ABSTRACT
The intestinal tract is the shared locus of intestinal epithelial cells, immune cells, nutrient digestion and absorption, and microbial survival. The gut in animals faces continuous challenges in communicating with the external environment. Threats from endogenous imbalance and exogenous feeds, especially pathogens, could trigger a disorder of homeostasis, leading to intestinal disease and even systematic disease risk. As a part of the intestinal protective barrier, endogenous host defense peptides (HDPs) play multiple beneficial physiological roles in the gut mucosa. Moreover, enhancing endogenous HDPs is being developed as a new strategy for resisting pathogens and commensal microbes, and to maintain intestinal health and reduce antibiotic use. In recent years, multiple nutrients such as branched-chain amino acids, SCFAs, lactose, zinc, and cholecalciferol (vitamin D3) have been reported to significantly increase HDP expression. Nutritional intervention has received more attention and is viewed as a promising means to defend against pathogenic infections and intestinal inflammation. The present review focuses on current discoveries surrounding HDP expression and nutritional regulation of mechanisms in the gut. Our aim is to provide a comprehensive overview, referable tactics, and novel opinions.
Keywords: nutrients, gut, pathogens, inflammation, host defense peptides, antibiotic alternative
Introduction
The gut is the junction and one of the most intimate zones between the body and the external environment. Many bacteria, fungi, viruses, and parasites live in the complex gut environment. As the largest immunity organ, the gut is at the forefront of the body's defense. Against this background, the gut has evolved multiple effective protection mechanisms to defend against endogenous and exogenous threats. However, a variety of factors inside and outside the gastrointestinal tract, such as diets (1), toxins (2), pathogens (3), and environmental factors (4), can seriously affect these defense mechanisms, upset the balance within the gut ecosystem, and destroy gut homeostasis. In turn, the damaged homeostasis further aggravates the unbalanced ecosystem and enhances the probability of infection by intestinal pathogens. Such infections eventually lead to local or systemic diseases such as inflammatory bowel disease, septicemia, and similar conditions (5).
Antibiotics are widely used in the treatment of illness and as feed supplements in animal husbandry because of their efficient antibacterial and somatotrophic properties. However, long-term, consistent heavy use of antibiotics has caused problems such as bacterial resistance, antibiotic residues, and environmental pollution, which have proved to be serious threats to human health. As a result of high selection pressure and development of resistance, ordinary antibiotics are losing their efficacy. Even though it is necessary to continue to develop new antibiotics with antimicrobial activities, host-directed therapies have emerged as an attractive alternative method to resist infectious diseases (6). Antimicrobial peptides (AMPs) are evolutionarily conserved components of the innate immune system and are widely distributed in animals, plants, and microbes. These peptides confer protection against infection. AMPs have long been considered ideal antibiotic substitutes based on their stable structure, wide-spectrum and high-efficiency antimicrobial activity, selective toxicity, and minimal side effects (7). Most of all, AMPs are less likely to contribute to drug resistance than are conventional antibiotics (8). In recent years, because they act as immunomodulators that mediate host immune responses, AMPs have been renamed host defense peptides (HDPs). The gastrointestinal tract is a major functional locus for HDPs. Recent research suggests that HDPs are a vital protective mechanism for the gut and their expression can be regulated to some degree.
Based on their properties and vital roles in the innate immune system, HDPs are being developed by genetic and fermentation engineering technologies to serve as drugs or additives. But the high cost and low production efficiency involved are temporarily limiting their widespread application. Furthermore, clinically therapeutic HDPs still bear the risk of drug resistance (9, 10) and may affect the protective function of endogenous HDPs in the innate immune system. However, many relevant studies have found some nutrients significantly contribute to the expression of endogenous HDPs in the gut. Generation of endogenous HDPs provides a more economical, effective, and promising pathway to protect intestinal health from invading pathogens and inflammatory responses.
Character and Functions of HDPs in the Gut
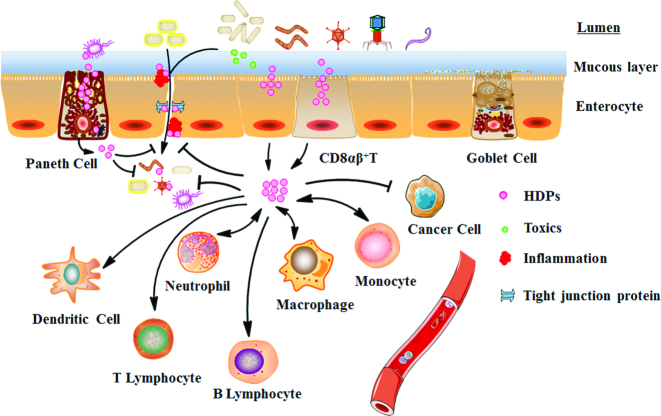
HDPs are short peptides of <50 amino acids that carry an average net charge of +3 because of the predominance of acidic amino acids (Arg, Lys, and His) (11). There are >2000 HDPs found in animals according to the antimicrobial peptide database, mainly including defensin, cathelicidin, lysozyme (LYZ), natural killer cell lysin, regenerating protein family, hepcidin, chemokines, and some RNA enzymes (12, 13). The types of HDPs present vary between different species. Defensin and cathelicidin are 2 major HDP families in vertebrate animals and there are multiple members in each family (14). Enterocytes, paneth cells, CD8αβ+T cells, and phagocytes in the gut can all synthesize and secrete HDPs (12, 15) (Figure 1). In healthy animals, HDPs produced by the gut mucosa take part in gut barrier functions to defend against pathogens. HDPs are active against a wide range of organisms covering bacteria, fungi, helminths, and viruses. HDPs kill bacteria by penetrating the cell wall and cytomembrane using a high proportion of acidic phospholipids or by inhibiting synthesis of biological macromolecules (16). In addition, HDPs can clear inflammation, suppress cancer cells, and promote wound healing in the gut (17). Without compromising the immune functions required to clear infections, HDPs simultaneously control inflammation responses (8). Therefore, HDPs are of special biological significance for intestinal protection by enhancing the innate and adaptive immune systems (17). This ability is particularly important for infants or other young of various species because of their immature immune systems. Corresponding to these views, decreased HDP expression is involved in pathogenesis of digestive diseases and bacterial translocation (7, 18).
FIGURE 1.
Multiple enterocytes and phagocytes synthesize and secrete HDPs in the intestine. The HDPs in the intestine can attach to and kill bacteria. Simultaneously, HDPs can enhance innate and adaptive immune functions to reduce inflammatory reactions and enhance antimicrobial capacity, can kill cancer cells, and can repair damage to intestinal tissues. HDP, host defense peptide.
Multiple Amino Acids Contribute to HDP Expression in Enterocytes
Amino acids have obvious first-pass metabolic effects in the intestinal tract. Long-term studies have revealed that a large number of amino acids are used in intestinal tissue metabolism and microbial utilization (19). Malnutrition, especially lack of amino acids, significantly restrains the immune function of gut tissue. The branched-chain amino acids (BCAAs), Arg, and Trp are important functional amino acids. Their metabolism boosts intestinal immune capacity in several ways, including enhancement of HDP expression (Figure 2).
FIGURE 2.
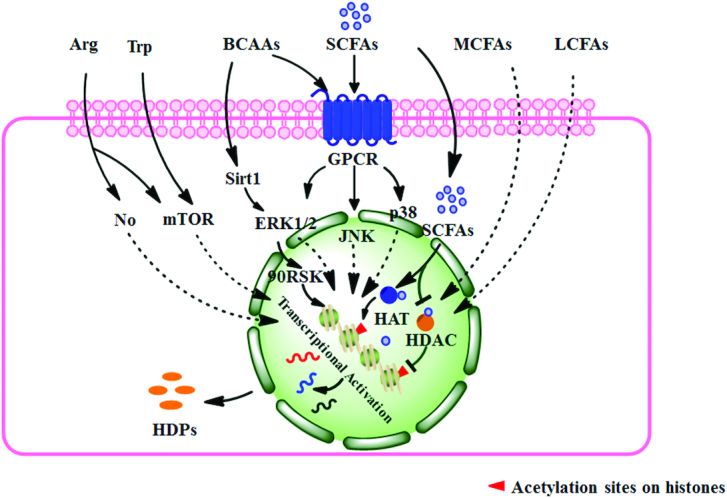
Multiple amino acids and fatty acids upregulate HDP expression in the gut. The BCAAs enhance HDP expression by the Sirt1–ERK1/2–90RSK and GPCR–MAPK pathways. Arg significantly enhances HDP expression and may be mediated by the NO signal or mTOR pathway. Trp may enhance HDP expression by the mTOR pathway. SCFAs, MCFAs, and LCFAs can all contribute to HDP expression and SCFAs affect the HDP expression conducted by directly influencing histone acetylation and the GPCR–MAPK signal pathway. BCAA, branched-chain amino acid; ERK, extracellular regulated protein kinase; GPCR, G protein-coupled receptor; HAT, histone acetyltransferase; HDAC, histone deacetylase; HDP, host defense peptide; JNK, c-Jun N-terminal kinase; LCFA, long-chain fatty acid; MAPK, mitogen-activated protein kinase; MCFA, medium-chain fatty acid; mTOR, mammalian target of rapamycin; Sirt1, sirtuin-1; 90RSK, 90-kDa ribosomal S6 kinase.
BCAAs induce HDPs in enterocytes by the G protein-coupled receptor–extracellular regulated protein kinase 1/2 and sirtuin-1–extracellular regulated protein kinase–90-kDa ribosomal S6 kinase pathways
In humans, the co-incubation of isoleucine and IL-1α induces human β-defensin-2 (hBD2) expression in colonic epithelial cells, which is mediated by the G protein–coupled receptor (GPCR)–extracellular regulated protein kinase-1/2 (ERK1/2) signaling pathway (20). This provides a method to mediate endogenous HDP expression. Considering their similarity in structure and immune function, leucine and valine may have similar induction capacity. Another piece of research more completely investigated the induction capacity and possible regulation mechanism of β-defensins gene expression by BCAAs (21). They found that leucine, isoleucine, and valine all significantly increased intestinal epithelial porcine β-defensin-1 (pBD1), pBD2, pBD3, pBD114, pBD129, and epididymis protein 2 splicing variant C (pEP2C) in vivo and in vitro. Of these 3 amino acids tested, isoleucine appeared to be the most effective. Further investigation revealed that activation of the sirtuin-1–ERK–90-kDa ribosomal S6 kinase pathway was an important mechanism for the expression of β-defensins (21). Therefore, BCAAs, especially isoleucine, can promote expression of β-defensins to enhance intestinal immune function.
Arg may enhance HDP expression in enterocytes by the NO and mammalian target of rapamycin pathways
An experiment exploring immune-enhancing formulas found that Arg, Ile, and albumin all contributed to hBD1 expression in human colonic cells (22). By the same token, dietary Arg supplementation evidently enhanced pBD2 and pBD3 expression in ileum (23). Such trials in vitro and in vivo indicate Arg can protect intestinal epithelial cells through upregulation of HDPs. Growing evidence indicates that Arg and its metabolite NO are critical to maintain normal physiology, mucosal integrity, and defense of the gastrointestinal tract (24). The NO produced via NO synthase can prevent mucosal injury and reduce inflammatory responses (25). The NO signal pathway may be one of the functional mechanisms of Arg in the expression of HDPs. Moreover, protein synthesis in intestinal epithelial cells induced by Arg is mammalian target of rapamycin (mTOR)-dependent and independent of NO (26). Therefore, HDP expression induced by Arg may also be mediated by mTOR.
Trp may enhance HDP expression in enterocytes by the mTOR pathway
Trp is an essential amino acid and its metabolites from multiple metabolic pathways mediate crosstalk between the intestinal mucosal immune system and microflora. Trp may be a promising nutrient target for regulation of mucosal immune functions (27). The activated mTOR can promote the expression of HDPs (28). Several studies have found that Trp can directly activate the mTOR pathway but independently of the PI3K–AKT pathway (29, 30). Hence, there is speculation that dietary Trp can enhance HDP induction in gut tissue. More experiments are necessary to investigate the ability of Trp to alleviate intestinal inflammation through HDP expression. What is noteworthy is that in trials of amino acids inducing HDP expression, appropriate dose is the key element. In growing and adult pigs, it has been investigated whether moderate dietary protein restriction can optimize the gut microbiota and mucosal barrier (31–33). These suggest that an excessive concentration of amino acid may have the opposite effect.
Various Types of Fatty Acids Promote Intestinal HDP Expression
Repeated trials have found that fatty acids of differing lengths are closely connected with intestinal HDPs (Figure 2). According to their aliphatic chain length, fatty acids are broadly classified into 3 categories: SCFAs (1–5), medium-chain fatty acids (MCFAs, 6–11), and long-chain fatty acids (LCFAs, ≥12).
SCFAs, MCFAs, and LCFAs promote intestinal epithelial and phagocytic HDP expression
Besides possessing direct antimicrobial characteristics, lauric acid (12), palmitic acid (16), and oleic acid (18) in human sebum also disinfected by inducing hBD2 expression through CD36 and NF-κB (34). These findings suggest LCFAs can protect the epithelium from infection by inducing HDP expression. Similarly, in addition to direct antimicrobial effects, MCFAs also have HDP induction effects. MCFAs can prevent inflammation and intestinal barrier dysfunction, and reduce diarrhea triggered by pathogenic Escherichia coli (35). Exploring reasons for the beneficial effects of MCFAs, it was found that the pBD1, pBD2, and pBD3 significantly increased (35). In colonic epithelial cells and monocytic cells of humans, hexanoate (6:0) and heptanoate (7:0) were more potent in promoting gene expression of cathelicidin antimicrobial peptides (CAMP), which encodes the human cathelicidin antimicrobial peptide LL-37 (LL-37). However, free fatty acids with chains longer than 7 or shorter than 4 carbons showed only a marginal effect on CAMP expression (36). Therefore, MCFAs may be more active in individual types of HDP expression in the gut. However, long-term studies conclude that the category of fatty acids with the most potential to modulate HDP expression is SCFAs. Under normal conditions, SCFAs exist in the small and large intestines, but are most abundant in the cecum and colon (37). In the distal intestine, SCFA concentrations range from 70 to 140 mM in the proximal colon and from 20 to 70 mM in the distal colon. Acetate, propionate, and butyrate are present in an approximate molar ratio of 60:20:20 (38). In addition to providing energy to epithelial and peripheral tissues, SCFAs also promote HDP expression in the intestine. SCFA-induced synthesis of endogenous HDPs is a phylogenetically conserved innate host defense mechanism. In mammals and aves, SCFAs are able to remarkably induce the expression of genes of multiple HDPs in intestinal epithelial cells and phagocytes (39, 40).
To evaluate the effects of fatty acid chain length on induction of HDPs, SCFAs, MCFAs, and LCFAs were used to treat macrophages and monocytes of chickens. Results showed that these 3 groups of fatty acids can all significantly contribute to the expression of multiple HDP genes including avian β-defensin 9 (AvBD9) and cathelicidin B1 in a dose-dependent manner. But butyric acid seemed to have the greatest potential (41). Similar results occurred in a porcine intestinal epithelial cell model (40). Therefore, butyric acid is confirmed as the most powerful inducer of SCFAs. Moreover, sodium butyrate shows excellent capacity to modulate intestinal permeability and change the bacterial community to decrease postweaning diarrhea in animals (41). Supplementing chickens with a combination of acetate, propionate, and butyrate in water resulted in a further reduction of Salmonella enteritidis in the cecum (39). Combinations of SCFAs seemed to exert a synergistic effect on HDP induction. This observation indicates the possibility to augment HDP expression by the synergy of combinations of different inducers. As for the level of fatty acid saturation, when comparing acids of the same carbon chain length, the oleic acid (18:1), linoleic acid (18:2), conjugated linoleic acid (18:2), and α-linoleic acid (18:3) treatment groups showed higher AvBD9 mRNA levels than the stearic acid (18:0) group in monocytes of chicken (42). But PUFAs do not increase cathelicidin expression (43). It is possible that the species of HDPs evoked by different inducers are selective. These studies suggest that SCFAs and unsaturated fatty acids may have stronger capacity to induce synthesis of multiple but not all HDPs. Similar effects are widespread in animals including mammals and birds.
The mechanisms of SCFAs on HDP expression in enterocytes
Based on their superior ability to induce HDP expression, many studies have investigated the mechanisms of SCFAs. SCFA-mediated regulation is mainly achieved by inhibition of histone deacetylase (HDAC), activation of GPCRs, stimulation of histone acetyltransferase (HAT) activity, and stabilization of the hypoxia-inducible factors (44–47). In humans, one of the main mechanisms for SCFAs to maintain intestinal health is through inhibition of HDACs (48); butyrate is the most potent HDAC inhibitor, whereas valerate and propionate are moderate and acetate is the least potent (49). HDAC inhibition enhances histone acetylation and is closely related to the transcriptionally active chromatin. However, the effects of HAT are contrary. Elevated levels of histone H2, H3, and H4 acetylation at particular sites such as H3K9 are active marks and often associated with ongoing transcription (50, 51). The individual treatment of chickens with acetate, propionate, or butyrate can inhibit the HDAC activity. In line with the synergy for HDP expression, cotreatment with 3 SCFAs showed the greatest HDAC inhibition (42). In vivo or in vitro trials with piglets demonstrated that butyrate upregulated the expression of multiple HDPs to promote clearance of pathogens and alleviate inflammation by depending on HDAC inhibition in the gut (51). Therefore, the acetylation of the histones is one of the key control factors.
However, besides directly inhibiting HDACs, the exact signal transduction mechanism contributing to HDP gene transcription is still unclear. The GPCR signaling pathway is another main mechanism of SCFAs. GPCR41 [free fatty acid receptor (FFAR)3], GPCR43 (FFAR2), GPCR109, and olfactory factor 78 are normally expressed in intestinal epithelial cells, adipocytes, and immune cells (52, 53). GPCRs are involved in SCFA-induced alleviation of inflammation (46). These receptors present specificities for different SCFAs, for example butyrate preferentially binds to GPCR41, yet GPCR43 has higher affinity for acetate and propionate (47, 54). In the colon of neonatal piglets, butyric acid–induced HDP expression was accompanied by increase in the mRNA level of GPCR41 (55). GPCRs and the mitogen-activated protein kinase (MAPK) pathway present a close correlation. Combined treatment with butyrate and cAMP showed a stronger effect on promoting AvBD9 expression in macrophages and primary jejunal explants by MAPK pathways (56). Inhibition of the mitogen-activated protein kinase kinase (MEK)–ERK pathway augmented AvBD9 expression, whereas blocking c-Jun N-terminal kinase (JNK) or the p38 MAPK pathway diminished expression (56). However, the upregulation of cathelicidin gene expression depended on the activation of ERK1/2 (57). In some reports, the role of ERK1/2 in induction of HDPs is contradictory. Maybe, the regulatory roles of ERK1/2 in different tissue cells and HDP types are inconsistent. Therefore, the GPCR–MAPK pathway plays an important role in mediating SCFA-induced HDP expression in the gut. Next, we still need further research to clarify SCFA induction mechanisms, including the GPCR, MAPK, and other pathways.
Saccharides of Different Sources Facilitate the Expression of HDPs in the Intestine
Lactose and polysaccharides from plants enhance intestinal HDP expression
A study reported that lactose can upregulate the transcript of the CAMP gene encoding the LL-37 in human colon cancer cell line (HT-29) and human monocytic cell line (THP-1) in a dose- and time-dependent manner. This active substance from milk protected the gut of infants from invasion of pathogens (58), and indicated the importance of bioactive compounds in breast milk for the intestinal health of newborn animals. Some kinds of active polysaccharides in plants have broad areas of bioactivity involving immune adjunction; antibacterial, antivirus, and antitumor effects; and reducing heart and cerebral vessel diseases, in which several may act by HDP expression. For example, astragalus polysaccharides were found to enhance LYZ expression in spleen of carp (59). A lot of immune cells in the intestinal mucosa originate from bone marrow and may have similar HDP induction capacity. Besides immune cells, the astragalus polysaccharides significantly increased respiratory epithelial cells’ expression of LL-37, which was attributed to activation of the p38 MAPK, JNK, and NF-κB pathways (60). Some dietary fiber, such as the macromolecules polysaccharides and prebiotics, can be fermented into SCFAs by intestinal flora together with other undigested carbohydrates. The induction capacity of SCFAs on HDP expression was discussed in the previous section. In different intestinal regions of the same or different species, the same or different types of fermentable fiber can produce various contents and components of SCFAs (61). Divergent SCFA combinations may have differential effects on HDP expression. This implies that different types of fermentable fiber may induce divergent expressions of HDP. Whether there are other mechanisms from dietary fiber on HDP expression is unclear.
Polysaccharides from bacteria trigger intestinal self-protection by enhanced HDPs
To some extent, specific components carried by some pathogens such as Citrobacter, Giardia, and Salmonella can contribute to intestinal HDP expression (62). For self-protection, the gut can respond to the stimulation of pathogenic bacteria polysaccharides by significantly enhancing HDP expression. For example, peptidoglycan can induce a protease cascade reaction through peptidoglycan recognition proteins-SA and lead the Toll-like receptors (TLRs) to form dimers that promote the expression of HDPs (63). Normal expression of the regenerating islet-derived protein 3γ gene depends on the TLRs (64). LPS can stimulate TLRs and nucleotide-binding oligomerization domain protein (NOD) signaling. And activation of the TLR and NOD signaling pathway can enhance HDP expression (63–65). The upregulation of HDPs neutralizes exotoxins and endotoxins (66). But, when the influence from bacterial polysaccharides lasts for a long time or is overly severe, the body cannot resist the excessive stimulation and endogenous defense elements are downregulated (67). So even though some components of pathogens can induce the HDPs in the gut, it is not desirable to stimulate HDP expression by special pathogenic molecular structures because of the possible concomitant inflammatory response and other side effects. However, exopolysaccharides from bacteria and fungus, mostly the probiotics, have beneficial bioactivity in modulating innate antiviral responses (68), pathogen-induced inflammatory responses (69), and microbial diversity in the gut with no toxic effects (70). By feeding of exopolysaccharides, functional immunity of the gut is enhanced. But whether HDP expression is one of their functions is still unknown and worthy of attention.
Micronutrients Augment Intestinal HDP Expression to Inhibit Infection
Multiple trace elements like zinc and iron, and vitamins such as cholecalciferol (vitamin D3), vitamin A, and niacin, are recognized as regulators of both innate and adaptive immune responses in intestinal mucosa, and command the intestinal immune defensive capacity (Figure 3). Improper micronutrients contribute to decreased mucosal immune responses against infection in the intestine (71).
FIGURE 3.
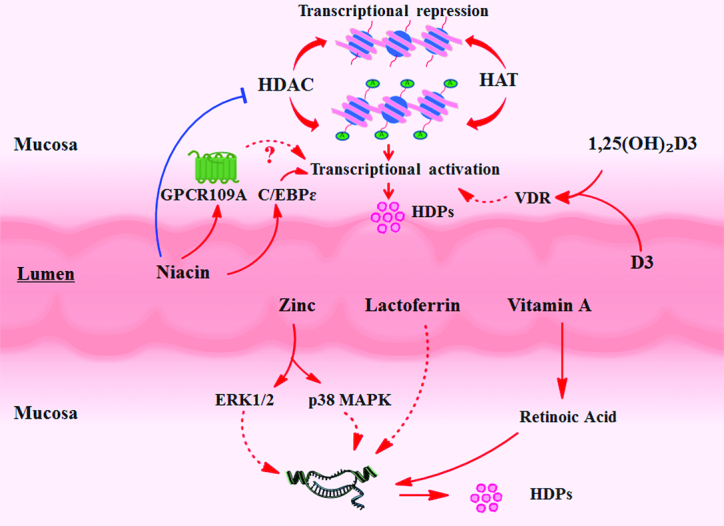
Multiple trace elements and vitamins enhance HDP expression in intestinal mucosa. Zinc contributes to HDP expression by the ERK1/2 and p38 MAPK pathways. Lactoferrin can increase intestinal HDP expression, but the study about its mechanism of action is limited. Cholecalciferol (vitamin D3) and its metabolite 1,25-dihydroxycholecalciferol [1,25(OH)2-D3] and vitamin B-3 increase histone acetylation levels to enhance transcription of HDP genes. Vitamin D3 and 1,25(OH)2-D3 regulate increased histone acetylation level via the VDR. Vitamin A can contribute to HDP expression by activating the retinoic acid response element. Niacin can restrain the activity of class III HDACs and increase C/EBPε to enhance antibacterial capacity. GPCR109A also mediates the regulation of niacin. C/EBPε, CCAAT/enhancer binding protein ε; ERK, extracellular regulated protein kinase; GPCR109A, G protein-coupled receptor 109A; HAT, histone acetyltransferase; HDAC, histone deacetylase; HDP, host defense peptide; p38 MAPK, p38 mitogen-activated protein kinase; VDR, vitamin D receptor.
Some kinds of trace elements augment HDP expression to inhibit intestinal infection
Mineral elements play a major role in nearly all metabolism processes and some mineral elements have beneficial effects for the intestinal mucosa barrier. Some studies have found mineral elements have positive impacts in regulating HDP expression. In the Caco-2 cell line, zinc >20 μM was found to significantly increase LL-37 secretion in a dose- and time-dependent manner, and the regulation was mediated by the ERK1/2 and p38 MAPK pathways (72). A high level of zinc supplementation in the diet shows effective mitigation of diarrhea in young animals. For weaned pigs, dietary ZnO at the concentration of 164 mg/kg induced the greatest expression of pBD3 and a decreased diarrhea ratio (73). These trials suggest that at least part of the intestinal protection effects of zinc is mediated by contributing to HDP expression. However, an exorbitant concentration of dietary ZnO (2425 mg Zn/kg) decreased pBD3 expression (73). Lactoferrin, a nonheme iron binding glycoprotein in milk, effectively contributes to the development of the gut and defends against intestinal infections. In weaned pigs, 1% lactoferrin in diets increased the cathelicidin proline-arginine rich-39 (PR-39) and Protegrin-1 mRNA levels (74). This once more demonstrates the importance and potential value of milk-derived bioactive substances to intestinal health. In addition, enhanced expression of HDPs can compete with bacteria for essential trace elements such as iron, manganese, and zinc to restrain the proliferation of bacteria in the gut (12).
Vitamin D3 and 1,25(OH)2-D3 enhance HDP expression in the gut by the vitamin D receptor and histone acetylation
Vitamin D3 is a sterol that can be obtained from feed. After absorption into the body, vitamin D3 gets 2 hydroxylations in the liver and kidney, respectively, and is metabolized into 1,25-dihydroxycholecalciferol [1,25(OH)2-D3]. It is the most biologically active form of vitamin D3. Previous studies focused on its major functions in maintenance of calcium and phosphorus homeostasis. However, it is also able to promote HDP expression in many animal species. In humans, vitamin D3 or 1,25(OH)2-D3 increases the expression of multiple HDPs in multiple cells including enterocytes and immune cells (75). In bovines and chickens, vitamin D3 or 1,25(OH)2-D3 stimulates the production of many kinds of HDPs such as cathelicidins and β-defensin in gut and other tissues (76, 77).
Mechanistic research indicated that 1,25(OH)2-D3 can increase histone acetylation level, and activation of the histone acetylation enzyme is mediated by the vitamin D receptor (VDR) (78). The VDR is a valid target to modulate HDP expression by histone acetylation. However, 1 report showed that the VDR did not respond to the induction of 1,25(OH)2-D3 in the intestinal epithelial cells of chickens (77). The response may be diverse across different doses. In different tissues and species, there may be other mechanisms involving 1,25(OH)2-D3. Some new mechanisms have recently been discovered and researched, such as the PPAR-γ–mediated induction of 1,25(OH)2-D3 in hBD3 and cathelicidin expression through the regulation of AP-1 and p38 MAPK activity in human keratinocytes (79). Moreover, IL-17A can enhance induction by 1,25(OH)2-D3 of cathelicidin through activation of Act1 and the MEK–ERK pathway in keratinocytes (80). In contrast, the glucocorticoid, dexamethasone, downregulated induction in human monocytes and bronchial epithelial cells (81). In addition, butyrate synergized with 1,25(OH)2-D3 to contribute to LL-37 expression and enhance the antimicrobial function of keratinocytes (78). Similarly to butyrate, 4-phenylbutyrate also enhanced vitamin D in LL-37 induction possibly through the VDR pathway in human bronchial epithelial cells and macrophages (82). The mechanisms of butyrate and vitamin D3 in modulation of HDP synthesis show potential value in antibiotic-free livestock production (83).
Vitamin A enhances HDP expression in the gut
Vitamin A plays a crucial role in maintaining the function of epithelial tissue of the digestive tract and genital tract, and retinoic acid is its active metabolite (84). In intestine, retinoic acid plays a special role in maintaining immune homeostasis. Some studies reported that retinoic acid can induce gene expression of porcine PR-39and enhance human cathelicidin antimicrobial protein 18 promoter activity in bone marrow cells (85). Using stably integrated reporter constructs, retinoic acid was found to activate α-defensin 1 gene expression directly through a proximal and distal element within a minimal promoter in a dose-dependent manner (86). In some HDP promoter regions, there is a retinoic acid response element (87). Considering the origin of immune cells in the intestinal mucosa, phagocytes in gut tissue may have similar functions. However, 1 report found retinoic acid inhibited the expression of hBD2 in human keratinocytes (88). This unexpected result may result from the dose of retinoic acid and specificity in the induction of HDP species.
Niacin enhances HDP expression in the gut
Niacin, also called vitamin B-3, can relieve pellagra characterized by inflammation of the skin and digestive system (89). In epithelial and nervous tissue, nicotinamide can be generated from niacin. The nuclear transcription factor CCAAT/enhancer binding protein ε (C/EBPε) family is closely associated to prevention of bacterial infection. C/EBPε deficiency led to severe infections by Staphylococcus aureus in mice, and C/EBPε deficiency in neutrophils contributed to the infectious phenotype. In contrast, exposure to nicotinamide significantly increased expression of C/EBPε in myeloid cells (90). Therefore, it can be sure that niacin has an important antibacterial capacity in relation to immune cells. Nicotinamide acts as a competitive inhibitor of class III HDACs, also called sirtuins (91). As an epigenetic modulator, nicotinamide enhances protein expression of a select number of its downstream targets in human neutrophils, such as cathelicidin (92). Moreover, activation of the receptor GPCR109A by nicotinamide suppressed intestinal inflammation and enhanced mechanical barrier functions similarly to butyric acid and β-hydroxybutyric acid (93). Thus, inhibition of HDAC and activation of GRCP109A may be additional mechanisms of the effects of niacin on HDP expression in the gut. However, excessive vitamin supplementation may lead to elevated intestinal inflammation as observed with vitamin D (94).
Additional Active Substances
Natural or synthetic nonnutrients are found to enhance intestinal HDP expression
There are some nonnutrients that can play a positive role in HDP induction. For example, bile salts, sulforaphane, curcumin, forskolin, resveratrol, pterostilbene, and polydatin can enhance HDP induction (95). Interestingly, some of these nonnutritive activators are able to amplify nutritive HDP induction capacity. A report has indicated that foskolin synergized butyric acid to enhance AvBD9 expression in macrophages and primary jejunal explants (56). Lithocholic acid, metabolized from bile acid by bacteria in the gut, enhanced transcription of the CAMP gene and synergized butyrate in colonic epithelial cells (96). Stilbenoids can synergistically induce human cathelicidin gene expression with vitamin D in monocytes and keratinocytes (97).
Probiotics and their metabolites enhance intestinal HDP expression
In a time- and dose-dependent manner, some probiotics significantly promote HDP expression in intestinal epithelial cells. Escherichia coli Nissle 1917 contributed to hBD2 expression in human Caco-2 cells by flagellin and JNK pathway (98). Not only a single probiotic, but incubation of a mixture of multiple lactobacillus strains obviously increased hBD2 expression via the NF-κB, AP-1, and MAPK pathways in Caco-2 cells (99). Besides the probiotic bacteria themselves, metabolites from these probiotic strains may be another reason for enhanced HDP expression. Lactobacillus reuteri I5007 increased the expression of multiple β-defensins in intestinal epithelial cells of neonatal piglets through increased butyric acid (55). HDP expression is partly responsible for the immunomodulatory function of probiotics.
Research Prospects
Currently, we lack sufficient data on the spatiotemporal expression rules of HDPs in the gut of humans and economically important animals. This information will guide when to intervene, which nutrients are to be used, and which species of HDPs will perform the main defensive functions. Previous studies showed that in the short term, HDPs were not easy to develop drug resistance against because they possessed multiple mechanisms to kill bacteria. But drug resistance may still occur in the long term. If so, high doses and direct supplements of exogenous HDPs through feed may be adverse. Some bacteria have evolved resistance mutations to HDPs, which can resist HDP expression (100). Moreover, another report showed that enteric α-defensin 5 can promote the adhesion and invasion of Shigella to host in humans (101). There is no proof explaining whether this is an innate characteristic or a mutational result of Shigella. However, these results indicate that the security and use-pattern of HDPs need further evaluations. Several researches confirmed that HDPs are found as novel antibiofilm agents to inhibit the growth of bacterial communities (102). The precise mechanism is not clear. Otherwise, previous reports have suggested endogenous HDPs may be insufficient at physiological concentration to kill bacteria, and their function is mainly based on an anti-inflammatory effect (103). This evidence shows that present knowledge about endogenous HDPs is still limited. A comprehensive study of HDP properties and functions is necessary.
Previous studies have found some highly effective HDP inducers. Most inducers functionally behave as HDAC inhibitors. However, it is surprising that some stronger HDAC inhibitors are less efficient in the induction of HDPs. Even stronger HDAC inhibition induced by higher concentrations of HDAC inhibitors conversely shows decreased HDP induction (83). Moreover, transcriptional genes of HDPs mediated by different inducers are selective. The mechanism is indistinct. Besides HDAC inhibition, the research about other functional mechanisms is insufficient. Other epigenetic modifications such as the histone modifications and accurate signal transduction mechanisms in the gut need to be researched. Comprehensively clarifying the networks governing HDP expression is necessary.
Conclusions
Intestinal endogenous HDP expression can be modulated by specific nutrients in the diet. As an important part of disease-resistant nutrition research, enhanced expression of endogenous HDPs could fortify immune defensive capacity, alleviate tissue inflammation, and shape gut microbes to maintain intestinal homeostasis. Some amino acids, fatty acids, saccharides, trace elements, vitamins, and other nonnutritional active substances show excellent potential to induce HDPs and may have crucial value in clinical application. The synergy of multiple inducers may be leveraged. Intestinal health ensures nutrient conversion and growth in humans and in animals of economic importance. Regulation of endogenous HDPs to maintain intestinal health by dietary nutrients may effectively promote replacement of antibiotics in the future.
Acknowledgments
The authors’ responsibilities were as follows—XM: mainly designed the review; JW: mainly wrote the manuscript; NM, LJJ, and XM: edited the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by National Key R&D Program of China grants 2018YFD0500601 and 2017YFD0500501 (both to XM), National Natural Science Foundation of China grants 31829004 and 31722054 (to XM), College of Animal Science and Technology “Young Talents Program” at China Agricultural University grant 2017DKA001, Beijing Nova Programme Interdisciplinary Cooperation Project grant xxjc201804, 111 Project grant B16044, and the Developmental Fund for Animal Science by Shenzhen Jinxinnong Feed Co., Ltd.
Author disclosures: JW, NM, LJJ, and XM, no conflicts of interest.
Abbreviations used: AMP, antimicrobial peptide; AvBD9, avian β-defensin 9; BCAA, branched-chain amino acid; CAMP, cathelicidin antimicrobial peptides; C/EBPε, CCAAT/enhancer binding protein ε; ERK, extracellular signal regulated protein kinase; FFAR, free fatty acid receptor; GPCR, G protein–coupled receptor; HAT, histone acetyltransferase; hBD, human β-defensin; HDAC, histone deacetylase; HDP, host defense peptide; JNK, c-Jun N-terminal kinase; LCFA, long-chain fatty acid; LL-37, cathelicidin antimicrobial peptide LL-37; LYZ, lysozyme; MAPK, mitogen-activated protein kinase; MCFA, medium-chain fatty acid; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; NOD, nucleotide-binding oligomerization domain protein; pBD, porcine β-defensin; PR-39, cathelicidin proline-arginine-rich-39; TLR, Toll-like receptor; VDR, vitamin D receptor; 1,25(OH)2-D3, 1,25-dihydroxycholecalciferol.
References
- 1. Varady J, Eder K, Ringseis R. Dietary oxidized fat activates the oxidative stress-responsive transcription factors NF-κB and Nrf2 in intestinal mucosa of mice. Eur J Nutr. 2011;50:601–9. [DOI] [PubMed] [Google Scholar]
- 2. Liew WP, Mohd-Redzwan S. Mycotoxin: its impact on gut health and microbiota. Front Cell Infect Microbiol. 2018;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Awad WA, Hess C, Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel). 2017;9:E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearce SC, Mani V, Weber TE, Rhoads RP, Patience JF, Baumgard LH, Gabler NK. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J Anim Sci. 2013;91:5183–93. [DOI] [PubMed] [Google Scholar]
- 5. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. [DOI] [PubMed] [Google Scholar]
- 6. Zumla A, Rao M, Wallis RS, Kaufmann SH, Rustomjee R, Mwaba P, Vilaplana C, Yeboah-Manu D, Chakaya J, Ippolito G et al.. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis. 2016;16:e47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansour SC, Pena OM, Hancock RE. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35:443–50. [DOI] [PubMed] [Google Scholar]
- 8. Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol. 2012;113:723–36. [DOI] [PubMed] [Google Scholar]
- 9. Bechinger B, Gorr SU. Antimicrobial peptides: mechanisms of action and resistance. J Dent Res. 2016;96:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung GYC, Fisher EL, McCausland JW, Choi J, Collins JWM, Dickey SW, Otto M. Antimicrobial peptide resistance mechanism contributes to Staphylococcus aureus infection. J Infect Dis. 2018;217:1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mishra B, Wang GS. The importance of amino acid composition in natural AMPs: an evolutional, structural, and functional perspective. Front Immunol. 2012;3:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung LK, Raffatellu M. G.I. pros: antimicrobial defense in the gastrointestinal tract. Semin Cell Dev Biol. 2018;88:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niyonsaba F, Kiatsurayanon C, Ogawa H. The role of human β-defensins in allergic diseases. Clin Exp Allergy. 2016;46:1522–30. [DOI] [PubMed] [Google Scholar]
- 15. Chen B, Ni X, Sun R, Zeng B, Wei H, Tian Z, Wei H. Commensal bacteria-dependent CD8αβ+T cells in the intestinal epithelium produce antimicrobial peptides. Front Immunol. 2018;9:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varney KM, Bonvin AM, Pazgier M, Malin J, Yu W, Ateh E, Oashi T, Lu W, Huang J, Diepeveen-de Buin M et al.. Turning defense into offense: defensin mimetics as novel antibiotics targeting lipid II. PLoS Pathog. 2013;9:e1003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci. 2011;68:2161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154–63. [DOI] [PubMed] [Google Scholar]
- 19. Dai ZL, Zhang J, Wu G, Zhu WY. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 2010;39:1201–15. [DOI] [PubMed] [Google Scholar]
- 20. Konno Y, Ashida T, Inaba Y, Ito T, Tanabe H, Maemoto A, Ayabe T, Mizukami Y, Fujiya M, Kohgo Y. Isoleucine, an essential amino acid, induces the expression of human β defensin 2 through the activation of the G-protein coupled receptor-ERK pathway in the intestinal epithelia. Food Nutr Sci. 2013;3:548–55. [Google Scholar]
- 21. Ren M, Zhang S, Liu X, Li S, Mao X, Zeng X, Qiao S. Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous β-defensin expression through the Sirt1/ERK/90RSK pathway. J Agric Food Chem. 2016;64:3371–9. [DOI] [PubMed] [Google Scholar]
- 22. Sherman H, Chapnik N, Froy O. Albumin and amino acids upregulate the expression of human beta-defensin 1. Mol Immunol. 2006;43:1617–23. [DOI] [PubMed] [Google Scholar]
- 23. Osei-Boadi K, Gordon E, Melgarejo T. Select amino acids induced expression of human beta-defensin in Caco-2 cells. FASEB J. 2013;27(1 Suppl):866.8. [Google Scholar]
- 24. Dijkstra G, van Goor H, Jansen PL, Moshage H. Targeting nitric oxide in the gastrointestinal tract. Curr Opin Investig Drugs. 2004;5:529–36. [PubMed] [Google Scholar]
- 25. Leitão RF, Brito GA, Oriá RB, Braga-Neto MB, Bellaguarda EA, Silva JV, Gomes AS, Lima-Júnior RC, Siqueira FJ, Freire RS et al.. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol. 2011;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauchart-Thevret C, Cui L, Stoll B, Burrin D. Arginine-mediated stimulation of intestinal epithelial cell protein synthesis is mTOR-dependent but NO-independent. FASEB J. 2009;23(1 Suppl):227.7. [Google Scholar]
- 27. Ma N, Guo P, Zhang J, He T, Kim SW, Zhang G, Ma X. Nutrients mediate intestinal bacteria–mucosal immune crosstalk. Front Immunol. 2018;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Powell JD, Pollizzi KM, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang H, Ji Y, Wu G, Sun K, Sun Y, Li W, Wang B, He B, Zhang Q, Dai Z et al.. L-tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J Nutr. 2015;145:1156–62. [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S et al.. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen X, Song P, Fan P, He T, Jacobs D, Levesque CL, Johnston LJ, Ji L, Ma N, Chen Y et al.. Moderate dietary protein restriction optimized gut microbiota and mucosal barrier in growing pig model. Front Cell Infect Microbiol. 2018;8:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan P, Liu P, Song P, Chen X, Ma X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci Rep. 2017;7:43412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma N, Ma X. Dietary amino acids and the gut-microbiome-immune axis: physiological metabolism and therapeutic prospects. Compr Rev Food Sci Food Saf. 2018;18:221–42. [DOI] [PubMed] [Google Scholar]
- 34. Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating β-defensin-2 expression. J Invest Dermatol. 2010;130:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang J, Lu J, Xie X, Xiong J, Huang N, Wei H, Jiang S, Peng J. Blend of organic acids and medium chain fatty acids prevents the inflammatory response and intestinal barrier dysfunction in mice challenged with enterohemorrhagic Escherichia coli O157:H7. Int Immunopharmacol. 2018;58:64–71. [DOI] [PubMed] [Google Scholar]
- 36. Jiang W, Sunkara LT, Zeng X, Deng Z, Myers SM, Zhang G. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides. 2013;50:129–38. [DOI] [PubMed] [Google Scholar]
- 37. Haenen D, Zhang J, Souza da Silva C, Bosch G, van der Meer IM, van Arkel J, van den Borne JJGC, Pérez Gutiérrez O, Smidt H, Kemp B et al.. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr. 2013;143:274–83. [DOI] [PubMed] [Google Scholar]
- 38. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, Lamont S, Lillehoj HS, Beker A, Teeter RG et al.. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6:e27225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeng X, Sunkara LT, Jiang W, Bible M, Carter S, Ma X, Qiao S, Zhang G. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS One. 2013;8:e72922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang C, Song P, Fan P, Hou C, Thacker P, Ma X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr. 2015;145:2774–80. [DOI] [PubMed] [Google Scholar]
- 42. Sunkara LT, Jiang W, Zhang G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS One. 2012;7:e49558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo C, Rosoha E, Lowry MB, Borregaard N, Gombart AF. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr Biochem. 2013;24:754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin MY, de Zoete MR, van Putten JP, Strijbis K. Histone deacetylase inhibition and dietary short-chain fatty acids. Front Immunol. 2015;6:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vinolo MA, Hirabara SM, Curi R. G-protein-coupled receptors as fat sensors. Curr Opin Clin Nutr Metab Care. 2012;15:112–6. [DOI] [PubMed] [Google Scholar]
- 46. Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A et al.. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: a double-edged sword for health?. Adv Nutr. 2018;9:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 49. Cousens LS, Gallwitz D, Alberts BM. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979;254:1716–23. [PubMed] [Google Scholar]
- 50. Gates LA, Shi J, Rohira AD, Feng Q, Zhu B, Bedford MT, Sagum CA, Jung SY, Qin J, Tsai MJ et al.. Acetylation on histone H3 lysine 9 mediates a switch from transcription initiation to elongation. J Biol Chem. 2017;292:14456–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonçalves P, Araújo JR, Di Santo JP. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:558–72. [DOI] [PubMed] [Google Scholar]
- 52. Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv Nutr. 2012;3:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu H, Hou C, Wang G, Jia H, Yu H, Zeng X, Thacker PA, Zhang G, Qiao S. Lactobacillus reuteri I5007 modulates intestinal host defense peptide expression in the model of IPEC-J2 cells and neonatal piglets. Nutrients. 2017;9:E559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sunkara LT, Zeng X, Curtis AR, Zhang G. Cyclic AMP synergizes with butyrate in promoting β-defensin 9 expression in chickens. Mol Immunol. 2014;57:171–80. [DOI] [PubMed] [Google Scholar]
- 57. D'Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C et al.. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435–43. [DOI] [PubMed] [Google Scholar]
- 58. Cederlund A, Kai-Larsen Y, Printz G, Yoshio H, Alvelius G, Lagercrantz H, Strömberg R, Jörnvall H, Gudmundsson GH, Agerberth B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS One. 2013;8:e53876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yuan C, Pan X, Gong Y, Xia A, Wu G, Tang J, Han X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int Immunopharmacol. 2008;8:51–8. [DOI] [PubMed] [Google Scholar]
- 60. Zhao L, Tan S, Zhang H, Liu P, Tan YZ, Li JC, Jia D, Shen XF. Astragalus polysaccharides exerts anti-infective activity by inducing human cathelicidin antimicrobial peptide LL-37 in respiratory epithelial cells. Phytother Res. 2018;32:1521–9. [DOI] [PubMed] [Google Scholar]
- 61. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- 62. Manko A, Motta JP, Cotton JA, Feener T, Oyeyemi A, Vallance BA, Wallace JL, Buret AG. Giardia co-infection promotes the secretion of antimicrobial peptides beta-defensin 2 and trefoil factor 3 and attenuates attaching and effacing bacteria-induced intestinal disease. PLoS One. 2017;12:e0178647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P et al.. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal α-defensin expression. Gut. 2004;53:1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosenfeld Y, Papo N, Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem. 2006;281:1636–43. [DOI] [PubMed] [Google Scholar]
- 67. Wu H, Zhang G, Minton JE, Ross CR, Blecha F. Regulation of cathelicidin gene expression: induction by lipopolysaccharide, interleukin-6, retinoic acid, and Salmonella enterica serovar typhimurium infection. Infect Immun. 2000;68:5552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kanmani P, Albarracin L, Kobayashi H, Iida H, Komatsu R, Humayun Kober AKM, Ikeda-Ohtsubo W, Suda Y, Aso H, Makino S et al.. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol Immunol. 2018;93:253–65. [DOI] [PubMed] [Google Scholar]
- 69. Jones SE, Paynich ML, Kearns DB, Knight KL. Protection from intestinal inflammation by bacterial exopolysaccharides. J Immunol. 2014;192:4813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Salazar N, Gueimonde M, Hernández-Barranco AM, Ruas-Madiedo P, de los Reyes-Gavilán CG. Exopolysaccharides produced by intestinal Bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl Environ Microbiol. 2008;74:4737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang Y, Yuan Y, Tao Y, Wang W. Effects of vitamin A deficiency on mucosal immunity and response to intestinal infection in rats. Nutrition. 2011;27:227–32. [DOI] [PubMed] [Google Scholar]
- 72. Talukder P, Satho T, Irie K, Sharmin T, Hamady D, Nakashima Y, Kashige N, Miake F. Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int Immunopharmacol. 2011;11:141–4. [DOI] [PubMed] [Google Scholar]
- 73. Liu P, Pieper R, Tedin L, Martin L, Meyer W, Rieger J, Plendl J, Vahjen W, Zentek J. Effect of dietary zinc oxide on jejunal morphological and immunological characteristics in weaned piglets. J Anim Sci. 2014;92:5009–18. [DOI] [PubMed] [Google Scholar]
- 74. Wang Y, Shan T, Xu Z, Liu J, Feng J. Effect of lactoferrin on the growth performance, intestinal morphology and expression of PR-39 and protegrin-1 genes in weaned piglets. J Anim Sci. 2006;84:2636–41. [DOI] [PubMed] [Google Scholar]
- 75. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C et al.. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. [DOI] [PubMed] [Google Scholar]
- 76. Merriman KE, Kweh MF, Powell JL, Lippolis JD, Nelson CD. Multiple β-defensin genes are upregulated by the vitamin D pathway in cattle. J Steroid Biochem Mol Biol. 2015;154:120–9. [DOI] [PubMed] [Google Scholar]
- 77. Zhang L, Lu L, Li S, Zhang G, Ouyang L, Robinson K, Tang Y, Zhu Q, Li D, Hu Y et al.. 1,25-Dihydroxyvitamin-D3 induces avian β-defensin gene expression in chickens. PLoS One. 2016;11:e0154546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schauber J, Oda Y, Büchau AS, Yun QC, Steinmeyer A, Zügel U, Bikle DD, Gallo RL. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–24. [DOI] [PubMed] [Google Scholar]
- 79. Dai X, Sayama K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, Yang L, Hirakawa S, Hashimoto K. PPARγ mediates innate immunity by regulating the 1α,25-dihydroxyvitamin D3 induced hBD-3 and cathelicidin in human keratinocytes. J Dermatol Sci. 2010;60:179–86. [DOI] [PubMed] [Google Scholar]
- 80. Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, Ruzicka T, Gallo RL, Schauber J. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Petrillo MG, Bortner CD, Cidlowski JA. Glucocorticoids: inflammation and immunity. In: Geer E, editor. The hypothalamic-pituitary-adrenal axis in health and disease. Cham: (Switzerland): Springer International Publishing; 2017. pp. 43–63. [Google Scholar]
- 82. Rekha RS, Rao Muvva SS, Wan M, Raqib R, Bergman P, Brighenti S, Gudmundsson GH, Agerberth B. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy. 2015;11:1688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Robinson K, Ma X, Liu Y, Qiao S, Hou Y, Zhang G. Dietary modulation of endogenous host defense peptide synthesis as an alternative approach to in-feed antibiotics. Anim Nutr. 2018;4:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30. [DOI] [PubMed] [Google Scholar]
- 85. Elloumi HZ, Holland SM. Complex regulation of human cathelicidin gene expression: novel splice variants and 5’UTR negative regulatory element. Mol Immunol. 2008;45:204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang N, Su Q, Boeckh-Herwig S, Yaneva M, Tempst P. Delayed-late activation of a myeloid defensin minimal promoter by retinoids and inflammatory mediators. Leuk Res. 2004;28:879–89. [DOI] [PubMed] [Google Scholar]
- 87. Zhao C, Ganz T, Lehrer RI. Structures of genes for two cathelin-associated antimicrobial peptides: prophenin-2 and PR-39. FEBS Lett. 1995;376:130–4. [DOI] [PubMed] [Google Scholar]
- 88. Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schröder JM. Differential gene induction of human β-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–9. [DOI] [PubMed] [Google Scholar]
- 89. Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol. 2011;164:1188–200. [DOI] [PubMed] [Google Scholar]
- 90. Kyme P, Thoennissen NH, Tseng CW, Thoennissen GB, Wolf AJ, Shimada K, Krug UO, Lee K, Müller-Tidow C, Berdel WE et al.. C/EBPε mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice. J Clin Invest. 2012;122:3316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee AR, Kishigami S, Amano T, Matsumoto K, Wakayama T, Hosoi Y. Nicotinamide: a class III HDACi delays in vitro aging of mouse oocytes. J Reprod Dev. 2013;59:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bassett SA, Barnett MP. The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients. 2014;6:4273–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH et al.. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ghaly S, Kaakoush NO, Lloyd F, McGonigle T, Mok D, Baird A, Klopcic B, Gordon L, Gorman S, Forest C et al.. High dose vitamin D supplementation alters faecal microbiome and predisposes mice to more severe colitis. Sci Rep. 2018;8:11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lyu W, Deng Z, Sunkara LT, Becker S, Robinson K, Matts R, Zhang G. High throughput screening for natural host defense peptide-inducing compounds as novel alternatives to antibiotics. Front Cell Infect Microbiol. 2018;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Termén S, Tollin M, Rodriguez E, Sveinsdóttir SH, Jóhannesson B, Cederlund A, Sjövall J, Agerberth B, Gudmundsson GH. PU.1 and bacterial metabolites regulate the human gene CAMP encoding antimicrobial peptide LL-37 in colon epithelial cells. Mol Immunol. 2008;45:3947–55. [DOI] [PubMed] [Google Scholar]
- 97. Guo C, Sinnott B, Niu B, Lowry MB, Fantacone ML, Gombart AF. Synergistic induction of human cathelicidin antimicrobial peptide gene expression by vitamin D and stilbenoids. Mol Nutr Food Res. 2014;58:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human β-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75:2399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and vsl#3 induce enterocyte β-defensin 2. Clin Exp Immunol. 2008;151(3):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chaili S, Cheung AL, Bayer AS, Xiong YQ, Waring AJ, Memmi G, Donegan N, Yang SJ, Yeaman MR. The GraS sensor in Staphylococcus aureus mediates resistance to host defense peptides differing in mechanisms of action. Infect Immun. 2015;84:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xu D, Liao C, Zhang B, Tolbert WD, He W, Dai Z, Zhang W, Yuan W, Pazgier M, Liu J et al.. Human enteric α-defensin 5 promotes Shigella infection by enhancing bacterial adhesion and invasion. Immunity. 2018;48:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hongwei C, Wubbolts RW, Haagsman HP, Veldhuizen EJA. Inhibition and eradication of Pseudomonas aeruginosa biofilms by host defence peptides. Sci Rep. 2018;8:10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Choi KY, Mookherjee N. Multiple immune-modulatory functions of cathelicidin hos t defense peptides. Front Immunol. 2012;3:149. [DOI] [PMC free article] [PubMed] [Google Scholar]