Abstract
While visual assessment by a clinician is the standard of care for burn severity evaluations, new technologies at various stages of development are attempting to add objectivity to this practice by quantifying burn severity. Assessment accuracy generally improves after the burn injury has progressed, but early assessments that correctly identify superficial partial and deep partial burns have the potential to lead to more prompt treatments and shorter recovery times. To date, Spatial Frequency Domain Imaging (SFDI) has only been used in animal models of burns, but has shown the potential to categorize burns accurately at earlier time points. Here we examine the potential for SFDI to assess burn severity in clinical patients. We also utilize Laser Speckle Imaging (LSI), an FDA cleared non-invasive imaging technology that typically measures blood perfusion in order to evaluate burns in clinical patients. We present a case series of two patients, both with partial thickness burns of varying severity. Partial thickness burns are often difficult for clinicians to categorize based on visual appearance alone. SFDI and LSI were both performed on each patient at approximately 24 and 72 h after their respective burn incidents. Each technique was able to render spatially resolved information that enabled improved assessment accuracy for each burn. This represents the first publication of SFDI applied to clinical burn patients after being successfully utilized in animal models, and highlights the potential for SFDI as a feasible tool for the timely categorization of burn severity.
Keywords: Burns, Clinical, Humans, Spatial Frequency Domain Imaging, Laser Speckle Imaging
1. Introduction
More than 11 million reports of injuries due to burns are made annually worldwide [1]. If these wounds are not treated in a timely manner, they can lead to scarring that is damaging both functionally and cosmetically. Being able to accurately grade the severity of a given burn or burn region earlier than is typically done is one way to improve outcomes. Delays in the decision for surgical intervention can lead to the progression of burn injuries and typically increase costs associated with wound management and hospital stay [2].
Several imaging techniques have been utilized to help clinicians address issues related to expedite accurate burn diagnosis. Laser Doppler (LD) has been a popular technology to measure the perfusion of burned tissue in order to successfully assess severity at a single point [3]. This has subsequently led to imaging techniques like Laser speckle imaging (LSI) [4,5], that have the ability to examine perfusion over a large area, providing clinicians with a easy to interpret perfusion map that can be generated quickly. Areas of the map with high perfusion indicate the vasculature remains intact and the tissue will likely heal on its own while lower perfusion areas are an indication of damage that may require surgery. The accuracy of LSI, while consistently higher than clinical impression, is time dependent. Thus, LSI/LD burn severity classification is not optimal until 48–72 h post-burn occurrence [3], as measurements before 48 h have yielded inconclusive results [6].
Spatial Frequency Domain Imaging (SFDI) is an emerging imaging technology that has the ability to measure absorption and scattering properties of biological tissue [7–10]. Using different wavelengths of light, as well as different spatial patterns, allows the technique to quantify both hemodynamic [11,12] and structural [13–15] properties of skin. Our earlier research using SFDI to study burns in animal models of rats and pigs has shown an ability to noninvasively categorize burns as early as 24 h after injury [13–15]. Specifically we saw that structural changes associated with collagen damage seen in histology correlated with measured changes in scattering properties [13–15].
Here, for the first time, we use a research grade SFDI device in a clinical setting to determine the feasibility of using this approach in human burn patients. We also utilized a commercial grade LSI device that has FDA clearance to assess burn severity in humans. This is a case series consisting of two patients with partial thickness burns, which are among the most difficult for clinicians to accurately categorize [16], that are imaged with both technologies and assessed by clinicians at 24 and 72 h.
2. Methodology
UCI IRB approved protocol (HS #2009–7322) was used to enroll subjects in to the study. Subjects were recruited from patients seen in the burn and surgical intensive care unit at the University of California Irvine Medical Center and consented to participating in the study. Patients were imaged approximately 24 and 72 h after the burn injury occurred. The PeriCam PSI (Perimed AB, Sweden), a commercial LSI device, was used to collect all of the LSI data. The distance between the imaging device and the patient was approximately 25 cm, and the field of view was limited to 15 × 15 cm. The imaging session lasted approximately 60 s at an acquisition rate of 5 images/second. The Perimed PimSoft v2 software was used to handle all data collection as well as well as analysis for specific regions where it calculated the average value in Perfusion Units (PU) [17]. The OxImager RS™ (Modulim, Inc., Irvine, CA), a commercially available SFDI device, was used to collect all of the SFDI data. The distance between the imaging device and the patient was approximately 32 cm, and the field of view was limited to 20 cm × 15 cm. The imaging session lasted approximately 90 s and acquired 3 sets of images at 5 spatial frequencies (0–0.2 mm−1) and 8 wavelengths (471–850 nm). For all cases, the SFDI and LSI devices were used one after another in the same position, and all reasonable efforts were made to keep ambient conditions consistent between measurements. All body parts in Cases 1 and 3 were elevated approximately 1 foot during both imaging sessions. These setups have been described previously [8,18], as well as the process for analyzing the data [7,19]. In order to analyze the SFDI data, a calibration phantom that came with the OxImager RS™ system was measured after patient data was collected. The MI Acquire v1.34.00 software package (Modulim, Inc., Irvine, CA) was then used to process the images collected from the patient and the calibration phantom in order to generate images of tissue optical properties at different wavelengths (absorption coefficient, μa and reduced scattering coefficient, μ’s). For both imaging devices, a small region of interest (1 cm × 1 cm) on the patient was selected to calculate average values with standard deviation. The attending clinician remained blinded to the study images to prevent any potential bias.
3. Case 1
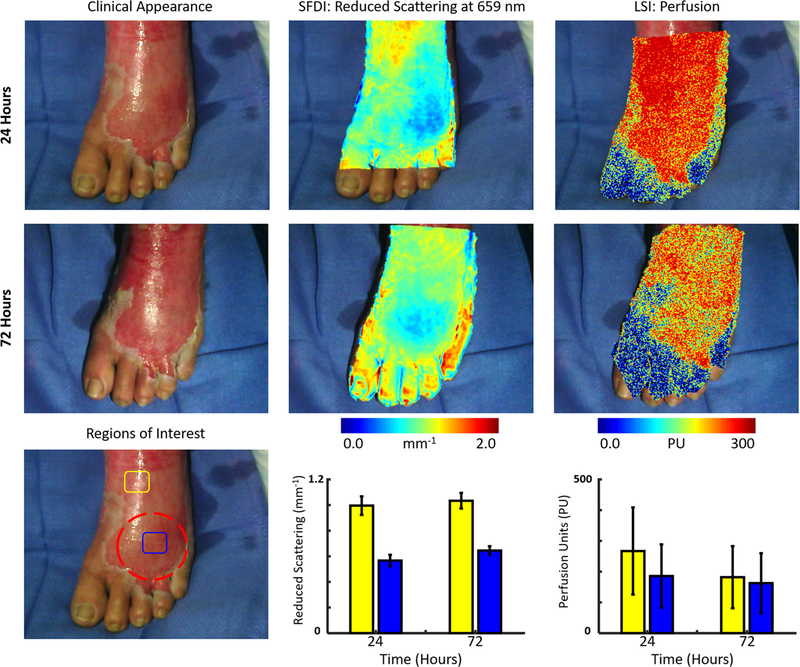
A 69 year-old female sustained partial thickness burns to the foot due to a scald burn from hot water and was transferred to the UCI burn unit. Fig. 1 depicts the clinical appearance, SFDI-derived reduced scattering coefficient and LSI-derived perfusion images obtained at 24 and 72 h. It also highlights two distinct regions of interest (blue and yellow) on the foot and reports values from both instruments at 24 and 72 h post-burn in those regions. The blue region depicts an area that is relatively distal on the foot, while the yellow region depicts a region that is more proximal. At 24 h, LSI showed high perfusion values throughout the burned region particularly in the proximal (yellow) region. SFDI also showed a difference in scattering values at the proximal (yellow) and distal (blue) portions of the burn with less variation (smaller error bars). Earlier animal studies that have utilized SFDI to study burns in rats [15] and pigs [13,14,19] have shown that the reduced scattering coefficient typically decreases in severe burns that require surgical intervention. At 72 h, the clinical appearance of the burn remained unchanged. LSI still showed elevated perfusion throughout the burn region, although values had decreased slightly in the proximal regions from the initial measurements at 24 h. SFDI scattering values remained relatively unchanged at the 72-hour time point, still showing decreased reduced scattering coefficient values in the distal portions of the burn. The visual appearance at 96 h was concerning to the clinician (dashed red region), but ultimately it was decided the patient would not require surgery. The patient did not display clinical signs of peripheral vascular disease, however she was a diabetic patient taking insulin, so there was concern for slower than normal healing if debridement was performed. She remained in the hospital for 144 h post-burn and was then discharged with only a dressing applied to the wound after non-excisional debridement.
Fig. 1.
(Top row, left to right) Case 1. The clinical appearance, SFDI derived reduced scattering image and LSI derived perfusion image obtained at 24 h. (Middle row, left to right) The clinical appearance, SFDI derived reduced scattering image and LSI derived perfusion image obtained at 72 h. (Bottom row) Average SFDI and LSI values at 24 and 72 h post-burn in two regions (blue and yellow). The concerning region (dashed red) received non-excisional debridement. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Case 2
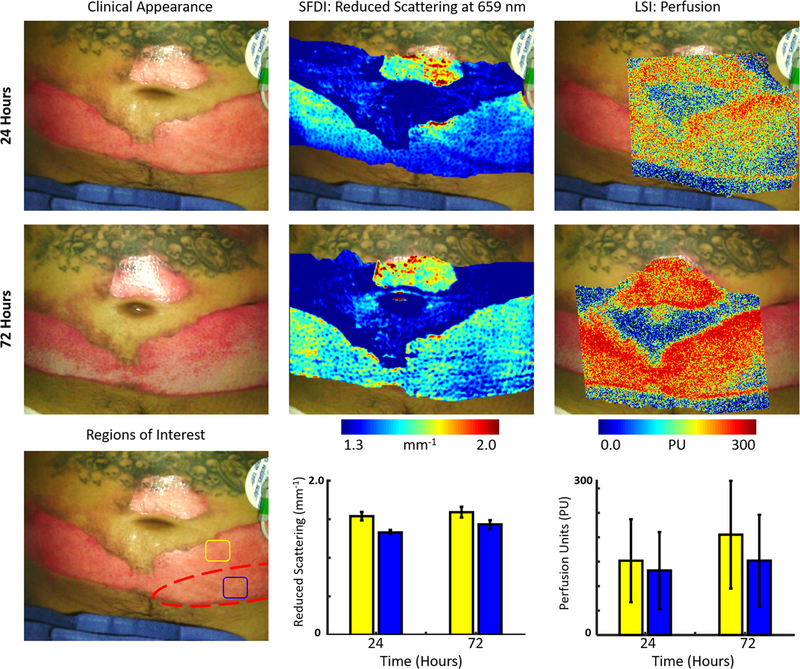
A 38-year-old male sustained partial thickness burns to the abdomen region following a flash burn endured when refilling a butane lighter and was transferred to the UCI burn unit. Fig. 2 shows the clinical appearance, SFDI-derived reduced scattering coefficient and LSI-derived perfusion images obtained at 24 and 72 h. It also depicts two regions of interest (blue and yellow) on the burned portion of the abdomen. The blue region is an area on the lower portion of the burned region while the yellow region is on an upper portion of the burned region. The patient was taken to the operating room at 72 h post-burn and the left lower portion of the burn on the lower abdomen was covered by a skin autograft. The patient was discharged at 240 h post-burn.
Fig. 2.
(Top row, left to right) Case 2. The clinical appearance, SFDI derived reduced scattering image and LSI derived perfusion image obtained at 24 h. (Middle row, left to right) The clinical appearance, SFDI derived reduced scattering image and LSI derived perfusion image obtained at 72 h. (Bottom row) Average SFDI and LSI values at 24 and 72 h post-burn in two regions (blue and yellow). The final debrided regions (dashed red) were determined by clinicians 72 h after burn. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The clinical appearance of the burn is relatively homogeneous at 24 h post-burn. SFDI showed a small difference between the upper and lower regions of the burned area, as did LSI although with larger error bars. The clinical appearance of the burn had changed significantly at 72 h and there was an obvious need to graft the lower portion of the burned region. SFDI showed similar results in the reduced scattering coefficient at the 24 and 72 h time point. LSI showed an increase in perfusion to the upper region of the burn at the 72-h time point that is indicative of an area expected to heal. This was in contrast to the lower region, which still showed an area of lower perfusion.
5. Case 3
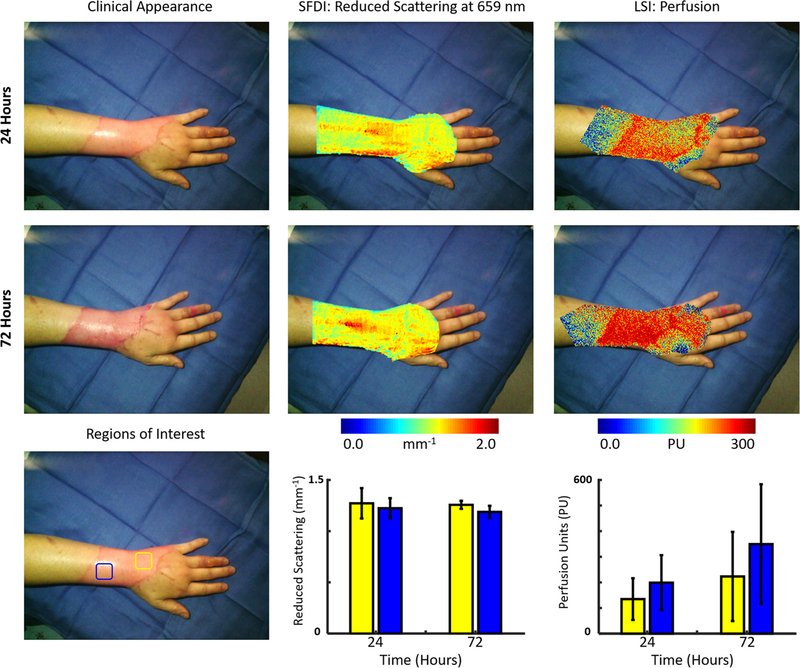
A 53 year-old female sustained partial thickness burns to the wrist due to a scald burn from hot oil and was transferred to the UCI burn unit. Fig. 3 depicts the clinical appearance, SFDI-derived reduced scattering coefficient and LSI-derived perfusion images obtained at 24 and 72 h. It also highlights two distinct regions of interest (blue and yellow) on the wrist and reports values from both instruments at 24 and 72 h post-burn in those regions. The yellow region depicts an area that is relatively distal on the wrist, while the blue region depicts a region that is more proximal. At 24 h, LSI showed high perfusion values throughout the burned region particularly in the proximal (blue) region. SFDI also showed high scattering values at both the proximal (blue) and distal (yellow) portions of the burn. At 72 h, the clinical appearance of the burn showed more erythema throughout. LSI showed elevated perfusion through the entirety of the burn region. SFDI scattering values remained relatively unchanged at the 72-h time point, although with less a spatial variation (smaller error bars). The visual appearance led to the decision to perform non-excisional debridement of the wrist 168 h after the burn. The wound was covered with porcine intestinal submucosal matrix xenograft.
Fig. 3.
(Top row, left to right) Case 3. The clinical appearance, SFDI derived reduced scattering image and LSI derived perfusion image obtained at 24 h. (Middle row, left to right) The clinical appearance, SFDI derived reduced scattering image and LSI derived perfusion image obtained at 72 h. (Bottom row) Average SFDI and LSI values at 24 and 72 h post-burn in two regions (blue and yellow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
6. Discussion
Commercialized LSI devices have frequently shown an ability to successfully characterize burns around 48–72 h after injury human patients [20] similar to these results. The ability to accurately categorize burns even earlier at the 24 h time point would be an important advancement since this has the potential to enable clinicians to make decisions earlier about whether a patient requires surgical intervention. SFDI has previously been demonstrated to accurately characterize burn severity in animal models [13,19,21] earlier than perfusion based techniques. The reason for this is likely due to the fact that the changes in the reduced scattering coefficient measured by SFDI is sensitive to the structural changes in damaged collagen that occur immediately after injury. We have illustrated this in porcine burns for which we also collected histology [15]. In case 2, SFDI measured lower reduced scattering in the distal region of the lower abdomen. The difference in reduced scattering between this region and elsewhere within the burn remained constant across the 24 h and 72 h imaging time points. In this case, the debrided areas determined by the clinicians correspond to the low scattering regions indicated by SFDI in Fig. 2. However, the area was debrided 72 h after the initial imaging. Alternately, case 1 showed little to no spatial variation and high scattering throughout the burn wound. This wound would not necessitate an allograft, but instead was eventually dressed 144 h after the initial imaging timepoint and the pateient was discharged. While this patient did not display clinical signs of peripheral vascular disease, it is possible that this would affect interpreting perfusion changes. This would be another potential benefit for utilizing SFDI data that is sensitive to structural changes in tissue instead. Here, application of SFDI to identify low scattering could have expidited the decision for surgery, thereby decreasing patient length of stay and hospital operation cost. Additionally, it has been shown that earlier intervention in the case of deep partial and full-thickness burns leads to improved wound healing and immune response when compared to delayed grafting [22,23].
The time scale for the perfusion changes measured by LSI are known to take place several days after the initial injury [3]. These clinical cases highlight the typical fluctuations that are seen in LSI images at 24 and 72 h after the initial burn. In case 1, a patient with diabetese and hypertension, a greater difference in perfusion was measured between identified regions of interest at 24 h post-burn than 72 h post-burn, contrary to previous literature. Work with LSI in diabetic foot ulcers indicate that baseline LSI values are lower in patients with ischemia, although the difference is not significant [24]. This difference in baseline perfusion may effect the ability of LSI to categorize burn severity several days earlier than what is typically decided clinically. In case 2, low perfusion measured by LSI corresponds to regions of low scattering from SFDI, but, as the literature suggests, the difference in perfusion between superficial partial burn (higher abdomen) and deep partial burn (lower left abdomen) increases at 72 h post-burn. Still, in this case both measurements indicate the need for intervention before the final outcome was determined by the clinician. In case 3, the LSI measurements showed higher perfusion throughout the burn at both the 24 and 72 h time-points, indicative of a partialthickness burn that does not require an allograft, long before the actual clinical decision was made. Again, use of the device in these instances could have reduced patient stay and improved wound healing times.
Here we have only shown results from one parameter (e.g. reduced scattering coefficient) collected using SFDI, but there is potential to improve accuracy by examining some combination of parameters. As shown in previous works, SFDI can be used to measure hemodynamic parameters, such as total hemoglobin and oxygen saturation, that correspond directly with the perfusion measurements from LSI [19,25]. However, these parameters would also be effected by the same physiological response to a burn that causes fluctuation in LSI accuracy up to 72 h after burn. The data analysis associated with SFDI also has room for optimization to specifically focus on burn wounds, by combining multiple wavelengths and structured illumination patterns through machine learning [26]. Here, utilization of the changes in depth penetration across the different measured parameters, along with a simplification of the output from a gradient of scattering values to a discrete index, could provide a burn severity classifier informed by SFDI measurements of underlying structure.
7. Patient consent
Subjects were recruited from patients seen in the burn and surgical intensive care unit at the University of California Irvine Medical Center and consented to participating in the study.
Acknowledgments
We gratefully acknowledge primary support from NIGMS grant R01GM108634. In addition, we acknowledge the NIBIB, including P41EB015890 (A Biomedical Technology Resource) and ASLMS Grant PG11831. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS, NIBIB or NIH. We also acknowledge the Arnold and Mabel Beckman Foundation.
Footnotes
Declaration of Competing Interest
Except for AJD, the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. AJD is a co-founder of Modulated Imaging Inc.. However, he does not have any active role in that company and is in compliance with UCI IRB and NIH policy regarding conflicts of interest.
References
- [1].Peck MD. Epidemiology ofburns throughout the world. Part I: Distribution and risk factors. Burns 2011;37:1087–100. [DOI] [PubMed] [Google Scholar]
- [2].Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31:849–73. [DOI] [PubMed] [Google Scholar]
- [3].Hoeksema H, Van de Sijpe K, Tondu T, Hamdi M, Van Landuyt K, Blondeel P, et al. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns 2009;35:36–45. [DOI] [PubMed] [Google Scholar]
- [4].Burke-Smith A, Collier J, Jones I. A comparison of non-invasive imaging modalities: infrared thermography, spectrophotometric intracutaneous analysis and laser Doppler imaging for the assessment of adult burns. Burns 2015;41:1695–707. [DOI] [PubMed] [Google Scholar]
- [5].Kaiser M, Yafi A, Cinat M, Choi B, Durkin AJ. Noninvasive assessment of burn wound severity using optical technology: a review of current and future modalities. Burns 2011;37:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nguyen K, Ward D, Lam L, Holland AJ. Laser Doppler Imaging prediction of burn wound outcome in children: is it possible before 48 h?. Burns 2010;36:793–8. [DOI] [PubMed] [Google Scholar]
- [7].Cuccia DJ, Bevilacqua F, Durkin AJ, Ayers FR, Tromberg BJ. Quantitation and mapping of tissue optical properties using modulated imaging. J Biomed Opt 2009;14:024012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cuccia DJ, Bevilacqua F, Durkin AJ, Tromberg BJ. Modulated imaging: quantitative analysis and tomography of turbid media in the spatial-frequency domain. Opt Lett 2005;30:1354–6. [DOI] [PubMed] [Google Scholar]
- [9].Torabzadeh M, Park IY, Bartels RA, Durkin AJ, Tromberg BJ. Compressed single pixel imaging in the spatial frequency domain. J Biomed Opt 2017;22:30501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wilson RH, Crouzet C,Torabzadeh M,Bazrafkan A, Farahabadi MH, Jamasian B, et al. High-speed spatial frequency domain imaging of rat cortex detects dynamic optical and physiological properties following cardiac arrest and resuscitation. Neurophotonics 2017;4:045008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ponticorvo A, Taydas E, Mazhar A, Scholz T, Kim HS, Rimler J, et al. Quantitative assessment of partial vascular occlusions in a swine pedicle flap model using spatial frequency domain imaging. Biomed Opt Express 2013;4:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yafi A, Vetter TS, Scholz T, Patel S, Saager RB, Cuccia DJ, et al. Postoperative quantitative assessment of reconstructive tissue status in a cutaneous flap model using spatial frequency domain imaging. Plast Reconstr Surg 2011;127:117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ponticorvo A, Burmeister DM, Yang B, Choi B, Christy RJ, Durkin AJ. Quantitative assessment of graded burn wounds in a porcine model using spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI). Biomed Opt Express 2014;5:3467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burmeister DM, Ponticorvo A, Yang B, Becerra SC, Choi B, Durkin AJ, et al. Utility of spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI) to non-invasively diagnose burn depth in a porcine model. Burns 2015;41:1242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nguyen JQ Crouzet C, Mai T, Riola K, Uchitel D, Liaw LH, et al. Spatial frequency domain imaging of burn wounds in a preclinical model of graded burn severity. J Biomed Opt 2013;18:66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns 2008;34:761–9. [DOI] [PubMed] [Google Scholar]
- [17].Krezdorn N, Limbourg A, Paprottka FJ, Konneker, Ipaktchi R, Vogt PM. Assessing burn depth in tattooed burn lesions with LASCA imaging. Ann Burns Fire Disasters 2016;29:223–7. [PMC free article] [PubMed] [Google Scholar]
- [18].Ponticorvo A, Rowland R, Baldado M, Burmeister DM, Christy RJ, Bernal NP, et al. Evaluating clinical observation versus Spatial Frequency Domain Imaging (SFDI), Laser Speckle Imaging (LSI) and thermal imaging for the assessment of burn depth. Burns 2019;45(2):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mazhar A, Saggese S, Pollins AC, Cardwell NL, Nanney L, Cuccia DJ. Noncontact imaging of burn depth and extent in a porcine model using spatial frequency domain imaging. J Biomed Opt 2014;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lindahl F, Tesselaar E, Sjoberg F. Assessing paediatric scald injuries using Laser Speckle Contrast Imaging. Burns 2013;39:662–6. [DOI] [PubMed] [Google Scholar]
- [21].Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, et al. Burn wound healing and treatment: review and advancements. Crit Care 2015;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Singer AJ, Toussaint J, Chung WT, McClain SA, Raut V, Rosenberg L. Early versus delayed excision and grafting of full-thickness burns in a porcine model: a randomized study. Plast Reconstr Surg 2016;137:972e–9e. [DOI] [PubMed] [Google Scholar]
- [23].Fear VS, Poh WP, Valvis S, Waithman JC, Foley B, Wood FM, et al. Timing of excision after a non-severe burn has a significant impact on the subsequent immune response in a murine model. Burns 2016;42:815–24. [DOI] [PubMed] [Google Scholar]
- [24].Mennes OA, Netten JJ, van Baal JG, Steenbergen W. Assessment of microcirculation in the diabetic foot with laser speckle contrast imaging. Physiol Meas 2019;40. [DOI] [PubMed] [Google Scholar]
- [25].Ponticorvo A, Burmeister DM, Rowland R, Baldado M, Kennedy GT, Saager R, et al. Quantitative long-term measurements of burns in a rat model using Spatial Frequency Domain Imaging (SFDI) and Laser Speckle Imaging (LSI). Lasers Surg Med 2017;49:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rowland R, Ponticorvo A, Baldado M, Kennedy GT, Burmeister DM, Christy RJ, et al. A simple burn wound severity assessment classifier based on spatial frequency domain imaging (SFDI) and machine learning. SPIE 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]