Abstract
Background
We have limited knowledge about the effects of antipsychotic exposure on the development of gestational diabetes mellitus (GDM). Aim of this study is to perform a systematic review and meta-analysis to assess GDM risk associated with antipsychotic exposure in pregnancy.
Methods
Systematic literature search was performed using PubMed, Science Direct, Scopus, and Web of Science databases up to August 22, 2018. No restrictions to language or date were applied. Randomized, controlled trials, case–control, or cohort studies reporting GDM risk in antipsychotic-exposed, healthy controls or antipsychotic-ceased patients were included in the meta-analysis. The primary outcomes were study defined GDM, including number of events, odds ratios, and/or risk ratios (RR) with confidence intervals (CI).
Results
Ten studies were included in the meta-analysis. The total number of subjects was 6213 for the antipsychotic-exposed group, 6836 for antipsychotic-ceased control group, and 1 677 087 for the healthy control group. Compared with the healthy controls, the unadjusted cumulative RR for GDM associated with antipsychotic use was 1.63 (95% CI = 1.20–2.22). Adjusted risk for GDM was significantly higher in antipsychotic exposure group than in healthy controls (RR = 1.30, 95% CI = 1.023–1.660). The adjusted RR for GDM was similar between the antipsychotic-exposed group and the antipsychotic-ceased group (RR = 0.78, 95% CI = 0.281–2.164). No significant association was found between study quality, smoking, alcohol use, gestational age, and cumulative GDM risk.
Discussion
Our results indicate an increased risk of GDM with antipsychotic exposure in pregnant women, who may benefit from close pregnancy monitoring, early testing for GDM, targeting modifiable risk factors, and lifestyle modifications.
Keywords: antipsychotic, gestational diabetes, pregnancy
Introduction
Gestational diabetes mellitus (GDM) is a common complication of pregnancy, defined as glucose intolerance with onset or first recognition during pregnancy.1 The increasing prevalence of GDM is a growing public health concern.2 GDM is associated with several maternal and fetal adverse health consequences, including macrosomia, gestational hypertension, preeclampsia, neonatal hypoglycemia, and development of diabetes mellitus and serious mental illnesses (SMI) later in life.1,3–5
Some of the well-known risk factors for GDM are older maternal age, family history of diabetes mellitus, and obesity.1,6 In addition to these risk factors, women with SMI, namely psychotic spectrum disorders, bipolar disorder, and depression, form a unique and high-risk population for GDM development. First expression of SMI often occurs during young adulthood, ie, at women’s childbearing years,7,8 and over 50% of women with SMI become pregnant during their lives.9,10
Recent findings indicate that patients with SMI may have an innate vulnerability to insulin resistance and glucose imbalance.11–16 Research from treatment-naive first-episode patients with SMI have shown higher insulin resistance, fasting insulin, and glucose levels, and increased rates of diabetes mellitus compared with healthy controls.11–15 Evidence has indicated that pregnancy itself reduces insulin sensitivity, most likely related to placental hormones. This is overcome by increased insulin levels.1 However, the combination of metabolic changes in pregnancy and risk factors, such as obesity before pregnancy, render women with SMI prone to develop GDM.1,6
The management of SMI during pregnancy and the postpartum period poses significant challenges to mothers, infants, and clinicians.7,8 Key in the management in SMI is adequate dosing of antipsychotic medication during active and remission phases of the illness. Discontinuation of antipsychotic treatment leaves women with SMI at high risk of relapse during pregnancy and postpartum period.17–20 Anticipation to the risk of relapse and related dire outcomes have led to an increase in antipsychotic medication use in pregnancy.21,22 However, antipsychotic medications can cause metabolic side effects including weight gain, insulin resistance, and diabetes mellitus.23,24
A small number of studies and case reports evaluating the relationship between antipsychotic exposure during pregnancy and GDM have revealed inconsistent results.8,25–33 Several studies have found that antipsychotic exposure during pregnancy is associated with the development of GDM.8,26,31,33 Antipsychotic exposure during pregnancy is reported to increase the risk of GDM development between 1.78- and 2.44-folds.26,31 Conversely, some studies reported no increase in risk of GDM development with antipsychotic exposure.25,28,29,32
In addition to SMI, antipsychotic medications are also being used in other psychiatric disorders for symptom management.28 Use of antipsychotic medications during pregnancy has increased from 3 per 1000 pregnancies in 2001 to 8 per 1000 pregnancies in 2007.21,33 Despite the increase in antipsychotic medication use in pregnancy, we still have limited information about the effects of antipsychotic exposure on the development of GDM.7 Previous research yielded inconsistent results and to date, no meta-analysis has been investigated the antipsychotic-exposure-related risk of GDM development. As antipsychotic treatment-related adverse effects may increase the risk of deleterious health outcomes for mother and infant, it is critical to understand the risk of developing GDM with these types of medications. The aim of this study is to conduct a systematic review and meta-analysis of existing literature to investigate the risk of developing GDM in pregnant patients treated with antipsychotic medication.
Methods
Search Strategy
A systematic search was performed to find relevant articles through August 22, 2018. The following databases were searched for title, abstract, and index terms of reference: PubMed, Science Direct, Scopus, and Web of Science. In addition, a manual search was carried out to identify relevant references by verifying references in retrieved articles, related review articles, and meta-analyses. Two reviewers (S.K. and K.C.) independently conducted the literature search using the following MESH and free-text search terms: antipsychotic, major tranquilizers, tranquilizing agents, neuroleptic medications, pregnancy, gestation, gestational diabetes, side effects, insulin, insulin resistance, glucose, metabolic disturbances, and diabetes.
Selection Criteria
The following inclusion criteria were applied: (1) only randomized, controlled trials, case–control, or cohort studies that included an antipsychotic-exposed and -nonexposed group during pregnancy were allowed; (2) studies had to report antipsychotic exposure during pregnancy and (3) on gestational diabetes outcomes; and (4) we limited findings to human research. This meta-analysis was conducted and reported according to the PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-analysis) guidelines (supplementary table 1).34 The quality of the cohort and case–control studies was peer-reviewed using the Newcastle-Ottawa Scale (NOS).35
Data Extraction
Two reviewers (S.K. and K.C.) independently extracted all data. After data extraction, a third reviewer (C.T.) checked all extracted data to clarify missing data. Any conflicts were discussed with the third reviewer (C.T.). The following data were extracted: study design, sample size, data source, gestational diabetes outcomes including number of events, odds ratios, and/or risk ratios (RR) with confidence intervals (CI). To obtain any missing information, we contacted the authors to request relevant data. Outcomes of overlapping samples were extracted from the more detailed report.
Statistical Analysis
We examined the differences on GDM outcomes between antipsychotic-exposed and healthy controls, and antipsychotic-exposed and antipsychotic-ceased groups by calculating unadjusted and adjusted RR estimates using the software Comprehensive Meta-Analysis Version 2 (Biostat). Statistical heterogeneity was assessed using Q and I2 tests, in which I2 ≥ 50% was considered to indicate heterogeneity. When heterogeneity was present between studies, the antipsychotic-exposure period was examined. We examined the effects of early antipsychotic exposure (first- and/or second-trimester exposure) and anytime antipsychotic exposure on GDM outcomes by grouping the studies reporting outcomes during the relevant time of pregnancy. Publication bias was assessed with Egger’s regression test.36 A random-effects model was used due to the methodological and sampling differences between studies. The results for a fixed-effects model were also presented as a sensitivity analysis. We performed meta-regression analyses to examine the relationship between study quality (NOS), smoking and alcohol use rates, gestational age, and unadjusted GDM outcomes. Statistical significance was defined as P < .05.
Results
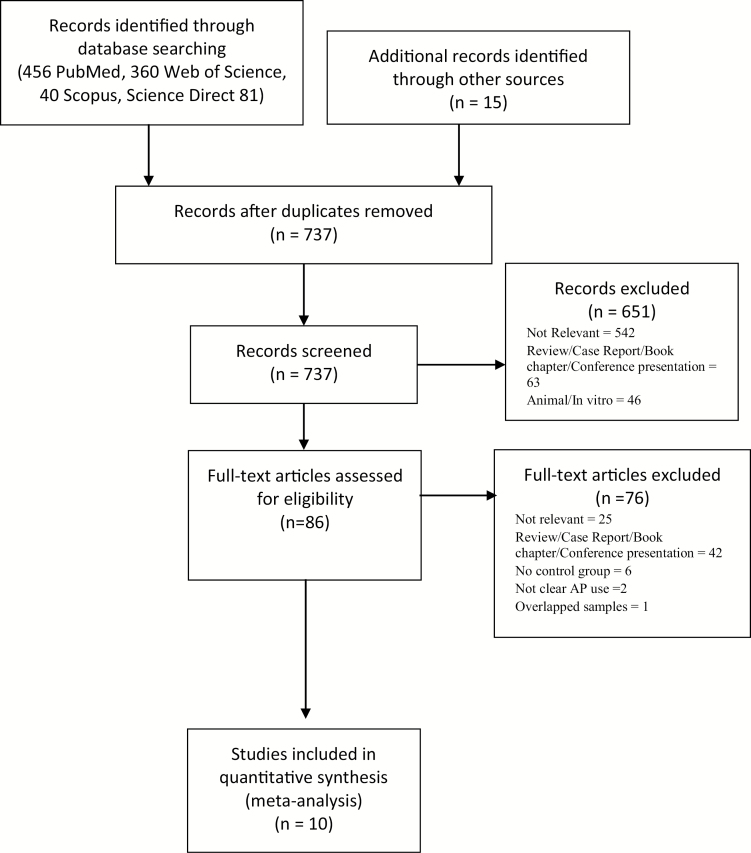
Our search resulted in a total of 737 articles, and 651 studies were excluded based on the title and/or abstract as they did not deal with the topic under study here. Of the remaining 86 articles, 76 were excluded after full-text review because they did not fulfill inclusion criteria, resulting in 10 eligible studies for inclusion in this meta-analysis (figure 1, supplementary tables 2 and 3).8,25–33 Of those, 6 studies8,26,27,30,31,33 were retrospective database investigation and 4 studies were prospective cohort investigation.25,28,29,32 Eight of the studies compared antipsychotic-exposed group with healthy controls.25,26,28–33 Three studies had an antipsychotic ceased group as a control group.8,27,30 The total number of subjects was 6213 for the antipsychotic-exposed group, 6836 for antipsychotic-ceased control group, and 1 677 087 for the healthy control group. The study quality was high overall, with scores between 6 and 9 on the NOS (supplementary table 2). The mean (SD) NOS score was 8.3 (1.06), and the median NOS score was 9.
Fig. 1.
PRISMA flow diagram.
Outcome Definitions
The time of antipsychotic exposure in the included studies was defined as either at least one exposure during first trimester,25,27–29,31 or at least one exposure during first or second trimester,8,30,33 or exposure any time during pregnancy.26,32
All included studies provided general information about antipsychotic treatment class. Most studies investigated more than one antipsychotic classified as second-generation antipsychotics (SGAs), such as clozapine, olanzapine, and risperidone.8,28,29,32 Some studies investigated both SGAs and first-generation antipsychotics (FGAs) medication exposure.26,30,31,33 A few studies investigated only one specific antipsychotic exposure during pregnancy.25,27
GDM in Antipsychotic-Exposed Group vs Healthy Controls
In our overall meta-analysis, the crude risk for developing GDM in the antipsychotic exposure group was significantly higher than in healthy controls. Compared with the healthy controls, the unadjusted cumulative RR for GDM associated with antipsychotic use was 1.63 (95% CI = 1.20 to 2.22, P = .02) (figure 2). No evidence of publication bias was found for antipsychotic-exposed and health control comparison (Egger’s test = 0.805, CI = −1.75 to 3.36). There was significant heterogeneity on the unadjusted risk for GDM outcomes across the studies included in this analysis (I2 = 59.64, df = 7, P = .01).
Fig. 2.
Meta-analysis of unadjusted GDM risk between antipsychotic users and healthy controls. Heterogeneity: Tau2 = 0.09; I2 = 59.64%; Q = 17.34, df = 7, P = .015. Overall effect (fixed): 1.48 (95% CI = 1.27–1.72), Z = 5.089, P < .001.
Four studies reported adjusted differences between antipsychotic exposure individuals and healthy controls.26,30,31,33 The adjusted risk for GDM was significantly higher in antipsychotic exposure group than in healthy controls (estimated RR = 1.30, 95% CI = 1.023 to 1.660, P = .032) (figure 3). There was no significant heterogeneity in RRs across 3 studies (I2 = 14.37, df = 3, P = .32).
Fig. 3.
Meta-analysis of adjusted GDM risk between antipsychotic users and healthy controls. Heterogeneity: Tau2 = 0.009; I2 = 14.37%; Q = 3.504, df = 3, P = .32. Overall effect (fixed): 1.29 (95% CI = 1.04–1.61), Z = 2.321, P = .02.
We tested the effect of reported antipsychotic-exposure period (early exposure vs anytime exposure during pregnancy) across studies. Six studies reported outcomes from patients who had been exposed to antipsychotics during early pregnancy (at least once in the first and/or second trimester).25,28–31,33 The antipsychotic-exposure period in the remaining 2 studies26,32 was specified as anytime during pregnancy. Compared with the healthy controls, the unadjusted RR estimate for GDM in patients with early antipsychotic exposure (estimated RR = 1.42, 95% CI = 1.07 to 1.89, P = .015) and anytime antipsychotic exposure (estimated RR = 2.533, 95% CI = 1.73 to 3.71, P < .001) was significantly higher. There was no significant heterogeneity in RR estimates of GDM risk in early antipsychotic-exposure and anytime antipsychotic-exposure groups (supplementary figure 1).
GDM in Antipsychotic-Exposed Group vs Antipsychotic-Ceased Group
We identified 7 outcomes from 3 studies that examined the risk of GDM in the antipsychotic-exposed and the antipsychotic-ceased patients.8,27,30 The unadjusted RR estimate for GDM was significantly higher in the antipsychotic-exposed group compared with the antipsychotic-ceased group (estimated RR = 1.55, 95% CI = 1.187 to 2.034, P = .001) (supplementary figure 2). Only 2 studies8,30 reported adjusted risk of GDM in the antipsychotic-exposed and the antipsychotic-ceased patients. The adjusted RR estimate for GDM was similar between the antipsychotic-exposed group and the antipsychotic-ceased group (estimated RR = 0.78, 95% CI = 0.28 to 2.16, P = .633) (supplementary figure 3). Significant heterogeneity was detected on RRs across outcomes (I2 = 85.43, df = 1, P = .009).
Meta-regression
In meta-regression analyses, no significant association was found between study quality (measured with NOS), smoking and alcohol use rates, gestational age, and cumulative crude GDM risk (supplementary table 4).
Discussion
This meta-analysis of GDM risk in antipsychotic-exposed pregnant women indicates that the unadjusted, cumulative risk of GDM was 1.6-fold higher compared with healthy women. Compared with healthy controls, the adjusted risk of GDM was 1.3 in antipsychotic-exposed patients. The adjusted risk of GDM was similar between antipsychotic-exposed pregnant women and antipsychotic-ceased controls diagnosed with SMI.
Overall, unadjusted and adjusted results from our meta-analyses are in line with previous cohort studies and systematic reviews, which suggest an increase in risk of GDM with antipsychotic exposure during pregnancy.8,26,31,33 Although alterations in glucose metabolism are a part of the natural course of pregnancy, increased risk of developing GDM with antipsychotic treatment can be explained by the nature of SMI and antipsychotic medication-related adverse effects. For example, recent meta-analyses have shown that having an SMI already increases the risk for glucose metabolism impairments even before introducing antipsychotic medications.11,12,37 Although the exact mechanism has not been fully unraveled yet, research suggests that alterations in systemic inflammation and shared genetic liability may underlie associations between SMI and glucose metabolism disturbances,37 and may also contribute to the risk of developing GDM in pregnant patients with SMI. In addition, early environmental factors can explain the part of the relationship between SMI and GDM. As suggested by the “thrifty psychiatric phenotype” concept, an epigenetic pathway initiated during gestation may interact with genetic programing and lead to SMIs and glucose metabolism disturbances in adulthood.38
Our findings show significant heterogeneity across studies evaluating the unadjusted risk of GDM between antipsychotic-exposed and healthy pregnant women. A number of limitations pertinent to the studies and possible confounders can (at least in part) explain this heterogeneity. First, only a few studies reported adjusted of GDM between antipsychotic-exposed and healthy pregnant women.26,30,31 However, GDM development was associated with various risk factors, including maternal age, family history of diabetes mellitus, obesity, smoking, and care during pregnancy term.1,6 For instance, women treated with antipsychotic medications before pregnancy appear to be more overweight or obese when they became pregnant.18 Hence, advanced pre-pregnancy body weight is one of the well-known risk factors for GDM development.1,6 However, our meta-regression results did not reveal any significant relationship between the risk of GDM and smoking, alcohol use rates, and gestational age. Adjusted results from limited number of studies26,30,31,33 indicate that pre-pregnancy confounding factors (eg, body mass index levels, smoking, additional medication use) may contribute to the risk of GDM development. Although the risk of GDM development was still significant in antipsychotic users after adjustments, considerable attenuation on the RR suggests that controlling pre-pregnancy confounders in this patient population may help to prevent GDM development during pregnancy.
Although the unadjusted outcomes show lower risk, cumulative results from adjusted outcomes suggest that cessation of antipsychotic medications during pregnancy may not affect the risk of GDM development in pregnant women with SMI. However, these findings are based on a very limited number of studies. From the outcomes provided by Park et al,8 it should be noted that continued use of particular antipsychotic medications (olanzapine RR = 1.61; quetiapine RR = 1.28) may increase the risk of GDM development. This relationship, as Park et al noted, may be related to metabolic adverse-effect profiles of the specific antipsychotic medications. Antipsychotic medication-related adverse effects, particularly weight gain and alterations in glucose utilization, usually worsen glucose metabolism impairment and contribute to the development of diabetes in patients with SMI.18,37,39 Because different antipsychotic medications have varying metabolic profiles, it may be beneficial to switch to another antipsychotic medication with better metabolic adverse-effect profile.24 This meta-analysis, however, does not answer this question. Excess body weight gain related to antipsychotic treatment in pregnancy most likely contributes to the development of GDM.18 Although tapering antipsychotic treatment during pregnancy may decrease body weight gain, and decrease the risk of GDM development, it should be noted that this can also incur exacerbations in psychiatric symptoms, sometimes resulting in harm for the mother and even the baby.17–20 Therefore, a careful review of individual patient-related factors and shared clinical decision making on a case by case basis is crucial in managing GDM risk among antipsychotic users.
An important limitation of current literature is the lack of antipsychotic-specific reports. Although most studies included in this meta-analysis reported cumulative outcomes from SGAs and/or FGAs,26,28–32 only a handful reported on antipsychotic-specific GDM risks.8,25,27,33 Furthermore, studies also reported that included patients were on more than one class of psychotropic medications, such as antidepressants or anxiolytics. Current literature on the risk of GDM development with antidepressants or anxiolytics is (thus far) limited, but these medications may also contribute to body weight gain and development of diabetes.40–42 Without antipsychotic medication-specific data, it is currently not possible to determine the true risk of GDM development for users of distinct antipsychotic medications, and this of course warrants further research.
We need to acknowledge the diversity of psychiatric diagnoses in the studies that included this meta-analysis. Despite most of these studies having been conducted in patients with SMI, some also included patients with various other psychiatric diagnoses, such as personality disorders, sleep disorders, and alcohol/substance use disorders.28,29,32,33 As mentioned earlier, patients with SMI may be more prone to develop glucose metabolism abnormalities related to nature of mental illness, as well as medication adverse effects. Although a number of studies have also suggested a relationship between diabetes and other mental illnesses and sleep disorders,43–45 the nature and extent of this relationship is not clear. Future studies with psychiatric diagnosis specified outcomes are necessary to evaluate the risk of antipsychotic treatment-related GDM.
In addition to the caveats of the current literature, it is important to note the limitations of this meta-analysis. The meta-analysis included only a limited number of studies. Therefore, we were not able to carry out analyses for publication bias on meta-analyses with fewer than 10 outcomes. Furthermore, some of these studies were carried out with a small number of patients. Determining outcomes from small sample sizes can reduce the precision of outcome estimations. After adjustment for several confounding factors (ie, lifestyle factors, maternal age, smoking), some studies included in this meta-analysis indicated no increased risk for GDM development with antipsychotic medication use.29,30,33 It is possible that the unadjusted RR reported in this meta-analysis may also partially reflect the effects of other confounding variables on GDM. Finally, we carried out our literature search only on PubMed, Science Direct, Scopus, and Web of Science. Although these databases cover most scientific literature, not including other databases (ie, Psych Info, Embase) in our search may limit our final article numbers.
Women receiving antipsychotic medications in pregnancy are of higher risk of developing GDM. Although terminating antipsychotic medication during pregnancy may reduce the risk of GDM somewhat, tapering may also lead to increased risk of exacerbations in psychiatric symptoms. Because GDM can lead to adverse consequences for both mother and offspring, antipsychotic medications should not be used without proper indication. Clinicians should carefully evaluate the benefits and drawbacks of antipsychotic medication use during pregnancy and engage in shared decision making on this topic. If antipsychotic medication is needed during pregnancy, using medications with lower weight gain liabilities, while considering patients’ clinical stability, may decrease the risk of GDM development. This specific population may benefit from close pregnancy monitoring, targeting modifiable risk factors (ie, smoking, weight reduction), and lifestyle modifications.9,18 In addition, testing for GDM during first trimester rather than between 24 and 28 gestational weeks, as suggested by guidelines, can prevent the development of GDM in patients with SMI.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK093924.
Supplementary Material
Acknowledgments
S.K., S.G., J.J.L., B.P.F.R., and C.T. contributed to study conception, data interpretation, and writing of the manuscript. S.K., K.C., and C.T. contributed to data collection and analysis. S.K., M.O.B., S.G., and C.T. contributed to study conception and data interpretation. All authors were involved in critically revising the article for important intellectual content and gave final approval of the version to be published. B.P.R.F. and C.T. were responsible for supervision of the meta-analysis. All authors declare no competing interests.
References
- 1. Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care. 2007;30(suppl 2):S105–S111. [DOI] [PubMed] [Google Scholar]
- 2. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;11:CD010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. [DOI] [PubMed] [Google Scholar]
- 5. Van Lieshout RJ, Voruganti LP. Diabetes mellitus during pregnancy and increased risk of schizophrenia in offspring: a review of the evidence and putative mechanisms. J Psychiatry Neurosci. 2008;33(5):395–404. [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(suppl 2):S141–S146. [DOI] [PubMed] [Google Scholar]
- 7. Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park Y, Hernandez-Diaz S, Bateman BT, et al. Continuation of atypical antipsychotic medication during early pregnancy and the risk of gestational diabetes. Am J Psychiatry. 2018;175(6):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coughlin CG, Blackwell KA, Bartley C, Hay M, Yonkers KA, Bloch MH. Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet Gynecol. 2015;125(5):1224–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCauley-Elsom K, Gurvich C, Elsom SJ, Kulkarni J. Antipsychotics in pregnancy. J Psychiatr Ment Health Nurs. 2010;17(2):97–104. [DOI] [PubMed] [Google Scholar]
- 11. Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perry BI, McIntosh G, Weich S, Singh S, Rees K. The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry. 2016;3(11):1049–1058. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Rizo C, Kirkpatrick B, Fernandez-Egea E, Oliveira C, Bernardo M. Abnormal glycemic homeostasis at the onset of serious mental illnesses: a common pathway. Psychoneuroendocrinology. 2016;67:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Rizo C, Fernandez-Egea E, Miller BJ, et al. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naïve patients with depression. Brain Behav Immun. 2013;28:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guha P, Bhowmick K, Mazumder P, Ghosal M, Chakraborty I, Burman P. Assessment of insulin resistance and metabolic syndrome in drug naive patients of bipolar disorder. Indian J Clin Biochem. 2014;29(1):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenhalgh AM, Gonzalez-Blanco L, Garcia-Rizo C, et al. Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naïve patients with nonaffective psychosis. Schizophr Res. 2017;179:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulkarni J, McCauley-Elsom K, Marston N, et al. Preliminary findings from the National Register of Antipsychotic Medication in Pregnancy. Aust N Z J Psychiatry. 2008;42(1):38–44. [DOI] [PubMed] [Google Scholar]
- 18. Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother. 2015;16(9):1335–1345. [DOI] [PubMed] [Google Scholar]
- 19. Howard LM, Goss C, Leese M, Appleby L, Thornicroft G. The psychosocial outcome of pregnancy in women with psychotic disorders. Schizophr Res. 2004;71(1):49–60. [DOI] [PubMed] [Google Scholar]
- 20. Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789–1799. [DOI] [PubMed] [Google Scholar]
- 21. Toh S, Li Q, Cheetham TC, et al. Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: a population-based study of 585,615 deliveries. Arch Womens Ment Health. 2013;16(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitworth AB. Psychopharmacological treatment of schizophrenia during pregnancy and lactation. Curr Opin Psychiatry. 2017;30(3):184–190. [DOI] [PubMed] [Google Scholar]
- 23. Annamalai A, Kosir U, Tek C. Prevalence of obesity and diabetes in patients with schizophrenia. World J Diabetes. 2017;8(8):390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tek C, Kucukgoncu S, Guloksuz S, Woods SW, Srihari VH, Annamalai A. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry. 2016;10(3):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellet F, Beyens MN, Bernard N, Beghin D, Elefant E, Vial T. Exposure to aripiprazole during embryogenesis: a prospective multicenter cohort study. Pharmacoepidemiol Drug Saf. 2015;24(4):368–380. [DOI] [PubMed] [Google Scholar]
- 26. Bodén R, Lundgren M, Brandt L, Reutfors J, Kieler H. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry. 2012;69(7):715–721. [DOI] [PubMed] [Google Scholar]
- 27. Galbally M, Frayne J, Watson SJ, Snellen M. Aripiprazole and pregnancy: a retrospective, multicentre study. J Affect Disord. 2018;238:593–596. [DOI] [PubMed] [Google Scholar]
- 28. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444–449; quiz 546. [DOI] [PubMed] [Google Scholar]
- 29. Panchaud A, Hernandez-Diaz S, Freeman MP, et al. Use of atypical antipsychotics in pregnancy and maternal gestational diabetes. J Psychiatr Res. 2017;95:84–90. [DOI] [PubMed] [Google Scholar]
- 30. Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016;176(2–3):349–356. [DOI] [PubMed] [Google Scholar]
- 31. Reis M, Källén B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28(3):279–288. [DOI] [PubMed] [Google Scholar]
- 32. Sadowski A, Todorow M, Yazdani Brojeni P, Koren G, Nulman I. Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ Open. 2013;3(7):e003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vigod SN, Gomes T, Wilton AS, Taylor VH, Ray JG. Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study. BMJ. 2015;350:h2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 35. Wells GSB, O’Connell B, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed June 1, 2017).
- 36. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kucukgoncu S, Kosir U, Zhou E, Sullivan E, Srihari VH, Tek C. Glucose metabolism dysregulation at the onset of mental illness is not limited to first episode psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia-Rizo C, Fernandez-Egea E, Bernardo M, Kirkpatrick B. The thrifty psychiatric phenotype. Acta Psychiatr Scand. 2015;131(1):18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Liu Y, Su Y, et al. The metabolic side effects of 12 antipsychotic drugs used for the treatment of schizophrenia on glucose: a network meta-analysis. BMC Psychiatry. 2017;17(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salvi V, Grua I, Cerveri G, Mencacci C, Barone-Adesi F. The risk of new-onset diabetes in antidepressant users – a systematic review and meta-analysis. PLoS One. 2017;12(7):e0182088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oyebode F, Rastogi A, Berrisford G, Coccia F. Psychotropics in pregnancy: safety and other considerations. Pharmacol Ther. 2012;135(1):71–77. [DOI] [PubMed] [Google Scholar]
- 42. Lopez-Yarto M, Ruiz-Mirazo E, Holloway AC, Taylor VH, McDonald SD. Do psychiatric medications, especially antidepressants, adversely impact maternal metabolic outcomes? J Affect Disord. 2012;141(2–3):120–129. [DOI] [PubMed] [Google Scholar]
- 43. Bathla M, Singh M, Anjum S, Kulhara P, Jangli SI. Metabolic syndrome in drug naive patients with substance use disorder. Diabetes Metabolic Synd. 2017;11(3):167–71. [DOI] [PubMed] [Google Scholar]
- 44. Quirk SE, El-Gabalawy R, Brennan SL, et al. Personality disorders and physical comorbidities in adults from the United States: data from the National Epidemiologic Survey on Alcohol and Related Conditions. Soc Psychiatry Psychiatr Epidemiol. 2015;50(5):807–820. [DOI] [PubMed] [Google Scholar]
- 45. Ryan S. Sleep and diabetes. Curr Opin Pulm Med. 2018;24(6):555–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.