Abstract
Background:
The Health Measures at Home Study was a study designed to evaluate the feasibility of incorporating dried blood spots (DBS) collection into the National Health Interview Survey and to compare the proficiencies between field interviewers and health technicians in obtaining DBS.
Methods:
DBS collection and venipuncture were attempted on 125 participants. The DBS were collected in the participant’s home and venous blood was collected in the National Health and Nutrition Examination Survey (NHANES) mobile examination center. The DBS results were compared to venous results in the NHANES for the measurements of hemoglobin A1c (HbA1c) and total and high-density lipoprotein (HDL) cholesterol.
Results:
Field interviewers and health technicians were able to collect the DBS for greater than 95% of participants. For DBS, health technicians and field interviewers were highly correlated for HbA1c (r = 0.92) and total cholesterol (r = 0.89), but not for HDL cholesterol (r = 0.72). The DBS results of interviewers and health technicians compared to the venous method for HbA1c (r = 0.90), but did not compare well for HDL cholesterol (r = 0.64–0.66) and total cholesterol (r = 0.65–0.67).
Conclusion:
DBS was comparable to venous HbA1c, but not for total and HDL cholesterol. Health technicians and field interviewers had similar performance for DBS methods, except HDL cholesterol.
Keywords: Dried blood spots, Survey methods, Laboratory method comparison, Diabetes, Lipids
1. Introduction
In the United States, cardiovascular disease is the leading cause of death [1]. The HHS Million Hearts Initiative is a national initiative to prevent 1 million heart attacks and strokes by 2017 [2]. Diabetes, hyper-tension, obesity, smoking, and hyperlipidemia are significant risk factors for cardiovascular disease. The National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) is monitoring the prevalence of these risk factors with multiple population-based surveys including the National Health and Nutrition Examination Survey and the National Health Interview Survey (NHIS) [3,4].
NHANES is a series of national cross-sectional representative surveys of the non-institutionalized US population conducted since 1960. NHANES includes a household interview followed by a physical examination at a mobile examination center (MEC) where blood and urine samples are collected. Since 1999, NHANES has been collecting data annually on approximately 5000 participants. Survey questions and laboratory measurements for hyperlipidemia and diabetes are included in the NHANES [3].
The NHIS collects data by conducting face-to-face household interviews in about35,000 households per year covering a wide variety of health issues [4]. There are no examination components or laboratory measurements included in the NHIS.
The NCHS Health Measures at Home Study (HMHS) was a pilot study that examined the ability of field interviewers and health technicians to collect physical measurements including blood pressure, anthropometry, and dried blood spots in the homes of participants [5]. The HMHS used dried blood spots for the measurement of hemoglobin A1c, total and HDL cholesterol.
McDade et al. reviewed the advantages and disadvantages of DBS methods compared to venous methods [6]. DBS does not need to be immediately refrigerated or frozen following collection compared to serum or plasma samples. Also, DBS does not require skilled phlebotomists and separation of blood by centrifugation, and is a minimally invasive procedure. DBS cards can be stored at ambient temperatures and allows for convenient shipment and storage of samples. Some disadvantages of DBS are assay development that includes the validation of novel non-standardized dried blood spot methods when compared with established standardized venous methods. Additional validation of DBS methods, including the effect of drying of the analytes on DBS cards, elution efficiency and a small sample size requiring sensitive analytical methods, can be challenging for DBS assay development.
2. Materials and methods
2.1. Survey and participants
The Health Measures at Home Study used a subsample of the 2011–2012 National Health and Nutrition Examination Survey participants aged ≥18 years. NHANES constitutes a series of cross-sectional, nationally representative surveys of the US non-institutionalized population. NHANES exams are conducted in a mobile examination center using standard equipment and procedures conducted by trained staff [3]. The HMHS study was conducted in the NHANES MEC and the participant’s home in 2012. NHANES participants were eligible to participate in the HMHS if they received a household interview, and then completed an examination in the MEC which included a blood draw, height, weight, arm circumference and blood pressure measurements. Participants who agreed to participate in the HMHS signed an electronic informed consent form, and the home visits were scheduled within 1 to 3 weeks after the MEC exam at approximately the same time of day as the MEC exam. The field interviewers and health technicians conducted the home exams on the same visit. The order of examination by the field interviewers and health technicians was randomized. Specific details about recruitment and study design of the HMHS can be found elsewhere [5].
HMHS participants received a NHANES report of findings in the MEC that included results for height and weight, blood pressure, and venous laboratory results. At the conclusion of the home exam, they received a blood pressure report. Participants were remunerated once the home exam had been completed. The dried blood spot results were not reported to the participants. The NCHS Research Ethics Review Board approved the HMHS study protocol.
There were 6 examiners who worked on the HMHS. Four field interviewers had no prior health sciences training and 2 health technicians had a B.S. degree in health sciences. The venous draw was collected by certified NHANES phlebotomists in the MEC prior to the DBS collection in the home.
There were 133 NHANES participants who were successfully recruited into the HMHS and 130 participants completed a home exam. Due to contract limitations with the laboratory, DBS collection could only be performed on the first 125 participants.
2.2. Venous specimen collection
The laboratory in the NHANES MEC is CLIA-certified. The MEC laboratory centrifuged, aliquoted and shipped specimens to laboratories for analyses of the specimens for the NHANES. Specific details about the NHANES laboratory protocols are described elsewhere [7].
2.3. Dried blood spot collection
The ZRT Laboratory (Beaverton, OR) processed the DBS and provided the dried blood spot collection kits for the HMHS. ZRT is a CLIA-certified laboratory that specializes in DBS testing. The ZRT DBS kit was packaged in a clear plastic container and included an Ahlstrom (Greenville, SC) 226 DBS card, 2 Becton Dickinson (Franklin Lakes, NJ) Microcontainer contact-activated lancets, an alcohol pad, bandage, gauze, and a ZRT labeled shipping bag.
There were two dried blood spot collections per home visit. Prior to collecting the dried blood spots, field interviewers and health technicians were instructed to ask participants to stimulate blood flow to the fingers by rubbing their hands together. They then asked participants to hang their hands by their sides and pump their fists, to further stimulate blood flow. Hand-warmers were also available for participants with cold hands.
The examiners were allowed to prick fingers on both hands if necessary. However, each examiner was allowed a maximum of two finger pricks from the same hand to obtain the dried blood spot collection. If 2 finger pricks had been initially obtained by the first examiner, the second examiner would then begin the DBS collection with the opposite hand.
The participant’s finger was pricked using the lancet. The first drop of blood was wiped away, and subsequent drops of blood were collected to fill five 1/2″ discs on the filter card. Only one drop of blood was obtained per disc on the DBS card. The DBS discs were required to be filled at least 80% to be considered valid. The time of the DBS exam duration was recorded.
The DBS cards were dried for 30 min at ambient room temperature in a plastic storage container with the lid left slightly ajar. After 30 min, the lids were closed and placed in the bag provided by ZRT along with a dessicant. The bags were dried overnight. The following day, multiple DBS cards were shipped priority overnight in FedEx boxes to the ZRT laboratory with each DBS card stored inside its own plastic containers and wrapped individually with a Ziploc bag and Tyvek® envelopes.
At the ZRT laboratory, an initial visual inspection of the DBS cards for quality purposes was performed including inspecting the number of blood spots, proper labeling, and the lack of blood permeation through the card and the complete filling of spots by the blood. DBS cards were processed at ambient temperatures.
2.4. Dried blood spot analyses
DBS was analyzed in the following priority order: total cholesterol, HDL cholesterol, and hemoglobin A1c. A Wallac Perkin Elmer (Austin, TX) automatic puncher punched either 3 or 6 mm punches out of the DBS cards into a 96-well microtiter plate and then analyzed for the specific assay.
All DBS tests were performed on the Siemens Dimension Xpand Plus System. There were three 6 mm spots punched for total cholesterol that were eluted with 300 μl of methanol for 2 h at room temperature. The eluates were then tested using a Sekisui Diagnostics Cholesterol-SL test kit. The DBS total cholesterol used coupled enzymatic reactions including cholesterol esterase, cholesterol oxidase and peroxidase to form a quinoneimine dye and was measured at 505 nm.
HDL cholesterol used two 3 mm spots that were eluted with 300 μl of methanol for 2 h at room temperature. The Trinity Biotech (Jamestown, NY) EZ HDL cholesterol kit was used for DBS. The HDL cholesterol test used an anti-human β-lipoprotein antibody that binds to lipoproteins (LDL cholesterol, VLDL cholesterol, and chylomicrons) other than HDL cholesterol. The antigen–antibody complex blocks enzyme reactions when cholesterol esterase and cholesterol oxidase are added, which reacts only with HDL cholesterol. Hydrogen peroxide yields a blue color complex upon oxidase condensation with FDAOS [N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3, 5-dimethoxy-4 fluoroanaline, sodium salt] and 4-aminoantipyrine in the presence of peroxidase and was measured at 600 nm.
Hemoglobin A1c used two 3 mm spots that were eluted with deionized water for 1 h at room temperature. The Kamiya Biomedical (Seattle, WA) hemoglobin A1c test kit was used for DBS. The determination of hemoglobin A1c utilizes an antigen–antibody complex to determine the hemoglobin A1c in whole blood. Mouse anti-human hemoglobin A1c monoclonal antibody is added to latex hemoglobin A1c mouse anti-human hemoglobin A1c antibody to form a complex. The amount of agglutination was measured at 620 nm.
The inter-assay imprecision coefficients of variation (CVs) of the DBS methods were: hemoglobin A1c 4.1% (mean HbA1c 5.8%), 8.3% (mean HbA1c 8.8%); total cholesterol 7.4% (mean 3.02 mmol/l), 7.5% (mean 5.35 mmol/l); and HDL cholesterol 10.5% (mean 0.78 mmol/l), 11.0% (mean 1.66 mmol/l).
2.5. Venous assay analyses
The serum and venous assays were performed by NHANES contract laboratories [8]. NHANES used labs certified by the National Glycohemoglobin Standardization Program [9] and CDC Lipid Standardization Program [10]. The NHANES hemoglobin A1c was performed using the Tosoh Medics G7 ion-exchange HPLC analyzer. The serum total cholesterol used a coupled enzymatic method including cholesterol esterase, cholesterol oxidase and peroxidase to produce a quinoneimine dye, measured spectrophotometrically on a Roche/Hitachi Modular P analyzer. The serum HDL cholesterol was a direct assay where magnesium/dextran sulfate forms complexes with non-HDL cholesterol fractions. A coupled enzymatic method was performed for cholesterol using polyethylene glycol (PEG)-cholesterol esterase, PEG-cholesterol oxidase, and peroxidase to form a dye measured on a Roche/Hitachi Modular P analyzer [8].
The inter-assay imprecision CVs of the venous methods were: hemoglobin A1c 0.98% (mean HbA1c 5.3%), 0.56% (mean HbA1c 10.2%); total cholesterol 1.2% (mean 5.10 mmol/l), 1.3% (mean 6.56 mmol/l); and HDL cholesterol 3.8% (mean 0.73 mmol/l), 2.5% (mean 1.30 mmol/l).
2.6. Statistical analysis
Descriptive and inferential statistics were conducted using SAS software version 9.3 (SAS Institute, Inc., Cary, NC). Linear regression was performed for DBS vs. venous methods for bivariate plots. Nonsignificant intercepts (including zero within 95% confidence intervals) were suppressed. Pearson correlation coefficients were calculated with 95% confidence intervals. Bland–Altman plots were performed using Analyse-It software (Analyse-It, Ltd., Leeds, United Kingdom) version 2.26. Statistical hypotheses were tested using the paired t-test at α = 0.05. Pearson correlation and bivariate plots were used to evaluate the relationship between DBS and venous methods for health technicians and interviewers separately.
3. Results
3.1. DBS performance for health technicians and interviewers
The performance of the health technicians and field interviewers collecting DBS were compared. Five spots filling at least 80% of each DBS spot on the filter card by visual inspection were required to be considered complete for analysis of hemoglobin A1c, total and HDL cholesterol. Overall, the field interviewers and health technicians were similarly able to collect complete dried blood spot cards in the home. The field interviewers collected all 5 spots for 95.2% of the DBS collections and the health technicians collected all 5 spots for 94.4% of the DBS collections. Hand warming techniques used prior to collecting DBS were significantly different between the health technicians and field interviewers. The health technicians used hand warming techniques at 83.2% of the DBS collections compared to 30.4% for the field interviewers’ collections.
There were 3 DBS specimens that had insufficient blood collected to perform all 3 tests. One DBS specimen had insufficient blood for all 3 tests, while 2 DBS specimens had insufficient blood for total cholesterol but contained enough blood to perform testing for hemoglobin A1c and HDL cholesterol. Of the total DBS specimens collected, 98.4% of DBS collections performed by health technicians and 99.2% of DBS collections performed by field interviewers collected enough blood on the DBS to perform all three tests.
The mean (SD) DBS collection time for health technicians was 12.5 (3.7) min, shorter than the 15.3 (6.4) min for interviewers (p > 0.05). The DBS collection times were different for health technicians compared to interviewers: 31.0% of DBS collections performed by health technicians and 11.9% of DBS collections by field interviewers collected DBS in b10 min, while 43.7% of DBS collections performed by field interviewers and 21.4% of DBS collections performed by health technicians collected DBS for ≥15 min.
3.2. Comparison of field interviewer and health technician DBS laboratory results to venous results
The mean, SD, minimum and maximum values, selected percentiles and paired t-test for DBS and venous methods for hemoglobin A1c, HDL cholesterol, and total cholesterol are shown in Table 1. The means (SD) of hemoglobin A1c for DBS were comparable between the health technicians (5.55% (0.86)) and field interviewers (5.58% (0.92)), and DBS means were comparable to venous hemoglobin A1c (5.57% (0.81)).
Table 1.
Distribution of dried blood spots (DBS) by examiner compared to venous methods.
| Selected percentiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte (unit) | n | Specimen | Mean (SD)a | Min | 5% | 25% | 50% | 75% | 95% | Max |
| Hemoglobin A1c (%) | 125 | Venous | 5.57 (0.81) | 4.4 | 4.9 | 5.2 | 5.4 | 5.7 | 6.9 | 11.5 |
| 125 | DBS - health tech | 5.55 (0.86) | 4.0 | 4.6 | 5.1 | 5.4 | 5.8 | 6.8 | 10.9 | |
| 125 | DBS - interviewer | 5.58 (0.92) | 4.2 | 4.4 | 5.1 | 5.4 | 5.9 | 7.3 | 11.1 | |
| HDL cholesterol (mmol/l) | 125 | Venous | 1.30 (0.38) | 0.54 | 0.78 | 1.04 | 1.24 | 1.53 | 1.97 | 2.95 |
| 125 | DBS - health tech | 1.12 (0.34)b | 0.41 | 0.67 | 0.91 | 1.11 | 1.32 | 1.71 | 2.33 | |
| 125 | DBS - interviewer | 1.15 (0.36)b | 0.44 | 0.65 | 0.91 | 1.11 | 1.32 | 1.76 | 2.28 | |
| Total cholesterol (mmol/l) | 124 | Venous | 4.85 (1.07) | 1.53 | 3.37 | 4.07 | 4.78 | 5.36 | 6.58 | 8.34 |
| 124 | DBS - health tech | 4.33 (0.86)b | 1.84 | 3.34 | 3.74 | 4.08 | 4.87 | 5.80 | 7.30 | |
| 124 | DBS - interviewer | 4.40 (0.85)b | 2.54 | 3.26 | 3.78 | 4.25 | 4.87 | 5.93 | 6.81 | |
SD, standard deviation.
Paired t-test: p < 0.05 for difference between venous and DBS means.
The health technicians’ and field interviewers’ means for DBS HDL cholesterol (1.12 and 1.15 mmol/l, respectively) were not significantly different from each other but were significantly lower compared to the venous HDL cholesterol (1.30 mmol/l, p < 0.01). The mean venous total cholesterol (4.85 mmol/l) was higher (p < 0.01) when compared to the DBS method from the health technicians (4.33 mmol/l) and the interviewers (4.40 mmol/l). The DBS distributions for hemoglobin A1c were generally comparable to venous HbA1c (Table 1). The venous values for total and HDL cholesterol were higher than the DBS values for the health technicians and field interviewers across the entire distribution.
A sensitivity and specificity analysis was performed on DBS methods using venous methods as a reference at selected clinically important cut-points. Hemoglobin A1c has a sensitivity of 100% for DBS performed by health technicians and interviewers, and a specificity of 98.3% for health technicians and 99.1% for interviewers was seen at a HbA1c cut-point of 6.5%. For an HDL cholesterol cut-point of 1.04 mmol/l, the sensitivity was 81.3% and the specificity was 66.7% for health technicians and interviewers. Total cholesterol, using a cut-point of 6.22 mmol/l, showed a sensitivity of 27.3% for health technicians and 36.4% for interviewers. The specificity for total cholesterol was 99.1% for health technicians and 98.2% for interviewers.
The clinical acceptability of DBS values, using venous results as a reference, was examined using the College of American Pathologists (CAP) evaluation limits for proficiency testing. For hemoglobin A1c, using a ±6% evaluation limit, 65% and 58% of health technician and interviewer DBS results, respectively, were acceptable. For HDL cholesterol, using a ±30% evaluation limit, 79% of health technician results and 75% of interviewer values were acceptable. For total cholesterol, 30% of health technician and 46% of interviewer results were acceptable using an evaluation limit of ±10%.
The Pearson correlations between DBS and venous results were high for hemoglobin A1c for health technicians (r = 0.90) and field interviewers (r = 0.90). Also, the DBS health technicians’ and interviewers’ hemoglobin A1c results had a correlation of 0.92. The DBS and venous correlations were low for HDL and total cholesterol. For HDL cholesterol, the correlation between DBS and venous results was 0.66 for health technicians and 0.64 for the field interviewers. The DBS health technicians and field interviewers HDL cholesterol results had a correlation of 0.72. The correlation between DBS and venous results was 0.67 for health technicians and 0.65 for interviewers for total cholesterol. The DBS correlation between the health technician and interviewer total cholesterol results was 0.89.
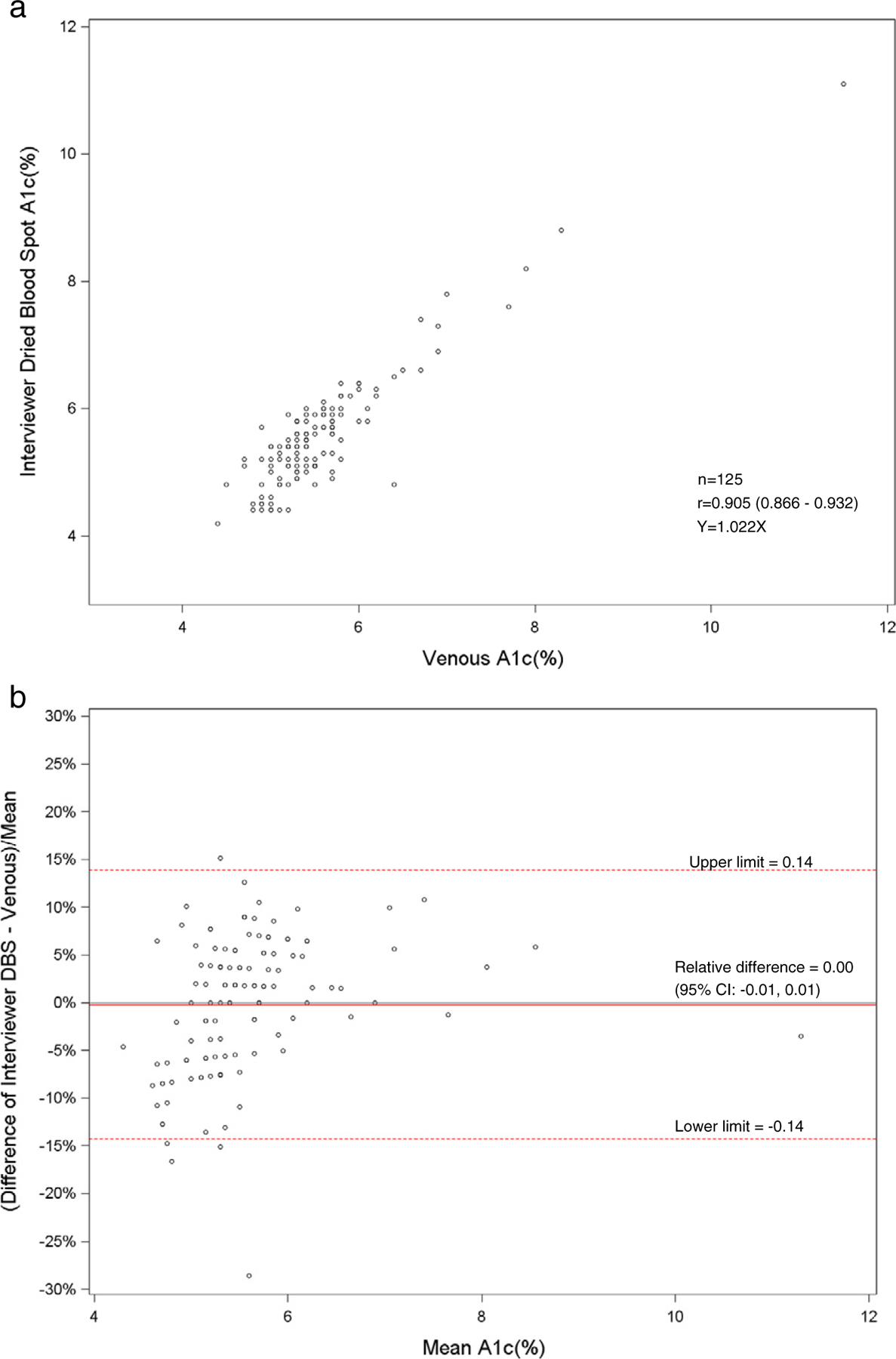
The bivariate plots showing the linear relationship of DBS and venous methods for the health technicians and interviewers and the linear relationship between health technicians and interviewers for DBS methods for hemoglobin A1c, HDL and total cholesterol are seen in Figs. 1a–9a. The corresponding Bland–Altman plots of relative differences of the DBS and venous methods and differences between health technicians and interviewers for DBS methods are seen in Figs. 1b–9b. The Bland–Altman plots for hemoglobin A1c showed no concentration dependent bias with any relative difference between DBS and the venous method. The Bland–Altman plots for HDL cholesterol showed no concentration dependent bias with a relative difference (−0.13 to −0.15) between DBS and the reference venous method. However, the Bland–Altman plots for total cholesterol showed concentration dependent bias at total cholesterol concentrations above 5.18 mmol/l with a relative difference (−0.09 to −0.10) between DBS and the reference venous method. (Figs. 7b, 8b.)
Fig. 1.
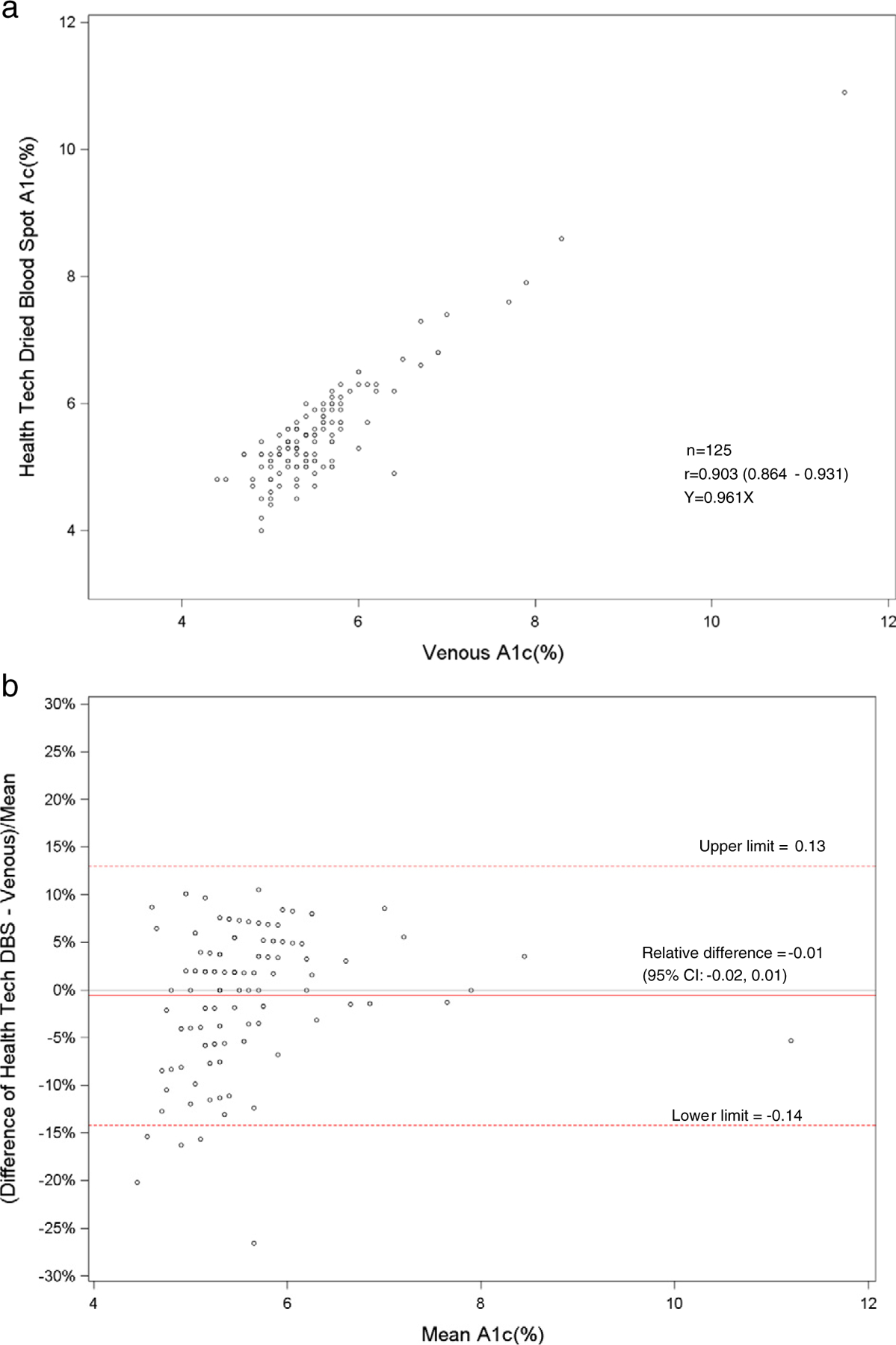
a. Scatterplot of dried blood spot of health technician versus venous hemoglobin A1c. b. Bland–Altman plot of hemoglobin A1c showing relative difference between the health technician DBS and venous methods.
Fig. 9.
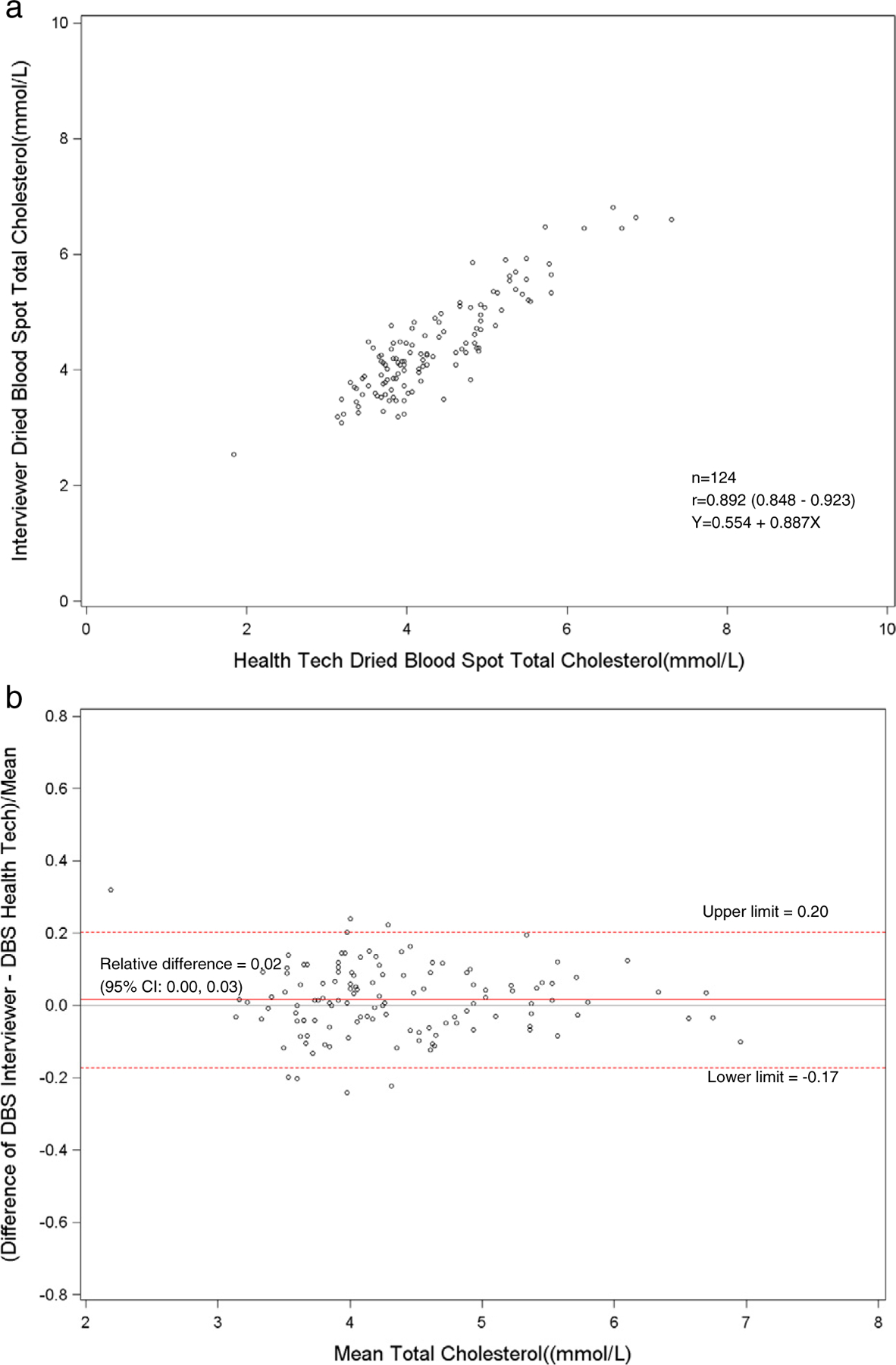
a. Scatterplot of dried blood spot of interviewer versus the health technician DBS for total cholesterol. b. Bland–Altman plot of total cholesterol showing relative difference between the interviewer DBS versus the health technician DBS.
Fig. 7.
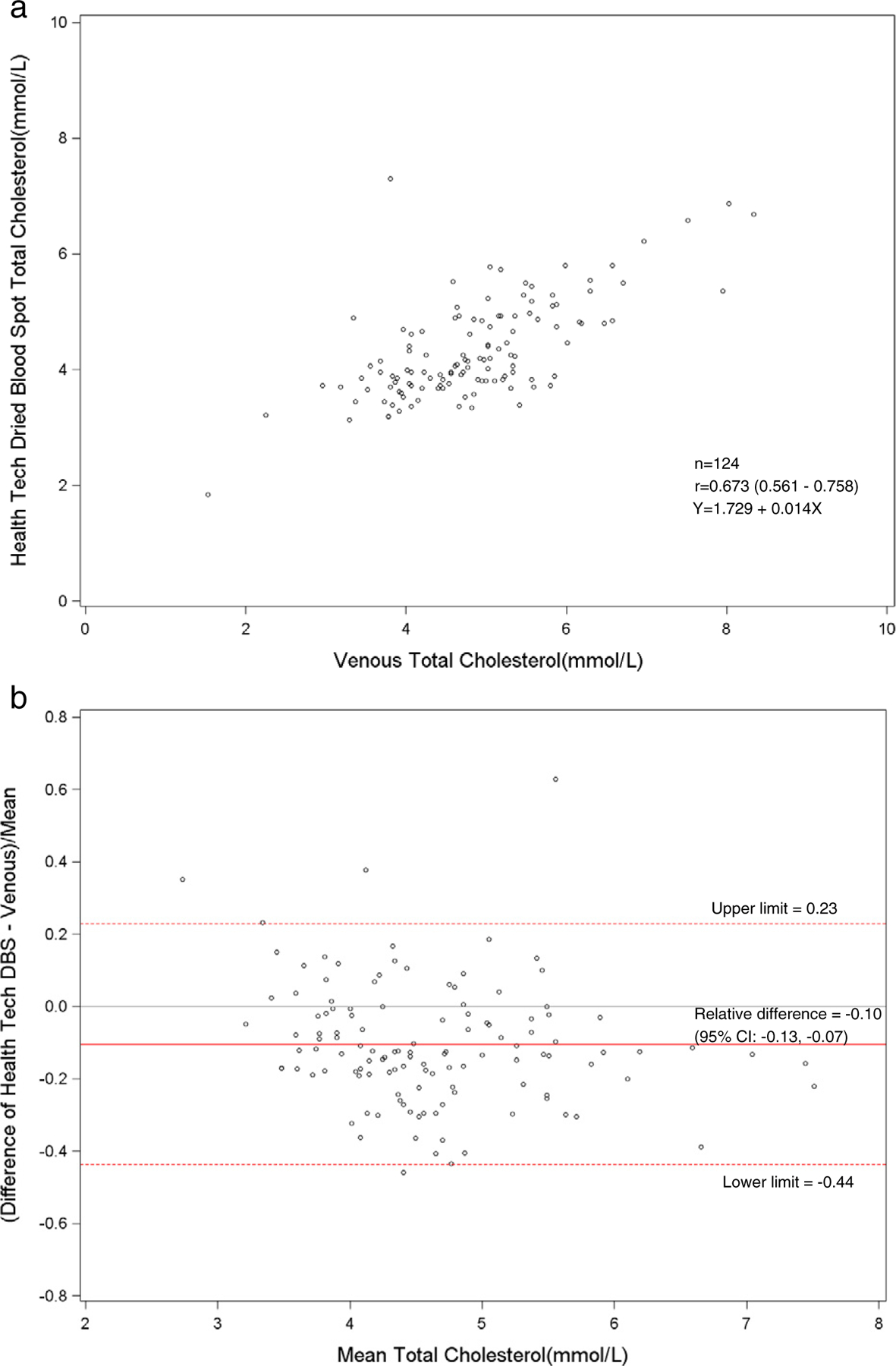
a. Scatterplot of dried blood spot of health technician versus venous total cholesterol. b. Bland–Altman plot of total cholesterol showing relative difference between the health technician DBS and venous methods.
Fig. 8.
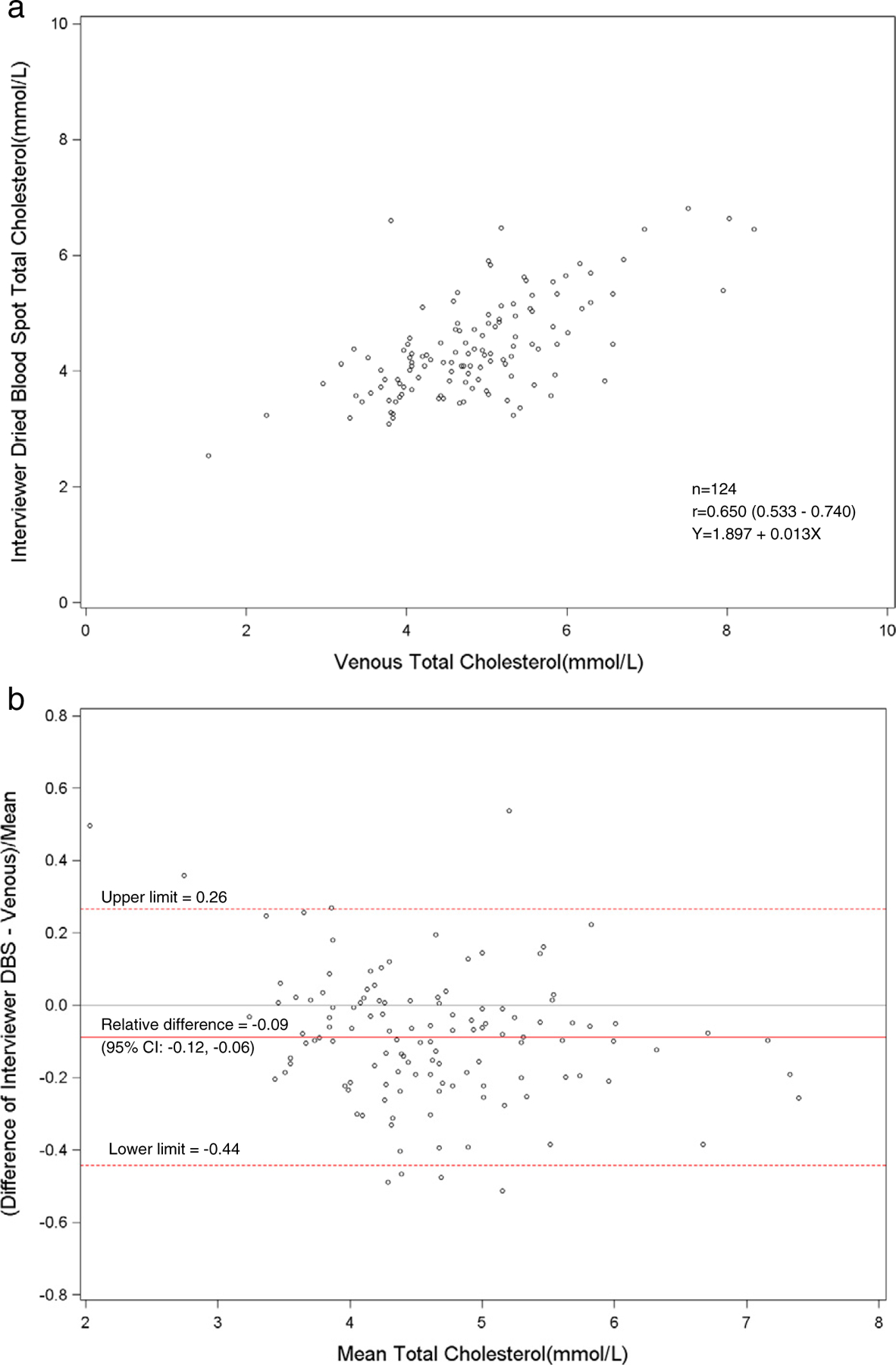
a. Scatterplot of dried blood spot of interviewer versus venous total cholesterol. b. Bland–Altman plot of total cholesterol showing relative difference between the interviewer DBS and venous methods.
4. Discussion
Dried blood spots are a promising substitute for venipuncture in population-based epidemiology studies because DBS can be collected by non-phlebotomists in non-clinical settings, and can be easily transported and stored. The disadvantages of DBS are that some assays are difficult to develop and validate against standardized venous methods. Lacher et al. compared DBS methods performed at the University of Washington to venous methods for hemoglobin A1c, glucose, total and HDL cholesterol and C-reactive protein in the NHANES mobile examination center using 2 expert phlebotomists [11]. In that study, the DBS was collected within 15 min after the phlebotomy in the MEC by the phlebotomists. The DBS compared well with venous methods for hemoglobin A1c, glucose and C-reactive protein (r > 0.90), but DBS was poorly correlated to venous methods for total and HDL cholesterol (r < 0.58). The poor correlation between the DBS and venous lipid assays was a laboratory method issue and not due to the time between the DBS and phlebotomy collection.
The HMHS was designed to compare the values obtained by DBS collection in the home to venous blood collected at the MEC. The HMHS study was also designed to evaluate the performance of field interviewers with no health sciences training and health technicians with health sciences training.
The HMHS showed that the DBS method for hemoglobin A1c correlated well to the standardized venous hemoglobin A1c method (r = 0.90). For hemoglobin A1c, linear regression analyses between DBS and venous methods revealed slopes of 0.96 for health technicians (Fig. 1a) and 1.02 for interviewers (Fig. 2a). There was excellent sensitivity (100%) and specificity (98.3–99.1%) of DBS methods compared with venous methods at a cut-point of 6.5%. However, only 58–65% of DBS values were acceptable using a CAP evaluation limit of ±6%. Most of the HbA1c values were just beyond the CAP evaluation limit, which has recently been tightened. Also, the low percentage of clinically acceptable HbA1c results may have been due to matrix differences between DBS and venous samples. The means and distributions of the DBS and venous hemoglobin A1c were similar. Also, the hemoglobin A1c results between the health technicians and field interviewers were highly correlated (r = 0.92). The venous A1c method was standardized by the NGSP program [9]. The HbA1c DBS method would optimally be standardized by the NGSP program to compare to venous A1c methods.
Fig. 2.
a. Scatterplot of dried blood spot of interviewer versus venous hemoglobin A1c. b. Bland–Altman plot of hemoglobin A1c showing relative difference between the interviewer DBS and venous methods.
The total and HDL cholesterol DBS methods did not correlate well with the standardized venous total and HDL cholesterol methods (r = 0.64–0.67). The means and distributions of the DBS methods were significantly (p < 0.001) lower than the venous methods for total and HDL cholesterol. The correlation between the health technicians and the field interviewers for DBS methods was high for total cholesterol (r = 0.89), but low for HDL cholesterol (r = 0.72). The sensitivity (81%) and specificity (67%) were fairly low for DBS HDL cholesterol methods at a cut-point of 1.04 mmol/l. For total cholesterol, the sensitivity was poor (27–36%), but the specificity was high (N98%) at a cut-point of 6.22 mmol/l. The sensitivities and specificities of the HDL and total cholesterol methods reflected the poor correlation of DBS with venous methods. Also, this was most likely due to the relative imprecision (CVs 10.5–11.0%) of the DBS HDL cholesterol method. The clinically acceptable DBS values for HDL cholesterol (75%) were higher than total cholesterol (<47%) that reflected the wide CAP evaluation limits for the HDL cholesterol method (±30%) compared with the total cholesterol method (±10%).
Previously, Lacher et al. had found that total and HDL cholesterol for DBS methods, performed at the University of Washington laboratory, correlated poorly with venous methods (r < 0.59) [11]. In the current study, there was some time (1–3 weeks) between the phlebotomy and the subsequent DBS collection. This could have affected the comparability of the DBS and venous results due to biological variability. However, hemoglobin A1c and total and HDL cholesterol would be relatively stable between phlebotomy and DBS collection unless therapy and/or diet had changed. The poor correlation seen in the lipid methods was likely due to a laboratory method issue. Potter et al. suggested that oxidative changes of lipid components may occur for DBS cards over time [12]. The total and HDL cholesterol DBS methods were more imprecise than venous methods. The NHANES venous methods were performed by laboratories using standardized protocols but the DBS lipid methods were not standardized. DBS lipid methods still need to be carefully established and confirmed against standardized venous methods suggesting that additional assay developments for dried blood spot methods are needed.
Affan et al. did a systematic review and meta-analysis of the comparability of dried blood spot methods vs. venous methods for hemoglobin A1c and lipids [13]. In general, Affan et al. found good agreement for 16 studies of HbA1c DBS methods compared to venous methods. However, there were very few studies comparing DBS to venous methods for triglycerides, total and HDL cholesterol. Total cholesterol DBS methods gave lower results than venous methods for total cholesterol. Affan et al. recommended standardizing DBS collection, storage, and transportation of samples and standardizing the method analyses [13].
The performance of staff conducting DBS was generally good because the field interviewers and health technicians were able to collect the dried blood spots in the home for greater than 90% of the sample participants, with more than a 95% completed collection of five dried blood spots. However, the health technicians were faster and collected more dried blood spots. The HMHS showed that DBS collection can be conducted in the home environment for population-based epidemiology surveys by lay interviewers, with minimal differences in DBS collection when compared to health technicians. The hemoglobin A1c results obtained using the DBS method were similar to results obtained using the venous method. However, there was a poor correlation for total and HDL cholesterol between DBS and venous methods due to the DBS methods and further DBS method development is needed.
Fig. 3.
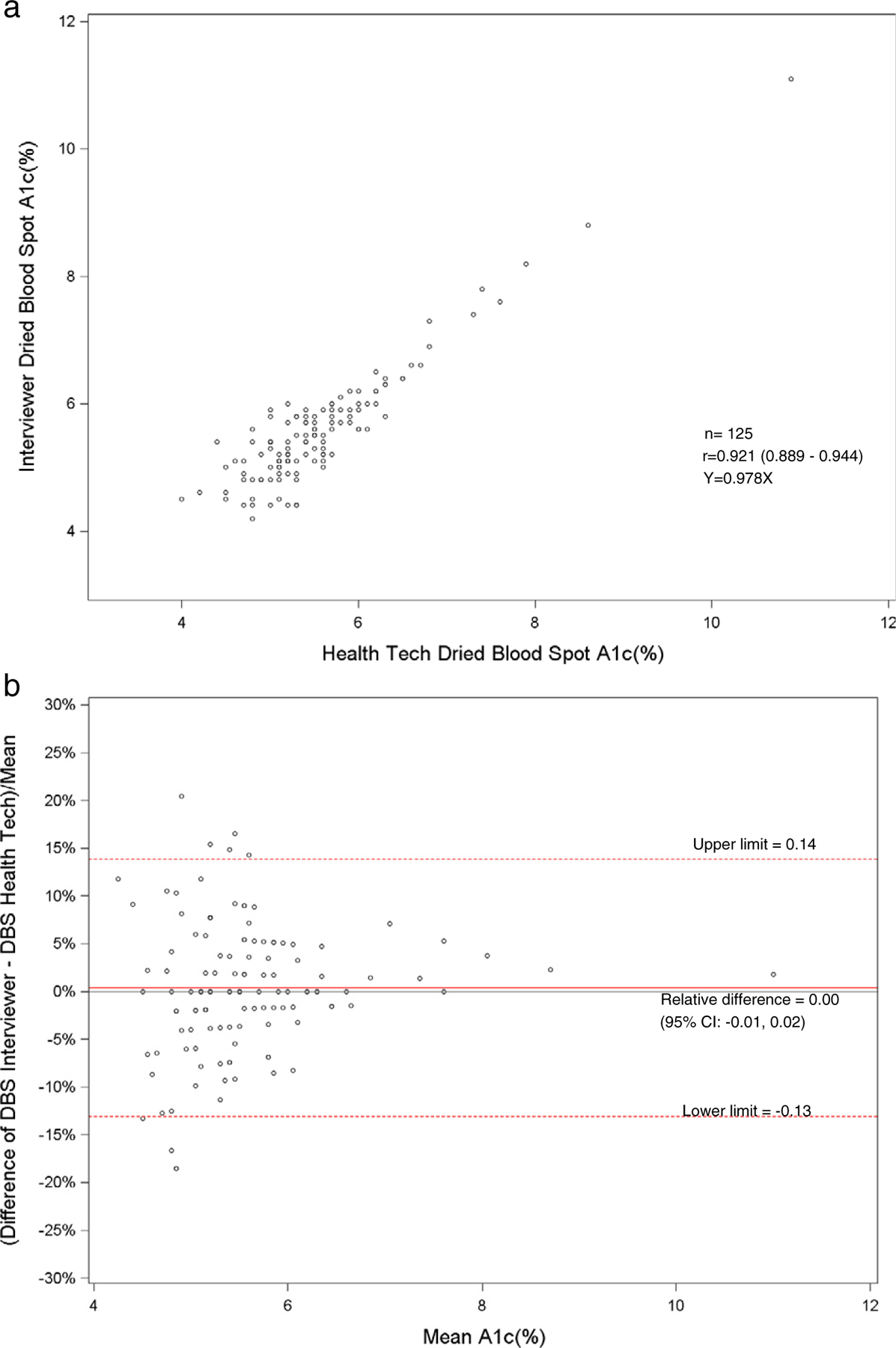
a. Scatterplot of dried blood spot of interviewer versus health technician for hemoglobin A1c. b. Bland–Altman plot of hemoglobin A1c showing relative difference between the interviewer DBS versus the health technician DBS.
Fig. 4.
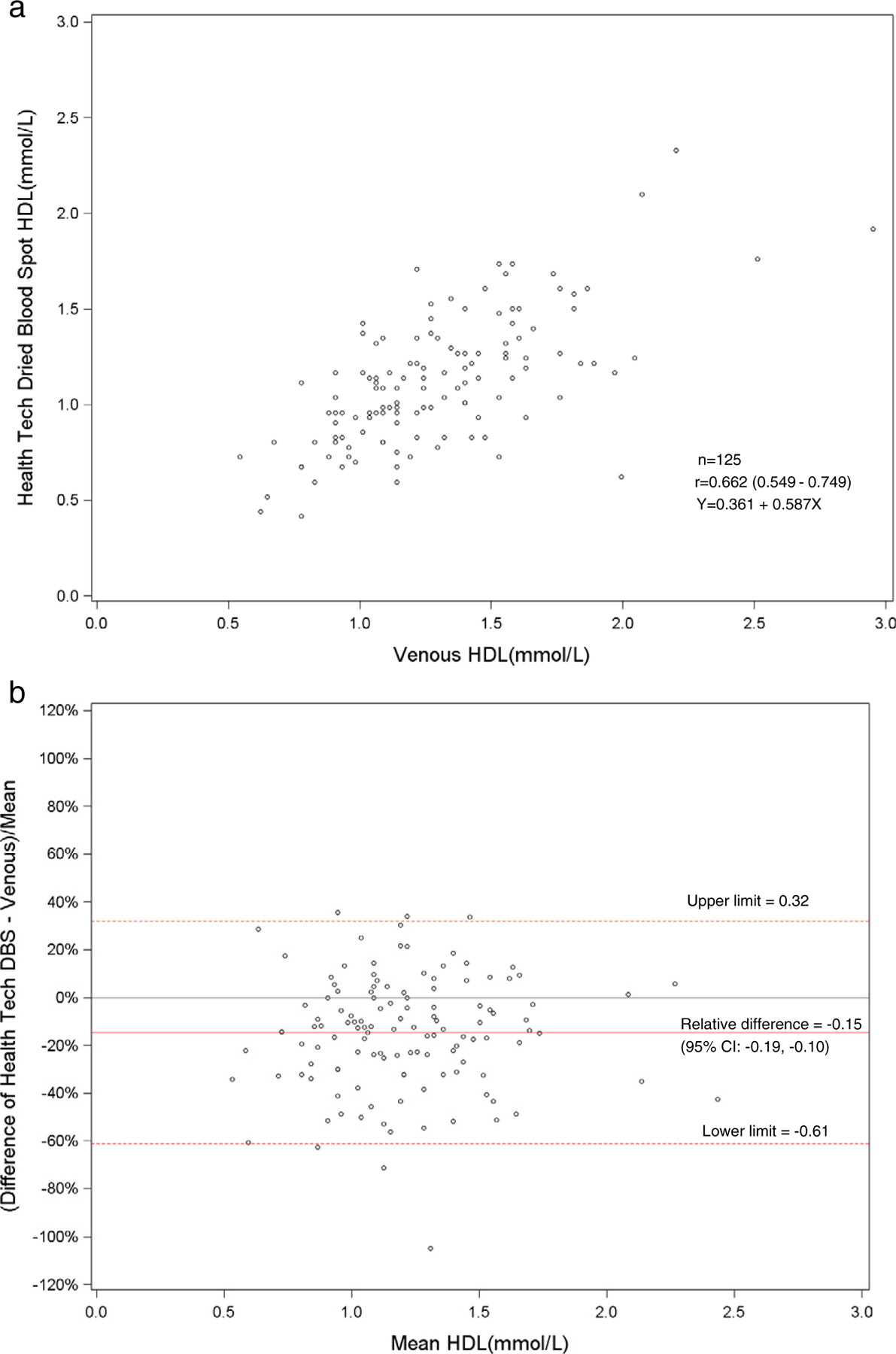
a. Scatterplot of dried blood spot of health technician versus venous HDL cholesterol. b. Bland–Altman plot of HDL cholesterol showing relative difference between the health technician DBS and venous methods.
Fig. 5.
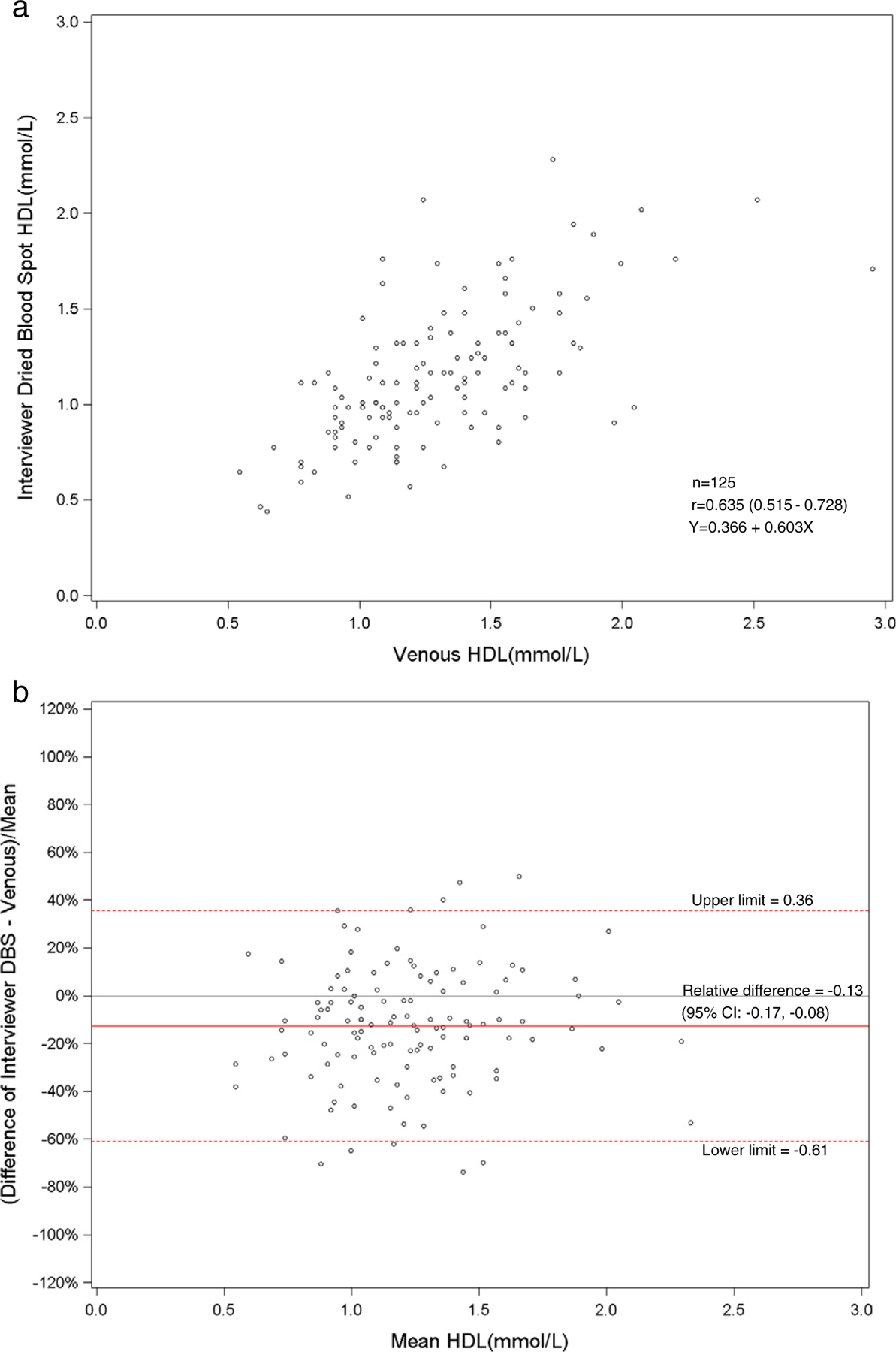
a. Scatterplot of dried blood spot of interviewer versus venous HDL cholesterol. b. Bland–Altman plot of HDL cholesterol showing relative difference between the interviewer DBS and venous methods.
Fig. 6.
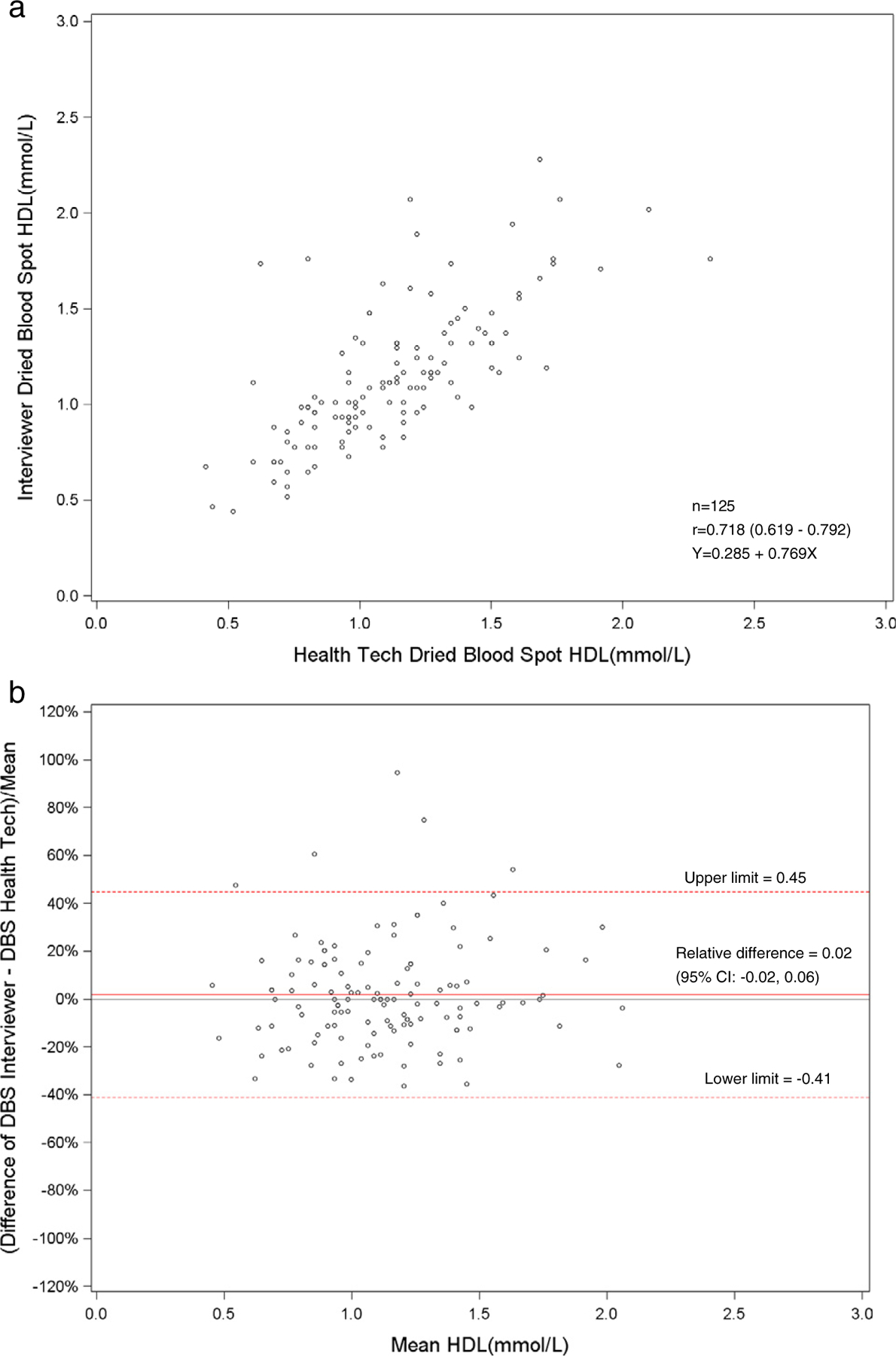
a. Scatterplot of dried blood spot of interviewer versus health technician for HDL cholesterol. b. Bland–Altman plot of HDL cholesterol showing relative difference between the interviewer DBS versus the health technician DBS.
Abbreviations
- DBS
dried blood spot
- HMHS
Health Measures at Home Study
- MEC
mobile examination center
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- NHIS
National Health Interview Survey
- CAP
College of American Pathologists
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics, Centers for Disease Control and Prevention.
References
- [1].Hoyert DL, Xu JQ. Deaths: preliminary data for 2011 National vital statistics reports, vol. 61 no 6. Hyattsville, MD: National Center for Health Statistics; 2012. [http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf, [Accessed September 2014]]. [PubMed] [Google Scholar]
- [2].Million Hearts. Strategies to reduce the prevalence of leading cardiovascular disease risk factors—United States 2011. MMWR 2011;60(36):1248–51. [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention/National Health and Nutrition Examination Survey. About the National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Accessed September 2014].
- [4].Centers for Disease Control and Prevention/National Health Interview Survey. About the National Health Interview Survey. http://www.cdc.gov/nchs/nhis/about_nhis.htm. [Accessed September 2014].
- [5].Gindi RM, Zipf G, Galinsky A, et al. Series 2, No. 164. Comparison of in-home collection of physical measures and bio-specimens by non-medically trained interviewers with clinical measurements: the Health Measures at Home Study. NHSR; 2014. [http://www.cdc.gov/nchs/data/series/sr_02/sr02_164.pdf, [Accessed September 2014]]. [Google Scholar]
- [6].McDade TW, Williams S, Snodgrass JS. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 2007;44:899–925. [DOI] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention/National Health and Nutrition Examination Survey. Laboratory procedures manual. http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_Laboratory_Procedures_Manual.pdf. [Accessed September 2014].
- [8].Centers for Disease Control and Prevention/National Health and Nutrition Examination Survey. 2011–2012 Lab Methods. http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/lab_methods_11_12.htm. [Accessed September 2014].
- [9].National Glycohemoglobin Standardization Program. http://www.ngsp.org. [Accessed September 2014].
- [10].The Centers for Disease Control and Prevention/lipid Standardization Program. Laboratory quality assurance and standardization programs. http://www/cdc/gov/labstandards/lsp.html;2012. [Accessed September 2014].
- [11].Lacher DA, Berman LE, Chen T-C, Porter KS. Comparison of dried blood spot to venous methods for hemoglobin A1c, glucose, total cholesterol, high-density lipoprotein cholesterol, and C-reactive protein. Clin Chim Acta 2013;422:54–8. [DOI] [PubMed] [Google Scholar]
- [12].Potter A, Wener M, Edenfield M. Dried blood spot assays: recent work. http://gero.usc.edu/CBPH/network/files/5_2_2012_PAA_SanFran/ALAN_POTTER.pdf. [Accessed September 2014].
- [13].Affan ET, Praveen D, Chow CK, Neal BC. Comparability of HgA1c and lipids measured with dried blood spot versus venous samples: a systematic review and meta-analysis. BMC Clin Pathol 2014;14(21):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


