Abstract
Honey bees and bumble bees harbour a small, defined set of gut bacterial associates. Strains matching sequences from 16S rRNA gene surveys of bee gut microbiotas were isolated from two honey bee species from East Asia. These isolates were mesophlic, non-pigmented, catalase-positive and oxidase-negative. The major fatty acids were iso-C15 : 0, iso-C17 : 0 3-OH, C16 : 0 and C16 : 0 3-OH. The DNA G+C content was 29–31 mol%. They had ∼87 % 16S rRNA gene sequence identity to the closest relatives described. Phylogenetic reconstruction using 20 protein-coding genes showed that these bee-derived strains formed a highly supported monophyletic clade, sister to the clade containing species of the genera Chryseobacterium and Elizabethkingia within the family Flavobacteriaceae of the phylum Bacteroidetes. On the basis of phenotypic and genotypic characteristics, we propose placing these strains in a novel genus and species: Apibacter adventoris gen. nov., sp. nov. The type strain of Apibacter adventoris is wkB301T ( = NRRL B-65307T = NCIMB 14986T).
Bacteria from the phylum Bacteroidetes are often constituents of animal microbiotas. In humans, members of the genera Prevotella and Bacteroides are among the most numerically abundant gut symbionts, and appear to anchor different states of stability (enterotypes) within the community (Arumugam et al. 2011). Honey bees and bumble bees have also been found to harbour members of the phylum Bacteroidetes: culture-independent 16S rRNA gene surveys of their gut communities have identified sequences falling within the order Flavobacteriales, with >10 % divergence from described species. Although their prevalence appears sporadic compared with the core bee gut microbiota (Babendreier et al., 2007; Koch & Schmid-Hempel, 2011; Ahn et al., 2012; Moran et al., 2012; Lim et al., 2015), the bee Flavobacteriales sequences form a monophyletic clade, suggesting they are specific to bee guts, or to other environments (e.g. plants, nectar, hive material) where they are prone to be ingested by bees. Here, we provide the first description of members of this novel group.
In July and August of 2014, samples of the Asian honey bee (Apis cerana) and the giant honey bee (Apis dorsata) were collected from Singapore and Kuala Lumpur, Malaysia (Table S1, available in the online Supplementary Material). The entire intestinal tract was removed and crushed in 19 % (v/v) glycerol and frozen. Bacteria were recovered by plating out the frozen samples onto heart infusion agar (Hardy Diagnostics) supplemented with 5 % defibrinated sheep blood and incubating at 35 °C in 5 % CO2. After 2–3 days, small ( < 0.5 mm) white, non-haemolytic colonies appeared. Three isolates were chosen for further characterization: wkB180, wkB309 and wkB301T.
Strains wkB180, wkB309 and wkB301T grew on heart infusion agar, brain heart infusion agar, trypticase soy agar, Columbia agar and lysogeny broth (LB) agar, but exhibited poor or no growth on nutrient agar, MacConkey agar, Lactobacillus MRS agar or R2A agar (all media from BD Difco). No growth was observed under anaerobic conditions (90 % N2, 5 % CO2, 5 % H2) or in ambient air. In liquid culture, growth was observed in Insectagro DS2 (Corning), Columbia broth, trypticase soy broth, and LB (without NaCl), but not in Lactobacillus MRS. On solid media, the strains formed smooth, round, pale/semi-translucent colonies. Strain wkB309 exhibited rapidly spreading margins of growth extending from colony edges, consistent with gliding motility.
DNA was extracted as described by Powell et al. (2014) with the following modifications. RNase A was added directly to cetyltrimethylammonium bromide (CTAB) buffer prior to bead-beating, a final concentration of 0.5 % 2-mercaptoethanol was used, and the lysate was incubated at 56 °C for 1 h instead of overnight. Samples were submitted to the Genome Sequencing and Analysis Facility at the University of Texas at Austin for Illumina MiSeq 2 × 300 bp library preparation and whole genome sequencing. Sequence reads were assembled with CLC Genomics Workbench 5.5 (CLC bio). Genome sizes and DNA G+C content were inferred from draft assemblies.
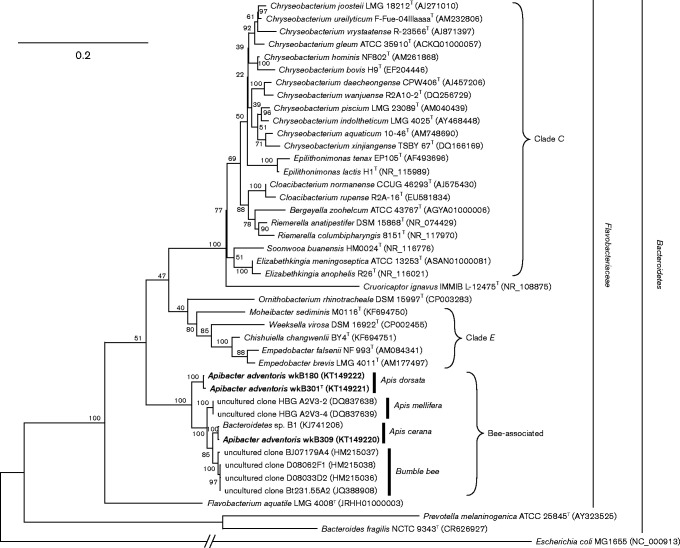
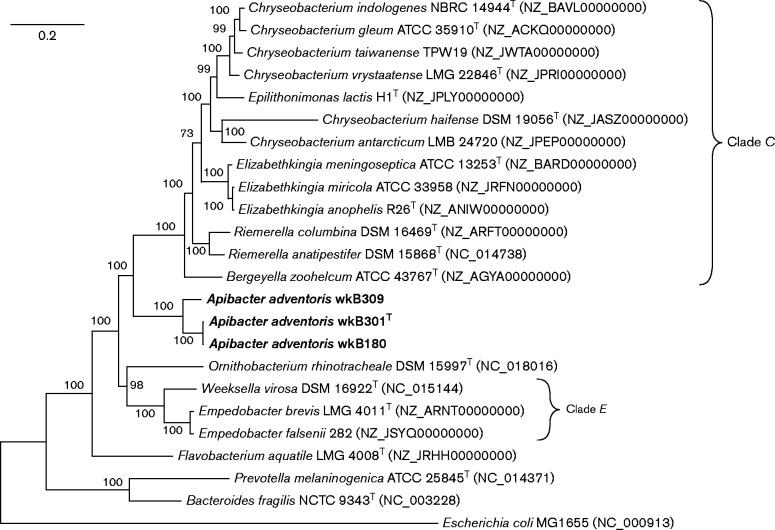
Phylogenetic analysis based on 16S rRNA sequences (1250 bp) showed that strains wkB180, wkB309 and wkB301T clustered together and were only distantly related to previously described species (Fig. 1). Strain wkB301T had 87.6, 87.5, 87.2 and 85.5 % 16S rRNA gene sequence identity to the type strains of Empedobacter brevis, Ornithobacterium rhinotracheale, Chryseobacterium gleum and Flavobacterium aquatile, respectively (Table S1). However, our strains could not be confidently placed in relation to the clades encompassing the genera Empedobacter and Chryseobacterium (clades E and C, respectively) using 16S rRNA gene sequences alone (Fig. 1). To obtain better phylogenetic resolution, we extracted and aligned 20 protein sequences from the strains' genomes and that of sequenced relatives to produce a tree based on 9746 total residues (Fig. 2). This tree strongly suggested that the bee gut strains are sister to clade C, and not to clade E. All phylogenetic analysis was done in mega 6 (Tamura et al. 2013).
Fig. 1.
16S rRNA gene phylogeny of strains wkB301T, wkB309, wkB180 and close relatives. Hosts of origin for bee-derived sequences or strains are highlighted. The tree was built with a maximum-likelihood algorithm using the Generalized Time Reversible substitution model with gamma-distributed rates and invariant sites. Escherichia coli MG1655 is the outgroup. Bootstrap percentages (based on 1000 replicates) are shown at nodes. Bar, 0.2 substitutions per site.
Fig. 2.
Phylogenetic tree of strains wkB301T, wkB309, wkB180 and related taxa, based on 20 conserved proteins (encoded by genes alaS, atpA, dnaA, dnaN, ftsZ, fusA, groEL, gyrB, lepA, metK, nusG, pfkA, pyrG, recA, rplA, rplB, rpoB, rpsB, sdhA and secA). Accession numbers for these genes are listed in Table S2. Proteins were concatenated and aligned with muscle and the tree was built with a maximum-likelihood algorithm using the Jones–Taylor–Thornton substitution model with gamma-distributed rates and invariant sites. Bootstrap percentages (1000 replicates) are shown at nodes. Bar, 0.2 substitutions per site.
We conducted further assays to characterize the phenotypic traits of our strains. Scanning electron micrographs were taken to determine cell size and morphology. Strains were grown on Columbia blood agar plates for 48 h, then fixed with 2.5 % (v/v) glutaraldehyde in 0.1 M sodium cacodylate buffer (SCB) overnight at 4 °C. After three rinses in 0.1 M SCB, they were post-fixed with 1 % osmium tetroxide in 0.1 M SCB for 2 h. Cells were rinsed with water and dehydrated with increasing concentration of ethanol, followed by critical-point drying (Samdri-795; Tousimis). Cells were air-dried, mounted on scanning electron microscope (SEM) stubs and sputter coated with gold-palladium in a Cressington 108auto. Images were acquired on a Zeiss Sigma Field-Emission SEM (University of Texas Southwestern Electron Microscopy Facility). Cells of strain wkB301T were approximately 2.0 × 0.3 μm in size and formed extensive filaments (Fig. 3a); when grown in liquid media, filamentation was reduced. Strain wkB309 did not filament, but instead consisted of short, rounded rods approximately 0.9–1.5 μm long and 0.4 μm wide (Fig. 3b).
Fig. 3.
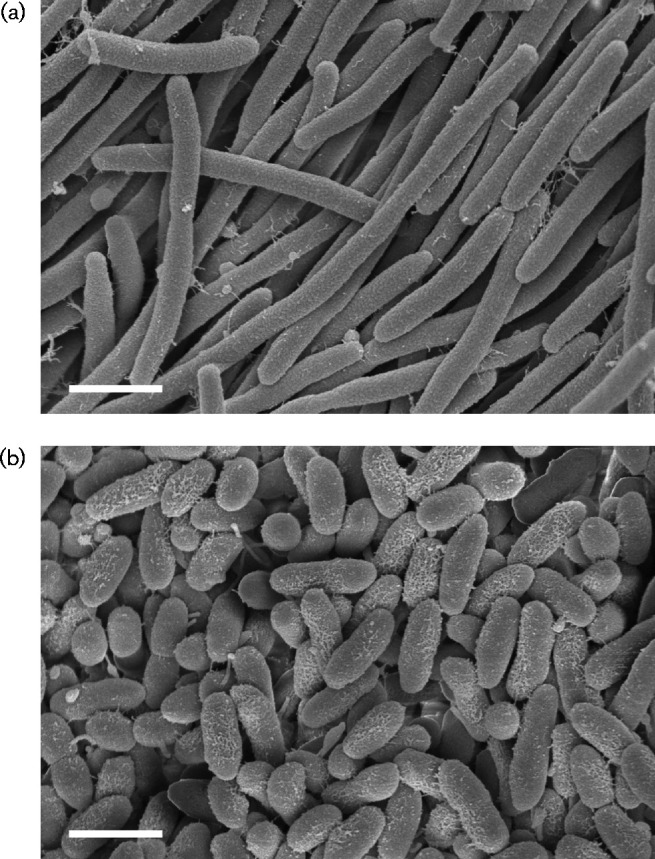
Scanning electron micrographs of cells of strains wkB301T (a) and wkB309 (b). Bars, 1 μm.
Biochemical properties of strains wkB301T and wkB309 were determined with API 20 NE and API 50 CH kits (bioMérieux) according to manufacturer's protocols. Catalase activity was tested by addition of cells directly to 3 % H2O2 and observing for generation of gas. Presence of cytochrome c oxidase was determined with OxiStrips (Hardy Diagnostics). Acetoin production was assayed by addition of Voges–Proskauer reagents (Hardy Diagnostics) to strains cultured in Methyl-Red Voges–Proskauer broth for 5–7 days. We tested for flexirubin-type pigments using the KOH method as recommended by Bernardet et al. (2002). Antibiotic susceptibility tests against ampicillin, chloramphenicol, gentamicin, kanamycin, spectinomycin, streptomycin and tetracycline were performed with MIC test strips (Table S3). Salt tolerance was tested by cultivation in LB with NaCl added in 0.5 % increments.
Cellular fatty acid composition of strains wkB301T and wkB309 was determined by fatty acid methyl ester (FAME) analysis (Sherlock MIS-MIDI; Microbial ID). Strains were plated on 5 % sheep blood trypticase soy agar at 35 °C for 48 h under 3–5 % CO2, and cells from the late-exponential phase portion were harvested for FAME analysis. Respiratory quinone and polar lipid analysis of strain wkB301T was performed on lyophilized cells by the Identification Service of the DSMZ, according to the protocols of Tindall (1990) and Tindall et al. (2007) (Fig. S1), respectively.
Differential characteristics of strains wkB301T and wkB309 and those of their closest relatives are summarized in Tables 1 and S4. Compared with most species in clades E, C and the genus Ornithobacterium, strains wkB301T and wkB309 had distinctive fatty acid profiles (containing substantial C16 : 0 and C16 : 0 3-OH), low DNA G+C content (29–31 mol%), and lack of flexirubin-type pigment production and cytochrome oxidase activity. Phylogenetic reconstruction showed that these strains comprise a divergent monophyletic lineage (Fig. 2). These data indicate that strains wkB180 and wkB301T are representatives of a novel species and genus within the family Flavobacteriaceae of the phylum Bacteroidetes, for which we propose the name Apibacter adventoris gen. nov., sp. nov. Additionally, at 95.8 % 16S rRNA gene sequence identity and 77.5 % average nucleotide identity (by the method of Goris et al., 2007) to wkB301T, strain wkB309 is sufficiently distinct to warrant eventual classification as a representative of its own species within the genus Apibacter. Further characterization will be needed to delineate the taxonomic status of strain wkB309 and other similar isolates.
Table 1.
Differential characteristics of strains wkB301T, wkB309 and closely related members of the family Flavobacteriaceae
Taxa: 1, wkB301T; 2, wkB309; 3, Empedobacter brevis CCUG 7320T; 4, O. rhinotracheale (multiple strains); 5, C. gleum (multiple strains). +, Positive; − , negative; v, variable between strains; v(+), majority positive; v( − ), majority negative; nd, no data. All strains were negative for acid production from d-mannitol and d-sorbitol. No data for O. rhinotracheale and C. gleum were available for acid production from erythritol, l-arabinose, l-xylose, methyl β-d-xylopyranoside, l-sorbose, methyl α-d-mannopyranoside, methyl α-d-glucopyranoside, amygdalin, arbutin, inulin, melezitose, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, potassium gluconate, potassium 2-ketogluconate or potassium 5-ketogluconate, but these were negative in the other strains. No data for O. rhinotracheale were available for acid production from l-rhamnose, dulcitol, inositol, cellobiose or raffinose, but these were negative in the other strains. No data for acid production from l-fucose in C. gleum, but this was negative in the other strains.
| Characteristic | 1 | 2 | 3* | 4† | 5‡ |
|---|---|---|---|---|---|
| Isolated from: | Giant honey bee (Apis dorsata) | Asian honey bee (Apis cerana) | Diseased humans, water | Avian respiratory tract | Diseased humans, water, soils, hospitals |
| Genome size§ | 2.7 Mb | 2.3 Mb | 3.79 Mb | 2.40 Mb | 5.57 Mb |
| DNA G+C content (mol%) | 29.0 | 30.6 | 32.7 | 37–39 | 36–39 |
| Gliding motility | − | + | − | − | − |
| Flexirubin pigment production | − | − | + | + | + |
| Growth on: | |||||
| MacConkey agar | − | − | + | − | + |
| Nutrient agar | − | − | + | nd | nd |
| Enzymic activity: | |||||
| Arginine dihydrolase | − | − | − | v( − ) | − |
| β-Galactosidase (PNPG) | − | + | − | + | v(+)|| |
| β-Glucosidase (aesculin) | + | + | + | − | + |
| Catalase | + | + | + | − | + |
| Cytochrome oxidase | − | − | + | + | + |
| Indole production | − | − | + | − | + |
| Gelatinase | − | − | + | − | + |
| Nitrate reduction | + | − | − | − | v |
| Urease | − | − | − | v(+) | v |
| Acid production from: | |||||
| Glycerol | + | − | − | nd | v |
| d-Arabinose | − | − | − | nd | v(+) |
| d-Ribose | − | − | − | v(+) | nd |
| d-Xylose | − | − | − | − | v( − ) |
| d-Adonitol | + | − | − | nd | − |
| d-Galactose | − | − | − | v(+) | nd |
| d-Glucose | + | + | − | v(+) | + |
| d-Fructose | + | + | − | v(+) | + |
| d-Mannose | + | + | − | v(+) | nd |
| N-Acetylglucosamine | − | − | − | v(+) | nd |
| Salicin | − | + | − | nd | − |
| Maltose | − | − | + | v(+) | + |
| Lactose | − | − | − | v(+) | − |
| Sucrose | − | − | − | v( − ) | − |
| Trehalose | − | − | − | − | + |
| Starch | − | − | + | + | nd |
| Glycogen | − | − | + | nd | nd |
| d-Arabitol | + | − | − | nd | nd |
| l-Arabitol | + | − | − | nd | nd |
| Major cellular fatty acids | iso-C15 : 0 (23.2 %), iso-C17 : 03-OH (18.0 %), C16 : 0(15.1 %), C16 : 0 3-OH (11.0 %) | iso-C17 : 0 3-OH (22.2 %), C16 : 0 (21.6 %), iso-C15 : 0(15.4 %), C16 : 0 3-OH (13.8 %) | iso-C15 : 0 (24.5 %), iso-C17 : 0 3-OH (17.6 %), C16 : 1ω7c and/or C16 : 1ω6c (15.5 %) | iso-C15 : 0 (57.4 %), iso-C17 : 0 3-OH (20.2 %), iso-C15 : 03-OH (8.1 %) | iso-C15 : 0 (35.0 %), iso-C17 : 0 3-OH (22.0 %), iso-C17 : 1ω9c (20.0 %) |
Data from Zhang et al. (2014).
Data from van Empel et al. (1997) and Vandamme et al. (1994), where 21 and 443 strains were tested, respectively.
Data from Holmes et al. (1984), for 12 strains, and Hugo et al. (2003), for two strains. Additional data from Bernardet et al. (2002, 2006).
Size information from NCBI genomes.
ONPG assay.
The ecological niche of Apibacter adventoris gen. nov., sp. nov. is not well understood, although current evidence suggests it is a bee gut specialist. Like other members of the bee gut microbiota, our strains did not readily grow in ambient air, but instead required a microaerobic atmosphere (Kwong & Moran, 2013; Engel et al., 2013). A smaller genome size compared with free-living relatives (Table 1), and the concomitant reduced functional capabilities, suggests Apibacter adventoris has a host-restricted, rather than a heterotrophic, free-living lifestyle. The limited 16S rRNA sequence data available thus far (Fig. 1) show clustering of sequences according to host species, indicating that different bee hosts may harbour their own specialized species or strains of the genus Apibacter. The clustering observed is independent of geographical origin: the Apis dorsata-derived strains wkB180 and wkB301T are from Singapore and Malaysia, respectively, while the Apis cerana-derived strains wkB309 and Bacteroidetes sp. B1 are from Malaysia and South Korea, respectively. Greater sampling is thus needed to demonstrate the ecological ranges of various species and strains within the genus Apibacter gen. nov., and the nature of their relationship with bee hosts.
Description of Apibacter gen. nov.
Apibacter (a.pi.bac′ter. L. fem. n. apis bee; N.L. masc. n. bacter a rod; N.L. masc. n. Apibacter a rod-shaped bacterium first isolated from bees).
Cells are Gram-staining-negative, microaerophilic, mesophilic, salt-intolerant and rod-shaped. Positive for catalase and negative for oxidase. Flexirubin-type pigments are not produced. The major fatty acids are iso-C15 : 0, iso-C17 : 0 3-OH, C16 : 0 and C16 : 0 3-OH. Menaquinone 6 (MK-6) is the only respiratory quinone detected. The major polar lipids include phosphatidylethanolamine, an unidentified lipid, two phospholipids, three aminolipids, a phosphoaminolipid and a glycolipid. The DNA G+C content is 29–31 mol%. The genus belongs to the family Flavobacteriaceae in the phylum Bacteroidetes. The type species is Apibacter adventoris.
Description of Apibacter adventoris sp. nov.
Apibacter adventoris (ad.ven.to′ris. L. gen. n. adventoris of a visitor, of a guest, referring to the affiliation with the gut of bee hosts).
In addition to the characteristics of its genus, the following are displayed. Cells are approximately 2.0 × 0.3 μm in size, but may form longer filaments. Grows on heart infusion agar, brain heart infusion agar, trypticase soy agar and Columbia agar at 20–40 °C (optimum 30–35 °C) in 5 % CO2. No growth in anaerobic or normal atmospheric conditions. Grows in LB medium with 0–1.0 % NaCl (optimum, 0–0.5 %), pH 6.0–8.0. Positive for Voges–Proskauer reaction and aesculinase (β-glucosidase). Positive for nitrate reduction (strain wkB309 is negative). Negative for arginine dihydrolase, β-galactosidase (strain wkB309 is positive), indole production, gelatinase and urease. Acid is produced from d-adonitol, arabitol and glycerol (strain wkB309 is negative), and d-glucose, d-fructose and d-mannose. Resistant to the aminoglycosides kanamycin and gentamicin. Fatty acid composition of the type strain, wkB301T, is as follows: iso-C15 : 0 (23.2 %), iso-C17 : 0 3-OH (18.0 %), C16 : 0 (15.1 %), C16 : 0 3-OH (11.0 %), C16 : 1ω7c and/or C16 : 1ω6c (9.6 %), iso-C15 : 0 3-OH (4.5 %), C18 : 2ω6,9c and/or anteiso-C18 : 0 (4.0 %), C18 : 1ω9c (2.5 %), iso-C17 : 0 (1.8 %) and anteiso-C15 : 0 (1.5 %).
The type strain is wkB301T ( = NRRL B-65307T = NCIMB 14986T), which was isolated from the gut of an adult worker Apis dorsata collected from Kuala Lumpur, Malaysia. The DNA G+C content of the type strain is 29.0 mol%.
Supplementary Data
Supplementary Data
Acknowledgements
We thank Gary Kong-Wah Sing and Eunice Soh for their assistance in collecting bee specimens, and Luis Medina for strain cultivation. This work was supported by Yale University, the Yale University Department of Ecology and Evolutionary Biology Chair's Fund, Sigma Xi Grants-in-Aid of Research, and a Canadian Natural Sciences and Engineering Research Council Postgraduate Scholarship (to W. K. K.), and the US National Science Foundation Dimensions of Biodiversity awards 1046153 and 1415604 (to N. A. M.).
Footnotes
The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequences of strains wkB301T, wkB309 and wkB180 are KT149221, KT149220 and KT149222 respectively. Protein-coding genes from these strains are deposited under the accession numbers KT149223–KT149282.
Four supplementary tables and one supplementary figure are available with the online Supplementary Material.
References
- Ahn J.-H., Hong I.-P., Bok J.-I., Kim B.-Y., Song J., Weon H.-Y. Pyrosequencing analysis of the bacterial communities in the guts of honey bees Apis cerana and Apis mellifera in Korea. J Microbiol. 2012;50:735–745. doi: 10.1007/s12275-012-2188-0. [DOI] [PubMed] [Google Scholar]
- Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D. R., Fernandes G. R., Tap J., Bruls T., other authors Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babendreier D., Joller D., Romeis J., Bigler F., Widmer F. Bacterial community structures in honeybee intestines and their response to two insecticidal proteins. FEMS Microbiol Ecol. 2007;59:600–610. doi: 10.1111/j.1574-6941.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Bernardet J. F., Nakagawa Y., Holmes B., Subcommittee on the taxonomy of Flavobacterium and Cytophaga-like bacteria of the International Committee on Systematics of Prokaryotes Proposed minimal standards for describing new taxa of the family Flavobacteriaceae and emended description of the family. Int J Syst Evol Microbiol. 2002;52:1049–1070. doi: 10.1099/00207713-52-3-1049. [DOI] [PubMed] [Google Scholar]
- Bernardet J.-F., Hugo C., Bruun B. The genera Chryseobacterium and Elizabethkingia . In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., editors. The Prokaryotes: a Handbook on the Biology of Bacteria. 3rd edn. vol. 7. New York: Springer; 2006. pp. 638–676. [Google Scholar]
- Engel P., James R. R., Koga R., Kwong W. K., McFrederick Q., Moran N. A. Standard methods for research on Apis mellifera gut symbionts. J Apic Res. 2013;52:1–24. doi: 10.3896/IBRA.1.52.4.07. [DOI] [Google Scholar]
- Goris J., Konstantinidis K. T., Klappenbach J. A., Coenye T., Vandamme P., Tiedje J. M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- Holmes B., Owen R. J., Steigerwalt A. G., Brenner D. J. Flavobacterium gleum, a new species found in human clinical specimens. Int J Syst Bacteriol. 1984;34:21–25. doi: 10.1099/00207713-34-1-21. [DOI] [Google Scholar]
- Hugo C. J., Segers P., Hoste B., Vancanneyt M., Kersters K. Chryseobacterium joostei sp. nov., isolated from the dairy environment. Int J Syst Evol Microbiol. 2003;53:771–777. doi: 10.1099/ijs.0.02232-0. [DOI] [PubMed] [Google Scholar]
- Koch H., Schmid-Hempel P. Bacterial communities in central European bumblebees: low diversity and high specificity. Microb Ecol. 2011;62:121–133. doi: 10.1007/s00248-011-9854-3. [DOI] [PubMed] [Google Scholar]
- Kwong W. K., Moran N. A. Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria . Int J Syst Evol Microbiol. 2013;63:2008–2018. doi: 10.1099/ijs.0.044875-0. [DOI] [PubMed] [Google Scholar]
- Lim H. C., Chu C. C., Seufferheld M. J., Cameron S. A. Deep sequencing and ecological characterization of gut microbial communities of diverse bumble bee species. PLoS One. 2015;10:e0118566. doi: 10.1371/journal.pone.0118566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A., Hansen A. K., Powell J. E., Sabree Z. L. Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS One. 2012;7:e36393. doi: 10.1371/journal.pone.0036393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. E., Martinson V. G., Urban-Mead K., Moran N. A. Routes of acquisition of the gut microbiota of the honey bee Apis mellifera . Appl Environ Microbiol. 2014;80:7378–7387. doi: 10.1128/AEM.01861-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. mega6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall B. J. Lipid composition of Halobacterium lacusprofundi . FEMS Microbiol Lett. 1990;66:199–202. doi: 10.1111/j.1574-6968.1990.tb03996.x. [DOI] [Google Scholar]
- Tindall B. J., Sikorski J., Smibert R. M., Krieg N. R. Phenotypic characterization and the principles of comparative systematics. In: Reddy C. A., Beveridge T. J., Breznak J. A., Marzluf G., Schmidt T. M., Snyder L. R., editors. Methods for General and Molecular Microbiology. 3rd edn. Washington, DC: American Society for Microbiology; 2007. pp. 330–393. [Google Scholar]
- van Empel P., van den Bosch H., Loeffen P., Storm P. Identification and serotyping of Ornithobacterium rhinotracheale . J Clin Microbiol. 1997;35:418–421. doi: 10.1128/jcm.35.2.418-421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme P., Segers P., Vancanneyt M., van Hove K., Mutters R., Hommez J., Dewhirst F., Paster B., Kersters K., other authors Ornithobacterium rhinotracheale gen. nov., sp. nov., isolated from the avian respiratory tract. Int J Syst Bacteriol. 1994;44:24–37. doi: 10.1099/00207713-44-1-24. [DOI] [PubMed] [Google Scholar]
- Zhang R. G., Tan X., Zhao X. M., Deng J., Lv J. Moheibacter sediminis gen. nov., sp. nov., a member of the family Flavobacteriaceae isolated from sediment, and emended descriptions of Empedobacter brevis, Wautersiella falsenii and Weeksella virosa . Int J Syst Evol Microbiol. 2014;64:1481–1487. doi: 10.1099/ijs.0.060178-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data