Abstract
Behavioral health issues such as smoking and overweight are risk factors for a variety of adverse health outcomes, including mortality. Over the past decade, a growing number of randomized controlled trials have examined the efficacy of acceptance- and mindfulness-based interventions for smoking cessation and weight loss. The purpose of the current meta-analytic reviews was to quantitatively synthesize the existing literature comparing these interventions to controls for a) smoking cessation and b) weight loss outcomes. Searches identified 17 smoking cessation studies and 31 weight loss studies eligible for inclusion. Meta-analytic results indicated a non-significant effect favoring acceptance- and mindfulness-based interventions over controls for smoking cessation (OR = 1.13) and a small, significant effect favoring these interventions over controls for weight loss outcomes (Hedge’s g = 0.30). Statistical heterogeneity and risk of bias were assessed. Subgroup and meta-regression analyses were conducted to examine moderating variables (e.g., sample and intervention characteristics). The findings indicated that acceptance- and mindfulness-based interventions were at least as efficacious as active control conditions. Given the significant health risks associated with smoking and overweight, these findings have important clinical and public health implications. Limitations (e.g., relative infancy of the literature; lack of diversity in sample demographics) and future directions (e.g., further exploration of mediators and moderators of change) are discussed.
Keywords: mindfulness, experiential avoidance, health behavior change, smoking cessation, weight loss, meta-analysis
Many of the most prominent physical health problems and causes of death in the United States can be linked to problematic health behavior (Mokdad et al., 2018). Thus, healthcare professionals and behavioral health specialists routinely target behavior change outcomes in an attempt to improve patient health. Specifically, smoking and overweight may be common targets in clinical practice as both are important risk factors for a variety of adverse outcomes. For example, research has indicated that behavioral risk factors such as tobacco use, diet and nutrition, and physical activity are causally associated with some of the leading causes of death in the United States (Johnson, Hayes, Brown, Hoo, & Ethier, 2014; Murray et al., 2013). Seminal work by Mokdad and colleagues (2004) examining actual causes of death in the United States identified smoking and overweight as the two leading causes of mortality, with smoking accounting for approximately 18% of deaths and overweight accounting for approximately 15.2% (Mokdad, Marks, Stroup, & Gerberding, 2004; Mokdad, Marks, Stroup, & Gerberding, 2005). Similarly, newly published work by Mokdad and colleagues (2018) examining burden of disease (from 1990-2016) identified dietary risks and tobacco use as the two leading risk factors for mortality in the United States, while tobacco use, high body mass index, and dietary risks were the top three risk factors for disability-adjusted life years. Additionally, smoking and overweight are associated with significant economic costs including direct healthcare expenditures and the indirect costs associated with loss of productivity (e.g., workplace absenteeism; Centers for Disease Control and Prevention; CDC, 2017a, 2017b; US Department of Health and Human Services; USDHHS, 2014). Even still, 15.1% of United States adults are current smokers (Jamal et al., 2016), and 70.2% are overweight or obese (Body Mass Index; BMI ≥ 25), with 37.7% falling into the obese category (BMI ≥ 30; National Institutes of Diabetes and Digestive and Kidney Diseases; NIDDK, 2017).
Health risks that are associated with modifiable behaviors such as smoking and weight loss present a valuable opportunity for risk reduction through intervention. Existing individual-level interventions targeting smoking cessation and weight loss include nonpharmacological and pharmacological approaches, as well as combined methods (Berkel, Poston, Reeves, & Foreyt, 2005; Patnode et al., 2015; Saunders, Shukla, Igel, Kumar, & Aronne, 2016). Nonpharmacological approaches consist largely of cognitive-behavioral therapeutic interventions (Berkel at al., 2005; Niaura, 2008). Traditional behavior modification interventions include goal-setting, self-monitoring, stimulus control, problem solving, and relapse prevention (Abraham & Michie, 2008; Berkel et al., 2005). Additionally, Motivational Interviewing (Miller & Rollnick, 2013), which aims to elicit an individual’s own reasons for change, has been shown to be effective across a variety of health-related outcomes, including smoking cessation and weight loss (Armstrong et al., 2011; Lindson-Hawley, Thompson, & Begh, 2015; Rollnick, Miller, & Butler, 2008).
Although there are many approaches targeting these two leading causes of mortality, there is room for improvement in weight loss and smoking cessation outcomes. Approximately 70% of US adults express a desire to quit smoking, and 55% report a quit attempt within the last year, yet only 7.4% report recently quitting successfully (Babb, Malarcher, Schauer, Asman, & Jamal, 2017). Abstinence rates are improved by the addition of behavioral and pharmacological interventions (West et al., 2015), yet quit rates for behavioral interventions are still fairly low, with some recent estimates indicating quit rates between approximately 7-13% for behavioral interventions vs. approximately 5-11% for control groups (Patnode et al., 2015; Siu, 2015). Similarly, for weight loss, comprehensive behavioral interventions generally lead to initial clinically relevant weight loss, yet these outcomes are difficult to maintain after treatment ceases; on average, individuals regain one-third of weight lost within one year (Butryn, Webb, & Wadden, 2011; Wadden, Butryn, & Wilson, 2007). The significant health risks associated with smoking and overweight, in combination with suboptimal intervention outcomes, present a challenge for the development of novel behavior change approaches.
One target for behavioral health changes such as smoking cessation and weight loss is flexible (adaptable, contextually-sensitive) responding to external cues (e.g., patterns of smoking after mealtime, eating while watching television) and internal cues (e.g., cravings, urges, anxiety, stress, boredom; Brewer et al., 2011; Forman et al., 2016; Gifford & Lillis, 2009). These cues can become associated with opportunities for positive reinforcement (e.g., reward associated with food or nicotine) or negative reinforcement (e.g., stress reduction), thus maintaining and strengthening the short-term reinforcement contingencies associated with smoking or overeating (Brewer et al., 2011; Gifford & Lillis, 2009). Traditional behavioral approaches typically either do not rigorously target the internal cues involved in these reinforcement loops, or they aim to control, reduce, or avoid any sort of internal or external cue that may trigger the behavior (Brewer et al., 2011; Forman et al., 2013, 2016). Recently, acceptance-and mindfulness-based interventions have been implemented for health behavior change efforts, including smoking cessation and weight loss. The premise of utilizing these interventions for health behavior change efforts is as follows: by learning to relate to internal experiences in a different way (nonjudgmental acceptance, rather than avoidance), an individual can build behavioral flexibility (ability to adaptively engage behavior that is most workable in a given context) to respond to long-term rather than short-term contingencies (e.g. long-term health outcomes associated with not smoking a cigarette or eating a carrot vs. short-term reward of smoking or eating a donut; Brewer et al., 2011; Forman et al., 2013; Lee, An, Levin, & Twohig, 2015; Olson & Emery, 2015; Tapper et al., 2009).
Acceptance- and Mindfulness-Based Interventions
Acceptance- and mindfulness-based interventions include therapies such as mindfulness-based stress reduction (MBSR; Kabat-Zinn, 1990), mindfulness-based cognitive therapy (MBCT; Segal, Williams, & Teasdale, 2002), dialectical behavior therapy (DBT; Linehan, 1993), and acceptance and commitment therapy (ACT; Hayes, Strosahl, & Wilson, 1999), as well as closely-related or specialized variations (Baer, 2015, pp. 4-5). These interventions typically target processes (e.g., mindfulness, acceptance), rather than specific symptoms, and thus have important transdiagnostic value. Indeed, these interventions have demonstrated empirical support across a variety of targets (see Hofmann, Sawyer, Witt, & Oh, 2010; Öst, 2014 for reviews).
Increasingly, acceptance- and mindfulness-based interventions have been utilized in health behavior change efforts. Research has shown positive outcomes for these interventions with smoking cessation (Davis, Manley, Goldberg, Smith, & Jorenby, 2014b; Gifford et al., 2004) and weight loss (Daubenmier et al., 2016; Forman et al., 2016; Lillis, Hayes, Bunting, & Masuda, 2009), as well as with substance use (Hayes et al., 2004; Li, Howard, Garland, McGovern, & Lazar, 2017), diabetes management (Gregg, Callaghan, Hayes, & Glenn-Lawson, 2007), and physical activity (Butryn, Forman, Hoffman, Shaw, & Juarascio, 2011). Most clinical research studies examining interventions for psychological disorders measure efficacy in terms of symptom reduction. The primary aim of acceptance- and mindfulness-based interventions, however, is not to control or get rid of unpleasant internal experiences, but rather to relate to these experiences in a way that allows for greater behavioral flexibility. Given this, these therapies may be particularly well-suited for behavior change efforts, and likewise, behavior change or related outcomes may be an especially appropriate measure of efficacy for acceptance- and mindfulness-based interventions.
Previous Reviews and Objectives of the Current Study
In addition to the theoretical rationale for implementing acceptance- and mindfulness-based interventions for health behavior change outcomes, there is growing empirical evidence that these interventions may be efficacious. Given that healthcare professionals and behavioral health specialists often work with a heterogeneous population of patients presenting a range of behavior change targets, it is useful to systematically explore the empirical support for the implementation of these interventions for a variety of health behavior change outcomes. As referenced, smoking and overweight are two leading risk factors for adverse health consequences and mortality. Additionally, there is a burgeoning literature exploring acceptance- and mindfulness-based interventions for the specific health behavior change outcomes of smoking cessation and weight loss. As such, the purpose of the current review is to quantitatively synthesize the evidence from randomized controlled trials (RCTs) examining the efficacy of acceptance- and mindfulness-based interventions for these two particularly common and important health behavior change targets: smoking cessation and weight loss.
Previous smoking cessation reviews.
A recent literature review by de Souza and colleagues (2015) concluded that mindfulness-based interventions may be useful for smoking cessation efforts, though the review did not include a quantitative component. Additionally, a recent meta-analysis exploring mindfulness compared to standard treatment for smoking cessation found significant effects for mindfulness training over and above other standard treatments for smoking cessation at 17-24 weeks (relative risk = 1.88, 95% CI = 1.04, 3.40), and non-significant effects at 4-6 weeks (relative risk = 1.52, 95% CI = 0.95, 2.45); however, the review included only four randomized controlled trials (RCTs; Oikonomou, Arvanitis, & Sokolove, 2016). A more recent meta-analysis of RCTs explored the use of Mindfulness Meditation (MM)-specific interventions for smoking cessation, finding no significant effects for MM over and above controls for smoking cessation behavior (odds ratio at 2-4 weeks = 1.61, 95% CI = 0.98, 2.65, 5 RCTs; odds ratio at longest measured follow-up = 2.52, 95% CI = 0.76, 8.29, 6 RCTs; Maglione et al., 2017). The authors noted the limited number of RCTs, the heterogeneity in interventions, and the variation in study quality as limitations. Li et al. (2017) recently conducted a review and meta-analysis examining mindfulness-based interventions for substance misuse, including smoking, indicating effects approaching significance for mindfulness treatment vs. control for smoking cessation at post-treatment (odds ratio = 1.76, 95% CI = 0.98, 3.15, p = 0.056, 4 RCTs). Finally, Lee et al. (2015) conducted a similar meta-analysis examining ACT for substance use and reported a significant effect favoring ACT over control groups for smoking cessation (Hedge’s g = 0.42, 95% CI = 0.19, 0.64, p < .001, 5 RCTs). Notably, studies included in previous smoking cessation reviews commonly compared acceptance- or mindfulness-based interventions to active control conditions.
Previous weight loss reviews.
Two recent literature reviews outlining the current state of the literature on mindfulness-based interventions for weight loss (Olson & Emery, 2015) and obesity-related behaviors (O’Reilly, Cook, Spruijt-Metz, & Black, 2014), reported initial support for the efficacy of these interventions. Katterman et al. (2014b) examined the specific strategy of Mindfulness Meditation for binge-eating, emotional eating, and weight loss, finding only mixed support for MM for weight loss. None of the aforementioned reviews included a quantitative meta-analytic component. More recently, three meta-analyses have examined the efficacy of acceptance- and/or mindfulness-based interventions for weight loss and associated behaviors. Rogers et al. (2017) reported small post-treatment effects for acceptance- and mindfulness-based interventions on BMI in overweight and obese populations (Hedge’s g = 0.47, 95% CI = 0.30, 0.65, p < .01, 8 total studies); these effects were similar when including only RCTs in analyses (Hedge’s g = 0.43, 95% CI = 0.21, 0.65, 5 studies). Another meta-analysis conducted by Ruffault and colleagues (2017) showed no significant advantage for mindfulness and acceptance-based interventions vs. controls on BMI change baseline to post-intervention (Mean Difference = −0.15kg/m2, 95% CI = −0.59, 0.29, p = 0.50, 9 RCTs). Finally, a third meta-analysis by Carrière and colleagues (2018) examined mindfulness (but not acceptance-based) interventions and demonstrated small effects for these interventions for weight loss from pre- to post-intervention (Hedge’s g = 0.42, 95% CI = 0.26, 0.59, p < .001, 16 studies). The authors also reported that mindfulness interventions showed small effects on weight loss when only including controlled studies (Hedge’s g = 0.35, 95% CI = 0.02, 0.67, p < .05, 13 studies; Carrière, Khoury, Günak, & Knäuper, 2018). Notably, for results examining controlled studies specifically, previous reviews typically included studies with both active and non-active control groups.
Need for the current review.
Overall, the state of the evidence for acceptance- and mindfulness-based interventions for smoking cessation and weight loss to date has been mixed. Reviews have often focused on one specific type of mindfulness strategy. Furthermore, some reviews have included brief laboratory-based interventions that may not be representative of the impact of therapeutic programs, and some of the smoking cessation reviews have discussed studies that did not explicitly recruit participants who were aiming to quit smoking. Additionally, numerous reviews have included populations that may have increased heterogeneity (e.g., individuals with Binge Eating Disorder -- BED, alcohol abuse problems, or mild intellectual disability, bariatric surgery patients), which may have influenced outcomes. Moreover, most reviews have not included doctoral dissertations or searched clinical trial registries for unpublished data, introducing an increased risk of the “file drawer problem.” Additionally, several recent reviews have not included a meta-analytic component. Quantitative reviews can provide unique insight into the magnitude of an effect. Furthermore, while reviews examining weight loss have been promising in terms of pre- to post-intervention weight loss, the outcomes for reviews that have compared acceptance and/or mindfulness-based interventions to controls have been mixed and have generally reported substantial heterogeneity. Thus, there is a need for further exploration of whether these interventions provide an advantage over other interventions or the passage of time. Finally, given the relative infancy of the literature examining these interventions for health behavior change outcomes, any one study adds valuable information to meta-analytic results. Multiple new (and in some cases large-scale) RCTs have been published since the publication of previous reviews, and thus updated meta-analyses will provide useful information regarding current evidence for efficacy. In sum, recent reviews have provided important information, however, given the characteristics of recent reviews, the mixed evidence to date, and newly published studies, a rigorous quantitative exploration of the efficacy of acceptance- and mindfulness-based interventions compared to controls for smoking cessation and weight loss is warranted.
There are clear conceptual and theoretical similarities as well as operational overlap between acceptance- and mindfulness-based interventions (Vøllestad, Nielsen, & Nielsen, 2012). As such, the present review will include both acceptance- and mindfulness-based interventions. Furthermore, because providers often work with a variety of patients and target a variety of health behavior change outcomes, the review will provide an updated quantitative synthesis of the efficacy of these interventions for two particularly prominent behavioral health risk factors: smoking cessation and weight loss, and will thus provide unique practical utility for healthcare and behavioral health professionals. Additionally, by examining only RCTs, the current review provides insight into whether these interventions are superior to the passage of time, treatment-as-usual, or other interventions. Finally, by including a quantitative meta-analytic component, the current review provides insight into the magnitude of the potential effect for these interventions compared to controls.
Method
Search Strategy
Database search strategies were developed with the assistance of a health sciences librarian with expertise in searches for systematic reviews. The final search was conducted in February 2019 in the following databases: PubMed, CINAHL (EBSCO), PsycINFO, and ProQuest Dissertations and Theses. Both index and keyword methods were used in order to maximize sensitivity. There was no time frame exclusion based upon publication date. The English language filter was applied, and customized filters were utilized to identify randomized controlled trials. The PubMed search strategy can be found in Appendix A, and detailed search strategies for all other databases are available by request. First, titles and abstracts were screened for potential inclusion. Next, relevant articles were obtained in full-text and were assessed for eligibility. Finally, in an effort to reduce the impact of publication bias, we searched ClinicalTrials.gov (and emailed investigators of potentially relevant studies) and emailed the Association for Contextual Behavioral Science listserv in an attempt to obtain quality unpublished data that may be relevant to the current meta-analyses. Total yield and duplicate count can be found in Figure 1, PRISMA flow diagram.
Figure 1.
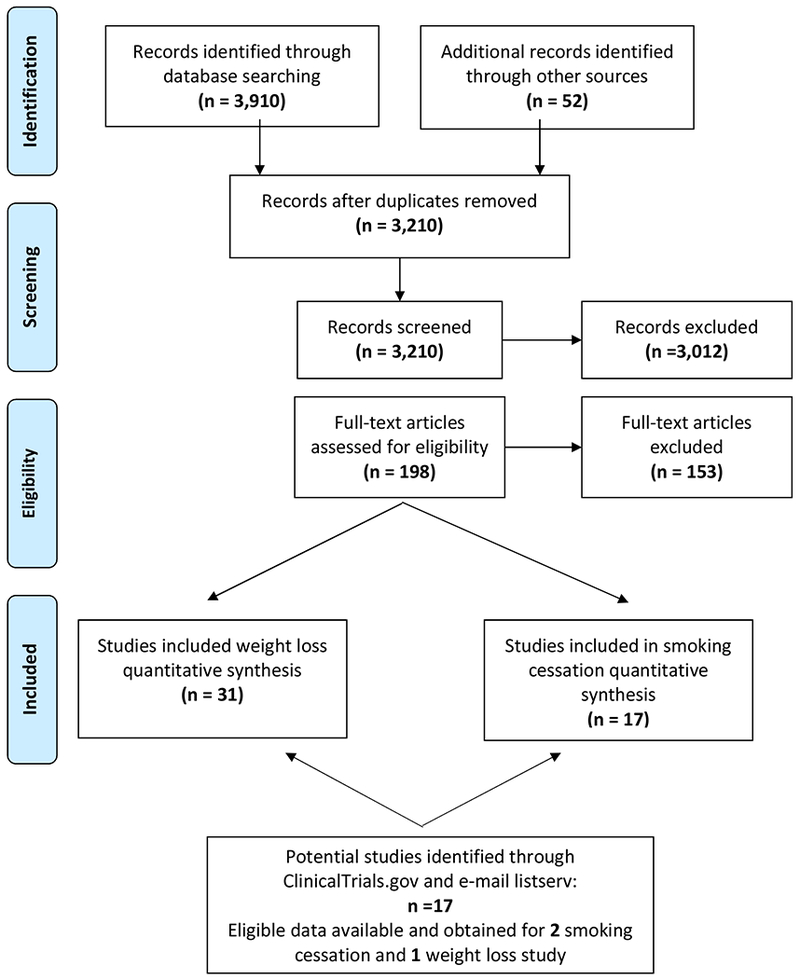
PRISMA flow diagram
Eligibility Criteria
In order to be included in the current review, the study had to: be an RCT; include an acceptance- or mindfulness-based intervention compared to a control; include change in weight/BMI or smoking abstinence as an outcome; have at least one pre- and post-intervention measure; be a peer-reviewed publication, a doctoral dissertation, or be registered on ClinicalTrials.gov; be reported in English.
Studies were excluded from the current review if the study: was targeted at a group with a specific medical diagnosis other than obesity, diabetes, or cardiovascular disease (medical conditions reasonably associated with the outcome variables); was targeted at a group with a specific psychological diagnosis (e.g., substance use, binge drinking, intellectual disability, chronic pain, elevated depressive or anxiety, specific eating pathology – this included studies that were primarily focused on patients with elevated BED symptoms, even if subthreshold); was targeted at pregnant women; was targeted at post-bariatric surgery patients; was lab- or experimental-based rather than intervention-based; evaluated other meditation interventions (e.g., yoga, tai chi, quigong, transcendental meditation, integrative body-mind training, Buddhist walking); was targeted at children/adolescents; was a masters or medical thesis; (if smoking cessation study) the intervention was not framed as an attempt to quit smoking.
Reference sections of eligible studies and previous reviews were used to identify additional studies that potentially met inclusion criteria. See Figure 1 for PRISMA flow diagram and Appendix A (Table A.1) for detailed explanations for exclusion of the studies that were screened in full-text.
Data Collection
Coded data included: participant characteristics (age, sex, % white, baseline BMI for weight loss studies, and cigarettes smoked per day for smoking studies), intervention characteristics (primarily mindfulness-focused vs. primarily acceptance-based behavioral intervention, duration of intervention), control type, outcome data (change in BMI, change in weight, point-prevalence abstinence, prolonged abstinence, continuous abstinence), and time to follow-up (months). Two reviewers independently abstracted and coded data from all studies using a standardized extraction protocol. Any coding discrepancies were discussed and resolved by consensus.
Outcome data were extracted based on the reported follow-up measurement closest to one month post-intervention. The time frame of one month post-intervention was chosen in advance in an attempt to gather outcome data that would be from similar time points for all studies. Many studies analyzed outcomes based on an intention-to-treat (ITT) sample, including all randomized participants in the final analysis, regardless of whether or not the participants completed treatment and provided assessment data. For smoking cessation studies, this typically meant classifying missing data as “smoking” or “not abstinent.” For weight loss studies, this often involved an imputation process. Intention-to-treat (ITT) data were utilized if available, and in these cases sample size was entered into calculations as the size of the ITT sample. If multiple outcome measures were reported for smoking cessation studies, we utilized the most stringent measure reported. This meant prioritizing outcome data as follows: sustained/continuous abstinence, prolonged abstinence, repeat point prevalence abstinence, and point prevalence abstinence (longest point prevalence measure reported). Additionally, we utilized biologically confirmed abstinence when reported. If full data for both change in weight and change in BMI were reported in weight loss studies, we used change in BMI as the outcome variable, which is consistent with previous research (Armstrong et al., 2011).
When a study included multiple intervention arms, we utilized the intervention arm that most purely represented a mindfulness- or acceptance-based intervention and that provided the most relevant comparison to the control condition. When a study used multiple control arms, we included the control arm that provided the strongest comparison.
If the study did not provide sufficient data for calculating an effect size, authors were contacted in an attempt to obtain the necessary data. Depending on the data available, calculating an effect size for change scores (e.g., change in BMI/weight from baseline to the follow-up time point) can require a pre-post correlation, a statistic that is rarely reported, so all authors were contacted for weight loss studies. When data were provided directly from the author, we prioritized using these data over the data reported in the manuscript. Therefore, if the author directly provided all requested relevant data on study “completers,” but not on all participants with ITT, we used the completer data.
Quality Assessment
The first author assessed risk of bias using the Cochrane risk of bias tool (Higgins et al., 2011). This tool assesses risk of bias across seven domains: 1) Selection bias – random sequence generation; 2) Selection bias – allocation concealment; 3) Performance bias – blinding (participants and personnel); 4) Detection bias – blinding (outcome assessment); 5) Attrition bias – incomplete outcome data; 6) Reporting bias – selective reporting; and 7) Other bias – other sources of bias. Each domain is rated as either Low risk of bias, High risk of bias, or Unclear risk of bias based upon reported study protocol and characteristics.
Statistical Analyses
Data were analyzed using Comprehensive Meta-Analysis (CMA) software. The primary outcomes were: a) smoking abstinence and b) change in BMI/weight.
For smoking cessation studies, effect sizes were calculated using odds ratios (OR). If an OR reported in the manuscript was in a format that could be directly entered into CMA, the reported effect size was used in the analyses. If not, available data were utilized to calculate an OR in CMA. One study (Bricker et al., 2014b) reported an OR with a markedly asymmetrical confidence interval. Because CMA uses the confidence interval to calculate the standard error of the effect size, raw outcome data from this study were used to calculate the study’s effect size. Overall, this resulted in seven studies for which an OR was directly reported, eight studies for which raw data were entered, and two studies for which a chi-square value was entered and converted to an OR.
For weight loss studies, effect sizes were calculated using a Hedge’s g, standardized by the change score standard deviation. In order to calculate a standardized difference in means or a Hedge’s g statistic for a difference score, CMA requires the pre-post correlation for the outcome variable (i.e., BMI or weight), though it is not directly used in all calculations. If the pre-post correlation for the total sample was not obtained from the study author, the correlations were estimated, as follows. First, if a pre-post correlation could be calculated for treatment and control groups based on available study data, a Fisher’s z transformation was used for each individual correlation, and the average of the two Fisher’s z values was calculated to estimate a total sample pre-post correlation for the study (Silver & Dunlap, 1987). Ultimately, there were 17 studies for which pre-post correlations were obtained or calculated. These 17 studies were used to estimate a pre-post correlation value for the remaining 14 studies. In order to estimate the correlation values, a Fisher’s z transformation was used on all raw correlation values. Then, the average of the Fisher’s z values was calculated. Finally, the average Fisher’s z value was transformed back to a correlation (Silver & Dunlap, 1987). Based on these calculations, all unavailable pre-post correlations were thus estimated to be 0.967.
For 28 of the weight loss studies, the Hedge’s g was calculated by obtaining the mean and standard deviation change in BMI/weight (from pre-intervention to the relevant follow-up) for intervention and control groups and standardizing using the pooled change score standard deviation. For the three remaining weight loss studies, for which the mean and/or standard deviation of the change scores were not reported, the pre- and follow-up means and standard deviations and the estimated pre-post correlation were entered. These were then used to calculate the standard deviation of the change score and subsequently the Hedge’s g standardized by the change score standard deviation. Standardizing using change scores and pooled standard deviation was preferred to standardization by post-scores because our aim was to assess the effect of the intervention on change in BMI/weight relative to change that occurred in control groups. Additionally, this approach was preferred because in studies with smaller sample sizes, intervention and control participants may have important pre-intervention differences even after randomization, and a change score is less impacted by these potential differences than a post-score would be (Johnsen & Friborg, 2015). This approach has been utilized in previous meta-analytic work (McGuire et al., 2014). If any relevant standard deviations were not directly reported, we calculated them based on reported statistics (i.e., standard error, 95% CI). All effect sizes were standardized so that positively keyed results (i.e., smoking cessation, weight loss) were indicative of the intervention group performing better than the control. Conventional interpretations of Hedge’s g effect sizes are as follows: small = 0.2, medium = 0.5, large = 0.8 (Cohen, 1988).
Effect size estimates for both meta-analyses were calculated using a random-effects model due to the variability in intervention type, treatment modality, and population, among other characteristics (Borenstein, Hedges, Higgins, & Rothstein, 2009). Heterogeneity in effect sizes was assessed using Q and I2 statistics. Moderation and subgroup analyses were used to explore moderation by sample and methodological features that were hypothesized to have an impact on effect size outcomes. Moderation analyses were performed using mixed-effects subgroup analyses for categorical variables and method of moments meta-regression for continuous variables. Moderation and subgroup analyses excluded studies that did not report on the relevant moderator variable. Subgroup analyses only included studies with three or more studies classified within the same subgroup. Publication bias was assessed through visual inspection of a random-effects funnel plot, Duval and Tweedie’s (2000) trim-and-fill analyses (based on the random-effects model), and an estimate of Fail-safe N.
Results
Smoking Cessation Meta-Analysis
Study and participant characteristics.
Search strategy and selection criteria yielded 17 smoking cessation studies for inclusion. Studies were completed between 2004 and 2019. Fourteen studies were peer-reviewed publications, one study was a doctoral dissertation, and two studies were unpublished studies registered on ClinicalTrials.gov for which investigators provided data. Fifteen of the studies were conducted in the United States, one was conducted in Scotland, and one was conducted in Ireland. All studies compared an acceptance- or mindfulness-based intervention to an active control group. Eleven studies examined a primarily acceptance-based behavioral intervention, and six studies examined a primarily mindfulness-focused intervention. For outcome, nine studies used 7-day point prevalence abstinence as the outcome measure, four studies used 30-day point prevalence abstinence, two studies used continuous abstinence, one study used 24-hour point prevalence abstinence, and one study used prolonged abstinence. Ten studies confirmed abstinence biologically, and seven studies did not. The seventeen studies had a total of 5,195 participants included in analyses with sample sizes ranging from 49 to 2,637. Participant characteristics were estimated based on demographic characteristics reported in each study, which often led to utilizing demographic information reported on all randomized participants (e.g., including completers and non-completers), rather than only those who were included in analyses (e.g., only completers). Additionally, not all studies reported on all demographic characteristics, and thus overall estimates are based on reported characteristics. Participants had a mean age of 45.52 years, were 32.17% male, 75.21% Caucasian, and smoked 19.13 cigarettes per day on average. See Appendix A (Table A.2) for detailed study characteristics.
Effect size analyses.
A mean effect size (odds ratio, [OR]) was computed to compare acceptance- and mindfulness-based interventions to controls. Random-effects modeling indicated that the overall effect size favored acceptance- and mindfulness-based interventions over controls, but was not statistically significant (OR = 1.13, 95% CI = 0.86, 1.48, z = 0.89, p = .373, k = 17). The aggregated odds ratio demonstrates that, on average, the acceptance- or mindfulness-based conditions had a 13% higher odds of being abstinent from smoking at follow-up measurement than control conditions. Figure 2 summarizes treatment effects for smoking cessation.
Figure 2.
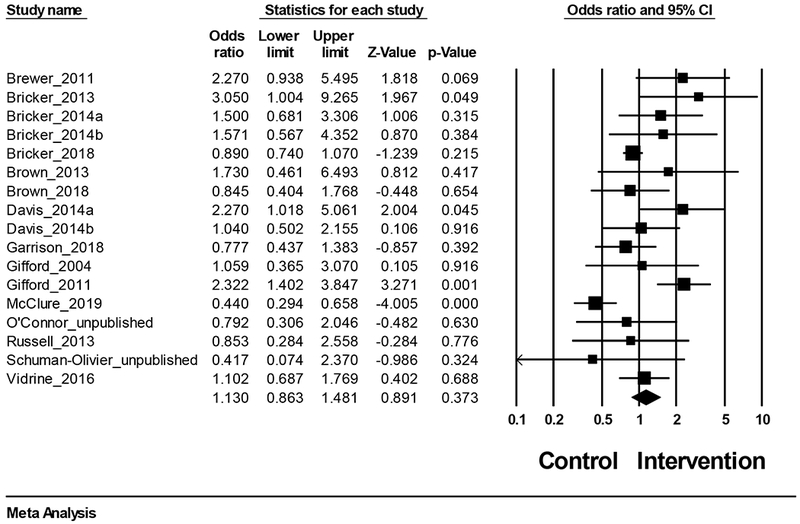
Treatment effects for smoking abstinence
Heterogeneity analyses.
The Q statistic (a measure of heterogeneity) indicated significant heterogeneity (Q(16) = 44.10, p < .001). The I2 statistic, which demonstrates the percentage of total variance due to heterogeneity and which is not directly impacted by the number of studies, was also calculated (Borenstein et al., 2009). The I2 statistic (I2 = 63.72) indicated that a sizeable variation in effect size across studies was due to heterogeneity. Thus, moderation analyses were justified.
Subgroup and meta-regression analyses.
Sample characteristics were examined as potential moderators of effect size. Meta-regression analyses indicated no significant moderation by sample age (Q = 0.61, df= 1, p > .10, k = 17; β = −0.03, z = −0.78, p > .10), percentage of the sample that was male (Q = 0.31, df=1, p > .10, k = 17; β = 0.73, z = 0.55, p > .10), percentage of the sample that was Caucasian (Q = 0.05, df = 1, p > .10, k = 15; β = −0.23, z = −0.23, p > .10), or sample baseline cigarettes smoked per day (Q = 1.75, df=1, p > .10, k = 12; β = 0.07, z = 1.32, p >.10). Additionally, intervention characteristics were examined using meta-regression and mixed-effects subgroup analyses. Meta-regression analyses indicated no significant moderation by intervention duration in months (Q = 0.03, df = 1, p > .10, k = 16; β = −0.01, z = −0.18, p >.10) or length of time to follow-up measurement in months (Q = 1.15, df = 1, p > .10, k = 16; β = −0.11, z = −1.07, p > .10). Mixed-effects subgroup analyses revealed no significant differences in effect size between primarily acceptance-based behavioral interventions and primarily mindfulness-focused interventions (Q = 0.08, df = 1, p > .10, k = 17) or between in-person and remotely-delivered interventions (Q = 0.08, df = 1, p > .10, k = 17). Additionally, mixed-effects subgroup analyses revealed no significant difference based on type of abstinence measurement (Q = 0.54, df = 1, p > .10, k = 13), although this analysis only included 30-day and 7-day point-prevalence abstinence measurements (13 studies) as the other measurement types were not utilized in a sufficient number of studies to be included in moderation analyses. Further, mixed-effects subgroup analyses revealed no significant difference in effect size between studies that were published and those that were not (Q = 1.27, df = 1, p > .10, k = 17), though the estimated effect was larger for those that were published (OR = 1.20 for published, k = 14 vs. OR = 0.72 for unpublished, k = 3). Finally, mixed-effects subgroup analyses revealed a significant difference in effect size between studies reporting on full intention-to-treat (ITT) samples vs. not (Q = 4.07, df = 1, p < .05, k = 17), indicating that effect sizes based on full ITT samples (OR = 0.94, p > .10, k = 11) were lower than those that were not based on full ITT samples (OR = 1.60, p < .05, k =6), as might be expected given penalized imputation procedures. We did not perform subgroup analyses based on type of control group, as nearly all controls were comprised of other commonly used approaches to smoking cessation, and thus were difficult to meaningfully categorize. We did not perform subgroup analyses based on country (United States [U.S.] vs. other) because only two studies were conducted outside of the United States.
Publication bias.
Potential publication bias was examined through visual inspection of a funnel plot and through trim-and-fill analyses. Given that the overall effect was already non-significant, estimation of a Fail-safe N for the smoking cessation meta-analysis was not appropriate. As shown in Figure 3, the random-effects funnel plot of standard error by log odds ratio appears to be symmetrical, providing little evidence of publication bias. Trim-and-fill analyses based on the random-effects model did not reveal a need for adjustment of the effect size estimate, and no studies were imputed, again indicating little evidence of publication bias. Even still, current results should be interpreted in the context of the still-existing potential for publication bias.
Figure 3.
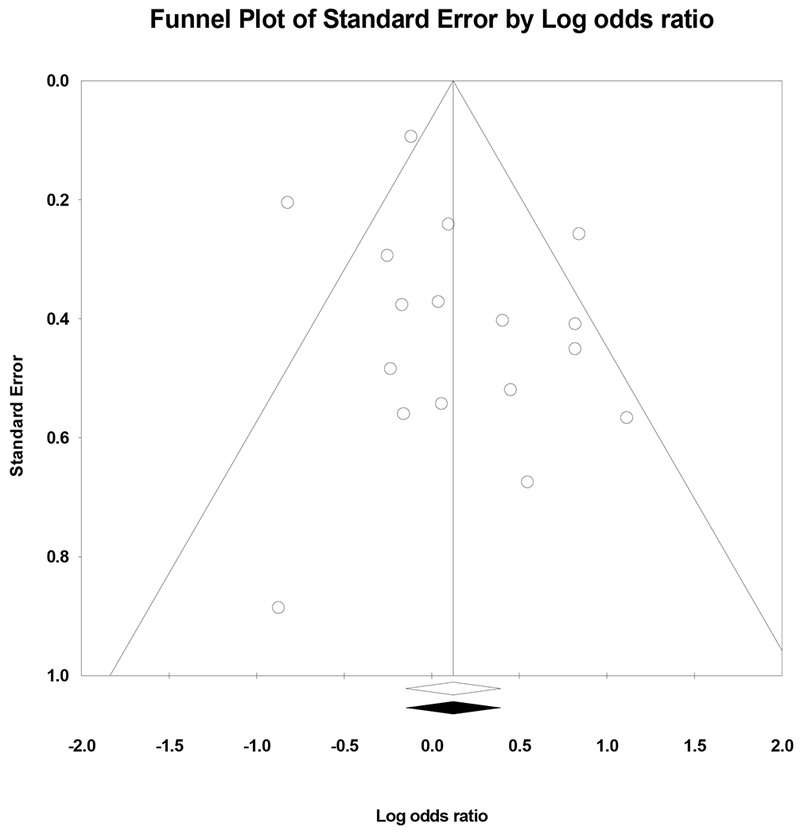
Random-effects funnel plot indicating the association between Log odds ratio and standard error in studies examining smoking cessation.
Study quality: Risk of bias.
Study quality was assessed using the Cochrane risk of bias tool. Study quality was assessed using the Cochrane risk of bias tool. Approximately half of the smoking cessation studies described methods used for random sequence generation, resulting in a low risk of bias rating for this category. Many studies did not describe allocation concealment processes, resulting in an unclear rating in this category for over half of the included studies. Performance bias was rated as either unclear or high in the majority of studies. The nature of psychological intervention studies often results in participants being aware of their condition (particularly if the intervention compares treatment vs. waitlist control), which increases the risk of potential performance bias. Detection bias was rated as low for all studies that utilized biologically-confirmed abstinence and as unclear for studies that used self-reported abstinence. The majority of studies were classified as low risk for attrition bias, as most studies had a comparable dropout rate for treatment and control groups, and many studies reported no significant differences on relevant baseline variables between drop-outs and treatment completers. Additionally, some studies reported on ITT samples. The majority of studies appeared to report on the outcomes that they planned to measure and were therefore rated as low risk of reporting bias. Finally, there were a variety of other study characteristics and methodological properties that may have presented a risk of bias, which resulted in numerous studies receiving an unclear risk of bias rating for the “other risk of bias” category. Notably, all unpublished studies were rated as unclear risk of bias for this category as they were not subjected to the peer review process. Overall results for risk of bias assessments are depicted in Figure 4.
Figure 4.
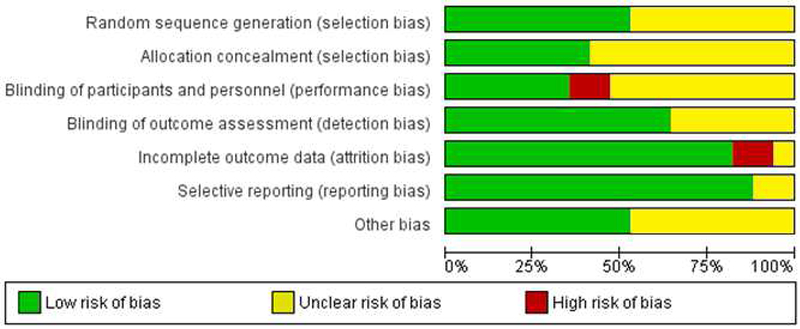
Risk of bias assessment for smoking cessation studies
Weight loss meta-analysis
Study and participant characteristics.
The search strategy yielded 31 weight loss studies to be included in the meta-analysis. Studies were completed between 2008 and 2019. Twenty-five studies were peer-reviewed publications, five studies were doctoral dissertations, and one study was an unpublished study registered on ClinicalTrials.gov for which the investigator provided data. Twenty-one studies were conducted in the United States, two in the Netherlands, two in Greece, and one in each of the following countries: Portugal, India, Finland, Austria, Canada, and the United Kingdom. Seventeen studies were classified as examining a primarily mindfulness or mindfulness-meditation-focused intervention, and 14 studies were classified as examining an acceptance-based behavioral intervention. Twenty-two studies compared an acceptance- or mindfulness-based intervention to an active control group (14 active but “non-psychological” interventions and eight “other psychological” interventions), and nine studies compared the intervention to a waitlist control group. Fifteen studies reported change in BMI over time, and thus BMI was used to calculate effect sizes for these studies. The remaining 16 studies reported change in weight over time (but did not report change in BMI in a manner that allowed for usable data)1, and thus change in weight was used to calculate an effect size for these studies. The 31 studies had a total of 2,076 participants included in analyses with sample sizes ranging from 19 to 194. Participant characteristics were estimated based on demographic characteristics reported in each study, which often led to utilizing demographic information reported on all randomized participants (rather than only those who were included in analyses). Additionally, not all studies reported on all demographic characteristics, and thus overall estimates are based on reported characteristics. Participants had a mean age of 45.38, a mean BMI of 33.45, were 72.48% Caucasian, and 14.39% male, with ten studies including only female participants. See Appendix A (Table A.3) for detailed study characteristics.
Effect size analyses.
A mean effect size (Hedge’s g) was computed to compare acceptance- and mindfulness-based interventions to controls. Random-effects modeling indicated that the overall effect size demonstrated a small, statistically significant effect favoring acceptance- and mindfulness-based interventions over controls (Hedge’s g = 0.30, 95% CI=0.15, 0.45, z = 3.97, p < .001, k = 31). Figure 5 summarizes weight loss treatment effects for each of the 31 studies.
Figure 5.
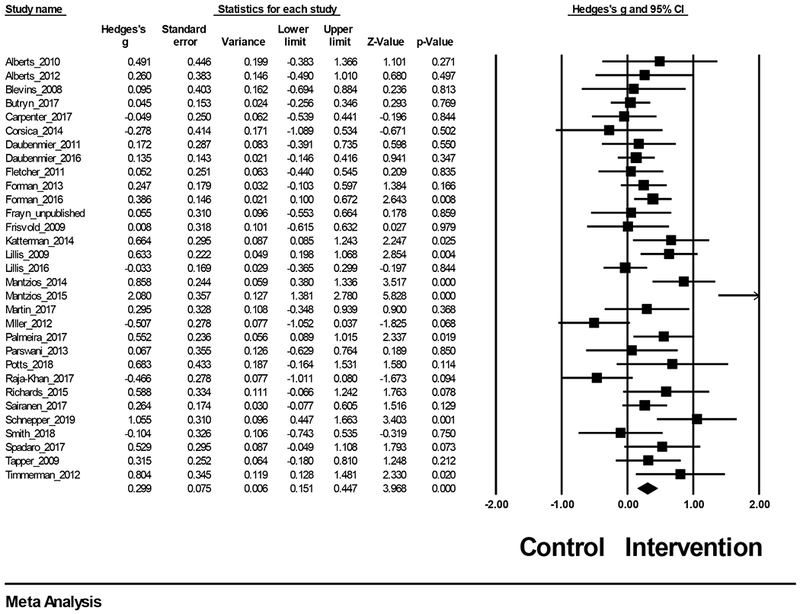
Treatment effects for weight loss
Heterogeneity analyses.
The Q statistic indicated significant heterogeneity (Q(30) = 78.03, p < .001). Similarly, the I2 statistic indicated that a sizeable variation in effect size across studies was due to heterogeneity (I2 = 61.55). Thus, moderation analyses were justified.
Subgroup and meta-regression analyses.
Sample characteristics were examined as potential moderators of effect size. Meta-regression analyses indicated that sample mean age significantly moderated effect size (Q = 16.47, df = 1, p < .001, k = 31, R2 = .50), indicating that younger sample age was associated with larger effect size (β = −0.03, z = −4.06, p < .001). Additionally, sample baseline BMI significantly moderated effect size (Q = 16.20, df = 1, p < .001, k = 30, R2 = .49), indicating that lower sample BMI was associated with larger effect size (β = −0.07, z = −4.03, p < .001). Meta-regression analyses indicated no significant moderation by percentage of the sample that was male (Q = 0.20, df = 1, p > .10, k = 29; β = 0.16, z = 0.45, p > .10) or percentage of the sample that was Caucasian (Q = 0.01, df = 1, p > .10, k = 21; β = 0.04, z = 0.08, p > .10). Finally, meta-regression analyses indicated no significant moderation by length of time to follow-up measurement in months (Q = 0.80, df = 1, p > .10, k =31; β = −0.07, z = −0.89, p >.10), but did indicate that intervention duration in months approached significance as a moderator (Q = 3.73, df = 1, p = .0536, k =31, R2 = .08) such that shorter interventions were associated with larger effect sizes (β = −0.04, z = −1.93, p = .0536).
Mixed-effects subgroup analyses revealed a significant difference in effect size between studies conducted in the United States as compared to other countries (Q = 6.78, df = 1, p < .01, k = 31), indicating that the studies conducted outside the United States resulted in greater effect size outcomes than those conducted in the United States (non-U.S. studies: Hedge’s g = 0.58 ,p < .001, k = 10; U.S. studies: Hedge’s g = 0.18, p < .05, k = 21). Mixed-effects subgroup analyses revealed no significant differences between studies that used primarily mindfulness-based interventions and primarily acceptance-based interventions (Q = 0.03, df = 1, p > .10, k = 31). Mixed-effects subgroup analyses did not indicate that effect size varied significantly by type of control group (classified as: waitlist, active/non-psychological, or other psychological intervention; Q = 2.82, df = 2, p > .10, k = 31). Notably, however, exploring subgroups indicated that there was only significant evidence for the effect of acceptance- or mindfulness-based interventions vs. control when the control type was waitlist (Hedge’s g = 0.50, p < .001, k = 9), while the effects when compared to active/nonpsychological controls (Hedge’s g = 0.20, p =.083, k = 14) and other psychological interventions (Hedge’s g = 0.25, p = .074, k = 8) only approached significance. Additionally, mixed-effects subgroup analyses did not indicate that effect size varied significantly by type of measurement (weight vs. BMI; Q = 0.66, df = 1, p > .10, k = 31). Further, mixed effects subgroup analyses revealed no significant differences in effect size between studies that were published and studies that were unpublished (Q = 0.22, df= 1, p > .10, k = 31), though those that were published reflected a greater overall and significant effect (Hedge’s g = 0.32, p < .001, k = 25) than those that were unpublished, which reflected a smaller and non-significant effect (Hedge’s g = 0.22, p > .10, k = 6). Finally, mixed-effects subgroup analyses revealed no significant difference in effect size between studies reporting on full intention-to-treat samples vs. not (Q = 0.48, df = 1, p > .10, k = 31).
Exploratory analyses.
Given the substantial heterogeneity across studies and moderation findings based on variables that were determined to be examined a priori, additional analyses were conducted in an effort to explore the results in further detail.
First, given the visibly large overall effect size in the Mantzios (2015) study, we conducted one-study removed analyses to gain insight into the extent to which this single study may have impacted the overall effect size outcome. When the study was removed from analyses, the weighted mean effect size was smaller, but still statistically significant (Hedge’s g = 0.25, 95% CI = 0.13, 0.37, z = 3.96, p < .001). Additionally, because the Frayn (unpublished) study had wide-ranging durations of intervention length (intervention length was based on sessions rather than time), we also ran one-study removed analyses for this study. When the study was removed from analyses, the weighted mean effect size was slightly larger, but comparable to overall results (Hedge’s g = 0.31, 95% CI = 0.16, 0.46, z = 3.97, p < .001).
Finally, given the moderation findings, we conducted a meta-regression model that included both age and baseline BMI as moderators of effect size outcome. When both variables were added to the model, the overall model significantly moderated effect size outcomes (Q = 20.76, df = 2, p < .001, k = 30, R2 = .57). Baseline BMI was a significant moderating variable in the model (β = −0.05, z = −1.97, p = .0489), while age only approached significance (β = −0.02, z = −1.89, p = .0586). Given that these results indicated that baseline BMI may have been the primary driver of moderation outcomes, meta-regression was then used to explore a model that included baseline BMI and country (United States vs. not). The overall model significantly moderated effect size outcome (Q = 16.93, df = 2, p < .001, k = 30, R2 = .47), but indicated that baseline BMI was the only significant moderating variable (β = −0.06, z = −2.73, p < .01), while country was non-significant (p > .10).
Publication bias.
Potential publication bias was examined through visual inspection of a funnel plot, trim-and-fill analyses, and an estimate of Fail-safe N. As shown in Figure 6, the random-effects funnel plot of standard error by Hedge’s g appears to be relatively symmetrical. Trim-and-fill analyses based on the random-effects model resulted in no imputed studies and no adjustment to effect size. The fail-safe N estimate indicated that an additional 287 studies with a mean effect size of zero would be needed to nullify the results, though this number should not be over-interpreted given that its purpose is to estimate the number of zero-effect studies necessary to bring statistical significance to zero rather than to understand how zero or low effect size studies may impact clinical relevance of the meta-analytic effect size estimate (Borenstein, 2009). As noted above, current results should be interpreted in the context of the still-existing potential for publication bias.
Figure 6.
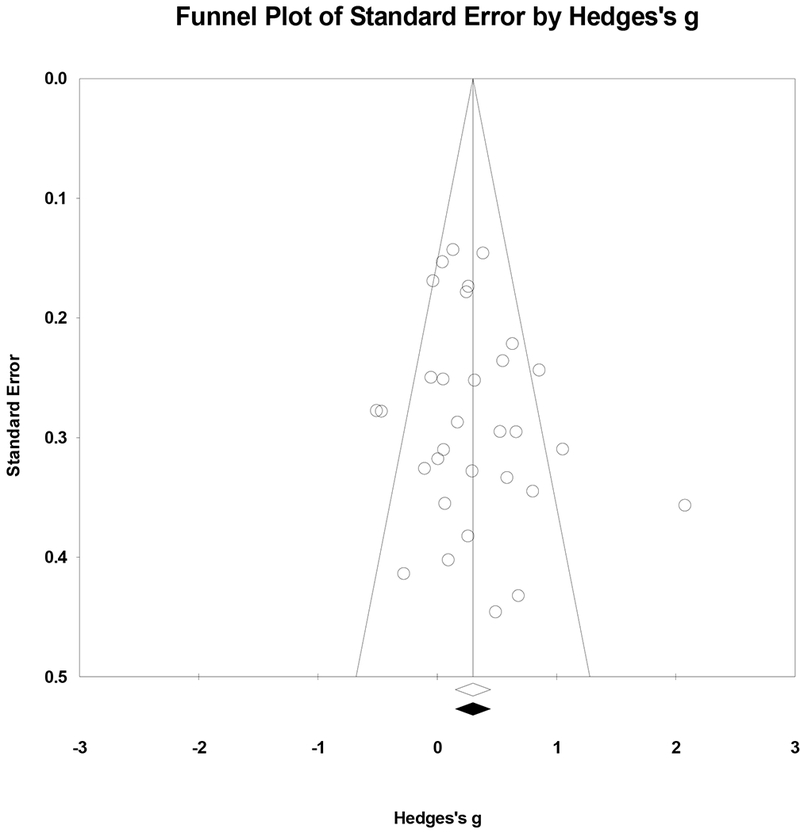
Random-effects funnel plot indicating the association between Hedge’s g and standard error in studies examining weight loss.
Study quality: Risk of bias.
Study quality was assessed using the Cochrane risk of bias tool. Approximately half of the weight loss studies described methods used for random sequence generation, resulting in a low risk of bias rating for this category. The majority of studies did not describe allocation concealment processes, resulting in an unclear selection bias rating in this category for many studies. Nearly half of the studies were classified as high risk for performance bias. As referenced above, the nature of psychological intervention studies often increases the risk of potential performance bias. Most studies were rated as low risk for detection bias because of the objective nature of weight as an outcome measure; self-report weight measures were rated as unclear. The majority of studies were classified as low risk for attrition bias, as most studies had a comparable dropout rate for treatment and control groups, and many studies reported no significant differences on relevant baseline variables between drop-outs and treatment completers. Additionally, numerous studies reported on ITT samples. The majority of studies appeared to report on the outcomes they planned to measure and were therefore rated as low risk of reporting bias.
There were a variety of other study characteristics and methodological properties that may have presented a risk of bias, which resulted in numerous studies receiving an unclear risk of bias rating for the “other risk of bias” category. Notably, participants of the Mantzios (2015) study were Greek soldiers who typically chose to eat a fairly regimented military meal plan, though this was not required. Although controls were provided the same meal plan, this study characteristic is notable given that the study resulted in the largest overall effect size. Finally, as with the smoking cessation studies referenced above, all unpublished studies were rated as unclear risk of bias for this category as they were not subjected to the peer review process. Overall results for risk of bias assessments are depicted in Figure 7.
Figure 7.
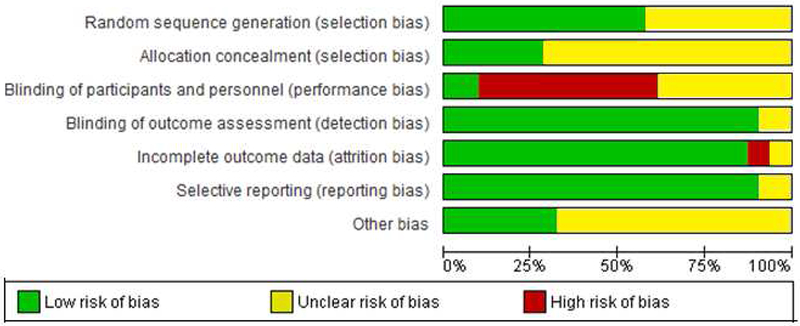
Risk of bias assessment for weight loss studies
Discussion
Acceptance- and mindfulness-based interventions aim to alter the way an individual relates to internal experiences. Instead of trying to avoid or get rid of unpleasant internal experiences, these interventions attempt to cultivate an open and nonjudgmental awareness and acceptance of thoughts, feelings, physical sensations, and urges in an effort to promote greater behavioral flexibility. Theoretically, these interventions are well-suited for health behavior change efforts. Specifically, smoking cessation and weight loss require a capacity to respond to long-term contingencies associated with improved health rather than responding to short-term contingencies prompted by an internal urge or craving. The present review and meta-analyses aimed to provide updated quantitative synthesizes of the existing evidence for the utility of acceptance- and mindfulness-based interventions for the important public health outcomes of smoking cessation and weight loss. It is our hope that the review has important practical utility for healthcare and behavioral health professionals.
Smoking Cessation
The meta-analytic results indicated a non-significant effect favoring acceptance- and mindfulness-based interventions over control conditions for smoking cessation outcomes (OR = 1.13). Notably, the majority of studies included in the current smoking cessation meta-analysis compared an acceptance- or mindfulness-based intervention to an active control, many of which were commonly implemented, empirically supported intervention options (e.g., CBT, nicotine replacement therapy, standard behavioral treatment, Smokefree.gov). These results indicate that acceptance- and mindfulness-based interventions are likely as efficacious as many commonly used and well-supported smoking cessation treatment options. Though the evidence herein does not support incremental utility for acceptance- and mindfulness-based interventions over and above these existing treatments, it is practically useful for behavioral health providers to have multiple treatment approaches in their repertoire when working with patients.
There was substantial heterogeneity in effect size across studies, and subgroup and meta-regression analyses identified only one significant moderating variable (intention-to-treat [ITT] vs. not). The finding that studies reporting on a full ITT sample resulted in lower effect sizes is not surprising, as missing data is often classified as “smoking” in these cases. Thus, dropouts (even if similar in both groups) could make it more difficult to detect a significant effect.
Though the current findings are generally in line with previous reviews indicating support for mindfulness-based (de Souza et al., 2015; Li et al., 2017; Maglione et al., 2017; Oikonomou et al., 2016) or ACT-based (Lee et al., 2015) interventions individually for smoking cessation outcomes, the non-significant meta-analytic OR effect size (1.13) indicated in the current results is slightly lower than those indicated in previous reviews. This may be due to the addition of two recently-published studies: Bricker et al. (2018) and McClure et al. (2019) which included 2,637 and 450 participants respectively, compared ACT-based interventions to strong control conditions (Smokefree.gov and CBT), and used ITT analyses. The Odds Ratios reported in these studies (0.89 and 0.44) were weighted heavily in the current meta-analysis. Finally, the present results are smaller than the significant effects found in meta-analyses examining various widely accepted treatments for smoking cessation, including pharmacotherapy. For example, Heckman et al. (2010) found significant effects for motivational interviewing over controls for smoking cessation (OR = 1.45, 95% CI = 1.14, 1.83). Additionally, Wu et al. (2006) found significant effects for nicotine replacement therapy (NRT) vs. controls at 3-months (OR = 1.98, 95% CI = 1.77, 2.21) and 1-year (OR = 1.71, 95% CI = 1.55, 188), and Eisenberg et al. (2008) found significant effects for various forms of pharmacotherapy (OR = 1.71-2.41).
Weight Loss
The results of the weight loss meta-analysis indicated a small significant effect for acceptance-and mindfulness-based interventions over controls (Hedge’s g = 0.30). Notably, a minority of the individual included studies reported a statistically significant effect for acceptance- or mindfulness-based interventions over controls, yet the overall aggregate effect was significant. Meta-analyses provide improved power and precision when summarizing effects. In this case, meta-analytic techniques may have been particularly valuable to summarize an effect given the early stage of the literature which led to the inclusion of trials with smaller sample sizes that may have resulted in larger confidence intervals and a diminished ability to detect significant effects.
There was significant heterogeneity across studies. Meta-regression analyses indicated that sample age and sample baseline BMI moderated the effect such that younger sample size and lower baseline sample BMI were associated with larger effects. It may be that younger samples and those with lower BMIs may have more potential to benefit from acceptance- and mindfulness-based strategies as behavioral patterns may not yet be as solidly entrenched as those of older age or greater baseline BMI. Biological explanations for these differences may also be sensible (e.g., it may be more difficult for older individuals or those with a higher BMI to lose weight). Additionally, subgroup analyses indicated that studies conducted outside of the United States demonstrated larger effect sizes than those conducted in the United States. The finding that effect sizes for studies conducted outside of the United States were larger than those conducted in the United States is not unprecedented in that it aligns with a meta-analysis by Öst (2014) examining the efficacy of ACT. The Öst review found that studies conducted in the European Union resulted in larger effects than studies conducted in the United States and other countries. Finally, an effect approaching significance indicated that shorter interventions may be associated with larger effect size outcomes. Though this was unexpected, previous literature has suggested that briefer psychotherapy may result in greater initial improvement in psychological symptoms than longer-term therapy (Knekt et al., 2008) and that gains in therapy may not be best represented by a continuous and linear dose-response relationship (Howard, Kopta, Krause, & Orlinsky, 1986). It may also be that interactions with other potential sample and study characteristics influenced this outcome.
Additional exploratory meta-regression analyses examining a model including both age and baseline BMI indicated that BMI was likely the stronger moderating variable. Another meta-regression model including both country and baseline BMI as moderators indicated that country was no longer a significant moderator. Thus, it is possible that the moderation findings were being driven primarily by baseline BMI status. It is possible that individuals with a higher BMI have a greater difficulty with distress tolerance and delayed gratification or may have more solidly entrenched behavioral patterns. This may result in greater difficulty successfully implementing acceptance- or mindfulness-based strategies, leading to a lower magnitude of the effect for these interventions, especially over and above other active control comparisons. Alternatively, it could be hypothesized that those with a higher BMI are more likely to benefit from any intervention in general, but do not differentially benefit from acceptance- and mindfulness-based strategies (i.e., they benefit similarly when engaged in an active control comparison condition).
Finally, previous reviews and meta-analyses examining acceptance- and mindfulness-based interventions for weight loss and associated behaviors have been somewhat mixed and have generally reported substantial heterogeneity. Additionally, quantitative syntheses to date have been limited in the examination of the efficacy of these interventions vs. controls. Rogers et al. (2017) reported small, but significant effects on BMI change for acceptance- and mindfulness-based interventions (Hedge’s g = 0.43 for RCTs), while Ruffault and colleagues reported non-significant effects for these interventions compared to controls (Mean Difference = −0.15kg/mg2). Similarly, Carrière and colleagues (2018) reported small, but significant effects on weight loss for mindfulness-based interventions when including only controlled studies (Hedge’s g = 0.35). The results of the present meta-analysis therefore align with the gradually building evidence for the efficacy of these interventions and specifically contribute to the field’s understanding of the efficacy of these approaches in comparison to controls. Notably, the present meta-analytic results demonstrated a smaller overall effect size (Hedge’s g = .30) for acceptance- and mindfulness-based interventions compared to controls than the medium effect size (standardized mean difference = 0.51) reported in a meta-analysis by Armstrong et al. (2011) which compared motivational interviewing interventions to controls. The Armstrong meta-analysis, however, included mostly non-active controls, whereas the present meta-analysis included a majority of RCTs that utilized an active control group.
Clinical and Research Implications
Given that smoking and overweight are two leading causes of death in the US, the current meta-analyses have important research, clinical, and public health implications. Results are promising for acceptance- and mindfulness-based interventions, as they demonstrate that these approaches appear to be at least as efficacious as active treatment comparisons. Given the difficulty of quitting smoking or losing weight, behavioral health professionals significantly benefit from having a variety of evidence-based strategies at their disposal. Acceptance- and mindfulness-based approaches provide clinicians with another intervention strategy that can be implemented with reasonable confidence. Moreover, these interventions have the potential to be delivered in a group format (allowing for efficiency within a primary care or behavioral health setting) or remotely (potentially allowing both greater access to care and reduced cost). Given the magnitude of the public health impact associated with smoking and overweight, any improvements upon standard approaches could have a substantial influence on health outcomes and cost savings at a societal level.
From a clinical research perspective, future work may benefit from further exploration of the potential functional processes underlying topographically different health behaviors (e.g., smoking, weight loss). For example, experiential avoidance, defined as any attempt to alter or change unpleasant internal experiences, such as thoughts, emotions, bodily sensations, or urges (Hayes, Wilson, Gifford, Follette, & Strosahl, 1996), has been identified as a common process involved in the development of maladaptive behaviors associated with various forms of psychopathology (Chawla & Ostafin, 2007). Specifically, previous work has proposed that a variety of risky or problem behaviors may serve a common avoidant function and may be characteristic of a tendency to respond to short-term rather than long-term contingencies (Cooper, Wood, Orcutt, & Albino, 2003; Kingston, Clarke, & Remington, 2010; Kingston, Clarke, Ritchie, & Remington, 2011; Lewis & Naugle, 2017). Given that acceptance- and mindfulness-based therapies aim to foster a willing, nonjudgmental awareness and acceptance of internal experiences, they may be particularly useful in targeting functionally avoidant behaviors. Of particular importance to the health behaviors explored in the current review, previous research has demonstrated that smoking cessation and reductions in BMI after acceptance-based interventions are mediated by reductions in avoidance, suggesting a common clinical pathway for these behaviors that may be targeted through intervention (Gifford & Lillis, 2009). Future RCTs should continue to conduct mediation analyses to understand relevant functional processes that may be driving behavior change outcomes.
Finally, many of the trials included in the current review had relatively small sample sizes that may have limited power to detect effects, and thus, findings may be considered exploratory. Future research would benefit from larger RCTs with more demographically diverse populations. Both smoking cessation and weight loss studies demonstrated substantial heterogeneity, indicating that future work examining moderators of treatment outcome will be important in order to best match treatment strategies to individual participant characteristics.
Limitations
Although the present meta-analyses provide important quantitative syntheses, several limitations should be acknowledged. The research in this area is still young, as all studies were published after year 2000. This resulted in a limited number of studies generally, as well as in the inclusion of a number of potentially underpowered pilot studies and doctoral dissertations. These studies may not be as methodologically rigorous as larger, potentially pre-registered trials, and thus their contribution to effect size estimates based on random-effects modeling could be deemed excessive. Additionally, most of the studies were conducted in the United States and included limited ethno-racial diversity. Similarly, weight loss studies targeted primarily female participants, though overweight and obesity is also an important health issue for men. The lack of diversity in demographic features may limit claims of generalizability. Further, though the inclusion/exclusion criteria were developed in an attempt to limit heterogeneity, strict criteria can also result in a lack of generalizability to other populations. In addition, the analyses conducted varied between studies, with some studies reporting on intention-to-treat samples and others reporting on study completers only. Individual studies also accounted for different covariates (or no covariates) in analyses.
Outcomes examined were those reported closest to one month post-intervention. Although this was an attempt to obtain similar follow-up time points, there was still variability in the time points examined, though length to follow-up did not appear to moderate effect size outcomes. Previous research has indicated that the benefits of acceptance-based interventions over controls may be more prominent over time (Arch et al., 2012; Lee et al., 2015; Luoma, Kohlenberg, Hayes, & Fletcher, 2012). This theorized “sleeper effect” hypothesizes that these interventions promote flexibility and build skills that can be useful in addressing challenges and persisting in values-based behavior even after interventions are complete (Lee et al., 2015; Lillis et al., 2016). Several studies included in the current review reported only a post-treatment follow-up measure, however, multiple studies also included longer-term follow-up measures, and some demonstrated results that may align with the theorized effect (e.g., Gifford et al., 2004; Lillis et al., 2016). Given the importance of maintaining outcomes, future research should examine outcomes at more distant follow-ups to further our understanding of the incremental benefits of these interventions in the long-term.
With regard to study quality, the majority of studies were rated as either low or unclear risk of bias across most categories using the Cochrane tool. The greatest risk of bias was performance bias. With these types of trials, participants are often aware of their group assignment, particularly if the trial compares the intervention to a wait-list control. Finally, as noted, while acceptance- and mindfulness-based interventions target hypothesized mechanisms of change (e.g., acceptance, mindfulness), a minority of studies conducted formal mediation analyses to examine these process-based mechanisms. Thus, future research could benefit from larger trials that would accommodate formal mediation analyses to identify specific mechanisms of change.
Conclusion
In conclusion, the results of the current review indicate that acceptance- and mindfulness-based interventions show promise for the important behavioral health outcomes of smoking cessation and weight loss. Although somewhat exploratory in nature given the relative infancy of the literature, the meta-analyses found effect sizes indicating that these interventions are at least as efficacious as active controls for these outcomes. Given that smoking and overweight are two leading causes of mortality in the United States, these findings have important clinical and research implications. Further work is needed to deepen the understanding of how, why, and in what contexts these interventions are efficacious. Future research will benefit from high-quality RCTs of diverse samples that examine mediators, moderators, and maintenance of change in outcome variables.
Highlights.
Problematic health behaviors are risk factors for adverse health outcomes
Acceptance- and mindfulness- interventions may be efficacious for smoking and weight
Meta-analyses show these interventions are similar to established control conditions
Current meta-analyses have important clinical and public health implications
Acknowledgments:
Many thanks to Sydney K. Kroska (S.K.K.) for her hard work on study coding. Thanks also to Jennifer DeBerg, health sciences librarian, for helping to develop search strategies. Finally, thanks to Dr. Michael W. O’Hara, Dr. Marin Schweizer, Dr. Kristian Markon, Dr. Mark Vander Weg, Dr. Ernest O’Boyle, and Dr. Michelle L. Miller for their guidance and support throughout the development and execution of this project.
Funding: This work was supported in part by the National Institute of Health T32 pre-doctoral training grant: T32GM108540. The NIH had no role in the study design, collection, analysis, or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication.
Appendix A
PubMed search strategy:
(((Dialectical[Title/Abstract] OR Mindfulness[Title/Abstract] OR Meditation[Title/Abstract] OR acceptance[Title/Abstract] OR MBSR[Title/Abstract] OR MBCT[Title/Abstract] OR DBT[Title/Abstract]) AND (smoking[Title/Abstract] OR smoke[Title/Abstract] OR smoker[Title/Abstract] OR smokers[Title/Abstract] OR nicotine[Title/Abstract] OR exercise[Title/Abstract] OR tobacco[Title/Abstract] OR weight[Title/Abstract] OR obesity[Title/Abstract] OR cigarette[Title/Abstract] OR cigarettes[Title/Abstract] OR body mass index[Title/Abstract] OR BMI[Title/Abstract])) OR ((“Smoking Cessation”[Mesh] OR “Smoking”[Mesh] OR “Tobacco Products” [Mesh] OR “Tobacco Use Disorder”[Mesh] OR “Tobacco Use Cessation”[Mesh] OR “Tobacco Use”[Mesh] AND “Weight Loss”[Mesh] OR “Obesity”[Mesh] OR “Body Mass Index”[Mesh] OR “Body Weight”[Mesh]) AND (“Acceptance and Commitment Therapy”[Mesh] OR “Meditation”[Mesh] OR “Mindfulness”[Mesh]))) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR placebo[tiab] OR “clinical trials as topic”[MeSH Terms:noexp] OR randomly[tiab] OR trial[ti]) AND English[lang]
Table A.1:
Reasons for exclusion of full-text screened articles
| Reason for Exclusion | Number Excluded |
|---|---|
| Not acceptance/mindfulness-based intervention | 34 |
| Presented same data as another study that was already included in the meta-analysis (e.g., process examination, dissertation that was subsequently published) | 27 |
| Not RCT | 32 |
| Weight loss/smoking cessation not reported as outcome | 30 |
| Targeted at a population with potential medical or psychological confound | 10 |
| Lab-based intervention/manipulation | 9 |
| Not dissertation, peer-reviewed publication, or registered on ClinicalTrials.gov (i.e., thesis) | 5 |
| Not framed as smoking cessation intervention (for smoking studies) | 2 |
| Not acceptance/mindfulness vs. control | 2 |
| Study design only | 1 |
| Could not access | 1 |
| =153 excluded | |
Note. RCT = Randomized Controlled Trial
Table A.2:
Characteristics of smoking cessation studies
| Study | Population | Intervention n analyzed/ Total N analyzed; ITT or Not | Mean age | % male | Baseline cigarettes/day | Intervention | Comparison group | Follow-up time (months) | Country |
|---|---|---|---|---|---|---|---|---|---|
| Brewer_2011 | Treatment-seeking, nicotine-dependent adults | 33/71 Not |
45.9 | 62.1% | 20 | 4-week Mindfulness Training for smoking cessation group | 4-week American Lung Association’s Freedom from smoking group treatment | 0 months | USA |
| Bricker_2013 | Adult smokers interested in quitting | 57/115 Not |
45.05 | 38% | Not reported | 3-month web-delivered ACT intervention | 3-month web-delivered National Cancer Institute Smokefree.gov | 0 months | USA |
| Bricker_2014a | South Carolina State Quitline callers who were adult smokers | 59/121 ITT |
39.08 | 31% | Not reported | Telephone-based ACT intervention for smoking cessation (5 sessions, total length not reported) + 2 weeks NRT | Telephone-delivered CBT-based intervention delivered through South Carolina Quiteline (5 sessions, total length not reported) + 2 weeks NRT | 6 months post-randomization (unclear how long after intervention) | USA |
| Bricker_2014b | Adult smokers interested in learning skills to quit smoking | 80/164 Not |
41.55 | 48% | Not reported | 8-week smartphone application ACT intervention | 8-week National Cancer Institute’s smartphone application for smoking cessation (QuitGuide) | 0 months | USA |
| Bricker_2018 | Adults who smoked at least 5 cigarettes per day | 1,319/2,637 ITT |
46.2 | 21% | Not reported | 12-month web-delivered ACT intervention | 12-month web-delivered Smokefree.gov | 0 months | USA |
| Brown_2013 | Adult smokers with a history of early lapse in prior quit attempts | 27/49 ITT |
47.68 | 51% | 21.65 | 8-week distress tolerance treatment that incorporated ACT and exposure-based principles (individual and group sessions) & 8 weeks transdermal nicotine patch | 6-week Standard behavioral smoking cessation treatment (group sessions and one individual phone session) + 8 weeks transdermal nicotine patch | 0.92 months | USA |
| Brown_2018 | Adult smokers who smoked at least 10 cigarettes per day for the past 3 years | 62/116 ITT |
46.06 | 59% | 20.10 | 9-week distress tolerance treatment that incorporated ACT-based content (individual, group, & phone components) + 8 weeks transdermal nicotine patch | 9-week Standard behavioral smoking cessation treatment (individual, group, & phone components) + 8 weeks transdermal nicotine patch | 0.92 months | USA |
| Davis_2014a | Moderately low socioeconomic status smokers who report high motivation to quit | 105/196 ITT |
41.65 | 50% | 15.75 | 4-week group mindfulness training for smokers (MBSR-based) + 3 additional weeks of optional meditation groups + 4 weeks NRT | Availability of Tobacco telephone quitline + 4 weeks NRT | 0.92 months | USA |
| Davis_2014b | Smokers living in low SES areas and who report high motivation to quit | 68/135 ITT |
44.5 | 53.3% | 17.67 | 7-week group mindfulness training for smokers & 2 weeks nicotine patch | 7-week group American Lung Association’s Freedom From Smoking group program (“enhanced”) + 2 weeks nicotine patch | 0.58 months | USA |
| Garrison_2018 | Adults who smoked at least 5 cigarettes per day | 143/325 No |
41.28 | 28.30% | 16.11 | 22-day (5-15 minute session) Mobile Mindfulness Training with Experience Sampling (Craving to Quit) | 22 days of Experience Sampling only | 5.28 months | USA |
| Gifford_2004 | Self-identified nicotine-dependent smokers | 26/62 Not |
43.00 | 41% | 21.4 | 7-week ACT for smoking cessation (group and individual) | 7-weeks of NRT in addition to an education session provided by a psychiatrist | 0 months | USA |
| Gifford_2011 | Self-identified nicotine-dependent smokers | 122/212 Not |
45.99 | 41.3% | 24 | 10-week ACT and FAP-based behavioral intervention (group and individual) + slow release bupropion & medication instruction group | Slow release bupropion & medication instruction group | 0 months | USA |
| McClure_2019 | Adults who smoked at least 10 cigarettes per day | 224/450 ITT |
51.3 | 47.3% | Not reported | 5- week group ACT for smoking cessation + nicotine patch following health care system’s standard dosing protocol | 5-week group CBT for smoking cessation + nicotine patch following health care system’s standard dosing protocol | 0.23 months | USA |
| O’Connor_unpublished | Adults who smoked at least 10 cigarettes per day for the past 12 months | 50/100 ITT |
36.94 | 44% | 16.88 | 6-week group ACT for smoking cessation | 6-week group behavioral support (including core skills of Motivational Interviewing) | 0 months | Ireland |
| Schuman-Olivier_unpublished | Adults who smoked at least 10 cigarettes per day and CO > 9 | 25/54 ITT |
45 | 48.1 | 21.5 | 4-week (twice-weekly) group Mindfulness Training for smoking cessation | 4-week (twice weekly) American Lung Association’s Freedom from smoking group treatment | USA | |
| Russell_2013 | Nicotine-dependent and motivated-to-quit adult smokers | 42/79 ITT |
34.86 | 26.6% | 21.2 | 3-week group ACT for smoking abstinence | 3- week group Cognitive-Behavioral Coping Skills Training | 2.76 months | Scotland |
| Vidrine_2016 | Adult smokers motivated to quit | 154/309 ITT |
48.60 | 45.60% | 20.10 | 8-week group Mindfulness-Based Addiction Treatment for smoking cessation (modeled on MBCT) + nicotine patch therapy (adjusted to fit number of cigarettes per day) | 8-week group CBT for smoking cessation + naticotine patch therapy (adjusted to fit number of cigarettes per day) | 0.23 months | USA |
Note. USA = United States of America; US = United States; ACT = Acceptance and Commitment Therapy; MBSR = Mindfulness-Based Stress Reduction. MBCT = Mindfulness-Based Cognitive Therapy; CBT = Cognitive Behavioral Therapy; FAP = Functional Analytic Psychotherapy; NRT = Nicotine Replacement Therapy; SES = Socioeconomic Status; ITT = Intention to Treat.
Table A.3:
Characteristics of weight loss studies
| Study | Population | Intervention n analyzed/total N analyzed ITT or Not | Mean Age | % male | Baseline BMI | Intervention | Comparison Group | Follow-up time (in months) | Country |
|---|---|---|---|---|---|---|---|---|---|
| Alberts_2010 | Overweight and obese Dutch adults participating in a dietary group treatment for overweight | 10/19 ITT |
51.88 | 10.53% | 31.3 | 7-week manual-based acceptance and mindfulness-based training in combination with a 10-week dietary group treatment for overweight | 10-week dietary group treatment for overweight | 0 months | Netherlands |
| Alberts_2012 | Adult Dutch women reporting problematic eating behavior | 12/26 ITT |
48.50 | 0% | 32.7 | 8-week group MBCT-based intervention for problematic eating | Wait-list control | 0 months | Netherlands |
| Blevins_2008 | Overweight US college-age women | 12/23 Not |
21.00 | 0% | 30 | 8-week group MBSR-based mindfulness intervention adjusted for eating in combination with a standard behavioral group treatment | 8-week Standard behavioral group treatment | 0 months | USA |
| Butryn_2017 | Overweight and obese adults | 77/170 Not |
53.32 | 20.38% | 35.30 | 12-month group Acceptance-based behavior therapy + environmental change | 12-month group Behavior therapy + environmental change | 0 months | USA |
| Carpenter_2017 | Overweight and obese US adults reporting high emotional eating | 45/69 Not |
47.3 | 8% | 31.5 | 6-month, 11-session phone-based Mind Your Weight program (weight loss program with mindfulness addition) | 6-month, 11-session phone-based standard Weight Talk program | 0 months | USA |
| Corsica_2014 | US adults who reported a high level of stress and were at high risk for weight gain/obesity and reported problematic eating behaviors | 10/22 Not |
45.4 | 2% | 35 | 6-week MBSR intervention adjusted to eating | 6-week Cognitive behavioral and exposure-based stress-eating intervention | 1.38 months | USA |
| Daubenmier_2011 | Overweight and obese US women who reported being stressed | 24/47 ITT |
40.89 | 0% | 31.09 | 4-month group MBSR, MBCT, and MB-EAT-based intervention for stress eating and one 2-hour nutrition and exercise information session | 2-hour nutrition and exercise information session | 0 months | USA |
| Daubenmier_2016 | Obese US adults | 100/194 ITT |
47.49 | 17.61% | 35.50 | 5.5-month group MBEAT-based mindfulness intervention combined with diet and exercise guidelines | 5.5 month group diet and exercise program | 0.5 months | NR (USA imputed) |
| Fletcher_2011 | US adults who were currently or had previously enrolled in a weight-loss program | 29/62 Not |
53.37 | 17.7% | 35.45 | 1-day group ACT-based intervention | Wait-list control | 3 | USA |
| Frayn_unpublilshed | Overweight or obese Canadian adults considered to be emotional eaters | 19/40 Not |
46.91 | 7.6% | Not Reported | 8-session (5-10 minutes per session) ACT-based intervention delivered by physician in weight loss clinic (16.3 weeks on average for both conditions combined, range from 7-<50) | 8-session (5-10 minutes per session) standard diet and exercise counseling and psychoeducation delivered by physician in weight loss clinic (16.3 weeks on average for both conditions combined, range from 7-<50) | 0 months | Canada |
| Forman_2013 | Overweight and obese US adults | 74/128 ITT |
45.69 | NR | 34.1 | 40-week group Acceptance-based behavioral treatment | 40-week group Standard behavioral treatment | 0 months | USA |
| Forman_2016 | Overweight and obese US adults | 100/190 ITT |
51.64 | 17.9% | 36.93 | 1-year group Acceptance-based behavioral | 1-year group Standard behavioral group treatment | 0 months | USA |
| Frisvold_2009 | US women nurses | 20/38 Not |
48.35 | 0% | 31 | 8-week MBSR intervention followed by 8-week diet and exercise program | 8-week group perimenopausal education followed by 8-week diet and exercise program | 1.84 months after MBSR intervention (post-diet and exercise program) | USA |
| Katterman_2014 | US undergraduate or graduate student women | 22/47 Not |
22.35 | 0% | 26.63 | 16-week group acceptance-based behavioral intervention | Assessment-only control | 0 months | USA |
| Lillis_2009 | US adults who had completed at least 6-months of a weight-loss program | 40/84 Not |
50.80 | 9.71% | 33.02 | 1-day group ACT-based intervention | Wait-list control | 3 | USA |
| Lillis_2016 | US adults with overweight or obesity and reporting high internal disinhibition | 68/138 Not |
50.20 | 12% | 37.6 | 1-year group Acceptance-based behavioral intervention | 1-year group Standard behavioral treatment | 0 months | USA |
| Mantzios_2014 | Greek college undergraduates | 36/72 Not |
21.11 | 58.33% | 25.55 | 5-week mindful concrete construal diary | 5-week abstract construal diary | 0 months | Greece |
| Mantzios_2015 | Greek soldiers | 19/49 Not |
22.28 | Not reported | 26.47 | 5-week mindfulness meditation intervention with group and individual practice components | Brief psychoeducation on weight loss | 0 months | Greece |
| Martin_2017 | Overweight or obese US adults | 17/36 Not |
40.75 | 26.31 | 32.53 | 4-hour Mindful Decision-Making group workshop for weight loss + 2- & 4-week 1-hour booster sessions | 4-hour Standard behavioral group workshop for weight loss + 2- & 4-week 1-hour booster sessions | 0.46 months | USA |
| Miller_2012 | Overweight or obese US adults with Type 2 diabetes but no insulin therapy | 27/52 Not |
53.95 | 36.52% | 36.14 | 3-month group MB-EAT-based mindfulness intervention + 1 & 3-month follow-up sessions (6 months total) | 3-month Diabetes group self-management education (Smart Choices) + 1 & 3-month follow-up sessions | 0 months (i.e. post-final booster session) | USA |
| Palmeira_2017 | Overweight or obese Portuguese adult women enrolled in nutritional treatment for weight loss in primary care units | 36/73 ITT |
42.36 | 0% | 34.23 | 3.5 month group mindfulness, ACT, and compassion-based intervention | Treatment as usual (medical and nutritional appointments) | 0 months | Portugal |
| Parswani_2013 | Indian adult males with coronary heart disease | 15/30 Not |
48.94 | 100% | 24.99 | 8-week individual MBSR intervention + health education session | Treatment as usual (routine cardiac care + health education session) | 0 months | India |
| Potts_2018 | Overweight or obese US adults who reported experiencing weight self-stigma | 10/21 Not |
40.07 | 6.65% | 37.03 | 8-week ACT-based guided self-help with phone prompting | Waitlist | 0 months | USA |
| Raja-Khan_2017 | Overweight or obese US adult women | 31/53 Not |
44.5 | 0% | 38.9 | 8-week group MBSR intervention | 8-week group health education intervention | 1.84 | USA |
| Richards_2015 | Overweight or obese US young adults | 19/36 Not |
22.8 | 25% | 32.84 | 4-week group ACT-based intervention + brief psychoeducational supplement | Brief psychoeducational supplement | 0 months | USA |
| Sairanen_2017 | Overweight or obese Finnish adults reporting symptoms of perceived psychological distress | 64/132 Not |
49.5 | 15.5% | 31.10 | 8-week group ACT-based intervention | Assessment-only control | 0 months | Finland |
| Schnepper_2019 | Austrian adults motivated to improve eating behavior or lose weight | 23/46 ITT |
35.45 | 26.1% | 27.7 | 8-week, 4-session (2 group, 2 individual) Mindfulness & Prolonged Chewing Intervention | Waitlist control | 0 months | Austria |
| Smith_2018 | Obese post-menopausal US women | 18/36 Not |
58.46 | 0% | 36.46 | 6-week group MEAL intervention + monthly refresher sessions | 6-week group active control intervention with relevant health professionals + monthly refresher sessions | 0 months | USA |
| Spadaro_2017 | Overweight and obese US adults | 22/46 Not |
45.2 | 13% | 32.5 | 6-month group Standard behavioral weight loss program + Mindfulness meditation | 6-month group Standard behavioral weight loss program | 0 months | USA |
| Tapper_2009 | UK adult women attempting to lose weight | 31/62 ITT |
41 | 0% | 31.57 | 3-week group ACT-based intervention + follow-up session 3 months later (3.69 months total) | Assessment-only control | 3 | UK |
| Timmerman_2012 | Healthy perimenopausal US women who ate out frequently | 19/35 Not |
49.6 | 0% | 31.8 | 6-week group mindful restaurant eating intervention | Wait-list control | 0 months | USA |
Note. USA = United States of America; US = United States; UK = United Kingdom; ACT = Acceptance and Commitment Therapy; MBSR = Mindfulness-Based Stress Reduction; MBCT = Mindfulness-Based Cognitive Therapy; MB-EAT = Mindfulness-Based Eating Awareness Training; MEAL = Mindful Eating And Living ITT = Intention to Treat. Daubenmier et al., 2016 neglected to report the location in which the study was carried out, however, Daubenmier et al., 2011 was conducted in the United States, and we therefore classified Daubenmier et al., 2016 as having been conducted in the United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: none.
Note: We used change in weight data from the dissertation of Spadaro et al., 2018 as this data was most comparable to data used from other studies.
References
References marked with an asterisk indicate studies included in the meta-analysis.
- Abraham C, & Michie S (2008). A taxonomy of behavior change techniques used in interventions. Health Psychology, 27(3), 379–387. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Eifert GH, Davies C, Vilardaga JCP, Rose RD, & Craske MG (2012). Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. Journal of Consulting and Clinical Psychology, 80(5), 750–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Alberts HJ, Mulkens S, Smeets M, & Thewissen R (2010). Coping with food cravings. Investigating the potential of a mindfulness-based intervention. Appetite, 55(1), 160–163. [DOI] [PubMed] [Google Scholar]
- *Alberts HJ, Thewissen R, & Raes L (2012). Dealing with problematic eating behaviour. The effects of a mindfulness-based intervention on eating behaviour, food cravings, dichotomous thinking and body image concern. Appetite, 58(3), 847–851. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, & Hemmelgarn BR (2011). Motivational interviewing to improve weight loss in overweight and/or obese patients: A systematic review and meta-analysis of randomized controlled trials. Obesity Reviews, 12(9), 709–723. [DOI] [PubMed] [Google Scholar]
- Babb S, Malarcher A, Schauer G, Asman K, & Jamal A (2017). Quitting smoking among adults – United States, 2000-2015. MMWR. Morbidity and Mortality Weekly Report, 65, 1457–1464. [DOI] [PubMed] [Google Scholar]
- Baer RA (Ed.). (2015). Mindfulness-based treatment approaches: Clinician’s guide to evidence base and applications. Elsevier. [Google Scholar]
- Berkel LA, Poston WSC, Reeves RS, & Foreyt JP (2005). Behavioral interventions for obesity. Journal of the American Dietetic Association, 105(5), 35–43. [DOI] [PubMed] [Google Scholar]
- *Blevins NC (2008). Mindfulness meditation as an intervention for body image and weight management in college women: A pilot study (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses Global. (UMI No. 3334447). [Google Scholar]
- Borenstein M, Hedges LV, Higgins J, & Rothstein HR (2009). Introduction to meta-analysis. John Wiley & Sons, Ltd. [Google Scholar]
- *Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, … & Carroll KM (2011). Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug and Alcohol Dependence, 119(1), 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bricker JB, Bush T, Zbikowski SM, Mercer LD, & Heffner JL (2014a). Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: A pilot study. Nicotine & Tobacco Research, 16(11), 1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bricker JB, Mull KE, Kientz JA, Vilardaga R, Mercer LD, Akioka KJ, & Heffner JL (2014b). Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug and Alcohol Dependence, 143, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bricker JB, Mull KE, McClure JB, Watson NL, & Heffner JL (2018). Improving quit rates of web-delivered interventions for smoking cessation: Full scale randomized trial of WebQuit.org versus Smokefree.gov. Addiction, 113(5), 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bricker J, Wyszynski C, Comstock B, & Heffner JL (2013). Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine & Tobacco Research, 15(10), 1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Brown RA, Palm Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, … & Hayes SC (2018). A randomized controlled trial of distress tolerance treatment for smoking cessation. Psychology of Addictive Behaviors, 32(4), 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Brown RA, Reed KMP, Bloom EL, Minami H, Strong DR, Lejuez CW, … & Hayes SC (2013). Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine & Tobacco Research, 15(12), 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Forman E, Hoffman K, Shaw J, & Juarascio A (2011). A pilot study of acceptance and commitment therapy for promotion of physical activity. Journal of Physical Activity and Health, 8(4), 516–522. [DOI] [PubMed] [Google Scholar]
- *Butryn ML, Forman EM, Lowe MR, Gorin AA, Zhang F, & Schaumberg K (2017). Efficacy of environmental and acceptancebased enhancements to behavioral weight loss treatment: The ENACT trial. Obesity, 25(5), 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Webb V, & Wadden TA (2011). Behavioral treatment of obesity. The Psychiatric Clinics of North America, 34(4), 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Carpenter KM, Vickerman KA, Salmon EE, Javitz HS, Epel ES, & Lovejoy JC (2017). A randomized pilot study of a phone-based mindfulness and weight loss program. Behavioral Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière K, Khoury B, Günak MM, & Knäuper B (2018). Mindfulness-based interventions for weight loss: A systematic review and meta-analysis. Obesity Reviews, 19(2), 164–177. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Adult Obesity Causes & Consequences. (2017a). https://www.cdc.gov/obesity/adult/causes.html/ Accessed 18 Nov 2017.
- Centers for Disease Control and Prevention. Smoking and Tobacco Use, Fast Facts. (2017b). https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm Accessed 17 Feb 2018.
- Chawla N, & Ostafin B (2007). Experiential avoidance as a functional dimensional approach to psychopathology: An empirical review. Journal of Clinical Psychology, 63(9), 871–890. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Comprehensive Meta-Analysis (Version 3) [Computer Software]. Englwood, NJ: Biostat; Available at https://www.meta-analysis.com/. [Google Scholar]
- Cooper ML, Wood PK, Orcutt HK, & Albino A (2003). Personality and the predisposition to engage in risky or problem behaviors during adolescence. Journal of Personality and Social Psychology, 84(2), 390–410. [DOI] [PubMed] [Google Scholar]
- *Corsica J, Hood MM, Katterman S, Kleinman B, & Ivan I (2014). Development of a novel mindfulness and cognitive behavioral intervention for stress-eating: A comparative pilot study. Eating Behaviors, 15(4), 694–699. [DOI] [PubMed] [Google Scholar]
- *Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, … & Epel E (2011). Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: An exploratory randomized controlled study. Journal of Obesity, 2011, Article ID 651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Daubenmier J, Moran PJ, Kristeller J, Acree M, Bacchetti P, Kemeny ME, … & Milush JM (2016). Effects of a mindfulness-based weight loss intervention in adults with obesity: A randomized clinical trial. Obesity, 24(4), 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, & Baker TB (2014a). Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Substance Use & Misuse, 49(5), 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Davis JM, Manley AR, Goldberg SB, Smith SS, & Jorenby DE (2014b). Randomized trial comparing mindfulness training for smokers to a matched control. Journal of Substance Abuse Treatment, 47(3), 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza ICW, de Barros VV, Gomide HP, Miranda TCM, de Paula Menezes V, Kozasa EH, & Noto AR (2015). Mindfulness-based interventions for the treatment of smoking: A systematic literature review. The Journal of Alternative and Complementary Medicine, 21(3), 129–140. [DOI] [PubMed] [Google Scholar]
- Duval S, & Tweedie R (2000). Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta- analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, … & Pilote L (2008). Pharmacotherapies for smoking cessation: A meta-analysis of randomized controlled trials. Canadian Medical Association Journal, 179(2), 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Fletcher L (2011). A mindfulness and acceptance-based intervention for increasing physical activity and reducing obesity (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses Global. (UMI No. 3490761). [Google Scholar]
- *Forman EM, Butryn ML, Juarascio AS, Bradley LE, Lowe MR, Herbert JD, & Shaw JA (2013). The mind your health project: A randomized controlled trial of an innovative behavioral treatment for obesity. Obesity, 21(6), 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Forman EM, Butryn ML, Manasse SM, Crosby RD, Goldstein SP, Wyckoff EP, & Thomas JG (2016). Acceptance-based versus standard behavioral treatment for obesity: Results from the mind your health randomized controlled trial. Obesity, 24(10), 2050–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Frayn M, Carrière K, & Knäuper B (personal communication). An ACT-based physician-delivered weight loss intervention: A pilot RCT demonstrates limits to feasibility.
- *Frisvold MH (2009). The “Midlife Study” mindfulness as an intervention to change health behaviors in midlife women (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses Global. (UMI No. 3389316). [Google Scholar]
- *Garrison KA, Pal P, O’Malley SS, Pittman BP, Gueorguieva R, Rojiani R, … & Brewer JA (2018). Craving to quit: A randomized controlled trial of smartphone app–based mindfulness training for smoking cessation. Nicotine & Tobacco Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, & Palm KM (2004). Acceptance-based treatment for smoking cessation. Behavior Therapy, 35(4), 689–705. [DOI] [PubMed] [Google Scholar]
- *Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, & Palm KM (2011). Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy, 42(4), 700–715. [DOI] [PubMed] [Google Scholar]
- Gifford EV, & Lillis J (2009). Avoidance and inflexibility as a common clinical pathway in obesity and smoking treatment. Journal of Health Psychology, 14(7), 992–996. [DOI] [PubMed] [Google Scholar]
- Gregg JA, Callaghan GM, Hayes SC, & Glenn-Lawson JL (2007). Improving diabetes self-management through acceptance, mindfulness, and values: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 75(2), 336–343. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, & Wilson KG (1999). Acceptance and commitment therapy: An experiential approach to behavior change. Guilford Press. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Bissett R, Piasecki M, Batten SV, … & Gregg J (2004). A preliminary trial of twelve-step facilitation and acceptance and commitment therapy with polysubstance-abusing methadone-maintained opiate addicts. Behavior Therapy, 35(4), 667–688. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, & Strosahl K (1996). Experiential avoidance and behavioral disorders: A functional dimensional approach to diagnosis and treatment. Journal of Consulting and Clinical Psychology, 64(6), 1152–1168. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Egleston BL, & Hofmann MT (2010). Efficacy of motivational interviewing for smoking cessation: A systematic review and meta-analysis. Tobacco Control, 19(5), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, … & Sterne JA (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. British Medical Journal, 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, & Oh D (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard KI, Kopta SM, Krause MS, & Orlinsky DE (1986). The dose–effect relationship in psychotherapy. American psychologist, 41(2), 159. [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, & Graffunder CM (2016). Current cigarette smoking among adults—United States, 2005-2015. MMWR. Morbidity and Mortality Weekly Report, 65, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Johnsen TJ, & Friborg O (2015). The effects of cognitive behavioral therapy as an antidepressive treatment is falling: A meta-analysis. Psychological Bulletin, 141(4), 747–768. [DOI] [PubMed] [Google Scholar]
- Johnson NB, Hayes LD, Brown K, Hoo EC, & Ethier KA CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005-2013. MMWR 2014:63(4): 1–27. [PubMed] [Google Scholar]
- Kabat-Zinn J, & University of Massachusetts Medical Center/Worcester. Stress Reduction Clinic. (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness: the program of the Stress Reduction Clinic at the University of Massachusetts Medical Center. New York: Dell. [Google Scholar]
- *Katterman SN, Goldstein SP, Butryn ML, Forman EM, & Lowe MR (2014a). Efficacy of an acceptance-based behavioral intervention for weight gain prevention in young adult women. Journal of Contextual Behavioral Science, 3(1), 45–50. [Google Scholar]
- Katterman SN, Kleinman BM, Hood MM, Nackers LM, & Corsica JA (2014b). Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eating Behaviors, 15(2), 197–204. [DOI] [PubMed] [Google Scholar]
- Kingston J, Clarke S, & Remington B (2010). Experiential avoidance and problem behavior: A mediational analysis. Behavior Modification, 34(2), 145–163. [DOI] [PubMed] [Google Scholar]
- Kingston J, Clarke S, Ritchie T, & Remington B (2011). Developing and validating the gcomposite measure of problem behaviorsh. Journal of Clinical Psychology, 67(7), 736–751. [DOI] [PubMed] [Google Scholar]
- Knekt P, Lindfors O, Härkanen T, Välikoski M, Virtala E, Laaksonen MA, … & Helsinki Psychotherapy Study Group. (2008). Randomized trial on the effectiveness of long-and short-term psychodynamic psychotherapy and solution-focused therapy on psychiatric symptoms during a 3-year follow-up. Psychological Medicine, 38(5), 689–703. [DOI] [PubMed] [Google Scholar]
- Lee EB, An W, Levin ME, & Twohig MP (2015). An initial meta-analysis of Acceptance and Commitment Therapy for treating substance use disorders. Drug and Alcohol Dependence, 155, 1–7. [DOI] [PubMed] [Google Scholar]
- Lewis M, & Naugle A (2017). Measuring experiential avoidance: evidence toward multidimensional predictors of trauma sequelae. Behavioral Sciences, 7(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard MO, Garland EL, McGovern P, & Lazar M (2017). Mindfulness treatment for substance misuse: A systematic review and meta-analysis. Journal of Substance Abuse Treatment, 75, 62–96. [DOI] [PubMed] [Google Scholar]
- *Lillis J, Hayes SC, Bunting K, & Masuda A (2009). Teaching acceptance and mindfulness to improve the lives of the obese: A preliminary test of a theoretical model. Annals of Behavioral Medicine, 37(1), 58–69. [DOI] [PubMed] [Google Scholar]
- *Lillis J, Niemeier HM, Thomas JG, Unick J, Ross KM, Leahey TM, … & Wing RR (2016). A randomized trial of an acceptance-based behavioral intervention for weight loss in people with high internal disinhibition. Obesity, 24(12), 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindson-Hawley N, Thompson TP, & Begh R (2015). Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No.: CD006936. DOI: 10.1002/14651858.CD006936.pub3. [DOI] [PubMed]
- Linehan M (1993). Cognitive-behavioral treatment of borderline personality disorder. Guilford Press. [Google Scholar]
- Luoma JB, Kohlenberg BS, Hayes SC, & Fletcher L (2012). Slow and steady wins the race: A randomized clinical trial of acceptance and commitment therapy targeting shame in substance use disorders. Journal of Consulting and Clinical Psychology, 80(1), 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione MA, Maher AR, Ewing B, Colaiaco B, Newberry S, Kandrack R, … & Hempel S (2017). Efficacy of mindfulness meditation for smoking cessation: A systematic review and meta-analysis. Addictive Behaviors, 69, 27–34. [DOI] [PubMed] [Google Scholar]
- *Mantzios M, & Wilson JC (2014). Making concrete construals mindful: A novel approach for developing mindfulness and self-compassion to assist weight loss. Psychology & Health, 29(4), 422–441. [DOI] [PubMed] [Google Scholar]
- *Mantzios M, & Wilson JC (2015). Exploring mindfulness and mindfulness with selfcompassion-centered interventions to assist weight loss: Theoretical considerations and preliminary results of a randomized pilot study. Mindfulness, 6(4), 824–835. [Google Scholar]
- *Martin LM, Espel-Huynh HM, Marando-Blanck S, Evans BC, Forman EM, Butryn ML, … & Herbert JD (2017). Trusting homeostatic cues versus accepting hedonic cues: A randomized controlled trial comparing two distinct mindfulness-based intervention components. Journal of Contextual Behavioral Science, 6(4), 409–417. [Google Scholar]
- *McClure JB, Bricker J, Mull K, & Heffner JL (2019). Comparative effectiveness of group-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: A randomized controlled trial. Nicotine & Tobacco Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Brennan EA, Lewin AB, Murphy TK, Small BJ, & Storch EA (2014). A meta-analysis of behavior therapy for Tourette syndrome. Journal of Psychiatric Research, 50, 106–112. [DOI] [PubMed] [Google Scholar]
- *Miller CK, Kristeller JL, Headings A, Nagaraja H, & Miser WF (2012). Comparative effectiveness of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: A pilot study. Journal of the Academy of Nutrition and Dietetics, 112(11), 1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2013). Motivational interviewing: Helping people change. Guilford Press. [Google Scholar]
- Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, … & Kasaeian A (2018). The State of US Health, 1990-2016: Burden of Diseases, Injuries, and Risk Factors Among US States. Journal of the American Medical Association, 319(14), 1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the United States, 2000. Journal of the American Medical Association, 291(10), 1238–1245. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2005). Correction: Actual causes of death in the United States, 2000. Journal of the American Medical Association, 293(3), 293–294. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Abraham J, Ali MK, Alvarado M, Atkinson C, Baddour LM, … & Bolliger I (2013). The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. Jama, 310(6), 591–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Diabetes and Digestive and Kidney Diseases. Overweight and Obesity Statistics. (2017). https://www.niddk.nih.gov/health-information/healthstatistics/overweight-obesity/ Accessed 18 November 2017.
- Niaura R (2008). Nonpharmacologic therapy for smoking cessation: Characteristics and efficacy of current approaches. The American Journal of Medicine, 121(4), S11–S19. [DOI] [PubMed] [Google Scholar]
- *O’Connor M, Whelan R, Bricker J, & McHugh L (personal communication). Randomized controlled trial of a smartphone application as an adjunct to acceptance and commitment therapy for smoking cessation.. [DOI] [PubMed]
- Oikonomou MT, Arvanitis M, & Sokolove RL (2016). Mindfulness training for smoking cessation: A meta-analysis of randomized-controlled trials. Journal of Health Psychology, 22(14), 1841–1850. [DOI] [PubMed] [Google Scholar]
- Olson KL, & Emery CF (2015). Mindfulness and weight loss: A systematic review. Psychosomatic Medicine, 77(1), 59–67. [DOI] [PubMed] [Google Scholar]
- O’Reilly GA, Cook L, Spruijt-Metz D, & Black DS (2014). Mindfulness-based interventions for obesity-related eating behaviours: A literature review. Obesity Reviews, 15(6), 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öst LG (2014). The efficacy of Acceptance and Commitment Therapy: An updated systematic review and meta-analysis. Behaviour Research and Therapy, 61, 105–121. [DOI] [PubMed] [Google Scholar]
- *Palmeira L, Pinto-Gouveia J, & Cunha M (2017). Exploring the efficacy of an acceptance, mindfulness & compassionate-based group intervention for women struggling with their weight (Kg-Free): A randomized controlled trial. Appetite, 112, 107–116. [DOI] [PubMed] [Google Scholar]
- *Parswani MJ, Sharma MP, & Iyengar SS (2013). Mindfulness-based stress reduction program in coronary heart disease: A randomized control trial. International Journal of Yoga, 6(2), 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, & Whitlock EP (2015). Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: A review of reviews for the US preventive services task force interventions for smoking cessation. Annals of Internal Medicine, 163(8), 608–621. [DOI] [PubMed] [Google Scholar]
- *Potts SA (2018). Putting weight in context: Acceptance and commitment therapy (ACT) guided self-help for weight self-stigma (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses Global. (UMI No.10845767).. [Google Scholar]
- *Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, … & Legro RS (2017). Mindfulness-based stress reduction in women with overweight or obesity: A randomized clinical trial. Obesity, 25(8), 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Richards SM (2015). Lifestyle intervention in emerging adulthood: A brief acceptance-based behavioral intervention with young adults (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses Global. (UMI No. 3712880). [Google Scholar]
- Rogers JM, Ferrari M, Mosely K, Lang CP, & Brennan L (2017). Mindfulness-based interventions for adults who are overweight or obese: A meta-analysis of physical and psychological health outcomes. Obesity Reviews, 18(1), 51–67. [DOI] [PubMed] [Google Scholar]
- Rollnick S, Miller WR, Butler C (2008). Motivational interviewing in health care: Helping patients change behavior. New York: Guilford Press. [Google Scholar]
- Ruffault A, Czernichow S, Hagger MS, Ferrand M, Erichot N, Carette C, … & Flahault C (2017). The effects of mindfulness training on weight-loss and health-related behaviours in adults with overweight and obesity: A systematic review and meta-analysis. Obesity Research & Clinical Practice, 11(5), 90–111. [DOI] [PubMed] [Google Scholar]
- *Russell C (2013). A randomised controlled study of the relative efficacy and mechanisms of action of cognitive-behavioural coping skills training (CBST) and acceptance and commitment therapy (ACT) for smoking abstinence (Doctoral dissertation). University of Strathclyde, Glasgow, Scotland, United Kingdom. [Google Scholar]
- *Sairanen E, Tolvanen A, Karhunen L, Kolehmainen M, Järvelä-Reijonen E, Lindroos S, … & Lappalainen R (2017). Psychological flexibility mediates change in intuitive eating regulation in acceptance and commitment therapy interventions. Public Health Nutrition, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders KH, Shukla AP, Igel LI, Kumar RB, & Aronne LJ (2016). Pharmacotherapy for obesity. Endocrinology and Metabolism Clinics of North America, 45(3), 521–538. [DOI] [PubMed] [Google Scholar]
- *Schnepper R, Richard A, Wilhelm FH, & Blechert J (2019). A combined mindfulness prolonged chewing intervention reduces body weight, food craving, and emotional eating. Journal of Consulting and Clinical Psychology, 87(1), 106. [DOI] [PubMed] [Google Scholar]
- *Schuman-Olivier Z, Pachas G, Hoeppner BB, & Eden Evins A (personal communication). A randomized controlled trial of mindfulness training for smokers.
- Segal ZV, Williams JMG & Teasdale JD (2002). Mindfulness-based cognitive therapy for depression A new approach to preventing relapse. New York: Guilford Press. [Google Scholar]
- Silver NC, & Dunlap WP (1987). Averaging correlation coefficients: Should Fisher’s z transformation be used?. Journal of Applied Psychology, 72(1), 146. [Google Scholar]
- Siu AL (2015). Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine, 163(8), 622–634. [DOI] [PubMed] [Google Scholar]
- *Smith BW, Shelley BM, Sloan AL, Colleran K, & Erickson K (2018). A preliminary randomized controlled trial of a mindful eating intervention for post-menopausal obese women. Mindfulness, 9(3), 836–849. [Google Scholar]
- Spadaro KC (2008). Weight loss: Exploring self-regulation through Mindfulness Meditation (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses Global. (UMI No. 3335832). [Google Scholar]
- *Spadaro KC, Davis KK, Sereika SM, Gibbs BB, Jakicic JM, & Cohen SM (2017). Effect of mindfulness meditation on short-term weight loss and eating behaviors in overweight and obese adults: a randomized controlled trial. Journal of Complementary and Integrative Medicine, 15(2). [DOI] [PubMed] [Google Scholar]
- *Tapper K, Shaw C, Ilsley J, Hill AJ, Bond FW, & Moore L (2009). Exploratory randomised controlled trial of a mindfulness-based weight loss intervention for women. Appetite, 52(2), 396–404. [DOI] [PubMed] [Google Scholar]
- *Timmerman GM, & Brown A (2012). The effect of a mindful restaurant eating intervention on weight management in women. Journal of Nutrition Education and Behavior, 44(1), 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2014). The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 17. [Google Scholar]
- *Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, … & Tindle HA (2016). Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. Journal of Consulting and Clinical Psychology, 84(9), 824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vøllestad J, Nielsen MB, & Nielsen GH (2012). Mindfulness-and acceptance-based interventions for anxiety disorders: A systematic review and meta-analysis. British Journal of Clinical Psychology, 51(3), 239–260. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Butryn ML, & Wilson C (2007). Lifestyle modification for the management of obesity. Gastroenterology, 132(6), 2226–2238. [DOI] [PubMed] [Google Scholar]
- West R, Raw M, McNeill A, Stead L, Aveyard P, Bitton J, … & Borland R (2015). Health-care interventions to promote and assist tobacco cessation: A review of efficacy, effectiveness and affordability for use in national guideline development. Addiction, 110(9), 1388–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wilson K, Dimoulas P, & Mills EJ (2006). Effectiveness of smoking cessation therapies: A systematic review and meta-analysis. BMC Public Health, 6(1), 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen R, Sairanen E, Järvelä E, Rantala S, Korpela R, Puttonen S, … & Kaipainen K (2014). The effectiveness and applicability of different lifestyle interventions for enhancing wellbeing: The study design for a randomized controlled trial for persons with metabolic syndrome risk factors and psychological distress. BMC Public Health, 14(1), 310. [DOI] [PMC free article] [PubMed] [Google Scholar]


