Abstract
Magnetic seizure therapy (MST) is a noninvasive neuromodulation therapy under investigation for the treatment of severe neuropsychiatric disorders. MST involves inducing a therapeutic seizure under anesthesia in a setting similar to electroconvulsive therapy (ECT). To date, randomized controlled trials suggest that MST has similar antidepressant efficacy as ECT, but without significant cognitive adverse effects. Large scale clinical trials are currently underway to confirm these preliminary findings. So far, there has only been one study evaluating the clinical predictors of response to MST and more research is needed. This study found that patients with fewer episodes of depression and a positive family history of depression had a better response to MST. Overall, the ability of MST to focus the delivery of the electric field and the resultant seizure makes targeting seizure therapy to specific brain regions possible, and further research will be helpful in identifying personalized targets to maximize clinical benefit. In this review, we describe MST methodology and how it could be individualized to each patient. We also summarize the clinical and cognitive effects of MST and provide indications of which patients may be most likely to benefit. Finally, we summarize the studied neurophysiological predictors of response.
Keywords: Magnetic seizure therapy, MST, neuromodulation, depression, neurocognition
1. Introduction
Electroconvulsive therapy (ECT) has been used to treat neuropsychiatric disorders since the late 1930s and it remains one of the most effective antidepressant therapies for major depressive disorder (MDD) and treatment-resistant depression (TRD; patient with TRD has failed two or more antidepressant treatments with adequate dose and duration) [1]. Other indications for ECT include MDD with psychotic features, bipolar disorder, and catatonia. ECT is also beneficial when a rapid antidepressant response is needed, for example, due to imminent suicidal ideation. Although ECT has been consistently found to have rapid antidepressant effects with high response (typically defined as at least a 50% reduction in the depression scores from baseline) and remission rates (minimal symptoms, typically 10 or less than in depression rating scales such as Hamilton Depression Rating Scale (HDRS)), many patients treated with ECT experience cognitive adverse effects [2]. Such ECT-induced cognitive effects include disorientation, decreased attention, executive dysfunction, anterograde amnesia, and retrograde amnesia [2]. Research has suggested that the ECT-induced cognitive adverse effects are transient, though can persist for up to 6-months or longer after the last ECT treatment [2]. In addition to cognitive adverse effects, ECT is associated with a negative social stigma, and collectively these limit the use of ECT for the severely depressed patients and leave them with chronic, disabling, and often life-threatening depression.
The idea of using magnetic fields to induce antidepressant therapeutic seizures was theorized in the 1990s but required extensive technological development and preclinical testing before the conceptualization of magnetic seizure therapy (MST) [3]. Preclinical studies and computational modeling provided the initial technological and safety information for MST, which then proceeded to clinical investigations [3]. The rationale underlying MST is predicated on the fact that magnetic field induced electric fields can be better controlled and are more focal than directly applied electric fields on the scalp [4]. This is because the magnetic fields pass through the scalp and skull unimpeded and the induced electric field is confined to regions near the stimulation coil [5]. Furthermore, magnetic fields attenuate rapidly, thereby limiting and containing the effects of the induced electric field to the superficial layers of the cortex (see Figure 1). To date, MST has been found to have antidepressant benefits with benign neurocognitive effects [6]. If the preliminary findings across multiple clinical studies are confirmed in large randomized controlled-clinical trials currently underway, MST may provide a new safe and efficacious non-invasive neuromodulation antidepressant therapeutic option.
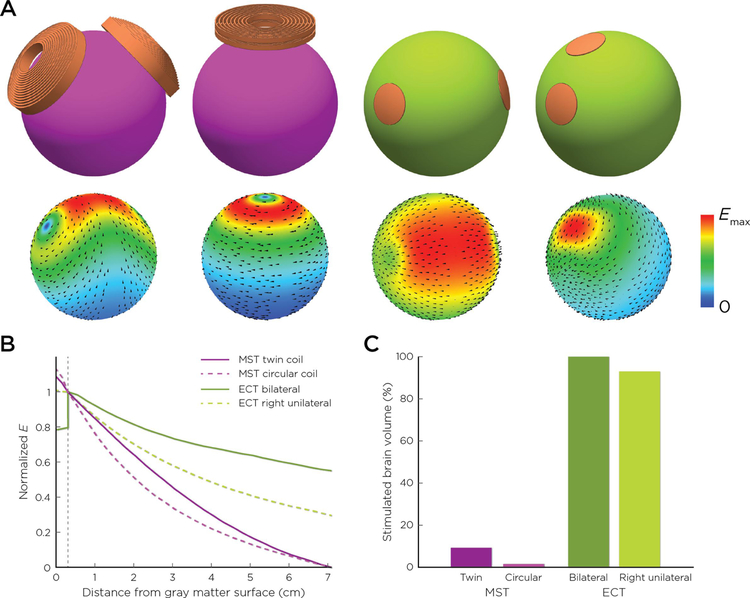
Figure 1.
A. Magnetic seizure therapy (MST)/electroconvulsive therapy (ECT) coil and electrode configurations and corresponding electric field distribution on a spherical head model (from left to right: MagVenture twin coil MST, MagStim circular coil MST, bilateral ECT, right unilateral ECT). B. Electric field as a function of depth in the brain from the gray matter surface (field magnitude is normalized to unity at the gray matter-white matter interface). C. Stimulated brain volume.
2. MST methodology and current treatment procedure
MST technology is based on repetitive transcranial magnetic stimulation (rTMS); however, as the aim is to induce a seizure, the MST stimulation parameters are administered at higher frequencies, intensities, and train durations than in conventional rTMS. MST mainly impacts the cortex directly under the stimulation coil (see Figure 1) with minimal effects to the medial temporal lobe structures (i.e., hippocampus). The applied stimulation frequencies have varied between 25 and 100Hz, and commonly the maximum stimulator output is used as the stimulation intensity. However, it should be noted that the optimal dosing for MST, and how best to individualize the dose, remains an open question.
In general, the MST treatment procedure is similar to that of ECT. Before the first treatment, a complete psychiatric, mental status, neurologic and medical examination are performed. Furthermore, in many research settings comprehensive neurocognitive evaluations are performed. Because MST is a convulsive treatment, it is always administered while the patient is under general anesthesia. The MST dosage is individualized to each patient by finding the patient specific seizure threshold, based on the common practice in ECT. This is accomplished by increasing the duration of MST stimulation train until visible motor activity in a cuffed limb and ictal electroencephalography (EEG) activity can be observed. At subsequent treatment sessions, the therapeutic treatment is administered at a supra-threshold duration (typically six times the seizure threshold). One preclinical study has suggested that seizure threshold in MST could also be titrated based on resting motor threshold (rMT) measured with transcranial magnetic stimulation (TMS), as rMT and amplitude-titrated seizure threshold have been shown to correlate [7]. To date, pulse amplitude titration of seizure threshold has not been investigated in clinical studies.
In research, the MST treatments have been provided 2–3 times per week, every other day, until the depressive symptoms have abated and the patient has remitted, or the maximum allowed treatment number is reached [8, 9]. Also, an accelerated MST protocol was investigated in one clinical trial [10]. In the accelerated MST protocol, treatments were administered once a day for six consecutive week days (e.g., Monday-Friday, and the following Monday). Thus, the MST treatment course was completed in eight days.
3. Seizure characteristics
The ECT-induced seizure is associated with characteristic phases including a recruitment phase where the ictal EEG increases in amplitude, followed by high amplitude polyspike activity. This is followed by slow wave ictal activity that finally leads to a robust suppression of EEG activity after the seizure end (post-ictal suppression). The two most common seizure characteristics that have been reported to be associated with remission after ECT have been ictal slow-wave amplitude and postictal suppression [11, 12].
MST initiates the seizure more focally than ECT, although after initiation the seizure generalizes [13]. Previous studies that compared the seizure characteristics between ECT and MST have been inconclusive mainly due to varying MST parameters across studies as the MST stimulation frequency has been observed to significantly influence the seizure characteristics [9]. To date, no clear trends in seizure characteristics with increasing frequency have been found [9]. Rather, the stimulation frequency seems to influence the seizure characteristics in a non-linear fashion. The only exception is post-ictal suppression, which is dependent on the stimulation frequency as higher frequencies induce greater post-ictal suppression [9]. Interestingly, increasing MST dosage may have no effect on the seizure characteristics [14]. A limitation of these studies is that they have typically used 2-channels of fronto-mastoid EEG, which may not be optimally positioned to record more focal seizure expression depending on their location relative to the MST coil.
In general, it seems that seizure characteristics in MST differ from those of ECT in both ictal and post-ictal phases. Preclinical models have suggested that MST relative to ECT induces a seizure with less theta, alpha and beta power [14, 15], though both show similar EEG power in the delta band. Topographical EEG analysis in humans showed that MST-induced seizures had less robust ictal expression. For MST, there appeared to be local increases in the delta and beta power during the ictal period, consistent with more focal seizure activity of MST compared to ECT. On the other hand, ECT showed greater increase from baseline in all frequency bands, particularly the beta power during the ictal period [16]. Further, the post-ictal suppression associated with MST is less evident and has been reported in both human and non-human primate studies [8, 9, 14, 16]. MST may also induce shorter seizures than ECT [8], but conflicting results have been found [17].
A preliminary clinical study used bispectral index (BIS), which is an index that reflects the anesthetic depth based on EEG activity, to evaluate the level of anesthesia during ECT and MST [18]. The study showed that according to BIS, post-ictal recovery was faster in MST when the assessment was started from the same pre-ictal BIS levels. MST has also been connected with a decreased need for muscle relaxant anesthetic agent dosage relative to what is needed for ECT [19]. The recovery and reorientation times after treatment have consistently been found to be shorter with MST relative to ECT [8, 20]. Indeed, recovery and reorientation typically occur within a few minutes after MST [8, 20].
As ECT is known to influence the autonomic nervous system that may result in cardiac effects, one study compared the effects of MST and ECT to heart-rate variability during the treatment in a preclinical model [21]. That study found bradycardia right after stimulation with ECT, but not with MST. Also, ECT, relative to MST, was found to produce greater rates of tachycardia during the ictal and post-ictal phases. However, MST resulted in significantly increased heart-rate variability during the post-ictal phase in comparison to ECT. Also, both ECT and MST have been associated with acute hemodynamic responses [19].
4. Clinical outcome
Preliminary data has suggested that MST is similar to ECT in terms of antidepressant efficacy. To date, a few dozen clinical trials and case reports have been published that reported MST induced antidepressant effects. From these, most have been open-label studies and only a few have directly compared the clinical efficacy of ECT and MST. Collectively, most of the studies have applied MST in patients with unipolar and bipolar depression, and two in patients with schizophrenia. However, most of the studies have only included a small patient sample and none have included a sham condition. Since MST is still under investigation, previous studies also include some variability in methodology and design. Therefore, large scale randomized controlled clinical trials are warranted to confirm MST antidepressant effects.
In the first study that compared ECT and MST in 2003, Lisanby et al., recruited a total of 10 inpatients with MDD [22]. Three of these patients had bipolar disorder and one had psychotic features. In this study, the acute side effects of MST and ECT were compared in a within-subjects study in which two MST treatments were administered within the right unilateral (RUL) ECT treatment course, i.e., two ECT treatments were replaced with MST. The study was not designed to assess clinical efficacy of MST and ECT. MST was applied with a stimulation frequency of 60Hz with various coil geometry (e.g., round) types. This study demonstrated that MST was safe and feasible. Also, adverse cognitive and subjective side effects with MST were reported to be lower than those after ECT.
After these encouraging results, a larger two-center study compared the clinical efficacy between ECT and MST [19]. In this study, 10 patients with severe MDD treated with MST were compared to 10 patients with severe MDD treated with ECT. MST was provided with a frequency of 50Hz. In both groups, HDRS reduced, however, the reduction was significantly bigger in ECT than in MST group (mean pre-treatment HDRS ECT: 30 ± 6 (standard deviation, SD), mean pre-treatment HDRS MST: 32 ± 4 SD, difference between ECT and MST p>0.05; mean post-treatment HDRS ECT: 6 ± 6 SD, mean post-treatment HDRS MST: 14 ± 10 SD, difference between ECT and MST p<0.05).
Soon after this, high-frequency (i.e., 100Hz) MST was developed in an attempt to optimize the dosage of MST [20]. In 2011 Kayser et al., compared high-frequency MST to ECT in 20 patients with TRD [23]. Both treatment groups included two patients with bipolar disorder. Twelve treatments were administered twice a week and antidepressant response was evaluated with the Montgomery Åsberg Depression Rating Scale (MADRS). The study found no difference in the antidepressant efficacy between the ECT and MST groups (ECT response rate: 40%, remission rate: 40%; MST response rate: 60%, remission rate: 30%; difference between ECT and MST p>0.05). Similar clinical outcomes were also found in a slightly larger study with 37 patients with MDD [17]. This study also evaluated the number of treatments needed for an adequate clinical response between ECT and MST and found no significant differences between the two treatment conditions [17].
Promising results have been reported using the accelerated MST protocol, in which three patients with TRD received six MST treatments [10]. After the treatment course, two of the patients responded (one of these remitted) and third showed decreased depression severity, but not enough to reach the response criteria. These patients also showed decreased anxiety symptom severity as documented with the Hamilton Anxiety Rating Scale (mean pre-treatment score: 33.3 ± 13.6 SD, mean post-treatment score: 11.3 ± 7.8 SD).
A case study evaluated MST in an adolescent with refractory bipolar disorder [24]. The patient remitted after 18 MST treatments that were followed by nine maintenance MST treatments over six months. The remission was maintained for up to 11 months after the last acute MST treatment.
MST has also demonstrated encouraging results in patients with schizophrenia [25, 26], but has not yet been directly compared with ECT in that disorder. In a study with eight patients with treatment resistant schizophrenia, MST was administered with a stimulation frequency between 25 to 100Hz and a maximum of 24 treatments [25]. The Brief Psychiatric Rating Scale (BPRS) was used for clinical evaluation. Of the four patients that completed the trial, three remitted and one responded to MST. In another study, patients with schizophrenia were provided with 10 MST sessions at a frequency of 25Hz [26]. Reduction in clinical symptoms was evaluated with the Positive and Negative Syndrome Scale (PANSS). Six of the patients completed at least five treatments. Of these patients, three responded and five had a reduction in their PANSS scores.
Suicidal ideation is a significant symptom of TRD, bipolar disorder and schizophrenia. In a study with 27 patients with TRD, suicidal ideation was assessed before and after MST with the Scale for Suicide Ideation (SSI) [27]. SSI ranges from 0 to 38 and the higher the SSI score, the greater the severity of suicide ideation and a score of six or more is considered clinically significant. Before the MST treatment, the mean SSI score was 9.0 ± 6.8 SD with 30% of the patients having an SSI of 0. After an average of 16.9 ± 7.0 SD MST treatments, the mean post MST treatment SSI score was 4.2 ± 6.3 SD, and 67% had a score of 0. This difference between pre- and post-values was statistically significant (p<0.001). Similar findings were observed in another study with 23 patients with TRD [28]. The SSI scores decreased significantly (p<0.005) from a mean pre-MST value of 9.3 ± 6.3 SD (22% had a score of 0) to a mean post value of 4.3 ± 5.6 SD (57% had a score of 0). The average number of treatments was not provided in this study.
5. Predictors of response
There is still very limited knowledge regarding which patient groups and which symptoms, or biological markers are most likely to show a response to MST. According to a study of 38 patients with TRD who were treated with MST, the main clinical predictors for response were the number of previous major depressive episodes and a family history of MDD [29]. Specifically, patients who had fewer previous episodes of depression had a better response with MST [29]. Positive family history of depression predicted a good response to MST indicating that genetic factors may play a role in treatment outcome [29]. In addition, patients who responded to MST had less anxiety and melancholic symptoms, and lower levels of somatic anxiety and anhedonia [29]. This study, however, did not provide the full extent of possible clinical predictors as the selected variables were based on predictors identified for ECT [29]. Previously, MST has been applied to only a few patients with TRD and psychotic symptoms. Therefore, no conclusions about the effectiveness of MST in psychotic depression can be made, although this phenotype is known to respond well to ECT [30]. Also, even patients with atypical depression can benefit from ECT [31], indications for this have not yet been observed with MST [29].
As in ECT, positive treatment outcome to MST may potentially be predicted from the seizure characteristics measured with EEG [9]. There is some preliminary evidence that seizure characteristics; however, predict treatment outcome differently in MST than in ECT [9]. In MST, low slow-wave amplitude and short polyspike duration in the ictal EEG have been associated with good clinical outcome. Also, even though post-ictal EEG suppression indicates positive outcome in ECT [32], such association has not been found in MST [9]. These findings imply that ECT and MST may have different biological mechanisms of action and therefore the biomarkers associated with outcome may be distinct.
The clinical and cognitive treatment outcomes of MST may also be associated with brain region and timescale specific complexity of the EEG [33]. A study that included 34 patients with TRD found that a reduction in EEG complexity after the treatment course, evaluated by multiscale entropy measures with resting-state EEG, correlated with positive treatment outcome [33]. Especially, a reduction in EEG complexity in the occipital regions indicated positive clinical outcome. On the contrary, an increase in complexity in the parieto-central regions was associated with greater impairment in neurocognitive function. Interestingly, the study included patients who received either ECT or MST, but the results were the same despite the treatment method.
Promising evidence has suggested that remission from suicidal ideation after the MST treatment course could be predicted from the baseline TMS-EEG findings [27]. Specifically, cortical inhibitory indices such as N100 and long-interval cortical inhibition (LICI) measured from the dorsolateral prefrontal cortex (DLPFC) may identify patients who most likely benefit from MST. The study found that a greater N100 amplitude and LICI at baseline were associated with a greater decrease in suicidal ideation [27].
6. Neurocognitive effects
Relative to ECT, in MST the seizure can be induced with greater focality and the MST-induced electric field influences a smaller amount of brain volume [4]. These features are relevant and important especially when considering neurocognitive effects, as non-focality and electric field spreading to deeper brain structures (e.g., hippocampus) have been thought to be associated with ECT-induced cognitive adverse effects [2]. To date, MST relative to ECT has been found to have a superior neurocognitive profile [6], though this awaits confirmation in future randomized controlled-clinical studies.
In the early studies of MST when the stimulation frequency was around 50Hz or below, MST was found to be superior to ECT regarding neurocognitive outcomes. A within-subject study that included 10 patients with MDD found that after treatment with MST relative to ECT, patients showed better performance on measures of attention, retrograde memory, and semantic fluency [22]. Another case study in a patient with TRD evaluated verbal and visuospatial learning and memory, and autobiographical memory [34]. That study found no decline or impairment in neurocognitive performance on those selected measures [34].
After technological advancements in MST, researchers were able to induce seizures with higher stimulation frequencies (up to 100Hz) that were also found to be cognitively benign. For example, a study that enrolled 37 patients with TRD compared the neurocognitive effects of MST and ECT on domains of attention, processing speed, verbal and visuospatial memory, and executive function and found no significant decline in performance within the cohort treated with MST [17]. Another study with 26 patients with TRD assessed the neurocognitive effects of MST via verbal and visuospatial learning and memory, working memory, language, attention, visual perception, and executive function measures [35]. The study found that performance across all neurocognitive measures remained stable after MST. Additional research has suggested that MST may even be associated with improvement in cognitive functions relative to pre-treatment levels in domains such as visuospatial learning, and verbal learning and memory [22, 23, 34, 35]. Also, neurocognitive improvement was observed in patients with TRD who completed an accelerated MST protocol [10].
Furthermore, ECT and MST have been found to differ in their effects on acute memory retrieval [36]. In a clinical study of 20 patients with TRD who were asked to recall a set of words that was memorized at baseline before the treatment, patients treated with MST had no differences in recalling the set of words across both treatment and non-treatment days [36]. However, after ECT, patients showed significantly worse recall on treatment days relative to non-treatment days. In both ECT and MST groups, patients showed stable recall of the words when they were provided with cues.
In addition to neurocognitive studies of patients with TRD treated with MST, to date there have been two preliminary studies that examined the MST associated neurocognitive effects in patients with schizophrenia [25, 26]. In one study, two of three patients showed improved performance after MST on measures of immediate and delayed recall memory [26]. In the other study, eight patients with schizophrenia showed no change in performance on neurocognitive measures of processing speed, working memory, verbal learning, verbal fluency, and executive function [25]. However, patients showed a slight decrease in consistency of autobiographical memory recall that may have been more related to time than to the treatment.
Collectively, preclinical and clinical evidence suggests that MST has benign neurocognitive effects and based on preliminary evidence could possibly have neurocognitive enhancing effects. Should MST have neurocognitive enhancement effects, that would be useful given that neurocognitive difficulties are central to many neuropsychiatric illnesses. Future research is warranted to determine the neurocognitive effects of MST across various technical parameters (e.g., coil type, stimulation frequency) and neuropsychiatric conditions (e.g., TRD, schizophrenia, bipolar disorder). Given the more benign cognitive profile, MST may in future prove to be a useful option for those patients with pre-existing cognitive decline.
7. Mechanisms of action and neurophysiological and metabolic effects
The mechanisms of antidepressant action of MST remain unknown; however, it is thought that the mechanisms are similar to those in ECT. In a preliminary study, MST was found to induce neuroplasticity in excitatory and inhibitory circuits in the frontal areas measured through TMS-induced EEG responses [28]. MST has also been observed to induce changes in functional connectivity, especially in the beta-frequency band [37]. Changes in functional connectivity after MST have also been found through EEG microstate analysis, which is a method that evaluates the spatial stability of the brain network dynamics with time [38]. Microstate changes following MST were not specific to any network, but a decrease of relative power above 17Hz was detected [38]. The clinical and cognitive meaning of these changes remains unclear.
Metabolic changes have also been associated with MST [34, 35, 39]. Particularly, a study that included 12 patients with TRD found an increase in metabolic activity in the frontal cortex and a decrease in the left striatum with fluorodeoxyglucose positron emission tomography (FDG-PET) [35]. Another study with 10 patients with TRD used FDG-PET to evaluate metabolic changes produced by MST and found increased metabolism in the basal ganglia, orbitofrontal cortex, medial frontal cortex and DLPFC [39]. Furthermore, a case study of a single patient with TRD observed increased metabolism in the left fronto-parietal region and the brainstem with 99mTc-hexamethylpropyleneamineoxime single photon emission computed tomography (HMPAO SPECT) [34]. Overall, these metabolic changes found were in brain regions closely associated with MDD.
8. Towards Personalized Seizure Therapy
MST allows the clinician to focus the delivered electric field and resultant seizure to targeted regions of the brain. Using frameless stereotaxic methodology, coil placement can be guided with millimeter resolution to the patient’s own anatomy in real-time using structural and functional imaging. This spatial precision enables for the first time the ability not only to focus seizure therapy precisely, but also the ability to individualize its delivery based on individual circuitry. Research has generated emerging biomarkers of response to ECT [40, 41] which represent potential therapeutic targets for MST, informing where to target the MST coil and also providing measures of target-engagement to evaluate whether clinical benefit is mechanistically linked to changes in the target. Most of the work to date to optimize MST has focused on avoiding non-targets linked to cognitive side effects, such as the hippocampus. The benign cognitive profile of MST demonstrates that hippocampal sparing can be cognition sparing. What remains to be determined is how to optimize efficacy of MST by selecting circuits essential to antidepressant response and target those based on individual anatomy and physiology. In so doing, not only may we achieve a means of effectively treating each patient, we will also be able to test the causal mechanisms of antidepressant response to seizure therapy, which remains an unanswered question not only for MST but for ECT as well.
9. Summary and future directions
MST is a promising non-invasive neuromodulation treatment with demonstrated antidepressant properties and relatively benign neurocognitive adverse effects (see Table 1). Despite this, MST field has advanced slowly because MST is a new technology and we still need research to replicate and confirm the positive findings. To date, MST has been tested in computational models, preclinical studies, and clinical investigations in patients with unipolar and bipolar depression, and in schizophrenia. Many questions remain, including mechanisms of action, optimal treatment paradigms and predictors of response. As such, future research is warranted to identify optimal treatment parameters, mechanisms of action, applicability across neuropsychiatric disorders, neurocognitive effects, and optimal means to select those most likely to respond as well as how to personalize the treatment for each individual. Given its spatial precision and existing tools to target the treatment to individual anatomy and physiology, MST has an exciting potential to translate the emerging biomarkers of response to ECT into selective and precisely delivered treatments for each patient.
Table 1.
Comparison between electroconvulsive therapy (ECT) and magnetic seizure therapy (MST).
| Convulsive method | ECT | MST |
|---|---|---|
| Stimulating method | Electric fields | Magnetic fields |
| Focality | Non-focal | Relatively focal |
| Stimulating tool | Electrodes | Coil |
| Antidepressant effects | Yes | Yes |
| Neurocognitive effects | Transient cognitive adverse effects that can last up to 6 months or longer, magnitude depends on the stimulation parameters | Little or no cognitive adverse effects, may also enhance cognition, magnitude depends on the stimulation parameters |
| Other adverse effects | Headache Nausea Anesthesia related adverse effects | Relatively less headache than associated with ECT. Relatively less nausea than associated with ECT. Similar anesthesia related adverse effects as in ECT. |
Highlights.
Magnetic seizure therapy (MST) is a non-invasive neuromodulation therapy under investigation.
MST has antidepressant effects and benign neurocognitive effects.
MST has been found to be safe and feasible in neuropsychiatric disorders.
Acknowledgments
Funding: This work was partially supported by National Institute of Mental Health (NIMH) (Project Number: 1R01MH112815–01). The funding source had no role in the writing of this review article and in the decision to submit this review for publication. Z.-D. Deng is supported by the Intramural Research Program at NIMH, and by a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation.
Financial disclosures:
Elisa Kallioniemi: None.
Shawn M. McClintock: Dr. McClintock has received teaching honorarium from TMS Health Solutions. He has received research support from the National Institutes of Health. He is a consultant to Pearson.
Mustafa M. Husain: Dr. Husain has received research support from the National Institutes of Health, National Alliance for Research on Schizophrenia and Depression, Stanley Medical Foundation, Cyberonics, Neuronetics, St. Jude Medical/Abbott, Brainsway, NeoSync, Alkermes, Assurex, and Avanir Pharmaceuticals. Furthermore, Dr. Husain has received equipment support from MagStim and MagVenture.
Zhi-De Deng and Sarah H. Lisanby: Dr. Deng and Dr. Lisanby are inventors on patents/patent applications related to magnetic stimulation technology owned by Columbia University. They are employed by the National Institutes of Health. The views expressed are their own and do not necessarily represent the views of the National Institutes of Health or the United States Government.
Abbreviations
- BIS
bispectral index
- BPRS
Brief Psychiatric Rating Scale
- DLPFC
dorsolateral prefrontal cortex
- ECT
electroconvulsive therapy
- EEG
electroencephalography
- FDG
fluorodeoxyglucose
- HDRS
Hamilton Depression Rating Scale
- LICI
long-interval cortical inhibition
- MADRS
Montgometry Åsberg Depression Rating Scale
- MDD
major depressive disorder
- MST
magnetic seizure therapy
- PANSS
Positive and Negative Syndrome Scale PET positron emission tomography
- rMT
resting motor threshold
- rTMS
repetitive transcranial magnetic stimulation
- RUL
right unilateral
- SD
standard deviation
- SPECT
single-photon emission computed tomography
- SSI
Scale for Suicidal Ideation
- TMS
transcranial magnetic stimulation
- TRD
treatment-resistant depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association, The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association 2nd ed. Washington, D.C.: American Psychiatric Association; 2002. 10.1176/appi.ajp.159.2.331 [DOI] [Google Scholar]
- 2.McClintock SM, Choi J, Deng ZD, Appelbaum LG, Krystal AD, Lisanby SH. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J ECT 2014;30(2):165–176. 10.1097/YCT.0000000000000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lisanby SH, Schlaepfer TE, Fisch HU, Sackeim HA. Magnetic seizure therapy of major depression. Arch Gen Psychiatry 2001;58(3):303–5. 10.1001/archpsyc.58.3.303 [DOI] [PubMed] [Google Scholar]
- 4.Deng ZD, Lisanby SH, Peterchev AV. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J Neural Eng 2011;8(1):016007 10.1088/1741-2560/8/1/016007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilmoniemi RJ, Ruohonen J, Karhu J. Transcranial magnetic stimulation--a new tool for functional imaging of the brain. Crit Rev Biomed Eng 1999;27(3–5):241–84. [PubMed] [Google Scholar]
- 6.McClintock SM, Tirmizi O, Chansard M, Husain MM. A systematic review of the neurocognitive effects of magnetic seizure therapy. Int Rev Psychiatry 2011;23(5):413–23. 10.3109/09540261.2011.623687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterchev AV, Krystal AD, Rosa MA, Lisanby SH. Individualized Low-Amplitude Seizure Therapy: Minimizing Current for Electroconvulsive Therapy and Magnetic Seizure Therapy. Neuropsychopharmacol 2015;40(9):2076–84. 10.1038/npp.2015.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayser S, Bewernick BH, Hurlemann R, Soehle M, Schlaepfer TE. Comparable seizure characteristics in magnetic seizure therapy and electroconvulsive therapy for major depression. Eur Neuropsychopharmacol 2013;23(11):1541–50. 10.1016/j.euroneuro.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Backhouse FA, Noda Y, Knyahnytska Y, Farzan F, Downar J, Rajji TK, et al. Characteristics of ictal EEG in Magnetic Seizure Therapy at various stimulation frequencies. Clin Neurophysiol 2018;129(8):1770–79. 10.1016/j.clinph.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Vila-Rodriguez F, Jiang W, Ren YP, Wang CM, Ma X. Accelerated Magnetic Seizure Therapy for Treatment of Major Depressive Disorder: A Report of 3 Cases. J ECT 2018. Epub 2018 Jul 3. 10.1097/YCT.0000000000000521 [DOI] [PubMed] [Google Scholar]
- 11.Perera TD, Luber B, Nobler MS, Prudic J, Anderson C, Sackeim HA. Seizure expression during electroconvulsive therapy: relationships with clinical outcome and cognitive side effects. Neuropsychopharmacol 2004;29(4):813–25. 10.1038/sj.npp.1300377 [DOI] [PubMed] [Google Scholar]
- 12.Minelli A, Abate M, Zampieri E, Gainelli G, Trabucchi L, Segala M, et al. Seizure Adequacy Markers and the Prediction of Electroconvulsive Therapy Response. J ECT 2016;32(2):88–92. 10.1097/YCT.0000000000000274 [DOI] [PubMed] [Google Scholar]
- 13.Morales OG, Sackeim HA, Berman RM, Lisanby SH. Magnetic seizure therapy: development of a novel intervention for treatment resistant depression. Clin Neurosci Res 2004;4(1–2);59–70. 10.1016/j.cnr.2004.06.005 [DOI] [Google Scholar]
- 14.Cycowicz YM, Rowny SB, Luber B, Lisanby SH. Differences in Seizure Expression Between Magnetic Seizure Therapy and Electroconvulsive Shock. J ECT 2018;34(2):95–103. 10.1097/YCT.0000000000000470 [DOI] [PubMed] [Google Scholar]
- 15.Cycowicz YM, Luber B, Spellman T, Lisanby SH. Differential neurophysiological effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS) in non-human primates. Clin EEG Neurosci 2008;39(3):144–9. 10.1177/155005940803900309 [DOI] [PubMed] [Google Scholar]
- 16.Deng Z, Peterchev AV, Krystal AD, Luber B, McClintock SM, Husain MM, et al. Topography of seizures induced by electroconvulsive therapy and magnetic seizure therapy. 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER). 2013:577–80. https://doi:10.1109/NER.2013.6696000 [Google Scholar]
- 17.Fitzgerald PB, Hoy KE, Elliot D, McQueen S, Wambeek LE, Chen L, et al. A pilot study of the comparative efficacy of 100 Hz magnetic seizure therapy and electroconvulsive therapy in persistent depression. Depress Anxiety 2018;35(5):393–401. 10.1002/da.22715 [DOI] [PubMed] [Google Scholar]
- 18.Soehle M, Kayser S, Ellerkmann RK, Schlaepfer TE. Bilateral bispectral index monitoring during and after electroconvulsive therapy compared with magnetic seizure therapy for treatment-resistant depression. Br J Anaesth 2014;112(4):695–702. 10.1093/bja/aet410 [DOI] [PubMed] [Google Scholar]
- 19.White PF, Amos Q, Zhang Y, Stool L, Husain MM, Thornton L, et al. Anesthetic considerations for magnetic seizure therapy: a novel therapy for severe depression. Anesth Analg 2006;103(1):76–80. 10.1213/01.ane.0000221182.71648.a3 [DOI] [PubMed] [Google Scholar]
- 20.Kirov G, Ebmeier KP, Scott AI, Atkins M, Khalid N, Carrick L, et al. Quick recovery of orientation after magnetic seizure therapy for major depressive disorder. Br J Psychiatry 2008;193(2):152–5. 10.1192/bjp.bp.107.044362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowny SB, Cycowicz YM, McClintock SM, Truesdale MD, Luber B, Lisanby SH. Differential heart rate response to magnetic seizure therapy (MST) relative to electroconvulsive therapy: a nonhuman primate model. Neuroimage 2009;47(3):1086–91. 10.1016/j.neuroimage.2009.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacol 2003;28(10):1852–65. 10.1038/sj.npp.1300229 [DOI] [PubMed] [Google Scholar]
- 23.Kayser S, Bewernick BH, Grubert C, Hadrysiewicz BL, Axmacher N, Schlaepfer TE. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res 2011;45(5):569–76. 10.1016/j.jpsychires.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Noda Y, Daskalakis ZJ, Downar J, Croarkin PE, Fitzgerald PB, Blumberger DM. Magnetic seizure therapy in an adolescent with refractory bipolar depression: a case report. Neuropsychiatr Dis Treat 2014;10:2049–55. 10.2147/NDT.S71056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang VM, Blumberger DM, McClintock SM, Kaster TS, Rajji TK, Downar J. Magnetic Seizure Therapy in Treatment-Resistant Schizophrenia: A Pilot Study. Front Psychiatry 2018;8:310 10.3389/fpsyt.2017.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J, Li Q, Sheng J, Yang F, Cao X, Zhang T, et al. 25 Hz Magnetic Seizure Therapy Is Feasible but Not Optimal for Chinese Patients With Schizophrenia: A Case Series. Front Psychiatry 2018;9:224 10.3389/fpsyt.2018.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Farzan F, Mulsant BH, Rajji TK, Fitzgerald PB, Barr MS, et al. Indicators for Remission of Suicidal Ideation Following Magnetic Seizure Therapy in Patients With Treatment-Resistant Depression. JAMA Psychiatry 2016;73(4):337–45. 10.1001/jamapsychiatry.2015.3097 [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Blumberger DM, Mulsant BH, Rajji TK, Fitzgerald PB, Barr MS, et al. Magnetic seizure therapy reduces suicidal ideation and produces neuroplasticity in treatment-resistant depression. Transl Psychiatry 2018;8(1):253 10.1038/s41398-018-0302-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayser S, Bewernick BH, Wagner S, Schlaepfer TE. Clinical Predictors of Response to Magnetic Seizure Therapy in Depression: A Preliminary Report. J ECT 2018. Epub 2018 Apr 2. 10.1097/YCT.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 30.Pinna M, Manchia M, Oppo R, Scano F, Pillai G, Loche AP, et al. Clinical and biological predictors of response to electroconvulsive therapy (ECT): a review. Neurosci Lett 2018;669:32–42. 10.1016/j.neulet.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 31.Husain MM, McClintock SM, Rush AJ, Knapp RG, Fink M, Rummans TA, et al. The efficacy of acute electroconvulsive therapy in atypical depression. J Clin Psychiatry 2008;69(3):406–11. 10.4088/JCP.v69n0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azuma H, Fujita A, Sato K, Arahata K, Otsuki K, Hori M, et al. Postictal suppression correlates with therapeutic efficacy for depression in bilateral sine and pulse wave electroconvulsive therapy. Psychiatry Clin Neurosci 2007;61(2):168–73. 10.1111/j.1440-1819.2007.01632.x [DOI] [PubMed] [Google Scholar]
- 33.Farzan F, Atluri S, Mei Y, Moreno S, Levinson AJ, Blumberger DM, et al. Brain temporal complexity in explaining the therapeutic and cognitive effects of seizure therapy. Brain 2017;140(4):1011–1025. 10.1093/brain/awx030 [DOI] [PubMed] [Google Scholar]
- 34.Kosel M, Frick C, Lisanby SH, Fisch HU, Schlaepfer TE. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacol 2003;28(11):2045–8. 10.1038/sj.npp.1300293 [DOI] [PubMed] [Google Scholar]
- 35.Kayser S, Bewernick BH, Matusch A, Hurlemann R, Soehle M, Schlaepfer TE. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med 2015;45(5):1073–92. 10.1017/S0033291714002244 [DOI] [PubMed] [Google Scholar]
- 36.Polster JD, Kayser S, Bewernick BH, Hurlemann R, Schlaepfer TE. Effects of electroconvulsive therapy and magnetic seizure therapy on acute memory retrieval. J ECT 2015;31(1):13–9. 10.1097/YCT.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 37.Deng ZD, McClinctock SM, Lisanby SH. Brain network properties in depressed patients receiving seizure therapy: A graph theoretical analysis of peri-treatment resting EEG. Conf Proc IEEE Eng Med Biol Soc 2015;2015:2203–6. 10.1109/EMBC.2015.7318828 [DOI] [PubMed] [Google Scholar]
- 38.Atluri S, Wong W, Moreno S, Blumberger DM, Daskalakis ZJ, Farzan F. Selective modulation of brain network dynamics by seizure therapy in treatment-resistant depression. Neuroimage Clin 2018;20:1176–1190. 10.1016/j.nicl.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoy KE, Thomson RH, Cherk M, Yap KS, Daskalakis ZJ, Fitzgerald PB. Effect of magnetic seizure therapy on regional brain glucose metabolism in major depression. Psychiatry Res 2013;211(2):169–75. 10.1016/j.pscychresns.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 40.Leaver AM, Wade B, Vasavada M, Hellemann G, Joshi SH, Espinoza R, et al. Fronto-Temporal Connectivity Predicts ECT Outcome in Major Depression. Front Psychiatry 2018;9:92 10.3389/fpsyt.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Njau S, Joshi SH, Espinoza R, Leaver AM, Vasavada M, Marquina A, et al. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J Psychiatry Neurosci 2017;42(1):6–16. 10.1503/jpn.150177 [DOI] [PMC free article] [PubMed] [Google Scholar]