Abstract
Buprenorphine is an effective treatment for opioid use disorder. As a high affinity, partial agonist for the mu opioid receptor, buprenorphine suppresses opioid withdrawal and craving, reduces illicit opioid use, and blocks exogenous opioid effects including respiratory depression. Other pharmacologic benefits of buprenorphine are its superior safety profile compared to full opioid agonists and its long half-life that allows for daily or less-than-daily dosing. New and innovative buprenorphine formulations, with pharmacokinetic profiles that differ from the original tablet formulation, continue to be developed. These include higher bioavailability transmucosal tablets and films as well as 6-month implantable and monthly injectable products. This growing array of available formulations allows for more choices for patients and increased opportunity for clinicians to individualize treatment; thus, it is important for buprenorphine prescribers to understand these differences.
Keywords: sublingual buprenorphine, buprenorphine implants, sustained-release, buprenorphine depot injections, opioid use disorder, clinical pharmacology, Pharmaceutical Technology, Pharmacokinetics
Introduction
Buprenorphine, a semi-synthetic opioid, was developed in 1970s by Reckitt & Colman, as an analgesic (Cowan et al. 1977) and subsequently investigated by clinical researchers at the Addiction Research Center in Lexington, Kentucky. Their work resulted in a landmark paper (Jasinski et al. 1978), which correctly predicted that buprenorphine had potential for the treatment of opioid use disorder (OUD) due to its unique pharmacology. Despite this, buprenorphine was not approved in the United States (US) for OUD until 2002, although a parenteral formulation was approved for analgesic use in 1989. Prior to 2000, Food and Drug Administration (FDA) and Drug Enforcement Administration regulations limited delivery of treatment with opioid agonist therapy (i.e., methadone) to highly regulated federally licensed clinics, and the pharmaceutical company was hesitant to bring buprenorphine to the US market under those regulations (it was already marketed abroad for OUD at this time [Auriacombe et al. 1994]). In 2000, the Drug Addiction Treatment Act (DATA 2000) was passed—allowing qualified physicians to obtain a federal waiver to prescribe Schedule III-V opioids FDA-approved for OUD treatment in settings outside of opioid treatment programs (i.e., methadone clinics)—introducing a new US treatment paradigm. Sublingual (SL) buprenorphine was placed in Schedule III and approved for opioid dependence treatment (the contemporary DSM-IV nomenclature). Buprenorphine products have undergone continual innovation, leading to development and marketing of SL films, other transmucosal products, 6-month implants, and a monthly subcutaneous (SC) depot injection formulation. Another injection depot formulation is in late-phase development.
Utilization of FDA-approved medication for OUD treatment in general, and buprenorphine specifically, is associated with positive outcomes for both patients and society. In a recent analysis of persons prescribed buprenorphine in France, patients who discontinued treatment were ~29 times more likely to die than those who remained on buprenorphine (Dupouy et al. 2017). Likewise, heroin-related overdose deaths in Baltimore from 1995–2009 decreased significantly as access to methadone and buprenorphine treatment expanded (Schwartz et al. 2013). Moreover, utilization of buprenorphine treatment is associated with reductions in illicit opioid-related crime and decreased transmission of communicable diseases, such as HIV and HCV (Sullivan and Fiellin 2005, Volkow et al. 2014). Buprenorphine is efficacious and effective for OUD because it provides relief of craving and withdrawal, produces opioid blockade, has an excellent safety profile, has reduced abuse liability compared to full opioid agonists, and is suitable for daily or less-than-daily dosing. Each of these treatment outcomes and beneficial characteristics can be directly explained by its pharmacology. As innovative buprenorphine formulations are marketed, it is important for providers to have a complete understanding of buprenorphine formulations and pharmacology in order to make well-informed prescribing and therapeutic decisions.
The aims of this narrative review are to synthesize available published evidence (peer-reviewed journal articles, guidance documents, drug monographs, etc.) in order to:
Review buprenorphine pharmacology (of both transmucosal and long-acting formulations),
Explain how aspects of buprenorphine pharmacology manifest as clinical effects, and
Provide practical information about each buprenorphine formulation, with a focus on how these formulations differ.
Buprenorphine Pharmacology
This section will describe the physiological basis for the clinical effects of buprenorphine. The properties of buprenorphine contributing to its efficacy in OUD treatment include: mu opioid receptor-related factors (e.g., high affinity, low efficacy, & slow dissociation kinetics) and non-mu opioid receptor-related factors (e.g., long terminal half-life, lipophilicity). While buprenorphine also binds the delta and opioid-receptor-like 1 receptors and is a high affinity kappa opioid receptor antagonist, the contribution of these interactions in OUD treatment is unknown and likely minimal; however, buprenorphine is under investigation for depression treatment (Karp et al. 2014) due to kappa opioid receptor involvement in stress systems implicated in depression pathophysiology (Crowley and Kash 2015). For each pharmacologic property discussed below, the corresponding clinical effects are summarized and differences (or lack thereof) in pharmacology between transmucosal and long-acting buprenorphine formulations are noted.
Buprenorphine interaction with mu opioid receptors (pharmacodynamics)
Formulation differences? No. Buprenorphine exhibits the same interaction with the mu opioid receptor regardless of the route by which it is administered.
Low efficacy agonist:
As an agonist (a compound that can elicit cellular response) at the mu opioid receptor, buprenorphine produces typical opioid effects (e.g., euphoria, analgesia, decreased gastrointestinal motility, miosis, respiratory depression, etc.). This results in both beneficial (e.g., suppression of withdrawal and craving) and adverse (e.g., constipation, sedation) clinical effects. Buprenorphine is a partial (or low efficacy) agonist, meaning that the maximal effect produced by buprenorphine will be less than that produced by a full (or high efficacy) mu opioid receptor agonist. This is illustrated in Figure 1: buprenorphine does not reach the same peak effect as measured on the y-axis as full agonists (fentanyl and morphine). Clinically, this partial agonism translates to a ceiling effect for mu opioid receptor-mediated effects of buprenorphine, such as respiratory depression and euphoria (Walsh et al. 1994). In pre-clinical models, buprenorphine exhibits a bell-shaped dose response curve for both respiratory depression and analgesia (i.e., at high enough doses, respiratory depression and analgesia decrease), but the descending limb of this curve has not been reproduced in human studies (for review, see [Cowan, 2003]). This could be due, in part, to the fact that much higher relative doses can be administered in animal models than in humans. This The ceiling effect observed clinically is the basis for its improved safety profile and reduced abuse potential compared to full mu opioid receptor agonists. The exact dose at which this ceiling is observed may vary among persons (see pharmacokinetics below), but clinical laboratory studies have shown that intravenous doses of up to 0.6mg/70kg did not significantly decrease respiration more than 0.2mg/70kg (Dahan et al. 2005), and respiratory depression was no greater after 32mg than 16mg SL among persons without physical dependence on opioids (Walsh et al. 1994). The best evidence for buprenorphine safety perhaps lies in the relative dearth of buprenorphine overdose deaths compared to those with full opioid agonists. While the abuse potential of buprenorphine is less than that of high efficacy agonists (Jasinski et al. 1978), it still has intrinsic reinforcing effects as a partial agonist and can be misused and diverted (for review, see [Lofwall and Walsh, 2014]).
Figure 1.
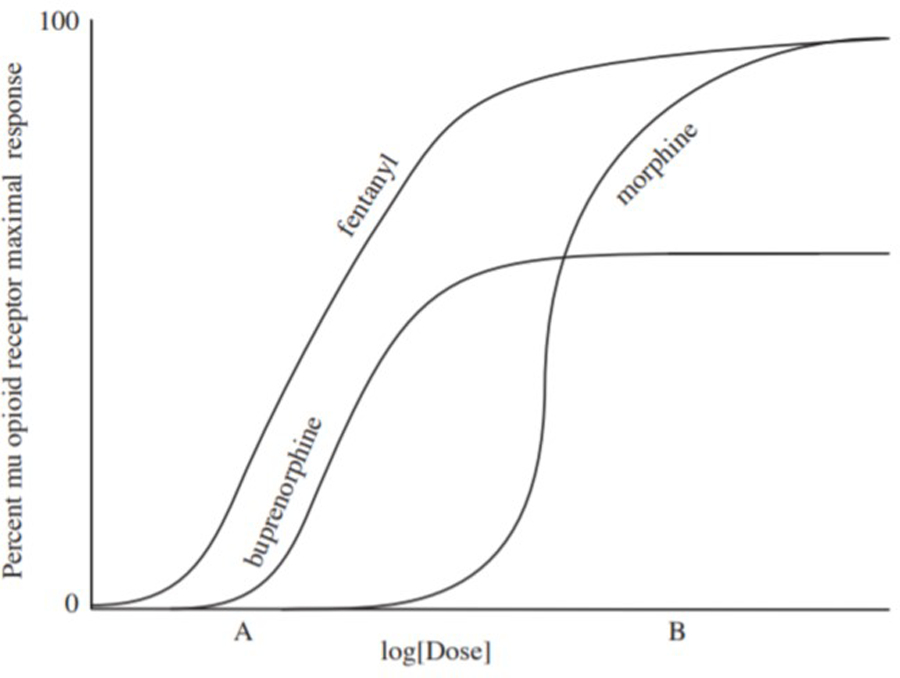
Dose-response curve schematic of three opioid agonists. At a low dose (Dose A), fentanyl and buprenorphine produce significantly greater responses than morphine (i.e., fentanyl and buprenorphine are more potent than morphine). While fentanyl response is dose-related until reaching 100% maximal response, buprenorphine effects reach a ceiling, at which point further increases in dose do not increase the magnitude of response. Because buprenorphine is a partial agonist, it cannot not produce a 100% response like a full agonist (i.e., fentanyl) can. At higher doses (Dose B), morphine (a full agonist with low potency) produces greater response than buprenorphine.
High affinity:
Buprenorphine affinity (a measure of the attractive force between a compound and a receptor) for the mu opioid receptor is approximately 1.7 times that of hydromorphone, 5.4 times that of morphine, 6.2 times that of fentanyl, and 120 times that of oxycodone (Volpe et al. 2011). This high affinity means that buprenorphine is difficult (but not impossible) to displace from the mu opioid receptor, which explains its ability to block subjective and physiological effects of other opioids. As receptor theory suggests and clinical observation confirms, blockade of the mu opioid receptor by buprenorphine is surmountable with higher doses (Bickel et al. 1988, Strain et al. 2002) or with high-affinity opioids, such as fentanyl. Its high affinity is also the primary reason that buprenorphine can precipitate withdrawal when given to individuals physically dependent on opioids. Precipitated withdrawal can be avoided, particularly among persons dependent on short-acting opioids, by waiting to administer buprenorphine until signs of opioid withdrawal emerge (a time of low receptor occupancy)— this is the common clinical strategy for buprenorphine induction.
High potency:
Buprenorphine is a high-potency medication. Potency is simply a measure of drug activity expressed as the absolute dose required to produce a given effect. For example, if Drug A produces effect X at 10mg and Drug B produces X at 100mg, Drug A is 10x more potent than Drug B. Potency depends on both efficacy and affinity. While comparatively low doses of buprenorphine may elicit some degree of respiratory depression compared to morphine, the potency of buprenorphine does not significantly increase with doses within the clinical range (see Figure 1). This concept of potency is important for understanding why buprenorphine should not be converted to morphine milligram equivalents (MME) either for purposes of opioid analgesic rotations or for assessing overdose risk based on daily opioid dose. While the Centers for Disease Control and Prevention (CDC) Guidelines for Prescribing Opioids for Chronic Pain (Dowell 2016) caution against daily opioid doses >90 MME due to overdose risk, buprenorphine dose escalation does not pose increasing overdose risk like the full opioid agonists due to its low efficacy. This explain why CDC guidelines do not include buprenorphine in the MME conversion table and why the American Society of Addiction Medicine does not support legislation limiting buprenorphine prescribing based upon MMEs (ASAM 2016).
Slow dissociation from mu opioid receptor:
Buprenorphine has slow dissociation kinetics (~166min [(Boas and Villiger 1985), contributing to its long duration of action and allowing for daily or less-than-daily dosing. Studies testing the efficacy of administering higher-than-normal daily buprenorphine doses on less-than-daily dosing schedules suggest that patients can be maintained on alternate-day or thrice-weekly dosing with minimal withdrawal and similar rates of illicit opioid use compared to daily dosing (Amass et al. 1994, Eissenberg et al. 1997, Bickel et al. 1999). Duration of opioid blockade closely mirrors that of withdrawal suppression. Subjects maintained on SL buprenorphine (8, 12, 16, or 32mg) for two weeks, and then administered placebo under blinded conditions displayed attenuated subjective responses (e.g., drug liking) to hydromorphone (6, 12 & 18mg IM) up to 98h after the last active buprenorphine dose. Withdrawal increased, but was mild, with time since last dose, but severity of withdrawal was not related to buprenorphine dose (Correia et al. 2006).
Buprenorphine Pharmacokinetics:
Formulation differences? Yes. Buprenorphine PK are altered when taken by different routes of administration.
Bioavailability:
Inter-person variability in transmucosal buprenorphine pharmacokinetics (PKs) is high, with estimates of bioavailability (the amount of parent drug to reach systemic circulation) commonly ranging by 3-fold or more after both acute and chronic administration (Kuhlman et al. 1996, Strain et al. 2004, Chawarski et al. 2005, Compton et al. 2007). These differences may be partly due to individual variability in absorption. Buprenorphine bioavailability is high after IV or SC administration, considerably lower by the sublingual and buccal (transmucosal products) routes and very low orally.
Half-life:
The half-life (t½) of buprenorphine is variable after transmucosal administration, ranging from 24–42h reported in buprenorphine product package inserts. This long t½ allows for daily or less-than daily transmucosal dosing schedules, but the variability underscores the need for individualized dosing based on clinical response. The t½ is much longer after transmucosal compared to IV (3h) administration (Kuhlman et al. 1996), possibly due to sequestration of buprenorphine in the oral mucosa and lipid storage sites when administered transmucosally (Welsh and Valadez-Meltzer 2005). The reported t½ of SC and implantable buprenorphine formulations also exceeds transmucosal, but this is due to continuous release and absorption of buprenorphine from the indwelling implant and/or depot matrix— not because of a fundamental change in metabolism.
Metabolism:
When administered via routes that undergo first-pass metabolism (i.e., sublingual/buccal), buprenorphine is metabolized to norbuprenorphine via CYP450 3A4/5-mediated N-dealkylation, and both buprenorphine and norbuprenorphine undergo glucuronidation to buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide—metabolites that are generally considered inactive (Kuhlman et al. 1998, Elkader and Sproule 2005). While norbuprenorphine is a potent mu-opioid receptor agonist (Huang et al. 2001), brain concentrations of norbuprenorphine are very low (Brown et al. 2012) suggesting that norbuprenorphine does not contribute to the clinical effects of buprenorphine. Because this metabolite is found in high concentrations in urine, urine toxicology for patients receiving buprenorphine frequently includes testing norbuprenorphine to ensure patients are not simply adding buprenorphine directly into the urine sample. Routes of administration that bypass first-pass metabolism (e.g., IV, IN, SC) result in significantly lower norbuprenorphine formation (Kuhlman et al. 1996, Harris et al. 2000). If using urine norbuprenorphine concentrations as a marker of medication adherence, it must be understood that patients receiving SC or subdermal buprenorphine may have low urine concentrations of norbuprenorphine.
Drug-drug interactions:
Concomitant use of CYP450 inhibitors and inducers can affect the metabolism of buprenorphine—leading to possible over- or under-medication (especially relevant for patients with moderate-to-severe hepatic impairment). This is one reason that monitoring of liver function enzymes prior to initiation and during treatment is recommended in all buprenorphine product package inserts. Notably, the NIDA Clinical Trials Network START (Starting Treatment With Agonist Replacement Therapies) study enrolled patients with opioid use disorder who had AST and ALT values less than 5 times the upper limits of normal and alkaline phosphatase levels less than 3 times the upper limits of normal and randomized them to either SL buprenorphine/naloxone or methadone for 24 weeks (Saxon et al. 2013). Liver function tests were evaluated over time. There was no evidence of liver damage induced by SL buprenorphine doses up to 32 mg SL daily. Extreme increases in liver function tests were uncommon and associated with 1) seroconversion to hepatitis B and C and 2) illicit drug use during the first two months of treatment. Thus, this study suggests that monitoring when prescribing transmucosal buprenorphine among patients with normal to elevated (but not more than 5x AST / ALT or 3x alkaline phosphatase) may be clinically relevant as a screening measure to suggest newly contracted hepatitis C and/or B or ongoing illicit drug use (if urine tests and clinical manifestation of hepatitis are undetected). Because the SC route bypasses first-pass metabolism, buprenorphine interactions with CYP450 inducers and/or inhibitors should be limited.
The concomitant use of benzodiazepines with buprenorphine does increase the risk of serious adverse events and death, but the mechanism of this interaction remains unclear. Benzodiazepines alone do not cause respiratory depression, but there is synergistic effect that occurs when they are combined with opioids. It has been speculated that both benzodiazepines (via GABA) and opioids (via mu opioid receptor agonism) depress medullary controls for respiration (White and Irvine 1999); however, in a Drug Safety Communication (FDA 2017c), the FDA has advised that careful medication management can reduce these risks and that buprenorphine should not be uniformly withheld from OUD patients taking benzodiazepines Drug Safety Communication (FDA 2017c). Harms caused by withholding effective medication treatment with buprenorphine may outweigh the risks of concomitant prescribed and supervised use of these two medications.
Naloxone in Buprenorphine Combination Products
The incorporation of naloxone to transmucosal buprenorphine products was designed to decrease misuse of buprenorphine products via IN and IV routes of administration. Naloxone is a high affinity mu opioid receptor antagonist with a short half-life and rapid dissociation (~6.5 min) from the mu opioid receptor. When buprenorphine/naloxone is ingested via prescribed routes, naloxone is essentially inert due to poor oral and sublingual bioavailability followed by first-pass metabolism and elimination; however, when insufflated or injected, naloxone is bioavailable and can precipitate withdrawal. Sublingual bioavailability of naloxone has been estimated to be about 3% in the first FDA-approved buprenorphine/naloxone formulation (FDA, 2002), while naloxone intranasal bioavailability (i.e., of the crushed and snorted SL tablet) is estimated to be 30% (Middleton et al. 2011). Laboratory studies reliably report reduced abuse liability of buprenorphine/naloxone compared to buprenorphine alone (Comer and Collins 2002, Comer et al. 2010, Middleton et al. 2011, Walsh et al. 2016). A review of available epidemiological evidence also reports that buprenorphine/naloxone is injected less frequently than the buprenorphine monoproducts (Lofwall and Walsh 2014).
Overview of Sublingual and Other Transmucosal Buprenorphine Products
The safety of transmucosal buprenorphine formulations has been well established and is similar among products. Side effects listed in transmucosal buprenorphine package inserts include common mu opioid receptor agonist effects (e.g., constipation, abdominal pain, nausea, sweating, headache, drowsiness, insomnia, dizziness, respiratory depression). While transmucosal products vary modestly in bioavailability, Cmax, AUC, dissolve time, and flavoring, they are otherwise largely comparable. Year of FDA approval, available doses, cost per month, and dissolution time are listed in Table 1.
Table 1.
Approved Buprenorphine Products
| Formulations | FDA approval year | Available doses (mg) | Total cost per montha | Dissolution time (min) |
|---|---|---|---|---|
| Buprenorphine/Naloxone Tablet | 2002 for brand name productsb | 2/0.5 and 2 | $154 | 7 – 12.4 |
| 2013 and after for generics | 8/2 and 8 | $164 | ||
| Buprenorphine/Naloxone Film | 2010 | 2/0.5 | $131 | 5 – 6.6 |
| 4/1 | $222 | |||
| 8/2 | $222 | |||
| 12/3 | $432 | |||
| Buprenorphine/Naloxone Tablet (Zubsolv®) | 2013 | 0.7/0.18 | $131 | 5 |
| 1.4/0.36 | $131 | |||
| 2.9/0.71 | $254 | |||
| 5.7/1.4 | $254 | |||
| 8.6/2.1 | $377 | |||
| 11.4/2.9 | $499 | |||
| Buprenorphine/Naloxone Film (Bunavail®) | 2014 | 2.1/0.3 | $252 | 30 |
| 4.2/0.7 | $252 | |||
| 6.3/1.0 | $495 | |||
| 6-month Buprenorphine Implant (Probuphine®) | 2016 | 320 | $825 | N/A |
| Monthly Buprenorphine Depot(Sublocade®) | 2017 | 100 | $1,580 | N/A |
| 300 | $1,580 | |||
N/A not applicable
Total cost for transmucosal products is assuming once daily use for 30 days. Total cost for the 6-month product is $4,950. Prices taken from drugs.com on February 1, 2018.
The brand name mono-product tablet was no longer marketed after 2011, but the generic mono-product remains available and is still used for patients with naloxone sensitivities and for pregnant women. The brand name combination product is also no longer available.
Prior to development and FDA approval of the SL buprenorphine tablet in 2002, research was conducted using a SL liquid consisting of buprenorphine dissolved in an ethanol/water solution (Kuhlman et al. 1996) and administered directly under the tongue. It was never commercially available. This solution had greater bioavailability than the subsequently marketed SL tablets. The first FDA-approved buprenorphine SL tablets have a relative bioavailability of .70 to the ethanol/water solution (FDA, 2002) that was used in early buprenorphine studies (e.g. Walsh et al. 1994).
After the initial buprenorphine tablet products were approved (buprenorphine [Subutex®, Reckitt Benckiser Healthcare, Slough, UK] and buprenorphine/naloxone [Suboxone®, Indivior UK Limited, Slough, UK]), others entered the market through the FDA 502b pathway. This pathway allows new medications with the same active ingredient as an approved product to use safety and efficacy data from that product as long as the new product is bioequivalent (i.e., no significant difference between the drugs in acute dosing pharmacokinetic parameters, such as AUC0-inf and Cmax using a 90% confidence interval (FDA 2013). Consequently, chronic dosing pharmacokinetics are unavailable for most of the transmucosal buprenorphine products marketed subsequent to the original.
As there are small differences in transmucosal products’ PK parameters, it is possible that patients switching formulations (e.g., when insurance coverage changes) may note differences in response and dose adjustments could be needed. For example, if a patient maintained comfortably on 4.2mg of the higher bioavailability film is switched to 8mg of the generic tablet and complains of mild withdrawal, the physician can consult Table 2 and see that the new medication may produce lower buprenorphine plasma concentrations than the previous one; thus, the report of withdrawal is not unexpected. Of course, individual differences in absorption and metabolism mean that PK values can only be used as guidelines and ultimately dosing regimens should be titrated to individual patient needs. This next section discusses each transmucosal formulation and how it compares to the original buprenorphine/naloxone tablet formulation approved in 2002.
Table 2.
Pharmacokinetics of Buprenorphine Formulations
| Formulations | Doses (mg) | Single Dose |
Steady State |
|||
|---|---|---|---|---|---|---|
| Cmax (ng/mL) | AUC0-inf (h*ng/mL) | Cmin (ng/mL) | Cavg (ng/mL) | Cmax (ng/mL) | ||
| Buprenorphine/Naloxone Tablet | ||||||
| Brand namea | 2/0.5 and 2 | -- | -- | -- | -- | -- |
| 8/2 and 8 | 3.00 | 20.22b | 0.65 | 1.19 | 4.74 | |
| 12 | -- | -- | 0.81 | 1.71 | 5.35 | |
| 24 | -- | -- | 1.54 | 2.91 | 8.27 | |
| Generic group 1c | 2/0.5 | 0.95 | 8.65 | -- | -- | -- |
| 8/2 | 3.37 | 30.45 | -- | -- | -- | |
| Generic group 2d | 2/0.5 | 0.78 | 7.65 | -- | -- | -- |
| 8/2 | 2.58 | 25.31 | -- | -- | -- | |
| Generic group 3e | 2/0.5 | 1.25 | 10.93 | -- | -- | -- |
| 8/2 | 2.88 | 28.39 | -- | -- | -- | |
| 16/4 | 4.70 | 47.09 | -- | -- | -- | |
| Buprenorphine/Naloxone Film | 2/0.5 | 1.07 | 8.43 | -- | -- | -- |
| 4/1 | 1.66 | 14.62 | -- | -- | -- | |
| 8/2 | 3.55 | 30.66 | -- | -- | -- | |
| 12/3 | 4.80 | 41.74 | -- | -- | -- | |
| Buprenorphine/Naloxone Tablet (Zubsolv®) | 0.7/0.18 | -- | -- | -- | -- | -- |
| 1.4/0.36 | 0.81 | 7.01 | -- | -- | -- | |
| 2.9/0.71 | -- | -- | -- | -- | -- | |
| 5.7/1.4 | 2.66 | 23.51 | -- | -- | -- | |
| 8.6/2.12 | 3.68 | 32.27 | -- | -- | -- | |
| 11.4/2.9 | 4.58 | 41.51 | -- | -- | -- | |
| Buprenorphine/Naloxone Film (Bunavail®) | 2.1/0.3 | -- | -- | -- | -- | -- |
| 4.2/0.7 | 3.41 | 27.17 | -- | -- | -- | |
| 6.3/1.04 | 4.90 | 38.47 | -- | -- | -- | |
| Buprenorphine Implant (Probuphine®) | 320 | ~2.6f | 19.6g | ~0.6f | ~0.82 | ~2.6f |
| Monthly Buprenorphine Depot (Sublocade®) | 100 | 1.54 | 1557.40 | 2.48 | 3.21 | 4.88 |
| 300 | 5.37 | -- | 5.01 | 6.54 | 10.12 | |
| Buprenorphine Depot (CAM2038) | ||||||
| Weekly | 16 | 3.08 | 335 | 0.84 | 2.09 | 4.30 |
| 24 | 3.64 | -- | -- | -- | -- | |
| 32 | 5.27 | 638 | 2.63 | 4.17 | 6.87 | |
| Monthly | 64 | 3.81 | 1360 | -- | -- | -- |
| 96 | 5.47 | 1830 | -- | -- | -- | |
| 128 | 6.59 | 2550 | 2.09 | 3.89 | 11.1 | |
| 160 | -- | -- | 2.66 | 5.27 | 15.4 | |
Unless otherwise noted, data are taken from product package inserts and Center on Drug Evaluation Research Clinical Biopharmaceutical Review Packages
The brand name product is no longer marketed. The generic products are bioequivalent; thus, pharmacokinetic parameters are similar for all buprenorphine/naloxone tablets.
AUC0–48, not 0-inf
Manufacturers using these pharmacokinetic data in their package inserts are Amneal Pharmaceuticals, LLC and Ethypharm SA
Manufacturers using these pharmacokinetic data in their package inserts are Kremers Urban Pharmaceuticals and SpecGx LLC.
Manufacturers using these pharmacokinetic data in their package inserts are: Actavis Pharma Inc.; Sun Pharmaceutical Industries Limited; Teva Pharmaceuticals USA, Inc.; and West-Ward Pharmaceuticals Corp.
Estimated based on graph from Beebe et al. (2009) poster: Safety, Efficacy, and Pharmacokinetics of Probuphine®, a 6-Month Implantable Sustained-Release Formulation of Buprenorphine, for the Treatment of Opioid Addiction
AUC0–24, not 0-inf
Buprenorphine/naloxone Film (Suboxone®, Indivior Inc. Richmond, VA) delivers higher peak and plasma buprenorphine concentrations compared to the 2/0.5 and 8/2 tablet product (see Table 2).
Buprenorphine/naloxone sublingual tablets (Zubsolv®, Orexo US, Inc. Morristown, NJ) have higher bioavailability than the original buprenorphine/naloxone tablets. A 5.4/1.7mg and 1.4/0.36 dose produce exposure bioequivalent to 8/2mg and 2/0.5 buprenorphine/naloxone tablet, respectively (per package insert). Notably, the 0.7/0.18mg buprenorphine/naloxone dose is the lowest dose available.
Buprenorphine/naloxone buccal film (Bunavail®, Biodelivery Sciences International, Inc., Raleigh, NC) has higher bioavailability than all other transmucosal products. According to the FDA, a 4.2/0.69mg dose produces buprenorphine concentrations approximately equivalent (3.4 ng/ml Cmax) to 8mg buprenorphine/naloxone tablet (3.0 ng/ml Cmax, see Table 2).
Overview of Implantable and Injectable Buprenorphine Formulations
Novel buprenorphine products with unique delivery systems are being developed at a rapid pace. Two long-acting products were approved in the past two years, a 6-month subdermal implant and a monthly injectable sustained-release formulation. Another weekly and monthly injectable is in late-stage development (see Table 3 for indications, common adverse events, excipient toxicology).
Table 3.
Long-acting buprenorphine formulations
| Indication (s) | Adverse events occurring in >5% of patients | Excipients & toxicology | Delivery system | Storage and administration | |
|---|---|---|---|---|---|
| 6-month Buprenorphine Implant (Prohuphinc®) | Maintenance for patients who have achieved prolonged stability on ≤ 8mg TM buprenorphine | Headache, depression, constipation, nausea, vomiting, back pain, toothache, oropharyngeal pain and injection site reactions (pain, pruritus, & erythema) | N/A | Ethylene vinyl acetate (EVA) implants | Subdermal insertion of 4 flexible matchstick-sized rods in upper arm under local anesthetic by trained provider. |
| 11 FDA-approved productsa | |||||
| Monthly Buprenorphine Depot (Suhlocade®) | Maintenance treatment (to be used after initiation on TM buprenorphine) | Constipation, nausea, vomiting, fatigue, elevated liver enzymes, headache, and injection site reactions (pain, pruritus, & erythema) | NMP - At doses equivalent to those in the depot, reproductive harmsb were reported in animal studies. | ATIRGEL® (Tolmar Inc.) | Pre-filled syringe (refrigeration required). SC abdominal injection by trained provider. Injection volume of .5 and 1.5mL. |
| 7 FDA-approved productsc | |||||
| Weekly and Monthly Buprenorphine Depot (CAM 2038) | PROPOSED: Induction and maintenance treatment | Headache, nausea, urinary tract infection, constipation, nasopharyngitis, and injection site reactions (pain, swelling & erythema) | NMPb - dose used has a 3-fold safety margin for reproduction | FluidCyrstal® Technology (Camurus) | Pre-filled syringe (no refrigeration required) with injection volume of .16 to .64mL. SC injection into buttock by trained provider. |
| 1 FDA-approved productc | |||||
BUP— Buprenorphine, TM— Transmucosal, SC—Subcutaneous, NMP— N-methyl-2-pyrrolidase (a biocompatible solvent)
Examples of approved products with EVA: etonogestrel/ethinyl estradiol vaginal ring [NuvaRing®, Organon/Merck & Co, Kenilworth, NJ], birth control implants [Implanon®, Organon/Merck & Co. Kenilworth, NJ], and pilocarpine - ophthalmic ocular system [Ocusert® Alza Mountain View, CA]
Preimplantation losses, delayed ossification, reduced fetal weight, developmental delays and reduced cognitive function
Examples of approved products with Atrigel®: leuprolide acetate suspension for subcutaneous injection [Eligard®, Tolmar Inc. Fort Collins, CO] and doxycycline hyclate [Atridox®, Tolmar Inc. Fort Collins, CO] and doxycycline hyclate [Atridox®, Tolmar Inc. Fort Collins, CO]
NMP is present in the monthly formulation only
Oral spray for oral mucositis pain [Episil®, Camurus Lund, Sweden]
Buprenorphine Subdermal Implant (Probuphine®, Titan Pharmaceuticals, San Francisco, CA) This product consists of four matchstick-sized rods each containing 80mg (total 320mg). A short office-based procedure for insertion and removal is needed, and training is required by the pharmaceutical company. A provider can be certified to prescribe, insert or both prescribe and insert the implant; both require a waiver. The steady-state plasma buprenorphine concentration is slightly less than that produced by 8mg daily SL buprenorphine (Ling et al. 2010); thus, it is recommended for patients already stable on ≤8mg buprenorphine. While clinical judgement can be used to extend treatment duration, the package insert recommends only two successive implants (i.e., one year of treatment) before transitioning back to transmucosal buprenorphine—primarily because there is inadequate experience with repeated insertions and removals to date.
Efficacy:
An initial study in new-to-treatment patients with OUD demonstrated that the implant was superior to placebo at suppressing withdrawal and craving and reducing illicit opioid use (Ling et al. 2010). A second small study with new-to-treatment patients compared the active implant vs. placebo implant vs. open-label SL buprenorphine and similarly found superiority over placebo and no differences between the active implant and SL buprenorphine (Rosenthal et al., 2013). A larger 6-month, double-blind, double-dummy, non-inferiority study enrolled patients who were already in treatment and stable on transmucosal buprenorphine at ≤8mg and randomized to either the active implant (plus placebo SL tablets) or 8mg SL buprenorphine/naloxone (and placebo implants). The implant was non-inferior to SL buprenorphine/naloxone at suppressing withdrawal and craving and reducing illicit opioid use, and more implant patients maintained illicit opioid abstinence for the duration of the study (81%) compared to those on SL buprenorphine/naloxone (67%) (Rosenthal et al. 2016).
Because the implant reduces patient burden by limiting reliance on daily medication, treatment adherence may be higher with the implant than with SL dosing regimens. Retention rates for the 6-month studies (which required monthly visits) were 65.7% and 96.4% (Rosenthal et al. 2013, Rosenthal et al. 2016), with the observed difference likely attributable to the earlier study enrolling unstable patients.
Safety:
Implant site adverse events (AEs) occurred in 23 (Rosenthal et al. 2016) to 56.5% (Ling et al. 2010) of subjects. In the outpatient trials, there were no AEs related to respiratory depression or overdose in the persons receiving implants. In the control groups receiving SL buprenorphine/naloxone and placebo, two volunteers were admitted to inpatient treatment programs, one hospitalized for circumstances related to illicit opioid use, and one experienced non-fatal respiratory failure. While improper implant insertion/removal can theoretically lead to complications including nerve damage, implant migration, and protrusion and expulsion, none of these events occurred in the outpatient trials (n=309 and n=198 active and placebo implants, respectively) (Ling et al. 2010, Rosenthal et al. 2013, Rosenthal et al. 2016).
Monthly Subcutaneous Buprenorphine Injection Depot (Sublocade®, formerly RBP-6000 [Indivior Inc. Richmond, VA]) was approved by the FDA in late 2017 and is now available in two doses: 100mg and 300mg. The recommended treatment regimen is two 300mg monthly doses followed by 100mg doses thereafter; however, the FDA is requiring the pharmaceutical company to conduct a post-marketing study to determine if the depot is effective when administered at inter-dose intervals exceeding the currently approved 1-month interval. Its long t½ (~38 days [FDA 2017a]) and the observation that the depot produces higher buprenorphine plasma concentrations with each subsequent dose suggests that patients may be adequately treated at longer dosing intervals.
Efficacy:
In a Phase II trial (Nasser et al. 2016), assessing the ability of the depot to block effects of an opioid agonist, non-treatment seeking volunteers with OUD were inducted and stabilized on 8 to 24mg SL buprenorphine and then administered two doses of the 300mg depot 4 weeks apart. Hydromorphone (6 and 18mg, IM) was administered before and up to 8 weeks after the second depot injection. The 300mg depot produced complete blockade of 6mg hydromorphone for 4 weeks after the first injection and complete blockade of 18 mg hydromorphone for 3 weeks (with partial blockade during the 4th week). During Phase III outpatient trials evaluating the efficacy of the depot to reduce withdrawal, craving, and illicit opioid use, participants with OUD were inducted onto SL buprenorphine for up to 2 weeks and were then randomized to receive six once-monthly injections of i) 300mg depot, ii) two doses of 300mg followed by four doses of 100mg, iii) or matched-volume placebo injections. Withdrawal severity, as measured by the COWS, was low throughout the 24-week treatment period for all participants (<2 with active buprenorphine, <4 with placebo). The low withdrawal scores in the placebo group may be explained by the ongoing use of illicit opioids.
Significantly more patients receiving either active dosing regimen (300/300 or 300/100) of the depot provided illicit-opioid-negative urine samples with corroborating self-report on at least 80% of testing days compared to placebo (active: 28.4%, placebo: 2%). There were no differences in efficacy between the two dosing regimens (i.e., no dose-dependency in abstinence rates or reduction in withdrawal), but adverse events were more frequent with the higher dose. These findings suggested that the lower dosing regimen is likely appropriate for most patients, and this is what is recommended in the package insert. Secondary analyses suggested that certain sub-populations (e.g., people who inject drugs) may benefit from the higher dose regimen, and the pharmaceutical company is required to conduct a post-marketing study to identify those for whom the benefits of the 300mg per month dose outweigh the risks of the higher dose (i.e., clinically meaningful hepatotoxicity—see below) (FDA 2017d).
Safety:
The overall safety profile of this monthly depot product is generally consistent with the safety profile of buprenorphine, with the addition of injection site adverse events and buprenorphine exposures for the 300mg dose that exceed what is recommended by the FDA (i.e., equivalent up to 24mg SL buprenorphine). Long-term safety data for patients administered multiple doses of the 300mg injection are not available. The local tolerability of the abdominal injection was similar to other approved products using the sustained-release delivery system, and most injection site reactions were of mild-to-moderate severity. The 300mg regimen produced greater drop-out and more AEs related to injection site reactions and elevations in hepatic enzymes compared to the 100mg dosing regimen. Thirty percent of participants receiving the 300mg depot in the Phase III study required dose reductions to 100mg; a quarter of these dose reductions were necessary because of AEs. The most common AEs leading to dose reduction included abnormal liver function tests, sedation, constipation, nausea, fatigue and headache. Specifically, the incidence of elevations 3x the upper limits of normal in AST were 11.4% (300mg depot doses each month for 3 months), 7.9% (300mg depot dose first two months then 100mg in the third month), and 1.0% (placebo depot dose each month). Results for incident ALT elevations 3x the upper limits of normal were similar (300mg depot doses each month for 3 months: 12.4%, 300 mg depot dose first two months then 100 mg in third month: 5.4%, and placebo: 4.0%). The most common AEs leading to medication discontinuation included elevated liver enzymes, injection site reactions, sedation, constipation, somnolence, lethargy, and drug withdrawal syndrome. In two cases, surgical removal of the depot was necessary. The FDA package insert states “Liver function tests, prior to initiation of treatment, are recommended to establish a baseline. Monthly monitoring of liver function during treatment, particularly with the 300mg maintenance dose, is also recommended. An etiological evaluation is recommended when a hepatic event is suspected…If signs and symptoms of toxicity or overdose occur within 2 weeks of administration, removal of the depot may be required.” Surgical removal of the depot is possible within 14 days of injection: after 14 days, the depot is likely not removable.
Weekly and Monthly Subcutaneous Buprenorphine Injection Depot (CAM2038 [Braeburn Pharmaceuticals, Princeton, NJ]) This product is in development and not yet FDA approved although a FDA Advisory Committee voted in favor of approval in November 2017. The company received a Complete Response Letter from the FDA on January 19, 2018 indicating that the agency had outstanding questions related to the CAM2038 product (PRNewswire 2018). While the letter is not publicly available, no further clinical trials were mandated, making it possible that this product could still be approved in the near future. Doses in development and PK parameters are listed in Table 2.
Efficacy:
In an inpatient laboratory study, non-treatment seeking adults with OUD and physical dependence received two SC weekly injections of either 24 or 32mg (estimated equivalence to 16 and 24mg daily SL buprenorphine, respectively) and were challenged with hydromorphone (0, 6 & 18mg, IM) before and up to 6 days after each injection. Both doses produced complete blockade of 18mg hydromorphone for 6 days (Walsh et al. 2017). In a 6-month outpatient trial evaluating the efficacy of the SC depot compared to SL buprenorphine/naloxone (Lofwall et al. 2018), patients with OUD were randomized to receive 12 injections of the weekly depot followed by 3 injections of the monthly depot or to receive daily SL buprenorphine/naloxone. Both SL and SC depot dosing was flexible and based on patient needs using clinical judgment, as would occur in clinical practice (FDA 2017b). In both groups, withdrawal and craving were suppressed on Day 1 and remained low throughout the study, including during the transition to monthly injections. Retention was similar between groups, and a significantly greater percentage of patients who received the SC depot provided illicit-opioid-negative urine samples in weeks 4 through 24 of the study than those who received SL buprenorphine/naloxone. The authors concluded that CAM was an efficacious treatment for OUD with potential advantages over SL buprenorphine/naloxone.
Safety:
Among patients with OUD, the SC depot produced a safety profile consistent with that of transmucosal buprenorphine, with additional AEs related to the injections. Localized injection site reactions occurred in ~7 (Haasen et al. 2017) to 9% (Walsh et al. 2017) of subjects. The highest doses of the depot produced buprenorphine exposures that exceed what is recommended by the FDA (i.e., ~ 24mg SL buprenorphine; see Table 2) and long-term safety data for patients exposed to these doses are lacking. No incidences of serious respiratory depression were reported for persons receiving the SC depot. Notably, in the 6-month outpatient trial comparing the SC depot to SL buprenorphine/naloxone, five drug overdoses occurred in the group randomized to receive SL buprenorphine/naloxone while none occurred in the SC depot group (Lofwall et al. 2018).
Conclusions
Buprenorphine can now be administered in daily, monthly, and twice-yearly doses, and clinicians have a growing number of treatment options for patients new to treatment and those who are more stable. Theoretically, implantable and injectable buprenorphine products should increase adherence and reduce diversion or misuse but empirical data are not yet available to confirm these potentially important patient and public health benefits. These products may also obviate several patient concerns, including the risk of having their prescription stolen, traveling while carrying a controlled substance (this is illegal in some countries), needing to safely store their medication away from children, and the daily reminder of their OUD that comes with daily medication. It will be important for clinicians to consider patient status in choosing the most beneficial option. Because the buprenorphine implant produces comparatively lower plasma concentrations, it is most suited for patients already stabilized on buprenorphine at ≤8mg. While it may be very convenient because of low patient burden, acquiring, inserting and removing the implant places more initial burden on the provider. The approved monthly injectable produces the highest buprenorphine plasma concentrations of the marketed products; therefore, it may be most appropriate for those with greater physical dependence or already on higher daily doses. The injectable product may be helpful too for patients transitioning between different treatment settings. For example, patients leaving the hospital after treatment for a complication related to OUD (e.g., endocarditis) and stabilized on buprenorphine in-hospital may have a delay connecting to outpatient care after discharge, and the injectable could be provide coverage during the treatment gap. This product provides higher buprenorphine concentrations and could be beneficial for patients who are at high risk of illicit opioid use and overdose. As this product has been used only after stabilization on SL buprenorphine, it may not be suitable for direct induction, (e.g., in an emergency department setting) unless further data verify that direct induction is safely tolerated. The as-yet unapproved depot product is being developed in multiple doses and as both weekly and monthly formulations—which would provide further flexibility for both patients and providers. Experience to date with that product has shown that direct induction onto the weekly injectable is well tolerated as is initiation of treatment after a test dose of SL buprenorphine (i.e., prior stabilization on SL buprenorphine is not required). The highest doses of both injectable depot products do raise some concerns as they expose patients to buprenorphine at much higher concentrations than what has been previously deemed safe by the FDA (see Table 2). Because buprenorphine exhibits a ceiling effect (due to its low efficacy), increasing doses beyond a certain point may provide no additional clinical benefit. That ceiling effect has been suggested to occur at or near the equivalent of 24mg/day transmucosal buprenorphine by both the FDA and ASAM.
Treatment outcomes (and the corresponding buprenorphine dose prescribed) must be determined on a case-by-case basis, and not all outcomes are equally important in all phases of treatment. Reducing the risk of relapse to illicit opioid use by buprenorphine is achieved through withdrawal suppression, craving suppression, and blockade of agonist effects but the relative importance of each may change depending on patient status. For example, withdrawal suppression is critical throughout treatment as the anticipation and experience of withdrawal are highly stressful and key risk for relapse. Likewise, reducing a patient’s desire to use illicit opioids (i.e., craving) is critically important but, with time in treatment, the frequency or intensity of craving may diminish. Opioid blockade is an important benefit of treatment especially for patients new to treatment and unstable or for those who continue to use illicit opioids intermittently while in treatment. Blockade may be less critical for those who are stable and abstaining from illicit use successfully. For example, it would be expected that the implant product would produce limited blockade because it yields lower plasma concentrations, but it was quite effective in patients already stable and abstaining. Similarly, treatment with lower daily SL buprenorphine (mean =9.6mg [Dupouy, private communication]) was associated with an ~30 times lower mortality rate among persons with OUD compared to those not in treatment (Dupouy et al. 2017). Thus, both lower and higher doses of buprenorphine have been shown to be efficacious, and doses can vary considerably based on patient needs. Prescribers should, as always, use the lowest effective dose to achieve the desired treatment outcomes and now have more options from which to choose.
Sources of funding:
Over the last three years, Dr. Lofwall has had research funding from Braeburn, has received consulting fees from Braeburn and Indivior, travel support from Braeburn and Camurus, and has received honorarium from PCM Scientific for developing and giving educational talks about opioid use disorder. Dr. Walsh has received research funding and consulting fees and travel support from Braeburn and Camurus and travel support and honoraria from Indivior.
Footnotes
Conflicts of interest: Ms. Coe reports no disclosures.
References
- 1.U.S. Food and Drug Administration/Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review: Suboxone® (Buprenorphine HCl/Naloxone HCl) 2mg/0.5mg, 8mg/2mg Sublingual Tablets. September 10, 2002.
- 2.Amass L, Bickel WK, Higgins ST, Badger GJ. Alternate-day dosing during buprenorphine treatment of opioid dependence. Life Sci 1994;54:1215–28. [DOI] [PubMed] [Google Scholar]
- 3.The American Society of Addiction Medicine. Public Policy Statement on Morphine Equivalent Units/Morphine Milligram Equivalents. Available at: https://www.asam.org/advocacy/find-a-policy-statement/view-policy-statement/public-policy-statements/2016/10/11/public-policy-statement-on-morphine-equivalent-units-morphine-milligram-equivalents. Accessed December 10, 2017.
- 4.Auriacombe M, O’Brien CP, Tignol J. Buprenorphine in the treatment of opiate dependence. Ann Med Interne (Paris) 1994;145 Suppl 3:27. [PubMed] [Google Scholar]
- 5.Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology (Berl) 1999;146:111–8. [DOI] [PubMed] [Google Scholar]
- 6.Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther 1988;247:47–53. [PubMed] [Google Scholar]
- 7.Boas RA, Villiger JW. Clinical actions of fentanyl and buprenorphine. The significance of receptor binding. Br J Anaesth 1985;57:192–6. [DOI] [PubMed] [Google Scholar]
- 8.Brown SM, Campbell SD, Crafford A, Regina KJ, Holtzman MJ, Kharasch ED. P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception. J Pharmacol Exp Ther 2012;343:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawarski MC, Moody DE, Pakes J, O’Connor PG, Schottenfeld RS. Buprenorphine tablet versus liquid: a clinical trial comparing plasma levels, efficacy, and symptoms. J Subst Abuse Treat 2005;29:307–12. [DOI] [PubMed] [Google Scholar]
- 10.Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther 2002;303:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comer SD, Sullivan MA, Vosburg SK, et al. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction 2010;105:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compton P, Ling W, Chiang CN, et al. Pharmacokinetics of buprenorphine: a comparison of sublingual tablet versus liquid after chronic dosing. J Addict Med 2007;1:88–95. [DOI] [PubMed] [Google Scholar]
- 13.Correia CJ, Walsh SL, Bigelow GE, Strain EC. Effects associated with double-blind omission of buprenorphine/naloxone over a 98-h period. Psychopharmacology (Berl) 2006;189:297–306. [DOI] [PubMed] [Google Scholar]
- 14.Cowan A Buprenorphine: new pharmacological aspects. Int J Clin Pract Suppl 2003;3–8; discussion 23–4. [PubMed] [Google Scholar]
- 15.Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 1977;60:537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley NA, Kash TL. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 2015;62:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 2005;94:825–34. [DOI] [PubMed] [Google Scholar]
- 18.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain. MMWR Recomm Rep 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 19.Dupouy J, Palmaro A, Fatseas M, et al. Mortality Associated With Time in and Out of Buprenorphine Treatment in French Office-Based General Practice: A 7-Year Cohort Study. Ann Fam Med 2017;15:355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eissenberg T, Johnson RE, Bigelow GE, et al. Controlled opioid withdrawal evaluation during 72 h dose omission in buprenorphine-maintained patients. Drug Alcohol Depend 1997;45:81–91. [DOI] [PubMed] [Google Scholar]
- 21.Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet 2005;44:661–80. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration/Center for Drug Evaluation and Research. Guidance for Industry: Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA. December 2013. Available at: http://academy.gmp-compliance.org/guidemgr/files/UCM377465.PDF. Accessed November 4, 2017.
- 23.U.S. Food and Drug Administration/Center for Drug Evaluation and Research. FDA Briefing Document on NDA 209819 for RBP-6000 (buprenorphine injectable) for treatment of opioid dependence. October 31, 2017a.
- 24.U.S. Food and Drug Administration/Center for Drug Evaluation and Research. FDA Briefing Document on NDA 210136 for CAM2038 (buprenorphine injectable) for treatment of opioid dependence. November 1, 2017b.
- 25.U.S. Food and Drug Administration. Drug Safety Communication: FDA urges caution about withholding opioid addiction medications from patients taking benzodiazepines or CNS depressants: careful medication management can reduce risks. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm575307.htm. Accessed February 10, 2018.
- 26.U.S. Food and Drug Administration/Center for Drug Evaluation and Research. NDA Approval: SUBLOCADE. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/209819Orig1s000ltr.pdf. Accessed February 10, 2018.
- 27.Haasen C, Linden M, Tiberg F. Pharmacokinetics and pharmacodynamics of a buprenorphine subcutaneous depot formulation (CAM2038) for once-weekly dosing in patients with opioid use disorder. J Subst Abuse Treat 2017;78:22–29. [DOI] [PubMed] [Google Scholar]
- 28.Harris DS, Jones RT, Welm S, Upton RA, Lin E, Mendelson J. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend 2000;61:85–94. [DOI] [PubMed] [Google Scholar]
- 29.Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 2001;297:688–95. [PubMed] [Google Scholar]
- 30.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry 1978;35:501–16. [DOI] [PubMed] [Google Scholar]
- 31.Karp JF, Butters MA, Begley AE, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry 2014;75:e785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhlman JJ Jr., Lalani S, Magluilo J Jr., Levine B, Darwin WD. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol 1996;20:369–78. [DOI] [PubMed] [Google Scholar]
- 33.Kuhlman JJ Jr., Levine B, Johnson RE, Fudala PJ, Cone EJ. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction 1998;93:549–59. [DOI] [PubMed] [Google Scholar]
- 34.Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA 2010;304:1576–83. [DOI] [PubMed] [Google Scholar]
- 35.Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med 2014;8:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and Monthly Buprenorphine Depots for Outpatient Opioid Use Disorder Treatment: A Randomized Clinical Noninferiority Trial. JAMA Intern Med 2018;178:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction 2011;106:1460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasser AF, Greenwald MK, Vince B, et al. Sustained-Release Buprenorphine (RBP-6000) Blocks the Effects of Opioid Challenge With Hydromorphone in Subjects With Opioid Use Disorder. J Clin Psychopharmacol 2016;36:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.PRNewswire. Braeburn Receives Complete Response Letter for CAM2038 Injectable Buprenorphine Depot for the Treatment of Opioid Use Disorder. Available at: https://www.prnewswire.com/news-releases/braeburn-receives-complete-response-letter-for-cam2038-injectable-buprenorphine-depot-for-the-treatment-of-opioid-use-disorder-300585623.html. Accessed Febraury 2, 2018.
- 40.Rosenthal RN, Ling W, Casadonte P, et al. Buprenorphine implants for treatment of opioid dependence: randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction 2013;108:2141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenthal RN, Lofwall MR, Kim S, et al. Effect of Buprenorphine Implants on Illicit Opioid Use Among Abstinent Adults With Opioid Dependence Treated With Sublingual Buprenorphine: A Randomized Clinical Trial. JAMA 2016;316:282–90. [DOI] [PubMed] [Google Scholar]
- 42.Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/Naloxone and Methadone Effects on Laboratory Indices of Liver Health: a Randomized Trial. Drug Alcohol Depend 2013; 128:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz RP, Gryczynski J, O’Grady KE, et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am J Public Health 2013;103:917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GE. Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug Alcohol Depend 2004;74:37–43. [DOI] [PubMed] [Google Scholar]
- 45.Strain EC, Walsh SL, Bigelow GE. Blockade of hydromorphone effects by buprenorphine/naloxone and buprenorphine. Psychopharmacology (Berl) 2002;159:161–6. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan LE, Fiellin DA. Buprenorphine: its role in preventing HIV transmission and improving the care of HIV-infected patients with opioid dependence. Clin Infect Dis 2005;41:891–6. [DOI] [PubMed] [Google Scholar]
- 47.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med 2014;370:2063–6. [DOI] [PubMed] [Google Scholar]
- 48.Volpe DA, McMahon Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol 2011;59:385–90. [DOI] [PubMed] [Google Scholar]
- 49.Walsh SL, Comer SD, Lofwall MR, et al. Effect of Buprenorphine Weekly Depot (CAM2038) and Hydromorphone Blockade in Individuals With Opioid Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2017;74:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh SL, Nuzzo PA, Babalonis S, Casselton V, Lofwall MR. Intranasal buprenorphine alone and in combination with naloxone: Abuse liability and reinforcing efficacy in physically dependent opioid abusers. Drug Alcohol Depend 2016;162:190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clinical Pharmacol Ther 1994;55:569–80. [DOI] [PubMed] [Google Scholar]
- 52.Welsh C, Valadez-Meltzer A. Buprenorphine: a (relatively) new treatment for opioid dependence. Psychiatry 2005;2:29–39. [PMC free article] [PubMed] [Google Scholar]
- 53.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction 1999;94:961–72. [PubMed] [Google Scholar]


