & Matsushima GK (2001). Interleukin-1beta promotes repair of the CNS. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 21(18), 7046–7052. doi:21/18/7046 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, … Maffei L (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science (New York, N.Y.), 320(5874), 385–388. doi: 10.1126/science.1150516 [doi] [DOI] [PubMed] [Google Scholar]
- Meininger V, Pradat PF, Corse A, Al-Sarraj S, Rix Brooks B, Caress JB, … Wurthner J (2014). Safety, pharmacokinetic, and functional effects of the nogo-a monoclonal antibody in amyotrophic lateral sclerosis: A randomized, first-in-human clinical trial. PloS One, 9(5), e97803. doi: 10.1371/journal.pone.0097803 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL 2nd, … Hays SA (2018). Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke, 49(3), 710–717. doi: 10.1161/STROKEAHA.117.019202 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, … Robinson RG (2014). Prevention of post-stroke generalized anxiety disorder, using escitalopram or problem-solving therapy. The Journal of Neuropsychiatry and Clinical Neurosciences, 26(4), 323–328. doi: 10.1176/appi.neuropsych.11020047 [doi] [DOI] [PubMed] [Google Scholar]
- Minnerup J, Strecker JK, Wachsmuth L, Hoppen M, Schmidt A, Hermann DM, … Schabitz WR (2018). Defining mechanisms of neural plasticity after brainstem ischemia in rats. Annals of Neurology, 83(5), 1003–1015. doi: 10.1002/ana.25238 [doi] [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, … ffrench-Constant C (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature Neuroscience, 16(9), 1211–1218. doi: 10.1038/nn.3469 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostany R, Chowdhury TG, Johnston DG, Portonovo SA, Carmichael ST, & Portera-Cailliau C (2010). Local hemodynamics dictate long-term dendritic plasticity in peri-infarct cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 30(42), 14116–14126. doi: 10.1523/JNEUROSCI.3908-10.2010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert JP, Koch MW, Heerings M, Heersema DJ, & De Keyser J (2008). Therapeutic potential of fluoxetine in neurological disorders. CNS Neuroscience & Therapeutics, 14(2), 153–164. doi: 10.1111/j.1527-3458.2008.00040.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, & Cohen LG (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology, 55(3), 400–409. doi: 10.1002/ana.10848 [doi] [DOI] [PubMed] [Google Scholar]
- Muresanu DF, Heiss WD, Hoemberg V, Bajenaru O, Popescu CD, Vester JC, … Guekht A (2016). Cerebrolysin and recovery after stroke (CARS): A randomized, placebo-controlled, double-blind, multicenter trial. Stroke; a Journal of Cerebral Circulation, 47(1), 151–159. doi: 10.1161/STROKEAHA.115.009416 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, & Corbett D (2009). Plasticity during stroke recovery: From synapse to behaviour. Nature Reviews Neuroscience, 10(12), 861–872. doi: 10.1038/nrn2735 [doi] [DOI] [PubMed] [Google Scholar]
- Murray CJ, & Lopez AD (2013). Measuring the global burden of disease. The New England Journal of Medicine, 369(5), 448–457. doi: 10.1056/NEJMra1201534 [doi] [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, … Pascual-Leone A (2005). Improved picture naming in chronic aphasia after TMS to part of right broca’s area: An open-protocol study. Brain and Language, 93(1), 95–105. doi:S0093–934X(04)00227–5 [pii] [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, & Olsen TS (1994). Recovery of upper extremity function in stroke patients: The copenhagen stroke study. Archives of Physical Medicine and Rehabilitation, 75(4), 394–398. [DOI] [PubMed] [Google Scholar]
- Netz J, Lammers T, & Homberg V (1997). Reorganization of motor output in the non-affected hemisphere after stroke. Brain : A Journal of Neurology, 120 (Pt 9)(Pt 9), 1579–1586. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Hagemann G, & Witte OW (1995). Cellular correlates of neuronal hyperexcitability in the vicinity of photochemically induced cortical infarcts in rats in vitro. Neuroscience Letters, 193(2), 101–104. doi:0304–3940(95)11677-O [pii] [DOI] [PubMed] [Google Scholar]
- Ng KL, Gibson EM, Hubbard R, Yang J, Caffo B, O’Brien RJ, … Zeiler SR (2015). Fluoxetine maintains a state of heightened responsiveness to motor training early after stroke in a mouse model. Stroke, 46(10), 2951–2960. doi: 10.1161/STROKEAHA.115.010471 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MF, Tong RK, & Li LS (2008). A pilot study of randomized clinical controlled trial of gait training in subacute stroke patients with partial body-weight support electromechanical gait trainer and functional electrical stimulation: Six-month follow-up. Stroke, 39(1), 154–160. doi:STROKEAHA.107.495705 [pii] [DOI] [PubMed] [Google Scholar]
- Nijboer TC, Kollen BJ, & Kwakkel G (2013). Time course of visuospatial neglect early after stroke: A longitudinal cohort study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 49(8), 2021–2027. doi: 10.1016/j.cortex.2012.11.006 [doi] [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, Karbe H, & Fink GR (2008). Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Archives of Neurology, 65(6), 741–747. doi: 10.1001/archneur.65.6.741 [doi] [DOI] [PubMed] [Google Scholar]
- Nudo RJ (2011). Neural bases of recovery after brain injury. Journal of Communication Disorders, 44(5), 515–520. doi: 10.1016/j.jcomdis.2011.04.004 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, & Milliken GW (1996). Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. Journal of Neurophysiology, 75(5), 2144–2149. doi: 10.1152/jn.1996.75.5.2144 [doi] [DOI] [PubMed] [Google Scholar]
- Ohab JJ, & Carmichael ST (2008). Poststroke neurogenesis: Emerging principles of migration and localization of immature neurons. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 14(4), 369–380. doi:1073858407309545 [pii] [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, & Carmichael ST (2006). A neurovascular niche for neurogenesis after stroke. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 26(50), 13007–13016. doi:26/50/13007 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah M, Amor S, Brouwer N, Vinet J, Eggen B, Biber K, & Boddeke HW (2012). Identification of a microglia phenotype supportive of remyelination. Glia, 60(2), 306–321. doi: 10.1002/glia.21266 [doi] [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, & Jannell S (1993). The results of clinical trials in stroke rehabilitation research. Archives of Neurology, 50(1), 37–44. [DOI] [PubMed] [Google Scholar]
- Overman JJ, & Carmichael ST (2014). Plasticity in the injured brain: More than molecules matter. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(1), 15–28. doi: 10.1177/1073858413491146 [doi] [DOI] [PubMed] [Google Scholar]
- Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, … Carmichael ST (2012). A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proceedings of the National Academy of Sciences of the United States of America, 109(33), E2230–9. doi: 10.1073/pnas.1204386109 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SJ, Levine P, & Leonard A (2007). Mental practice in chronic stroke: Results of a randomized, placebo-controlled trial. Stroke, 38(4), 1293–1297. doi:01.STR.0000260205.67348.2b [pii] [DOI] [PubMed] [Google Scholar]
- Park KI, Teng YD, & Snyder EY (2002). The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nature Biotechnology, 20(11), 1111–1117. doi: 10.1038/nbt751 [doi] [DOI] [PubMed] [Google Scholar]
- Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, & Olsen TS (1995). Aphasia in acute stroke: Incidence, determinants, and recovery. Annals of Neurology, 38(4), 659–666. doi: 10.1002/ana.410380416 [doi] [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, & Maffei L (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science (New York, N.Y.), 298(5596), 1248–1251. doi: 10.1126/science.1072699 [doi] [DOI] [PubMed] [Google Scholar]
- Podda MV, Cocco S, Mastrodonato A, Fusco S, Leone L, Barbati SA, … Grassi C (2016). Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of bdnf expression. Scientific Reports, 6, 22180. doi: 10.1038/srep22180 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, … Krakauer JW (2008). Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabilitation and Neural Repair, 22(1), 64–71. doi:1545968307305302 [pii] [DOI] [PubMed] [Google Scholar]
- Que M, Witte OW, Neumann-Haefelin T, Schiene K, Schroeter M, & Zilles K (1999). Changes in GABA(A) and GABA(B) receptor binding following cortical photothrombosis: A quantitative receptor autoradiographic study. Neuroscience, 93(4), 1233–1240. doi:S0306–4522(99)00197–9 [pii] [DOI] [PubMed] [Google Scholar]
- Raivich G, & Banati R (2004). Brain microglia and blood-derived macrophages: Molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Research.Brain Research Reviews, 46(3), 261–281. doi:S0165017304000785 [pii] [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, & Altschuler EL (2009). The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain : A Journal of Neurology, 132(Pt 7), 1693–1710. doi: 10.1093/brain/awp135 [doi] [DOI] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Broetz D, Rea M, Laer L, Yilmaz O, Brasil FL, … Birbaumer N (2013). Brain-machine interface in chronic stroke rehabilitation: A controlled study. Annals of Neurology, 74(1), 100–108. doi: 10.1002/ana.23879 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Bennion DM, & Sumners C (2014). Cerebroprotective action of angiotensin peptides in stroke. Clinical Science (London, England : 1979), 126(3), 195–205. doi: 10.1042/CS20130324 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Biseko MR, Shayo AF, Mmbando TN, Grundy SJ, Xu A, … Okeng’o K (2019). Opportunities for intervention: Stroke treatments, disability and mortality in urban tanzania. International Journal for Quality in Health Care : Journal of the International Society for Quality in Health Care, 31(5), 385–392. doi: 10.1093/intqhc/mzy188 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Das AS, Lo EH, & Caplan LR (2018). Advances in understanding the pathophysiology of lacunar stroke: A review. JAMA Neurology, doi: 10.1001/jamaneurol.2018.1073 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Das AS, Ohtomo R, Lo EH, Ayata C, & Gurol ME (2019). Pathophysiology of lacunar stroke: History’s mysteries and modern interpretations. Journal of Stroke and Cerebrovascular Diseases : The Official Journal of National Stroke Association, 28(8), 2079–2097. doi:S1052–3057(19)30226–5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Das AS, Stapleton CJ, Chandra RV, Rabinov JD, Patel AB, … Leslie-Mazwi TM (2017). Blood pressure and penumbral sustenance in stroke from large vessel occlusion. Frontiers in Neurology, 8, 317. doi: 10.3389/fneur.2017.00317 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, & Sumners C (2013). Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke. Neuropharmacology, 71, 154–163. doi: 10.1016/j.neuropharm.2013.03.025 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, … Sumners C(2014). Centrally administered angiotensin-(1-7) increases the survival of stroke-prone spontaneously hypertensive rats. Experimental Physiology, 99(2), 442–453. doi: 10.1113/expphysiol.2013.075242 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Mecca AP, Flavin SA, Boulouis G, Lauer A, Zachrison KS, … Leslie-Mazwi TM (2018). Delays in the air or ground transfer of patients for endovascular thrombectomy. Stroke, 49(6), 1419–1425. doi: 10.1161/STROKEAHA.118.020618 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, McQueen J, Searcy L, Scullion G, Zonta B, Desmazieres A, … Horsburgh K (2011). Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(49), 18185–18194. doi: 10.1523/JNEUROSCI.4936-11.2011 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESTORE Collaborators. (2018). Randomized efficacy and safety trial with oral S 44819 after recent ischemic cerebral event: International, multi-centre, randomized, double blind placebo-controlled phase II study. Retrieved from https://clinicaltrials.gov/ct2/show/NCT02877615
- Ringelstein EB, Thijs V, Norrving B, Chamorro A, Aichner F, Grond M, … AXIS 2 Investigators. (2013). Granulocyte colony-stimulating factor in patients with acute ischemic stroke: Results of the AX200 for ischemic stroke trial. Stroke, 44(10), 2681–2687. doi: 10.1161/STROKEAHA.113.001531 [doi] [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, & Attwell D (2011). Regulation of oligodendrocyte development and myelination by glucose and lactate. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(2), 538–548. doi: 10.1523/JNEUROSCI.3516-10.2011 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, … Arndt S (2008). Escitalopram and problem-solving therapy for prevention of poststroke depression: A randomized controlled trial. Jama, 299(20), 2391–2400. doi: 10.1001/jama.299.20.2391 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig S, & Carmichael ST (2015). The axon-glia unit in white matter stroke: Mechanisms of damage and recovery. Brain Research, 1623, 123–134. doi: 10.1016/j.brainres.2015.02.019 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, & Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 120(12), 2008–2039. doi: 10.1016/j.clinph.2009.08.016 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel BA, Hamid AIA, Borrmann C, Speck O, & Antal A (2019). Transorbital alternating current stimulation modifies BOLD activity in healthy subjects and in a stroke patient with hemianopia: A 7 tesla fMRI feasibility study. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, doi:S0167–8760(18)31055–9 [pii] [DOI] [PubMed] [Google Scholar]
- Sanin V, Heess C, Kretzschmar HA, & Schuller U (2013). Recruitment of neural precursor cells from circumventricular organs of patients with cerebral ischaemia. Neuropathology and Applied Neurobiology, 39(5), 510–518. doi: 10.1111/j.1365-2990.2012.01301.x [doi] [DOI] [PubMed] [Google Scholar]
- Savitz SI, Cramer SC, Wechsler L, & STEPS 3 Consortium. (2014). Stem cells as an emerging paradigm in stroke 3: Enhancing the development of clinical trials. Stroke, 45(2), 634–639. doi: 10.1161/STROKEAHA.113.003379 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, … Sommer C (2004). Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke, 35(4), 992–997. doi: 10.1161/01.STR.0000119754.85848.0D [doi] [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Laage R, Vogt G, Koch W, Kollmar R, Schwab S, … Schneider A (2010). AXIS: A trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke, 41(11), 2545–2551. doi: 10.1161/STROKEAHA.110.579508 [doi] [DOI] [PubMed] [Google Scholar]
- Scheidtmann K, Fries W, Muller F, & Koenig E (2001). Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: A prospective, randomised, double-blind study. Lancet (London, England), 358(9284), 787–790. doi:S0140–6736(01)05966–9 [pii] [DOI] [PubMed] [Google Scholar]
- Schiene K, Bruehl C, Zilles K, Qu M, Hagemann G, Kraemer M, & Witte OW (1996). Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 16(5), 906–914. doi: 10.1097/00004647-199609000-00014 [doi] [DOI] [PubMed] [Google Scholar]
- Schneider UC, Schilling L, Schroeck H, Nebe CT, Vajkoczy P, & Woitzik J (2007). Granulocyte-macrophage colony-stimulating factor-induced vessel growth restores cerebral blood supply after bilateral carotid artery occlusion. Stroke, 38(4), 1320–1328. doi:01.STR.0000259707.43496.71 [pii] [DOI] [PubMed] [Google Scholar]
- Senesh MR, & Reinkensmeyer DJ (2019). Breaking proportional recovery after stroke. Neurorehabilitation and Neural Repair, , 10.1177/1545968319868718 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Baron JC, & Rowe JB (2009). Motor imagery after stroke: Relating outcome to motor network connectivity. Annals of Neurology, 66(5), 604–616. doi: 10.1002/ana.21810 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan CM, & Kertesz A (1984). Effects of speech and language treatment on recovery from aphasia. Brain and Language, 23(2), 272–299. doi:0093–934X(84)90068–3 [pii] [DOI] [PubMed] [Google Scholar]
- Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, & Arai K (2016). Subcortical ischemic vascular disease: Roles of oligodendrocyte function in experimental models of subcortical white-matter injury. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 36(1), 187–198. doi:jcbfm201580 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R, Veit R, Stevens B, Caria A, Gerloff C, Birbaumer N, & Hummel F (2012). Acquired control of ventral premotor cortex activity by feedback training: An exploratory real-time FMRI and TMS study. Neurorehabilitation and Neural Repair, 26(3), 256–265. doi: 10.1177/1545968311418345 [doi] [DOI] [PubMed] [Google Scholar]
- Sofroniew MV (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neurosciences, 32(12), 638–647. doi: 10.1016/j.tins.2009.08.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2012). Transgenic techniques for cell ablation or molecular deletion to investigate functions of astrocytes and other GFAP-expressing cell types. Methods in Molecular Biology (Clifton, N.J.), 814, 531–544. doi: 10.1007/978-1-61779-452-0_35 [doi] [DOI] [PubMed] [Google Scholar]
- Sozmen EG, & Carmichael ST (2014). White matter repair in subcortical stroke In Baltan S, Carmichael ST, Matute C & Zhang JH (Eds.), White matter injury in stroke and CNS disease (pp. 262). New York: Springer. [Google Scholar]
- Sozmen EG, Kolekar A, Havton LA, & Carmichael ST (2009). A white matter stroke model in the mouse: Axonal damage, progenitor responses and MRI correlates. Journal of Neuroscience Methods, 180(2), 261–272. doi: 10.1016/j.jneumeth.2009.03.017 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozmen EG, Rosenzweig S, Llorente IL, DiTullio DJ, Machnicki M, Vinters HV, … Carmichael ST (2016). Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proceedings of the National Academy of Sciences of the United States of America, 113(52), E8453–E8462. doi: 10.1073/pnas.1615322113 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey ML, & Schwab ME (2014). How plastic is the brain after a stroke? The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(4), 359–371. doi:1073858413514636 [pii] [DOI] [PubMed] [Google Scholar]
- Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, … Schwartz NE (2016). Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke, 47(7), 1817–1824. doi: 10.1161/STROKEAHA.116.012995 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Fleming MK, & Byblow WD (2008). Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain : A Journal of Neurology, 131(Pt 5), 1381–1390. doi: 10.1093/brain/awn051 [doi] [DOI] [PubMed] [Google Scholar]
- Stinear CM, Smith MC, & Byblow WD (2019). Prediction tools for stroke rehabilitation. Stroke, 50(11), 3314–3322. doi: 10.1161/STROKEAHA.119.025696 [doi] [DOI] [PubMed] [Google Scholar]
- Sulzer J, Haller S, Scharnowski F, Weiskopf N, Birbaumer N, Blefari ML, … Sitaram R (2013). Real-time fMRI neurofeedback: Progress and challenges. NeuroImage, 76, 386–399. doi: 10.1016/j.neuroimage.2013.03.033 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland A, Tinson D, & Bradley L (1994). Differences in recovery from constructional apraxia after right and left hemisphere stroke? Journal of Clinical and Experimental Neuropsychology, 16(6), 916–920. doi: 10.1080/01688639408402703 [doi] [DOI] [PubMed] [Google Scholar]
- Suntrup-Krueger S, Ringmaier C, Muhle P, Wollbrink A, Kemmling A, Hanning U, … Dziewas R (2018). Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Annals of Neurology, 83(2), 328–340. doi: 10.1002/ana.25151 [doi] [DOI] [PubMed] [Google Scholar]
- Taguohi J, Yamada K, Hayakawa T, Katoka K, Komura E, Nakao K, … Kanai N (1989). Ischemic axonal injury and its recovery after focal cerebral ischemia. No to Shinkei = Brain and Nerve, 41(8), 813–818. [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, & Yong VW (2003). Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Annals of Neurology, 53(5), 588–595. doi: 10.1002/ana.10519 [doi] [DOI] [PubMed] [Google Scholar]
- Takase H, Kurihara Y, Yokoyama TA, Kawahara N, & Takei K (2017). LOTUS overexpression accelerates neuronal plasticity after focal brain ischemia in mice. PloS One, 12(9), e0184258. doi: 10.1371/journal.pone.0184258 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuru Y, Fukumoto D, Yoshitomo M, Nemoto T, Tsukada H, & Nabekura J (2009). Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(32), 10081–10086. doi: 10.1523/JNEUROSCI.1638-09.2009 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, & Ikoma K (2005). Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke, 36(12), 2681–2686. doi:01.STR.0000189658.51972.34 [pii] [DOI] [PubMed] [Google Scholar]
- Tanaka T, Murakami K, Bando Y, & Yoshida S (2013). Minocycline reduces remyelination by suppressing ciliary neurotrophic factor expression after cuprizone-induced demyelination. Journal of Neurochemistry, 127(2), 259–270. doi: 10.1111/jnc.12289 [doi] [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, & Morris DM (2006). The learned nonuse phenomenon: Implications for rehabilitation. Europa Medicophysica, 42(3), 241–256. doi:R33061687 [pii] [PubMed] [Google Scholar]
- Teasell RW, & Kalra L (2004). What’s new in stroke rehabilitation. Stroke, 35(2), 383–385. doi: 10.1161/01.STR.0000115937.94104.76 [doi] [DOI] [PubMed] [Google Scholar]
- Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, … Kokaia Z (2013). Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain : A Journal of Neurology, 136(Pt 12), 3561–3577. doi: 10.1093/brain/awt278 [doi] [DOI] [PubMed] [Google Scholar]
- Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, … Carmichael ST (2006). A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 26(4), 1269–1274. doi:26/4/1269 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrin NP, & Rivest S (2006). Molecular and cellular immune mediators of neuroprotection. Molecular Neurobiology, 34(3), 221–242. doi:MN:34:3:221 [pii] [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, & Lemon RN (1996). Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalography and Clinical Neurophysiology, 101(4), 316–328. [DOI] [PubMed] [Google Scholar]
- Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, & Dekker J (2004). The impact of physical therapy on functional outcomes after stroke: What’s the evidence? Clinical Rehabilitation, 18(8), 833–862. doi: 10.1191/0269215504cr843oa [doi] [DOI] [PubMed] [Google Scholar]
- Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, Meskers CG, & Kwakkel G (2017). Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabilitation and Neural Repair, 31(2), 107–121. doi: 10.1177/1545968316666957 [doi] [DOI] [PubMed] [Google Scholar]
- Volpe BT, Huerta PT, Zipse JL, Rykman A, Edwards D, Dipietro L, … Krebs HI (2009). Robotic devices as therapeutic and diagnostic tools for stroke recovery. Archives of Neurology, 66(9), 1086–1090. doi: 10.1001/archneurol.2009.182 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu Q, Xie K, Eckert M, Zhao W, & Cramer SC (2014). Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology, 82(14), 1277–1286. doi: 10.1212/WNL.0000000000000278 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DT, Parker V, & Langton Hewer R (1986). Memory disturbance after stroke: Frequency and associated losses. International Rehabilitation Medicine, 8(2), 60–64. [DOI] [PubMed] [Google Scholar]
- Wade DT, Skilbeck CE, Hewer RL, & Wood VA (1984). Therapy after stroke: Amounts, determinants and effects. International Rehabilitation Medicine, 6(3), 105–110. [DOI] [PubMed] [Google Scholar]
- Wahl AS, & Schwab ME (2014). Finding an optimal rehabilitation paradigm after stroke: Enhancing fiber growth and training of the brain at the right moment. Frontiers in Human Neuroscience, 8, 381. doi: 10.3389/fnhum.2014.00381 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, & Dore S (2007). Inflammation after intracerebral hemorrhage. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 27(5), 894–908. doi:9600403 [pii] [DOI] [PubMed] [Google Scholar]
- Wang L, Conner JM, Nagahara AH, & Tuszynski MH (2016). Rehabilitation drives enhancement of neuronal structure in functionally relevant neuronal subsets. Proceedings of the National Academy of Sciences of the United States of America, 113(10), 2750–2755. doi: 10.1073/pnas.1514682113 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, & Chopp M (2004). Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke, 35(7), 1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4 [doi] [DOI] [PubMed] [Google Scholar]
- Ward NS (2017). Restoring brain function after stroke - bridging the gap between animals and humans. Nature Reviews.Neurology, 13(4), 244–255. doi: 10.1038/nrneurol.2017.34 [doi] [DOI] [PubMed] [Google Scholar]
- Ward NS, & Cohen LG (2004). Mechanisms underlying recovery of motor function after stroke. Archives of Neurology, 61(12), 1844–1848. doi:61/12/1844 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster BR, Celnik PA, & Cohen LG (2006). Noninvasive brain stimulation in stroke rehabilitation. NeuroRx : The Journal of the American Society for Experimental NeuroTherapeutics, 3(4), 474–481. doi:S1545–5343(06)00130–1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, & Frackowiak RS (1993). Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Annals of Neurology, 33(2), 181–189. doi: 10.1002/ana.410330208 [doi] [DOI] [PubMed] [Google Scholar]
- Wieloch T, & Nikolich K (2006). Mechanisms of neural plasticity following brain injury. Current Opinion in Neurobiology, 16(3), 258–264. doi:S0959–4388(06)00062–6 [pii] [DOI] [PubMed] [Google Scholar]
- Winship IR, & Murphy TH (2008). In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(26), 6592–6606. doi: 10.1523/JNEUROSCI.0622-08.2008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters C, van Wegen EE, Daffertshofer A, & Kwakkel G (2015). Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabilitation and Neural Repair, 29(7), 614–622. doi: 10.1177/1545968314562115 [doi] [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, … EXCITE Investigators. (2006). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. Jama, 296(17), 2095–2104. doi:296/17/2095 [pii] [DOI] [PubMed] [Google Scholar]
- Wu H, Zhou Y, & Xiong ZQ (2007). Transducer of regulated CREB and late phase long-term synaptic potentiation. The FEBS Journal, 274(13), 3218–3223. doi:EJB5891 [pii] [DOI] [PubMed] [Google Scholar]
- Xiao G, & Hinman JD (2016). Concepts and opportunities for repair in cerebral microvascular disease and white matter stroke. Neural Regeneration Research, 11(9), 1398–1400. doi: 10.4103/1673-5374.191203 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang HT, & O’Donnell JM (2011). Phosphodiesterases in the central nervous system: Implications in mood and cognitive disorders. Handbook of Experimental Pharmacology, (204):447–85. doi(204), 447–485. doi: 10.1007/978-3-642-17969-3_19 [doi] [DOI] [PubMed] [Google Scholar]
- Zai L, Ferrari C, Dice C, Subbaiah S, Havton LA, Coppola G, … Benowitz LI (2011). Inosine augments the effects of a nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(16), 5977–5988. doi: 10.1523/JNEUROSCI.4498-10.2011 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai L, Ferrari C, Subbaiah S, Havton LA, Coppola G, Strittmatter S, … Benowitz LI (2009). Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(25), 8187–8197. doi: 10.1523/JNEUROSCI.0414-09.2009 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Alon L, Ryan SL, Lazar RM, Vry MS, Weiller C, … Krakauer JW (2011). Prediction of motor recovery using initial impairment and fMRI 48 h poststroke. Cerebral Cortex (New York, N.Y.: 1991), 21(12), 2712–2721. doi: 10.1093/cercor/bhr047 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler SR (2019). Should we care about early post-stroke rehabilitation? not yet, but soon. Current Neurology and Neuroscience Reports, 19(3), 13–019-0927-x. doi: 10.1007/s11910-019-0927-x [doi] [DOI] [PubMed] [Google Scholar]
- Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O’Brien RJ, & Krakauer JW (2013). Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke, 44(2), 483–489. doi: 10.1161/STROKEAHA.112.676940 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler SR, Hubbard R, Gibson EM, Zheng T, Ng K, O’Brien R, & Krakauer JW (2016). Paradoxical motor recovery from a first stroke after induction of a second stroke: Reopening a postischemic sensitive period. Neurorehabilitation and Neural Repair, 30(8), 794–800. doi: 10.1177/1545968315624783 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, & Chopp M (2013). Cell-based therapy for ischemic stroke. Expert Opinion on Biological Therapy, 13(9), 1229–1240. doi: 10.1517/14712598.2013.804507 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Y, Xing S, Liang Z, & Zeng J (2012). Secondary neurodegeneration in remote regions after focal cerebral infarction: A new target for stroke management? Stroke, 43(6), 1700–1705. doi: 10.1161/STROKEAHA.111.632448 [doi] [DOI] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Meier DH, Winter S, Wang L, Szalad A, … Zhang ZG (2013). Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke, 44(7), 1965–1972. doi: 10.1161/STROKEAHA.111.000831 [doi] [DOI] [PubMed] [Google Scholar]
- Zhang Q, Gao T, Luo Y, Chen X, Gao G, Gao X, … Dai, J. (2012). Transient focal cerebral ischemia/reperfusion induces early and chronic axonal changes in rats: Its importance for the risk of alzheimer’s disease. PloS One, 7(3), e33722. doi: 10.1371/journal.pone.0033722 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, … Chopp M (2000). VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. The Journal of Clinical Investigation, 106(7), 829–838. doi: 10.1172/JCI9369 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng MX, Hua XY, Feng JT, Li T, Lu YC, Shen YD, … Xu WD (2018). Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. The New England Journal of Medicine, 378(1), 22–34. doi: 10.1056/NEJMoa1615208 [doi] [DOI] [PubMed] [Google Scholar]


