ABSTRACT
This systematic review and landscape analysis describes patterns in dietary meat (skeletal muscle and associated tissues from mammalian, avian, and aquatic species; i.e., muscle foods) categories (CAT) and descriptions (DESCR) used throughout nutrition-related chronic disease literature, as there is anecdotally noted variation. A total of 1020 CAT and 776 DESCR were identified from 369 articles that assessed muscle food consumption and primary prevention of cardiovascular disease, obesity, type 2 diabetes, or cancer in adults ≥19 y from PubMed, Cochrane, and CINAHL up to March 2018. Specificity of CAT was analyzed on an empirical 1–7 ordinal scale as: 1) broad/undescriptive, “fish”; 2) muscle food type, “red meat”; 3) species, “poultry”; 4) broad + 1 descriptor, “processed meat”; 5) type/species + 1 descriptor, “fresh red meat”; 6) broad/type + 2 descriptors, “poached lean fish”; and 7) specific product, “luncheon meat.” Median CAT specificity for randomized controlled trials (RCTs) and observational studies (OBSs) was 3 and 2 points out of 7, respectively, with no differences between chronic disease types. Specificity of OBS CAT was higher in recent articles but RCT CAT became less specific starting in the 2000s. RCT CAT were 400% more likely to include species, 500% more likely to include leanness, but 400% less likely to include processing degree compared with OBS CAT. A DESCR was included for 76% and 82% of OBS and RCT CAT, respectively. Researchers described processed meat, red meat, and total meat CAT more commonly than poultry or fish CAT. Among processed meat DESCR, 31% included a common term used in public regulatory definitions. In conclusion, muscle food categories and descriptions are substantively different within and between experimental and observational studies and do not match regulatory definitions. A practical muscle food classification system is warranted to improve interpretation of evidence regarding muscle food consumption and chronic disease.
Keywords: muscle foods, red meat, white meat, fish, poultry, dietary guidance, food group terminology, dietary intake assessment, animal proteins, flesh foods
Introduction
Dietary meat is a fundamental component of eating patterns within many populations, yet the term “meat” is disparately described in regulatory and scientific settings. The American Meat Science Association developed a lexicon to provide standardized descriptions of selected meat and poultry products (1). The American Meat Science Association defines “meat” as “skeletal muscle and associated tissues derived from mammalian, avian, reptilian, amphibian, and aquatic species harvested for human consumption” (1). However, the term “meat” is often equated to mammalian species by nutrition researchers, health practitioners, and the public. For example, the 2015–2020 Dietary Guidelines for Americans describes meat as: “Meat (also known as “red meat”) – all forms of beef, pork, lamb, veal, goat, and nonbird game (e.g. venison, bison, elk)” (2). The distinctly different ways in which the American Meat Science Association (1) and 2015–2020 Dietary Guidelines for Americans (2) describe the term “meat” highlight the varied use of this term and creates confusion among health researchers, clinical professionals, and policymakers. Due to the noted misconception that “meat” is equated with mammalian sources only, the term “muscle food” is used for the present systematic review and landscape analysis to be inclusive of all species.
Although the scientific community is aware of inconsistent muscle food categorizations and descriptions in chronic disease research (2), the types and magnitude of inconsistencies lack systematic assessment. Understanding and documenting variation in muscle food categories and descriptions has meaningful implications in accurately measuring dietary intakes and inferring causal associations between intake and disease. Therefore, this systematic review and landscape analysis describes patterns from published articles regarding muscle food consumption and chronic disease outcomes. Patterns assessed included changes in the content or specificity of muscle food categories and descriptions across publication date, between observational and experimental research designs, and among chronic disease types [i.e., obesity, cardiovascular disease (CVD), type 2 diabetes mellitus (T2DM), and various types of cancer].
Methods
Search process
Research question, search strategy, and data extraction
This systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (https://www.crd.york.ac.uk/prospero/) before the search commenced (ID# CRD42018078994) and is in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (3). The Population, Intervention, Comparator Outcome, and Study design (PICOS) criteria used to define our research question is presented in Table 1. This systematic review included observational studies (OBSs), randomized controlled trials (RCTs), and systematic reviews/meta-analyses of OBSs or RCTs that assessed associations between or effects of, respectively, consuming muscle foods (1, 4) on human chronic disease risk or commonly recognized risk factors. The overarching term “muscle food” is used throughout this article to refer to skeletal muscle and associated tissues from livestock, poultry, or seafood (1, 4). Articles were included if: 1) primary independent variable(s) of interest described in the article purpose statement included consumption of muscle food(s); 2) primary dependent variable(s) of interest described in the article purpose statement included the primary prevention of ≥1 chronic disease outcome or associated risk factor(s), i.e., prevention of the first occurrence of the main chronic disease assessed by researchers; and 3) included participants aged ≥19 y and not pregnant or lactating. Chronic disease outcomes included obesity, CVD, T2DM, and cancer, as well as various risk factors including body weight, BMI, waist circumference, body composition, fasting blood lipids, lipoproteins, apolipoproteins, inflammatory markers, and markers of glycemic control. Postulated cancer risk factors were not included due to lack of validation and consensus in the field; hence, only articles assessing cancer cases were included.
TABLE 1.
Description of PICOS criteria used for a systematic review and landscape analysis of muscle food categorizations and description used throughout chronic disease literature1
| Population | Adults aged ≥19 y; males and females; females not pregnant or lactating |
| Intervention | Muscle food consumption (for example: meat, fish, poultry) |
| Comparator | Not applicable |
| Outcome | Primary prevention of nutrition-related chronic disease, i.e., outcomes and/or associated risk factors of obesity, cardiovascular disease, type 2 diabetes, and cancer |
| Study design | Observational studies (prospective cohorts, case-controls, and cross-sectional analyses) and randomized controlled trials (acute and longitudinal feeding trials) |
| Research question | How are muscle foods categorized and described in research assessing primary prevention of nutrition-related chronic diseases? |
PICOS, Population, Intervention, Comparator Outcome, and Study design.
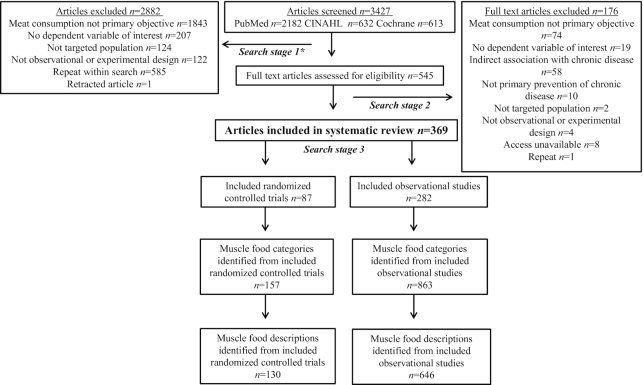
Potentially eligible articles were identified via a search of 3 electronic health research databases [PubMed, Cochrane, and CINAHL (Cumulative Index of Nursing and Allied Health Literature)] up to March 2018. Database and search terms were selected with assistance from Purdue University's Health and Life Sciences Library Division and are described in Supplemental Table 1. Each article identified during the search process (n = 3427) was independently assessed by 2 reviewers (LEO and CLG) to determine eligibility. A third reviewer (KEB or WWC) was consulted if the 2 primary reviewers could not reach a consensus on article inclusion or exclusion. The search and data extraction processes consisted of the following 3 stages: 1) potential eligibility based on information provided in the title and abstract, 2) confirmation of eligibility based on information provided in the purpose statement of the full text of qualified abstracts, and 3) data extraction from full text articles if deemed qualified. The following information was extracted from all qualified full text articles: 1) author; 2) year of publication; 3) country where research was conducted; 4) purpose statement as reported by the researchers in the introduction; 5) muscle food categorization(s) as independent variable(s); 6) description(s) of muscle food categories if provided; 7) research design; 8) chronic disease-dependent variables categorized into obesity, CVD, T2DM, and cancer; and 9) categorizations and descriptions of other pertinent muscle foods assessed by researchers. All doubly extracted data were crosschecked and confirmed for accuracy. Article authors were not contacted for additional information, risk of bias for included articles was not assessed, and strength of evidence was not graded as the purpose of this present analysis was to assess how muscle food categories and descriptions were reported in published articles. Extracted data regarding the author, publication year, PubMed ID, category name, and category description are presented in Supplemental Tables 2 and 3. Results of the search are presented in Figure 1.
FIGURE 1.
Search process of a systematic review and landscape analysis assessing muscle food categorization and descriptions in chronic disease literature. *The search process and data extraction consisted of the following 3 stages: 1) potential eligibility based on information provided in the abstract, 2) confirmation of eligibility based on information provided in the full text if abstract qualified, 3) data extraction from full text articles once deemed qualified.
Assessment of muscle food categories
Coding
The term that researchers used to refer to a muscle food or muscle food grouping was considered a “category” in this analysis. The amount of detail, i.e., the “specificity,” included in a category term was coded from 1 (most broad) to 7 (most specific) on an ordinal scale, as described further in Table 2 (5–15). The text mining package (16) was used in RStudio 3.4.4 (The R Foundation for Statistical Computing) to build word clouds and visually represent the most common muscle food categories identified by researchers in the included articles. The mapping global data package, rworldmap (17), was used to develop figures displaying frequency of country of origin of included articles.
TABLE 2.
Overview of muscle food category specificity and description scales with examples from a systematic review and landscape analysis assessing muscle food categorization and descriptions in chronic disease literature
| Scale | Example | |
|---|---|---|
| Ordinal muscle food category resolution scale | ||
| 1 | Broad or undescriptive category | “Meat” (5)1 |
| 2 | Type of meat | “Red meat” (6) |
| 3 | Species-specific | “Beef” (7) |
| 4 | Broad category plus 1 descriptor2 | “Processed meat” (8) |
| 5 | Type or species plus 1 descriptor | “Fresh red meat” (9) |
| 6 | Broad, type, or species with ≥2 descriptors | “Poached lean fish” (10) |
| 7 | Specific product | “Luncheon meats” (11) |
| Discrete muscle food description factor scale | ||
| 1 | One factor in a description3 | “Beef, lamb, and pork” (12)1 |
| 2 | Two factors in a description | “All types of cold cuts, bacon, ham, hotdogs, and sausages from red and white meats” (13) |
| 3 | Three factors in a description | “Cooked lean red meat with all visible fat removed” (14) |
| 4 | Four factors in a description | “Select grade top round, chuck shoulder pot roast and 95% lean ground beef” or “prepared via braising, grilling, or frying (95% lean ground beef only)” (7) |
| 5 | Five factors in a description | “Poultry (chicken, cold cuts, ground, turkey), fish (fresh, frozen, canned), and low-fat hotdogs and sausages, which are usually made from turkey” (15) |
References correspond to the article reference list and are examples of descriptions used by researchers in observational studies and randomized controlled trials identified in this systematic review.
"Descriptors" included leanness, degree of processing, origin, size, or cooking method.
Descriptive factors included the following: 1) species, e.g., “beef” or “chicken”; 2) specific cut or product, e.g., “sirloin” or “ham”; 3) food dish, e.g., “mixed dish”; 4) processing method, e.g., “salted” or “cured”; 5) muscle food type, e.g., “white meat” or “fish”; 6) specification of fat content or using a description of leanness; 7) nonspecific or broad description, e.g., “all meat” or “total meat”; 8) cooking method or doneness; 9) preparation method, e.g., “sliced” or “ground”; 10) quality grade, e.g., “choice” or “select”; 11) geographical origin or location; 12) animal specification, e.g., animal age; 13) phrases, e.g., “processed” or “other.”
Statistics
A generalized linear mixed model (GLMM; PROC GLIMMIX, SAS 9.4, SAS Institute Inc.) was used to model muscle food category specificity (as an ordinal categorical variable) by publication date for OBSs and RCTs independently. The number of muscle food categories assessed per article was included as a covariate because a higher number of muscle food categories per article could represent categories that were more specific (i.e., the contrast of assessing 1 category of “meat” compared with 2 categories of “processed meat” and “fresh meat”). World region was also included as a covariate to adjust for potentially unmeasurable differences in muscle food types and intake assessment methods. Article ID was used as a random effect to control for articles that identified >1 muscle food category. When statistical significance was identified on an ordinal scale, a weighted least squares regression model was used to model muscle food category specificity as a continuous outcome variable, weighted by the covariate of number of included articles published per year and adjusted for yearly average count of muscle food categories. All analyses were performed independently for OBSs and RCTs for all muscle food categories. In a separate analysis, a 1-factor ANOVA was performed to assess differences in muscle food category specificity among OBSs and RCTs, world regions, and chronic disease types. The main analyses assess the totality of muscle food groups extracted from included studies. Supplementary material presents independent analyses for water-based muscle foods (such as fish or shellfish) and land-based muscle food (such as beef, pork, and poultry) when appropriate.
Assessment of muscle food descriptions
Coding
In this analysis, ‘muscle food descriptions’ were considered the explanation of muscle food categories provided to human subjects or assessed by researchers. Terminology used in researchers’ descriptions of muscle foods, such as species (e.g., “beef”) or leanness (e.g., “95% lean”) were considered description factors and are further described in Table 2. The number of muscle food description factors used to describe each muscle food category name was quantified on a discrete 1–5 scale to assess the level of detail provided by researchers. A combination of any 5 description factors was the maximum number of factors observed among all descriptions. The mapping global data package, rworldmap (17), was used to develop figures displaying frequency of country origin of extracted muscle food category descriptions. Among description factors in this analysis, the degree of processing was considered a physical change of the product (i.e.', “unprocessed” or “processed,” “fresh” or “frozen,” “ground” or “minced”). Processing methods were considered as preservative processes (i.e., the addition of ingredients such as salt, phosphate, or nitrite; using the terms “smoking,” “curing,” etc.) and leanness was considered the identification and/or designation of fat content in muscle food products (i.e., using the term “lean,” “85% lean,” etc.).
Statistics
Frequency of description factors (quantity of each level of the discrete scale), frequency of experimental design, and chronic disease outcomes assessed in articles were determined (PROC FREQ, SAS 9.4, SAS Institute Inc.). A binary logit model (PROC GLIMMIX, SAS 9.4, SAS Institute Inc.) was used to estimate probabilities of each description factor (dependent variable: presence/absence of description or description factor) included in muscle food categories (independent variable; e.g., “fish,” “poultry,” “red meat,” “processed meat”). Muscle food category mean conditional probabilities (95% confidence limits) were calculated and compared using the PDIFF function at α = 0.05. A GLMM (PROC GLIMMIX, SAS 9.4, SAS Institute Inc.) was used to model muscle food description specificity (as a discrete categorical variable) by publication date for OBS and RCT in the same manner as described previously for category specificity.
Results
Search process
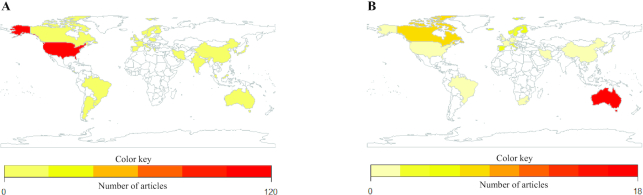
All extracted categories and descriptions including first author, publication date, and PubMed ID number are presented in Supplemental Tables 2 and 3, which also serves as the reference list for all articles included in this systematic review. A total of 369 articles (n = 3427 originally reviewed) met inclusion criteria for this systematic review, including 282 OBS (165 prospective, 72 case controls, 35 cross-sectional assessments, and 10 meta-analyses) and 87 RCTs (7 acute feeding trials, 78 longitudinal trials, and 2 meta-analyses). The US and Canada/Australia produced the most OBS and RCT articles in the final data set, respectively (Figure 2). The most common outcomes assessed for OBS and RCT were cancer and CVD, respectively, [OBS: cancer n = 163, 58%, (Supplemental Table 4); CVD n = 68, 24%; T2DM n = 30, 11%; obesity n = 5, 2%; and ≥2 outcomes n = 16, 6% and RCT: cancer n = 1, 1%; CVD n = 58, 67%; T2DM n = 10, 12%; obesity n = 6, 7%; and ≥2 outcomes n = 12, 14%].
FIGURE 2.
Country of publication for observational studies (A) and randomized controlled trials (B) included in a systematic review and landscape analysis assessing muscle food categorization and descriptions in chronic disease literature.
Muscle food categories
The 25 most common OBS and RCT muscle food categories identified from the included articles are shown in Figure 3 (see Supplemental Figures 1 and 2 for land- and water-based subgroups, respectively). The median category specificity score for OBS and RCT, identified on a 7-point ordinal scale (higher numbers indicating higher specificity) as described in Table 2, were 2 (IQR = 2) and 3 (IQR = 3) points, respectively. Mean muscle food category specificity, for either OBS or RCT, did not differ among chronic disease types. Specificity levels 2 and 5 were most common among all chronic disease outcomes for OBS and RCT, respectively (except RCT T2DM risk factors were level 3; Supplemental Figure 3). Articles from Norway and the UK contained more specific OBS muscle food categories than several other countries (an average of ∼1.9 and ∼1.4 points higher, respectively). Norway was ∼2 points more specific than other countries for OBS water-based muscle food category resolution, but not land-based. There were no differences in RCT muscle food category specificity among countries or world regions. Among all muscle food categories identified, specificity of OBS categories increased with more recent publication dates but specificity of RCT categories began to decrease in the 2000s (Figure 4). Muscle food categories from RCTs were 400% more likely to be species specific, 500% more likely to specify leanness, but 400% less likely to specify degree of meat processing compared with OBS.
FIGURE 3.
Top 25 most commonly used muscle food categories in observational studies (A) and randomized controlled trials (B) from a systematic review and landscape analysis assessing muscle food categorization and descriptions in chronic disease literature. The larger text implies more frequent use. Land-based and water-based muscle food frequency word clouds are presented in Supplemental Figures 1 and 2.
FIGURE 4.
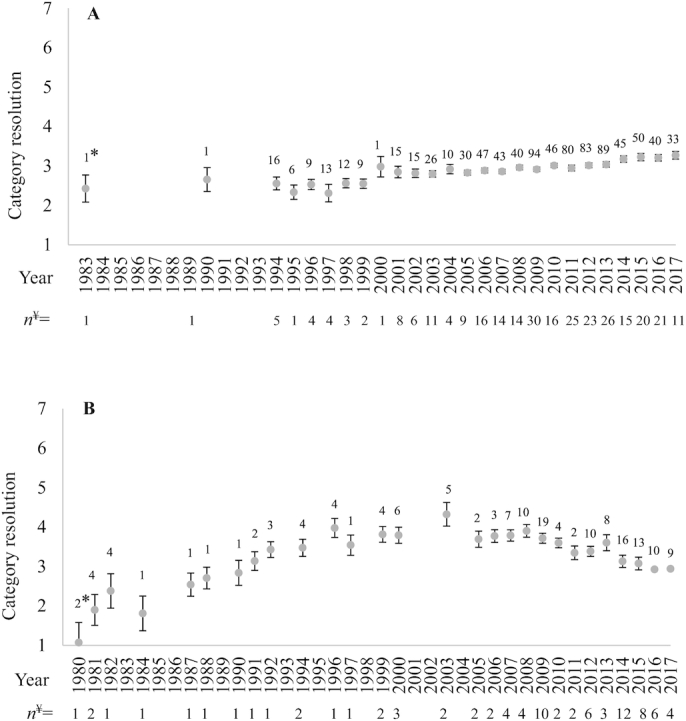
Trends in muscle food category specificity over time for observational studies (A) and randomized controlled trials (B) from a systematic review assessing muscle food categorization and descriptions in chronic disease literature. Results were analyzed via a least squares regression model weighted by total number of muscle food categories assessed per year and adjusted by the yearly average count of muscle food categories per article. *Total number of muscle food categories assessed that year, ¥number of articles published that year from the final data set. Land-based and water-based muscle food category resolution showed similar trends. Observational studies adjusted and weighted R2 = 0.39, coefficient for year = 0.03 (0.01 to 0.05). Randomized controlled trials adjusted and weighted quadratic R2 = 0.54, transformed coefficients nonsensical.
Muscle food descriptions
Overview
A total of 1020 total muscle food categories were identified. Of OBS and RCT articles, 75% (n = 646) and 83% (n = 130) of categories were accompanied by a description, respectively. Articles from the United States and Australia for OBSs and RCTs, respectively, were most likely to provide a muscle food category description (Supplemental Figure 4). The number of factors included in muscle food descriptions increased for RCTs per increasing year of publication date but was unchanged for OBS (Supplemental Figure 5). Less than 1% and 3% of OBSs and RCTs, respectively, reported the maximum of 5 description factors per muscle food. Overall, species was most commonly used by researchers to describe muscle food categories, more so for RCTs than OBSs. Beef and pork were the most commonly identified land-based muscle food species for both OBSs and RCTs, and mackerel and cod were the most commonly identified water-based species for OBSs and RCTs, respectively (Supplemental Table 5). Muscle food cut name or product name was included in ≥40% of OBS and RCT descriptions (Table 3). Processing descriptors (“fresh” or “frozen,” “unprocessed” or “processed,” “ground” or “minced”) were more commonly reported for OBSs, whereas leanness descriptors (using the term “lean” or including a fat specification) were more commonly reported for RCTs.
TABLE 3.
Counts and percentages of muscle food description factors of observational studies and randomized controlled trials from a systematic review and landscape analysis assessing muscle food categorization and descriptions in chronic disease literature
| Observational studies | Randomized controlled trials | |||
|---|---|---|---|---|
| Terminology in description | n | %1 | n | %2 |
| Species | 404 | 63 | 112 | 86 |
| Processing methods | ||||
| “Smoking” or “smoked” terms | 38 | 6 | 2 | 2 |
| Preservation by use of other ingredients3 | 26 | 4 | 4 | 3 |
| Degree of processing | ||||
| “Fresh” or “frozen” terms | 56 | 9 | 12 | 9 |
| “Unprocessed” term | 15 | 2 | 2 | 2 |
| “Processed” term | 124 | 19 | 6 | 5 |
| “Ground” or “minced” terms | 48 | 7 | 19 | 15 |
| Leanness | ||||
| “Lean” term | 24 | 4 | 25 | 19 |
| Fat specification | 15 | 2. | 12 | 9 |
| Muscle cut or product name | 337 | 52 | 53 | 41 |
| Cookery method or “cooked” term | 35 | 5 | 6 | 5 |
| Other terminology4 | 487 | 75 | 78 | 60 |
Percentage calculated as frequency of terminology used/646 descriptions from observational studies provided × 100.
Percentage calculated as frequency of terminology used/130 descriptions from randomized controlled trials provided × 100.
Includes chemical preservatives such as nitrates.
Other terminology includes specifying a local term, using “all” or “total” to describe muscle foods, or using “products” in the description.
Among OBSs, the probability (P) that a description was provided was higher for processed meat (P = 0.87), red meat (P = 0.85), and total meat (P = 0.91) categories than for fish (P = 0.60) or poultry (P = 0.63) categories (Table 4). Articles including red meat and poultry were most likely to list a species, whereas articles incorporating processed meat were most likely to include a product term or to list cut or product names. Small numbers of meat (n = 8), red meat (n = 11), poultry (n = 5), and fish (n = 10) categories were assessed in RCTs (conditional probabilities of description terminology are presented in Supplemental Table 6).
TABLE 4.
Conditional probabilities within each column of description factors included by authors for selected muscle food categories among observational studies from a systematic review and landscape analysis assessing muscle food categorization and descriptions in chronic disease literature1
| Components of descriptions | |||||||
|---|---|---|---|---|---|---|---|
| Category | n | Description provided | Included species name | Included muscle cut or product name | Included leanness term or fat specification | Included “all” or “total” terms | Included “product” term |
| Fish | 82 | 0.60b (0.49–0.70) | 0.34b,c (0.25–0.45) | 0.15d (0.08–0.24) | 0.07 (0.03–0.15) | 0.17a,b (0.10–0.27) | 0.21c (0.13–0.31) |
| Poultry | 52 | 0.63b (0.50–0.75) | 0.62a (0.48–0.74) | 0.21 c,d (0.12–0.34) | 0.02 (0–0.13) | 0.12b (0.05–0.23) | 0.21c (0.12–0.34) |
| Processed meat | 118 | 0.87a (0.80–0.92) | 0.18c (0.12–0.26) | 0.80a (0.71–0.86) | 0.06 (0.03–0.11) | 0.17a,b (0.11–0.25) | 0.80a (0.71–0.86) |
| Red meat | 123 | 0.85a (0.79–0.91) | 0.80a (0.72–0.86) | 0.49b (0.40–0.58) | 0.02 (0.01–0.08) | 0.20a,b (0.14–0.28) | 0.50b (0.41–0.58) |
| Total meat | 56 | 0.91a (0.80–0.96) | 0.46b (0.34–0.59) | 0.43b,c (0.31–0.56) | None2 | 0.38a (0.26–0.51) | 0.27b,c (0.17–0.40) |
| White meat | 44 | 0.84a,b (0.70–0.92) | 0.64a (0.49–0.76) | 0.25bc (0.14–0.40) | 0.20 (0.11–0.36) | 0.14a,b (0.06–0.27) | 0.25b,c (0.14–0.40) |
Data are presented as least squares means (95% confidence limits). a–cMeans within a column without a common superscript differ at P <0.05. A binary logit model was used to estimate probabilities of each description factor included in muscle food categories.
No probabilities were calculated for a component with 0 observations.
In general, the number of description factors did not increase as specificity of category name increased on each respective scale (Figure 5). Among OBS, categories with a specificity level 2 (type of muscle food) were most commonly described using 1, 2, or 3 description factors. The next most common combination were categories with a specificity level 4 (broad with 1 descriptor) which were described using 1 or 2 description factors. Among RCTs, categories with a specificity level 3 (species) were described with 3 description factors and categories with a specificity level 5 (type or species with 1 descriptor) were described with 2 description factors. Among OBSs and RCTs, muscle food categories were most commonly described using 1 or 2 description factors across chronic disease types (Supplemental Figure 6).
FIGURE 5.
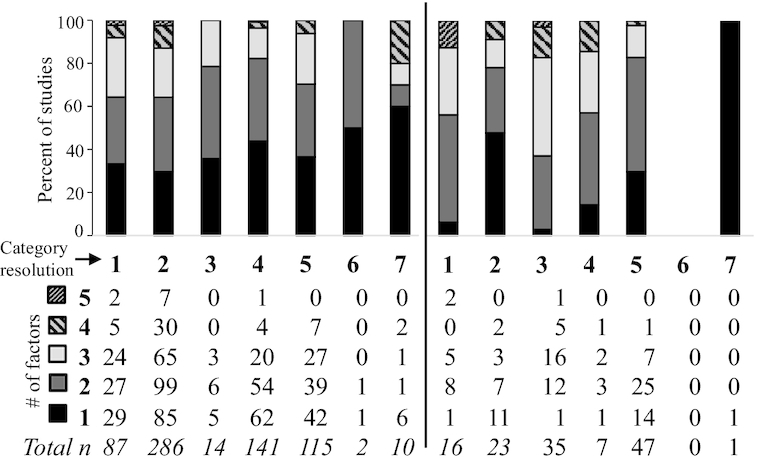
Distribution of description factors across category resolution from a systematic review and landscape analysis assessing muscle food categorization and descriptions in chronic disease literature. Category resolution, i.e., specificity, was analyzed on an empirical ordinal scale as 1) broad/undescriptive, e.g., “fish”; 2) type of muscle food, e.g., “red meat”; 3) species, e.g., “poultry”; 4) broad + 1 descriptor, e.g., “processed meat”; 5) type/species + 1 descriptor, e.g., “fresh red meat”; 6) broad/type + 2 descriptors, e.g., “poached lean fish”; and 7) specific product, e.g., “luncheon meat.” Number of factors included in the description were analyzed on a discrete 1–5 scale.
Red meat descriptions
Of articles that assessed red meat as a category, 10% (n = 12) of OBSs and 17% (n = 2) of RCTs used “unprocessed,” “fresh,” or muscle cut names to describe unprocessed red meat. Thirty-eight percent (n = 44) of OBSs described red meat categories using the term “processed” or included common processed meat product names such as “bacon,” “ham,” “sausage,” or “deli meats,” compared with 8% (n = 1) of RCTs. Twenty-seven percent (n = 33) of OBSs and 42% (n = 5) of RCTs described red meat using species names without providing additional information such as whether the form of red meat was unprocessed, processed, or lean.
Processed meat descriptions
Definitions of processed meat or further processing of meat provided by the USDA's Food Safety Inspection Service (4), 2015–2020 Dietary Guidelines for Americans (2), International Agency for Research on Cancer (18), and World Cancer Research Fund (19) vary. However, these organizations all include the terms “salting,” “smoking,” and “curing” as part of their definitions. Among studies that included an assessment of processed meat as a category, 31% (n = 39 OBS descriptions and n = 3 RCT descriptions) included a common term used by these organizations as part of the description. Among OBS studies, the probabilities that “salting,” “smoking,” or “curing” were included in a description were 0.19, 0.06, and 0.07, respectively. In comparison, a total of 81% (n = 109 of OBSs and n = 2 of RCTs) included common processed meat product names. A total of 26% (n = 34 of OBSs and n = 2 of RCTs) descriptions included both processed meat product names (i.e., “bacon,” “ham,” “sausage,” etc.) and processing methods (i.e., “curing,” “salting,” “smoking,” etc.). The probability that white meat or poultry terms were included in processed meat descriptions were 0.14 and 0.09, respectively.
White meat descriptions
Descriptions were provided for 84% (n = 37) of white meat categories for OBSs and the term “white meat” was not assessed as a category for RCTs. A total of 57% (n = 25) of OBS white meat descriptions included both chicken or poultry and fish together as part of the same description. Of these, 9 referenced leanness or a fat specification in the description. Fish, poultry (chicken and turkey), and low-fat sausages or hotdogs were included in all 9 descriptions of lean white meat. Six of these descriptions included canned fish or tuna and only 1 description included finfish or shellfish as a more specific descriptor of fish.
Discussion
Inconsistent muscle food categories and descriptions (such as meat, poultry, or seafood) are a recognized challenge for human chronic disease (20) and meat science researchers (1). To the best of our knowledge, this is the first systematic landscape assessment to characterize use of muscle food categories and descriptions in chronic disease literature. Our results provide quantitative support to the notion (2, 20) that muscle food categories and descriptions are inconsistent and substantively different within and between human observational and clinical research. Notably, ∼20% of researchers did not provide a description of the muscle food categories assessed in their articles. Further, there have not been meaningful increases in detail used to group or describe muscle food categories since the 1980s. Noted previously, inconsistent muscle food categories and descriptions hinder accurate measures of muscle food intakes, interpretations of associations between muscle food intakes and disease, as well as translation of research findings into public programs and policy (2, 20).
The amount of detail reported by OBS researchers, regarding muscle food categories and descriptions, has not increased meaningfully over time. The noted 0.03-point per publication year incremental improvement on our 7-point category resolution scale was likely trivial because category resolution remained below 4 points for all publication years. A 4-point score on our empirical scale disregards subcategories of “meat” and “fish,” particularly species-specific subcategories. This lack of granularity is likely attributable to frequent use of FFQs in OBSs. FFQs are the most commonly used dietary intake assessment method in OBSs (21), and assess broad food categories with little adaption to each research question, population of interest, or time period (21, 22). Although some FFQs have recent updates (23), others do not appear updated to reflect changes in the food supply over the past 10 y (24). Muscle food products are frequently changing based on evolving public health concerns, including lowered sodium, removal of nitrates, and even changing breeding systems to produce leaner animals (25, 26). Continuing to use dietary intake assessment methods that are unable to capture transient consumption trends are of limited value to improve muscle food methodologies, and this limits our understanding of relations between current muscle food products and health outcomes for future generations.
Researchers’ muscle food reporting methods were, on average, 1 point higher for RCT categories than OBSs. Researchers conducting RCTs on CVD or obesity-related outcomes used the most specific categorizations (score of 5 out of 7) of any subgroup assessed. However, muscle food categorizations in RCTs showed a downward trend of becoming less descriptive in the 2000s compared with those reported in previous decades. It is unclear whether a 0.02-point increase per publication year in number of factors included in descriptions counteracts the noted decline in categorization specificity. It is important to highlight that the muscle food groups assessed in RCTs were those allocated as part of treatment protocols, not from dietary intake assessments. Therefore, researchers were at liberty to describe muscle food protocols without the restraints of typical dietary intake assessment methods (21, 22). This was reflected by RCT categorizations and descriptions that contained more detail than OBSs about species and leanness. The finding that OBSs more commonly had muscle food processing descriptors whereas RCTs had more details about species and leanness likely reflects noted differences in experimental structure and dietary intake assessment methods.
A historical lack of muscle food reporting guidelines limits researchers’ abilities to accurately and consistently group and describe muscle foods in chronic disease literature. Researchers need to carefully consider species source and nutrient content when determining which muscle foods should be grouped together into broader categories for analysis (20). Although rabbit is not among the most commonly consumed muscle foods in the United States, there seemed to be a great misunderstanding of how to classify it worldwide. This review identified 19 US-based OBSs that classified “rabbit” as processed meat, processed red meat, or white meat; but OBS researchers in other countries classified “rabbit” as poultry or fresh red meat. By most definitions, rabbit would be considered a red meat, but may be inappropriately referred to as poultry due to similarities in fat content (27, 28). Another example, more relevant to the United States, was characterization of processed red meat products (i.e., “ham,” “sausage,” “hotdogs”) without indicating the degree of processing in the category name. This was problematic as commonly consumed muscle foods such as red meat should ideally be differentiated by degree and type of preservation method (18–20). Inconsistent muscle food grouping was also apparent among federal datasets such as the USDA's Food Availability Data System (29) and Food Patterns Equivalent Database (20, 30).
Conclusions regarding the effects of muscle food consumption on health outcomes are ambiguous and inconsistent (2, 20, 31–33), potentially because it was often unclear which muscle food(s) was assessed or included in analyses. For example, researchers who assessed red meat did not provide a description 15% and 18% of the time for OBSs or RCTs, respectively. Among studies that assessed “red meat” as a muscle food category, 26% and 42% of RCTs and OBSs, respectively, did not specify the degree of meat processing. Not specifying processing degree or method may contribute to unclear or erroneous conclusions regarding red meat and human health (33, 34). For example, from a previous article (35), when sandwich meats were classified as unprocessed meat, thereby disregarding that sandwich meats are generally processed, unprocessed red meat was associated with an increased risk of CVD. However, when sandwich meats were not included as unprocessed red meats, associations between unprocessed red meat and CVD risk were null (20, 34, 36–38). Similarly, grouping lean fish, fatty fish, and poultry as “white meat” poorly represent the nutritional diversity of these muscle foods (20). Including fatty fish in a white meat category may falsely attribute cardioprotective benefits (39) to lean white fish and poultry products. Muscle food descriptions developed without careful consideration of species source, nutrient content, or degree of processing can lead to confusion when interpreting results.
The collaborative interdisciplinary effort between human clinical researchers and meat scientists was a major strength of this extensive and comprehensive systematic review. This is the first systematic review, to the best of our knowledge, to characterize muscle food terminology used among chronic disease literature. This work identified muscle food categories described in diverse ways, highlighting the complexity for scientific audiences to interpret which foods were assessed. The exploratory nature of this descriptive, yet quantitative, landscape assessment limited the ability of using traditional hypothesis-driven meta-analytics. However, the descriptive statistics and probability calculations employed met the main objective of assessing variation and patterns in the use (or misuse) of muscle food categories and descriptions in chronic disease literature. Other limitations include that 1) the muscle food scoring systems were derived empirically for these data, 2) fish/seafood categories were not in the original aim or search terms, and 3) included article reference lists that were not hand-searched for potential additional articles. Fish and seafood data were largely extracted from articles that compared meat with fish/seafood intakes or assessed all together, but not fish/seafood independently.
Conclusions
The influx of health researchers adopting muscle food terminologies (such as meat, poultry, or seafood) has inconsistently evolved these terms away from the originally intended meanings (1). The main intention of this article was to provide a systematic assessment to inform regulatory agencies and nutrition-related policymakers about the stark variation, diversity, and misclassification of muscle food categories and descriptions used throughout chronic disease literature. In conclusion, muscle food categories and descriptions are substantively different within and between experimental and observational articles and do not match definitions provided by regulatory agencies. Assessing variation in terminology used to describe other food groups (40, 41) may be warranted in future work to develop a universal food classification system used consistently across nutrition-related chronic disease disciplines. We hope that this article will ignite discussion about muscle food research reporting guidelines, provide novel ways to dissect and compare results across articles, and lead to the development of a universal muscle food classification or reporting system for human nutrition and health research.
Supplementary Material
Acknowledgments
We thank Bethany McGowan from Purdue University's Health and Life Sciences Library Division for support in planning the search and choosing search terms; Yu Wang, a PhD student from Purdue University's Department of Nutrition Science for assisting with data entry and crosscheck; and Tianyang Hu and Arman Sabbaghi of Purdue University's Statistical Consulting Service for guidance with the statistical analysis. The authors’ responsibilities were as follows—LEO, CLG, DRW, KEB, and WWC: designed the research and obtained funding; LEO and CLG: conducted the search; LEO and CLG: analyzed the data with assistance from JLS; LEO and CLG: wrote the manuscript with editorial assistance from DRW, JLS, KEB, and WWC; and all authors read and approved the final manuscript.
Notes
This manuscript was sponsored by the Beef Checkoff.
Author disclosures: LEO, JLS, and CLG, no conflicts of interest. KEB and DRW, have received past funding from the National Cattlemen's Beef Association (NCBA) to conduct applied research in a number of topic areas and are nonvoting members of the NCBA. During the time this research was being conducted, WWC received funding for research grants (1), travel or honoraria for scientific presentations (2), or consulting services (3) from the following organizations: NCBA1,2,3, National Pork Board1,2, National Dairy Council3, North Dakota Beef Commission1, Foundation for Meat and Poultry Research and Education1, Barilla Group1,2, New York Beef Council2, and North American Meat Institute2.
Supplemental Tables 1–6 and Supplemental Figures 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
LEO and CLG contributed equally to this work.
Abbreviations used: CAT, muscle food category; CVD, cardiovascular disease; DESCR, muscle food description; GLMM, generalized linear mixed model; OBS, observational study; RCT, randomized controlled trial; T2DM, type 2 diabetes.
References
- 1. Seman DL, Boler DD, Carr CC, Dikeman ME, Owens CM, Keeton JT, Pringle TD, Sindelar JJ, Woerner DR, de Mello AS et al.. Meat science lexicon. Meat and Muscle Biology. 2018;2(1):127–41. [Google Scholar]
- 2. U.S. Department of Health and Human Services and U.S. Department of Agriculture. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. [Internet]. 1st Edition,February2015. Available from: https://health.gov/dietaryguidelines/2015-scientific-report/pdfs/scientific-report-of-the-2015-dietary-guidelines-advisory-committee.pdf (accessed 5 December, 2018). [Google Scholar]
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Department of Agriculture, Food Safety Inspection Service. Code of Federal Regulations, Title 9, Part 301, Section 2. Title, 2010. [Internet]. Available from: https://www.gpo.gov/fdsys/granule/CFR-2010-title9-vol2/CFR-2010-title9-vol2-sec301-2 (accessed 19 December, 2018). [Google Scholar]
- 5. Navarro A, Diaz MP, Munoz SE, Lantieri MJ, Eynard AR. Characterization of meat consumption and risk of colorectal cancer in Cordoba, Argentina. Nutrition. 2003;19(1):7–10. [DOI] [PubMed] [Google Scholar]
- 6. Cross AJ, Ward MH, Schenk M, Kulldorff M, Cozen W, Davis S, Colt JS, Hartge P, Cerhan JR, Sinha R. Meat and meat-mutagen intake and risk of non-Hodgkin lymphoma: results from a NCI-SEER case-control study. Carcinogenesis. 2006;27(2):293–7. [DOI] [PubMed] [Google Scholar]
- 7. Roussell MA, Hill AM, Gaugler TL, West SG, Ulbrecht JS, Vanden Heuvel JP, Gillies PJ, Kris-Etherton PM. Effects of a DASH-like diet containing lean beef on vascular health. J Hum Hypertens. 2014;28(10):600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez C, McCullough ML, Mondul AM, Jacobs EJ, Chao A, Patel AV, Thun MJ, Calle EE. Meat consumption among black and white men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2006;15(2):211–6. [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Zhang B, Zhai F, Wang H, Zhang J, Du W, Su C, Zhang J, Jiang H, Popkin BM. Fatty and lean red meat consumption in China: differential association with Chinese abdominal obesity. NMCD. 2014;24(8):869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engeset D, Andersen V, Hjartaker A, Lund E. Consumption of fish and risk of colon cancer in the Norwegian Women and Cancer (NOWAC) study. Br J Nutr. 2007;98(3):576–82. [DOI] [PubMed] [Google Scholar]
- 11. Hu J, La Vecchia C, DesMeules M, Negri E, Mery L. Meat and fish consumption and cancer in Canada. Nutr Cancer. 2008;60(3):313–24. [DOI] [PubMed] [Google Scholar]
- 12. O'Brien BC, Reiser R. Human plasma lipid responses to red meat, poultry, fish, and eggs. Am J Clin Nutr. 1980;33(12):2573–80. [DOI] [PubMed] [Google Scholar]
- 13. Kabat GC, Cross AJ, Park Y, Schatzkin A, Hollenbeck AR, Rohan TE, Sinha R. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int J Cancer. 2009;124(10):2430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashton E, Ball M.. Effects of soy as tofu vs meat on lipoprotein concentrations. Eur J Clin Nutr. 2000;54(1):14–9. [DOI] [PubMed] [Google Scholar]
- 15. Tasevska N, Cross AJ, Dodd KW, Ziegler RG, Caporaso NE, Sinha R. No effect of meat, meat cooking preferences, meat mutagens or heme iron on lung cancer risk in the prostate, lung, colorectal and ovarian cancer screening trial. Int J Cancer. 2011;128(2):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer D, Hornik K, Feinerer I. Text mining infrastructure in R. J Stat Softw. 2008;25(5):1–54. [Google Scholar]
- 17. South A. rworldmap: a new R package for mapping global data. R Journal. 2011;3(1):35–43. [Google Scholar]
- 18. International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Red Meat and Processed Meat, [Internet]. Volume114, March2018. Available from: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono114.pdf (accessed 2 October, 2018). [PubMed] [Google Scholar]
- 19. World Cancer Research Fund International. Animal Foods. [Internet]. Available from: http://www.wcrf.org/int/research-we-fund/cancer-prevention-recommendations/animal-foods (accessed 4 October, 2018). [Google Scholar]
- 20. Gifford CL, O'Connor LE, Campbell WW, Woerner DR, Belk KE. Broad and inconsistent muscle food classification is problematic for dietary guidance in the U.S. Nutrients. 2017;9(9):1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shim J-S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institutes of Health. Food Frequency Questionnaire at a Glance. [Internet]. Available from: https://dietassessmentprimer.cancer.gov/profiles/questionnaire/ (accessed 20 December, 2018). [Google Scholar]
- 23. National Institutes of Health. National Cancer Institute Diet History Questionnaire III. [Internet]. Available from: https://epi.grants.cancer.gov/dhq3/dhq3-past-year-with-serving-sizes-questionnaire.pdf (accessed 22 December, 2018). [Google Scholar]
- 24. U.S. Department of Agriculture, Food and Nutrition Services. SNAP-ED Connection, Harvard Willett Food Frequency Questionnaire. [Internet]. Available from: https://snaped.fns.usda.gov/materials/harvard-willett-food-frequency-questionnaire (accessed 22 December, 2018). [Google Scholar]
- 25. Higgs JD. The changing nature of red meat: 20 years of improving nutritional quality. Trends Food Sci Tech. 2000;11(3):85–95. [Google Scholar]
- 26. Berry D, Conroy S, Pabiou T, Cromie A. Animal breeding strategies can improve meat quality attributes within entire populations. Meat Sci. 2017;132:6–18. [DOI] [PubMed] [Google Scholar]
- 27. Dalle Zotte A. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest Prod Sci. 2002;75(1):11–32. [Google Scholar]
- 28. Cavani C, Petracci M, Trocino A, Xiccato G. Advances in research on poultry and rabbit meat quality. Ital J Anim Sci. 2009;8(sup2):741–50. [Google Scholar]
- 29. U.S. Department of Agriculture, Economic Research Service. Food Availability (Per Capita) Data System; Meat, Poultry, Fish, Eggs, and Nuts, [Internet] United States Department of Agriculture, Economic Research Service; Available from: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ (accessed 1 June, 2017). [Google Scholar]
- 30. U.S. Department of Agriculture, Agricultural Research Service. Food Patterns Equivalents Database, NHANES, WWEIA, FPED 2013–2014, Databases and SAS Data Sets. [Internet]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-databases/ (accessed 1 June, 2017). [Google Scholar]
- 31. Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, Capps NE. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332(7544):752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–57. [DOI] [PubMed] [Google Scholar]
- 33. O'Connor LE, Campbell WW.. Red meat and health: getting to the heart of the matter. Nutr Today. 2017;52(4):167–73. [Google Scholar]
- 34. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaluza J, Wolk A, Larsson SC. Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43(10):2556–60. [DOI] [PubMed] [Google Scholar]
- 39. American Heart Association. Fish and omega-3 Fatty Acids. [Internet]. Available from: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/fish-and-omega-3-fatty-acids (accessed 28 December, 2018). [Google Scholar]
- 40. Roark RA, Niederhauser VP. Fruit and vegetable intake: issues with definition and measurement. Public Health Nutr. 2013;16(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pereira PC. Milk nutritional composition and its role in human health. Nutrition. 2014;30(6):619–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.