Abstract
Given the general utility of lung ultrasound for the evaluation of respiratory failure in acutely ill patients, it is logical to consider its specific advantages in coronavirus disease 2019-related pulmonary disease. The authors, representing the extensive experience of the North American and European coronavirus disease 2019 epicenters, present an ultrasound scanning protocol and report on the common associated ultrasound findings.
Key Words: acute lung injury, critical care, lung ultrasound
Abbreviations: COVID-19, coronavirus disease 2019; CXR, chest radiograph; GGO, ground-glass opacities; LUS, lung ultrasound
An obese 62-year-old man with hypertension and type 2 diabetes presents to a New York City hospital with a 7-day history of progressive dyspnea and nonproductive cough. On presentation, he is hypoxemic (oxygen saturation of 82% on room air) and febrile (38.9°C), with a normal BP reading and a heart rate of 115 beats/min. Results of his laboratory tests, including coronavirus disease 2019 (COVID-19) testing, are pending.
Utility of Lung Ultrasound in COVID-19 Infection
It is useful for purposes of comparison to summarize the changes expected on chest CT scan and lung ultrasound (LUS). Findings from three recent coronavirus outbreaks (severe acute respiratory syndrome,1 Middle East respiratory syndrome-related,2 and COVID-193) are similar; each is associated with ground-glass opacities (GGO) with or without consolidation, a predilection for the lower lobes, typical peripheral/subpleural locations of GGO lesions, multifocal and bilateral lung involvement, and the absence of large pleural effusions. One large study3 reported the following specific patterns in COVID-19 disease: presence of GGO and consolidation (41% of 121 patients), presence of GGO alone (34%), presence of consolidation alone (2%), and absence of both GGO and consolidation (22%). The disease was bilateral or multifocal in most cases and favored the lower lobes.
In addition to its diagnostic utility (discussed later in detail), LUS has a potential role in monitoring patients for signs of improvement or deterioration, although the specific details remain unclear at present. Other parts of the point-of-care ultrasound toolkit, such as cardiac or vascular ultrasound, likely also have an important role to play, especially in severely ill patients in whom diagnostic uncertainty exists. Limitations of LUS generally can be grouped into operator factors (given its operator-dependent nature) and patient factors (with obesity and immobility being particularly relevant).
Scanning Protocol
There are differing opinions within the ultrasound community with respect to specific scanning protocols. Clinicians who are less familiar with LUS can refer to two related articles published in this issue of CHEST: a hands-on guide to performing basic LUS4 and a review of the utility of general LUS in acutely ill patients.5 Most providers will have access to a lower frequency transducer, either phased-array (“cardiac”) or convex/curvilinear (“abdominal”). Either can be used effectively, depending mostly on user preference.
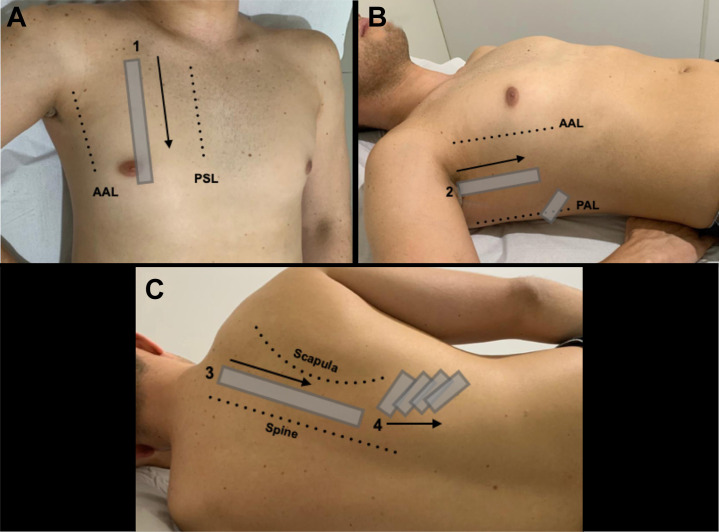
Depending on the clinical context, some providers favor a simple three-point-per-hemithorax sequence, essentially copying the seminal BLUE protocol.6 Others prefer a more comprehensive scanning protocol such as that described in the 2012 international evidence-based recommendations for point-of-care LUS.7 Specifically for patients with suspected COVID-19, it is appropriate to focus attention on the posterior and dependent lung areas, as the disease tends to manifest itself in these areas,3 which are not well covered by many traditional protocols. Although other similar protocols exist,8 the following scanning sequence is recommended:
-
(1)
At the second intercostal space in the mid-clavicular line, the operator examines the anterior pleural line for lung sliding. Some providers also use the high-frequency linear transducer here to examine pleural line morphology in more detail, although others use the single, lower frequency transducer for the entire examination.
-
(2)
Using the lower frequency transducer for the remainder of the examination, the operator examines the intercostal spaces of the anterior area (zone 1) (Fig 1 A). Steps 2 to 5 focus on the identification of common LUS findings, including A-lines, B-lines, pleural effusions, and consolidation.
-
(3)
The operator examines the intercostal spaces of the upper lateral area (zone 2) (Fig 1B).
-
(4)
The operator examines the costophrenic angle for pleural effusion or dependent areas of consolidation above the diaphragm.
-
(5)
The patient is placed in the lateral decubitus to scan the posterior thorax. The operator examines the area between the scapula and the spine (zone 3) (Fig 1C) and the basal area below the inferior margin of the scapula (zone 4).
-
(6)
Additional scans are performed as needed to further characterize focal areas of abnormality.
Figure 1.
Proposed scanning protocol, placing extra emphasis on the posterior lung regions given the tendency of coronavirus disease 2019 to manifest in these areas. A, Zone 1, the intercostal spaces in the mid-clavicular line. B, Zone 2, the intercostal spaces in the mid-axillary line, plus the area just above the diaphragm in the posterior axillary line. C, Zone 3 (the intercostal spaces between the spine and scapula) and Zone 4 (the area beneath the scapula). AAL = anterior axillary line; PAL = posterior axillary line; PSL = parasternal line.
Given the heterogeneous nature of this disease, a systematic examination remains paramount. As many intercostal spaces as possible should be scanned, and each point should be interrogated for a full respiratory cycle.
LUS Findings in COVID-19 Infection
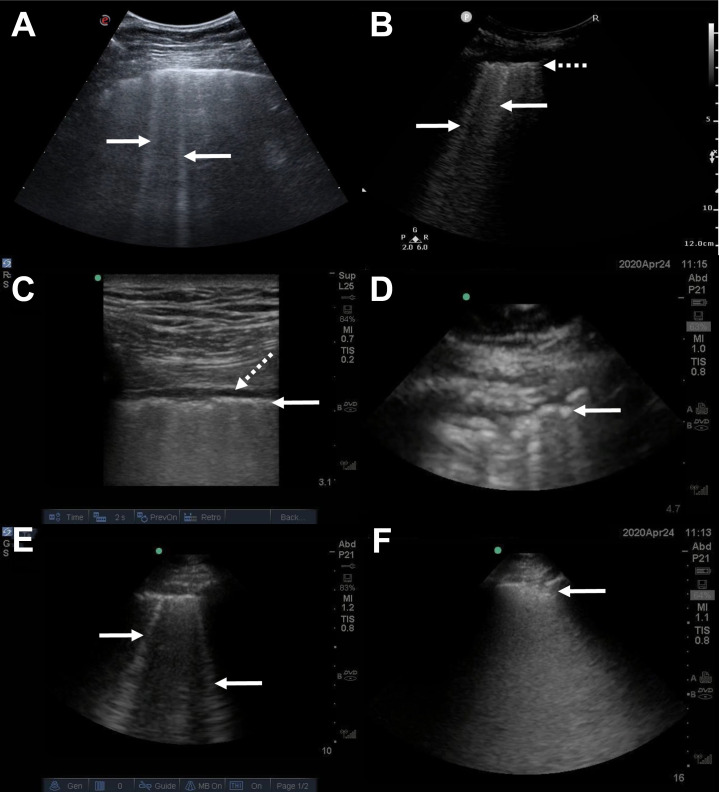
The typical LUS findings reported in COVID-19-infected patients include9 (Fig 2 ):
-
•
B-lines, usually multifocal, separated, and confluent, accompanied by segments of irregular pleural line alternating with small peripheral consolidations. B-lines are visualized in various intensities and distributions depending on the severity of illness
-
•
Bilateral disease affecting multiple lobes
-
•
Areas of patchy B-lines alternating with regular A-lines zones (spared areas or “skip” lesions)
-
•
Small peripheral consolidations present in any lung zone but more common at the lung bases
-
•
Less commonly larger consolidations with or without dynamic air bronchograms; large areas of consolidated lung should prompt consideration of a superimposed bacterial pneumonia
-
•
Absent or small pleural effusions
Figure 2.
A-F, Typical lung ultrasound findings in coronavirus disease 2019. A, Multiple B-lines (arrows), seen using a convex transducer oriented parallel to the ribs. B, Multiple B-lines (arrows) and a thickened irregular pleural line (dotted arrow), seen using a convex transducer oriented perpendicular to the ribs. C, Thickened irregular pleura (arrow) and an associated small pleural effusion (dotted arrow), seen using a linear transducer oriented perpendicular to the ribs. D, Thickened irregular pleura (arrow), seen using a phased-array transducer oriented perpendicular to the ribs. E, Multiple B-lines (arrows), seen using a phased-array transducer oriented perpendicular to the ribs. F, A subpleural consolidation (arrow), seen using a phased-array transducer oriented perpendicular to the ribs.
All of these findings are nonspecific, and therefore LUS cannot be used by itself to establish a definitive diagnosis of COVID-19 infection; the same problem applies, of course, to chest radiograph and chest CT findings.10 A patchy B-line pattern is typical for patients with ARDS, for example, and the subpleural consolidations can be found with other viral infections. As such, the integration of typical LUS findings with patient history, physical examination, laboratory results, and prevalence of the disease during the pandemic phases is required. A correlation between LUS findings and pathologic severity has been proposed,11 which also highlights the fact that milder cases may present more of a diagnostic challenge.
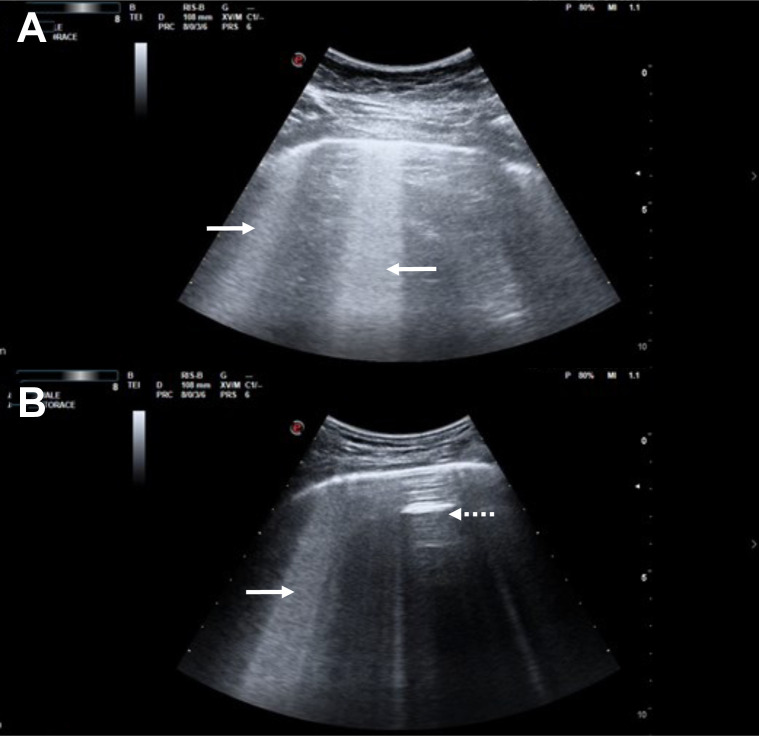
The authors have noted an unusual LUS finding that may be more specific to COVID-19: a thick hyperechoic band of confluent B-lines arising from a portion of the pleural line that appears relatively spared (comparatively smooth).12 , 13 This finding is best appreciated by using the convex transducer with the scanning plane oriented along the long-axis of an intercostal space (parallel to the ribs), such that a long uninterrupted length of pleura is visualized; it is worth noting that this is different from the transducer orientation preferred by many providers. Some clinicians refer to this finding using the descriptive but nonstandard term “light beam.” These areas of hyperechoic confluent B-lines stand out as they often alternate with spared areas of relatively normal lung, seen on ultrasound as A-lines and representing spared areas. Light beams commonly move back-and-forth in tune with the respiratory cycle (Fig 3 , Videos 1, 2, and 3). We speculate that this sign tends to occur in early disease because of the patchy nature of the GGO lesions seen on chest CT imaging, which are concentrated at the lung periphery and subject to respiratory movement. They may evolve, as the disease progresses, toward coalescent B-lines associated with pleural line irregularities and small subpleural consolidations. This evolution may correspond to progressive thickening of the interlobular septa (crazy paving as seen on chest CT imaging) and alveolar filling (consolidation as seen on CT imaging).
Figure 3.
A-B, Two examples of the “light beam” artifact, both generated using the convex ultrasound transducer oriented parallel to the ribs. Areas of thick confluent B-lines can be seen (arrows), emanating from a pleural line that is relatively spared from inflammation, along with interspaced regions of normally aerated lungs where A-lines are seen (spared areas or “skip” lesions; dotted arrows).
LUS Disease Patterns
The various combinations of signs typically observed in patients with COVID-19 disease allow LUS findings to be clustered into patterns. Each may help differentiate the probability of having the disease and can be classified as follows:
-
(1)
High probability LUS pattern
The most typical LUS pattern, as described earlier, consists of bilateral and multifocal clusters of B-lines alternating with normal lung (spared areas or “skip” lesions), with or without small peripheral consolidations. An associated light beam pattern may be present. Increased density of B-lines in posterior and inferior regions is typical.
-
(2)
Intermediate probability LUS pattern
A less typical pattern includes unilateral clusters of B-lines or focal multiple B-lines with or without peripheral consolidations.
-
(3)
Alternate LUS pattern
An isolated large lobar consolidation with air bronchograms, a large pleural effusion, or diffuse and very symmetrical B-lines with a gravity-related distribution are less common in COVID-19 lung disease (being more consistent with bacterial pneumonia, pleural effusion, and cardiogenic pulmonary edema, respectively). These findings alert the clinician to search for an alternate diagnosis.
-
(4)
Low probability LUS pattern
A generalized bilateral A-line pattern with lung sliding indicates normal lung aeration. Significant dyspnea presenting with this ultrasound pattern requires consideration of an alternate diagnosis other than COVID-19. This pattern is also seen in pulmonary embolism, potentially important due to the association between COVID-19 infection and thromboembolic disease.14 In patients who are well, a normal LUS pattern does not exclude infection with COVID-19 without pulmonary involvement.
Clinical COVID-19 Patient Phenotypes
Correlation of the LUS pattern with clinical information is crucial to increase diagnostic confidence while test results are pending, and the patient presentations can be divided as follows: (1) mild phenotype, patients with relatively mild nonrespiratory symptoms and no signs of respiratory failure; (2) severe phenotype, patients with significant dyspnea at rest or on exertion, or signs of respiratory failure; and (3) mixed phenotype, patients with dyspnea or respiratory failure but with preexisting significant chronic pulmonary or cardiac disease that may confound their presentation.
Another related variable to consider is timing of symptom onset. Patients with more severe clinical or LUS findings earlier in their disease course may be at higher risk of progression to respiratory failure.
Triage
Triaging patients with suspected COVID-19 is challenging due to the need for rapid decision-making in the absence of test results and in the presence of limited resources. Patients must be isolated and personal protective equipment donned where uncertainty exists, and decisions regarding admission to the hospital and potential treatments must be made quickly and prior to test results being available. These decisions are particularly challenging in a scenario in which patients suspected of COVID-19 flock to the ED during the height of an outbreak.
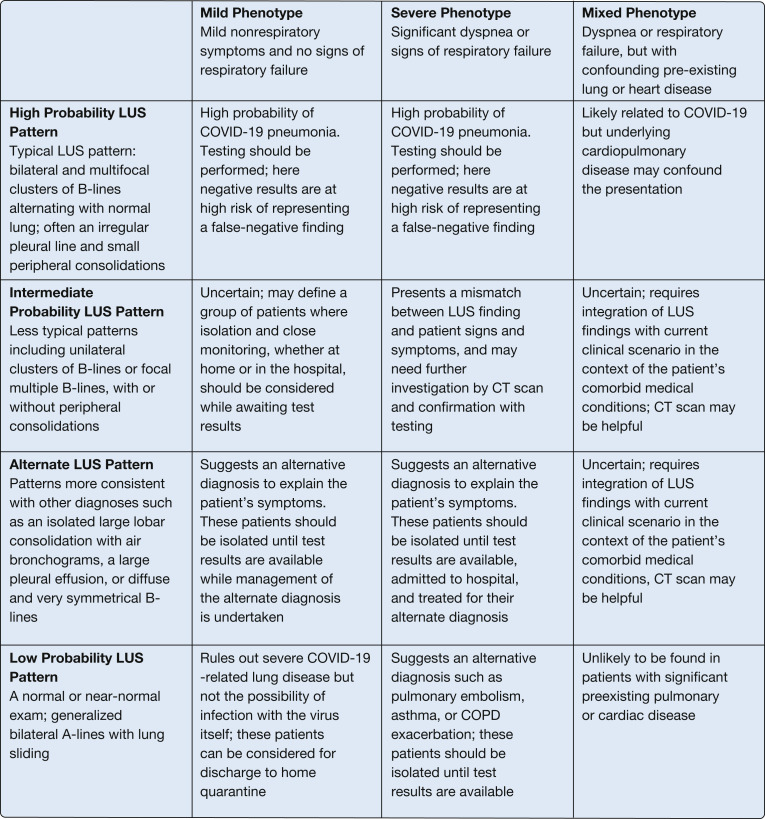
The interface between the patient phenotype (severity of clinical presentation) and the ultrasound pattern may be useful and will be immediately available assuming the presence of providers proficient in LUS. Immediate use of LUS for triage decisions may result in a lower risk of dissemination in crowded EDs compared with the routine use of chest radiograph or CT scan. Combining clinical phenotypes and LUS patterns can guide triage (Fig 4 ), as follows:
Figure 4.
Combining the clinical phenotype with the LUS pattern to aid in patient triage. COVID-19 = coronavirus disease 2019; LUS = lung ultrasound.
For the mild phenotype (patients with mild symptoms and no signs of respiratory failure):
-
•
A high probability LUS pattern indicates a high probability of COVID-19 pneumonia. Testing should be performed, but a negative result would be at high risk of representing a false-negative finding.
-
•
An intermediate probability LUS pattern may define a group of patients in which close monitoring, whether at home or in the hospital, should be considered.
-
•
An alternate LUS pattern, with signs such as a large consolidation or pleural effusion, suggests an alternative diagnosis to explain the patient’s symptoms. These patients should be isolated until test results are available while management of the alternate diagnosis is undertaken and a lower threshold for admission to hospital applied.
-
•
A normal LUS pattern rules out severe COVID-19-related lung disease but not the possibility of infection with the virus itself; these patients can be considered for discharge to home quarantine and monitoring while test results are pending, assuming they are otherwise well.
For the severe phenotype (patients with signs of significant dyspnea or respiratory failure, all of whom should be considered for hospital admission):
-
•
A high probability LUS pattern establishes a high probability of COVID-19 pneumonia. Testing should be performed, but here again a negative result may well represent a false-negative finding.
-
•
An intermediate probability LUS pattern presents a mismatch between LUS finding and patient signs and symptoms, and may need further investigation by using CT scanning and confirmation with testing.
-
•
An alternate LUS pattern, with signs such as a large consolidation or pleural effusion, suggests an alternate diagnosis as an explanation for the patient’s symptoms. These patients should be isolated until test results are available, admitted to the hospital, and treated for their alternate diagnosis.
-
•
A low probability LUS pattern in association with significant dyspnea or respiratory failure suggests an alternative diagnosis such as pulmonary embolism, asthma, or COPD exacerbation. In doubtful cases with cough and fever, these patients should be isolated until test results are available given the possibility of COVID-19 coinfection without pneumonia.
For the mixed phenotype (patients with respiratory failure and preexisting pulmonary or cardiac disease):
-
•
A high probability LUS pattern is likely to be related to COVID-19 but must be viewed in the context of an underlying disease that may confound the symptoms and severity of the clinical presentation.
-
•
The intermediate probability and alternative LUS patterns, as described earlier, require integration of LUS findings with a current clinical scenario in the context of the patient’s comorbid medical conditions; here great uncertainty with respect to the possibility of COVID-19 remains, and CT scanning may be crucial.
-
•
A low probability LUS pattern is unlikely to be found in patients with significant preexisting pulmonary or cardiac disease.
Conclusions
Although the specific role for LUS in the management of COVID-19 is still evolving, its utility in the management of patients with respiratory failure in general is very well established. Given the severity of illness associated with this particular pathogen and the need for strict isolation protocols, there is evidence to suggest that LUS will emerge as the best available imaging modality for these patients.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Additional information: The Videos can be found in the Multimedia section of the online article.
Supplementary Data
References
- 1.Antonio G.E., Wong K.T., Chu W.C.W. Imaging in severe acute respiratory syndrome (SARS) Clin Radiol. 2003;(58):825–832. doi: 10.1016/S0009-9260(03)00308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajlan A.M., Ahyad R.A., Jamjoom L.G. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJM. 2014;203:782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 3.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendin A., Koenig S., Millington S.J. Better with ultrasound: thoracic ultrasound. Chest. 2020;158(5):2082–2089. doi: 10.1016/j.chest.2020.04.052. [DOI] [PubMed] [Google Scholar]
- 5.Koenig S., Mayo P., Volpicelli G., Millington S.J. Lung ultrasound scanning for respiratory failure in acutely ill patients: a review. Chest. 2020;158(6):2511–2516. doi: 10.1016/j.chest.2020.08.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenstein D.A., Mezière G.A. The BLUE-points: three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure. Crit Ultrasound J. 2011;3:109–110. [Google Scholar]
- 7.Volpicelli G., Elbarbary M., Blaivas M. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS) (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 8.Soldati G., Smargiassi A., Inchingolo R., et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19. J Ultrasound Med. 2020;39(7):1413–1419. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Q.Y., Wang X.T., Zhang L.N., Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinga X., Xu J., Zhou J., Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur J Radiol. 2020;127:109009. doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida Monteiro R.A., de Oliveira E.P., Nascimento Saldiva P.H., Dolhnikoff M., Duarte-Neto A.N., BIAS-Brazilian Image Autopsy Study Group Histological-ultrasonographical correlation of pulmonary involvement in severe COVID-19. Intensive Care Med. 2020;46(9):1766–1768. doi: 10.1007/s00134-020-06125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpicelli G., Gargani L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020;12(1):22. doi: 10.1186/s13089-020-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpicelli G., Lamorte A., Villén T. What’s new in lung ultrasound during the COVID-19 pandemic. Care Med. 2020;46(7):1445–1448. doi: 10.1007/s00134-020-06048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bompard F., Monnier H., Saab I. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020;56(1):2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.