Abstract
Background:
Toxicological studies highlight the potential neurotoxicity of perfluoroalkyl substances (PFAS) during fetal development. However, few epidemiological studies have examined the impact of childhood PFAS on neurodevelopment.
Methods:
We employed data from 208 children in the Health Outcomes and Measures of the Environment Study, a birth cohort (Cincinnati, OH), to examine associations of six serum PFAS concentrations measured at 3 and 8 years with executive function assessed at 8 years using the validated parent-completed Behavior Rating Inventory of Executive Function survey. We used multiple informant models to identify susceptible windows of neurotoxicity to PFAS and executive function. We investigated trajectories of PFAS concentrations and whether sex modified these associations.
Results:
Each ln-increase in perfluorononanoate (PFNA) at 8 years was associated with a 3.4-point increase (95% CI 0.4, 6.3) in metacognition score, indicating poorer function. Children with PFNA above the median at 8 years had poorer global executive functioning compared to children with concentrations consistently below median levels (β=6.5, 95% CI 0.2, 12.9). Higher concurrent PFNA was associated with poorer behavior regulation among males, while associations among females were null (pPFNA×sex=0.018). Children with higher concurrent perfluorooctanoate (PFOA) had increased odds of being at risk of having clinical impairments in metacognition (OR=3.18, 95% CI 1.17, 8.60). There were no associations between perfluorooctane sulfonate and perfluorohexane sulfonate and executive function.
Conclusions:
PFNA and PFOA at 8 years, but not 3 years, may be related to poorer executive function at 8 years. Results need to be confirmed in cohort studies with larger sample sizes.
Keywords: Perfluoroalkyl substance (PFAS), executive function, childhood, postnatal, neurodevelopment
1. Introduction
Perfluoroalkyl substances (PFAS) have unique hydrophilic, oleophobic, and lipophobic physiochemical characteristics that render them ideal surfactants for food packaging, nonstick cookware, and textile surface treatments. Since their introduction in the 1950s, widespread use in an array of commercial products and industrial processes has resulted in their ubiquitous detection in wildlife and human tissue worldwide (Lau et al. 2007). Despite the voluntary phase-out by major manufacturers in the United States (Lau et al. 2007; Lindstrom et al. 2011), adverse health effects from PFAS remain a global concern due to their long half-lives (Olsen et al. 2007).
Laboratory studies highlight PFAS’ roles as potential neurotoxicants, with reports of pronounced effects on cognition and behavior in mice exposed to PFAS during development (Fuentes et al. 2007; Johansson et al. 2008; Johansson et al. 2009; Viberg et al. 2013). Biological mechanisms for PFAS neurotoxicity include alteration of levels of neuroproteins and neurotransmitters involved in synaptogenesis and synaptic plasticity, alteration of neurochemical signaling and homeostasis, disruption of neural cell differentiation, induction of neuronal cell apoptosis, promotion of reactive oxidative stress, and disruption of thyroid hormone homeostasis (Berntsen et al. 2017; Eggers Pedersen et al. 2015; Johansson et al. 2009; Lee and Viberg 2013; Lee et al. 2013; Lee et al. 2016; Liu et al. 2013; Liu et al. 2015; Long et al. 2013; Reistad et al. 2013; Slotkin et al. 2008; Yu et al. 2016). Few epidemiological studies have investigated the potential neurotoxicity of childhood PFAS exposures and reported mixed findings of increased impulsivity (Gump et al. 2011), contradictory conclusions regarding ADHD diagnoses (Hoffman et al. 2010; Stein and Savitz 2011), and higher full-scale IQ with increased PFAS concentrations (Stein et al. 2013). Contradictory findings have been reported for associations between childhood concentrations of PFAS and reading and language abilities, with better scores among children with higher PFAS concentrations in the Health Outcomes and Measures of the Environment (HOME) Study while null associations were noted in the C8 Health Project (Mid-Ohio Valley, US) (Stein et al. 2013; Zhang et al. 2018). The C8 Health Project also reported no relationship between childhood perfluorooctanoate (PFOA) and visual spatial processing, but observed that higher PFOA concentrations were associated with decreased ADHD characteristics (Stein et al. 2013).
The relationship between childhood PFAS and executive function, which is an interplay of constructs constituting cognitive and social-emotional control, has only been investigated in the C8 Health Project, with a focus solely on PFOA (Stein et al. 2014). The C8 investigators found no statistically significant association between PFOA and maternal reports of executive function; however, when they stratified by sex, they observed a favorable association among males (e.g., higher PFOA concentrations associated with better executive function) and an adverse association among females. Previously, prenatal concentrations of perfluorooctane sulfonate (PFOS) were significantly associated with poorer executive function scores based on parent assessments (Vuong et al. 2016). Effect modification by sex was also noted, with poorer scores on the metacognition index and global executive composite with higher prenatal PFOS concentrations in females, but not in males. However, no association was found between prenatal PFOA concentrations and executive function in the HOME Study. The aims of the current study were to examine the relationships of six PFAS measured at ages 3 and 8 years with executive function at age 8 years to identify susceptible windows of neurotoxicity during childhood and to investigate whether child sex modified these associations.
2. Methods
2.1. Population and study design
The study population was from the prospective pregnancy and birth cohort, the HOME Study (2003-2006, Cincinnati, OH, USA). Details on the HOME Study have been described previously (Braun et al. 2017). Briefly, inclusion criteria for pregnant women were: 1) ≥18 years of age; 2) 16±3 weeks of gestation; 3) living in housing built before 1978; 4) receiving and planning to continue prenatal care and deliver at one of the collaborating obstetric practices; 5) HIV negative status; and 6) not taking any medications related to seizures, thyroid disorders, or chemotherapy/radiation. After excluding children with missing information on PFAS and/or executive function assessment, we had 208 (53%) of the 390 live-born singletons available for the present study. This study was approved by the institutional review board (IRB) at the Cincinnati Children’s Hospital Medical Center (CCHMC), and collaborating institutions used the CCHMC IRB as the IRB of record.
2.2. Childhood PFAS concentrations
2.2.1. Measurement of child serum PFAS concentrations
We measured serum concentrations of PFOA, PFOS, perfluorohexane sulfonate (PFHxS), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluorooctane sulfonamide (PFOSA), 2-(N-methyl-perfluorooctane sulfonamide) acetate (Me-PFOSA-AcOH, also known as Me-FOSAA), and 2-(N-ethyl-perfluorooctane sulfonamide) acetate (Et-PFOSA-AcOH) at ages 3 and 8 years (2006-2014). Quantification was completed using on-line solid-phase extraction coupled to high-performance liquid chromatography-isotope dilution tandem mass spectrometry (Kato et al. 2011). To account for potential matrix effects, calibration standards were spiked into calf serum (Kato et al. 2014). Reagent blanks and low-concentration and high-concentration quality control (QC) materials were included in each analytical batch. The limit of detection (LOD) was 0.1 ng/mL, except for 0.2 ng/mL for PFOS. The coefficient of variation of the repeated measurements of the QC materials was ~6%.
2.2.2. PFAS trajectory characterization
Trajectories of childhood PFAS were categorized into four groups based on PFAS concentrations relative to the median value of each PFAS at 3 and 8 years: 1) consistently low; 2) low to high; 3) high to low; and 4) consistently high. For example, the median level of PFOA at ages 3 and 8 years were 5.4 and 2.4 ng/mL, respectively (Table S1). Children with a PFOA measurement at age 3 years that was <5.4 ng/mL who also had a PFOA measurement at age 8 years ≥2.4 ng/mL were considered to have a “low to high” PFOA trajectory. In addition, children with PFOS concentrations ≥6.1 ng/mL at age 3 years and ≥3.6 ng/mL at age 8 years were categorized as having a “consistently high” PFOS trajectory. Thus, a child would have different trajectories per PFAS compound.
2.3. Executive function
The Behavior Rating Inventory of Executive Function (BRIEF), a valid and reliable questionnaire to assess executive function (Gioia et al. 2000; Skogerbo et al. 2012), was completed by one parent (>95% were mothers) who had extensive contact with the child. Eight subscales are used to derive a summary measure of global executive composite along with two indices: 1) behavior regulation index [subscales: inhibit + shift + emotional control]; and 2) metacognition index [subscales: initiation + working memory + plan/organize + organization of materials + monitor]. BRIEF scores are standardized to T-scores based on sex- and age-specific norms, with higher scores indicating poorer executive function. T-scores 1.5 standard deviations (SDs) above the mean (50±10) are considered clinically significant (Gioia et al. 2000). However, given our modest sample size, we examined “at risk” BRIEF scores, defined as 1 SD above the mean (≥60) (Table S2).
2.4. Statistical analyses
We detected four PFAS (PFOA, PFOS, PFHxS, PFNA) in over 97% of serum samples at ages 3 and 8 years (Table S3). Values <LOD were replaced with LOD/√2 (Hornung and Reed 1990). Given the range of PFAS concentrations, we ln-transformed all PFAS concentrations to reduce the influence of outliers. We used multiple informant models to estimate βs and 95% confidence intervals (CIs) for repeated measures of ln-transformed PFAS in relation to BRIEF measures at age 8 years. Multiple informant models are non-standard versions of generalized estimating equations that can incorporate repeated measures of exposure in the model to identify potential windows of susceptibility (Sanchez et al. 2011). PFOA, PFOS, PFHxS, PFNA, PFDA, and Me-PFOSA-AcOH were modeled individually. Some interaction terms between PFAS and age at exposure had a p<0.10; therefore, we report age-specific βs. We also examined whether ln-transformed childhood PFAS concentrations were associated with increased risk of having an “at risk” BRIEF score (≥60) using multiple informant models to generate odds ratios (ORs) and corresponding 95% CIs.
Associations between trajectories of childhood PFAS concentrations and BRIEF scores were examined using multiple linear regression in 131 children who had PFAS measurements at both 3 and 8 years. We also examined whether the association between PFAS at 8 years and BRIEF scores were modified by child sex. We used multiple linear regression and included the interaction term between PFAS×child sex, with p<0.10 considered statistically significant. Lastly, we performed mutual adjustment for PFAS that were observed to be significantly associated with BRIEF scores in our main analyses between continuous measures of PFAS concentrations and continuous and dichotomized BRIEF scores. Covariates in the final models were based on a review of the literature and bivariate analyses (p<0.10) and included (categorized according to Table 1): maternal age, race/ethnicity, household income, child sex, maternal marijuana use, maternal serum cotinine (continuous), maternal blood lead (continuous), maternal depression, vitamin use, maternal IQ, marital status, Home Observation for Measurement of the Environment (HOME) score, and whether the child was ever breastfed.
Table 1.
Concurrent serum concentrations of PFAS (ng/mL) and global executive composite at 8 years in the Health Outcomes and Measures of the Environment Study by demographic characteristics.
| Child Serum Concentrations at age 8 years | GEC at 8 years Mean (SD) |
|||||
|---|---|---|---|---|---|---|
| n | PFOA GM (GSD) |
PFOS GM (GSD) |
PFHxS GM (GSD) |
PFNA GM (GSD) |
||
| Overall | 208 | 2.4 (1.5) | 3.9 (1.7) | 1.4 (2.1) | 0.8 (1.8) | 48.3 (10.6) |
| Age, years | ||||||
| <25 | 58 | 2.0 (1.5)* | 3.4 (1.6)* | 1.1 (1.8)* | 0.6 (1.8)* | 50.1 (10.6) |
| 25-34 | 119 | 2.5 (1.5) | 4.0 (1.7) | 1.5 (2.1) | 0.8 (1.9) | 47.3 (10.4) |
| ≥35 | 30 | 3.0 (1.6) | 4.8 (1.7) | 1.3 (2.3) | 0.8 (1.5) | 49.3 (11.3) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 123 | 2.8 (1.5)* | 4.5 (1.7)* | 1.6 (2.2)* | 0.8 (1.8) | 46.6 (10.1)* |
| Non-Hispanic Black and Others | 84 | 2.0 (1.5) | 3.2 (1.5) | 1.1 (1.8) | 0.7 (1.8) | 51.0 (10.9) |
| Household income | ||||||
| <$40,000 | 88 | 2.1 (1.5)* | 3.6 (1.7)* | 1.2 (2.0) | 0.7 (1.9) | 51.0 (10.2)* |
| $40,000-$79,999 | 67 | 2.5 (1.5) | 3.8 (1.6) | 1.4 (2.0) | 0.8 (1.9) | 46.9 (11.2) |
| ≥$80,000 | 52 | 3.0 (1.5) | 4.6 (1.7) | 1.6 (2.3) | 0.9 (1.7) | 45.9 (9.7) |
| Maternal marijuana use | ||||||
| No | 192 | 2.5 (1.5) | 4.0 (1.7)* | 1.4 (2.1) | 0.8 (1.8) | 47.7 (10.4)* |
| Yes | 15 | 2.0 (1.3) | 3.0 (1.4) | 1.1 (1.8) | 0.8 (1.9) | 57.5 (10.1) |
| Maternal depression | ||||||
| Minimal or mild | 185 | 2.5 (1.5)* | 4.0 (1.7) | 1.4 (2.1) | 0.8 (1.8) | 47.7 (10.7)* |
| Moderate or severe | 20 | 2.0 (1.4) | 3.1 (1.4) | 1.1 (1.7) | 0.7 (1.5) | 55.1 (7.8) |
| Maternal vitamin use | ||||||
| Daily | 159 | 2.5 (1.5) | 4.0 (1.7) | 1.4 (2.1) | 0.8 (1.8) | 47.4 (10.7)* |
| <Daily | 35 | 2.4 (1.5) | 3.5 (1.4) | 1.5 (2.0) | 0.8 (1.8) | 50.1 (10.6) |
| Never | 13 | 2.2 (1.9) | 3.5 (1.9) | 0.9 (1.7) | 0.8 (2.1) | 54.9 (6.9) |
| Marital status | ||||||
| Married or living with partner | 151 | 2.6 (1.5)* | 4.2 (1.7)* | 1.5 (2.2)* | 0.8 (1.8) | 46.8 (10.1)* |
| Not married or living alone | 56 | 2.0 (1.5) | 3.2 (1.5) | 1.1 (1.7) | 0.7 (1.9) | 52.6 (11.0) |
| HOME Score | ||||||
| ≥40 | 119 | 2.8 (1.5)* | 4.3 (1.7)* | 1.6 (2.1)* | 0.8 (1.9)* | 46.1 (10.7)* |
| 35-39 | 40 | 1.9 (1.4) | 3.3 (1.5) | 1.2 (2.0) | 0.6 (1.6) | 52.3 (10.3) |
| <35 | 34 | 2.2 (1.6) | 3.7 (1.8) | 1.1 (1.7) | 0.7 (1.9) | 50.2 (8.5) |
| Ever breastfed current child | ||||||
| No | 40 | 2.0 (1.4)* | 3.3 (1.7)* | 1.1 (1.8) | 0.7 (1.8) | 51.2 (10.4) |
| Yes | 166 | 2.5 (1.5) | 4.0 (1.7) | 1.4 (2.1) | 0.8 (1.8) | 47.7 (10.6) |
| Child Sex | ||||||
| Male | 93 | 2.3 (1.6) | 3.8 (1.7) | 1.3 (2.0) | 0.7 (1.9) | 48.0 (11.4) |
| Female | 115 | 2.5 (1.5) | 4.0 (1.7) | 1.4 (2.1) | 0.8 (1.8) | 48.6 (10.0) |
| GM (GSD) | Pearson r | Pearson r | Pearson r | Pearson r | Pearson r | |
| Maternal blood lead (µg/dL) | ||||||
| 0.6 (1.4) | −0.08 | −0.11 | −0.17* | 0.14* | 0.11 | |
| Maternal serum cotinine (ng/mL) | ||||||
| 0.07 (23.3) | −0.27* | −0.17* | −0.15* | −0.10 | 0.21* | |
| Maternal IQ | ||||||
| Mean±SD 105.8 (15.2) | 0.30* | 0.13 | 0.14 | 0.07 | −0.17* | |
Abbreviations: GEC, global executive composite; GM, geometric mean; GSD, geometric standard deviation.
p<0.05 (two-sided p values using ANOVA or t-test)
3. Results
3.1. Participant characteristics
Of the 208 children, 55% were females and 45% were males (Table 1). Mothers who were older, non-Hispanic white, married or living with a partner, and whose HOME score was ≥40 had children with significantly higher concentrations of PFOA, PFOS, and PFHxS at age 8 years. PFOA and PFOS concentrations were also significantly higher among children living in households with annual incomes ≥$80,000 and those who were ever breastfed. In contrast, children whose mothers were younger and who had lower HOME scores had significantly lower PFNA at 8 years. Children in the HOME Study had a global executive composite mean score (48.3±10.6) that was slightly below the population mean of 50±10 (higher scores indicate poorer global executive functioning). Significantly higher global executive composite scores were observed among children whose mothers were non-Hispanic black or others, used marijuana during pregnancy, did not take daily vitamin supplementation, and experienced moderate/severe depression. Global executive composite scores were also significantly higher among children living in households with low income and HOME scores <40.
3.2. Childhood PFAS concentrations
Serum concentrations of PFAS declined from age 3 to 8 years (Table S3). Concentrations of PFOS was the highest among PFAS in the HOME Study, followed by PFOA, PFHxS, and PFNA (Tables S1 and S3). Concentrations of PFOS were 6.6±1.9 ng/mL and 3.9±1.7 ng/mL at age 3 and 8 years, respectively. PFOA concentrations were lower, with concentrations of 5.4±1.7 ng/mL and 2.4±1.5 ng/mL at age 3 and 8 years, respectively. At age 3 years PFNA concentrations were 1.4±1.9 ng/mL, while at age 8 years concentrations declined to 0.8±1.8 ng/mL. PFAS measured at age 3 (rs=0.23-0.70) and 8 years (rs=0.15-0.65) were significantly correlated with each other (Table S4).
3.3. Childhood PFAS and executive function
3.3.1. Childhood PFAS and BRIEF scores
Positive associations were observed between PFNA concentrations at age 8 years and metacognition index scores (Figure 1). Each natural-log unit increase in PFNA was associated with a 3.4-point increase (95% CI 0.4, 6.3) on the metacognition index. Children who had higher PFNA concentrations at age 8 years had higher scores on several subscales of the metacognition index, including initiate, plan/organize, and organization of materials (Figure S1). In addition, PFNA at 8 years was significantly associated with poorer global executive functioning. In contrast, PFNA concentrations at age 3 years were associated with lower scores for metacognition and global executive functioning, albeit associations were not statistically significant.
Figure 1.
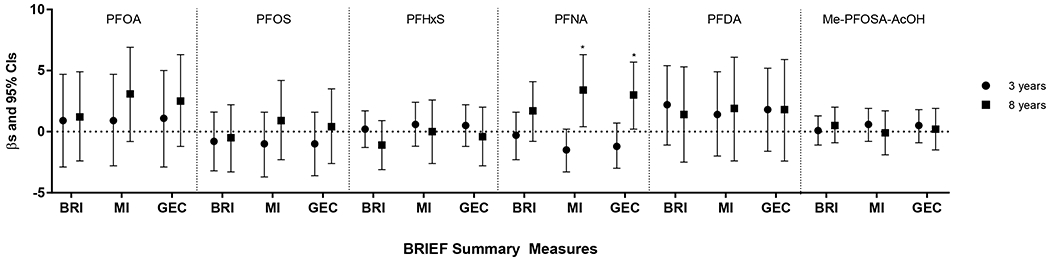
Estimated associations between childhood serum concentrations of PFAS (ng/mL) and BRIEF summary measures at 8 years, HOME Study. Adjusted by maternal age, race/ethnicity, household income, child sex, maternal marijuana use, maternal blood lead, maternal serum cotinine, maternal depression, vitamin use, maternal IQ, marital status, Home Observation for Measurement of the Environment Score, and whether the child was ever breastfed. Asterisks indicate associations which were statistically significant (p<0.05). Abbreviations: BRI, behavior regulation index; MI, metacognition index; GEC, global executive composite.
3.3.2. Childhood PFAS and “at risk” BRIEF scores
Children who had higher serum concentrations of PFNA at age 8 years had increased odds of having an “at risk” behavior regulation index score ≥60 (Table 2). This positive association was also present within the subscales of inhibit, shift, and emotional control of the behavior regulation index (Table S5). Similar associations were noted for the metacognition index and its subscales of working memory, plan/organize, and organization of materials. In addition, PFOA was associated with higher odds of having an “at risk” score on the shift, emotional control, and metacognition index. An increase in ln-PFDA at 3 years and ln-PFNA at 8 years was associated with ~3-fold greater odds of having an “at risk” score on global executive composite. No remarkable associations were observed for PFOS, PFHxS, and Me-PFOSA-AcOH.
Table 2.
Odds ratios and 95% confidence intervals of having a BRIEF summary measure score ≥60 at age 8 years by a ln-increase in childhood serum concentrations of perfluoroalkyl substances (ng/mL), HOME Studya
| PFAS Child Age |
Behavior Regulation Index | Metacognition Index | Global Executive Composite |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| PFOA | |||
| 3 years | 1.01 (0.29, 3.53) | 1.30 (0.47, 3.57) | 1.39 (0.45, 4.24) |
| 8 years | 1.56 (0.49, 4.92) | 3.18 (1.17, 8.60)* | 2.69 (0.92, 7.90) |
| PFOS | |||
| 3 years | 0.66 (0.29, 1.51) | 0.83 (0.42, 1.63) | 0.95 (0.45, 2.01) |
| 8 years | 0.40 (0.14, 1.14) | 1.53 (0.67, 3.52) | 1.04 (0.41, 2.68) |
| PFHxS | |||
| 3 years | 1.01 (0.60, 1.70) | 1.04 (0.69, 1.55) | 1.05 (0.67, 1.66) |
| 8 years | 0.54 (0.22, 1.32) | 1.10 (0.58, 2.09) | 0.65 (0.32, 1.32) |
| PFNA | |||
| 3 years | 1.48 (0.90, 2.43) | 0.88 (0.51, 1.53) | 1.43 (0.87, 2.33) |
| 8 years | 2.75 (1.30, 5.79)* | 2.94 (1.52, 5.69)* | 3.07 (1.60, 5.90)* |
| PFDA | |||
| 3 years | 1.95 (0.83, 4.62) | 1.73 (0.73, 4.09) | 2.95 (1.20, 7.23)* |
| 8 years | 1.70 (0.59, 4.88) | 2.11 (0.83, 5.35) | 2.69 (0.95, 7.60) |
| Me-PFOSA-AcOH | |||
| 3 years | 0.85 (0.59, 1.23) | 0.99 (0.69, 1.42) | 0.93 (0.64, 1.35) |
| 8 years | 0.79 (0.52, 1.19) | 1.16 (0.76, 1.77) | 1.13 (0.74, 1.72) |
n=185 (PFOA, PFOS, PFHxS, PFNA, Me-PFOSA-AcOH); n=161 (PFDA)
Adjusted by maternal age, race/ethnicity, household income, child sex, maternal marijuana use, maternal blood lead, maternal serum cotinine, maternal depression, vitamin use, maternal IQ, marital status, Home Observation for Measurement of the Environment Score, and whether the child was ever breastfed.
p<0.05
3.3.3. Trajectories of childhood PFAS and BRIEF scores
Among 122 children, children with a “high to low” trajectory of PFNA concentrations had poorer scores on the behavior regulation index and those with a “low to high” trajectory had poorer global executive composite scores (Figure 2). Although not statistically significant, we observed a pattern where children who had serum PFOA concentrations above the median at age 8 years had higher behavior regulation index, metacognition index, and global executive composite scores compared to those with consistently low serum PFOA concentrations. The analyses of PFHxS, PFDA, and Me-PFOSA-AcOH exposure trajectories did not suggest marked differences in BRIEF scores between the four groups. And while we observed a few statistically significant associations between trajectories of exposures for PFOS and a pattern of associations for PFOA, there is not sufficient evidence from our findings to indicate exposure trajectories of PFAS during childhood are associated with BRIEF-assessed executive function.
Figure 2.
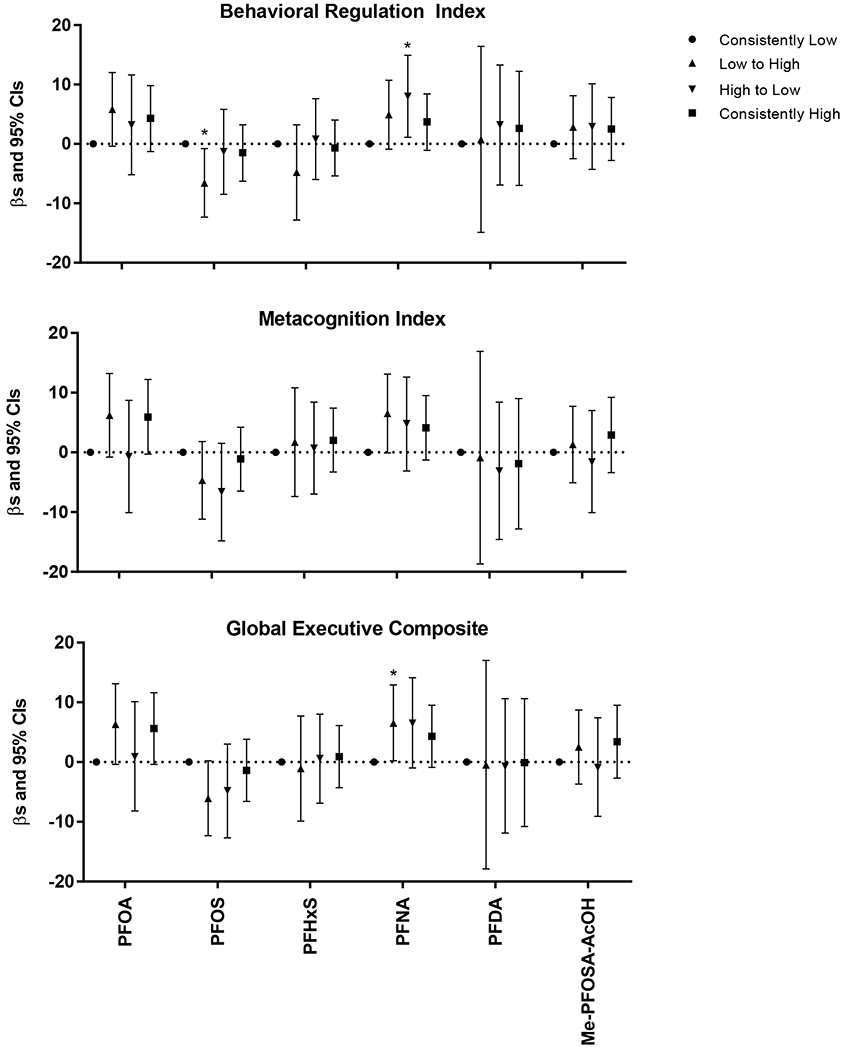
Estimated associations between trajectories of childhood serum concentrations of PFAS (ng/mL) from ages 3 and 8 years and BRIEF summary measures at 8 years, HOME Study. Asterisks indicate associations which were statistically significant (p<0.05). Adjusted by maternal age, race/ethnicity, household income, child sex, maternal marijuana use, maternal blood lead, maternal serum cotinine, maternal depression, vitamin use, maternal IQ, marital status, Home Observation for Measurement of the Environment Score, and whether the child was ever breastfed.
3.3.4. Mutual adjustment of PFAS
Mutual adjustment of PFOA and PFNA yielded similar conclusions (Table S6). Higher scores on metacognition index (β=3.1, 95% CI −0.1, 6.3) and global executive composite (β=2.8, 95% CI −0.1, 5.7) were observed with increased 8 year PFNA concentrations. The association between PFOA at 8 years and increased risk of having an emotional control score ≥60 (OR=4.44, 95% CI 1.61, 12.29) remained after mutual adjustment for PFNA; however, previous adverse associations between 8 year PFOA concentrations and metacognition index were not statistically significant.
3.4. Effect measure modification by child sex
We observed effect measure modification by child sex for serum PFNA concentrations at age 8 years and behavior regulation (pPFNA×sex=0.018) (Table 3). Males had significantly higher scores with an increase in ln-PFNA (β=5.4, 95% CI 1.5, 9.3), while null associations were observed among females (β=−0.7, 95% CI −4.1, 2.7). Child sex also modified the relationship between PFNA at age 8 years and shift and emotional control subscales (Table S7). We also noted poorer scores on these subscales among males, whereas non-significant associations were present among females.
Table 3.
β-coefficients and 95% CIs for associations of concurrent perfluoroalkyl substances (ng/mL) and BRIEF summary measures at 8 years by child sex.a
| PFAS | Interaction | Males | Females |
|---|---|---|---|
| p-value | β (95% CI) | β (95% CI) | |
| PFOA | |||
| Behavior regulation index | 0.222 | 3.4 (−1.9, 8.7) | −1.0 (−6.6, 4.5) |
| Metacognition index | 0.851 | 4.0 (−2.0, 10.1) | 3.2 (−3.1, 9.6) |
| Global executive composite | 0.551 | 4.1 (−1.7, 9.9) | 1.7 (−4.4, 7.8) |
| PFOS | |||
| Behavior regulation index | 0.795 | −0.1 (−4.7, 4.6) | −0.9 (−5.1, 3.3) |
| Metacognition index | 0.915 | 1.4 (−4.0, 6.7) | 1.0 (−3.8, 5.8) |
| Global executive composite | 0.847 | 0.9 (−4.2, 6.0) | 0.3 (−4.3, 4.9) |
| PFHxS | |||
| Behavior regulation index | 0.379 | −0.2 (−3.5, 3.1) | −2.1 (−5.1, 0.8) |
| Metacognition index | 0.323 | 1.2 (−2.6, 5.0) | −1.3 (−4.7, 2.1) |
| Global executive composite | 0.285 | 0.8 (−2.8, 4.4) | −1.8 (−5.0, 1.4) |
| PFNA | |||
| Behavior regulation index | 0.018 | 5.4 (1.5, 9.3)* | −0.7 (−4.1, 2.7) |
| Metacognition index | 0.512 | 5.0 (0.5, 9.5)* | 3.1 (−0.8, 7.0) |
| Global executive composite | 0.166 | 5.7 (1.4, 10.0)* | 1.8 (−2.0, 5.5) |
| PFDA | |||
| Behavior regulation index | 0.646 | 0.4 (−6.1, 7.0) | 2.5 (−3.4, 8.4) |
| Metacognition index | 0.372 | −0.1 (−7.3, 7.2) | 4.3 (−2.2, 10.9) |
| Global executive composite | 0.450 | 0.1 (−7.0, 7.2) | 3.8 (−2.6, 10.2) |
| Me-PFOSA-AcOH | |||
| Behavior regulation index | 0.060 | −1.6 (−3.8, 0.7) | 1.3 (−0.6, 3.2) |
| Metacognition index | 0.063 | −1.3 (−3.8, 1.2) | 1.9 (−0.2, 4.0) |
| Global executive composite | 0.052 | −1.4 (−3.8, 1.0) | 1.8 (−0.2, 3.8) |
n=122 (PFOA, PFOS, PFHxS, PFNA, Me-PFOSA-AcOH); n=96 (PFDA)
Adjusted by maternal age, race/ethnicity, household income, child sex, maternal marijuana use, maternal blood lead, maternal serum cotinine, maternal depression, vitamin use, maternal IQ, marital status, Home Observation for Measurement of the Environment Score, and whether the child was ever breastfed.
p<0.05
4. Discussion
We observed higher PFNA concentrations at age 8 years were associated with poorer metacognition and global executive functioning scores and increased odds of being “at risk” for behavior regulation and global executive functioning problems. A potential adverse association was also observed with 8 year concentrations of PFOA, but to a lesser extent. In particular, a positive association was observed between PFOA concentrations at age 8 years and “at risk” scores for inhibitory control, cognitive flexibility (shift), emotional control, as well as metacognition. Children who had high (≥median) concentrations of PFNA at any time or high concentrations of PFOA at age 8 years had poorer executive function. However, while poorer BRIEF scores were observed between PFNA and PFOA at 8 years in the multiple informant models, there is no clear evidence from the trajectory analysis that there is a window of susceptibility for childhood exposure to PFAS.
While few studies have examined PFNA neurotoxicity in children, there is evidence indicating that PFNA disrupts thyroid hormone homeostasis, which is important for brain development even after birth as thyroid hormones are involved in myelination of neurons. Maternal thyroid hormone disruption has been associated with poorer executive function in children (Andersen et al. 2018; Ghassabian et al. 2011). In addition, executive function has been reported to be influenced by thyroid hormones during adulthood (Constant et al. 2005; Grigorova and Sherwin 2012; Zhu et al. 2006). Long-term PFNA exposure in zebrafish from early life to adulthood resulted in elevated plasma triiodothyronine levels in adult zebrafish and histological changes in thyroid follicles of males (Liu et al. 2011). This may be due to PFNA’s ability to inhibit the UDP-glycosyltransferase pathway, thereby reducing biliary elimination of thyroid hormones. PFNA has also been reported to increase oxidative stress in zebrafish and green mussels (Liu et al. 2013; Liu et al. 2015). Oxidative stress can result in cellular dysfunction or even cell death. The brain is particularly vulnerable since it has high levels of oxygen coupled with a poor antioxidant defense system that can be overwhelmed by oxidative stress (Berntsen et al. 2017).
For PFOA, mice with neonatal exposure had increased levels of calcium/calmodulin-dependent protein kinase II, growth-associated protein-43, and synaptophysin in the hippocampus as well as increased synaptophysin and tau in the cerebral cortex (Johansson et al. 2009). These proteins are involved in the regulation of synaptogenesis, synaptic plasticity, axonal growth and modulation, and growth of neuronal processes. Neonatal exposure to PFOA also affected the cholinergic system in adult mice, manifesting as a hypoactive response to nicotine (Johansson et al. 2008). Metabolic profiles in the brain of juvenile male mice exposed to PFOA for 28 days had altered concentrations of neurotransmitters serotonin, dopamine, norepinephrine, and glutamate (Yu et al. 2016).
Only PFNA and PFOA demonstrated a potential adverse relationship with executive function in the present study. We previously reported prenatal exposure to PFOS was associated with poorer executive function in children (Vuong et al. 2016). However, associations between childhood concentrations of PFOS and executive function were null. This may be due to varying concentrations between windows of exposure. PFOS concentrations during gestation was 12.3±1.0 ng/mL compared to 6.6±1.9 ng/mL at age 3 years and 3.9±ng/mL at age 8 years. Structural differences between PFAS may provide clues as to why childhood PFNA and PFOA may be associated with executive function more than the other PFAS compounds. While PFOS has a greater potential for genotoxicity due to the sulfonate group having stronger chemical interactions with DNA molecules, PFNA accumulation in the brain might be much higher. The average PFNA concentration in the brain tissue of 20 deceased humans was 29.7 ng/g compared to 1.9 ng/g of PFOS (Perez et al. 2013). PFNA is more hydrophobic than PFOS and may have a stronger potential for binding and interacting with proteins and enzymes (Liu et al. 2013). In addition, PFOA and PFNA were observed to cause more membrane instability than the minor inductions of PFOS (Liu et al. 2013).
Only the C8 Health Study has examined childhood PFOA (2-8 years) and executive function (6-12 years), but they reported sex-modified associations between PFOA and executive function despite null findings in children overall (Stein et al. 2014). Females with PFOA concentrations in the highest quartile of exposure had marginally significant poorer global executive composite scores, while better scores were noted for males with comparable PFOA concentrations. In our study, we only found that sex modified associations between 8 year concentrations of PFNA and behavior regulation and its subscales, with males having worse scores.
Discrepancies between our findings and those reported in the C8 Health Study may be due to differences in the study population, executive function assessment, PFAS concentrations, statistical methods, and confounder adjustment. Compared with the C8 Health Study, which included predominantly Non-Hispanic whites (>95%) (Stein et al. 2014), the HOME Study was comprised of ~60% Non-Hispanic whites. Secondly, the C8 Health Study had a two-pronged approach with the BRIEF, relying on a parent and a teacher, whereas our study used only one parent’s assessment. Nevertheless, the C8 Health Study reported similar null associations with both maternal- and teacher-completed BRIEF assessments. Third, differences in PFAS concentrations between the children in the C8 Health Study and the HOME Study may play a role. The C8 Health Study population in the Mid-Ohio Valley was exposed to PFOA-contaminated drinking water, which is why the median PFOA concentration in the C8 Health Study children at age 2-8 years was 35.1 ng/mL compared to 5.4 and 2.4 ng/mL at ages 3 and 8 years, respectively, in the HOME Study. Differences in the timing of the measurements may have also contributed to the divergent conclusions. Lastly, we also adjusted for other potential neurotoxicants (lead and tobacco smoke), race/ethnicity, maternal depression, and whether the child was ever breastfed.
Effect modification by child sex may be due to differences in the elimination rate. The half-life of PFNA in rats is profoundly shorter in females (1.4 days) compared to males (30.6 days) (Tatum-Gibbs et al. 2011). In humans, there are marked differences in the clearance of PFHxS and PFOS between sexes, with PFHxS and PFOS having a significantly longer half-life in males than in females (Li et al. 2018). For PFOS, the half-life in males is 4.6 years compared to 3.1 years in females. The degree of variation in the elimination rate for PFNA in humans has yet to be determined.
The major strength in our study lies in its longitudinal design, allowing for the two PFAS measurements during childhood, which enabled us to explore trajectories of exposure. We were also able to test for susceptible windows of neurotoxicity during childhood using multiple informant models. While residual confounding is always a concern, we were able to adjust for maternal IQ and depression, whether the child was ever breastfed, socioeconomic status, the home environment, and other neurotoxicant exposures.
The current study has some limitations. First, selection bias may be a concern. However, no significant differences were noted between those included in the present study and those lost to follow-up aside from maternal blood lead levels. Outcome misclassification is possible, because we relied on one parent for the BRIEF survey. Further, the BRIEF survey is a parent-report measure and is only one of the assessment tools that can be used to measure executive function. We did not analyze associations between PFAS concentrations and executive function using different assessment tools. Third, we acknowledge that dichotomizing BRIEF scores with a cutoff at 1 SD from the mean is not clinically significant, but findings imply an underlying association that indicates PFNA and PFOA may adversely affect executive function. Fourth, while the usage of multiple informant models greatly reduced the number of models in our study, multiple comparisons continue to be a concern as there is no reduction in type 1 error. Lastly, we are limited by our sample size thus results for our trajectory analyses and effect measure modification by sex should be cautiously interpreted.
5. Conclusions
Evidence from the present study indicates that higher concentrations of PFNA at 8 years and, to a lesser extent, PFOA are associated with higher scores on the BRIEF survey at 8 years, indicating poorer parent-reported executive function in children. While associations were only observed with PFAS concentrations at age 8 years, this may not necessarily indicate age 8 as a sensitive window of susceptibility. Rather, it may be that PFAS’ cumulative exposure may play a role in executive function. The relationship between PFNA and executive function may be sexually dimorphic, with males more sensitive to PFNA neurotoxicity. The associations of childhood PFOS and PFHxS exposures with executive function were generally null. Further examination to determine PFAS’ potential neurotoxicity in humans is still warranted with regard to executive function, given the associations we found between PFNA and PFOA concentrations at age 8 years and poorer executive function. Limited number of epidemiological studies have examined PFAS and executive function, which is vital for daily complex activities, academic achievement, as well as social and behavioral interactions.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES020349, R01 ES024381, R01 ES014575, R00 ES020346, T32ES010957, P30ES006096; EPA P01 R829389). We acknowledge the technical assistance of K. Kato, and J. Tao (CDC). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
References
- Andersen SL, Andersen S, Liew Z, Vestergaard P, Olsen J. 2018. Maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age. J Clin Endocrinol Metab 103:660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen HF, Bjorklund CG, Audinot JN, Hofer T, Verhaegen S, Lentzen E, et al. 2017. Time-dependent effects of perfluorinated compounds on viability in cerebellar granule neurons: Dependence on carbon chain length and functional group attached. Neurotoxicology 63:70–83. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. 2017. Cohort profile: The health outcomes and measures of the environment (home) study. Int J Epidemiol 46:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant EL, Adam S, Seron X, Bruyer R, Seghers A, Daumerie C. 2005. Anxiety and depression, attention, and executive functions in hypothyroidism. J Int Neuropsychol Soc 11:535–544. [DOI] [PubMed] [Google Scholar]
- Eggers Pedersen K, Basu N, Letcher R, Greaves AK, Sonne C, Dietz R, et al. 2015. Brain region-specific perfluoroalkylated sulfonate (pfsa) and carboxylic acid (pfca) accumulation and neurochemical biomarker responses in east greenland polar bears (ursus maritimus). Environ Res 138:22–31. [DOI] [PubMed] [Google Scholar]
- Fuentes S, Colomina MT, Vicens P, Domingo JL. 2007. Influence of maternal restraint stress on the long-lasting effects induced by prenatal exposure to perfluorooctane sulfonate (pfos) in mice. Toxicol Lett 171:162–170. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, et al. 2011. Maternal thyroid function during pregnancy and behavioral problems in the offspring: The generation r study. Pediatr Res 69:454–459. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. 2000. Behavior rating inventory of executive function. Odessa, FL:Psychological Assessment Resources. [Google Scholar]
- Grigorova M, Sherwin BB. 2012. Thyroid hormones and cognitive functioning in healthy, euthyroid women: A correlational study. Horm Behav 61:617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Wu Q, Dumas AK, Kannan K. 2011. Perfluorochemical (pfc) exposure in children: Associations with impaired response inhibition. Environ Sci Technol 45:8151–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. 2010. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in u.S. Children 12-15 years of age. Environ Health Perspect 118:1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene 5:46–51. [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P. 2008. Neonatal exposure to perfluorooctane sulfonate (pfos) and perfluorooctanoic acid (pfoa) causes neurobehavioural defects in adult mice. Neurotoxicology 29:160–169. [DOI] [PubMed] [Google Scholar]
- Johansson N, Eriksson P, Viberg H. 2009. Neonatal exposure to pfos and pfoa in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci 108:412–418. [DOI] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218:2133–2137. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, et al. 2014. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of cincinnati, ohio pregnant women during 2003-2006. Environ Sci Technol 48:9600–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol Sci 99:366–394. [DOI] [PubMed] [Google Scholar]
- Lee I, Viberg H. 2013. A single neonatal exposure to perfluorohexane sulfonate (pfhxs) affects the levels of important neuroproteins in the developing mouse brain. Neurotoxicology 37:190–196. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee HG, Yang JH. 2013. Perfluorooctane sulfonate-induced apoptosis of cerebellar granule cells is mediated by erk 1/2 pathway. Chemosphere 90:1597–1602. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Choi SY, Yang JH. 2016. Amp-activated protein kinase is involved in perfluorohexanesulfonate-induced apoptosis of neuronal cells. Chemosphere 149:1–7. [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of pfos, pfhxs and pfoa after end of exposure to contaminated drinking water. Occup Environ Med 75:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: Past, present, and future. Environ Sci Technol 45:7954–7961. [DOI] [PubMed] [Google Scholar]
- Liu C, Chang VW, Gin KY. 2013. Environmental toxicity of pfcs: An enhanced integrated biomarker assessment and structure-activity analysis. Environ Toxicol Chem 32:2226–2233. [DOI] [PubMed] [Google Scholar]
- Liu H, Sheng N, Zhang W, Dai J. 2015. Toxic effects of perfluorononanoic acid on the development of zebrafish (danio rerio) embryos. J Environ Sci (China) 32:26–34. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang J, Fang X, Zhang H, Dai J. 2011. The thyroid-disrupting effects of long-term perfluorononanoate exposure on zebrafish (danio rerio). Ecotoxicology 20:47–55. [DOI] [PubMed] [Google Scholar]
- Long M, Ghisari M, Bonefeld-Jorgensen EC. 2013. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int 20:8045–8056. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Nadal M, Navarro-Ortega A, Fabrega F, Domingo JL, Barcelo D, et al. 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59:354–362. [DOI] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. 2013. Perfluoroalkylated compounds induce cell death and formation of reactive oxygen species in cultured cerebellar granule cells. Toxicol Lett 218:56–60. [DOI] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerbo A, Kesmodel US, Wimberley T, Stovring H, Bertrand J, Landro NI, et al. 2012. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG 119:1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. 2008. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect 116:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA. 2011. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5-18 years of age. Environ Health Perspect 119:1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC. 2013. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology 24:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC. 2014. Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6-12-year-old children. Paediatr Perinat Epidemiol 28:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum-Gibbs K, Wambaugh JF, Das KP, Zehr RD, Strynar MJ, Lindstrom AB, et al. 2011. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology 281:48–55. [DOI] [PubMed] [Google Scholar]
- Viberg H, Lee I, Eriksson P. 2013. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal pfhxs dose. Toxicology 304:185–191. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Webster GM, Sjodin A, Calafat AM, Braun JM, et al. 2016. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ Res 147:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Wei S, Li M, Yang J, Li K, Jin L, et al. 2016. Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Sci Rep 6:23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Ye X, Calafat AM, Dietrich KN, et al. 2018. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8years. Environ Int 111:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DF, Wang ZX, Zhang DR, Pan ZL, He S, Hu XP, et al. 2006. Fmri revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain 129:2923–2930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


