Abstract
Background:
Geniospasm is a rare genetic disorder characterized by paroxysmal rhythmic or irregular movements of the chin and lower lip due to repetitive contractions of the mentalis muscle. Pathophysiology is poorly understood, and optimal treatment has not been established.
Methods:
Geniospasm was characterized in a series of patients after evaluation in our clinics, and a comprehensive review of all cases in the medical literature was performed.
Results:
We evaluated four patients (1 female) in four families with geniospasm, aged 4 months to 9 years. Bothersome symptoms were present in one patient, who was treated with regular injections of onabotulinumtoxinA, with complete resolution of symptoms and no adverse effects. 9 patients in the literature have had similar outcomes.
Conclusions:
Limited data exist with regard to the effective treatment of geniospasm. Several treatments have been used historically, with variable outcomes. Our results, together with those of prior reported cases, demonstrate benefit of the use of botulinum toxin injections for management of this condition.
Keywords: geniospasm, chin trembling, tongue biting, botulinum toxin, hereditary chin tremor
Introduction
Geniospasm is a rare disorder characterized by paroxysms of rhythmic or irregular twitch-like, “quivering,” or “trembling” movements of the chin and lower lip due to involuntary repetitive contractions of the mentalis muscle bilaterally. It was first described in the Italian literature by Massaro in 1894 [1,2] and by Stocks in the English literature in 1922 [3], and since that time has been reported in fewer than 50 families worldwide. Symptoms can be mild and unimpairing, or can include more frequent and irksome chin movements or painful and sometimes bloody nocturnal tongue-biting. The disorder can be inherited in an autosomal dominant pattern, or can occur sporadically [4,5]. The genetic basis is as-yet poorly understood. Given the rarity of geniospasm, the literature to date is limited to small case series and case reports. As such, guidance on prognosis and management strategies can be difficult for providers to gather. We describe here four illustrative cases (1 female) and include a review of all reported cases to date in order to provide a concise review on this under-recognized disorder and provide a summary of the current understanding of geniospasm and treatment strategies.
Methods
We reviewed the medical records of two patients with geniospasm who presented for evaluation at our tertiary-care pediatric movement disorders clinic between 2015 and 2019. Two additional children were evaluated by video and chart review after being seen in the general child neurology clinic at our institution during this time period. Our review included the history, family history and phenomenology of the geniospasm events of the four affected individuals. Videos of all patients were obtained after signing a consent form approved by the Baylor College of Medicine Institutional Review Board. A systematic review of the medical literature was then performed, and all reported cases of geniospasm were reviewed.
Illustrative Cases
Patient 1
The patient is a male with borderline IQ, ADHD and mixed receptive-expressive language disorder who presented to our tertiary care pediatric movement disorders clinic at age 9 for evaluation of episodic chin quivering. Onset began at 3.5 months of age and would occur in bursts of 30 minutes to one hour in length. Triggers included excitement and anxiety but would also happen spontaneously. Initially the movements were intermittent with decreased frequency between ages 2–4 years with subsequent further increase in frequency at 7 years of age occurring in bouts lasting 60 minutes throughout the day (up to 15 hours of chin quivering per 24 hours) with resolution during sleep. These movements made him feel very embarrassed and sad as the movements occurred in front of his peers, which made him feel different and would often cause tearfulness related to wishing the movements would stop. There was no family history of similar movements. Home video revealed rhythmic chin trembling that was consistent with the diagnosis of geniospasm (Video Segment 1). Prior to evaluation in the movement disorder clinic, the family was offered low dose clonazepam, however family deferred. OnabotulinumtoxinA injections were pursued in our clinic and titrated to 30 units to each mentalis every 3 months with complete resolution of symptoms and no adverse effects. No other treatments were tried. Genetic material has been collected for comparative whole exome sequencing.
Video Segment 1.
Home video illustrating geniospasm in a child.
Patient 2
The patient is a typically-developing male presenting at 7 months of age with chin quivering occurring multiple times per day lasting between 30 minutes to 4 hours at a time since early infancy. No clear triggers reported. Family history is remarkable only for hypnic jerks in the father but no other members with chin quivering. Home video was provided that was consistent with the diagnosis of geniospasm. The movements subsided by 1 year of age, and as of 3 years of age have not recurred. No treatment was pursued and genetic testing has been deferred by the family.
Patient 3
The patient is a typically-developing male who presented at 14 months of age for evaluation of nocturnal tongue biting first noted at 11 months of age. He would repeatedly be awakened by oral pain, with blood found on the sheets, up to 25 times per night, leading to repeated ulceration of the tongue (Figure 1). He was also found to have quivering of the chin since his first day of life. It occurs intermittently in bursts of seconds over periods of 30 minutes. No clear triggers noted. His mother and maternal grandfather also report recurrent chin quivering of which persisted into adulthood, as well as recurrent hiccups. The onset of chin quivering in the family members was reported as young adulthood and stress seemed to trigger the movements. None of the family members have received treatment previously. The patient’s mother is planning to receive OnabotulinumtoxinA injections; injections for the patient have been discussed should the movements become bothersome. Genetic material from our patient and the other affected family members has been collected for comparative whole exome sequencing.
Figure 1.
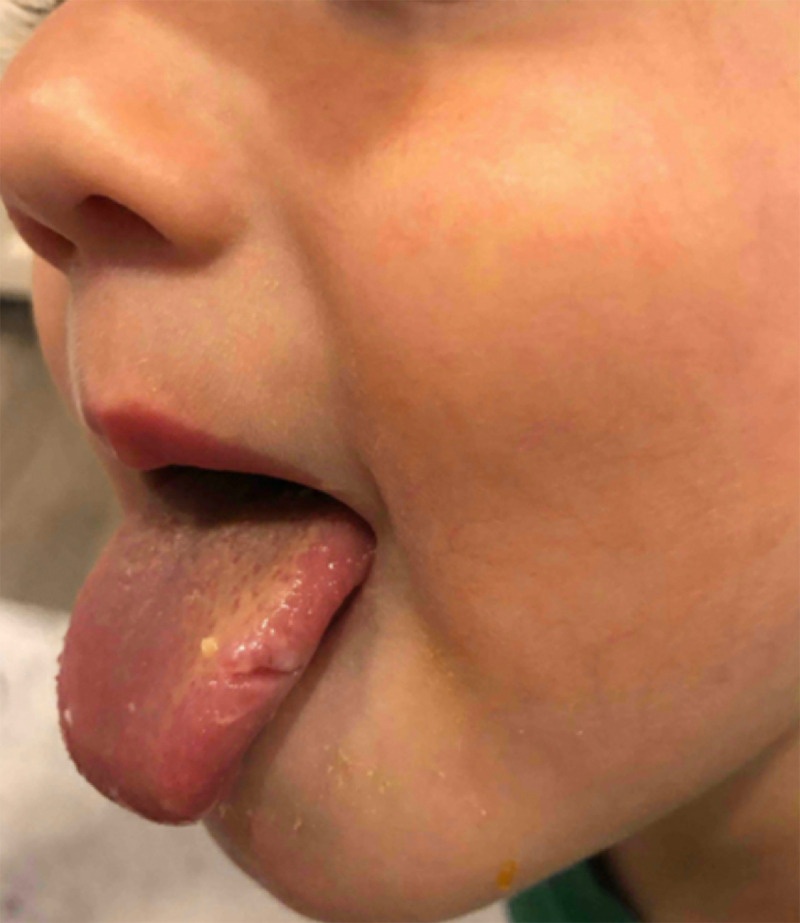
Tongue laceration due to nocturnal tongue biting associated with geniospasm.
Patient 4
The patient is a typically-developing female who presented at 4 months of age for chin quivering movements first noted on the first day of life which lasted seconds to minutes at a time. These movements occurred while awake and asleep with no clear triggers. She was born full term with an uncomplicated pregnancy and delivery. She has been developing appropriately. There is no family history of similar movements. The movements do not appear bothersome at this time and therefore no treatment discussed. Genetic testing was deferred by the family.
Results
Among the four patients with geniospasm evaluated at our institution (median age at presentation: 18 months, range: 4 months to 9 years), age of onset for all patients was within the first 6 months of life, and two (50%) had symptoms noted on the first day of life (Table 1). Three of our patients (75%) have no similar family history. One patient (25%) has associated nocturnal tongue-biting. One patient (25%) has cognitive impairments; other patients and their family members have typical development. No triggers have yet been noted in the patients under two years of age; our 9 year-old patient has noted strong emotion makes his events more likely, and the affected relatives of patient three have more events when they are feeling stressed. Affected relatives of patient three are both afflicted with recurrent bouts of hiccups. All patients have nonfocal neurologic exams apart from geniospasm.
Table 1.
Illustrative cases of geniospasm newly described in this article including pertinent historical details, diagnostics, treatment, and outcome.
| PATIENT | AGE OF PRESENTATION | SEX | AGE OF ONSET | TRIGGERS | ASSOCIATED SYMPTOMS | PAST MEDICAL HISTORY | FAMILY HISTORY | DIAGNOSTIC STUDIES | TREATMENT | OUTCOME |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 years | M | 3.5 months | Strong Emotions |
None | ADHD, Borderline IQ, Mixed receptive-expressive language disorder |
None | MRI Brain Normal | OnabotulinumtoxinA – 30 U to each mentalis | Complete resolution No adverse effects |
| 2 | 7 months | M | Early infancy | None | None | Healthy | None | None | Spontaneous remission by 1 year | |
| 3 | 14 months | M | First day of life | None | Recurrent Nocturnal tongue biting | Healthy | Mother and maternal grandfather with similar symptoms and recurrent hiccups | EEG normal | None | |
| 4 | F | Young adulthood | Stress | None | Recurrent hiccups | Mother of patient 3 | None | Plan for OnabotulinumtoxinA injections | ||
| 5 | M | Young adulthood | Stress | None | Recurrent hiccups | Maternal Grandfather of patient 3 | None | None | ||
| 6 | 4 months | F | First day of life | None | None | Healthy | None | None | None | |
To date, a total of 41 affected patients from 46 families have been clearly described in the English literature (Table 2). Of these, two had no family history of the disorder [4,5]. Age of onset ranged from shortly after birth to late childhood. Of these patients, 26 (63.4%) noted stress or heightened emotion made events more likely. Three (0.07%) were known to have associated tongue-biting. One patient noted improvement with alcohol. Not all cases reported were severe enough to warrant treatment. Of the patients that did receive treatment, 6 were given anticonvulsants. Results ranged from no improvement to some degree of decrease in frequency and duration of the attacks. Six patients were treated with sedatives, also with partial amelioration of symptoms. One patient was treated with a beta blocker, with similar outcome [6]. Botulinum toxin has been used in 9 patients in addition to our own. In all cases, there has been complete or near-complete resolution of symptoms. Injections were well-tolerated apart from one report of asymmetric smile which may have been due to addition of mylohyoid muscle injections [7]. Of note, in the 3 patients with geniospasm and associated tongue-biting, the nocturnal biting seemed to respond well to anticonvulsants or benzodiazepines without clear improvement in the geniospasm [6,8,9].
Table 2.
Geniospasm cases described in the literature including pertinent historical details, diagnostics, treatment, and outcome.
| YEAR OF PUBLICATION | AUTHOR | AGE AT PRESENTATION | SEX | AGE OF ONSET | TRIGGERS | ASSOCIATED SYMPTOMS | PAST MEDICAL HISTORY | FAMILY HISTORY | DIAGNOSTIC STUDIES | TREATMENT | OUTCOMES |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1923 | Stocks, P [3] | 18 years | M | – | Stress | None | Healthy | Two siblings, cousins, and niece with similar symptoms | None | None | |
| 1957 | Grossman, BJ [22] | 3 years | M | Infancy | Strong emotions | None | Healthy | Father, paternal uncle, paternal grandfather with similar symptoms Father, paternal uncle, and paternal grandmother with otosclerosis |
None | None | |
| 1958 | Wadlington, WB [25] | 40 years | F | Early childhood | Strong emotions | None | Healthy | Father and two sisters with same symptoms and two sonsA,B with similar symptoms | EEG normal | Phenytoin 100 mg BID Hydroxyzine 30 mg BID |
Some degree of Improvement Some degree of improvement No adverse effects |
| 9 years | M | 8 weeks | None | None | Healthy | SonA | None | Phenytoin 10 mg/kg/day Hydroxyzine 20 mg BID |
Some degree of improvement Some degree of improvement No adverse effects |
||
| 2 years | M | 4 months | During sleep, Strong emotions | None | Healthy | SonB | None | Phenytoin 10 mg/kg/day Hydroxyzine 10 mg BID |
Some degree of improvement Some degree of improvement No adverse effects |
||
| 1968 | Laurance et. al [34] | 5 years | M | Infancy | Strong emotions | Trembling impaired speech | Healthy | Maternal grandmother and maternal aunt with similar symptoms | EEG normal Needle EMG – rhythmic discharges of polyphasic complexes at 10 per second | None | |
| 6 years | M | 1 month | None | None | Bifid left kidney, strabismus | Sister, mother, maternal grandmother with similar symptoms | None | None | |||
| 1971 | Johnson et. al [8] | 13 months | M | Infancy | None | Tongue biting | Sleep myoclonus | Twin brotherC, older brother, father, paternal grandfather, and paternal uncle with similar symptoms Paternal aunt with seizures | Electrolytes, Urine organic acids, Skull films, and EEG normal | Valium | No improvement |
| 21 months | M | Infancy | None | None | Sleep myoclonus | Twin brotherC | None | None | |||
| 1984 | Fahn, S. [35] | 30 years | M | Early childhood | None | None | Healthy | SonD with similar symptoms | None | None | |
| 8 months | M | Infancy | None | None | Healthy | SonD | None | None | |||
| 1992 | Danek, A [16] | 13 years | M | Infancy | Stress | None | Somnambulism | Five other family members with similar chin movements One family member with Charcot-Marie-Tooth | Needle EMG – rhythmic polymorphic discharges in the mentalis | None | |
| 28 years | F | Early childhood | Stress, waking in the morning | None | Migraines | SonE and 10 other family members with similar symptoms | Needle EMG – rhythmic polymorphic discharges in the mentalis | None | |||
| 4 months | M | 2 weeks | Before and during breastfeeding | None | Healthy | SonE | Needle EMG – rhythmic polymorphic discharges in the mentalis | None | |||
| 1992 | Gordon et. al [26] | 28 years | M | 2 weeks | Strong emotions | None | Healthy | BrotherF, father*, and several paternal uncles with similar symptoms | None | 5 units botulinum toxin (Oculinum, Allergan) to each mentalis muscle q2–3 months | Complete resolution of symptoms for 2–3 months following each injection No adverse effects |
| 8 years | M | Infancy | None | None | Healthy | BrotherF | None | 5 units botulinum toxin (Oculinum, Allergan) to each mentalis muscle q2–3 months | Complete resolution of symptoms for 2–3 months following each injection No adverse effects | ||
| 1996 | Soland et. al [14] | 31 years | M | 4 years | During sleep, Strong emotions | Trembling impaired speech, drinking, and sleep | Action tremor | 16 family members with similar symptoms | CBC, peripheral smear, serum copper and ceruloplasmin normal EMG – during quivering showed motor units of normal morphology firing asynchronously | Variety of medications (unspecified) Botulinum toxin injection (Dysport 60 units) into mentalis on each side |
No improvement Complete resolution of symptoms within one week of injections No adverse effects |
| 38 years | F | Early childhood | Stress, strong emotions | None | Healthy | Sister with nocturnal episodes and tongue biting, 11 other family members with similar chin movements | None | Self resolved by late twenties | |||
| 1997 | Destee et. al [31] | 35 years | M | Infancy | Stress | None | Healthy | DaughterG, Brother, motherH, nephewI, and five cousins with similar symptoms | EEG normal Surface EMG – Sometimes bursts discharged in rhythmically but most often discharge frequency was irregular | None | |
| 4 years | F | Infancy | During sleep | None | Healthy | DaughterG | None | None | |||
| 62 years | F | Infancy | Stress | None | Healthy | MotherH | None | Self resolved with time | Occasionally felt shivering of the chin when stressed that was not visible | ||
| 11 years | M | Infancy | None | Trembling impaired speech | Healthy | NephewI | None | None | |||
| 1998 | Bakar et. al [7] | 28 years | M | Birth | Strong emotions | None | Healthy | Mother and maternal grandmother with similar symptoms | None | Sedatives and anticonvulsants Botulinum toxin(Botox) injections (25 units) in each mentalis and mylohyoid q4–5 months |
Unsatisfactory results Complete resolution of symptoms within two days of injections and lasting 5 months Adverse effects – abnormal appearance of mouth with corners depressing lower lip and center of lower lip elevated which lasted 30–45 days and resolved. Subsequent injection volumes reduced equal dose. No further adverse effects |
| 1999 | Diaz et. al [12] | 63 years | F | Early childhood | Stress, gazing at flying objects | None | Healthy | 28 family members with similar symptoms | Blood count, serum and urine copper, ceruloplasmin normal Surface EMG over mentalis – synchrony of motor unit firing without evidence of denervation Needle EMG – bursts of motor units of normal morphology firing pseudo-rhythmically throughout the muscle at 7–8 Hz | None | |
| 2002 | Grimes et. al [32] | 15 years | M | Infancy | Fatigue, stress | None | Healthy | Numerous other family members with similar symptoms | Evaluated for changes on the chromosome 9q13-q21 locus through sequencing analysis-Negative | 2.5 to 5 units botulinum toxin type A to each mentalis muscle q3–4 months | Complete resolution of symptoms No adverse effects |
| 2006 | Devetag et. al [36] | 16 years | M | Infancy | Anxiety, stress, tapping the chin | None | Healthy | Brother, grandmother, cousin, paternal aunt with similar symptoms | EEG, Median and trigeminal SEPs normal EMG – arrhythmic spontaneous activity from the mentalis muscle increased after tapping the muscle and disappeared during sleep | Clonazepam Botulinum toxin (Botox, Allergan) 5 units to each mentalis muscle q3–4 months |
No improvement Complete resolution of symptoms No adverse effects |
| 2006 | Goraya et. al [9] | 13 months | M | Infancy | During Sleep | Tongue biting during sleep | Healthy | Father with similar symptoms | EEG normal | Carbamazepine 100 mg BID Clonazepam 0.5 mg BID |
No improvement Mild improvement |
| 2007 | Erer, S and Jankovic, J [24] | 74 years | M | Early childhood | Stress | None | Parkinson’s disease | Two younger brothers with similar symptoms | None | Clonazepam 2 mg BID Bromocriptine 2.5 mg TID Carbidopa/levodopa 25/100 TID |
No improvement No improvement No improvement |
| 2007 | Papapetropolous, S and Singer, C [4]. | 15 years | F | Infancy | Feeding, Strong emotions Temper-ature changes | Impaired eating and drinking | Healthy | No family history of abnormal movements | CT/MRI brain, EEG normal | 25 units botulinum toxin type A to each mentalis muscle q 9 months | 95% improvement in symptoms No adverse effects |
| 2008 | Kharraz et. al [10] | 70 years | M | Early childhood | Strong emotions, physical stress | None | Healthy | Two daughtersJ,K with similar symptoms | EMG/NCS – no evidence of myopathic or neuropathic changes. Bilateral synchronous activity exclusively restricted to mentalis. Normal nerve conduction velocities to the chin. | Decreased in frequency with age | |
| 44 years | F | Early childhood | Strong emotions, physical stress | None | Healthy | DaughterJ | EMG/NCS as above | None | |||
| 43 years | F | Early childhood | Strong emotions, physical stress | None | Healthy | DaughterK | EMG/NCS as above Sleep study – chin trembling during sleep phase 2 | None | |||
| 2009 | Aggarwal et. al [20] | 42 years | M | Childhood | None | None | Healthy | Six family members with similar chin movements | EMG/NCS – spontaneous arrhythmic discharges of normal motor units in both mentalis muscle, no peripheral facial nerve hyperexcitability/denervation, presence of bilateral facial nuclear hyperexcitability demonstrated by spread of facial reflex response | Medications (not specified) Left lower peripheral facial nerve surgery 30 units botulinum toxin (Botox, Allergan) to each mentalis q8-10 months) |
No improvement No improvement Complete resolution of symptoms No adverse effects |
| 2014 | Mahmoudi, M and Kothare, SV [5] | 17 years | M | 12 years | Sleep | Tongue biting | Healthy | No family history of abnormal movements | CT/MRI brain normal Sleep study captured periods of tremor of chin and lower lip during sleep | Clonazepam 0.5 mg at bedtime | No improvement No adverse effects |
| 2014 | Macerollo, A et. al [37] | 68 years | M | Early childhood | Strong emotions, concentration | None | Healthy | DaughterL with similar symptoms | None | None | |
| 37 years | F | Early childhood | Strong emotions, concentration | None | Healthy | DaughterL | None | None | |||
| 32 years | F | Early childhood | Strong emotions, concentration | None | Healthy | Several family members with similar symptoms | None | None | |||
| 2015 | Ehm et. al [13] | 40 years | F | Early childhood | Strong emotions | None | Healthy | Six family members with similar symptoms | None | Clonazepam 0.5 mg TID Carbamazepine 100 mg TID |
Modest improvement Modest improvement No adverse effects |
| 2015 | Jain et. al [33] | 5 years | F | Early infancy | None | None | Healthy | Father with similar symptoms | EEG normal Neuroimaging normal |
None | |
| 2016 | Akiyama et. al [6] | 9 years | F | 1 week | None | None | Healthy | MotherM with similar symptoms | Electrolytes and thyroid studies normal CT/MRI normal EEG normal EMG – repetitive bursts of muscle activity that decreased during stage 1 sleep and disappeared during stage 2 sleep |
Arotinolol (peripherally acting beta blocker with weak alpha blockade) 2.5 mg titrated to 7.5 mg BID | Significant reduction with 2–3 days of symptom free days per week |
| 36 years | F | Early childhood | Stress | Impaired sleep | Healthy | MotherM | None | None | Noted improvement with alcohol | ||
| 2020 | This article | 9 years | M | 3.5 months | Strong emotions | None | ADHD, borderline IQ, mixed receptive-expressive language disorder | None | MRI brain normal | OnabotulinumtoxinA – 30 U to each mentalis | Complete resolution, no adverse effects |
| 7 months | M | Early infancy | None | None | Healthy | None | None | Spontaneous remission by 1 year | |||
| 14 months | M | First day of life | None | Nocturnal tongue biting | Healthy | MotherN and maternal grandfatherO with similar symptoms and recurrent hiccups | EEG normal | None | |||
| F | Young adulthood | Stress | None | Recurrent hiccups | MotherN | None | Plan for OnabotulinumtoxinA injections | ||||
| M | Young adulthood | Stress | None | Recurrent hiccups | Maternal GrandfatherO | None | None | ||||
| 4 months | F | First day of life | None | None | Healthy | None | None | None | |||
* Father was also injected with 5 units botulinum toxin (Oculinum, Allergan) to each mentalis muscle interdose interval 2–3 months with complete resolution of symptoms and no adverse effects.
Discussion
Geniospasm is a paroxysmal movement disorder of the mentalis muscle [10]. It has also been called familial trembling of the chin, hereditary quivering of the chin, hereditary chin trembling, and hereditary essential chin myoclonus [11]. Classically, the chin movements can be precipitated by stress or strong emotions, but can also occur spontaneously [12]. Improvement has been reported with alcohol consumption in at least one case [13]. Onset is in infancy or early childhood [1]. Events may become less frequent with age [14].
The presence of the mentalis muscle allows the lower lip to remain vertical to cover the lower incisors and prevent drooling. Contraction of the mentalis elevates the lower lip and chin, generating a pouty, thoughtful, or resolute facial expression, depending on concurrent actions of other facial muscles [15]. The mentalis is active during speech, pursing of the lips, smiling, whistling, kissing, and mastication [16]. Similar phenomenology to geniospasm can be seen as a prelude to crying [17]. The muscle arises from a circular area below the incisors, and its fibers spread to insert into the skin overlying the chin. The upper mentalis fibers lay horizontally, and lower fibers lay vertically [16]. The motor neurons of each of the two mentalis muscles originate ipsilaterally, and account for almost 10 percent of all motor neurons in each facial motor nucleus [18].
Goldsmith in 1927 described a family with hereditary geniospasm and suggested that the character of the chin movement in offspring appeared to be intensified by procreating with “high tempered mates” [19]. Since that time, little progress has been made to elucidate the precise genetic basis of the disorder. Most cases described in the literature have been hereditary with an autosomal dominant inheritance pattern and high penetrance [13]. There have been two sporadic cases described [4,5]. Three of our four cases were sporadic, suggesting that sporadic cases may be more common than previously appreciated. A genome-wide linkage study in 1997 suggested a causative locus of 9q13-q21 in one affected family, that did not appear causative in a second affected family [17,32]. Since these two studies utilizing linkage analysis, there have been no newer studies evaluating for causative genes using next-generation sequencing. It is possible that through utilization of more recent advances in genetic testing, we may find sequencing differences that account for the pathology of geniospasm. Other genetic etiologies that would not be captured by exome sequencing which could be causative in this disorder include trinucleotide repeats, deep intronic and regulatory element variants, or structural variants.
Electromyography (EMG) has demonstrated that each of the paired mentalis muscles contracts at the same time during geniospasm [18], with both rhythmic and arrhythmic discharges of normal motor units [10]. The origin of the movement is thought to occur from loss of inhibition or hyperexcitability of central projections to the facial nuclei [20]. It has been detected during sleep using EMG [5].
An association exists between geniospasm and recurrent nocturnal tongue-biting (RNTB), the latter of which is even less well-understood, and further studies including video polysomnography with EMG may further elaborate on underlying mechanisms. This symptom can be quite bothersome, awakening patients from sleep with painful lacerations, typically at the tip or sides of the tongue, compounded by repeated injury to the same area. Lacerations can be bloody, can lead to scarring in some, and in at least one case caused traumatic amputation of the tongue tip [8,9]. It has been described as the presenting symptom of geniospasm in several cases [5,8,9]. Biting tends to begin between 10 and 18 months and may abate or decrease during early childhood. It can occur during more than half the nights of the week and can occur more than once per night [9]. Patient three’s RNTB began at 11 months of age, at which time he began awakening multiple times per night (max 25 times) with resultant lacerations (Figure 1). It became less frequent two months later. His mother continues to have rare nocturnal tongue-biting in adulthood.
While benzodiazepines such as clonazepam generally are insufficient to treat the geniospasm itself, it appears to be helpful in treating the nocturnal biting [9]. We suggest using dosages between 0.01 and 0.1 mg/kg at bedtime in children, with gradual titration. RNTB in geniospasm has never been captured on video polysomnography, and the precise mechanism of tongue injury remains unclear. Johnson et al in 1971 reported that it appeared to be caused by hypnic jerks [8]. However, hypnic jerks in general are common and do not routinely cause such reliable tongue injury. The consistency with which patients bite their tongues during sleep suggests that the biting may be due to sleep-related faciomandibular myoclonus, a type of focal hypnic jerk that has since been described and is known to cause similar injury, and typically is responsive to treatment with clonazepam [21]. Worthy of note, patient three also is noted to regularly bite and lacerate his tongue when he sneezes.
Although for many with milder symptoms, geniospasm is an issue only of cosmesis, the movements can appear quite impressive and have been reported to cause significant social anxiety and embarrassment [20], as was the case with patient 1. There are reports of patients attempting to hide the movements by biting their lower lip or wearing a scarf [12]. Chin movements can occur of an amplitude and force sufficient to interfere with speech and drinking from a cup [9]. There have been cases described with other neurologic conditions associated such as nystagmus, strabismus, hereditary sensory motor neuropathy type 1 [16], otosclerosis [22,23], Parkinson disease [24], and REM behavior disorder [8], however the reports are limited and more likely reflect incidental associations [10].
Numerous treatments have been attempted for geniospasm. In 1930, Frey treated a family’s “quivering chins” with faradic current and ultraviolet light without much improvement. Family members also underwent a trial of psychotherapy, with some degree of improvement [25]. There is a single report of a patient who achieved remission after suffering a blow to the chin at age 13 [26]. Other treatments used have included dopamine receptor blocking agents, anticonvulsants, benzodiazepines, beta-blockers, antihistamines, and others with inconsistent results [14].
More recently, injections of botulinum neurotoxin (BoNT) have been utilized to treat geniospasm [7,26]. There are 7 major serotypes of BoNT (BoNT/A-G), each of which acts by inhibiting the release of acetylcholine from the presynaptic nerve terminal by interfering with fusion of the synaptic vesicle with the plasma membrane [27,28,30]. Botulinum toxin has been used clinically since 1977, when ophthalmologist Alan Scott first used it in the treatment of strabismus [29]. Since that time, it has been shown to be efficacious in the treatment of innumerable types of cramps, spasms, and involuntary movements, including dystonia, spasticity, and hemifacial spasm [30]. The first reported use of BoNT for geniospasm was in 1992 by Gordon, who injected a father and his two sons. Each patient received 5 units of onabotulinumtoxinA into each mentalis muscle, after which chin trembling completely resolved for periods of 2–3 months following injections. The family was followed for 5 years, during which the trembling was adequately controlled without need for increasing dosage, there were no adverse effects, and the family reported that treatment “significantly improved their quality of life” [26]. Effective doses of onabotulinumtoxinA for bothersome geniospasm range from 5 to 30 units to each mentalis muscle. The treatment appears to be beneficial and well-tolerated for those with bothersome geniospasm.
Conclusion
Due to the rarity of the condition, limited data exist with regard to the effective treatment of geniospasm. Several interventions have been tried historically, with variable results. Our results, together with those of prior reported cases, support the use of botulinum toxin injections for the management of this condition. We recommend the use of clonazepam for recurrent nocturnal tongue-biting if present. We suggest that the tongue-biting itself may be due to an association between geniospasm and sleep-related faciomandibular myoclonus; video polysomnography with EMG will be useful to determine this.
Funding Information
The authors have not received any external funding sources for this study.
Competing Interests
The authors have no competing interests to declare.
References
- 1.Massaro D. Ventisei casi di geniospasmo attraverso cinque geneazioni:contributo clinico allo studio dell’eredità fisiopatologica. I Pisani (Palermo). 1894; 1: 47–56. [Google Scholar]
- 2.Massaro D. Vingt-six cas de génio-spasme en cinq générations. Rev Neurol. 1894; 2: 534. [Google Scholar]
- 3.Stocks, P. Facial spasm inherited through four generations. Biometrika. 1923; 14(3): 311–315. DOI: 10.1093/biomet/14.3-4.311 [DOI] [Google Scholar]
- 4.Papapetropoulos S, Singer C. Sporadic geniospasm (chin trembling): report of a case. Movement Disorders. 2007; 22(3): 434 DOI: 10.1002/mds.21239 [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi M, Kothare SV. Tongue biting: a case of sporadic geniospasm during sleep. Journal of Clinical Sleep Medicine. 2014; 10(12): 1339–1340. DOI: 10.5664/jcsm.4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama T, Miyahara H, Waki K, Katsuhiro Kobayashi HY. A Japanese case of hereditary chin trembling responsive to arotinolol. Parkinsonism and Related Disorders. 2016; 29: 133–134. DOI: 10.1016/j.parkreldis.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 7.Bakar M, Zarifoglu M, Bora I, Turan F, Sen C, Ogul E. Treatment of hereditary trembling chin with Botulinum toxin. Movement Disorder Society. 1998; 13(5): 845–850. DOI: 10.1002/mds.870130516 [DOI] [PubMed] [Google Scholar]
- 8.Johnson LF, Kinsbourne M, Renuart AW. Hereditary chin-trembling with nocturnal myoclonus and tongue biting in dizygous twins. Develop. Med. Child Neurol. 1971; 13: 726–729. DOI: 10.1111/j.1469-8749.1971.tb08344.x [DOI] [PubMed] [Google Scholar]
- 9.Goraya JS, Virdi V, Parmar V. Recurrent nocturnal tongue biting in a child with hereditary chin trembling. Journal of Child Neurology. 2006; 21(11): 985–987. DOI: 10.1177/08830738060210111101 [DOI] [PubMed] [Google Scholar]
- 10.Kharraz B, Reilich P, Noachtar S, Danek A. An episode of geniospasm in sleep: toward new insights into pathophysiology. Movement Disorders. 2008; 23(2): 274–305. DOI: 10.1002/mds.21722 [DOI] [PubMed] [Google Scholar]
- 11.Frey E. Ein streng dominant erbliches Kinnmuskelzittern (Beitrag zur Erforschung der menschlichen Affektäu- ßerungen). Dtsch Z Nervenheilk. 1930; 115: 9–26. DOI: 10.1007/BF02196788 [DOI] [Google Scholar]
- 12.Diaz S, Scorticati MC, Micheli F. Hereditary chin tremor/myoclonus: a report from Latin America. Movement Disorders. 1999; 14(1): 180–181. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Ehm GH, Kim HJ, Jeon BS. Hereditary geniospasm in a Korean family. Parkinsonism and Related Disorders. 2015; 21: 665–666. DOI: 10.1016/j.parkreldis.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 14.Soland VL, Bhatia KP, Sheean GL, Marsden CD. Hereditary geniospasm: two new families. Movement Disorders. 1996; 11(6): 744–761. DOI: 10.1002/mds.870110626 [DOI] [PubMed] [Google Scholar]
- 15.Hur MS, Kim HJ, Choi BY, Hu KS, Kim HJ, Lee KS. Morphology of the mentalis muscle and its relationship with the orbicularis oris and incisivus labii inferioris muscles. J Craniofac Surg. 2013. March; 24(2): 602–4. DOI: 10.1097/SCS.0b013e318267bcc5 [DOI] [PubMed] [Google Scholar]
- 16.Danek, A. Geniospasm: hereditary chin trembling. Movement Disorders. 1992; 8(3): 335–338. DOI: 10.1002/mds.870080314 [DOI] [PubMed] [Google Scholar]
- 17.Jarman PR, Wood NW, Davis MT, Davis PV, Bhatia KP, Marsden CD, Davis MB. Hereditary geniospasm: linkage to chromosome 9q13-q21 and evidence for genetic heterogeneity. Am. J. Human Genetics. 1997; 61: 928–933. DOI: 10.1086/514883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalaupka FD, Bartholini F, Mandich G, Turro M. Two new families with hereditary essential chin myoclonus: clinical features, neurophysiological findings and treatment. Neurol. Sci. 2006; 27: 97–103. DOI: 10.1007/s10072-006-0607-x [DOI] [PubMed] [Google Scholar]
- 19.Goldsmith, JB. The inheritance of facial spasm and the effect of a modifying factor associated with high temper. Journal of Heredity. 1927; 18(4): 185–187. DOI: 10.1093/oxfordjournals.jhered.a102839 [DOI] [Google Scholar]
- 20.Aggarwal A, Warren JE, Warren JD, Thompson PD. Facial reflex hyperexcitability in geniospasm suggests a brainstem origin. Movement Disorders. 2009; 24(5): 783–790. DOI: 10.1002/mds.22454 [DOI] [PubMed] [Google Scholar]
- 21.Loi D, Provini F, Vetrugno R, D’Angelo R, Zaniboni A, Montagna P. Sleep-related faciomandibular myoclonus: A sleep-related movement disorder different from bruxism. Movement Disorders. 2007; 22(12): 1819–1822. DOI: 10.1002/mds.21661 [DOI] [PubMed] [Google Scholar]
- 22.Grossman, BJ. Trembling of the chin – an inheritable dominant character. Pediatrics. 1957; 19: 453–455. [PubMed] [Google Scholar]
- 23.Alsager DE, Bowen P, Bamforth JS. Trembling chin – a report of this inheritable dominant character in a four-generation Canadian family. Clinical Genetics. 1991; 40: 186–189. DOI: 10.1111/j.1399-0004.1991.tb03074.x [DOI] [PubMed] [Google Scholar]
- 24.Erer S, Jankovic J. Hereditary chin tremor in Parkinson’s disease. Clinical Neurology and Neurosurgery. 2007; 109: 784–785. DOI: 10.1016/j.clineuro.2007.05.020 [DOI] [PubMed] [Google Scholar]
- 25.Wadlington WB. Familial Trembling of Chin. Journal of Pediatrics. 1958; 53(3): 316–321. DOI: 10.1016/S0022-3476(58)80218-8 [DOI] [PubMed] [Google Scholar]
- 26.Gordon K, Cadera W, Hinton G. Successful treatment of hereditary trembling chin with botulinum toxin. J. Child Neurol. 1992; 7: 154–156. DOI: 10.1177/088307389300800208 [DOI] [PubMed] [Google Scholar]
- 27.Schantz EJ, Johnson EA. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol Rev. 1992; 56: 80–99. DOI: 10.1128/MMBR.56.1.80-99.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blasi J, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993; 365(6442): 160–3. DOI: 10.1038/365160a0 [DOI] [PubMed] [Google Scholar]
- 29.Scott AB. Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc. 1981; 79: 734–770. [PMC free article] [PubMed] [Google Scholar]
- 30.Jankovic J. Botulinum toxin: State of the art. Mov Disord. 2017; 32(8): 1131–1138. DOI: 10.1002/mds.27072 [DOI] [PubMed] [Google Scholar]
- 31.Destee A, Cassim F, Defebvre L, Guieu JD. Hereditary chin trembling or hereditary chin myoclonus. Journal of Neurology, Neurosurgery, and Psychiatry. 1997; 63: 804–807. DOI: 10.1136/jnnp.63.6.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimes DA, Han F, Bulman D, Nicolson ML, Suchowersky O. Hereditary chin trembling: a new family with exclusion of the chromosome 9q13-q21 locus. Movement Disorders. 2002; 17(6): 1390–1392. DOI: 10.1002/mds.10275 [DOI] [PubMed] [Google Scholar]
- 33.Jain P, Sharma S, Aneja S. Hereditary Chin-trembling. Indian Pediatrics. 2015; 52: 720 DOI: 10.1007/s13312-015-0706-y [DOI] [PubMed] [Google Scholar]
- 34.Laurance BM, Matthews WB, Diggle JH. Hereditary quivering of the chin. Arch. Dis. Childhood. 1968; 43: 249–251. DOI: 10.1136/adc.43.228.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahn S. Atypical tremors, rare tremors and unclassified tremors Movement Disorders: Tremor. 1984; 431–443. London: Macmillan Press; DOI: 10.1007/978-1-349-06757-2_33 [DOI] [Google Scholar]
- 36.Devetag Chalaupka F, Bartholini F, Mandich G, Turro M. Two new families with hereditary essential chin myoclonus: clinical features, neurophysiological findings and treatment. Neurol Sci. 2006; 27: 97–103. DOI: 10.1007/s10072-006-0607-x [DOI] [PubMed] [Google Scholar]
- 37.Macerollo, A, et al. Abnormalities of Masseteric Inhibitory Reflex in Hereditary Geniospasm: Evidence for a Brainstem Myoclonus. Movement Disorders Clinical Practice. 2014; 2(1): 49–52. DOI: 10.1002/mdc3.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]


