Abstract
Post-traumatic stress disorder (PTSD) is characterized by avoidance of trauma-associated stimuli and amygdala hyperreactivity, and is highly co-morbid with alcohol use disorder (AUD). Our lab uses a predator odor (bobcat urine) stress model that produces conditioned avoidance of an odor-paired context in a subset of rats, mirroring avoidance symptoms that manifest in some but not all humans exposed to trauma. We previously showed that after predator odor stress, Avoiders exhibit escalated operant alcohol self-administration (SA), higher aversion-resistant operant alcohol responding, hyperalgesia, and greater anxiety-like behavior compared to unstressed Controls. We also showed previously that systemic antagonism of corticotropin-releasing factor-1 receptors (CRFR1) reduced escalation of operant alcohol SA in rats not indexed for avoidance, that corticotropin-releasing factor (CRF) infusions into the central amygdala (CeA) produced conditioned place avoidance in stress-naïve rats, and that intra-CeA infusion of a CRFR1 antagonist reduced hyperalgesia in Avoiders. Here, we show that avoidance behavior is persistent after repeated predator odor exposure. In addition, Avoiders showed lower weight gain than Controls after predator odor re-exposure. In the brain, higher avoidance was correlated with higher number of c-Fos+ cells and CRF immunoreactivity in the CeA. Finally, we show that intra-CeA CRFR1 antagonism reversed post-stress escalation of alcohol SA and reduced avoidance behavior in Avoiders. Collectively, these findings suggest that elucidation of the mechanisms by which CRFR1-gated CeA circuits regulate avoidance behavior and alcohol SA may lead to better understanding of the neural mechanisms underlying co-morbid PTSD and AUD.
Keywords: Predator odor stress, operant alcohol self-administration, corticotropin-releasing factor
1. Introduction
Post-traumatic stress disorder (PTSD) has a prevalence rate of ~8% in the general population and ~20% among U.S. combat veterans (Hoge et al., 2004; Kessler et al., 1995) and costs the U.S. billions of dollars annually (Tanielian et al., 2008). According to the Diagnostic and Statistical Manual of Mental Disorders (5th edition), a PTSD diagnosis requires presence of symptoms from all four of the following symptom clusters for at least one month: 1) intrusive recollections of the traumatic event, 2) avoidance of trauma-related stimuli, 3) negative alterations in cognition, and 4) alterations in arousal and mood. Of these symptom clusters, avoidance of trauma-related stimuli may be the most detrimental to psychosocial functioning (Hendrix et al., 1994; Malta et al., 2009), is a strong predictor of future PTSD severity (Bryant et al., 2000; Perkonigg et al., 2005; Shin et al., 2015) and poor treatment response (Taylor et al., 2001), and is predicted by amygdala hyperreactivity (Sripada et al., 2013). Critically, PTSD is highly co-morbid with alcohol use disorder (AUD), with 22–43% of individuals with PTSD meeting criteria for AUD, compared to 8% in the general population (Blanco et al., 2013). Relative to either disorder alone, co-morbid AUD and PTSD produce worse outcomes, such as increased incidence of disease and greater loss of work productivity (McCarthy and Petrakis, 2010).
The use of animal models to investigate the neurobiological factors underlying traumatic stress effects is critical for the development of better prevention, diagnostic, and treatment strategies. Our lab uses a model in which a subset of rats exposed to predator odor (bobcat urine) show persistent avoidance (up to 6 weeks) of odor-paired stimuli (context with distinct tactile and visual cues), mirroring avoidance symptoms that manifest in some but not all humans exposed to traumatic stress (Albrechet-Souza and Gilpin, 2019; Breslau, 2009). After predator odor stress exposure, rats classified as Avoiders exhibit escalated operant alcohol self-administration (SA), more aversion-resistant operant alcohol responding (Edwards et al., 2013), hyperalgesia (Itoga et al., 2016), and higher anxiety-like behavior (also seen in Non-Avoiders) than unstressed Controls (Whitaker and Gilpin, 2015). Therefore, this animal model may be useful for understanding the neurobiology underlying behavioral changes after traumatic stress.
One goal of this study was to further characterize the avoidance phenotype in our predator odor stress model. In other predator stress models, rats exposed to a worn cat collar exhibit persistent and extinction-resistant reduction in locomotor activity after repeated exposure to the predator odor stress (Mackenzie et al., 2010). In addition, repeated cat exposures reduce weight gain and produce adrenal hypertrophy in rats (Figueiredo et al., 2003). Here, we tested avoidance behavior after repeated exposures to predator odor and the effect of predator odor exposure on body weight gain and adrenal and thymus weights in Avoiders, Non-Avoiders, and unstressed Controls.
Another goal of this study was to elucidate the neurobiological mechanisms contributing to predator odor-paired context avoidance and post-stress alcohol SA in the bobcat urine stress model. We previously found that intra-CeA corticotropin releasing factor (CRF) infusions produce dose-dependent avoidance of the CRF-paired chamber in stress-naïve rats, intra-CeA CRF-1 receptor (CRFR1) antagonism reduces hyperalgesia in Avoiders after stress (Itoga et al., 2016), and systemic CRFR1 antagonism reduces escalated alcohol SA in rats not indexed for avoidance (Roltsch et al., 2014). Here, we tested the hypotheses that Avoiders would exhibit greater CeA activation (as indicated by higher number of c-Fos-positive cells) and higher CeA CRF immunoreactivity (CRF-ir) than Non-Avoiders and unstressed Controls. We also tested the hypotheses that CRFR1 blockade would reduce avoidance of a predator odor-paired context and post-stress alcohol SA in Avoiders.
2. Materials and methods
2.1. General materials and methods
2.1.1. Animals
Specific pathogen free adult male Wistar rats (Charles River, Raleigh, NC) weighing 225–250 g at the time of arrival were housed in a humidity- and temperature-controlled (22 DC) vivarium on a 12 h light/ 12 h dark cycle (lights off at 8 a.m.). All rats were housed in groups of two. Rats were acclimated for one week before start of experiments and were handled daily prior to initiation of surgical and experimental protocols. Behavioral tests occurred during the dark period. All procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were in accordance with the National Institute of Health guidelines.
2.1.2. Drugs
R121919 (Neurocrine Inc., San Diego, CA) was solubilized in 1M HCl (10% final volume), diluted with 20% (w/v) 2-hydroxypropyl-β-cyclodextrin (HPC; Sigma-Aldrich, Catalog No. H107), and back-titrated with NaOH to pH 4.5. 20% HPC was used for the Vehicle group.
2.1.3. Predator odor place conditioning
Rats underwent a 5-day predator odor conditioned place avoidance (CPA) procedure as previously described (Schreiber et al., 2017). Briefly, on the first day, rats were allowed access to three chambers with distinct tactile (circles vs. grid. vs. rod floor) and visual (circles vs. white vs. stripes) cues (3-chamber Pretest). For each rat, the chamber with the most deviant time relative to the other two chambers (i.e., most extremely preferred or avoided chamber) was excluded from all subsequent testing. On day 2, rats were allowed 5 min to explore the two non-excluded chambers (2-chamber Pretest). Rats were assigned to predator odor stress or unstressed control groups that were counterbalanced for magnitude of baseline preference across the two chambers being used for each animal. For rats in the stress group, an unbiased and counterbalanced procedure was used to determine which chamber (i.e., more preferred or less preferred) would be paired with predator odor exposure in individual rats. On day 3, rats were placed in one chamber without odor for 15 min (neutral conditioning). On day 4, rats were placed in the other chamber for 15 min in the presence (or absence for unstressed Controls) of a bobcat urine-soaked sponge placed under the floor of the chamber (predator odor conditioning). On day 5, rats were again allowed to explore the two chambers for 5 min (Posttest) and were classified as Avoiders or Non-Avoiders based on their time spent in the odor-paired context. Rats that showed >10 s decrease in time spent in the odor-paired context between 2-chamber pretest and posttest were classified as ‘Avoiders,’ and all other odor-exposed rats were classified as ‘Non-Avoiders’. In some experiments, there was more than one Posttest, but Avoider/Non-Avoider phenotype was always assigned during Posttest 1 (see below).
2.1.4. Immunohistochemistry
Rats were placed under isoflurane anesthesia (5%) and transcardially perfused with saline followed by 4% paraformaldehyde (PFA). After perfusion, brains were harvested and post-fixed for 24 hours in 4% PFA, and cryoprotected by a 20% sucrose solution for 24–48 hours before being snap-frozen in 2-methylbutane (Thermo Fisher Scientific, Waltham, MA). Brains were stored at −80°C, then coronally sectioned at 40 μm on a cryostat. Sections were stored free-floating in a 0.1% sodium azide in 1X phosphate buffered saline (PBS) solution until immunolabeling.
Every 6th section collected from the cryostat that contains the CeA (~bregma −1.8 to −3.0; Paxinos & Watson 2007) were processed for immunolabeling. Sections were incubated in 1% H2O2 for 20 min at room temperature to quench endogenous peroxidase activity, then rinsed 3 × 10 min in 1X PBS. Slices were then incubated in blocking serum (0.3% Triton X 100; 5% normal donkey serum) and labeled with c-Fos (1:1000; Cell Signaling Technology, Danvers, MA) or CRF primary antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, sections were washed and labeled using horse-anti-goat ImmPress Kit (Vector Laboratories, Burlingame, CA). Signal was developed with DAB and nickel (Vector Laboratories, Burlingame, CA), and sections were mounted on slides for microscopic analysis.
To quantify the number of c-Fos+ cells, 20x images of 3 brain sections representative of the rostral-caudal axis of the CeA were analyzed using ImageJ (NIH; Schneider et al. 2012) by an experimenter blinded to experimental groups. The area of interest was traced and measured in pm2 using ImageJ, and the number of c-Fos+ cells was counted within the traced region. The number of c-Fos+ cells for each side and brain section was expressed as number of cells per μm2 and averaged across 3 or 4 brain sections for each animal.
To quantify CRF immunoreactivity (CRF-ir), 20x images of 3 brain sections representative of the rostral-caudal axis of the CeA were analyzed using ImageJ. For each image, immunoreactivity density of the CeA and a representative background area were quantified. Immunoreactivity is represented as the density of the area of interest divided by the density of the background area, averaged over 3 sections. All values were normalized to immunoreactivity of unstressed Controls.
2.1.5. Stereotaxic surgeries
Rats were anesthetized with 4% isoflurane and anesthesia was maintained with 1–3% isoflurane on the stereotaxic frame (Kopf Instruments, Tujunga, CA). Rats were treated with flunixin (2.5 mg/kg, s.c.), an analgesic, and cefazolin (20 mg/kg, i.m.), an antibiotic, immediately before surgery and once on the following day. Small burr holes were drilled bilaterally above the CeA and 26-gauge stainless steel guide cannulae (Plastic One, Roanoke, VA) were lowered into the brain, terminating 1 mm above the target coordinate for CeA (AP −2.5 mm, ML ±4.0 mm, DV - 8.4 mm). Guide cannulae were secured in place using dental cement anchored to the skull with metal screws. At the end of surgeries, internal cannulae fitted with screw caps (Plastics One, Roanoke, VA) were inserted into guide cannulae to prevent clogging. Rats were allowed to recover for 1 week before the start of experimental procedures.
2.1.6. Intra-CeA infusions
Rats were gently restrained and 33-gauge stainless steel injectors (Plastics One, Roanoke, VA) connected to 10 μL Hamilton syringes via polyethylene (PE-20) tubing were inserted into guide cannulae. Infusions were delivered using an electronic microinfusion pump (Harvard Apparatus, Holliston, MA) at a rate of 0.2 μL/min over the course of 2.5 min (final infusion volume = 0.5 μL/side) and injectors were left in place for 1 min to prevent backflow. All rats were habituated to handling and were given sham infusions before actual infusion procedures.
2.2. Experimental procedures
2.2.1. Repeated predator odor exposure effect on avoidance behavior
Rats underwent the predator odor CPA procedure and were indexed for avoidance (Controls, n=6; Non-Avoiders, n=9; Avoiders, n=6). Rats were re-exposed to the neutral chamber (5 days after Posttest) and to predator odor in the CS+ chamber (or no odor for Controls; 6 days after Posttest) and re-indexed for avoidance the next day (Fig. 1A).
Figure 1.
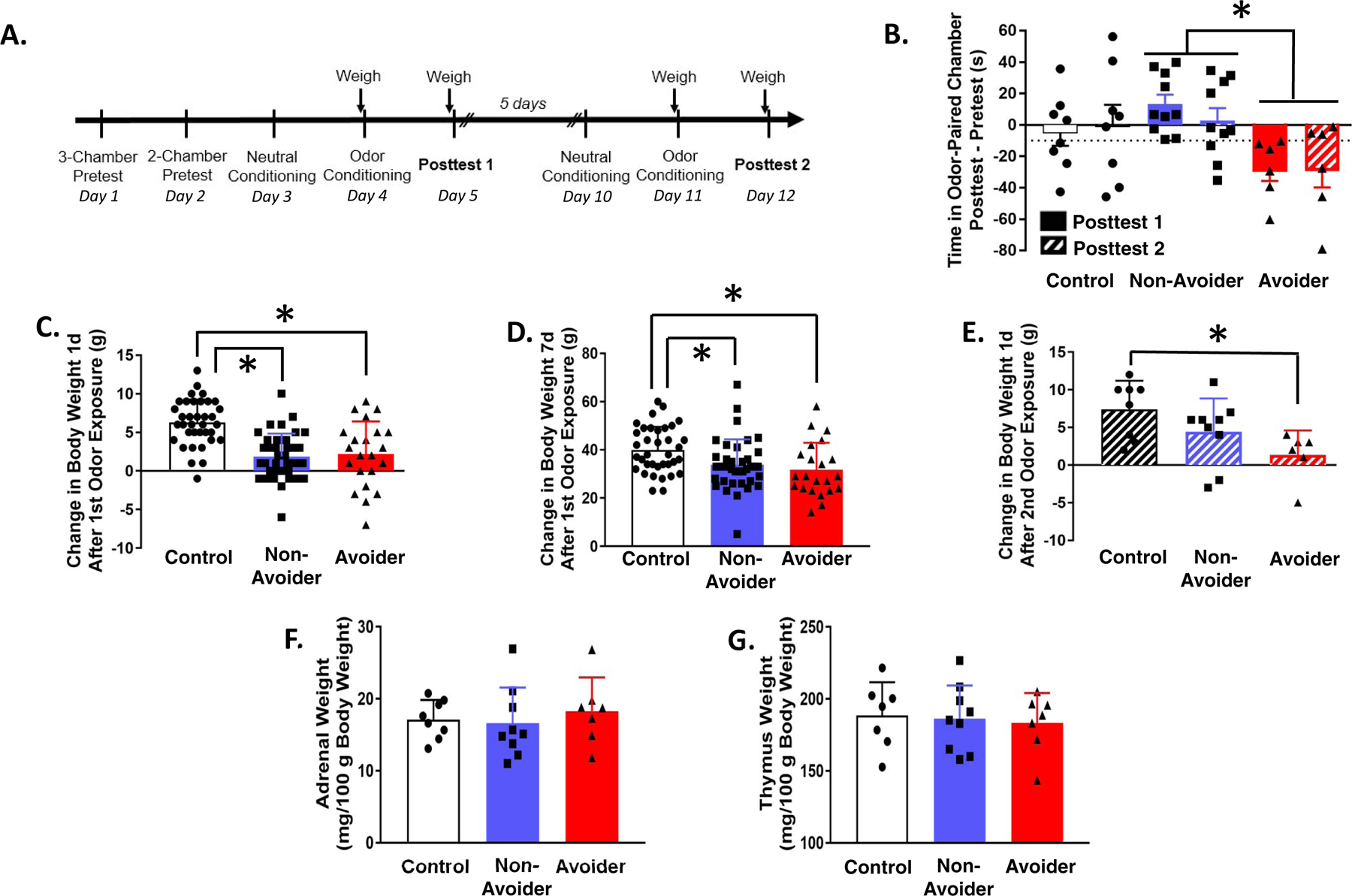
Predator odor stress produces avoidance behavior that is stable and changes in weight gain. (A) Timeline for testing the effect of repeated predator odor exposure on avoidance behavior and weight gain. (B) Time in odor-paired chamber during Posttests 1 (solid bars) and 2 (hatched bars) (after 1st and 2nd predator odor exposure). *p = 0.01, Avoiders vs. Non-Avoiders. (C) Change in body weight 1 day after predator odor exposure (*p’s < 0.001) and (D) 7 days after predator odor exposure (*p’s ≤ 0.05). (E) Change in body weight 1 day after 2nd predator odor exposure (*p = 0.04). (F) Adrenal and (G) thymus weights of Controls, Non-Avoiders, and Avoiders.
2.2.2. Predator odor exposure effect on body weight gain and adrenal and thymus weights
Rats underwent the predator odor CPA procedure and were indexed for avoidance (Controls, n=36; Non-Avoiders, n=38; Avoiders, n=22). A subset of rats (Controls, n=7; Non-Avoiders, n=9; Avoiders, n=6) was re-exposed to predator odor (or no odor for unstressed Controls) in the CS+ chamber (or no odor for Controls) 7 days after the 1st odor exposure (6 days after Posttest) (Fig. 1A). Rats were weighed immediately before and 1 day after the first predator odor exposure, then again immediately before and 1 day after the 2nd predator exposure. We calculated change in body weight 1 day and 7 days after the 1st odor exposure, as well as 1 day after the 2nd odor exposure. A subset of rats were sacrificed 7 days after the 1st odor exposure and their adrenal and thymus glands were weighed.
2.2.3. Predator odor exposure effect on c-Fos and CRF immunoreactivity in CeA
Rats were subjected to the predator odor CPA procedure and indexed for avoidance (Controls, n=12; Non-Avoiders, n=13; Avoiders, n=6). Six days after posttest, all Non-Avoiders and Avoiders were re-exposed to predator odor for 15 min in the CS+ chamber; half of the unstressed Controls (n=6) were also stressed that day (denoted as the “Single Stress” group) whereas the other half of unstressed Controls (n=6) were not exposed to odor and remained stress-naïve. The Single Stress group was included to test the effect of a single predator odor exposure on c-Fos and CRF measures in rats not-indexed for avoidance. All rats were sacrificed 90 min after 2nd odor exposure and brains were processed for immunohistochemical labeling for c-Fos and CRF.
2.2.4. Intra-CeA CRFR1 antagonism effect on post-stress avoidance and alcohol SA
Please refer to Figure 4A for experimental timeline. Rats were trained to orally self-administer 10% (w/v) ethanol or water as previously described (Schreiber et al., 2017). Upon stabilization of operant responding in 30 min sessions, rats were implanted with bilateral cannulae targeting the CeA. After ~1 week of recovery, rats were allowed to resume alcohol self-administration. Upon stabilization of responding, self-administration following intra-CeA vehicle infusion was recorded. Subsequently, rats underwent the predator odor CPA procedure and were indexed for avoidance (Control, n=14; Non-Avoider, n=27; Avoider, n=19). On days 2, 5, 8, 10, and 12 after posttest, rats were allowed to self-administer alcohol in 30-min operant sessions that were immediately preceded by intra-CeA infusion of a CRFR1 antagonist, R121919 (0.25 μg/0.5 μL/side), or vehicle (20% w/v 2-hydroxypropyl cyclodextrin in saline). One day after the last session of alcohol self-administration, rats were re-indexed for avoidance (Posttest 2) to test the effect of repeated intra-CeA R121919 or vehicle infusions on avoidance behavior. Two days later, a subset of rats (Control, n=6; Non-Avoiders, n=11 ; Avoiders, n=12) was re-indexed for avoidance (posttest 3) immediately following intra-CeA infusion of R121919 or vehicle to test the acute effect of CRFR1 antagonism on avoidance behavior. Rats with infusions outside the CeA were removed from all analyses.
Figure 4.
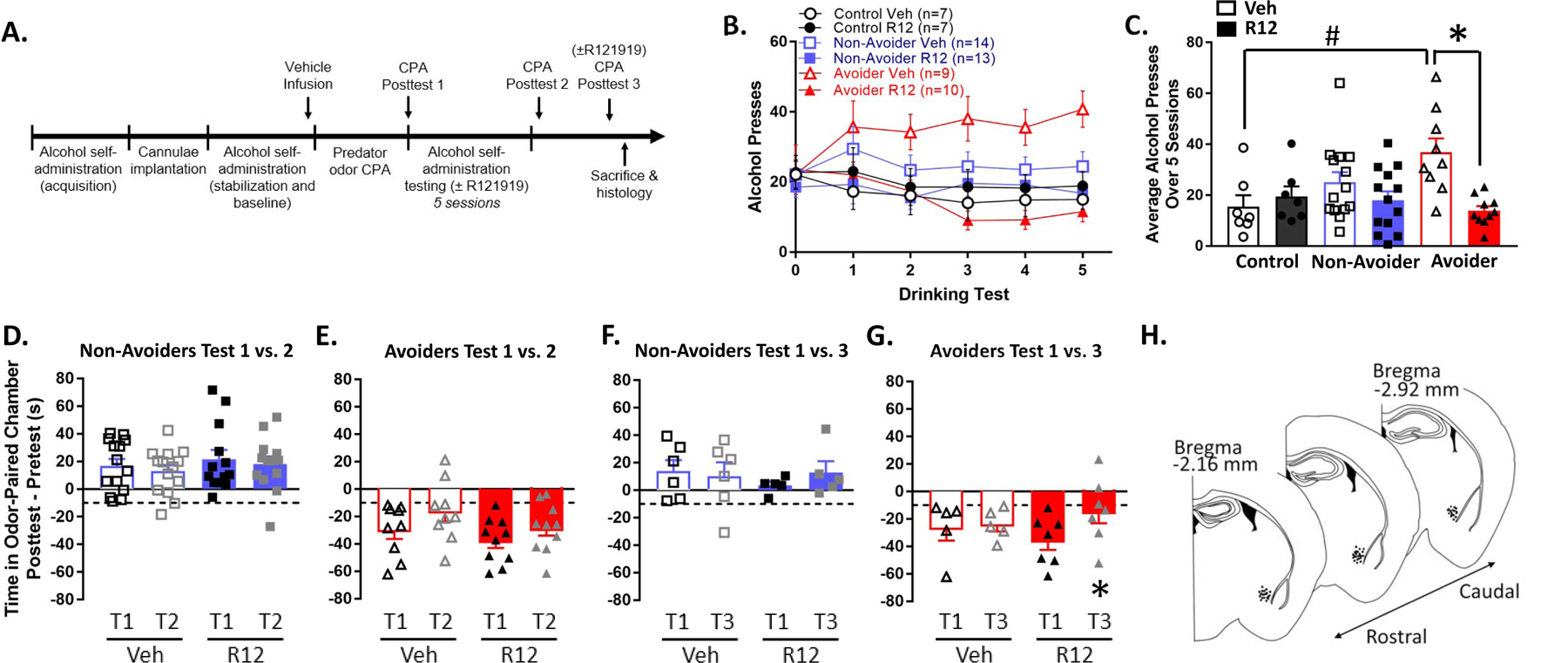
Intra-CeA CRFR1 antagonism reduces escalated alcohol drinking in Avoiders and avoidance behavior. (A) Timeline for testing the effect of intra-CeA CRFR1 antagonism on alcohol drinking and avoidance behavior. (B) Number of alcohol presses across 5 × 30 min operant alcohol oral self-administration test sessions. Rats were treated with R121919 (0.25 μg/side) or vehicle immediately before test sessions 1–5. All rats were treated with vehicle immediately before test session 0. (C) Number of alcohol presses averaged across test sessions 1–5. #p = 0.008, Avoiders vs. Controls in the Vehicle group. *p = 0.001, R121919 vs. Vehicle treatment in the Avoider group. (D) Time spent in odor-paired chamber during Posttests 1 and 2 by Non-Avoiders and (E) Avoiders. All rats received intra-CeA vehicle infusions 1 day prior to Posttest 1 and were treated repeatedly with intra-CeA R121919 or vehicle during the 5 intervening operant alcohol self-administration sessions between Posttests 1 and 2 (last infusion occurred 1 day prior to Posttest 2). (F) Time spent in odor-paired chamber during Posttests 1 and 3 by Non-Avoiders and (G) Avoiders. Rats were treated with intra-CeA R121919 or vehicle immediately before Posttest 3. *p = 0.003, Avoiders treated with R121919, Posttest 1 vs. 3. (H) Schematic of infusion sites within the CeA.
2.3. Statistical analysis
Data in Figures 1–4 are shown as mean ± SEM. All statistical analyses were performed using SPSS 25 (IBM Corp., Armonk, NY). Data were analyzed using omnibus factorial, ANOVAs with Stress Group and Treatment as between-subjects factors and Test Session as a within-subjects factor where appropriate, and significant effects were followed-up with Tukey’s post-hoc tests. The specific statistical tests used for each experiment are reported in the Results. Outliers were detected using the interquartile range rule (Jones, 2019) and animals with outlier values were excluded from all behavioral and molecular data analysis (n=12 across all experiments). Statistical significance was set at p ≤ 0.05.
3. Results
3.1. Avoidance behavior is similar after 1st and 2nd predator odor exposure
Rats were exposed to predator odor twice and indexed for avoidance after each exposure (Fig. 1A). Time spent in the odor-paired chamber during Posttest 1 (after first odor exposure; solid bars, Fig. 1B) and Posttest 2 (after 2nd odor exposure; hatched bars, Fig. 1B) were similar for Avoiders and Non-Avoiders (repeated measures Posttest x Stress Group ANOVA, p = 0.76). Across both Posttest sessions, time spent in odor-paired chamber was significantly different between Avoiders and Non-Avoiders (main effect of Stress Group, F2,21 = 4.93, p = 0.02; post-hoc test, p = 0.01) (Fig. 1B).
3.2. Predator odor exposure reduces body weight gain but does not affect adrenal or thymus weights
Both Avoiders and Non-Avoiders gained less weight than Controls 1 day (one-way ANOVA, F2,93 = 19.1, p < 0.001; post-hoc tests, p’s < 0.001; Fig. 1C) and 7 days (one-way ANOVA, F2,93 = 5.3, p = 0.01 ; post-hoc tests, p’s < 0.05; Fig. 1D) after the 1st predator odor exposure. One day after the 2nd predator odor exposure, only Avoiders showed reduced body weight gain relative to unstressed Controls (one-way ANOVA, effect of Stress Group, F2,20 = 4.1, p = 0.04; post-hoc test, p = 0.03; Fig. 1E). A subset of rats were sacrificed 7 days after odor exposure (Fig. 1A) and their adrenal and thymus glands were weighed. There were no differences in adrenal or thymus gland weights between Controls, Non-Avoiders, and Avoiders (Fig. 1F, G).
3.3. Avoiders have more c-Fos+ cells in CeA after odor re-exposure
Rats were exposed to predator odor, indexed for avoidance, re-exposed to predator odor, and sacrificed 90 min later for c-Fos immunostaining (Fig. 2A). Controls were never exposed to predator odor and Single Stress rats were exposed to predator odor once 90 min before sacrifice (see Methods). Avoiders had a higher number of c-Fos+ cells in the CeA than Non-Avoiders (one-way ANOVA, main effect of Stress Group, F1,25 = 3.1, p = 0.046; post-hoc test, p = 0.050; Fig. 2B). Analyses split by medial (CeM) and lateral (CeL) CeA subdivisions showed that in the CeM, c-Fos+ cell counts were higher in Avoiders compared to Non-Avoiders and rats that received Single Stress exposure (one-way ANOVA, main effect of Stress Group, F1,25 = 3.2, p = 0.039; post-hoc tests, p = 0.051 and 0.040, respectively; Fig. 2C). However, in the CeL, the effect of Stress group was not significant (one-way ANOVA, p = 0.089; Fig. 2D). Across Avoider and Non-Avoider groups (i.e., all rats exposed to odor twice), there was a significant inverse correlation between number of c-Fos+ cells in CeA and time spent in the odor-paired chamber during Posttest (Pearson’s r = −0.58, p = 0.016; Fig. 2E), such that high avoidance was correlated with high number of c-Fos+ cells in CeA. Representative images of c-Fos staining are shown in Fig. 2F–I.
Figure 2.
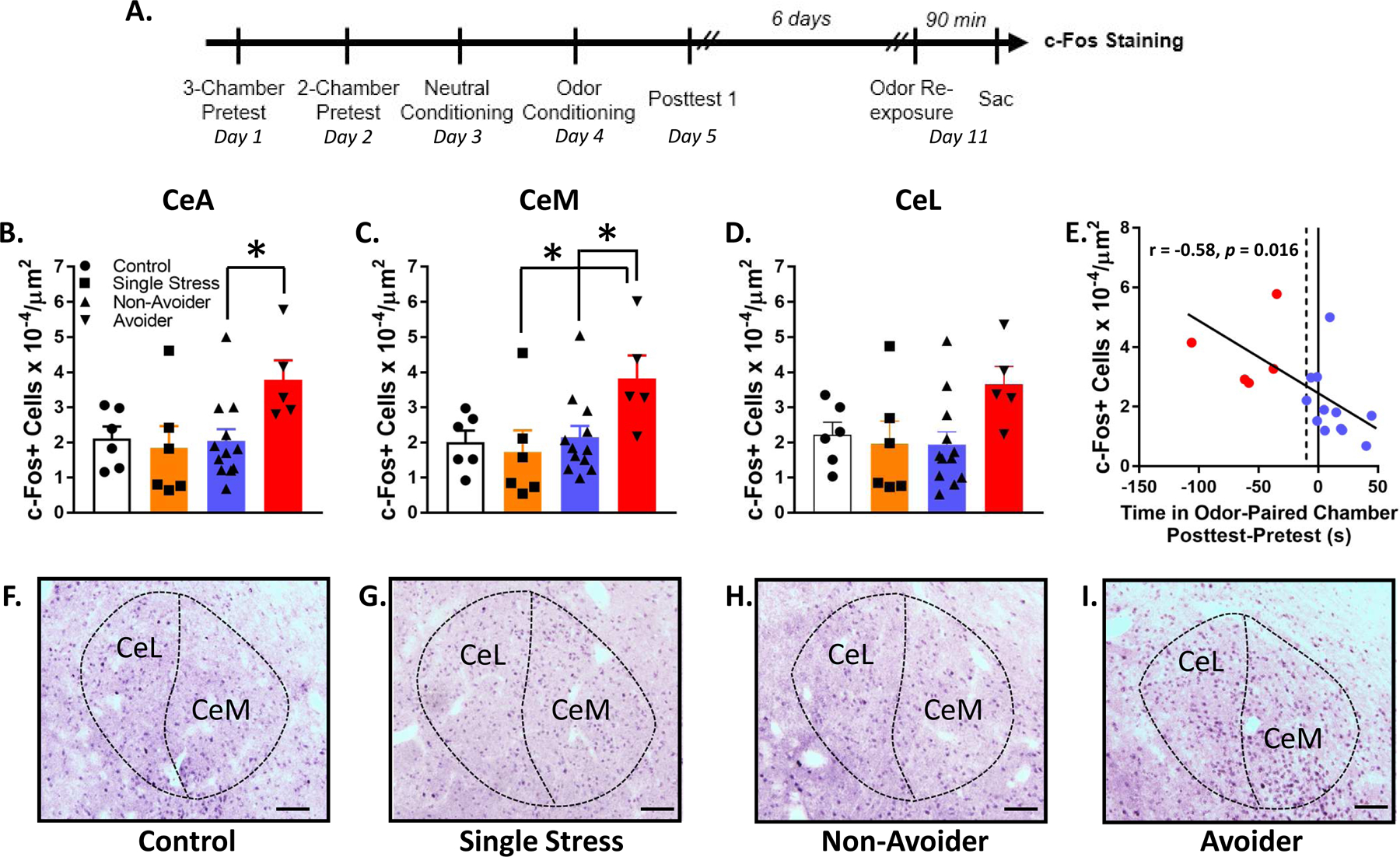
Avoiders have more c-Fos+ cells in CeA than Non-Avoiders. (A) Timeline for testing the effect of predator odor exposure on c-Fos induction. (B) c-Fos+ cells in CeA, (C) CeM, and (D) CeL (*p ≤ 0.05). (E) Correlation between avoidance and number of c-Fos+ cells in CeA. (F) Representative images of c-Fos staining in Control, (G) Single Stress, (H) Non-Avoider, and (I) Avoider rats. Scale bar = 100 μm.
3.4. Avoiders have higher CRF immunoreactivity in CeA after odor re-exposure
Rats were exposed to predator odor, indexed for avoidance, re-exposed to predator odor, and sacrificed 90 min later for CRF immunostaining (Fig. 3A). Avoiders showed greater CRF-ir in CeA than Non-Avoiders and rats that received Single Stress exposure (one-way ANOVA, main effect of Stress Group, F1,23 = 4.5, p = 0.012; post-hoc tests, p = 0.009 and 0.049, respectively; Fig. 3B). Analyses of CRF immunoreactivity in CeM and CeL individually did not yield statistically significant effects (Fig. 3C, D). In Avoiders and Non-Avoiders, there was a significant inverse correlation between CeA CRF immunoreactivity and time spent on the odor-paired chamber during Posttest (Pearson’s r = −0.67, p = 0.004; Fig. 3E), such that high avoidance was correlated with high CRF-immunoreactivity in CeA. This correlation was even stronger in Avoiders only (Pearson’s r = −0.83). Representative images of CRF staining are shown in Fig. 3F–I.
Figure 3.
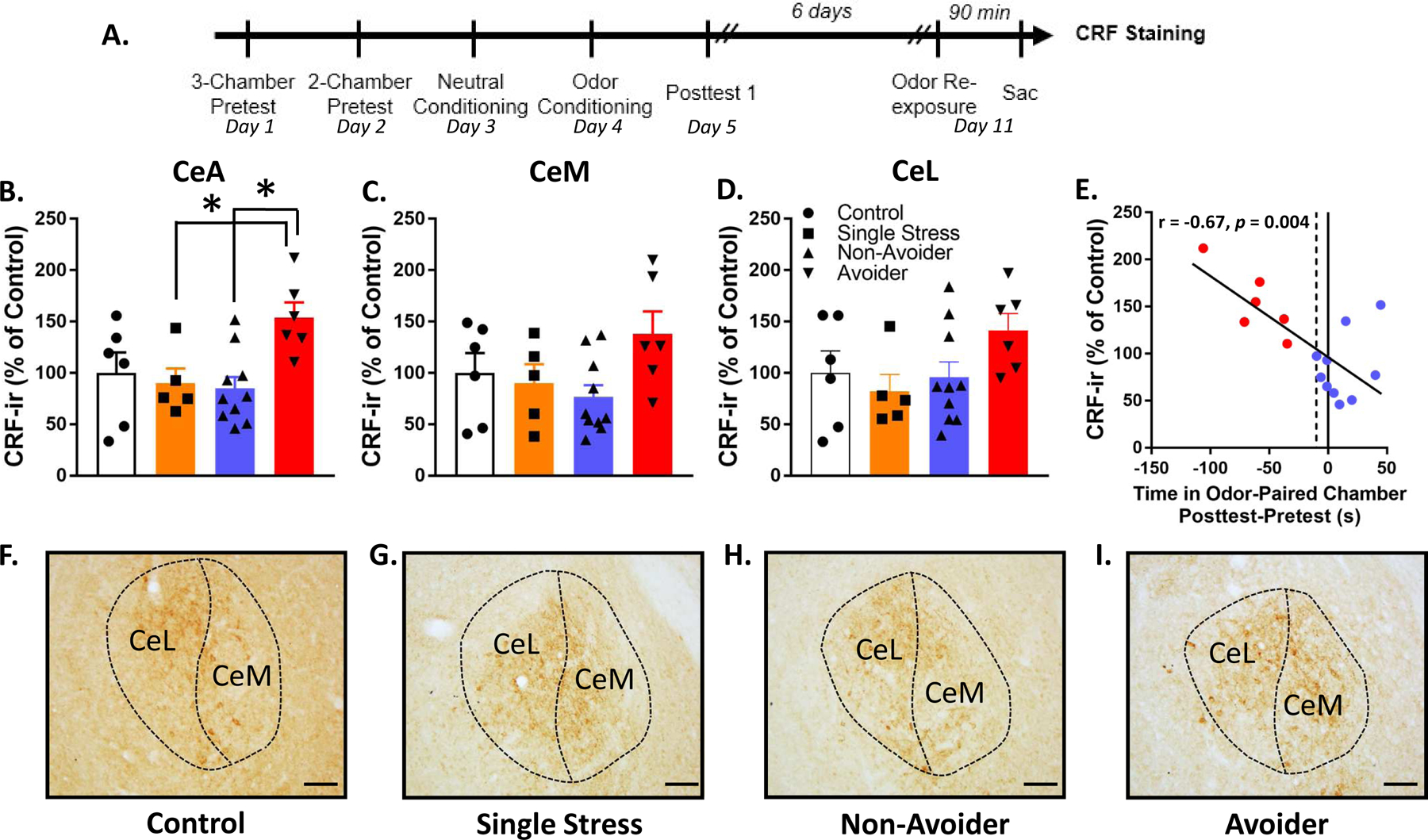
Avoiders have greater CRF immunoreactivity in CeA than Non-Avoiders. (A) Timeline for testing the effect of predator odor exposure on CRF immunoreactivity. (B) CRF immunoreactivity in CeA, (C) CeM, and (D) CeL (*p’s < 0.05). (E) Correlation between avoidance and CeA CRF immunoreactivity. (F) Representative images of CRF staining in CeA of Control, (G) Single Stress, (H) Non-Avoider, and (I) Avoider rats. Scale bar = 100 μm.
3.5. Intra-CeA CRFR1 antagonism reduces escalated alcohol SA in Avoiders
Rats were trained to orally self-administer alcohol, exposed to predator odor, indexed for avoidance, and allowed to self-administer alcohol during five 30-min sessions on days 2, 5, 8, 10, and 12 after Posttest (Fig. 4A). Control, Non-Avoider, and Avoider rats were pre-treated with intra-CeA infusions of R121919 (CRFR1 antagonist; 0.25 μg/side) or equivalent volume of vehicle immediately before operant self-administration testing sessions. A Stress Group x Treatment x Testing Session repeated measures ANOVA did not yield any significant main or interactive effects of Testing Session (Fig. 4B). A Stress Group x Treatment ANOVA and Tukey’s post-hoc tests showed that Avoiders treated with vehicle had a higher number of alcohol presses over 5 testing sessions than Controls treated with vehicle, demonstrating significant stress-induced escalation of alcohol intake in Avoiders (Stress Group x Treatment interaction, F2,54 = 4.7, p = 0.013; post-hoc test, p = 0.008). Within the Avoider group, R121919 treatment reduced the number of alcohol presses across 5 testing sessions relative to Vehicle-treated animals (effect of Treatment, F1,17 = 17.4, p = 0.001; Fig. 4C). R121919 treatment did not significantly reduce the number of alcohol lever presses in Non-Avoider or Control rats.
3.6. Intra-CeA CRFR1 antagonism reduces avoidance of the predator odor-paired context
Rats were re-indexed for avoidance behavior 1 day after the last alcohol self-administration testing session in the absence of drug treatment (Posttest 2; see timeline in Fig. 4A). Relative to Posttest 1, neither Avoiders nor Non-Avoiders showed changes in time spent in odor-paired chamber (Fig. 4D, E). Two days later, rats were indexed again for avoidance behavior immediately following intra-CeA infusions of R121919 (0.25 μg/side) or Vehicle (Posttest 3). Non-Avoiders did not show changes in time spent in odor-paired chamber between Posttest 1 and Posttest 3 (Fig. 4F). On the other hand, Avoiders treated with intra-CeA R121919 (but not vehicle) showed lower avoidance behavior in Posttest 3 relative to Posttest 1 (Posttest x Treatment repeated measures ANOVA, Posttest x Treatment interaction: F1,10 = 4.0, p = 0.062, ŋp2 = 0.3; significant effect of Posttest within Avoiders treated with R121919, F1,6 = 22.9, p = 0.003) (Fig. 4G).
4. Discussion
In these experiments, we characterized behavioral and biological correlates of an avoidance phenotype in our bobcat urine stress model. We found that avoidance behavior is persistent after predator odor re-exposure. In addition, body weight gain is lower in both Avoiders and Non-Avoiders (relative to unstressed Controls) 1 and 7 days after 1st predator odor exposure, but only in Avoiders 1 day after 2nd predator odor exposure. Predator odor exposure did not affect adrenal or thymus weights in Avoiders or Non-Avoiders. In the brain, we found that Avoiders have more c-Fos+ cells and CRF-ir than Non-Avoiders, Controls, and animals sacrificed after their 1st predator odor exposure (Single Stress). We also found that high avoidance was correlated with high number of c-Fos+ cells and high CRF immunoreactivity in CeA. Finally, we showed that intra-CeA CRFR1 antagonism reverses post-stress escalation of operant alcohol self-administration and reduces avoidance behavior in Avoiders.
The finding that both Avoiders and Non-Avoiders show decreased body weight gain relative to unstressed Controls suggests that all rats exposed to bobcat urine exhibit physiological signs of stress. Our lab previously reported that all rats exposed to predator odor exhibit increases in plasma ACTH and CORT (Whitaker et al., 2016). In the current study, predator odor-exposed rats displayed blunted weight gain over the ensuing 1 and 7 days (Fig. 1C, D). This blunted weight gain following predator odor exposure was not accompanied by decreases in thymus weight or adrenal hypertrophy, suggesting that neuroendocrine effects of predator odor exposure may not account for the observed body weight effects. Previous work by other labs demonstrated that acute and chronic (7–14 days) predator exposure robustly activates the HPA axis and suppresses weight gain in rats but does not produce adrenal hypertrophy or thymic involution (Figueiredo et al., 2003). In the current study, predator odor re-exposure reduced body weight gain only in Avoiders (Fig. 1E), suggesting that Avoiders may be more susceptible (i.e., less resilient) to the physiological effects of repeated stress. We also report that predator odor re-exposure did not significantly alter mean group avoidance of the predator odor-paired chamber. That said, predator odor re-exposure did significantly increase avoidance of the odor-paired chamber in a subset of animals initially classified as Non-Avoiders. Previous studies have shown that repeated exposure to a predator (cat) produces HPA axis activation in rats that does not habituate (Figueiredo et al., 2003). This is supported by our data showing equal or exaggerated suppression of body weight gain and equal or exaggerated avoidance of odor-paired stimuli after repeated versus single stress exposure.
The CeA is an important part of a brain network that regulates the behavioral and physiological effects of stress in rats (Ventura-Silva et al., 2013). However, whether or not acute stress exposure produces c-Fos activation in CeA seems to depend on the modality of the stressor. For instance, restraint stress, but not cat (Dielenberg et al., 2001) or ferret odor exposure (Butler et al., 2016), increases c-Fos activation in CeA (Kovács et al., 2018). Previous studies also show that stress-induced c-Fos activation in CeA may be predictive of coping strategy. For example, rats selectively bred for high freezing and low defensive burying behaviors when presented with a shock probe (low novelty responding rats; passive/reactive copers) show more CeA c-Fos activation following footshocks than their low freezing/high defensive burying counterparts (high novelty responding rats; active copers) (Cohen et al., 2017). Here, we found that Avoiders have more c-Fos+ cells in CeA than Non-Avoiders, Controls, and Single Stress rats not indexed for avoidance, and that the number of CeA c-Fos+ cells is positively correlated with avoidance of the predator odor-paired context (Fig. 2), suggesting that greater CeA activation supports an Avoider phenotype/coping strategy. Single Stress rats (stressed only once on the day of sacrifice but not indexed for avoidance) had CeA c-Fos levels similar to Non-Avoiders and Controls. We speculate that this is because more rats are reliably classified as Non-Avoiders than Avoiders in this procedure (Albrechet-Souza and Gilpin, 2019).
CRF-CRFR1 signaling has a role in mediating stress effects on physiology and behavior. For example, systemic CRFR1 antagonism 30 min prior to or immediately after predator stress (cat exposure) blocks escalated anxiety-like behavior after stress, suggesting that CRF-CRFR1 signaling plays an important role in stress reactivity (Adamec et al., 2010). Similarly, systemic CRFR1 antagonism reduces escalated operant alcohol self-administration following bobcat urine exposure in rats (Roltsch et al., 2014) or social defeat stress in mice (Newman et al., 2018), showing that CRF-CRFR1 signaling plays a role in stress-alcohol interactions. Within the brain, we predicted that the CeA is an important site in which CRF-CRFR1 signaling modulates stress reactivity and stress-induced escalation of operant alcohol self-administration. Prior work showed that footshock stress increases CRF mRNA in CeA (Iwasaki-Sekino et al., 2009), that intra-CeA CRF receptor blockade blocks consolidation of contextual fear conditioning (Pitts et al., 2009), and that acute or repeated intra-CeA CRFR1 antagonism blocks escalation of operant alcohol self-administration in rats chronically exposed to high doses of alcohol, a procedure that usually produces affective and somatic signs of alcohol dependence (Funk et al., 2007; Roberto et al., 2010). In our lab, we previously found that intra-CeA CRF infusions produce conditioned place avoidance in stress-naïve rats and that intra-CeA CRFR1 antagonism attenuates stress-induced hyperalgesia in Avoiders (Itoga et al., 2016). Here, we report that Avoiders have greater CeA CRF-ir, CeA CRF-ir is positively correlated with avoidance behavior (Fig. 3), and intra-CeA CRFR1 antagonism reduces operant alcohol self-administration and avoidance behavior in Avoiders, providing further evidence that CeA CRF-CRFR1 signaling plays a functionally relevant role in post-stress behavioral dysregulation in Avoiders.
The precise neural circuit mechanism by which CeA CRF-CRFR1 signaling contributes to avoidance behavior and escalated operant alcohol self-administration after predator odor stress is unclear. The CeA receives CRF afferents from distal brain regions (Uryu et al., 1992), but also contains local CRF neurons that release CRF within the CeA (Justice et al., 2008). We currently do not know which CRF source contributes to the greater CeA CRF immunoreactivity in Avoiders (Fig. 3), and we are using circuit-based manipulations to test this question. With regard to signaling mechanisms downstream of CRF release, it is well known that CRF increases GABAergic neurotransmission in putative CeA interneurons via a CRFR1-dependent mechanism, and effect that is reproduced by alcohol bath application and is exacerbated in alcohol-dependent rats (Roberto et al., 2010). Given that intra-CeA CRFR1 antagonism blocks escalation of operant alcohol self-administration in alcohol-dependent rats (Roberto et al., 2010), mapping the circuits engaged by CeA CRFR1-dependent GABAergic transmission may be useful for understanding the mechanism underlying avoidance behavior and escalated operant alcohol self-administration after predator odor stress.
In conclusion, the current study characterizes behavioral and biological correlates of avoidance behavior in our bobcat urine stress model and identifies CeA CRF-CRFR1 signaling as an important contributing factor to post-stress avoidance behavior and escalated alcohol SA. Future studies will characterize the contributions of specific CRFR1-gated CeA circuits to post-stress behavioral dysregulation and extend the current findings to females.
Highlights.
Predator odor stress produces differences in weight gain between Avoider and Non-Avoider rats
Predator odor stress increases c-Fos cell counts and corticotropin-releasing factor immunoreactivity in central amygdala of Avoider rats
Antagonism of corticotropin-releasing factor-1 receptors in central amygdala attenuates post-stress escalation of alcohol self-administration and avoidance behavior in Avoider rats
5. Acknowledgments
We thank Dr. Maureen Basha for her technical assistance and guidance in dissecting adrenal and thymus glands, Dr. Lisa Harrison-Bernard for microscope access, and Ms. Rosetta Shackett for technical assistance.
Funding and disclosures
This work was supported by NIH grants AA027145 (MMW), AA023696 (ALS), AA025831 (EMA), AA023305 (NWG), and AA026531 (NWG), and AA007577. NWG is a consultant for Glauser Life Sciences, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Adamec R, Fougere D, Risbrough V, 2010. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. International Journal of Neuropsychopharmacology 13, 747–757. 10.1017/S1461145709990496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L, Gilpin NW, 2019. The predator odor avoidance model of post-traumatic stress disorder in rats. Behav Pharmacol 30, 105–114. 10.1097/FBP.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S, 2013. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend 132, 630–638. https://doi.org/10.1016Zj.drugalcdep.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, 2009. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse 10, 198–210. 10.1177/1524838009334448 [DOI] [PubMed] [Google Scholar]
- Bryant RA, Marosszeky JE, Crooks J, Baguley I, Gurka J, 2000. Coping style and post-traumatic stress disorder following severe traumatic brain injury. Brain Inj 14, 175–180. [DOI] [PubMed] [Google Scholar]
- Butler RK, Oliver EM, Sharko AC, Parilla-Carrero J, Kaigler KF, Fadel JR, Wilson MA, 2016. Activation of corticotropin releasing factor-containing neurons in the rat central amygdala and bed nucleus of the stria terminalis following exposure to two different anxiogenic stressors. Behav. Brain Res. 304, 92–101. 10.1016/j.bbr.2016.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JL, Ata AE, Jackson NL, Rahn EJ, Ramaker RC, Cooper S, Kerman IA, Clinton SM, 2017. Differential stress induced c-Fos expression and identification of region-specific miRNA-mRNA networks in the dorsal raphe and amygdala of high-responder/low-responder rats. Behav. Brain Res. 319, 110–123. 10.1016/j.bbr.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS, 2001. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 104, 1085–1097. 10.1016/s0306-4522(01)00150-6 [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW, 2013. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry 3, e296 10.1038/tp.2013.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP, 2003. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur. J. Neurosci 18, 2357–2364. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF, 2007. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychiatry 61, 78–86. 10.1016/j.biopsych.2006.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix CC, Anelli LM, Gibbs JP, Fournier DG, 1994. Validation of the Purdue Post-Traumatic Stress Scale on a sample of Vietnam veterans. J Trauma Stress 7, 311–318. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL, 2004. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N. Engl. J. Med 351, 13–22. 10.1056/NEJMoa040603 [DOI] [PubMed] [Google Scholar]
- Itoga CA, Roltsch Hellard EA, Whitaker AM, Lu Y-L, Schreiber AL, Baynes BB, Baiamonte BA, Richardson HN, Gilpin NW, 2016. Traumatic Stress Promotes Hyperalgesia via Corticotropin-Releasing Factor-1 Receptor (CRFR1) Signaling in Central Amygdala. Neuropsychopharmacology 41, 2463–2472. 10.1038/npp.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T, 2009. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 34, 226–237. 10.1016/j.psyneuen.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Jones PR, 2019. A note on detecting statistical outliers in psychophysical data. Atten Percept Psychophys 81, 1189–1196. 10.3758/s13414-019-01726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W, 2008. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J. Comp. Neurol 511, 479–496. 10.1002/cne.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kovács LÁ, Schiessl JA, Nafz AE, Csernus V, Gaszner B, 2018. Both Basal and Acute Restraint Stress-Induced c-Fos Expression Is Influenced by Age in the Extended Amygdala and Brainstem Stress Centers in Male Rats. Front Aging Neurosci 10. 10.3389/fnagi.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Nalivaiko E, Beig MI, Day TA, Walker FR, 2010. Ability of predator odour exposure to elicit conditioned versus sensitised post traumatic stress disorder-like behaviours, and forebrain deltaFosB expression, in rats. Neuroscience 169, 733–742. 10.1016/j.neuroscience.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Malta LS, Wyka KE, Giosan C, Jayasinghe N, Difede J, 2009. Numbing symptoms as predictors of unremitting posttraumatic stress disorder. J Anxiety Disord 23, 223–229. 10.1016/j.janxdis.2008.07.004 [DOI] [PubMed] [Google Scholar]
- McCarthy E, Petrakis I, 2010. Epidemiology and management of alcohol dependence in individuals with post-traumatic stress disorder. CNS Drugs 24, 997–1007. 10.2165/11539710-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Newman EL, Albrechet-Souza L, Andrew PM, Auld JG, Burk KC, Hwa LS, Zhang EY, DeBold JF, Miczek KA, 2018. Persistent escalation of alcohol consumption by mice exposed to brief episodes of social defeat stress: suppression by CRF-R1 antagonism. Psychopharmacology (Berl.) 235, 1807–1820. 10.1007/s00213-018-4905-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkonigg A, Pfister H, Stein MB, Höfler M, Lieb R, Maercker A, Wittchen H-U, 2005. Longitudinal Course of Posttraumatic Stress Disorder and Posttraumatic Stress Disorder Symptoms in a Community Sample of Adolescents and Young Adults. AJP 162, 1320–1327. 10.1176/appi.ajp.162.7.1320 [DOI] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK, 2009. The Central Nucleus of the Amygdala and Corticotropin-Releasing Factor: Insights into Contextual Fear Memory. J. Neurosci 29, 7379–7388. 10.1523/JNEUR0SCI.0740-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH, 2010. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol. Psychiatry 67, 831–839. 10.1016/j.biopsych.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roltsch EA, Baynes BB, Mayeux JP, Whitaker AM, Baiamonte BA, Gilpin NW, 2014. Predator odor stress alters corticotropin-releasing factor-1 receptor (CRF1 R)-dependent behaviors in rats. Neuropharmacology 79, 83–89. 10.1016/j.neuropharm.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AL, Lu Y-L, Baynes BB, Richardson HN, Gilpin NW, 2017. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology 113, 323–330. 10.1016/j.neuropharm.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KM, Chang HY, Cho S-M, Kim NH, Kim KA, Chung YK, 2015. Avoidance symptoms and delayed verbal memory are associated with post-traumatic stress symptoms in female victims of sexual violence. J Affect Disord 184, 145–148. 10.1016/j.jad.2015.05.051 [DOI] [PubMed] [Google Scholar]
- Sripada RK, Rauch SAM, Tuerk PW, Smith E, Defever AM, Mayer RA, Messina M, Venners M, 2013. Mild traumatic brain injury and treatment response in prolonged exposure for PTSD. J Trauma Stress 26, 369–375. 10.1002/jts.21813 [DOI] [PubMed] [Google Scholar]
- Tanielian T, Jaycox LH, Adamson DM, Burnam MA, Burns RM, Caldarone LB, Cox RA, D’Amico EJ, Diaz C, Eibner C, Fisher G, Helmus T, Karney B, Kilmer B, Marshall GN, Martin LT, Meredith LS, Metscher KN, Osilla KC, Pacula RL, Ramchand R, Ringel J, Schell T, Sollinger J, Vaiana ME, Williams KM, Yochelson MR, 2008. Invisible Wounds of War [WWW Document]. URL https://www.rand.org/pubs/monographs/MG720.html (accessed 9.29.17).
- Taylor S, Fedoroff IC, Koch WJ, Thordarson DS, Fecteau G, Nicki RM, 2001. Posttraumatic stress disorder arising after road traffic collisions: patterns of response to cognitive-behavior therapy. J Consult Clin Psychol 69, 541–551. [PubMed] [Google Scholar]
- Uryu K, Okumura T, Shibasaki T, Sakanaka M, 1992. Fine structure and possible origins of nerve fibers with corticotropin-releasing factor-like immunoreactivity in the rat central amygdaloid nucleus. Brain Res. 577, 175–179. 10.1016/0006-8993(92)90554-m [DOI] [PubMed] [Google Scholar]
- Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, Pêgo JM, 2013. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci 7, 32 10.3389/fnbeh.2013.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AM, Farooq MA, Edwards S, Gilpin NW, 2016. Post-traumatic stress avoidance is attenuated by corticosterone and associated with brain levels of steroid receptor coactivator-1 in rats. Stress 19, 69–77. 10.3109/10253890.2015.1094689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AM, Gilpin NW, 2015. Blunted hypothalamo-pituitary adrenal axis response to predator odor predicts high stress reactivity. Physiol. Behav 147, 16–22. 10.1016/j.physbeh.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]


