Abstract
A phenomenon in schizophrenia patients that deserves attention is the high comorbidity rate with obsessive-compulsive disorder (OCD). Little is known about the neurobiological basis of schizo-obsessive comorbidity (SOC). We aimed to investigate whether specific changes in white matter exist in patients with SOC and the relationship between such abnormalities and clinical parameters. Twenty-eight patients with SOC, 28 schizophrenia patients, 30 OCD patients, and 30 demographically matched healthy controls were recruited. Using Tract-based Spatial Statistics and Probabilistic Tractography, we examined the pattern of white matter abnormalities in these participants. We also used ANOVA and Support Vector Classification of various white matter indices and structural connection probability to further examine white matter changes among the 4 groups. We found that patients with SOC had decreased fractional anisotropy (FA) and increased radial diffusivity in the right sagittal stratum and the left crescent of the fornix/stria terminalis compared with healthy controls. We also found changed connection probability in the Default Mode Network, the Subcortical Network, the Attention Network, the Task Control Network, the Visual Network, the Somatosensory Network, and the cerebellum in the SOC group compared with the other 3 groups. The classification results further revealed that FA features could differentiate the SOC group from the other 3 groups with an accuracy of .78. These findings highlight the specific white matter abnormalities found in patients with SOC.
Keywords: diffusion tensor imaging, schizo-obsessive comorbidity, schizophrenia, obsessive-compulsive disorder, support vector classification
Introduction
It is unclear whether schizo-obsessive comorbidity (SOC) is a subgroup of schizophrenia,1 although some researchers have reported that this subgroup may have diagnostic validity.2,3 Obsessive-compulsive disorder (OCD) occurs in up to 37.5% of schizophrenia patients and these patients typically present with more severe symptoms,4–6 emotional disturbance,7 social and neurocognitive deficits,8 and treatment resistance.9,10 Moreover, the incidence of SOC is much higher than other comorbid conditions, such as anxiety or depression in schizophrenia patients.11 This may be due to common neurobiological mechanisms,12,13 and genetic and environmental pathogenic factors.14–16 Previous evidence also suggests that positive symptoms of schizophrenia and OCD symptoms could exacerbate one another.17 Based on these studies, the “double jeopardy” hypothesis has been proposed,18 which suggests that OCD symptoms could be important exacerbating factors in the pathological development of schizophrenia.19 The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) also recognizes the existence of OCD in schizophrenia spectrum disorders,20 highlighting shared and overlapping clinical manifestations and implying a continuum between obsessions and delusions.21 There is an increased interest in improving our understanding of SOC and better characterizing patients with SOC clinically and neurobiologically.22,23
However, the etiology and neurobiological basis of SOC is not clear. Limited research suggests that the cause of SOC may be related to the many commonalities between schizophrenia and OCD, which include commonalities in pathogenic factors, impaired cognitive function, changed neural circuits,24 and therapeutic efficacy of serotonin reuptake inhibitors.25 The “disconnection” hypothesis considers aberrant integration of neuronal networks as a crucial deficit in schizophrenia and OCD patients.26 White matter changes in these patients could serve as important biomarkers.27–29 The majority of previous studies have reported that patients with schizophrenia and OCD both exhibit abnormalities in the cortico-striato-thalamo circuitry.4,30,31 Another study investigated white matter (WM) integrity in patients with schizophrenia only and patients with OCD only and found that both groups showed decreased fractional anisotropy (FA) in the corpus callosum.32 In our previous resting-state functional connectivity (rsFC) study, we found that patients with SOC showed the strongest rsFC within subregions of the Default Mode Network (DMN) and the weakest rsFC between the DMN and subregions of the Salience Network compared with schizophrenia patients, OCD patients, and healthy controls.27 However, little is known about whole brain white matter structural network abnormalities in patients with SOC.
In this study, we aimed to investigate tract-based white matter integrity and network-based biomarkers among patients with SOC, schizophrenia, OCD, and healthy controls. We first examined their WM fiber integrity including FA, Mean Diffusivity, Axial Diffusivity, and Radial Diffusivity using traditional Tract-based Spatial Statistics (TBSS). We then examined their Structural Connectivity Probability using Probabilistic Tractography in 264 gray matter regions of interest (ROIs). Traditional TBSS analysis could obtain FA values of white matter subregions within the skeletons of tract based on the Johns Hopkins stereotaxic atlas.33 Probabilistic Tractography can be used to track structural pathways between specific gray matter regions based on an estimate of the probability density of water diffusion direction,34 and previous studies have suggested that this approach may improve robustness and sensitivity compared with Deterministic Tractography,35 especially in tracking white matter with fiber crossings and undulations.36 Compared with TBSS indices, Probabilistic Tractography has advantages in microstructure and multi-pathway investigations.37 The combination of TBSS and Probabilistic Tractography could provide a more comprehensive understanding of white matter substrates.
The 264 gray matter ROIs used in Probabilistic Tractography analysis were 5-mm diameter spheres, as identified by Power et al,38 spanning the cerebral cortex, the cerebellum, and subcortical structures. These ROIs could represent whole brain gray matter structure for enhanced biological interpretability and data validity.38 Most of these ROIs belong to one of the following brain networks: the Default Mode Network (DMN), the Salience Network, the Dorsal Attention Network (DAN), the Ventral Attention Network (VAN), the Fronto-parietal Task Control Network (FPN), the Cingulo-opercular Task Control Network (CON), the Somatosensory Network, the Memory Retrieval Network (MRN), the Visual Network, the Auditory Network, the Subcortical Network, and the cerebellum. Abnormal connectivity both within and between these networks have been reported in schizophrenia and OCD studies, especially in the DMN,39,40 which is mainly associated with perceptual disturbances, a key factor in the development of schizophrenia and OCD symptoms.27
ANOVA of the TBSS and Probabilistic Tractography analysis were conducted to examine between-group differences. Since it is difficult to correct for multiple groups comparisons with the huge amount of raw data involved (such as 264 × 264 PT network matrix), machine learning may be a viable alternative method to identify biomarkers, predict new samples, and facilitate diagnosis. Therefore, we also adopted machine learning analysis in addition to ANOVA in this study.
We hypothesized that patients with SOC would exhibit extensive white matter changes in cortico-subcortical subregions, including changed FA and connection probability associated with the DMN, the Task-positive Network, and the Subcortical Network, which support the “double jeopardy” and “disconnection” hypotheses.
Materials and Methods
Participants
All participants were recruited from the outpatient clinics of the Department of Psychiatry, Second Xiangya Hospital of the Central South University and the local community in Changsha, China. Inclusion criteria of all participants were as follows: Han ethnicity; age ≥ 16; and IQ ≥ 70 (as estimated with the “common sense,” “arithmetic,” “similarity” and “digital span” subtests of the Chinese version of the Wechsler Adult Intelligence Scale-Revised).41 Exclusion criteria for all participants were any diagnosis of physical and neurological disorders/anxiety disorder/depression/autism/known genetic disorders/substance dependence and contraindications for MRI scanning. Healthy controls were excluded if there was a family history of psychiatric disorder. Patients with SOC were excluded if there were obvious drug-induced obsessive-compulsive symptoms. After diffusion tensor imaging (DTI) and T1 quality control procedures, 7 participants were excluded, leaving 116 participants in the final analysis.
The study was approved by the Ethics Committee of the Second Xiangya Hospital of the Central South University. All participants gave written informed consent.
Diagnostic Assessment
To determine schizophrenia and OCD diagnoses, patients were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorder, Patient Edition (SCID-IV) by 2 experienced psychiatrists.42 For SOC, participants needed to meet the diagnostic criteria of schizophrenia and OCD concurrently, while schizophrenia and OCD patients met the diagnostic criteria of schizophrenia and OCD separately. For healthy controls, the non-patient edition of the SCID was used to confirm the absence of mental disorders.43 The Positive and Negative Syndrome Scale (PANSS) was used to assess schizophrenia symptoms in the SOC and the schizophrenia groups.44 The Yale–Brown Obsessive-Compulsive Scale (Y-BOCS) was used to assess obsessive-compulsive symptoms in the SOC and the OCD groups.45
MRI Acquisition
MRI data were acquired on a 3.0T Siemens SKYRA MR scanner (Siemens Medical, Erlangen, Germany) at the Second Xiangya Hospital of the Central South University, Changsha, China. 64-direction diffusion weighted images with b-value of 1000 s/mm2 and 10 repetitions of images with a b-value of 0 s/mm2 were acquired with a twice-refocused spin echo pulse sequence: repetition time (TR) = 6400 ms, echo time (TE) = 86 ms, field of view (FOV) = 256 mm, flip angle = 90°, acquisition matrix = 128 × 128, slice thickness = 2.5 mm, slices = 74, and voxel size = 2 × 2 × 2.5 mm3. T1-weighted anatomical images were acquired with a sagittal-oriented magnetization prepared rapid gradient echo (MPRAGE) sequence. The parameters were as follow: TR = 1900 ms, TE = 2.01 ms, inversion time = 900 ms, FOV = 256 mm, flip angle = 9°, in-plane acquisition matrix = 256 × 256, slice thickness = 1 mm, no. slices = 176, and voxel size = 1 × 1 × 1 mm3.
Data Analysis
Details on MRI data preprocessing and Support Vector Classification can be found in the supplementary material. In brief, TBSS and Probabilistic Tractography of each participant were preprocessed using the PANDA 1.3.1 (http://www.nitrc.org/projects/panda) pipeline toolbox.46 After preprocessing and quality control, one-way ANOVA and post-hoc tests of the TBSS and Probabilistic Tractography results among the 4 groups were performed. TBSS results were corrected by Bonferroni correction, while Probabilistic Tractography results were corrected by the False Discovery Rate (FDR). Pearson correlation analysis was performed between FA and connection probability values of clusters with significant group differences and PANSS and Y-BOCS subscale scores within the relevant group with significance level set at PBonferroni <.05. Moreover, according to previous studies showing the influence of age, gender, and IQ on white matter,47,48 these 3 factors were entered as covariates in the analysis.
Results
Twenty-eight patients with SOC, 28 schizophrenia patients, 30 OCD patients, and 30 healthy controls participated in this study. The mean age of all participants was 21.87 years (SD = 4.66, range = 16–36 years). Demographic and clinical rating information are shown in table 1.
Table 1.
Demographic and Clinical Information of Patients and Healthy Controls
| SOC (n = 28) | SCZ ( n = 28) | OCD ( n = 30) | HCs ( n = 30) | F/χ 2/t | P | |
|---|---|---|---|---|---|---|
| Gender (no. M/F) | 17/11 | 16/12 | 15/15 | 12/18 | 2.92 | .40 |
| Age (years) | 22.32 (5.84) | 20.46 (3.78) | 22.17 (5.70) | 22.47 (2.46) | 1.15 | .33 |
| IQ | 97.29 (17.51) | 102.57 (15.55) | 110.03 (16.37) | 120.30 (10.16) | 12.70 | .001* |
| Duration of illness (years) | 3.56 (3.68) | 1.91 (2.59) | 3.85 (4.85) | NA | 2.04 | .14 |
| PANSS positive score | 14.25 (4.78) | 16.10 (4.46) | NA | NA | −1.50 | .14 |
| PANSS negative score | 9.39 (3.20) | 9.68 (3.94) | NA | NA | −0.30 | .77 |
| PANSS general score | 29.43 (6.76) | 30.61 (6.37) | NA | NA | −0.67 | .51 |
| Y-BOCS obsession score | 16.64 (2.90) | NA | 15.03 (3.66) | NA | 1.85 | .07 |
| Y-BOCS compulsion score | 12.43 (4.89) | NA | 13.07 (3.56) | NA | −0.57 | .57 |
Note: Data are presented as means (SD). HCs, healthy controls; NA, not applicable; OCD, obsessive-compulsive disorder; PANSS, Positive and Negative Syndrome Scale; SCZ, schizophrenia; SOC, schizo-obsessive comorbidity; Y-BOCS, Yale–Brown Obsessive-Compulsive Scale.
*Bonferroni .05 corrected.
The 4 groups differed significantly in estimated IQ (F = 12.70, df = 3, PBonferroni < .05). Post-hoc tests showed that the SOC group had lower IQ compared with the OCD group and healthy controls (to OCD: t = −3.21, PBonferroni < .05; to healthy controls: t = −5.80, PBonferroni < .05). The schizophrenia group had lower IQ compared with healthy controls (t = −4.47, PBonferroni < .05).
In addition, 11 patients with SOC, 7 schizophrenia patients, and 9 OCD patients were prescribed medications (see supplementary table S1). Sixty patients were medication-free. Due to the different types of medications prescribed for different disorders, we could not reliably calculate the equivalent dosage and did not include this as a covariate in the data analysis.
Tract-Based Spatial Statistics
The SOC group had significantly decreased mean FA value of whole brain white matter (t = −3.66, PBonferroni < .05) and increased RD values (t = 3.17, PBonferroni < .05) compared with HCs.
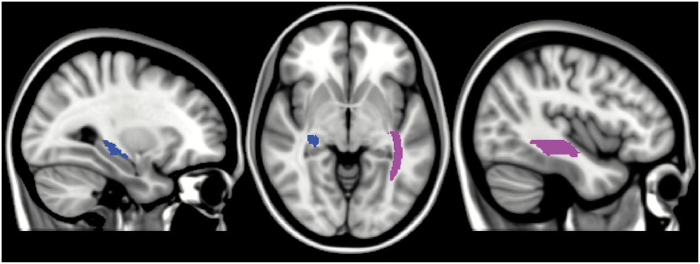
Post-hoc ANOVA in 50 white matter subregions with Bonferroni corrections showed that compared with healthy controls, the SOC group had significantly decreased FA and increased Radial Diffusivity in the right sagittal stratum and the left crescent of the fornix/stria terminalis (table 2 and figure 1). No correlation analysis result survived multiple comparison corrections.
Table 2.
Significant FA, MD, AD, and RD Differences Between the SOC Groups and HCs
| Regions | SOC | HCs | t | P | Cohen’s d | |
|---|---|---|---|---|---|---|
| FA | SS.R | 4.69E-01 (1.78E-02) | 4.88E-01 (2.62E-02) | −4.11 | .0001 | −0.93 |
| FX/ST.L | 4.43E-01 (2.04E-02) | 4.64E-01 (2.04E-02) | −4.09 | .0001 | −1.03 | |
| RD | SS.R | 6.16E-04 (2.32E-05) | 5.97E-04 (2.36E-05) | 3.76 | .0002 | 0.81 |
| FX/ST.L | 6.26E-04 (2.77E-05) | 5.95E-04 (2.84E-05) | 3.89 | .0002 | 1.11 |
Note: Bonferroni .05 corrected. AD, Axial Diffusivity; FA, Fractional Anisotropy; FX/ST, crescent of the fornix/stria terminalis; HCs, healthy controls; L, left; MD, Mean Diffusivity; R, right; RD, Radial Diffusivity; SOC, schizo-obsessive comorbidity; SS, sagittal stratum.
Fig. 1.
Significant FA differences between the SOC group and HCs. left regions, left FX/ST; right regions, right SS. FX/ST, the left crescent of the fornix/stria terminalis; HCs, healthy controls; L, left; R, right; SOC, schizo-obsessive comorbidity; SS, sagittal stratum (include inferior longitudinal fasciculus and inferior fronto-occipital fasciculus). For color, please see the figure online.
Classification results based on FA values showed an accuracy of .78 between the SOC group and healthy controls; an accuracy of .63 between the SOC group and the schizophrenia group; and an accuracy of .74 between the SOC group and the OCD group. The grand median weight figures are presented in supplementary figure S1.
Probabilistic Tractography
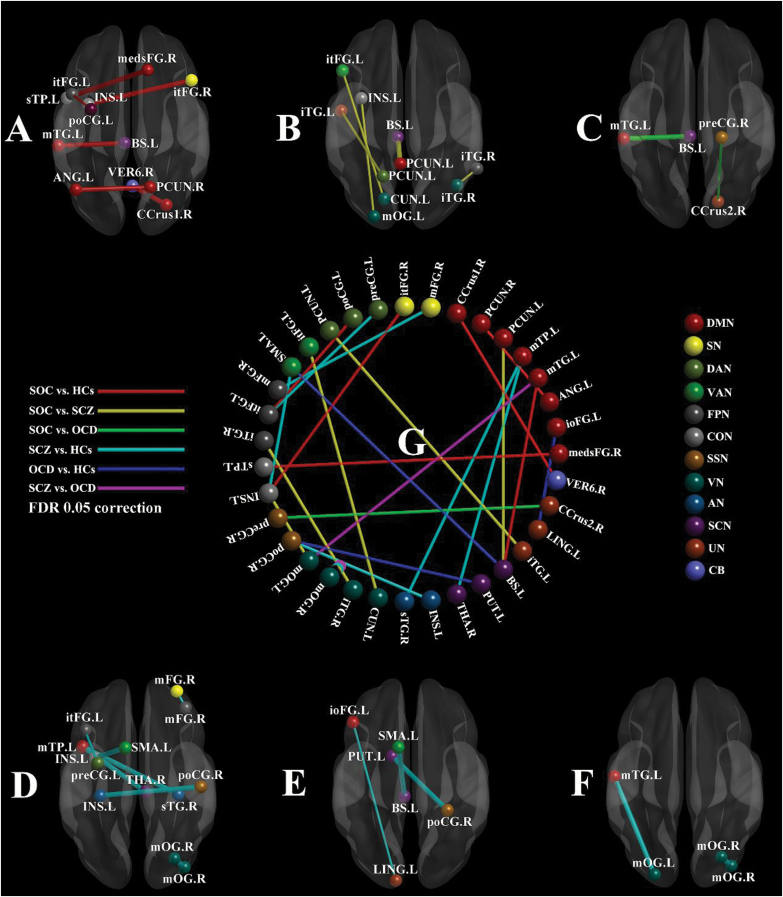
Post-hoc ANOVA with FDR corrections in valid connection probability features showed that compared with healthy controls, the SOC group had increased connection probability between the right precuneus and the left angular gyrus (within the DMN), between the right cerebellum crus1 and the right vermis 6 (the DMN and the cerebellum), between the right medial superior frontal gyrus (medsFG) and the left superior temporal pole (sTP, the DMN and the CON), between the left middle temporal gyrus (mTG) and the left brainstem (the DMN and the Subcortical Network), and between the left insula and the triangular part of right inferior frontal gyrus (itFG, the CON and the Salience Network). There was also significantly decreased connection probability between the left precentral gyrus and the left itFG (the DAN and the FPN).
Compared with the schizophrenia group, the SOC group had increased connection probability between the left precuneus and the left brainstem (the DMN and the Subcortical Network), decreased connection probability between the left middle occipital gyrus (mOG) and the left insula (the Visual Network and the CON), between the left cuneus and the left itFG (the Visual Network and the VAN), within the right inferior temporal gyrus (iTG, the Visual Network and the FPN), and between the left precuneus and the left iTG (the DAN and uncertain network).
Compared with the OCD group, the SOC group had increased connection probability between the left mTG and the left brainstem (the DMN and the Subcortical Network), and decreased connection probability between the precentral gyrus and the right cerebellum crus2 (the Somatosensory Network and the cerebellum)
Compared with healthy controls, the schizophrenia group had increased connection probability between the left middle temporal pole (mTP) and the right thalamus (the DMN and the Subcortical Network), between the left mTP and the right superior temporal gyrus (the DMN and the Auditory Network), between the right postcentral gyrus and the left insula (the Somatosensory Network and the Auditory Network), between the left supplementary motor area (SMA) and the left insula (the VAN and the CON), and within the right middle occipital gyrus (mOG, the Visual Network). There was also significantly reduced connection probability between the left precentral gyrus and the triangular part of left inferior frontal gyrus (itFG, the DAN and the FPN); and within the right middle frontal gyrus (mFG, the Salience Network and the FPN).
Compared with healthy controls, the OCD group had increased connection probability between the left SMA and the left brainstem (the VAN and the Subcortical Network), and between the postcentral gyrus and the left putamen (the Somatosensory Network and the Subcortical Network), and decreased connection probability between the orbital part of the inferior frontal gyrus (ioFG) and the left lingual gyrus (the DMN and uncertain network).
Compared with the OCD group, the schizophrenia group had increased connection probability within the right mOG (the Visual Network), and between the left mOG and the left middle temporal gyrus (mTG, the Visual Network and the DMN) (table 3 and figure 2). No correlation analysis result survived multiple comparison corrections.
Table 3.
Significant Structural Connectivity Probability Differences Between Groups
| A. SOC vs HCs | ||||||
|---|---|---|---|---|---|---|
| ROI | ROI | SCP in SOC | SCP in HCs | t | P | Cohen’s d |
| PCUN.R(DMN) (15 −63 26) |
ANG.L(DMN) (−44 −65 35) |
2.67E-03 (3.50E-03) |
0 (0) |
11.39 | .001 | 0.40 |
| CRUS1.R(DMN) (28 −77 −32) |
VER6.R(CB) (1 −62 −18) |
2.15E-03 (4.78E-03) |
4.69E-04 (8.45E-04) |
14.11 | .0004 | 0.48 |
| medsFG.R(DMN) (13 30 59) |
sTP.L(CON) (−51 8 −2) |
1.50E-03 (4.22E-03) |
1.55E-04 (5.94E-04) |
12.28 | .0009 | 0.42 |
| mTG.L(DMN) (−58 −30 −4) |
BS.L(SCN) (−5 −28 −4) |
1.64E-03 (2.20E-03) |
7.41E-04 (1.36E-03) |
11.53 | .001 | 0.74 |
| poCG.L(DAN) (−32 −1 54) |
itFG.L(FPN) (−47 11 23) |
8.72E-03 (1.48E-02) |
5.49E-02 (8.78E-02) |
−12.50 | .0008 | −0.61 |
| INS.L(CON) (−34 3 4) |
itFG.R(SN) (48 22 10) |
2.64E-03 (3.50E-03) |
6.82E-04 (1.16E-03) |
10.90 | .001 | 0.65 |
| B. SOC vs SCZ | ||||||
| ROI | ROI | SCP in SOC | SCP in SCZ | t | P | Cohen’s d |
| PCUN.L(DMN) (−3 −49 13) |
BS.L(SCN) (−5 −28 −4) |
1.52E-03 (1.69E-03) |
3.28E-04 (8.39E-04) |
10.83 | .001 | 0.73 |
| mOG.L(VN) (−24 −91 19) |
INS.L(CON) (−34 3 4) |
1.44E-04 (5.64E-04) |
3.07E-03 (5.08E-03) |
−11.27 | .001 | −0.47 |
| CUN.L(VN) (−16 −77 34) |
itFG.L(VAN) (−49 25 −1) |
7.60E-05 (2.79E-04) |
9.33E-04 (1.29E-03) |
−12.54 | .0008 | −0.58 |
| iTG.R(VN) (42 −66 −8) |
iTG.R(FPN) (58 −53 −14) |
2.18E-02 (2.27E-02) |
8.15E-02 (1.46E-01) |
−10.72 | .001 | −0.64 |
| PCUN.L(DAN) (−17 −59 64) |
iTG.L(UN) (−50 −7 −39) |
0 (0) |
4.54E-04 (8.24E-04) |
−11.53 | .001 | −0.44 |
| C. SOC vs OCD | ||||||
| ROI | ROI | SCP in SOC | SCP in OCD | t | P | Cohen’s d |
| mTG.L(DMN) (−58 −30 −4) |
BS.L (SCN) (−5 −28 −4) |
1.64E-03 (2.20E-03) |
8.74E-04 (1.63E-03) |
12.84 | .0007 | 0.51 |
| preCG.R(SSN) (20 −29 60) |
CRUS2.R(CB) (17 −80 −34) |
4.61E-03 (4.09E-03) |
9.85E-03 (7.88E-03) |
−16.76 | .0004 | −2.60 |
| D. SCZ vs HCs | ||||||
| ROI | ROI | SCP in SCZ | SCP in HCs | t | P | Cohen’s d |
| mTP.L(DMN) (−44 12 −34) |
THA.R(SCN) (6 −24 0) |
1.83E-04 (4.61E-04) |
0 (0) |
11.11 | .001 | 0.36 |
| mTP.L(DMN) (−44 12 −34) |
sTG.R(AN) (32 −26 13) |
9.66E-04 (1.67E-03) |
1.79E-04 (5.97E-04) |
10.82 | .001 | 0.52 |
| preCG.L(DAN) (−32 −1 54) |
itFG.L(FPN) (−42 25 30) |
4.14E-03 (5.72E-03) |
1.61E-02 (2.74E-02) |
−11.92 | .001 | −0.55 |
| mFG.R(SN) (31 56 14) |
mFG.R(FPN) (38 43 15) |
1.75E-02 (3.76E-02) |
3.05E-02 (4.87E-02) |
−11.75 | .001 | −0.80 |
| mOG.R(VN) (37 −84 13) |
mOG.R(VN) (29 −77 25) |
3.84E-01 (4.71E-01) |
1.50E-01 (2.16E-01) |
13.36 | .0006 | 0.88 |
| poCG.R(SSN) (50 −20 42) |
INS.L(AN) (−30 −27 12) |
2.15E-04 (6.38E-04) |
0 (0) |
11.07 | .001 | 0.29 |
| SMA.L(VAN) (−10 11 67) |
INS.L(CON) (−34 3 4) |
1.00E-01 (7.22E-02) |
5.61E-02 (4.36E-02) |
10.69 | .001 | 2.25 |
| E. OCD vs HCs | ||||||
| ROI | ROI | SCP in OCD | SCP in HCs | t | P | Cohen’s d |
| ioFG.L(DMN) (−46 31 −13) |
LING.L(UN) (−12 −95 −13) |
3.99E-03 (4.48E-03) |
1.15E-02 (1.12E-02) |
−13.10 | .0006 | −1.14 |
| BS.L(SCN) (−5 −28 −4) |
SMA.L(VAN) (−10 11 67) |
1.37E-02 (1.09E-02) |
7.34E-03 (6.55E-03) |
11.13 | .001 | 2.13 |
| PUT.L(SCN) (−15 4 8) |
poCG.R(SSN) (29 −39 59) |
3.34E-04 (7.00E-04) |
0 (0) |
10.94 | .001 | 0.35 |
| F. SCZ vs OCD | ||||||
| ROI | ROI | SCP in SCZ | SCP in OCD | t | P | Cohen’s d |
| mTG.L(DMN) (−56 −13 −10) |
mOG.L(VN) (−24 −91 19) |
3.35E-03 (4.29E-03) |
6.96E-04 (1.60E-03) |
10.69 | .001 | 0.61 |
| mOG.R(VN) (37 −84 13) |
mOG.R(VN) (29 −77 25) |
3.84E-01 (4.71E-01) |
1.19E-01 (1.16E-01) |
11.43 | .001 | 0.83 |
Note: FDR .05 corrected. Probability values are presented as means (SD). Regions are presented as name (network) (peak MNI coordinates). AN, Auditory Network; ANG, angular gyrus; BS, brainstem; CB, cerebellum; CCrus1, cerebellum crus1 region; CCrus2, cerebellum crus2 region; CON, Cingulo-opercular Task Control Network; CUN, cuneus; DAN, Dorsal Attention Network; DMN, Default Mode Network; FPN, Fronto-parietal Task Control Network; HCs, healthy controls; INS, insula; ioFG, inferior frontal gyrus, orbital part; itFG, inferior frontal gyrus, triangular part; iTG, inferior temporal gyrus; L, left; LING, lingual gyrus; medsFG, medial superior frontal gyrus; mFG, middle frontal gyrus; mOG, middle occipital gyrus; mTG, middle temporal gyrus; mTP, middle temporal pole; OCD, obsessive-compulsive disorder; PCUN, precuneus; poCG, postcentral gyrus; preCG, precentral gyrus; PUT, putamen; R, right; ROI, region of interest; SCN, subcortical network; SCP, structural connection probability; SCZ, schizophrenia; SMA, supplementary motor area; SN, Salience Network; SOC, schizo-obsessive comorbidity; SSN, Sensory/Somatomotor Network; sTG, superior temporal gyrus; sTP, superior temporal pole; THA, thalamus; UN, uncertain; VAN, Ventral Attention Network; VER6, cerebellum vermis 6 subregion; VN, Visual Network.
Fig. 2.
Significant structural connectivity probability differences between groups. (A) SOC vs HCs; (B) SOC vs SCZ; (C) SOC vs OCD; (D) SCZ vs HCs; (E) OCD vs HCs; (F) SCZ vs OCD; (G) differences between every 2 groups; the thickness of the line segment in A–F represents the sign of the t value, bold line is positive t value, thin line is negative t value. AN, Auditory Network; ANG, angular gyrus; BS, brainstem; CB, cerebellum; CCrus1, cerebellum crus1 region; CCrus2, cerebellum crus2 region; CON, Cingulo-opercular Task Control Network; CUN, cuneus; DAN, Dorsal Attention Network; DMN, Default Mode Network; FPN, Fronto-parietal Task Control Network; HCs, healthy controls; INS, insula; ioFG, inferior frontal gyrus, orbital part; itFG, inferior frontal gyrus, triangular part; iTG, inferior temporal gyrus; L, left; LING, lingual gyrus; medsFG, medial superior frontal gyrus; mFG, middle frontal gyrus; mOG, middle occipital gyrus; mTG, middle temporal gyrus; mTP, middle temporal pole; OCD, obsessive-compulsive disorder; PCUN, precuneus; poCG, postcentral gyrus; preCG, precentral gyrus; PUT, putamen; R, right; SCN, subcortical network; SCP, structural connection probability; SCZ, schizophrenia; SMA, supplementary motor area; SN, Salience Network; SOC, schizo-obsessive comorbidity; SSN, Sensory/Somatomotor Network; sTG, superior temporal gyrus; sTP, superior temporal pole; THA, thalamus; UN, uncertain; VAN, Ventral Attention Network;VER6, cerebellum vermis 6 subregion; VN, Visual Network. For color, please see the figure online.
Classification results based on connection probability showed an accuracy of .60 between the SOC group and healthy controls; an accuracy of .56 between the SOC group and the schizophrenia group; and an accuracy of .60 between the SOC group and the OCD group. The grand median weight figures are presented see in supplementary figure S2.
In addition, in order to address the potential confounding effect of medication and IQ on the results, we compared the FA and connection probability values between SOC participants on medication (n = 11) and not on medication (n = 17). We found no significant difference. We also compared the FA and connection probability values of SOC patients not on medication (n = 17) and healthy controls. This also did not alter the results. Correlation analysis showed that FA and connection probability values were not correlated with IQ and medication dosage in all patient groups.
Discussion
To the best of our knowledge, the present study is the first study to investigate changes in WM morphology in patients with SOC. We compared 4 indices of white matter integrity and 34 716 connection probability values among the SOC group, the schizophrenia group, the OCD group, and healthy controls, and used FA and connection probability results to classify the SOC group from other groups using machine learning methods. The comparison results revealed that the SOC group had significantly decreased FA and increased Radial Diffusivity in the whole brain, especially in the right sagittal stratum and the left stria terminalis compared with healthy controls; and the SOC group had altered connection probability in the DMN, the Subcortical Network, the Attention Network, the Task Control Network, the Visual Network, the Somatosensory Network, and the cerebellum compared with the other 3 groups. The classification results had an accuracy between .56 and .78 in distinguishing the SOC group from the other 3 groups. These findings may represent underlying neurobiological changes in patients with SOC and may provide insight into the similarities and differences of the structural connectivity patterns among patients with SOC, schizophrenia, and OCD.
In this study, the SOC group showed significantly decreased FA at the right sagittal stratum and the left stria terminalis, suggesting more severe white matter structural changes when schizophrenia and OCD symptoms co-occur, supporting the “double jeopardy” and “disconnection” hypotheses. The sagittal stratum is a cortico-subcortical WM bundle that mainly conveys fibers from the frontal, the parietal, and the occipital cortex to the thalamus and the basal ganglia.49,50 This finding is consistent with known changes in the cortical-thalamus pathway reported in previous OCD studies,30 and suggests that altered FA at the sagittal stratum may be associated with obsessive symptoms.51 In addition, previous behavioral studies have also shown that SOC patients exhibit more severe attentional set-shifting deficit52 and lower processing speed.53 Decreased FA at the sagittal stratum may be associated with these kinds of cognitive inflexibility and executive dysfunction.54–56 The stria terminalis is a pathway connecting the hippocampus, the amygdala, and the hypothalamus.57 Changes in the striatum terminalis have also been found in schizophrenia and OCD patients in previous studies.58,59 Decreased FA at the left stria terminalis in patients with SOC in our study may imply excessive anxiety in response to threat monitoring,60 more severe depression,61 impaired working memory,62 and attenuated behavioral inhibition when schizophrenia and OCD symptoms co-occur.63
Moreover, the sagittal stratum and the striatum terminalis also showed significantly increased Radial Diffusivity in the SOC group. While FA is usually regarded as an indicator of WM structural integrity,64 RD is thought to reflect the integrity and thickness of myelin sheets covering the axons and may be a marker of cell atrophy or loss.65,66 As such, the main underlying mechanism of white matter changes in the SOC group may be due to subtle demyelination rather than axonal alterations.
To further investigate the microstructure of white matter connection, we conducted Probabilistic Tractography analysis and the results also support the “disconnection” hypothesis in SOC patients. We found that patients with SOC had increased connection probability between the precuneus and the angular gyrus compared with healthy controls. The precuneus is the core subregion of the DMN, associated with self-consciousness and self-related mental representations.67 The angular gyrus is associated with abnormal semantic and episodic memory retrieval.68 In particular, the left angular gyrus is responsible for subjective memory retrieval.69 It is possible that the increased connection probability between the precuneus and the angular gyrus may strengthen the retrieval of memory related to pathological thinking in patients with SOC.27
We also found that SOC patients had increased connection probability between the mTG and the brainstem compared with the OCD group and healthy controls, and had increased connection probability between the precuneus and the brainstem compared with the schizophrenia group. Previous studies have demonstrated that the brainstem is involved in the development of psychotic disorders70 and may be associated with auditory hallucinations.71 Our results suggest that the disorganized relationship between interoception and sensory processing may be one possible reason for the more severe symptoms observed in patients with SOC.
At the same time, the SOC group and the schizophrenia group both exhibited decreased connection probability between the left precentral gyrus and the left inferior frontal gyrus compared with healthy controls. Previous studies have shown that these 2 regions may play important roles in social cognition, especially in facial emotion perception.72,73 The left inferior frontal gyrus is also extremely important for the comprehension of language and eye-expression.74 The impaired structural connection between the left precentral gyrus and the left inferior frontal gyrus in patients with SOC and schizophrenia may be associated with their poorer psychosocial functioning and performance.75
The SOC group exhibited decreased connection probability between the Visual Network including the mOG, the cuneus, and the task-positive network including the insula, the itFG, and the inferior temporal gyrus compared with the schizophrenia group. These results suggest that compared with SCZ patients, patients with SOC may have more severe disorganized sensory processing and integrative abnormalities.76–79
The SOC group also exhibited decreased connection probability between the precentral gyrus and the cerebellum compared with the OCD group, while the OCD group exhibited increased connection probability between the postcentral gyrus and the putamen compared with healthy controls. The precentral and postcentral gyrus mainly process sensory input and motor output.80 Our results suggest that compared with OCD patients, SOC patients may be more inclined to avoid the effort in integrating sensory information in a conflicted environment.81
From a network perspective, the DAN and the DMN are normally modulated by the FPN in maintaining dynamic balance between internal perception and the external environment.82 The decreased connection probability found in our study between the DAN and the FPN, together with the increased connection probability found within the DMN, between the DMN and the CON and the cerebellum, and between the CON and the Salience Network in patients with SOC may indicate dysfunctional self-regulation and disrupted homeostasis associated with pathological thinking and behavior.83 Patients with SOC may be more focused on internal states and lose control over the external environment.27,77 These results are consistent with previous rsFC research in SOC patients,9 further corroborating the homogeneity of DMN-related functional and structural connectivity changes in patients with SOC. However, we did not observe any abnormality in the striatum in the SOC group and the schizophrenia group. This may be due to the fact that the patients in the present study were in remission and the striatum is sensitive to antipsychotics.84,85
Lastly, the relatively high accuracy rate (.78 by FA features) in distinguishing SOC patients from healthy controls in our study supports the possibility of using machine learning in classifying schizophrenia spectrum disorders. The accuracy of classification using connection probability features was consistently lower than using FA features in the present study, indicating changes in FA features may be better regulated than changes in connection probability. Moreover, the fornix exhibited the largest weight in classification between the SOC group and the OCD group and healthy controls. This result suggests that the fornix may also play an important role in the neurobiological development of schizophrenia spectrum disorders.66
Important limitations of this study include the relatively small sample size and lack of investigation of cognitive function in our patient groups. The functional implications of the observed WM changes are speculative and require verification in future studies. We also cannot rule out the confounding effect of medications due to the inclusion of some medicated patients, although our results showed no correlation between antipsychotic dosage and Y-BOCS scores in the SOC group. We also compared the diffusion values between SOC patients not on medication and the other groups and found no significant changes in the results. Secondly, IQ is another important confounding factor in our study. A previous study has reported that IQ may be positively correlated with myelin water fraction in frontal white matter in healthy people.47 Although we took IQ as a covariate in our analysis and did not find any significant correlations between various white matter indices and IQ in all of our participants, the potential confounding effect of IQ on our results could not be completely excluded. Thirdly, although the TBSS results in the SOC group support the “double jeopardy” hypothesis, our connection probability results did not convincingly corroborate this hypothesis. The neurochemical basis of connection probability changes in patients with SOC needs further investigation. Fourthly, the weight of the classification results is not exactly the same as the ANOVA results. The practical physiological meaning behind this needs further investigation. More reliable and accurate classification should be carried out in the future with a much larger and a more homogeneous sample such as unmedicated first-episode patients characterized by a specific set of clinical symptoms. Fifthly, the Y-BOCS was not administered in the schizophrenia group and the PANSS was not administered in the OCD group, which might have limited the validity of our results. As all our participants were in clinical remission, it is also difficult to ascertain whether patients with SOC really had more severe symptoms. Sixthly, we did not examine the incidence of drug-induced OCD symptoms. A previous study which investigated 430 medicated schizophrenia patients reported that the incidence of drug-induced OCD is 1.4%, most of which were caused by clozapine.86 Drug-induced OC symptoms is a key confounding factor in all studies investigating SOC and should be addressed in future studies. Finally, recent studies suggest that free-water signal correction or elimination procedures should be included in DTI data preprocessing due to the complicated shape of the fornix and the partial volume effects of ventricular water.87,88 Neurite orientation dispersion and density imaging analysis is another advanced method for estimating the microstructural complexity of dendrites and axons. However, our imaging protocol and scanning parameters did not fit the requirement of these procedures.89 Future studies should consider incorporating these novel approaches.
In conclusion, despite these limitations, our main results are stable regardless of medication dosage and IQ differences. We found extensive cortico-subcortical and perceptual processing-related WM changes in patients with SOC. These changes may indicate specific superimposed effects and neural adaptation of combined schizophrenia and OCD symptoms associated with more far-ranging and longitudinally stable deficits in perception, cognition,90 emotion, and behavioral control in SOC patients compared with patients with only schizophrenia or only OCD,6 supporting the “disconnection” hypothesis of schizophrenia spectrum disorders and partially supporting the “double jeopardy” hypothesis of SOC.
Funding
This study was funded by the National Key Research and Development Programme (2016YFC0906402), the Beijing Municipal Science & Technology Commission Grant (Z161100000216138), the Beijing Training Project for the Leading Talents in S & T (Z151100000315020), the Strategic Priority Research Programme (B) of the Chinese Academy of Science (XDB02030002), and the CAS Key Laboratory of Mental Health, Institute of Psychology.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Sevincok L, Akoglu A, Arslantas H. Schizo-obsessive and obsessive-compulsive disorder: comparison of clinical characteristics and neurological soft signs. Psychiatry Res. 2006;145(2-3):241–248. [DOI] [PubMed] [Google Scholar]
- 2. Rahmatinejad P, Mohammadi SD, Noruzinejad G, Ashtian M, Haghverdi A. The abundance of obsessive-compulsive disorder in schizophrenia spectrum disorders: diagnostic validity of schizo-obsessive subtype. SSU_J. 2017;25:42–51. [Google Scholar]
- 3. Çeşmeci U, Nazik Yüksel R, Kaya H, Dilbaz N. Schizotypality and neurological soft signs in patients with obsessive–compulsive disorder. Psychiat Clin Psychopharmacol. 2017;27:234–240. [Google Scholar]
- 4. Faragian S, Fuchs C, Pashinian A, Weizman R, Weizman A, Poyurovsky M. Age-of-onset of schizophrenic and obsessive-compulsive symptoms in patients with schizo-obsessive disorder. Psychiatry Res. 2012;197(1-2):19–22. [DOI] [PubMed] [Google Scholar]
- 5. Owashi T, Ota A, Otsubo T, Susa Y, Kamijima K. Obsessive-compulsive disorder and obsessive-compulsive symptoms in Japanese inpatients with chronic schizophrenia - a possible schizophrenic subtype. Psychiatry Res. 2010;179(3):241–246. [DOI] [PubMed] [Google Scholar]
- 6. Cunill R, Huerta-Ramos E, Castells X. The effect of obsessive-compulsive symptomatology on executive functions in schizophrenia: a systematic review and meta-analysis. Psychiatry Res. 2013;210(1):21–28. [DOI] [PubMed] [Google Scholar]
- 7. Attademo L, Bernardini F, Quartesan R. Schizo-obsessive disorder: a brief report of neuroimaging findings. Psychopathology. 2016;49(1):1–4. [DOI] [PubMed] [Google Scholar]
- 8. Baytunca B, Kalyoncu T, Ozel I, Erermiş S, Kayahan B, Öngur D. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243–245. [DOI] [PubMed] [Google Scholar]
- 9. Lee KJ, Shin YW, Wee H, Kim YY, Kwon JS. Gray matter volume reduction in obsessive–compulsive disorder with schizotypal personality trait. Prog Neuro-Psychoph. 2006;30:1146–1149. [DOI] [PubMed] [Google Scholar]
- 10. Aoyama F, Iida J, Inoue M, et al. Brain imaging in childhood- and adolescence-onset schizophrenia associated with obsessive-compulsive symptoms. Acta Psychiatr Scand. 2000;102(1):32–37. [DOI] [PubMed] [Google Scholar]
- 11. Swets M, Dekker J, van Emmerik-van Oortmerssen K, et al. The obsessive compulsive spectrum in schizophrenia, a meta-analysis and meta-regression exploring prevalence rates. Schizophr Res. 2014;152(2-3):458–468. [DOI] [PubMed] [Google Scholar]
- 12. Tu PC, Lee YC, Chen YS, Li CT, Su TP. Schizophrenia and the brain’s control network: aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res. 2013;147(2-3):339–347. [DOI] [PubMed] [Google Scholar]
- 13. de Wit SJ, Alonso P, Schweren L, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171(3):340–349. [DOI] [PubMed] [Google Scholar]
- 14. Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci Biobehav Rev. 2015;51:15–30. [DOI] [PubMed] [Google Scholar]
- 15. Zai G, Zai CC, Arnold PD, et al. Meta-analysis and association of brain-derived neurotrophic factor (BDNF) gene with obsessive-compulsive disorder. Psychiatr Genet. 2015;25(2):95–96. [DOI] [PubMed] [Google Scholar]
- 16. Bilder RM, Volavka J, Czobor P, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52(7):701–707. [DOI] [PubMed] [Google Scholar]
- 17. Lysaker PH, Whitney KA. Obsessive-compulsive symptoms in schizophrenia: prevalence, correlates and treatment. Expert Rev Neurother. 2009;9(1):99–107. [DOI] [PubMed] [Google Scholar]
- 18. Lee MJ, Shin YB, Sunwoo YK, et al. Comparative analysis of cognitive function in schizophrenia with and without obsessive compulsive disorder. Psychiatry Investig. 2009;6(4):286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hur JW, Shin NY, Jang JH, et al. Clinical and neurocognitive profiles of subjects at high risk for psychosis with and without obsessive-compulsive symptoms. Aust N Z J Psychiatry. 2012;46(2):161–169. [DOI] [PubMed] [Google Scholar]
- 20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- 21. Scotti-Muzzi E, Saide OL. Schizo-obsessive spectrum disorders: an update. CNS Spectr. 2017;22(3):258–272. [DOI] [PubMed] [Google Scholar]
- 22. Opakunle T, Akinsulore A, Aloba OO, Fatoye FO. Obsessive-compulsive symptoms in schizophrenia: prevalence and associated factors in a Nigerian population. Int J Psychiatry Clin Pract. 2017;21(3):195–200. [DOI] [PubMed] [Google Scholar]
- 23. Grover S, Dua D, Chakrabarti S, Avasthi A. Obsessive compulsive symptoms/disorder in patients with schizophrenia: prevalence, relationship with other symptom dimensions and impact on functioning. Psychiatry Res. 2017;250:277–284. [DOI] [PubMed] [Google Scholar]
- 24. Damilou A, Apostolakis S, Thrapsanioti E, Theleritis C, Smyrnis N. Shared and distinct oculomotor function deficits in schizophrenia and obsessive compulsive disorder. Psychophysiology. 2016;53(6):796–805. [DOI] [PubMed] [Google Scholar]
- 25. Poyurovsky M, Koran LM. Obsessive-compulsive disorder (OCD) with schizotypy vs. schizophrenia with OCD: diagnostic dilemmas and therapeutic implications. J Psychiatr Res. 2005;39(4):399–408. [DOI] [PubMed] [Google Scholar]
- 26. Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Zou L, Xie W, et al. Altered functional connectivity of the default mode network in patients with schizo-obsessive comorbidity: a comparison between schizophrenia and obsessive-compulsive disorder. Schizophr Bull. 2019;45;199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alba-Ferrara LM, de Erausquin GA. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr Neurosci. 2013;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koch K, Reess TJ, Rus OG, Zimmer C, Zaudig M. Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): a review. J Psychiatr Res. 2014;54:26–35. [DOI] [PubMed] [Google Scholar]
- 30. Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. 2013;33(8):1163–1171. [DOI] [PubMed] [Google Scholar]
- 31. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hawco C, Voineskos AN, Radhu N, et al. Age and gender interactions in white matter of schizophrenia and obsessive compulsive disorder compared to non-psychiatric controls: commonalities across disorders. Brain Imaging Behav. 2017;11(6):1836–1848. [DOI] [PubMed] [Google Scholar]
- 33. Ge H, Yin X, Xu J, et al. Fiber pathways of attention subnetworks revealed with tract-based spatial statistics (TBSS) and probabilistic tractography. PLoS One. 2013;8(11):e78831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuchling J, Backner Y, Oertel FC, et al. Comparison of probabilistic tractography and tract-based spatial statistics for assessing optic radiation damage in patients with autoimmune inflammatory disorders of the central nervous system. Neuroimage Clin. 2018;19:538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu M, Tan X, Zhang X, et al. Alterations of white matter structural networks in patients with non-neuropsychiatric systemic lupus erythematosus identified by probabilistic tractography and connectivity-based analyses. Neuroimage Clin. 2017;13:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho KI, Shenton ME, Kubicki M, et al. Altered thalamo-cortical white matter connectivity: probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophr Bull. 2016;42(3):723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu ML, Zong XF, Mann JJ, et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 2017;33(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 2014;205(5):376–382. [DOI] [PubMed] [Google Scholar]
- 41. Gong Y. Manual of Wechsler Adult Intelligence Scale-Chinese Version. Changsha, China: Chinese Map; 1992. [Google Scholar]
- 42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 43. First MB, Spitzer RL, Gibbon M, Williams JB.. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 44. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 45. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. [DOI] [PubMed] [Google Scholar]
- 46. Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lang DJ, Yip E, MacKay AL, et al. 48 echo T₂ myelin imaging of white matter in first-episode schizophrenia: evidence for aberrant myelination. Neuroimage Clin. 2014;6:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cavallari M, Hshieh TT, Guttmann CR, et al. ; SAGES Study Group Brain atrophy and white-matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging. 2015;36(6):2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson’s disease. Hum Brain Mapp. 2014;35(4):1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter. Ann NY Acad Sci. 2008;1142:266–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fitzgerald KD, Liu Y, Reamer EN, Taylor SF, Welsh RC. Atypical frontal-striatal-thalamic circuit white matter development in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(11):1225–33, 1233.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patel DD, Laws KR, Padhi A, et al. The neuropsychology of the schizo-obsessive subtype of schizophrenia: a new analysis. Psychol Med. 2010;40(6):921–933. [DOI] [PubMed] [Google Scholar]
- 53. Michalopoulou PG, Konstantakopoulos G, Typaldou M, et al. Can cognitive deficits differentiate between schizophrenia with and without obsessive-compulsive symptoms? Compr Psychiatry. 2014;55(4):1015–1021. [DOI] [PubMed] [Google Scholar]
- 54. Rowland LM, de la Fuente-Sandoval C. Abnormal white matter integrity in antipsychotic-naïve first-episode psychosis patients assessed by a DTI principal component analysis. Schizophr Res. 2015;162:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim SG, Jung WH, Kim SN, Jang JH, Kwon JS. Alterations of gray and white matter networks in patients with obsessive-compulsive disorder: a multimodal fusion analysis of structural MRI and DTI using mCCA+jICA. PLoS One. 2015;10(6):e0127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benito-León J, Mato-Abad V, Louis ED, et al. White matter microstructural changes are related to cognitive dysfunction in essential tremor. Sci Rep. 2017;7:2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grieve SM, Korgaonkar MS, Clark CR, Williams LM. Regional heterogeneity in limbic maturational changes: evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage. 2011;55(3):868–879. [DOI] [PubMed] [Google Scholar]
- 58. Ikemoto K. Dopamine hypothesis is linked with neural stem cell (NSC) dysfunction hypothesis by D-Cell hypothesis (trace amine hypothesis) in etiology of schizophrenia. Biochem Physiol. 2015;4:2. [Google Scholar]
- 59. Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016;21(9):1272–1280. [DOI] [PubMed] [Google Scholar]
- 60. Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68(5):416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Viher PV, Stegmayer K, Giezendanner S, et al. Cerebral white matter structure is associated with DSM-5 schizophrenia symptom dimensions. Neuroimage Clin. 2016;12:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roos A, Kwiatkowski MA, Fouche JP, et al. White matter integrity and cognitive performance in children with prenatal methamphetamine exposure. Behav Brain Res. 2015;279:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30(20):7023–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41(2):223–232. [DOI] [PubMed] [Google Scholar]
- 65. Zhang J, Wang Y, Wang J, et al. White matter integrity disruptions associated with cognitive impairments in type 2 diabetic patients. Diabetes. 2014;63(11):3596–3605. [DOI] [PubMed] [Google Scholar]
- 66. Fitzsimmons J, Hamoda HM, Swisher T, et al. Diffusion tensor imaging study of the fornix in first episode schizophrenia and in healthy controls. Schizophr Res. 2014;156(2-3):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. [DOI] [PubMed] [Google Scholar]
- 68. Bellana B, Liu Z, Anderson JAE, Moscovitch M, Grady CL. Laterality effects in functional connectivity of the angular gyrus during rest and episodic retrieval. Neuropsychologia. 2016;80:24–34. [DOI] [PubMed] [Google Scholar]
- 69. Tune S, Asaridou SS. Stimulating the semantic network: what can TMS tell us about the roles of the posterior middle temporal gyrus and angular gyrus? J Neurosci. 2016;36(16):4405–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hirjak D, Wolf RC, Stieltjes B, et al. Neurological soft signs and brainstem morphology in first-episode schizophrenia. Neuropsychobiology. 2013;68(2):91–99. [DOI] [PubMed] [Google Scholar]
- 71. Källstrand J, Nehlstedt SF, Sköld ML, Nielzén S. Lateral asymmetry and reduced forward masking effect in early brainstem auditory evoked responses in schizophrenia. Psychiatry Res. 2012;196(2-3):188–193. [DOI] [PubMed] [Google Scholar]
- 72. Watanuki T, Matsuo K, Egashira K, et al. Precentral and inferior prefrontal hypoactivation during facial emotion recognition in patients with schizophrenia: a functional near-infrared spectroscopy study. Schizophr Res. 2016;170(1):109–114. [DOI] [PubMed] [Google Scholar]
- 73. Ji E, Weickert CS, Lenroot R, et al. Adjunctive selective estrogen receptor modulator increases neural activity in the hippocampus and inferior frontal gyrus during emotional face recognition in schizophrenia. Transl Psychiatry. 2016;6:e795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ou J, Lyu H, Hu M, et al. Decreased white matter FA values in the left inferior frontal gyrus is a possible intermediate phenotype of schizophrenia: evidences from a novel group strategy. Eur Arch Psychiatry Clin Neurosci. 2018;268(1):89–98. [DOI] [PubMed] [Google Scholar]
- 75. Dal Monte O, Schintu S, Pardini M, et al. The left inferior frontal gyrus is crucial for reading the mind in the eyes: brain lesion evidence. Cortex. 2014;58:9–17. [DOI] [PubMed] [Google Scholar]
- 76. Wang YM, Zou LQ, Xie WL, et al. Altered grey matter volume and cortical thickness in patients with schizo-obsessive comorbidity. Psychiatry Res Neuroimaging. 2018;276:65–72. [DOI] [PubMed] [Google Scholar]
- 77. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kantrowitz JT, Butler PD, Schecter I, Silipo G, Javitt DC. Seeing the world dimly: the impact of early visual deficits on visual experience in schizophrenia. Schizophr Bull. 2009;35(6):1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Green MF, Lee J, Wynn JK, Mathis KI. Visual masking in schizophrenia: overview and theoretical implications. Schizophr Bull. 2011;37(4):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brown JA, Seeley WW. Progressive supranuclear palsy and related parkinsonian disorders. Genomics Circuit Pathway Clin Neuropsychiatry. 2016;18:283–300. [Google Scholar]
- 81. Yun JY, Jang JH, Jung WH, et al. Executive dysfunction in obsessive-compulsive disorder and anterior cingulate-based resting state functional connectivity. Psychiatry Investig. 2017;14(3):333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49(1):865–874. [DOI] [PubMed] [Google Scholar]
- 84. Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry. 2001;50(10):729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24(1):47–54. [DOI] [PubMed] [Google Scholar]
- 86. Wu GJ, Yi ZH, Chen YM, et al. Investigation of drug-induced obsessive-compulsive symptoms caused by clozapine. Shanghai Psychiatry. 1999;2:81–126. [Google Scholar]
- 87. Hoy AR, Koay CG, Kecskemeti SR, Alexander AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. Neuroimage. 2014;103:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bergamino M, Pasternak O, Farmer M, Shenton ME, Hamilton JP. Applying a free-water correction to diffusion imaging data uncovers stress-related neural pathology in depression. Neuroimage Clin. 2016;10:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. [DOI] [PubMed] [Google Scholar]
- 90. Schirmbeck F, Rausch F, Englisch S, et al. Stable cognitive deficits in schizophrenia patients with comorbid obsessive-compulsive symptoms: a 12-month longitudinal study. Schizophr Bull. 2013;39(6):1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.