Abstract
This review covers the applications of mass spectrometry (MS) and its hyphenated techniques to characterize polyurethane (PU) synthetic polymers and their respective hard and soft segments. PUs are commonly composed of hard segments including methylene bisphenyl diisocyanate (MDI) and toluene diisocyanate (TDI), and soft segments including polyester and polyether polyols. This literature review highlights MS techniques such as electrospray ionization (ESI), matrix assisted laser/desorption ionization (MALDI), ion mobility-mass spectrometry (IM-MS), and computational methods that have been used for the characterization of this polymer system. Here we review specific case studies where MS techniques have elucidated unique features pertaining to the makeup and structural integrity of complex PU materials and PU precursors.
Keywords: polyurethanes, polyethers, polyesters, electrospray ionization, MALDI, ion mobility
1. INTRODUCTION
Polyurethane (PU) di-block copolymers are one of the most versatile polymeric materials, commonly manufactured in the form of flexible and rigid foams, thermoplastics, thermosets, coatings, adhesives, sealants, and elastomers [1]. In the 1930s, Otto Bayer and his co-workers at I.G. Farbenindustrie in Leverkusen, Germany developed PUs that were later used as a rubber alternative during World War II [2]. Since PU’s creation, the resourcefulness of this material has increased due to its ability to undergo synthetic alteration, which enhances PUs societal footprint. In a PU network, the polymer backbone is comprised of hard and soft segments. PU hard segments include aromatic or aliphatic diisocyanates (-NCO) and soft segments consist of aliphatic polyols (-OH). Hard segment isocyanates are formed by reaction between toluene diamine (TDA) or methylene dianiline (MDA) with phosgene to produce toluene diisocyanate (TDI) or methylene diphenyl diisocyanate (MDI), respectively [3]. Polyesters and polyethers are regularly used soft segment polyols which contribute to the polymer’s elasticity. The urethane (carbamate) moiety is the major repeat unit formed by random and/or block polyaddition between diisocyanates and polyols [4], as depicted in Figure 1. As a specific examples, PUs formed between poly(butylene adipate) and MDI are used as thermoplastic elastomers and adhesives. Their major structural segment is shown in Figure 1. PUs can also have customizable applications, where they contain other groups such as aromatic compounds, esters, ethers, and ureas [5,6]. They can also include additives such as flame retardants, pigments, cross-linkers, fillers, blowing agents, and surfactants which can enhance certain polymer properties [7]. Because of the structural and architectural complexity of this copolymer system, the demands for thorough characterization methods are necessary.
Figure 1.
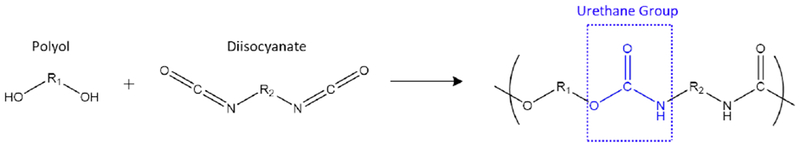
Basic polyurethane reaction scheme between polyol and diisocyanates to form a urethane group, highlighted in blue.
Mass spectrometry (MS) is a powerful analytical technique for the identification and characterization of many biopolymers (e.g. proteins, oligonucleotides, carbohydrates, etc.), aiding in the sequential identification and chemical analysis of ionized molecules based on their mass to charge (m/z) ratio [8,9]. Similar to biopolymer characterization, MS based strategies have been developed to investigate synthetic polymers complexity [10]. MS has become an indispensable tool for polymer analysis and often complements characterization data obtained through classical methods such as NMR, vibrational spectroscopy, size-exclusion chromatography (SEC), and liquid chromatography (LC) [10]. Although these classical methods have aided in the characterization of polymer systems, advanced analytical strategies such as MS-based techniques are needed to further elucidate polymer molecular structures. MS can be used for polymeric end-group analysis, direct mass measurement, molecular weight distribution (MWD), sequential identification, and detection of impurities or additives [11].
Soft ionization techniques such as electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) are frequently used for polymer characterization [11,12]. MALDI is used for direct desorption of ions, generating mostly singly charged species, which minimizes the complexity observed when there are overlapping charge states [12,13]. MALDI is relatively tolerant of contamination and salts, making high sensitivity and MS analysis accessible. ESI is typically considered a softer ionization technique compared to MALDI. For labile substances, ESI may be more appropriate for samples containing fragile end-groups, or supramolecular assemblies which are held together by noncovalent interactions. ESI is known for producing multiply charged species, therefore this ionization technique is useful for characterizing high mass species where the higher charge states shift the ion m/z down to the optimal measurement range for the mass spectrometer [14,15]. Another way to improve the characterization of complex polymer samples is through additional dimensions of separation combined with MS, such as LC-MS. For examples, LC-MS provides separations prior to MS that can aid in characterizing heterogeneous PUs [16,17].
Another multidimensional technique is tandem mass spectrometry (MS/MS), which can be performed in combination with either MALDI or ESI ionization. As its name implies, MS/MS utilizes two tandem mass spectrometers to perform mass-selected ion fragmentation experiments between the two MS stages, typically a low resolution mass filter (e.g., a quadrupole), coupled to a high resolution MS (e.g., a time-of-flight, TOF, or ion trap instrument). Collision-induced dissociation (CID) is the most commonly utilized ion activation technique for MS/MS. When conducting MS/MS experiments, fragmentation studies provide primary structural information about a precursor ion, the subsequent fragment ions generated, and the fragmentation pathways accessed at low and high activation energies [18–20]. Although, MS and MS/MS are useful for structural characterization, these mass analysis techniques are still limited when characterizing isomeric or isobaric species within a heterogeneous or polydisperse sample. An emerging analytical technique for characterizing isomeric species is ion mobility-mass spectrometry (IM-MS), which is a hyphenated gas-phase separation technique, comparable to gas chromatography (GC) or LC coupled to MS. While GC-MS and LC-MS methods separate molecules based on their volatility or polarity differences prior to mass analysis, IM-MS separates ions based on their size, shape, and charge [21,22]. In IM, ions are subject to many low energy collisions with a neutral buffer gas providing a drag force proportional to the ion’s surface area. Thus these ions are then separated by their effective gas-phase size and shape, and can be described by their collisional cross section (CCS) [23,24]. For example, IM-MS has been used to differentiate between linear and cyclic polymer topologies [10,25–28]. IM-MS data are often supplemented with computational studies to gain further insight about a molecule’s gas-phase conformation [29]. These studies are generally conducted in two steps: (1) computational sampling of conformational space, and (2) theoretical determination of CCS values for the generated conformations. IM-MS and computational methods are useful for characterizing PU precursors; however when it comes to fragmentation studies of complex polymers, computational methods are essential for chemical and structural interpretation [30–34].
This review focuses on literature pertaining to the characterization of PU polymers using MS techniques, mainly MALDI, ESI, IM-MS, and computational strategies. More specifically, we will highlight literature that has characterized either intact synthetic PU polymers or hard and soft segments used to make PUs. As illustrated in Figure 2, intact PUs surveyed in this review include flexible and rigid foams, thermoplastics, thermosets, coatings, adhesives, sealants, and elastomers. Hard segments reviewed include aromatic isocyanates, methylene diphenyl diisocyanate (MDI) 4,4’-MDI, and 2,4’-MDI and toluene diisocyanate (TDI); 2,4’-TDI and 2,6’-TDI. Hard segment precursors, such as methylene dianiline (MDA) and toluene dianiline (TDA) will also be considered. Soft segments reviewed include polyesters and polyethers. Our goal is to focus on recent literature characterizing intact PU networks and their precursors using MS. We will emphasize PUs and their precursors that are of large scale industrial importance.
Figure 2.

Example of important PU types and their common hard and soft segments to be reviewed.
2. POLYURETHANE HARD SEGMENT CHARACTERIZATION
2.1. Urethanes and Isocyanates
Given the urethane sequence -NH-C(O)-, isocyanates (-NCO) are essential for PU synthesis. Isocyanates can be di- or polyfunctional where two or more -NCO groups are represented per molecule. Isocyanates are electrophiles that react towards a variety of nucleophiles such as polyols (forming urethanes), amines (forming ureas), and water (producing CO2) [3]. Commonly used aromatic diisocyanates are TDI and MDI. TDI exists in the form of two isomers 2,4- and 2,6-TDI [35]. MDI can exist as 2,4’- and 4,4’-MDI, or short chain polymeric MDI (pMDI). While still important, aliphatic diisocyanates are not as commonly used. These include hexamethylene diisocyanate (HDI), hydrogenated MDI (H12MDI), and isophorone diisocyanate (IPDI) [3]. In this review, aliphatic isocyanates will be mentioned in the context of a specific case study.
2.2. MDI and TDI
MDI and TDI are used for various applications: MDI is used to make rigid foams, insulation, and automobiles and TDI is used to make flexible PU foams, coatings, adhesives, sealants, and elastomers. MDI is formed through the reaction between aniline and formaldehyde, using hydrochloric acid as a catalyst to produce a mixture of methylenedianiline (MDA) and multimeric MDA precursors [3]. When MDA is treated with phosgene, the isocyanate is formed, making pMDI and mixtures of MDI isomers. To make TDI, toluene is reacted with nitric acid to produce diaminotoluene (TDA) isomers. Upon treatment with phosgene, the TDA isomers form into TDI isomers and multimers [1]. Aromatic isocyanate derivatives such as 2,4’- and 4,4’-MDI, and 2,4- and 2,6-TDI that have been characterized using MS-based techniques are discussed below.
For investigating the urethane bond of a hard segment, Pasch and Maujana used MALDI-MS to probe the PU urethane backbone. This work highlighted how MS can be used to identify a polymer and its copolymer sequence, determine the end group functionality, determine MWD, and predict urethane fragmentation mechanisms [36,37]. Mass et al. later investigated the urethane backbone and studied isocyanate fragment ions and their respective fragmentation pathways using CID experiments [38]. The hard segments characterized were multimeric MDI-TDI copolymers, and multimer MDI and TDI homopolymers. These results indicated MDI-TDI copolymer fragmentation occurred at the single bonds near the carbonyl group, making it possible to determine fragmentation pathways. Figure 3 shows the MALDI-TOF mass spectrum of an MDI-TDI copolymer. The intense peaks alternating at mass increments of 148 (TDI) and 224 (MDI) Da, were observed up to masses of about 1000 m/z. The peak at 609 m/z was assigned to an oligomer with two MDI and one TDI repeat units, the peak at 682 m/z was assigned to an oligomer with one MDI and three TDI repeat units and so on [38].
Figure 3.

MALDI-TOF mass spectrum of the TDI-MDI sample. Mass distributions of 148 m/z and 224 m/z represent the addition of TDI and MDI respectively. Copyright 2009 Wiley. Used with permission from V Mass, W. Schrepp, B. Von Vacano, H. Pasch, Sequence analysis of an isocyanate oligomer by MALDI-TOF mass spectrometry using collision induced dissociation, Macromolecular Chemistry and Physics.
Carr et al. also investigated MDIs using MALDI-MS [39]. In this study, MALDI was used to monitor pMDI and MDI isomers treated with methanol, which produced stable urethanes. Warbuton et al. later investigated ways to characterize the derivatized PU isocyanate monomeric and prepolymeric species: MDI, HDI, 2,4-TDI and 2,6-TDI [40]. In their work, it was discovered that immediate derivatization prevented the decomposition of the isocyanates. Therefore, derivatization enhanced the detection and structural information gained regarding each precursor during MS/MS studies. Derivatizing agent 1-(2-methoxyphenyl) piperazine (1,2MP) was found to stabilize the monomeric and prepolymeric isocyanate mixtures [40]. Vangronsveld and Mandel also investigated a LC-UV-MS/MS method to chromatographically separate, detect, and identify the derivatized isocyanate species. As shown in Figure 4, LC was used for separations and MS/MS was used to characterize derivatized isocyanates: phenyl isocyanate (PI), 2,4’- and 4,4’-MDI, 2,4- and 2,6-TDI, trimer pMDI isomers (TRI) [41]. Of not is that their chromatographic method showed separation between all targeted isocyanate compounds.
Figure 4.

Extracted ion chromatograms for the m/z 193 fragment ion for all 1-2MP derivatives of isocyanates. LC separations showed good base peak resolution of derivatized isocyanates. Copyright 2003 Wiley. Used with permission from E. Vangronsveld, F. Mandel, Workplace monitoring of isocyanates using ion trap liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry.
2.3. MDA and TDA
MDA is a precursor used to synthesize MDI. Most formulations of industrial grade MDI are comprised of 2,4’-MDI and 4,4’-MDI isomers, or pMDI. This level of heterogeneity arises from structural isomers and multimeric species present within the MDA sample. In an in depth study, Hercules and coworkers structurally characterized pure MDA regioisomers and multimers using a variety of MS techniques (ESI, MALDI, MS/MS, IM-MS, and MS/MS) and interpreted the data with computational models [32–34,42]. The MDA species investigated include 2-ring MDA (2,2’-MDA, 2,4’-MDA, and 4,4’-MDA), 3-ring MDA, and 4-ring MDA. For each precursor species, the preferred protonation site was determined both experimentally and computationally. This helped elucidate fragmentation pathways for each MDA species during the CID experiments. IM-MS data was interpreted using computational models to provide structural information regarding each MDA precursor and their protonation sites [32–34]. ESI and MALDI ionization techniques were both investigated in the MDA study. In ESI, the [M+H]+ precursor was the only type observed for all MDA species [32,33]. However, when each MDA species was characterized using MALDI, three unique precursors were formed: [M+H]+, [M-]+, and [M-H]+. When comparing ESI MS, MALDI MS, and MS/MS spectra from both ionization techniques, each precursor was found to have a unique fragmentation pathway [34,42].
Fewer studies have characterized TDA isomers in great detail. Wang et al. investigated TDA’s degradation pathway using a biodegradable polyester urea-urethane [43]. In that study, the degradation of TDA from TDI-derived polyester urea-urethanes was investigated using a radioactive 14C label to enhance the TDA degradation pathway. The degradation products formed were separated using high performance liquid chromatography (HPLC) and structurally characterized by MS/MS fragmentation experiments [43]. In another study, TDA was determined to originate from unpolymerized TDI, or in the presence of water [44]. When moisture was present, TDI was found to convert back into TDA.
2.4. Carbodiimides and Polymeric MDI
During PU production, the formation of side products from isomeric MDI and pMDI can be of great hindrance to a polymeric material. These side products can occur at any step when making PUs and lead to viscosity buildup or the formation of solids which can alter the manufacturing process [3]. One modification to making MDI is the condensation reaction between isocyanate groups, which forms carbodiimides (CDI) and reversible uretonimines. The formation of CDI and uretonimines effectively breaks up the crystallinity and reduces the polymer viscosity. Using CDI-MDI materials is of increasing interest industrially, due to its ability to form into soft high resilient elastomers [45]. In a recent study, the formation of CDI-TDI side products was characterized using MALDI TOF/TOF and CID experiments [46]. To prepare the MALDI sample, a non-conventional evaporation-grinding method was utilized. The side products observed in CDI-TDI sample were identified as urethane links, branched species, uretone imine branched CDIs, and urea allophanates. In another study, evaporation-grinding was also used to prepare a pMDI sample for MALDI [47]. In this study, side products were determined to form in the presence of catalysts, iron (II), and iron (III) chlorides. In the mass spectra, small quantities of CDIs were observed which indicated the presence of viscosity buildup. This results in catalyzed side reactions between iron catalysts and CDIs causing extensive branching to occur within the sample.
2.5. Hard Segment Reaction Monitoring
Reaction monitoring between polyols and isocyanates are important to understand polymerization reaction rates. There have been several studies which have monitored the reactivity of isocyanates with primary and secondary polyol hydroxyl groups. For example, studies discern that secondary hydroxyl groups have a lower reactivity compared to primary hydroxyl groups [48–50]. In other studies, trifunctional polyol reactivity was studied, which found that hydroxyl reactivity decreases as the degree of substitution increases along the polyol soft segment [51,52]. In a study by He et al., the step growth polymerization reaction between TDI and trifunctional trimethylolpropane (TMP) was monitored at various temperatures using ESI-MS [53]. They determined the optimal -NCO/-OH reaction ratios, temperature conditions, and stoichiometry of each isocyanate/polyol component within the TDI-TMP product. The steric hindrance of TMP’s chemical structure was observed to reduce side reactions during the step growth process, which yielded a more desirable urethane product. Ahn et al. investigated two different PU polyaddition reactions: TDI and water [54], and TDI and ethylene glycol (EG) [55], using MALDI TOF-MS. The quantitative changes in the mass composition of each polymer during the polymerization reaction were determined. Uncatalyzed reaction monitoring between 2,4’-TDI and 4,4’-MDI with 1-butanol, 1,4-butanediol (BD), and diethylene glycol monomethylether was later performed by Nagy et al. using an HPLC-ESI-MS method [56]. Nagy monitored the urethane reaction rate at different temperatures, to determine the rate constants for each alcohol and diisocyanate. First-order rate constants were determined between the polyol-MDI reaction, (polypropylene glycol (PPG), polytetrahydrofuran (pTHF), poly(3-caprolactone)-diol (PCLD), and polypropylene glycol glycerol triether (PPG-GL)) [57]. Beldi et al. later monitored the changes within a polymer’s topology, the formation of linear and cyclic PU species using MALDI-MS [58]. Krol and Pilch-Pitera used computational simulations with MS strategies to determine the step-by-step polyaddition reaction between 2,4- and 2,6-TDI with BD, polyethers, and polyesters [59]. Computational modeling helped to interpret proposed structures and provided an understanding of reaction compositions formed during different polymerization stages.
3. POLYURETHANE SOFT SEGMENT CHARACTERIZATION
3.1. Polyols
There are two main types of polyurethane polyols: polyethers and polyesters. Both classes of polyols have elastic properties which relate to their unique range of industrial importance. Polyols used for PU synthesis typically consist of two or more -OH groups [1]. Polyether urethanes are considered to be a harder urethane, having superior dynamic properties. Polyester urethanes are typically softer and known for their tensile strength. Types of polyether polyols used in PU production include polypropylene oxide (PPO, also called polypropylene glycol, PPG), polytetramethylene oxide (PTMO, also known as polytetrahydrofurane, pTHF), and polyethylene glycol (PEG, also known as polyethylene oxide, PEO) [3]. Types of polyester polyols can be made from adipic acid and EGs (polyethylene adipate), or from butanediols and adipic acid known as polybutylene adipate (PBA). Additionally, copolyesters are often prepared from a mixture of glycols, adipic acid, and anhydrides [60].
3.2. Polyethers
There are many studies which have characterized polyethers using MS-based techniques [61], however a few studies will be highlighted in this section. Chattopadhyay et al. structurally investigated PPG and IPDI based PU prepolymers using MALDI-MS [62]. In this study, they monitored the reaction between PPG and IPDI at different time intervals to investigate mono-urethane transition into the di-urethane species. In another study led by Mehl et al., the degradation reaction between PU polyether (pTHF) and polyester (PBA) were also monitored by MALDI-MS [5]. To selectively degrade pTHF they used ethanolamine, and to degrade PBA they used phenyl isocyanate due to the high reactivity of the ester. In this study, SEC was coupled to MALDI to enhance accurate MW determination of each degraded ether-urethane and ester-urethane species.
IM is an emerging tool for separation and identification of synthetic polymers (linear, cyclic, starshaped, etc.). Recently, IM-MS has been used to investigate polydisperse samples, allowing for the identification and characterization of unique CCS trends, charge-dependent conformations, and different polymer topology [63,64]. IM-MS has been used to characterize the experimental and theoretical conformation of multicharged linear-chain PEGs [65–68], polylactic acid (PLA) [69], polyethylene terephthalate (PET) [70], PPG [71], PEO [72], and polycaprolactone (PCL) [72,73]. Sodium cationized PLA was investigated using IM-MS to experimentally determine the three-dimensional structure of the multiply charged PLA adducts. Experimental and theoretical observations were recorded to investigate how the polymer size and number of charged adducts effect the folding of the PLA through cation coordination [69]. Duez et al. used PLA and PEG polymers as reference calibrants for a traveling wave IM-MS. These polyethers were found to be good calibrants due to the polymer ions covering a large mass range and a large CCS window [74]. Previous studies have shown low-energy CID of PEG ionized with alkali metal cations to yield small fragment ions originating from hydrogen-rearrangement reactions. At high CID energies, 1,4-H2 elimination can be observed, producing two types of unsaturated fragment ions along with a homolytic cleavage reaction at both ends of the polymer [75]. Hilton et al. used IM-MS and IM-MS/MS to separate and differentiate between polyether oligomers with the same nominal molecular weights [76]. For instance, isobaric mixtures of PEGs with the same nominal m/z ratio were structurally characterized and separated using IM-MS/MS. Larriba et al., characterized the gas-phase structure of coulombically stretched PEG ions (Figure 5) [67]. Higher charge state PEG ions were observed to form a “beads on a string” motif. In this study, structural transitions for the ionized singly charged PEG species were interpreted through molecular dynamic simulations. Cody and Fouquet, in a recent study, used a paper spray ionization MALDI/TOF system to characterize 13 block and 2 random ethylene oxide and PPO copolymers and homopolymers using Kendrick mass defect analysis [77]. The Kendrick mass defect plots were used to estimate the percentage of the ethylene oxide and PPO in the copolymer materials. Girod et al. used MS/MS to characterize doubly charged PEO oligomers formed using ESI [78]. Similarly, Tintaru et al. experimentally and theoretically studied PEO-poly(amidoamine) polymer using H+ and Li+ cations [79]. IM-MS and MS/MS were employed to elucidate unique charge state trends (+2 and +4) of the adducted species. The experimental findings were coupled to molecular dynamics simulations to investigate conformational changes within different charge states. In other PEG studies, low energy CID pathways were probed using an assortment of monovalent cations (Li, Na, K, Rb, Cs) [80–82] and transition metal cations (Ag) [83]. In addition to gas-phase PEG folding, Ude et al. studied charge-induced unfolding of PEG species [66]. Kokudo et al. performed IM-MS on PEGs as model polymers to obtain the dielectric constant of their doubly charged species. Proposed mechanistic pathways were used to determine the fragmentation reactions of radical cationic species [84].
Figure 5.

Representation of transitional region of sphere equivalent diameters based on Z/z and m, respectively. MD simulations comparable to experimental values are included for several lengths of z = 4 ions. Not all the data points correspond to exact oligomer masses. Charge state trends and proposed structural molecular dynamic simulations are represented (A-E). Reprinted (adapted) with permission from C. Larriba, J. Fernandez De La Mora, The gas phase structure of coulombically stretched polyethylene glycol ions, J. Phys. Chem. B. 116 (2011) 593–598. Copyright 2012 American Chemical Society.
Over the years, there have been environmental concerns with producing polyethers for PU synthesis from petroleum sources. To address these concerns, researchers have investigated bio-renewable feedstocks to be used in place of petroleum based polyols. Li et al. performed LC-ESI-MS and MS/MS fragmentation experiments to detail an approach to the characterization of novel bio renewable polyols [85].
3.3. Polyesters
Polyesters, known as polycondensation polymers, can be used for a variety of applications, including fabrics, food-grade containers and packaging, electrically insulating films, and wood finishes. In a study by Williams et al., MALDI-MS was used to characterize a series of aliphatic polyesters and their derivatives [86]. The oligomeric peaks observed in the MS allowed for determination of each polymer repeat unit and the presence of cyclic species. Both the Mn and Mw values were determined by MS, and compared to consecutive NMR and GPC findings. GPC MW estimations were found to be larger than those determined by MALDI. In another study by Williams et al., MALDI-MS was used to study discrete mass polybutylene glutarate (PBG) oligomers having 8, 16, 32, and 64 degrees of polymerization [87]. These PBG derivatives were used to optimize MALDI and GPC analysis of PU-PBA species due to structural similarities between PBG and PBA. In this study, they investigated the effect of the MALDI matrix, laser intensity, and detector saturation. Rizzarelli et al. later probed the fragmentation pathways of four PBA isomers at relatively high collision energies using MALDI-TOF/TOF CID [88]. High collision energies were found to be less useful than low collision energies for studying native chemical alterations. Gies et al. later demonstrated a method for characterizing PBA species at low fragmentation energies using MALDI-TOF/TOF CID [89]. They were able to identify PBA fragment ions and unexpected side products from a complex mixture of melt polymerized PBA. Low energy fragmentation pathways were interpreted by computational models to verify the structure of the fragment ions. PBA oligomers were observed to undergo a number of low energy degradation pathways such as 1,5 H-shift (preferred), 1,3 H-shift, remote hydrogen abstraction, and multiple combinations of these reactions.
To study polyester reaction kinetics, Pretorius et al. developed a model system to study the reaction between phthalic acid and PEG based polyesters [90]. They monitored the reaction under different conditions to investigate the stages of polyesterification, MW, chemical composition, and end group analysis of these synthesized species using HPLC separations coupled to MALDI-TOFMS analysis. In related studies, Pretorius et al. investigated the same phthalic acid and PEG based polyesters using a combination of SEC, supercritical fluid chromatography (SFC), ESI-MS [91] and MALDI-MS [92]. The polyester samples in these studies were fractionated by SEC, analyzed by SFC (to determine degree of polymerization), then characterized by MS to determine the presence of linear or cyclic species. For a polyvinyl acetate (PVA) system, Gigurere and Mayer modeled the fragmentation pathways of ionized PVA using ESI-MS and MS/MS techniques to determine mechanistic dissociation reactions that could occur when PVA is used for PU adhesives or sealants in the textile industry [93].
Polyester polyols are typically formed from diacids and glycols. Usually polyester polyols are more viscous, as well as more expensive, compared to polyether polyols. Biodegradable and biocompatible urethane-macromolecules are a growing field even within PU polyester production. The biodegradation of PLA urethane copolymers are investigated to determine the rate of biodegradation as PLA content increases [94]. Borda et al. explored novel methods towards synthesizing biodegradable thermoplastic multiblock copolymers and characterizing this copolymer system using MALDI-TOFMS. In one study, linear-chain PUs were synthesized using PLA and 4,4’-MDI and TDI [95]. In another study, Borda et al. synthesized PLA, poly(ε-caprolactone (PCL), and PCL-PLA copolymers using TDI as chain extender and PEG as the intrinsic plasticizer [96]. They determined the chemical structure of each biodegradable PU polymer and the relative Mn using MALDI-TOFMS. In a study by Tang, telechelic hydroxyl-terminated polyesters were synthesized using biodegradable monomers in the MW range of 700 to 1,300 Da [97]. Other biodegradable PU routes have been explored by Baez et al. [98], where the reaction between PCL diols was monitored using MALDI-MS to determine formation of the ring opening ester-urethanes-ureas. Osaka et al. showed a detailed ESI and MALDI method for characterizing linear and cyclic PLAs and their solvolysis products [99]. Polyester urea-urethanes were synthesized with 14C labeled TDI and 14C labeled ethylene diamine chain extender to study cholesterol esterase [43]. The PU degradation products were characterized to determine the presence of unreacted TDI.
4. POLYURETHANE MATERIAL CHARACTERIZATION
4.1. Diisocyanate and Polyol Characterization
PU complexity arises from the addition reaction between isomeric diisocyanates and heterogeneous mixtures of varying polyol lengths. These synthetic complications lead to the formation of random, alternating, block, and graft copolymers. PU properties are greatly influenced by their structure, monomer functionality, hydroxyl group reactivity, polymerization temperature, and -NCO/-OH reaction ratio. Addressing PU complexity requires advanced analytical techniques such as MS to characterize the complexity of PU intact materials [100,101].
The reaction between diisocyanates and polyols (polyesters and polyethers) is a key process for making various PU products. Nagy et al. used HPLC-MS to determine reaction kinetics between diisocyanates (MDI and 2,4-TDI) and polyols: 1-butanol, 1,4-butanediol (BD), and diethylene glycol monomethylether (DEGME) [56]. These results indicated that the first isocyanate group on MDI reacted 1.5 times faster with the polyols compared to the second isocyanate, however the para isocyanate on 2,4-TDI was determined to react with the polyols faster than the first isocyanate on MDI. Both MDI and 2,4-TDI isocyanates reacted in a similar manner. Ferrerira et al. also used HPLC-UV and HPLC-ESI-MS/MS techniques to extensively characterize free monomeric MDI found in PU foams [102]. Ferrerira outlined the use of a new isocyanate derivatizing agent, N-benzylmethylamine (NBMA), which enhanced monomeric MDI solubility in the solvents routinely used for HPLC.
Diisocyanates and polyols are also known to react in the presence or absence of a catalyst; optimal reaction conditions have been previously studied [103–105]. However, Nagy et al. extensively studied the reaction between MDI and several polyols: pTHF, PCLD and PPG glycerol triether (PPG_GL) to determine intermediates, reaction products, and the reactivity of the polyols hydroxyl groups [57]. When, monitoring MDI and PPG_GL reaction using MALDI-MS, the formation of Bn, Cn, and Dn series appear, as shown in Figure 6. Series An represents the start of the reaction and series Dn represents the distribution at the end point of the reaction. As the reaction progresses, the appearance of additional series labelled Bn and Cn are noted, which correspond to mass shifts of 282 Da, indicating the addition of MDI to the PPG_GL hydroxyl groups. In this study, rate constants of the forming intermediates and final products were determined. MALDI-MS was also useful for monitoring the formation of cyclics and other side products formed during the reaction [58]. In a study led by Gies et al., a combination of MALDI, CID, and IM-MS experiments was used to study a polyester-based PU (Mn ~ 13,000 by GPC) prepared by melt polymerization; reactants were PBA and MDI, with BD as an extender [106]. Fragmentation studies were performed at relatively low collision energies to model pyrolysis reactions. Bond energy calculations were used to help to elucidate possible fragmentation pathways. The major species observed by MALDI were cyclic polyesters and PUs, linear polyesters (diol and acid terminated), and linear polyurethanes up to n ~ 20. PUs containing up to 10 hard blocks were observed. CID for linear PUs identified two major fragmentation pathways, 1,3 and 1,5 H-shift, the latter involving carbonyls both in the urethane group and in the polyester chain. Fragmentation of cyclics is a two-step process: (1) initial ring opening and (2) subsequent fragmentation. Both steps primarily involve 1,3 and 1,5 H-shift. IM-MS studies were performed to show the utility of this technique when characterizing complex polymeric mixtures. For example, it was shown that PU hard blocks capable of forming H-bonding were found to have a shorter drift times than hard blocks not capable of H-bonding [106].
Figure 6.

MALDI-TOF MS spectra of the quenched reaction mixture obtained in the reaction of PPG_GL with MDI at 1, 600 and 1560 minutes. Experimental conditions: [MDI]o = 0.32 M, [PPG_GL]o = 0.01 M and T = 80 °C. By monitoring the MDI and PPG_GL reaction using MALDI-MS, the formation of Bn, Cn, and Dn series appeared, series An represents the start of the reaction. Image adapted. Reproducted from T. Nagy, B. Antal, A. Dekany-Adamoczfy, J. Karger-Kocsis, M. Zsuga, S. Kéki, Uncatalyzed reactions of 4,4 ‘ - diphenylmethane-diisocyanate with polymer polyols as revealed by matrix-assisted laser desorption/ionization mass spectrometry, RSC Adv. With permission from the Royal Society of Chemistry.
4.2.1. Flexible and Rigid Foams: Biodegradable Importance
PU foams are classified as flexible or rigid foams depending on their flexibility and density. PU foams are complex engineered materials which can have customizable temperature or humidity control and visco-elastic behavior. The flexibility, morphology, and microstructure of PU foams are based on the degree of cross-linking and the -NCO/-OH ratio [3,107]. Flexible PU foams are known for their application as cushion materials, and rigid foams are known for their thermal stability and flame resistant properties. These foams are commonly made with PPO, however renewable alternatives for petrochemical polymer materials is a growing field [108]. Bio-based polyols, such as natural oils chemically modified to contain hydroxyl groups, are a new technique for integrating bio-based polyols in PU foam materials [109]. For example, Basso et al. studied renewable polyol alternatives in flexible-elastic copolymerized PU tannin foams using MALDI-MS [110]. The fatty amines were reacted with pMDI and tannins to make PU foams with highly flexible and elastic properties. MS based techniques were used to characterize the chemical and sequential arrangement of the amines, isocyanates, and tannins integrated within the PU foam matrix. Another method for monitoring the coreaction between amines, isocyanates, and tannins was to monitor the MWav Gaussian distributions of the coreacted species obtained by MALDI. The Gaussian distributions help to determine which coreaction and reaction conditions are preferred (amine-pMDI or tannin-pMDI) in forming the urethane bridge and assisted in detecting unreacted starting material [111].
4.2.2. Flexible Foams
When characterizing intact flexible foams, selective extraction processes are needed to investigate the chemical composition. During foaming, hard segments undergo chain-growth, whereas in hydrolysis a step-growth process occurs which contributes to the formation of cyclic species [107]. MALDI-MS is a common technique for PU foam reaction monitoring, hard segment distribution characterization, and detection of cyclic or hydrolysis products. In one study, the hydrolysis products of viscoelastic and conventional flexible foams were characterized using MALDI-MS [112]. MALDI characterization revealed the presence of cyclic species, hard segment chains with urea repeat units, and PPO based polyols [113]. The experimental hard segment length distributions were compared to theoretical Monte Carlo simulations. The simulations in this study agreed with the number-average degree of polymerization experimentally [112]. In a study by Yontz et al., MALDI-MS was also used to investigate water-blown PU foams by monitoring the addition of water in the poly(urea-urethane) formulation [114]. The water-blown PU foams were prepared with different hard segment lengths and at various temperatures. By using MS, they were able to determine the unique features related to each foams associated sample preparation method, including the number of hard segment repeat units and the formation of side products.
Chromatographic techniques integrated with MS, such as LC-MS, can increase the level of dimensional analysis for the characterization of aromatic amines (TDA/MDA). Marand was among the first to develop an LC-MS method for foam hydrolysis extracts containing TDI/TDA compounds and other oligomeric species [113]. Marand tested extraction solvents to observe hydrolysis products and found that organic solutions altered the physical properties of the foams and alcohol solutions caused free isocyanates to react and form urethane side products. Mild acid solutions were found to stabilize extracted aromatic amines from intact flexible foams [113]. More recently, Johnson et al. compared Marand’s LC-MS method to their hydrophilic interaction liquid chromatography (HILIC-MS) and MS/MS technique. In the Johnson study, the investigators focused on developing a separation method for HILIC-MS to distinguish TDA and MDA aromatic amines found in foam extracts. It was found that the free diamines required a derivatizing agent to prevent oxidation, and both HILIC-MS/MS and Marands LC-MS methods showed agreement for free amines detection in PU foams [6].
4.2.3. Rigid Foams
Rigid PU foams have not been extensively characterized using MS-based techniques. As such, there are only a few studies characterizing bio-based polyols that can be used to make rigid PU foams. In a recent study, MALDI-MS was used to monitor the cross linking between highly functional polyols that underwent epoxy ring opening reactions with glycerols to make a bio-based polyester [109]. In related work, MALDI-MS was used to characterize unique resins derived from natural products (polyflavonoid tannins-furfuryl alcohols). The urethane formation between flavonoid monomers, glyoxals, and isocyanate was monitored to determine the functionality of this PU natural product starting material [111].
4.3. Thermoplastic Polyurethanes
Thermoplastic PUs (TPUs) have numerous applications due to their diverse physical properties and processability. TPUs are known to be flexible, elastic, have resistance to impact, abrasion, weather, and become melt-processable. TPU materials are suitable for applications in the automotive, clothing, sports equipment, furniture, biomedical, and construction industries [115]. Conventionally, TPUs are synthesized from polymeric isocyanates and polyols with or without chain extenders. Common polyols used in TPUs include polyesters and polyethers; however specialty polyols such as polycarbonate, polysiloxane, and polyolefin diols can also be used [116]. Recently, non-conventional PUs such as non-isocyanate polyurethanes (NIPUs) have been developed. This novel type of PU can be prepared by ring opening reactions between bis-cyclic carbonates and diamines which enable the replacement of hazardous phosgene and isocyanates which are used in the conventional PU synthesis [117,118]. NIPUs can also be made through a fully bio- and CO2-sourced synthesis. In a recent study, Poussard monitored the formation of NIPUs under supercritical conditions using ESI-MS [119]. In another study, biodegradable TPU synthesis was monitored using MALDI-MS between TDI, PEG, and polylactic acid caprolactone to make a type of TPU multiblock polymer [96]. Oleochemicals are a type of vegetable oil that can be used as a TPU building block. To model the use of oleochemical, oleic acid and undecyclenic acid derivatives were used to synthesize PUs through a polycondensation reaction. The formation of linear TPUs was monitored using MS-based techniques and further characterized to test for thermal stability [120]. Due to environmental concerns, TPU chemical interaction with water is of interest. To study the water diffusion rate in TPUs, LC-MS was used to monitor water diffusion, and MS strategies can help elucidate structural cross-linking within the polymer back-bone [121].
Due to the increasing application for TPU materials, studying decomposition reactions with respect to time and temperature can enhance synthetic strategies, allowing for tailored polymeric design to match desired degradation patterns. One method used to study decomposition reactions and reaction products is pyrolysis [3]. Pyrolyzate products are formed during the decomposition process and can help to determine decomposition mechanisms and covalent/noncovalent chemical bond arrangement throughout a material. Lattimer was among the first to study TPU pyrolysis using MALDI-MS, to determine the structural properties pertaining to TPU and the pyrolysis conditions. Pyrolysis MS (Py-MS) is a method which heats PUs in a solid probe [122]. This method allows for slow pyrolysis to be monitored with respect to time or temperature, forming various pyrolyzate products. Py-MS has shown that segmented TPUs decompose at different stages. Ravey and Pearce previously studied the decomposition of TDI and complex polyether (glycerol, PPO, and polypropylene ethylene oxide) based PU foams, to better understand decomposition reactions [123]. Later, Lattimer et al. characterized segmented PU pyrolyzate products (MDI, BD, and PBA) using MALDI-MS. In their MALDI studies, higher mass pyrolyzate products were detected (800 to 10,000 Da species), elucidating pyrolysis mechanisms [124]. More generally, MALDI-MS indicates that the degradation products followed two primary pathways: (1) dissociation of the urethane linkage to form isocyanato and hydroxyl end groups and (2) an ester exchange where cyclic pyrolyzate oligomers were produced [122,124,125]. In 2002, Lattimer further characterized PEG pyrolysis products at different temperatures using MALDI-MS. At low temperatures, pyrolyzate products were detected to undergo C-O bond cleavage, forming hydroxyl and ethyl ether end groups. When temperatures were increased, methyl ether and vinyl ether end groups became more abundant [126]. They also studied thermal decomposition of pTHF pyrolyzates at low temperatures [127]. Although MALDI-MS has been widely used to analyze pyrolyzate products from PPG, PEG, pTHF, PBA, and bisphenol A (BPA) polycarbonate, the disadvantage of this technique lies within the insolubility of some pyrolyzates which increase characterization complications [126,128].
4.4. Thermoset Polyurethanes
Thermoset PUs are materials that undergo a curing chemical reaction and transform from a liquid to a solid. During the reaction, the PU forms a cross-linked network causing the material to solidify, which is irreversible. For example, shape memory foam is a type of thermoset PU that is manufactured for its desirable physical properties: amorphous, high crosslinking, and ultra-low density. Weems et al. performed an in-depth study on the degradation of porous shape memory foams. LC-MS techniques were used for absolute quantification and determination of degradation rate in biological materials [129]. In another study, Dopico-Garcia aimed to develop a robust HPLC-UV-MS method for separation of curing agent polyamines (isophorone diamine, IPDA; TCD-diamine; and triethylenetetramine, TETA). The chromatographic separations were coupled to UV detection of the aromatic compounds, and MS strategies aided in the determination of the exact chemical structures of the curing agents [130].
4.5. Polyurethane Coatings
PU coatings have many consumer uses such as: inner surface coatings for metallic food or beverage containers, steel hydraulic structures to prevent corrosion, and automotive clear coats, primers, and sealers [3,131]. The most common coatings are epoxy-resins which are based on bisphenol A (BPA). Due to safety concerns related to BPA exposure, alternative coatings have been sought [132]. Polyester and thermoset polyester-polyurethane (PEPU) coatings are an alternative to epoxy-based resins for metallic food containers. In PEPU coatings, polyisocyanates are used to facilitate the cross-linking of polymer networks. The high reactivity of the isocyanate moiety results in urethane linkages upon curing. Therefore the threedimensional polymer network can reduce small molecule migration. Many studies have explored a variety of analytical MS-based techniques (ESI-MS, LC-MS, and MS/MS) to monitor small molecule migration in PU coated food containers. Driffield et al. released an LC-MS/MS method for testing residual IPDI trimers in experimental formulations of thermoset PEPU coatings in food containers. This method involved extraction of IPDI trimers from the coated panels, and derivatization of the extracted IPDI trimer with dibutylamine (DBA) [133]. The curing kinetics of the PEPU coating and unreactive analytes were monitored using chromatography based MS techniques. Bradley et al. identified small molecule contaminants related to starting materials used in the formulation of coatings, reaction byproducts, and degradation products resulting from prolonged storage of the can. They used a variety of analytical techniques to detect volatile (GC-MS) and non-volatile substances (LC-MS) [134,135].
Recently, Omer et al. developed a method to predict non-intentionally added substances (NIAS) migrating from PEPU coatings [136]. They constructed a database which predicted a combination of known monomers based on their exact monoisotopic masses. They used a global untargeted approach, coupling LC to a high resolution MS (LC-HRMS) to elucidate lacquer extract fingerprints. Both positive and negative ionization modes were tested where more intense signals related to NIAS were found in the positive mode compared to the negative mode, and the findings were consistent with other research groups [135,137,138]. Omer’s confidence in peak identification was based on fragmentation patterns and chromatographic behavior of the lacquer samples tested [136].
4.6. Polyurethane Adhesives
PU adhesives are known for their high performance and range of applications. PU adhesives have been characterized using MS-based techniques, for example, adhesive tape for immobilized inorganic materials [139] and wood adhesives for engineered wood products such as plywood and particle board [140]. PU adhesives are also used for preparing multilayered laminates to coat the inner lining of food containers. When the adhesive is not properly cured, the polymerization reaction can yield unpolymerized aromatic isocyanates, causing primary aromatic amines (PAA) when reacted with water. There are several methods to isolate and chemically analyze the degradation products of PAAs from PU adhesives, for example TDA isomers can undergo derivatization with pentafluoropropionic anhydride (PFPA) prior to LC-MS characterization [140]. Marand et al. were among the first to implement this method of derivatization, showing the sensitivity for aromatic amines increased in TDI-based PU foams when derivatized prior to LC-MS analysis [113].
Adhesives are used to bond films together to form plastic laminates useful for manufacturing food storage products. Lawson et al. investigated the chemical migrants from a range of PU adhesives using MALDI-MS [141]. Due to rising concern for PAAs in manufactured plastic laminates; these PU adhesives were tested to determine if the adhesive was fully cured prior to use. Mortensen et al. developed an LC-MS/MS method to determine traces of 20 PAAs associated with PU adhesives [142]. In another study, Aznar et al. quantitatively determined 22 PAAs using cation-exchange solid-phase extraction and LC-MS methods for characterization [143]. Due to the relatively low detection limit of LC-MS, this method was advantageous for PAA detection in PU materials. Pezo et al. used a Q-TOFMS method for the identification and pattern recognition of PAAs in PU food packaging [144]. In a recent study, a non-targeted MS approach was used for the characterization of unexpected chemicals and NIAS found in food packaging materials [145]. They identified 26 potential migrants from the two packaging materials studied. Cyclic ester oligomers were found to migrate from the multilayer high barrier food contact material. Zhang et al. later completed an extensive study, performing a migration test on 537 commercial and/or developmental laminate samples [146]. MS techniques such as electron ionization (EI-MS), chemical ionization (CI-MS), and LC-MS were used to identify 56 short-chain cyclic oligoesters at both high and low concentration levels within the packaging material.
4.7. Encoded Polyurethanes
A new generation of high precision polymers is promising for a wide range of applications due to the synthetic ability to control radical polymerizations, stepwise syntheses, molecular structure and morphology. Development of polymeric material containing discrete encoded information and then recovering information by an MS sequencing technique is a growing field of research [147–149]. Applying the encoding technique to PU materials will likely be increasingly utilized for the identification of counterfeit goods. In a recent study by Gunay et al., uniform sequence-coded PUs were synthesized by a chemoselective multistep-growth process and sequenced using negative-mode MS/MS. The sequence-coded PUs are easily sequenced by MS due to their carboxylic acid deprotonation and predictable C-O carbamate fragmentation pathway [150]. In a complementary study, sequence-coded PUs, that contain digitally encoded oligourethanes with two monomers defined by arbitrarily binary units, 0 and 1 bits, can be used as molecular barcodes and blended in low amounts with other polymeric materials. These sequence-coded PUs were tested as anticounterfeiting tags for the labeling of methacrylate-based intraocular implants and were determined to be viable coding and labeling methods due to the ease of MS/MS identification by sequence decoding [151].
5. OTHER MS-BASED TECHNIQUES FOR PU CHARACTERIZATION
PUs are produced through a wide range of diisocyanates and polyols to make flexible and rigid foams, TPUs, thermosets, coatings, adhesives, sealants, elastomers, and many commercial products. However, in this review mainly soft ionization sources such as ESI and MALDI have been reviewed. There are many useful MS ionization sources that are appropriate in PU characterization. Ion sources specific for liquid sample introduction include atmospheric pressure chemical ionization (APCI), atmospheric pressure photoionization (APPI), desorption electrospray ionization (DESI), and electrosonic spray ionization (ESSI) [152–158]. For solid analysis, atmospheric solid analysis probe (ASAP) can be used, and for volatile samples GC is useful. A few studies have used alternative ionization sources to characterize PU samples. DESI and ESSI have previously been demonstrated to provide accurate average MW and MWD of industrial polymers, both as solids and in solution [156]. In a study by Bonnaire et al., PU films were analyzed on two instrumentation platforms: DESI-MS and ESI-MS. DESI-MS was found to generate mass spectral profiles of irradiated PU films with no sample preparation. When compared to conventional ESI-MS methods, the same species were observed, validating DESI-MS as a technique for PU film characterization [154]. Lebeau et al. used ASAP-MS applications for the characterization of PU copolymer material. ASAP-MS was found to require no sample pretreatment. ASAP-MS was able to differentiate between chemical structures within the PU, polyester and polyether monomers. ASAP-MS was easy for direct analysis of crude polymer samples [155].
6. CONCLUDING REMARKS
PUs are versatile materials contributing to a wide variety of societal and consumer applications. In this study, MS-based techniques used to characterize PU hard and soft segments, precursors, and intact PU materials have been extensively reviewed. The literature reviewed highlights a variety of techniques including soft ionization sources (ESI and MALDI), IM-MS, MS/MS, and computational methods for data interpretation. Additionally, this review highlights areas of PU research that are in need of further MS-based characterization studies.
Characterizing PU’s, and more generally, synthetic polymers using MS techniques provides several analytical advantages such as end-group analysis, hard and soft segment characterization with the polymer backbone, detection of impurities, and a route to monitor decomposition mechanisms. Similarly, the limitations for PU characterization by MS include the difficulty in ionizing and measuring high mass polymers using MS (ca. >10,000 Da), polymer solubility and compatibility with common ionization techniques such as MALDI and ESI, and challenges associated with distinguishing isomeric polymer structures. In regard to the latter, the addition of front-end chromatography (e.g., LC and GC), IM and tandem MS/MS measurements with MS provide additional capabilities for improving the structural elucidation and characterization of isomeric polymers. These new and updated MS capabilities will provide opportunities for fundamental MS investigation of PU-related systems as well as other polymeric species, for example, polyureas and uretidones. As novel solid-state MS sampling methods such as DESI continue to develop, the ability to make direct MS measurements on polymer films should further enable more comprehensive characterization of PUs across a wide variety of samples and applications.
Highlights:
Combination of MS-based techniques used to characterize PUs and their precursors.
Characterization techniques such as ESI, MALDI, IM-MS, and computational methods to interpret the data.
7. ACKNOWLEDGEMENTS
This work was supported in part by the resources of the Center for Innovative Technology (CIT) at Vanderbilt University. Financial support for this research was provided by the National Institutes of Health (NIH NIGMS R01GM092218 and NIH NCI R03CA222452).
Biographies

Tiffany M. Crescentini Ph.D., Candidate at Vanderbilt University. She completed her B.S. Chemistry degree at Waynesburg University in Waynesburg, PA. Her current works focus on utilizing a variety of mass spectrometry based techniques for the detailed characterization of polyurethane hard segments, soft segments and intact polymers. Her research also implements the utility of a stepwise synthetic approach to generate pure monodiscrete oligomers to be used as analytical standards within the polymer community.

David M. Hercules, Centennial Professor of Chemistry Emeritus and Research Professor at Vanderbilt University, he completed his Ph.D. at Massachusetts Institute of Technology. His research interest focuses on developing mass spectrometry based techniques such as electrospray, MALDI, and ion mobility-mass spectrometry for the characterization of intact polyurethanes and their precursors. He also seeks to develop synthetic methods to generate sequence defined polyester, polyether, and polyurethane based short chain oligomers.

Jody C. May, Associate Director of the Center for Innovative Technology and Research Assistant Professor, completed his B.S. degree in Chemistry at the University of Central Arkansas and his Ph.D. in analytical chemistry from Texas A&M University. His research interests focus on the development of next generation ion mobility-mass spectrometry instrumentation and allied developments.

John A. McLean, Director of the Center for Innovative Technology and Stevenson Professor of Chemistry, completed his B.S. Chemistry degree at the University of Michigan, his Ph.D. at the George Washington University, and completed postdoctoral training at Texas A&M University prior to joining the faculty at Vanderbilt University. His research focuses on the intersection of instrumentation and bioinformatics in support of systems, synthetic, and chemical biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- [1].Szycher M, Szycher’s handbook of polyurethanes, CRC press, 1999. [Google Scholar]
- [2].Bayer O, Das di-isocyanat-poluadditionsverfahren (polyurethane), Angew. Chemie. 59 (1947) 257–272. doi: 10.1002/ange.19470590901. [DOI] [Google Scholar]
- [3].Oertel G, Polyurethane handbook, Carl Hanser Verlag, 1985. [Google Scholar]
- [4].Hepburn C, Polyurethane elastomers, Springer Science & Business Media, 2012. [Google Scholar]
- [5].Mehl JT, Murgasova R, Dong X, Hercules DM, Nefzger H, Characterization of polyether and polyester polyurethane soft blocks using MALDI mass spectrometry, Anal. Chem 72 (2000) 2490–2498. doi: 10.1021/ac991283k. [DOI] [PubMed] [Google Scholar]
- [6].Johnson JR, Karlsson D, Dalene M, Skarping G, Determination of aromatic amines in aqueous extracts of polyurethane foam using hydrophilic interaction liquid chromatography and mass spectrometry, Anal. Chim. Acta. 678 (2010) 117–123. doi: 10.1016/j.aca.2010.08.020. [DOI] [PubMed] [Google Scholar]
- [7].Chattopadhyay DK, Webster DC, Thermal stability and flame retardancy of polyurethanes, Prog. Polym. Sci 34 (2009) 1068–1133. doi: 10.1016/j.progpolymsci.2009.06.002. [DOI] [Google Scholar]
- [8].McLean JA, Ruotolo BT, Gillig KJ, Russell DH, Ion mobility-mass spectrometry: A new paradigm for proteomics, Int. J. Mass Spectrom 240 (2005) 301–315. doi: 10.1016/j.ijms.2004.10.003. [DOI] [Google Scholar]
- [9].Fenn LS, Kliman M, Mahsut A, Zhao SR, McLean JA, Characterizing ion mobility-mass spectrometry conformation space for the analysis of complex biological samples, Anal. Bioanal. Chem 394 (2009) 235–244. doi: 10.1007/s00216-009-2666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Altuntaş E. Schubert US, “Polymeromics”: Mass spectrometry based strategies in polymer science toward complete sequencing approaches: A review, Anal. Chim. Acta. 808 (2014) 56–69. doi: 10.1016/j.aca.2013.10.027. [DOI] [PubMed] [Google Scholar]
- [11].Montaudo G, Robert P Lattimer, Mass spectrometry of polymers, CRC press, 2001. [Google Scholar]
- [12].Montaudo G, Samperi F, Montaudo MS, Characterization of synthetic polymers by MALDI-MS, Prog. Polym. Sci 31 (2006) 277–357. doi: 10.1016/j.progpolymsci.2005.12.001. [DOI] [Google Scholar]
- [13].Dey M, Castoro JA, Wilkins CL, Determination of molecular weight distributions of polymers by MALDI-FTMS, Anal. Chem 67 (1995) 1575–1579. doi: 10.1021/ac00105a016. [DOI] [Google Scholar]
- [14].Hart-Smith G, Lammens M, Du Prez FE, Guilhaus M, Bamer-Kowollik C, ATRP poly(acrylate) star formation: A comparative study between MALDI and ESI mass spectrometry, Polymer (Guildf). 50 (2009) 1986–2000. doi: 10.1016/j.polymer.2009.03.009. [DOI] [Google Scholar]
- [15].McLafferty FW, High-resolution tandem FT mass spectrometry above 10 kDa, Ace. Chem. Res 27 (1994) 379–386. doi: 10.1021/ar00047a009. [DOI] [Google Scholar]
- [16].Baumgaertel A, Altuntas E. Schubert US, Recent developments in the detailed characterization of polymers by multidimensional chromatography, J. Chromatogr. A. 1240 (2012) 1–20. doi: 10.1016/j.chroma.2012.03.038. [DOI] [PubMed] [Google Scholar]
- [17].Gruendling T, Guilhaus M, Bamer-Kowollik C, Quantitative LC-MS of polymers: Determining accurate molecular weight distributions by combined size exclusion chromatography and electrospray mass spectrometry with maximum entropy data processing, Anal. Chem 80 (2008) 6915–6927. doi: 10.1021/ac800591j. [DOI] [PubMed] [Google Scholar]
- [18].Hanton SD, Mass spectrometry of polymers and polymer surfaces, Chem. Rev 101 (2001) 527–570. doi: 10.1021/cr9901081. [DOI] [PubMed] [Google Scholar]
- [19].Bamer-Kowollik C, Gruendling T, Falkenhagen J, Weidner S, Mass spectrometry in polymer chemistry, John Wiley & Sons, 2012. [Google Scholar]
- [20].Pasch H, Schrepp W, MALDI-TOF mass spectrometry of synthetic polymers, Springer Science & Business Media, 2013. [Google Scholar]
- [21].May JC, Goodwin CR, Fareau NM, Feaptrot KF, Morris CB, Kurulugama RT, Mordehai A, Klein C, Barry W, Darland E, Ovemey G, Imatani K, Stafford GC, Fjeldsted JC, McFean JA, Conformational ordering of biomolecules in the gas phase: Nitrogen collision cross sections measured on a prototype high resolution drift tube ion mobility-mass spectrometer, Anal. Chem 86 (2014) 2107–2116. doi: 10.1021/ac4038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].May JC, McFean JA, Ion mobility-mass spectrometry: Time-dispersive instrumentation, Anal. Chem 87 (2015) 1422–1436. doi: 10.1021/ac504720m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eiceman GA, Karpas Z, Jr HHH., Ion mobility spectrometry, CRC press, 2013. [Google Scholar]
- [24].Wilkins CF, Trimpin S, Ion mobility spectrometry-mass spectrometry: theory and applications, CRC press, 2010. [Google Scholar]
- [25].Weidner SM, Trimpin S, Mass spectrometry of synthetic polymers, 82 (2010) 4811–4829. doi: 10.1021/ac8006413. [DOI] [PubMed] [Google Scholar]
- [26].Hoskins JN, Trimpin S, Grayson SM, Architectural differentiation of linear and cyclic polymeric isomers by ion mobility spectrometry-mass spectrometry, Macromolecules. 44 (2011) 6915–6918. doi: 10.1021/ma2012046. [DOI] [Google Scholar]
- [27].Montenegro-Burke JR, Bennett JM, McFean JA, Hercules DM, Novel behavior of the chromatographic separation of linear and cyclic polymers, Anal. Bioanal. Chem 408 (2016) 677–681. doi: 10.1097/NCN.0b013e318Ia91b58.Exploring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Trimpin S, Clemmer DE, Ion Mobility Spectrometry / Mass Spectrometry Snapshots for Assessing the Molecular Compositions of Complex Polymeric Systems, Anal. Chem 80 (2008) 9073–9083. [DOI] [PubMed] [Google Scholar]
- [29].Stow SM, Goodwin CR, Kliman M, Bachmann BO, McLean JA, Lybrand TP, Distance geometry protocol to generate conformations of natural products to structurally interpret ion mobility-mass spectrometry collision cross sections, J. Phys. Chem. B. 118 (2014) 13812–13820. doi: 10.1021/jp509398e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wesdemiotis C, Multidimensional mass spectrometry of synthetic polymers and advanced materials, Angew. Chemie Int. Ed 56 (2017) 1452–1464. doi: 10.1002/anie.201607003. [DOI] [PubMed] [Google Scholar]
- [31].Crotty S, Gcrislioglu S. Endres KJ, Wesdemiotis C, Schubert US, Polymer architectures via mass spectrometry and hyphenated techniques: A review, Anal. Chim. Acta. 932 (2016) 1–21. doi: 10.1016/j.aca.2016.05.024. [DOI] [PubMed] [Google Scholar]
- [32].Forsythe JG, Stow SM, Nefzger H, Kwiecien NW, May JC, McLean JA, Hercules DM, Structural characterization of methylenedianiline regioisomers by ion mobility-mass spectrometry, tandem mass spectrometry, and computational strategies: L electrospray spectra of 2-ring isomers, Anal. Chem 86 (2014) 4362–4370. doi: 10.1021/ac5001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stow SM, Onifer TM, Forsythe JG, Nefzger H, Kwiecien NW, May JC, McLean JA, Hercules DM, Structural characterization of methylenedianiline regioisomers by ion mobility-mass spectrometry, tandem mass spectrometry, and computational strategies. 2. electrospray spectra of 3-ring and 4-ring isomers, Anal. Chem 87 (2015) 6288–6296. doi: 10.1021/acs.analchem.5b01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stow SM, Crescentini TM, Forsythe JG, May JC, McLean JA, Hercules DM, Structural characterization of methylenedianiline regioisomers by ion mobility-mass spectrometry, tandem mass spectrometry, and computational strategies. 3. MALDI spectra of 2-ring isomers, Anal. Chem 89 (2017). doi: 10.1021/acs.analchem.7b02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vandenplas O, Malo JL, Saetta M, Mapp CE, Fabbri LM, Occupational asthma and extrinsic alveolitis due to isocyanates: Current status and perspectives, Br. J. Ind. Med 50 (1993) 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pasch H, Mautjana NA, Matrix-assisted laser desorption/ionization mass spectrometry of polymers, Encycl. Anal. Chem. Appl. Theory Instrum. (2006). [Google Scholar]
- [37].Mautjana NA, Pasch H, Matrix-assisted laser desorption ionization mass spectrometry of synthetic polymers, Macromol. Symp 313 (2012) 157–161. doi: 10.1002/masy.201250317. [DOI] [Google Scholar]
- [38].Mass V, Schrepp W, Von Vacano B, Pasch H, Sequence analysis of an isocyanate oligomer by MALDI-TOF mass spectrometry using collision induced dissociation, Macromol. Chem. Phys 210 (2009) 1957–1965. doi: 10.1002/macp.200900236. [DOI] [Google Scholar]
- [39].Carr RH, Jackson AT, Preliminary matrix-assisted laser desorption ionization time-of-flight and field desorption mass spectrometric analyses of polymeric methylene diphenylene diisocyanate, its amine precursor and a model polyether prepolymer, Rapid Commun. Mass Spectrom 12 (1998) 2047–2050. doi: . [DOI] [Google Scholar]
- [40].Warburton KE, Clench MR, Ford MJ, White J, Rimmer DA, Carolan VA, Characterisation of derivatised monomeric and prepolymeric isocyanates by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry and structural elucidation by tandem mass spectrometry, Eur. J. Mass Spectrom 11 (2005) 565–574. doi: 10.1255/ejms.778. [DOI] [PubMed] [Google Scholar]
- [41].Vangronsveld E, Mandel F, Workplace monitoring of isocyanates using ion trap liquid chromatography/tandem mass spectrometry, Rapid Commun. Mass Spectrom 17 (2003) 1685–1690. doi: 10.1002/rcm.1106. [DOI] [PubMed] [Google Scholar]
- [42].Crescentini TM, Stow SM, Forsythe JG, May JC, McLean JA, Hercules DM, Structural characterization of methylenedianiline regioisomers by ion mobility-mass spectrometry and tandem mass spectrometry. 4. 3-ring and 4-ring isomers, Anal. Chem 90 (2018). doi: 10.1021/acs.analchem.8b04103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang GB, Labow RS, Santerre JP, Biodegradation of a poly(ester)urea-urethane by cholesterol esterase: Isolation and identification of principal biodegradation products, J. Biomed. Mater. Res 36 (1998) 407–417. doi:doi:10.1002/. [DOI] [PubMed] [Google Scholar]
- [44].Lelah MD, Cooper SL, Polyurethanes in medicine, CRC press, 1986. [Google Scholar]
- [45].Ulrich H, Chemistry and technology of carbodiimides, John Wiley & Sons, West Sussex, England, 2008. [Google Scholar]
- [46].Gies AP, Heath WH, Keaton RJ, Jimenez JJ, Zupancic JJ, MALDI-TOF/TOF CID study of polycarbodiimide branching reactions, Macromolecules. 46 (2013) 7616–7637. doi: 10.1021/ma401481g. [DOI] [Google Scholar]
- [47].Gies AP, Stefanov Z, Rau NJ, Chakraborty D, Boopalachandran P, Chauvel JP, Iron(III)-catalyzed chain growth reactions of polymeric methylene diphenyl diisocyanate, Macromolecules. 49 (2016) 1201–1221. doi: 10.1021/acs.macromol.5b01973. [DOI] [Google Scholar]
- [48].Frisch KC, Reegen SL, Thir B, Kinetics of alcohol and polyether polyol-isocyanate reactions using metal catalysts, J. Polym. Sci. Part C. 16 (1967) 2191–2201. [Google Scholar]
- [49].Willeboordse F, The use of differential reaction kinetics in determining rate constants of hydroxyl-isocyanate reactions, J. Phys. Chem 74 (1970) 601–606. doi: 10.1021/j100698a020. [DOI] [Google Scholar]
- [50].Krol P, Galina H, Kaczmarski K, A three parameter kinetic model of formation of linear polyurethane from 2,4-toluenediisocyanate and butane-1,4-diol, Macromol. Theory Simulations. 8 (1999) 129–136. [Google Scholar]
- [51].Krol P, Experimental verification of the kinetic model for the reaction yielding linear polyurethanes, claiming dependence of oligomer reactivities on molecular weights. Ill, J. Appl. Polym. Sci 61 (1996) 2207–2219. [Google Scholar]
- [52].Krol P, Kinetic model for the process giving linear polyurethanes, with consideration of substitution effects and different chemical reactivities of functional groups in toluene 2,4-diisocyanate, J. Appl. Polym. Sci 69 (1998) 169–181. [Google Scholar]
- [53].He Y, Zhang X, Zhang X, Huang H, Chang J, Chen H, Structural investigations of toluene diisocyanate (TDI) and trimethylolpropane (TMP)-based polyurethane prepolymer, J. Ind. Eng. Chem 18 (2012) 1620–1627. doi: 10.1016/j.jiec.2012.02.023. [DOI] [Google Scholar]
- [54].Ahn YH, Kim JS, Kim SH, Reaction monitoring of toluenediisocyanate (TDI) polymerization on anon-mixable aqueous surface by MALDI mass spectrometry, Anal. Sci 29 (2013) 703–708. doi: 10.2116/analsci.29.703. [DOI] [PubMed] [Google Scholar]
- [55].Ahn YH, Lee YJ, Kim SH, MALDI MS-based composition analysis of the polymerization reaction of toluene diisocyanate (TDI) and ethylene glycol (EG), Anal. Sci 31 (2015) 513–520. doi: 10.2116/analsci.31.513. [DOI] [PubMed] [Google Scholar]
- [56].Nagy T, Antal B, Czifrak K, Papp I, Karger-Kocsis J, Zsuga M, Keki S, New insight into the kinetics of diisocyanate-alcohol reactions by high performance liquid chromatography and mass spectrometry, J. Appl. Polym. Sci 132 (2015) 42127–42136. [Google Scholar]
- [57].Nagy T, Antal B, Dekany-Adamoczky A, Karger-Kocsis J, Zsuga M, Keki S, Uncatalyzed reactions of 4,4’-diphenylmethane-diisocyanate with polymer polyols as revealed by matrix-assisted laser desorption/ionization mass spectrometry, RSC Adv 6 (2016) 47023–47032. doi: 10.1039/c6ra06671b. [DOI] [Google Scholar]
- [58].Beldi M, Medimagh R, Chatti S, Marque S, Prim D, Loupy A, Delolme L, Characterization of cyclic and non-cyclic poly-(ether-urethane)s bio-based sugar diols by a combination of MALDI-TOL andNMR, Eur. Polym. J 43 (2007) 3415–3433. doi: 10.1016/j.eurpolymj.2007.06.003. [DOI] [Google Scholar]
- [59].Krol P, Pilch-Pitera B, Urethane oligomers as raw materials and intermediates for polyurethane elastomers. Methods for synthesis, structural studies and analysis of chemical composition, Polymer (Guildf). 44 (2003) 5075–5101. doi: 10.1016/S0032-3861(03)00431-2. [DOI] [Google Scholar]
- [60].Akindoyo JO, Beg MDH, Ghazali S, Islam MR, Jeyaratnam N, Yuvaraj AR, Polyurethane types, synthesis and applications-a review, RSC Adv 6 (2016) 114453–114482. doi: 10.1039/c6ral4525f. [DOI] [Google Scholar]
- [61].Wesdemiotis C, Solak N, Polce MJ, Dabney DE, Chaicharoen K, Katzenmeyer BC, Fragmentation Pathways of Polymer Ions, Mass Spectrom. Rev 30 (2011) 523–559. doi: 10.1002/mas. [DOI] [PubMed] [Google Scholar]
- [62].Chattopadhyay DK, Raju NP, Vairamani M, Raju KVSN, Structural investigations of polypropylene glycol (PPG) and isophorone diisocyanate (IPDI) based polyurethane prepolymer by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF)-mass spectrometry, Prog. Org. Coatings. 62 (2008) 117–122. doi: 10.1016/j.porgcoat.2007.09.021. [DOI] [Google Scholar]
- [63].Von Helden G, Bowers MT, Wyttenbach T, Inclusion of a MALDI ion source in the ion chromatography technique: conformational information on polymer and biomolecular ions, Int. J. Mass Spectrom. Ion Process. 147 (1995) 349–364. [Google Scholar]
- [64].Haler JRN, Far J, Aqil A, Claereboudt J, Tomczyk N, Giles K, Jérôme C, De Pauw E, Multiple gas-phase conformations of a synthetic linear poly (acrylamide) polymer observed using ion mobility-mass spectrometry, J. Am. Soc. Mass Spectrom 28 (2017) 2492–2499. doi: 10.1007/s13361-017-1769-x. [DOI] [PubMed] [Google Scholar]
- [65].Trimpin S, Plasencia M, Isailovic D, Clemmer DE, Resolving oligomers from fully grown polymers with IMS-MS, Anal. Chem 79 (2007) 7965–7974. doi: 10.1021/ac071575i. [DOI] [PubMed] [Google Scholar]
- [66].Ude S, Fernández De La Mora J Thomson BA, Charge-induced unfolding of multiply charged polyethylene glycol ions, J. Am. Chem. Soc 126 (2004) 12184–12190. doi: 10.1021/ja0381306. [DOI] [PubMed] [Google Scholar]
- [67].Larriba C, Fernandez De La Mora J, The gas phase structure of coulombically stretched polyethylene glycol ions, J. Phys. Chem. B. 116 (2011) 593–598. doi: 10.1021/jp2092972. [DOI] [PubMed] [Google Scholar]
- [68].Gidden J, Wyttenbach T, Jackson AT, Scrivens JH, Bowers MT, Ts CV, August RV, Re V, Recei M, March V, Gas-phase conformations of synthetic polymers : polyethylene glycol), polypropylene glycol), and poly(tetramethylene glycol), J. Am. Chem. Soc 122 (2000) 4692–4699. [Google Scholar]
- [69].De Winter J, Lemaur V, Ballivian R, Chirot F, Coulembier O, Antoine R, Lemoine J, Comil J, Dubois P, Dugourd P, Gerbaux P, Size dependence of the folding of multiply charged sodium cationized polylactides revealed by ion mobility mass spectrometry and molecular modelling, Chem. - A Eur. J. 17 (2011) 9738–9745. doi: 10.1002/chem.201100383. [DOI] [PubMed] [Google Scholar]
- [70].Gidden J, Wyttenbach T, Batka JJ, Weis P, Bowers MT, Jackson AT, Scrivens JH, Polyethylene terephthalate) oligomers cationized by alkali ions: Structures, energetics, and their effect on mass spectra and the matrix-assisted laser desorption/ionization process, J. Am. Soc. Mass Spectrom 10 (1999) 883–895. doi: 10.1016/S1044-0305(99)00054-9. [DOI] [Google Scholar]
- [71].Wyttenbach T, von Helden G, Bowers MT, Conformations of alkali ion cationized polyethers in the gas phase: polyethylene glycol and bis[(benzo-15-crown-5)-l5-ylmethyl] pimelate, Int. J. Mass Spectrom. Ion Process. 165 (1997) 377–390. doi: 10.1016/SO168-1176(97)00179-1. [DOI] [Google Scholar]
- [72].Haler JRN, Morsa D, Lecomte P, Jérôme C, Far J, De Pauw E, Predicting ion mobility-mass spectrometry trends of polymers using the concept of apparent densities, Elsevier Methods. 144 (2018) 125–133. doi: 10.1016/j.ymeth.2018.03.010. [DOI] [PubMed] [Google Scholar]
- [73].Morsa D, Defize T, Dehareng D, Jerome C, De Pauw E, Polymer topology revealed by ion mobility coupled with mass spectrometry, Anal. Chem 86 (2014) 9693–9700. doi: 10.1021/ac502246g. [DOI] [PubMed] [Google Scholar]
- [74].Duez Q, Chirot F, Lienard R, Josse T, Choi CM, Coulembier O, Dugourd P, Cornil J, Gerbaux P, De Winter J, Polymers for traveling wave ion mobility spectrometry calibration, J. Am. Soc. Mass Spectrom 28 (2017) 2483–2491. doi: 10.1007/s13361-017-1762-4. [DOI] [PubMed] [Google Scholar]
- [75].Selby TL, Wesdemiotis C, Lattimer RP, Dissociation characteristics of [M+ X]+ ions (X= H, Li, Na, K) from linear and cyclic polyglycols, J. Am. Soc. Mass Spectrom 5 (1994) 1081–1092. [DOI] [PubMed] [Google Scholar]
- [76].Hilton GR, Jackson AT, Thalassinos K, Scrivens JH, Structural analysis of synthetic polymer mixtures using ion mobility and tandem mass spectrometry, Anal. Chem 80 (2008) 9720–9725. doi: 10.1021/ac801716c. [DOI] [PubMed] [Google Scholar]
- [77].Cody RB, Fouquet T, Paper spray and Kendrick mass defect analysis of block and random ethylene oxide/propylene oxide copolymers, Anal. Chim. Acta. 989 (2017) 38–44. doi: 10.1016/j.aca.2017.08.005. [DOI] [PubMed] [Google Scholar]
- [78].Girod M, Carissan Y, Humbel S, Charles L, Tandem mass spectrometry of doubly charged poly(ethylene oxide) oligomers produced by electrospray ionization, Int. J. Mass Spectrom 272 (2008) 1–11. [Google Scholar]
- [79].Tintaru A, Chendo C, Wang Q, Viel S, Quéléver G, Peng L, Posocco P, Pricl S, Charles L, Conformational sensitivity of conjugated poly(ethylene oxide)-poly(amidoamine) molecules to cations adducted upon electrospray ionization - A mass spectrometry, ion mobility and molecular modeling study, Anal. Chim. Acta. 808 (2014) 163–174. doi: 10.1016/j.aca.2013.08.030. [DOI] [PubMed] [Google Scholar]
- [80].Lattimer RP, Tandem mass spectrometry of lithium-attachment ions from polyglycols, J. Am. Soc. Mass Spectrom 3 (1992) 225–234. [DOI] [PubMed] [Google Scholar]
- [81].Memboeuf A, Vékey K, Lendvay G, Structure and energetics of poly (ethylene glycol) cationized by Li+, Na+, K+ and Cs+: a first-principles study, J. Mass Spectrom 17 (2011) 33–46. [DOI] [PubMed] [Google Scholar]
- [82].Shimada K, Nagahata R, Kawabata SI, Matsuyama S, Saito T, Kinugasa S, Evaluation of the quantitativeness of matrix-assistedlaser desorption/ionization time-of-flight massspectrometry using an equimolar mixture of uniformpoly(ethylene glycol) oligomers, J. Mass Spectrom 38 (2003) 948–954. doi: 10.1002/jms.508. [DOI] [PubMed] [Google Scholar]
- [83].Chen R, Li L, Lithium and transition metal ions enable low energy collision-induced dissociation of polyglycols in electrospray ionization mass spectrometry, J. Am. Soc. Mass Spectrom 12 (2001) 832–839. doi: 10.1016/S1044-0305(01)00261-6. [DOI] [PubMed] [Google Scholar]
- [84].Kokubo S, Vana P, Obtaining the Dielectric Constant of Polymers from Doubly Charged Species in Ion-Mobility Mass Spectrometry, Macromol. Chem. Phys 218 (2017) 1–8. doi: 10.1002/macp.201700126. [DOI] [Google Scholar]
- [85].Li J, Zhu M, Structural characterization of a vegetable oil-based polyol through liquid chromatography multistage mass spectrometry, J. Polym. Sci. Part A Polym. Chem 55 (2017) 255–262. doi: 10.1002/pola.28371. [DOI] [Google Scholar]
- [86].Williams JB, Gueev AL, Hercules DM, Characterization of polyesters by matrix-assisted laser desorption ionization mass spectrometry, Macromolecules. 30 (1997) 3781–3787. doi: 10.1021/ma970123c. [DOI] [Google Scholar]
- [87].Williams JB, Chapman TM, Hercules DM, Matrix-assisted laser desorption/ionization mass spectrometry of discrete mass poly(butylene glutarate) oligomers, Anal. Chem 75 (2003) 3092–3100. doi: 10.1021/ac030061q. [DOI] [PubMed] [Google Scholar]
- [88].Rizzarelli P, Puglisi C, Montaudo G, Matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectra of poly(butylene adipate), Rapid Commun. Mass Spectrom 20 (2006) 1683–1694. doi: 10.1002/rcm. [DOI] [PubMed] [Google Scholar]
- [89].Gies AP, Ellison ST, Chakraborty AK, Kwiecien NW, Hercules DM, MALDI-TOF/TOF CID study of poly(butylene adipate) fragmentation reactions, RSC Adv 2 (2012) 4135–4151. doi: 10.1039/c2ra20283b. [DOI] [Google Scholar]
- [90].Pretorius NO, Rhode K, Simpson JM, Pasch H, Characterization of complex phthalic acid/propylene glycol based polyesters by the combination of 2D chromatography and MAFDI-TOF mass spectrometry, Anal. Bioanal. Chem 407 (2015) 217–230. doi: 10.1007/s00216-014-7762-3. [DOI] [PubMed] [Google Scholar]
- [91].Pretorius NO, Willemse CM, de Villiers A, Pasch H, Combined size exclusion chromatography, supercritical fluid chromatography and electrospray ionization mass spectrometry for the analysis of complex aliphatic polyesters, J. Chromatogr. A. 1330 (2014) 74–81. doi: 10.1016/j.chroma.2014.01.018. [DOI] [PubMed] [Google Scholar]
- [92].Pretorius NO, Rode K, Simpson JM, Pasch H, Analysis of complex phthalic acid based polyesters by the combination of size exclusion chromatography and matrix-assisted laser desorption/ionization mass spectrometry, Anal. Chim. Acta. 808 (2014) 94–103. doi: 10.1016/j.aca.2013.07.030. [DOI] [PubMed] [Google Scholar]
- [93].Giguere MS, Mayer PM, Climbing the internal energy ladder: The unimolecular decomposition of ionized poly(vinyl acetate), Int. J. Mass Spectrom 231 (2004) 59–68. doi: 10.1016/j.ijms.2003.09.013. [DOI] [Google Scholar]
- [94].Owen S, Masaoka M, Kawamura R, Sakota N, Biodegradation of poly-D, L-lactic acid polyurethanes, J. Macromol. Sci. Part A Pure Appl. Chem 32 (1995) 843–850. [Google Scholar]
- [95].Borda J, Bodnár I, Kéki S, Sipos L, Zsuga M, Optimum conditions for the synthesis of linear polylactic acid-based urethanes, J. Polym. Sci. Part A Polym. Chem 38 (2000) 2925–2933. doi: . [DOI] [Google Scholar]
- [96].Borda J, Keki S, Bodnar I, Nemeth N, Zsuga M, New potentially biodegradable polyurethanes, Polym. Adv. Technol 17 (2006) 945–953. [Google Scholar]
- [97].Tang D, Noordover BA, Sablong RJ, Koning CE, Metal-free synthesis of novel biobased dihydroxyl-terminated aliphatic polyesters as building blocks for thermoplastic polyurethanes, J. Polym. Sci. Part A Polym. Chem 49 (2011) 2959–2968. [Google Scholar]
- [98].Báez JE, Marcos-Fernández A, Martínez-Richa A, Galindo-Iranzo P, Poly(ε-caprolactone) diols (HOPCLOH) and their poly(ester-urethanes) (PEUs): The effect of linear aliphatic diols [HO-(CH2)m-OH] as initiators, Polym. - Plast. Technol. Eng 56 (2017) 889–898. doi: 10.1080/03602559.2016.1247273. [DOI] [Google Scholar]
- [99].Osaka I, Watanabe M, Takama M, Murakami M, Arakawa R, Characterization of linear and cyclic polylactic acids and their solvolysis products by electrospray ionization mass spectrometry, J. Mass Spectrom 41 (2006) 1369–1377. doi: 10.1002/jms. [DOI] [PubMed] [Google Scholar]
- [100].Soares BP, Wood-Adams PM, Chemical composition distribution of multicomponent copolymers, Macromol. Theory Simulations. 12 (2003) 229–236. [Google Scholar]
- [101].Montaudo G, Lattimer RP, Mass spectrometry of polymers, CRC press, 2001. [Google Scholar]
- [102].Ferreira HE, Condêo JAD, Fernandes IO, Duarte DE, Bordado JCM, HPLC-UV and HPLC-ESI+-MS/MS analysis of free monomeric methylene diphenyl diisocyanate in polyurethane foams and prepolymers after stabilization with NBMA a new derivatizating agent, Anal. Methods. 6 (2014) 9242–9257. doi: 10.1039/c4ay01163e. [DOI] [Google Scholar]
- [103].Brock FH, Kinetics of the 2,4-tolylene diisocyanate-alcohol reaction, J. Phys. Chem 65 (1961) 1638–1639. doi: 10.1021/j100905a509. [DOI] [Google Scholar]
- [104].Dyer E, Taylor HA, Mason SJ, Samson J, The rates of reaction of isocyanates with alcohols. I. Phenyl issocyanate with 1- and 2-butanol, J. Am. Chem. Soc 71 (1949) 4106–4109. doi: 10.1021/ja01180a064. [DOI] [Google Scholar]
- [105].Burkus J, Eckert CF, The kinetics of the triethylamine-catalyzed reaction of diisocyanates with 1-butanol in toluene, J. Am. Chem. Soc 80 (1958) 5948–5950. doi: 10.1021/ja01555a015. [DOI] [Google Scholar]
- [106].Gies AP, Hercules DM, Collision induced dissociation study of ester-based polyurethane fragmentation reactions, Anal. Chim. Acta. 808 (2014) 199–219. doi: 10.1016/j.aca.2013.09.035. [DOI] [PubMed] [Google Scholar]
- [107].Saunders JH, Klempner D, Handbook of polymeric foams and foam technology, Munich: Hanser, 1991. [Google Scholar]
- [108].Guo A, Javni I, Petrovic Z, Rigid polyurethane foams based on soybean oil, J. Appl. Polym. Sci 77 (2000)467–473. [Google Scholar]
- [109].Kirpluks M, Kalnbunde D, Benes H, Cabulis U, Natural oil based highly functional polyols as feedstock for rigid polyurethane foam thermal insulation, Ind. Crops Prod 122 (2018) 627–636. doi: 10.1016/j.indcrop.2018.06.040. [DOI] [Google Scholar]
- [110].Basso MC, Giovando S, Pizzi A, Pasch H, Pretorius N, Delmotte L, Celzard A, Flexible-elastic copolymerized polyurethane-tannin foams, J. Appl. Polym. Sci 131 (2014) 2–7. doi: 10.1002/app.40499. [DOI] [Google Scholar]
- [111].Basso MC, Pizzi A, Lacoste C, Delmotte L, Al-Marzouki FM, Abdalla S, Celzard A, MALDI-TOF and 13C NMR analysis of tannin-furanic-polyurethane foams adapted for industrial continuous lines application, Polymers (Basel). 6 (2014) 2985–3004. doi: 10.3390/polym6122985. [DOI] [Google Scholar]
- [112].Aou K, Schrock AK, Ginzburg VV, Price PC, Characterization of polyurethane hard segment length distribution using soft hydrolysis/MALDI and monte carlo simulation, Polymer (Guildf). 54 (2013) 5005–5015. doi: 10.1016/j.polymer.2013.07.014. [DOI] [Google Scholar]
- [113].Marand A, Karlsson D, Dalene M, Skarping G, Extractable organic compounds in polyurethane foam with special reference to aromatic amines and derivatives thereof, Anal. Chim. Acta. 510 (2004) 109–119. doi: 10.1016/j.aca.2003.12.063. [DOI] [Google Scholar]
- [114].Yontz DJ, Hsu SL, A mass spectrometry analysis of hard segment length distribution in polyurethanes, Macromolecules. 33 (2000) 8415–8420. doi: 10.1021/ma000454g. [DOI] [Google Scholar]
- [115].Tang D, Macosko CW, Hillmyer MA, Thermoplastic polyurethane elastomers from bio-based poly(δ- decalactone) diols, Polym. Chem 5 (2014) 3231–3237. doi: 10.1039/c3py01120h. [DOI] [Google Scholar]
- [116].Holden G, Kricheldorf HR, Quirk RP, Thermoplastic elastomers, Munich: Hanser, 2004. [Google Scholar]
- [117].Guan J, Song Y, Lin Y, Yin X, Zuo M, Zhao Y, Tao X, Zheng Q, Progress in study of non-isocyanate polyurethane, Ind. Eng. Chem. Res 50 (2011) 6517–6527. doi: 10.1021/ie101995j. [DOI] [Google Scholar]
- [118].Lamarzelle O, Durand PL, Wirotius AL, Chollet G, Grau E, Cramail H, Activated lipidic cyclic carbonates for non-isocyanate polyurethane synthesis, Polym. Chem 7 (2016) 1439–1451. doi: 10.1039/c5py01964h. [DOI] [Google Scholar]
- [119].Poussard L, Mariage J, Grignard B, Detrembleur C, Jéroîme C, Calberg C, Heinrichs B, De Winter J, Gerbaux P, Raquez JM, Bonnaud L, Dubois P, Non-isocyanate polyurethanes from carbonated soybean oil using monomeric or oligomeric diamines to achieve thermosets or thermoplastics, Macromolecules. 49 (2016) 2162–2171. doi: 10.1021/acs.macromol.5b02467. [DOI] [Google Scholar]
- [120].More AS, Gadenne B, Alfos C, Cramail H, AB type polyaddition route to thermoplastic polyurethanes from fatty acid derivatives, Polym. Chem 3 (2012) 1594–1605. doi: 10.1039/c2py20123b. [DOI] [Google Scholar]
- [121].Prasad A, Xiang H, Wang J, Remsen EE, Analysis of pre- and post-conditioned polyurethane CMP pad surfaces as a function of conditioning temperature, ECS Trans 3 (2007) 31–44. [Google Scholar]
- [122].Lattimer RP, Muenster H, Budzikiewicz H, Pyrolysis tandem mass spectrometry (Py-MS/MS) of a segmented polyurethane, J. Anal. Appl. Pyrolysis. 17 (1990) 237–249. doi: 10.1016/0165-2370(90)85013-D. [DOI] [Google Scholar]
- [123].Ravey M, Pearce EM, Flexible polyurethane foam. I. Thermal decomposition of a polyether-based, water-blown commercial type of flexible polyurethane foam, J. Appl. Polym. Sci 63 (1997) 47–74. doi:. [DOI] [Google Scholar]
- [124].Lattimer RP, Polce MJ, Wesdemiotis C, MALDI-MS analysis of pyrolysis products from a segmented polyurethane, J. Anal. Appl. Pyrolysis. 48 (1998) 1–15. doi: 10.1016/S0165-2370(98)00092-8. [DOI] [Google Scholar]
- [125].Lattimer RP, Mass spectral analysis of low-temperature pyrolysis products from poly(ethylene glycol), J. Anal. Appl. Pyrolysis. 56 (2000) 61–78. [Google Scholar]
- [126].Lattimer RP, Williams RC, Low-temperature pyrolysis products from a polyether-based urethane, J. Anal. Appl. Pyrolysis. 63 (2002) 85–104. doi: 10.1016/S0165-2370(01)00143-7. [DOI] [Google Scholar]
- [127].Lattimer RP, Mass spectral analysis of low-temperature pyrolysis products from poly(tetrahydrofuran), J. Anal. Appl. Pyrolysis. 57 (2001) 57–76. doi: 10.1016/S0165-2370(00)00106-6. [DOI] [Google Scholar]
- [128].Ketata N, Sanglar C, Waton H, Alamercery S, Delolme F, Raffin G, Grenier-Loustalot MF, Thermal degradation of polyurethane bicomponent systems in controlled atmospheres, Polym. Polym. Compos 13 (2005) 1–26. doi: 10.1371/journal.pone.0159993. [DOI] [Google Scholar]
- [129].Weems AC, Wacker KT, Carrow JK, Boyle AJ, Maitland DJ, Shape memory polyurethanes with oxidation-induced degradation: In vivo and in vitro correlations for endovascular material applications, Acta Biomater 59 (2017) 33–44. doi: 10.1016/j.actbio.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Dopico-García MS, López-Vilariño JM, Fernández-Martínez G, González-Rodríguez MV, Liquid chromatography method to determine polyamines in thermosetting polymers, Anal. Chim. Acta. 667 (2010) 123–129. doi: 10.1016/j.aca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- [131].Simonsick WJ, Aaserud DJ, Modern mass spectrometry for coatings, Prog. Org. Coatings. 34 (1998) 206–213. [Google Scholar]
- [132].European Food Safety Authority (EFSA), Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission related to para hydroxybenzoates (E 214-219), EFSA J. 965 (2006) 1–7. doi: 10.2903/j.efsa.2007.428. [DOI] [Google Scholar]
- [133].Driffield M, Bradley EL, Castle L, Identification of unknown reaction by-products and contaminants in epoxyphenolic-based food can coatings by LC/TOF-MS, Wilmington, 2006. [Google Scholar]
- [134].Driffield M, Bradley EL, Castle L, A method of test for residual isophorone diisocyanate trimer in new polyester-polyurethane coatings on light metal packaging using liquid chromatography with tandem mass spectrometric detection, J. Chromatogr. A. 1141 (2007) 61–66. doi: 10.1016/j.chroma.2006.12.005. [DOI] [PubMed] [Google Scholar]
- [135].Bradley EL, Driffield M, Guthrie J, Harmer N, Thomas Oldring PK, Castle L, Analytical approaches to identify potential migrants in polyester-polyurethane can coatings, Food Addit. Contam 26 (2009) 1602–1610. [DOI] [PubMed] [Google Scholar]
- [136].Omer E, Cariou R, Remaud G, Guitton Y, Germon H, Hill P, Dervilly-Pinel G, Le Bizec B, Elucidation of non-intentionally added substances migrating from polyester-polyurethane lacquers using automated LC-HRMS data processing, Anal. Bioanal. Chem 410 (2018) 5391–5403. doi: 10.1007/s00216-018-0968-z. [DOI] [PubMed] [Google Scholar]
- [137].Schaefer A, Identification and quantification of migrants from can coatings, University of Hamburg, 2004. http://www.chemie.uni-hamburg.de/bibliothek/2004/DissertationSchaefer.pdf. [Google Scholar]
- [138].Schaefer A, Ohm VA, Simat TJ, Migration from can coatings: Part 2. Identification and quantification of migrating cyclic oligoesters below 1000 Da, Food Addit. Contam. Part A. 21 (2004)377–389. [DOI] [PubMed] [Google Scholar]
- [139].Ren SF, Zhang L, Cheng ZH, Guo YL, Immobilized carbon nanotubes as matrix for MALDI-TOF-MS analysis: Applications to neutral small carbohydrates, J. Am. Soc. Mass Spectrom 16 (2005) 333–339. doi: 10.1016/jjasms.2004.11.017. [DOI] [PubMed] [Google Scholar]
- [140].Wang GB, Santerre JP, Labow RS, High-performance liquid chromatographic separation and tandem mass spectrometric identification of breakdown products associated with the biological hydrolysis of a biomedical polyurethane, J. Chromatogr. B Biomed. Appl 698 (1997) 69–80. [DOI] [PubMed] [Google Scholar]
- [141].Lawson G, Bartram S, Fitchner S, Woodland ED, MALDI-MS and colorimetric analysis of diisocyanate and polyol migrants from model polyurethane adhesives used in food packaging, Analyst. 125 (2000) 115–118. doi: 10.1039/a906595d. [DOI] [PubMed] [Google Scholar]
- [142].Mortensen SK, Trier XT, Foverskov A, Petersen JH, Specific determination of 20 primary aromatic amines in aqueous food simulants by liquid chromatography-electrospray ionization-tandem mass spectrometry, J. Chromatogr. A. 1091 (2005) 40–50. doi: 10.1016/j.chroma.2005.07.026. [DOI] [PubMed] [Google Scholar]
- [143].Aznar M, Canellas E, Nerín C, Quantitative determination of 22 primary aromatic amines by cation-exchange solid-phase extraction and liquid chromatography-mass spectrometry, J. Chromatogr. A. 1216 (2009) 5176–5181. doi: 10.1016/j.chroma.2009.04.096. [DOI] [PubMed] [Google Scholar]
- [144].Pezo D, Fedelib M, Bosetti O, Nerina C, Aromatic amines from polyurethane adhesives in food packaging: The challenge of identification and pattern recognition using quadrupole-time of flight-mass spectrometry, Anal. Chim. Acta. 756 (2012) 49–59. [DOI] [PubMed] [Google Scholar]
- [145].Ramos MJG, Lozano A, Fernandez-Alba AR, High-resolution mass spectrometry with data independent acquisition for the comprehensive non-targeted analysis of migrating chemicals coming from multilayer plastic packaging materials used for fruit puree and juice, Elsevier Talanta. 191 (2019) 180–192. doi: 10.1016/j.talanta.2018.08.023. [DOI] [PubMed] [Google Scholar]
- [146].Zhang N, Kenion G, Bankmann D, Mezouari S, Hartman TG, Migration studies and chemical characterization of low molecular weight cyclic polyester oligomers from food packaging lamination adhesives, Packag. Technol. Sci 31 (2018) 197–211. doi: 10.1002/pts.2367. [DOI] [Google Scholar]
- [147].Lutz JF, Lehn JM, Meijer EW, Matyjaszewski K, From precision polymers to complex materials and systems, Nat. Rev. Mater 1 (2016) 16024. [Google Scholar]
- [148].Mutlu H, Lutz JF, Reading polymers: Sequencing of natural and synthetic macromolecules, Angew. Chemie Int. Ed 53 (2014) 13010–13019. doi: 10.1002/anie.201406766. [DOI] [PubMed] [Google Scholar]
- [149].Lutz JF, Coding macromolecules: inputting information in polymers using monomer-based alphabets, Macromolecules. 48 (2015) 4759–4767. doi: 10.1021/acs.macromol.5b00890. [DOI] [Google Scholar]
- [150].Gunay US, Petit BE, Karamessini D, Al Ouahabi A, Amalian JA, Chendo C, Bouquey M, Gigmes D, Charles L, Lutz JF, Chemoselective synthesis of uniform sequence-coded polyurethanes and their use as molecular tags, Chem. 1 (2016) 114–126. doi: 10.1016/j.chempr.2016.06.006. [DOI] [Google Scholar]
- [151].Karamessini D, Petit BE, Bouquey M, Charles L, Lutz JF, Identification-tagging of methacrylate-based intraocular implants using sequence defined polyurethane barcodes, Adv. Funct. Mater 27 (2017). doi: 10.1002/adfm.201604595. [DOI] [Google Scholar]
- [152].Takats Z, Wiseman JM, Gologan B, Cooks RG, Electrosonic spray ionization. A gentle technique for generating folded proteins and protein complexes in the gas phase and for studying ion-molecule reactions at atmospheric pressure, Anal. Chem 76 (2004) 4050–4058. doi: 10.1021/ac049848m. [DOI] [PubMed] [Google Scholar]
- [153].Takáts Z, Wiseman JM, Gologan B, Graham Cooks R, Mass spectrometry sampling under ambient conditions with desorption electrospray ionization, Science (80-.). 306 (2004) 471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- [154].Bonnaire N, Dannoux A, Pernelle C, Amekraz B, Moulin C, On the use of electrospray ionization and desorption electrospray ionization mass spectrometry for bulk and surface polymer analysis, Appl. Spectrosc 64 (2010) 810–818. doi: 10.1366/000370210791666318. [DOI] [PubMed] [Google Scholar]
- [155].Lebeau D, Ferry M, Direct characterization of polyurethanes and additives by atmospheric solid analysis probe with time-of-flight mass spectrometry (ASAP-TOF-MS), Anal. Bioanal. Chem 407 (2015) 7175–7187. doi: 10.1007/s00216-015-8881-1. [DOI] [PubMed] [Google Scholar]
- [156].Nefliu M, Venter A, Cooks RG, Desorption electrospray ionization and electrosonic spray ionization for solid- and solution-phase analysis of industrial polymers, Chem. Commun (2006) 888–890. doi: 10.1039/b514057a. [DOI] [PubMed] [Google Scholar]
- [157].Terrier P, Desmazieres B, Tortajada J, Buchmann W, APCI/APPI for synthetic polymer analysis, Mass Spectrom. Rev 30 (2011) 854–874. doi: 10.1002/mas. [DOI] [PubMed] [Google Scholar]
- [158].Whitson SE, Erdodi G, Kennedy JP, Lattimer RP, Wesdemiotis C, Direct probe-atmospheric pressure chemical ionization mass spectrometry of cross-linked copolymers and copolymer blends, Anal. Chem 80 (2008) 7778–7785. doi: 10.1021/ac801198g. [DOI] [PubMed] [Google Scholar]


