Abstract
CNS immune defenses are marshaled and dominated by brain resident macrophages and microglia, which are the innate immune sentinels and frontline host immune barriers against various pathogenic insults. These myeloid lineage cells are the predominant immune population in gliomas and can constitute up to 30–50% of the total cellular composition. Parenchymal microglial cells and recruited monocyte-derived macrophages from the periphery exhibit disease-specific phenotypic characteristics with spatial and temporal distinctions and are heterogeneous subpopulations based on their molecular signatures. A preponderance of myeloid over lymphoid lineage cells during CNS inflammation, including gliomas, is a contrasting feature of brain immunity relative to peripheral immunity. Herein we discuss glioma-associated macrophage and microglia immune biology in the context of their identity, molecular drivers of recruitment, nomenclature and functional paradoxes, therapeutic reprogramming and polarization strategies, relevant challenges, and our perspectives on therapeutic modulation.
Keywords: macrophages, microglia, immune suppression, glioma
The science of central nervous system (CNS) immunology has been enlivened in the last decade with the dismantling of the concept of “immune privilege” and the discovery of a lymphatic system within the brain1–3 that has triggered a new research outlook for CNS pathological states such as infections, autoimmune disorders, and brain cancer. Occasionally, resident neuronal cells of the CNS undergo mostly spontaneous malignant transformation due to failure of integrated genetic and epigenetic regulatory circuits that impact cell cycle and tumor suppressive mechanisms. This eventually leads to the formation of CNS tumors such as gliomas—a pathological and foreign entity necessitating elimination by an immune surveilling defense system. Glioma-induced immune dysregulation facilitates a reciprocal cellular and molecular crosstalk at pathologically breached physio-anatomical blood–brain barrier (BBB) interfaces. CNS immune defenses are marshaled and dominated by brain resident macrophages and microglia, which are the innate immune sentinels and frontline host immune barriers against various pathogenic insults.4,5 These myeloid lineage cells are the predominant immune population in glioma patients,6 and can constitute up to 30–50% of the total cellular composition.7,8 Parenchymal microglial cells and recruited monocyte-derived macrophages9 from the periphery exhibit disease-specific phenotypic characteristics with spatial and temporal distinctions10,11 and are heterogeneous subpopulations based on their molecular signatures. Macrophages, alongside monocytes and dendritic cells (DCs), form the trinity of the mononuclear phagocyte system,12 which are all extremely diverse subpopulations with distinct ontological origins and functional manifestations in steady state and glioma biology. However, we will largely confine our discussions to glioma-associated macrophages and microglia (GAMs). The downstream adaptive immune responses are mediated by rare CNS effector T cells, which exist in perivascular regions as reported in healthy human corpus callosum13 and within gliomas.14,15 A preponderance of myeloid over lymphoid lineage cells during CNS inflammation, including gliomas, is a contrasting feature of brain immunity relative to peripheral immunity which needs to be carefully revisited. Therefore, herein we discuss GAM immune biology, the major constituent immune cell in the tumor microenvironment (TME) in the context of their identity, molecular drivers of recruitment, nomenclature and functional paradoxes, therapeutic reprogramming and polarization strategies, relevant challenges, and our perspectives on the pretext of current knowledge and the way forward.
Glioblastoma (GBM) is the most aggressive brain malignancy. Clinically, T cell based immunotherapies have failed to make an impact on improving patient survival in the majority of patients relative to standard of care therapies comprising surgery, and chemoradiation, thus highlighting a paradoxical approach to targeting a rare or nonexistent T-cell population16 with rejuvenation strategies such as immune checkpoint blockade regimens such as programmed cell death 1 (PD-1) and/or cytotoxic T lymphocyte antigen 4. Although T cells can gain access to GBMs in the CNS, they are rare and are probably unable to exert significant effector responses.17 The function of these T cells is impaired by multiple tumor- and/or myeloid-cell (perivascular macrophages and monocyte-derived macrophages) derived immunosuppressive factors.16,18–20 Genetic and proteomic based immune phenotyping has revealed that GBM is uniquely enriched with macrophages relative to other types of malignancies, although other cancers such as lung,21 breast,22 and pancreatic cancers23 contain these macrophages, which are usually polarized to tumor-supportive and immunosuppressive phenotype. GAMs produce low levels of proinflammatory cytokines and lack expression of key molecules involved in T-cell costimulation (eg, CD86, CD80, CD40), indicating that they may be poor inducers of T-cell responses in glioma.6 Although the mere presence of macrophages within the TME is an indicator of poor outcome for GBM patients, there are possibilities for developing targeted immunotherapy due to their plasticity features, which provide opportunity for fine tuning their metabolic and transcriptional programs to a desirable phenotype.24,25
Identity and Ontogeny Conundrum of GAMs
Microglia are the resident macrophages of the CNS and are distributed throughout the brain. Their origin has been debated for decades as either arising from the yolk sac during embryogenesis or from the bone marrow. More recent sophisticated analyses of chimeras have shown that microglia arise mainly from the local expansion of existing resident microglia.26 Additionally, fate-mapping studies using congenic mice have identified immature yolk sac progenitors as the predominant source of brain microglia.27 The consensus in the scientific community is that microglia arise during embryogenesis and are long lived with limited capacity for local self-renewal and expansion. As such, microglia represent a distinct population of innate immune cells. In the glioma TME, GAMs include both tissue-resident microglia and the infiltrating macrophages derived from bone marrow,6,28 with the latter congregating in the perivascular and necrotic regions29 in response to areas of ischemia.30,31
The ability to distinguish between these 2 populations has been problematic due to the absence of a unique defining marker until recently, when transmembrane protein 119 (TMEM119) was proposed as a definitive microglia marker applicable for mouse and human.32 In contrast, CD49D/ITGA4, a marker specific to tumor infiltrating bone marrow derived macrophages but not microglia, has been identified in the context of brain malignancy of mouse and human.33 Another microglia marker, Sall1, is a key transcriptional regulator defining microglia identity and function in human and mouse.34 Membrane spanning 4-domains A335 may be a marker that distinguishes the relative contribution of blood monocytes to the tissue resident macrophage pool, but this claim needs to be validated during tumorigenesis. It should be noted that specific lineage marker claims have been made previously only to later be contested. Previously, an analysis of surface expression of CD11b and CD45low for microglia and CD45high for macrophages was used to define these populations from ex vivo rodent and human CNS tumors.6,36 Based on these markers, monocyte-derived macrophages would predominate in the glioma relative to microglia,37 but this would be influenced by the glioma subtype (eg, diffuse) and the location of the analysis (ie, infiltrating edge vs tumor core). However, it should be noted that microglia can upregulate CD45 expression.38 Alternatively, CX3CR1 and CCR2 have been used as markers to distinguish resident microglia and peripheral monocytes/macrophages, respectively, using CX3CR1GFP and CCR2RFP (knock in) lineage tracing murine models to investigate the fate of these cells in the TME.39 These tracer studies indicate that in mice, the predominant innate immune cells are derived from tissue-resident microglia rather than from peripheral macrophages.38 However, CX3CR1 can be expressed by peripheral monocytes and macrophages under a variety of situations.40–42 CCR2 can also be expressed in microglia,43 especially under the scenario of activation,44 and specifically within the context of glioma-immune cell interactions.45 In glioma associated inflammation, there could be empty microglial niches that are being compensated for by monocyte-derived macrophages, which have the propensity to behave like microglia, further compounding the origins and identity question. Clearly there is an unmet need for (i) identification of lineage-specific markers to distinguish these cell populations especially in glioma patients, (ii) functional assessment of cells of diverse origins, and (iii) delineation as to whether ontogeny bears functional implications during inflammatory scenarios including gliomas.
Macrophage Functional Classification System That Does Not Recapitulate In Vivo Biology
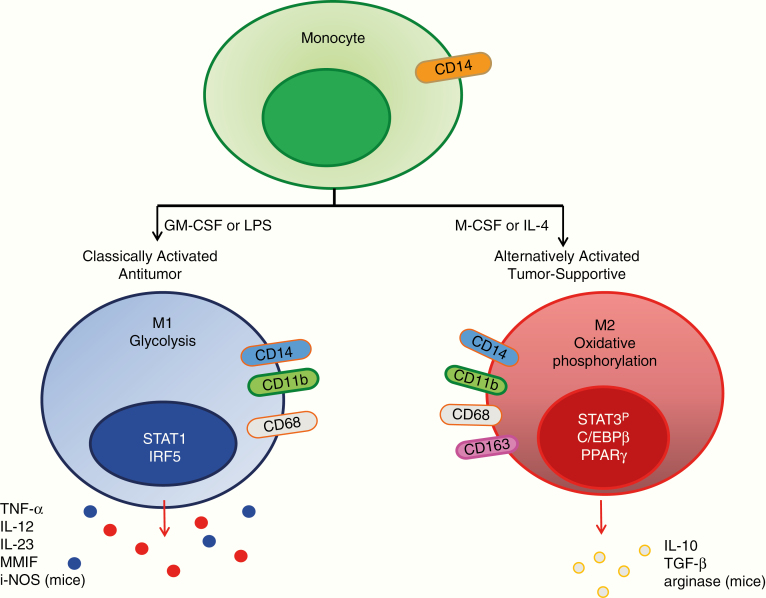
Researchers inspired by the T helper cell type 1 versus T helper cell type 2 functional dichotomization of T cells have attempted to define macrophage function after in vitro stimulation strategies. The naïve monocytes acquire proinflammatory M1 polarized phenotype (antitumor) in response to toll-like receptor 4 (TLR4) ligands and interferon (IFN)-gamma stimulation, whereas interleukin (IL)-4, IL-10, and/or IL-13 triggers polarization to alternative M2 phenotype (tumor-supportive/protumor).46 These polarized subpopulations have differential expression of receptors, cytokines, chemokines and effector function (Fig. 1). These functional polarization states depend on the extracellular environment. However, compelling evidence suggests that a distinct partitioning of the M1/M2 macrophage subtypes does not exist.47 Single-cell RNA-sequencing of the GAMs has shown frequent coexpression of both pro-inflammatory M1 and immune suppressive M2 genes in individual cells.29 Although this schematic provides a conceptual framework to describe potential phenotypic and functional roles, in pathological conditions such as gliomas, macrophages are highly plastic, and ex vivo analysis from gliomas demonstrates that these cells exist in a continuum, including in more undifferentiated states.48–51 In human GBM specimens, GAMs display a complex profile of both M1 and M2 polarization markers,52,53 and very likely there are yet undefined phenotypes.
Fig. 1.
Schematic demonstrating the cytokines for inducing polarized M1 versus M2 macrophages, markers used in their identification (oval), key transcriptional pathways that each uses (nucleus), metabolic preference (cytoplasm), and the elaborated immune effectors they secrete.
Comprehensive immune phenotyping, whole-genome microarray analysis, and microRNA expression profiling of human GAMs in alignment to the polarized subtypes of human macrophages demonstrated that the ex vivo macrophages exhibited a distinct phenotype of activation more related to the nonpolarized M0 (undifferentiated) status.49 The M0 phenotype is considered an attenuated M2 phenotype.54 Importantly, this study49 showed that the reliance on a single marker such as CD163 used to define the M2 state is not really reflective of the immune biology of the vast majority of GAMs. But it also has implications for the design of therapeutic modalities—if the vast majority of GAMs are not really polarized to M2, then therapeutics that are focused on either the elimination of this population or polarization to M1 will not really be effectual. Rather the field may need to redirect its focus to understanding the immune biology of M0 and more serious consideration of the therapeutic modulation of the M0 state. Currently, there is a clear need to provide a functional classification system that recapitulates diverse fluid states of GAMs in human gliomas and/or tumor biology.
Drivers of Monocyte/Macrophage Recruitment
Macrophage recruitment to the TME is driven by a variety of glioma-elaborated chemokines, including monocyte chemoattractant protein (MCP)-1,55 MCP-3,56 C-X-C motif chemokine 12 (CXCL12),57 C-X3-C motif ligand 1/fractalkine,58 colony-stimulating factor (CSF-1),59 lysyl oxidase (LOX),60 and glial cell–derived neurotrophic factor (GDNF).61 Several chemokines, such as GDNF, hepatocyte growth factor, CSF-1, and granulocyte-macrophage colony-stimulating factor have been claimed to be involved in GAM recruitment.59,61–65 These chemokines are heterogeneously expressed among gliomas and the pivotal drivers are contextual, probably due to the distinct glioma genetics. Transcriptional subtype-based classification revealed differences in immune infiltration in GBM. The mesenchymal subtype showed a greater frequency of macrophage/microglia compared with proneural or classical subtypes.66,67 NF1 deficiency was identified as a probable cause of differential chemotaxis in mesenchymal GBMs. Other alterations, such as phosphatase and tensin homolog (PTEN) deletion in glioma cells, have been shown to activate the transcription factor Yes-associated protein 1, which directly upregulates LOX expression. LOX is a potent chemokine recruiting macrophages via activation of the β1 integrin/proline-rich tyrosine kinase 2 pathway in macrophages. Inhibition of LOX suppresses macrophage infiltration and tumor progression specifically in PTEN-null glioma models.60 Amplification of the epidermal growth factor receptor (EGFR) gene and its truncation mutant EGFR variant (v)III is another common genetic alteration in glioblastoma. EGFR and EGFRvIII cooperate to recruit macrophages in GBM via induction of chemokine MCP-1.68
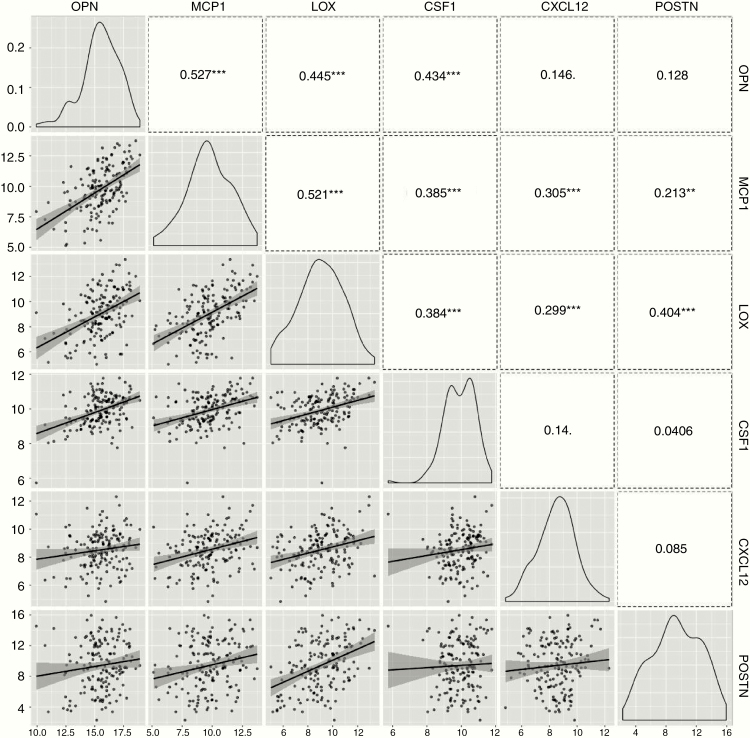
Cancer glioma stem cells (GSCs), in particular, may be a potent driver of macrophage infiltration.69 Chemokines elaborated by GSCs may be specific to the recruitment of specific macrophage subsets. For example, GSC-derived periostin (POSTN) recruits M2 GAMs via the integrin αvβ3 (ITGαvβ3) signaling. Disrupting POSTN in GSCs or inhibition of ITGαvβ3 specifically attenuates M2 GAM recruitment and inhibits glioma progression.70 Moreover, our group has recently shown that osteopontin (OPN) is a key driver of macrophage infiltration in gliomas and that the receptor ITGavβ5 is highly expressed on macrophages that have assumed a tumor supportive M2 phenotype. In addition to acting as a chemokine, glioma-elaborated OPN maintains the M2 macrophage gene signature and phenotype. OPN knock out results in a marked reduction of M2 macrophages, elevated T-cell effector activity in the infiltrating glioma, and sensitization of the glioma cells to direct CD8 T-cell cytotoxicity.71 Despite activity in other cancers, OPN-blocking antibodies and aptamers do not exert compelling biological responses in preclinical models of gliomas, probably because of the redundancy and plasticity of chemokines exploited by gliomas to recruit macrophages into the TME, and this is supported by a data analysis by The Cancer Genome Atlas (TCGA) that shows that the chemokine expression of OPN, MCP1, LOX, CSF1, CXCL12, and POSTN in GBM significantly correlates (Fig. 2).
Fig. 2.
Multiple major GAM chemokines are highly coexpressed in GBM and exhibit strong dual expression based on mRNA expression from TCGA. Pearson correlation analysis was used to evaluate the association of any 2 chemokines such as OPN, MCP1, LOX, CSF1, CXCL12, and POSTN. The number is the coefficient number r, and “**” or “ *** ” represents P < 0.01 or P < 0.001 associated to Pearson analysis.
GAM and Glioma Cell Interactions
The macrophages and microglia immune functionality are contextual and divergent. Their tumor-supportive roles have been demonstrated in mouse models of glioma in which the elimination of CD11b and/or CX3CR1 immune populations was associated with reduced tumor proliferation and/or in vivo tumor formation.72–74 In organotypic brain tumor-slice cultures and in vivo, the depletion of microglia decreases glioma growth and invasiveness.75–77 A variety of microglial-secreted factors such as stress inducible protein 1,78 epidermal growth factor,59 transforming growth factor beta (TGF-β),79,80 and matrix metallopeptidase-2 (MMP-2) play roles in glioma cell invasion. Toll-like receptor signal activation of microglia is a required component for at least the generation of MMP-2,81,82 and gliomas can also elaborate versican, which engages TLR2 signaling, thus supporting a feed-forward mechanism.83,84 Another feed-forward mechanism involves the release of glioma-elaborated CCL2, which triggers the release of IL-6 from microglia,45 which is, in turn, a ligand for the signal transducer and activator of transcription 3 (STAT3) pathway in gliomas that maintains the cancer stem-cell state and enhances invasiveness.85 Microglia can also directly reduce the sphere-forming ability of stem cells under normal conditions, but this is blocked when the microglia are obtained from glioma patients.86 Our group has also shown that supernatants from GSCs inhibit the phagocytic activity of macrophages and induce them to become immune suppressive as reflected by secretion of IL-10 and TGF-β.87 These GAMs also support angiogenesis through the elaboration of vascular endothelial growth factor.88 Intriguingly, the glioma-infiltrating macrophages and microglia may assume a proinflammatory immune function in specific scenarios such as during CD8 lymphopenia or depletion,89,90 which is probably an immune compensation mechanism.
GAMs and Outcome
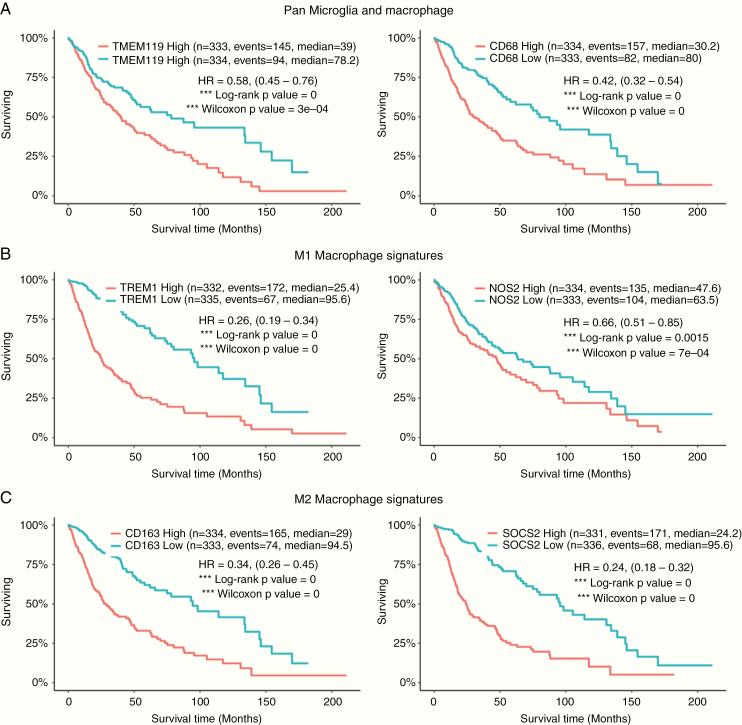
Almost all gliomas contain some level of GAMs.91 The number of CD68+ macrophages increases with glioma grade92 and is generally a negative prognostic factor for survival.93 A Kaplan‒Meier survival analysis linked to immune gene signatures extracted from the GlioVis web platform94 that subsumes TCGA and other glioma datasets reveals that the presence of the pan-microglia marker TMEM119 (Fig. 3A, left), the pan-macrophage marker CD68 (Fig. 3A, right), and the M2 macrophage signatures CD163 and SOCS2 (Fig. 3C) predicts poor survival in all gliomas inclusive of GBM. Notably, even the M1 macrophage signatures TREM1 (triggering receptor expressed on myeloid cells) and NOS2 (nitric oxide synthase 2), which are considered to be proinflammatory and antitumor, are negatively correlated with glioma patient survival (Fig. 3B). Therefore, we opine that such in silico analysis with M1 and M2 being defined as anti- and protumoral macrophages needs to be carefully interpreted and further validated.
Fig. 3.
Kaplan–Meier survival analysis of glioma associated macrophage gene signatures performed at GlioVis data portal. The GlioVis analysis predicts prognostic outcomes in patients with low-grade glioma and GBM based on the differential gene expression and corresponding Kaplan–Meier survival analysis for (A) pan-microglia (TMEM119-left) and pan macrophage (CD68-right) markers; (B) M1 macrophage signatures: Trem1 (left) and NOS2 (right); and (C) M2 macrophage signatures; CD163 (left) and SOCS2 (right). Kaplan–Meier estimates of survival time of patients (x-axis) is plotted against the percentage of patients surviving (y-axis) from glioma datasets of TCGA. The data for survival have been directly extracted from the GlioVis Portal (without any computation on our side), available as a freeware accessible at http://gliovis.bioinfo.cnio.es/. Designations for high and low expression used in the survival analysis are based on the median of the target gene expression in all samples. Similar outcome results are obtained when patients are stratified based on glioma grade.
Proinflammatory Immune Functions of Glioblastoma-Associated Macrophages
Conventionally, antigen presentation and immune activation are triggered in peripheral lymphatics. The activated T cell subsequently exits and travels to the TME, guided by a gradient of tumor-elaborated chemokines, to exert tumor destruction. In 2014, Klaus Ley put forth the second-touch hypothesis stating that full T-cell activation requires a second interaction with an antigen-presenting cell in the non-lymphoid, antigen-expressing target tissue. This initially marginalized concept has now been gaining increasing acceptance, in part supported by our work showing that DCs are present in the TME and are essential for T-cell effector responses.95 After encountering CD11c+ DCs, T cells are capable of undergoing proliferation—a hallmark of productive engagement with an antigen-presenting cell. Microglia under resting conditions typically do not express major histocompatibility complex (MHC) II or costimulatory molecules, but during inflammatory conditions they are capable of these functions.96 Macrophages can act as antigen-presenting cells but are considered to be generally less efficient at this process. However, Siglec-1+ macrophages97 can cross present tumor antigens to CD8+ T cells that probably repositions this specialized macrophage subset as antigen-presenting cells. In the bone marrow, hematopoietic stem cells produce myeloid- and lymphoid-committed precursors. The myeloid precursors give rise to monocyte, macrophage, and DC precursors. The distinction between DCs and macrophages is also confounded by marker lineage specificity. For example, CD11c is commonly used as a marker on DCs but can also be present on macrophages and microglia. In many instances, transcriptional profiling is necessary to distinguish these populations.98 Until the glioma immunologists clarify the nomenclature for these immune populations in both mouse and man in the context of phenotypes and functions, we are likely misidentifying populations and as a result present confounding interpretation. Currently, it is not clear whether macrophages and/or microglia within the TME are conducting antigen-presentation activities in gliomas.
Macrophages and microglia can mediate direct tumor killing through secreted products such as nitric oxide, and reactive oxygen species (ROS) may have limited cytolytic activity through antibody-dependent cellular cytotoxicity and can eliminate antibody-bound cells through antibody-dependent cellular phagocytosis. In addition, myeloid cells can facilitate tumor elimination indirectly through recruitment of cytotoxic immune cells. Notably, macrophage antitumor activity is not merely a function of phagocytosis, particularly in the absence of tumor-specific antibodies, and the implied connection to tumor cytotoxicity is not merited. Certainly, the presence of the macrophages and microglia as detected by markers such as IBA-1 (ionized calcium binding adaptor molecule 1) or CD68 does not directly imply either an immune suppressive or a proinflammatory function, which is often a misguided interpretation. Therapeutically, the macrophage population could be depleted using clodronate-filled liposomes,99 but this is not a scalable or practical strategy for human subjects and would not be specific to their immune functional role, including the elimination of proinflammatory cells supporting antitumor immunity.
Targeting GAM Polarization and Macrophage Subsets
Theoretically, an M2 immune suppressive population could be eliminated or depleted for a therapeutic effect based on the expression of CD163. Anti-CD163 antibody-conjugated lipid nanoparticles demonstrated a therapeutic response in mice with subcutaneous melanoma.100 However, there are several caveats to these data being extrapolated to glioma: (i) it is unknown if this strategy would impact the GAM population within the CNS, especially given the disassociation of CD163 expression with immune suppression function in GAMs; (ii) melanoma is enriched in T-cell responses, whereas gliomas are not; and (iii) it is unknown if this melanoma model recapitulates the degree of macrophage immune heterogeneity found in GAMs. As such, this strategy will require additional preclinical evaluation in immune competent models of gliomas before being more fully considered in human gliomas.
Although macrophages most commonly adopt a phenotype that supports tumor growth, theoretically their biology may be pliable and dependent on niche signals. However, repolarization claims of M2 to M1 need to be interpreted with caution unless there was comprehensive profiling,101 as these claims in the literature usually rely on a limited number of markers such as expression of MHC and costimulatory molecules. Bearing this caveat in mind, perhaps under the appropriate conditions, macrophages could be redirected to possess antitumor activity. One such therapeutic strategy that has been advanced into clinical trials is the use of inhibitors of CSF-1.102
BBB-permeable small molecular weight CSF-1R inhibitors including BLZ945 and PLX3397 significantly increased survival time in different models of GBM by reducing GAM accumulation within the tumor, inhibiting expression of M2 alternatively activated markers, and increasing phagocytic activity.102 A phase II study investigating the use of PLX3397 in patients with recurrent GBM has been completed, showing safety but no efficacy.103 Future studies are planned with the goal of exploring CSF-1R blockade in combination with other types of immunotherapies in hopes of improving therapeutic outcomes. Preclinical studies revealed that resistance is acquired by expression of macrophage-derived insulin-like growth factor 1 (IGF-1), and high IGF-1 receptor on tumor cells. The resulting IGF receptor activation induces the phosphatidylinositol-3 kinase pathway to enhance glioma cell survival and invasiveness,104 which may have been the case for these patients. Alternatively, the M2 macrophage phenotype may represent only a small minority of diverse macrophage functional states in the continuum as the M0 state predominates, and the result could be only a modest therapeutic response. Additionally, the role of CSF-1 inhibitors on the M0 state has thus far not been evaluated.
Other strategies that could impact polarization have included use of polyinosinic-polycytidylic acid [poly(I:C)]105 and IL-12106 to change the behavior of microglia from a tumor-supporting role to a tumor-suppressing function. Poly(I:C), a TLR3 agonist, has been shown to induce a strong proinflammatory response in primary human GAMs that leads to the inhibition of tumor growth and invasion.105 However, poly IC has not demonstrated therapeutic effects in clinical trials. IL-12 has complex immune stimulatory functions spanning both innate and adaptive immune components, and teasing out the contribution of inhibiting M2 polarization will be a challenge. Mammalian target of rapamycin inhibitors such as rapamycin can restrict the magnitude of M2 activation and polarize glioma-activated microglia to an M1 profile, conferring cytotoxic functions,107 but the contribution of the direct tumor effects versus the immune-modulatory properties is also not clear.
Macrophages downregulate mR-142-3p as they polarize from M1 toward M2, a critical step in prevention of apoptosis when acquiring M2 features. Delivery of this specific miRNA to peripheral monocyte-derived macrophages blocks TGF-β receptor 1 signaling, which triggers apoptosis in M2 macrophages as they acquire this phenotype. Systemic administration of miR142-3p demonstrated glioma growth inhibition and extended survival, with an associated decrease in glioma-infiltrating macrophages.108 However, clinical implementation of miR142-3p has thus far been limited by the development of an acceptable nanoparticle formulation of it.
Stimulator of interferon genes (STING) agonists can trigger a flood of T-cell infiltration into otherwise immunologically “cold” tumors through proinflammatory activation of suppressive tumor stroma. STING is a widely expressed sensor of cellular stress, specifically the presence of DNA in the cytoplasm that bridges the innate and adaptive immune systems, both by triggering interferon release and by cis-activation of myeloid cells. Distinct from most other innate immune agonists, STING activation can reeducate tumor-supportive M2 macrophages toward a proinflammatory M1 phenotype and can reverse the suppressive phenotype of myeloid-derived suppressor cells (MDSCs).109 STING is activated by cyclic dinucleotides, either originating directly from invading bacteria or generated by the protein cGAS (cyclic GMP-AMP synthase) upon binding to cytoplasmic DNA—a hallmark of viral infection. Furthermore, mouse gliomas grow faster in STING-knockout mice, demonstrating the critical role of this pathway in limiting tumor progression.110 Early clinical trials of viral therapy have achieved sporadic clinical responses in GBM patients, but these therapeutics are complex to manufacture, challenging to administer, and often limited to a single, direct intratumoral (ie, surgical) treatment. STING agonists activate many of the same innate pathways as oncolytic viruses but in a vastly more potent and focused fashion, free from the complexities and high costs of viral therapy. STING agonists are particularly compelling in diseases such as GBM, which have few infiltrating T cells and a preponderance of macrophages, because they (i) can simulate a foreign body reaction, thus providing a “target”; (ii) induce IFN, thereby providing potent T-cell effector action; (iii) induce chemokine production and thus T-cell trafficking to the tumor; and (iv) are scalable, inexpensive, and easy to generate as a Good Manufacturing Practices product. We have demonstrated a remarkable capacity for intratumorally injected STING agonists to eliminate not only the treated tumor but also distant, untreated sites of disease.111 Multiple STING agonists are being tested in canines with spontaneously arising gliomas at Texas A&M University in an effort to refine the logistics of delivery directly to the glioma TME as a prelude to trials in human subjects.
Enhancing Macrophage Function
Glioma cells can upregulate an antiphagocytic surface protein, CD47, which binds to its cognate receptor SIRPα (signal regulatory protein alpha) on phagocytic cells, thereby inhibiting their phagocytic activity.112 Blockade of the CD47- SIRPα myeloid checkpoint has been shown to effectively enhance tumor phagocytosis and hence reduce tumor burden.113,114 CD47 blockade can also elicit a therapeutic effect in preclinical models of GBM115 via macrophage-triggered cytotoxicity and phagocytosis.116 Cytosine-phosphate-guanine oligodeoxynucleotide, a TLR9 agonist, can also potentiate clearance of cancer cells and overcome the tumor-expressed CD47-mediated “don’t-eat-me” signal through metabolic rewiring.117 Chimeric antigen receptor (CAR) strategies could also be used to trigger phagocytosis.118 More specifically, macrophages can be engineered with a CAR construct that incorporates the cytosolic domains from Megfl10 and FcRγ, which are independent of extracellular signaling, to robustly engulf cancer cells based on a specific antigen. However, a key limitation for any type of CAR strategy in gliomas has been the identification of ubiquitously and homogenously expressed antigens. Phagocytosis is more robust in M1-polarized macrophages relative to M2 macrophages,119 but we have shown that phagocytic function is profoundly impaired upon exposure to GSC supernatants and GBM-elaborated MIC-1.87 As such, therapeutic strategies that rely on phagocytosis may be problematic in subsets of glioma patients. More specifically, GBM patients who have tumor Quaking (Qki) deficiency, which is present in over 60% of GBM,120 may demonstrate therapeutic resistance to such strategies because Qki loss impairs migration of monocytes and differentiation into macrophages,121 and it probably also impedes phagocytic functions. Moving forward, clinical trials of modulators that rely on phagocytosis function may need to consider stratification based on Qki expression.
Metabolic and Transcription Reprogramming of Macrophages
The STAT3 pathway is a potent regulator of glioma tumorigenesis122 and tumor-mediated immune suppression.87,123–125 A wide variety of growth factors and cytokines, including IL-6 produced by reactive astrocytes, can activate the STAT3 pathway. Upon activation, STAT3 translocates into the nucleus and induces the expression of a variety of target genes. STAT3 expression in the glioma cell propagates tumorigenesis by preventing apoptosis and enhancing proliferation, angiogenesis, and invasion,126 along with maintaining GBM stem cells85 and driving glioma-mediated immune suppression.124 STAT3 upregulation is linked with the mesenchymal GBM subtype,127 which is preferentially enriched with macrophages and microglia.67 STAT3 is also a key hub of tumor-mediated immune suppression. As there are multiple redundant mechanisms of glioma-mediated immune suppression, key molecular hubs such as STAT3, which control tumor-mediated immune suppression in a comprehensive manner, are particularly appealing targets.
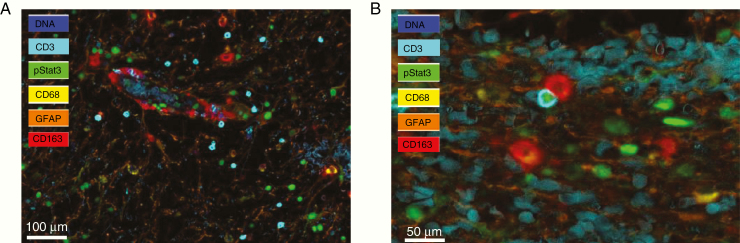
The STAT3 pathway becomes activated in diverse immune cells when they encounter either the glioma microenvironment or glioma-secreted products, resulting in profound global immune suppression.128–130 Specifically, STAT3 expression in macrophages has been shown to limit their activation,131–133 and we have shown that STAT3 inhibitors can reverse this state and restore proinflammatory potential.123 CD163-expressing macrophages line the GBM tumor vasculature, and as the T cells gain entry to the tumor from the peripheral circulation, they encounter these immune suppressive macrophages and make direct contact. These macrophages induce STAT3 activation in the infiltrating T cells, triggering a cascade of suppressive signaling which compromises their antitumor potential (Fig. 4).
Fig. 4.
(A) Multiplex immunohistochemical analysis of a GBM showing p-STAT3 expression (green) in CD3 T cells (blue) as they exit from the tumor vasculature that is lined with CD163 M2 macrophages (red). Glial fibrillary acidic protein (GFAP)+ glioma cells are shown in orange, and the nuclei are counterstained with 4′,6′-diamidino-2-phenylindole in blue (100 µm scale bar). (B) M2 macrophage (denoted in red) is directly interacting with a CD3 T cell (blue) that has p-STAT3 in its nucleus within the GBM microenvironment (50 µm scale bar).
Ablating STAT3 in only the hematopoietic cells in tumor-bearing mice results in marked enhancement of activated and functional T cells, natural killer (NK) cells, and DCs, and this in turn yields marked antitumor effects in vivo, indicating that STAT3 expression in immune cells restrains antitumor immune eradication.128 More recently, STAT3 has been shown to play a key role in modulating members of the immune checkpoint family. First, STAT3 has a well-established role in upregulating PD-1 ligand (PD-L1) expression and in inducing tolerogenic innate antigen-presenting cells.134 Furthermore, M2 polarization and B7-H3 have been shown to be induced by STAT3.135 Finally, B7-H4, which is associated with a poor prognosis in GBM and is expressed on both GBM stem cells and macrophages/microglia, is transcriptionally regulated by STAT3.136 STAT3 expression is also associated with M2 skewing.137,138 Specifically, STAT3 is a key transcriptional programmer that drives tumor-supportive macrophages.139 STAT3 inhibition activates antitumor M1-like GAMs, resulting in glioma growth inhibition.87 In addition to the regulatory role for immune checkpoint inhibitors on innate immune cells described above, we found that STAT3-activated macrophages become directly tumor supportive to gliomas. Specifically, we have shown that GBM-resident macrophages and microglia become polarized to the M0 and M2 phenotypes, which inhibits their phagocytosis and induces them to secrete IL-10 and TGF-β1. In autologous T-cell assays, glioma-conditioned macrophages directly inhibited T-cell proliferation; this macrophage dysfunction can be reversed by inhibiting phosphorylated (p-)STAT3.87
STAT3 pharmacological inhibitors such as WP1066 are capable of reversing the immune-tolerant microenvironment by activating microglia cells through production of lymphocyte-stimulating cytokines and upregulation of costimulatory molecules.87,123 Currently, there is a registered phase I trial investigating use of WP1066 against recurrent glioblastoma (NCT01904123). An alternative agent for targeting the STAT3 pathway is miR-124, which has been shown to be capable of regulating macrophage polarization.140 We have shown that this agent has robust preclinical efficacy in multiple models of glioma. STAT3 inhibition in combination with radiation therapy in preclinical models of other malignancies, but not as a single agent, improved tumor growth delay, decreased levels of regulatory T cells and M2 macrophages, and enhanced levels of effector T cells and M1 macrophages. Similar experiments conducted in nude mice negated this benefit of STAT3 inhibition and radiation therapy.141 We have found that the combination of WP1066 in combination with radiation reprograms the immune responses in the glioma microenvironment specifically affecting antigen presentation and T-cell effector functions (Ott, Neuro-Oncology, NOD19-00518).
Another potential metabolic pathway that could be exploited for targeting GAMs involves arginase 1 (Arg1), the final enzyme of the urea cycle that converts L-arginine to urea and L-ornithine.142 During infection, macrophages initiate ROS production to clear pathogens and dead cells through upregulation of inducible nitric oxide sythnase (iNOS) and NO production. After 3 to 5 days, Arg1 is upregulated by poorly understood mechanisms. This upregulation of arginase has three effects: (i) increasing L-ornithine, which is shunted into polyamine and collagen synthesis pathways and may support a growth-conducive microenvironment for tumors; (ii) overcompetition with iNOS for the shared substrate of arginine, which yields uncoupling of iNOS, producing superoxide molecules and elaboration of peroxynitrite, which is immunosuppressive to both myeloid cells and T cells; and (iii) reduction of extracellular arginine concentration below the minimum necessary to support effector T-cell persistence.143 An extensive library of compounds targeting Arg1 exists, including natural or derived α-amino acids, as well as boronohexanoic acid and its derivatives.144 Therapeutic implementation limitations for systemic administration to patients include off-target inhibition of Arg1 in the liver, which leads to hyperammonemia in patients, although such side effects can be ameliorated with compounds such as sodium benzoate and inhibition of Arg2—a structurally very similar enzyme with different tissue distribution, cellular localization, and function. Compounds have been synthesized with Arg1 selectivity and are being evaluated in other cancer types in a phase I clinical trial (NCT02903914).
There is metabolic polarization associated with skewed M1 versus M2 macrophages that provides several potential therapeutic opportunities. M1 macrophages predominantly use aerobic glycolysis upon activation, which is associated with increased glucose uptake and the conversion of pyruvate to lactate. At the same time, the activities of the respiratory chain are attenuated, allowing for ROS production145,146 needed for cytotoxic activity. In contrast, M2 macrophages obtain their energy from fatty acid oxidation and oxidative metabolism, and blocking oxidative metabolism. Blockade of oxidative metabolism may not only block polarization to the M2 phenotype, but also drive the macrophage back into an M1 state. Similarly, forcing oxidative metabolism in an M1 macrophage could potentiate the M2 phenotype.147,148 There are 2 oxidative phosphorylation inhibitors currently being evaluated in human subjects, but they also possess direct antitumor effects, meaning that teasing out the mechanism of activity will be a challenge.
Immune Checkpoint Regulation of GAMs
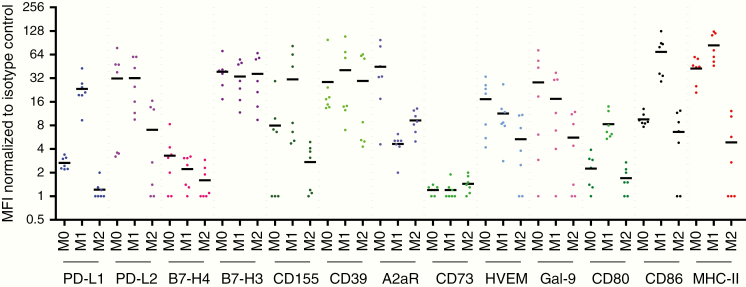
The use of immune checkpoint inhibitors is an area of ongoing therapeutic modulation for macrophages. PD-L1 expression has been shown to be associated with M2 macrophages.149 We have shown that GBM cancer stem cells induce M2 macrophages and PD-L1 expression in human monocytes through transfer of exosomes.150 However, as we have previously indicated, most of the macrophages/microglia characterized immediately ex vivo from GBMs are more aligned with an M0 phenotype. To clarify whether there are specific therapeutic opportunities from the available therapeutic compendium for targeting the M0- and/or M2-polarized macrophages, we obtained peripheral blood mononuclear cells from normal donors (n = 7), isolated their CD14+ monocytes, and then performed a standard skewing procedure.49 Notably, there are no M0- or M2-exclusive targets that do not also target M1 skewed macrophages. Some, such as PD-L1, are in fact preferentially enriched in the M1 macrophage (Fig. 5). Notably, many of the immune checkpoint ligands such as PD-L1, PD-L2, B7-H3, and B7-H4 are heterogeneously expressed on CD11b+ macrophages isolated directly ex vivo from glioma patients and in some instances not at all expressed.
Fig. 5.
Summarized flow cytometry data of human monocytes that are polarized to the M0, M1, or M2 (n = 7) states and then profiled for expression of immune markers for which therapeutic modalities are available. The mean fluorescence intensity (MFI) of each designated immune marker was normalized to the associated isotype control. The mean is denoted by bold horizontal bars.
Both mouse and human tumor-associated macrophages (TAMs) have been shown to express PD-1. The expression of PD-1 on the TAMs increases over time in murine models of malignancy and with disease stage in human cancers. The PD-1 expression on the TAMs correlates negatively with phagocytic activity against tumor cells, and blockade of PD-1/PD-L1 in vivo reduces tumor growth and increases survival in mouse models of cancer. These data indicate that PD-1/PD-L1 therapies may function through a direct effect on macrophages.151 Anti–PD-1 therapy has also been suggested to impact the polarization of M2 to M1 macrophages.152 The use of currently available immune checkpoint ligand therapeutics on modulation of macrophages/microglia is an underdeveloped area of opportunity.
GAMs as an Unappreciated Confounder of Response in Glioma Immunotherapy Clinical Trials
Although a phase III clinical study employing immune checkpoint inhibitors failed to demonstrate an improvement in the median survival time of GBM patients, certain groups of patients may respond secondary to the unique genetic features of their malignancy such as POLE (polymerase epsilon) mutations or in a neoadjuvant setting.18,153 In addition to the immune checkpoint inhibitors not being able to reverse immune exhaustion in GBM patients,17 and the relative paucity of these cells in the TME owing to their sequestration in the bone marrow,16 we have also shown in a clinical trial of anti–PD-1 in GBM patients with mass cytometry time-of-flight analysis that 72.6% of the leukocytes in the TME were macrophages.154 Although MHC is expressed on most of the CD68+ macrophages that would indicate they are capable of antigen presentation, many subpopulations expressed multiple markers of immune suppression such as VISTA (V-domain immunoglobulin suppressor of T cell activation) and B7-H3. These macrophages line the walls of the vasculature of the GBM, where they probably serve an immunological role in suppressing antitumor immune effector activity as the T cell migrates into the TME from the systemic circulation. These data would indicate that immune suppressive macrophages likely are a key confounder for attenuating the T-cell response, and it seems unlikely that activated effector T cells could really persist in this immune environment. Although the aforementioned clinical trial was for an anti–PD-1 therapeutic strategy, GAMs will likely serve as a hindrance for other immune therapeutic strategies and immune checkpoint inhibitors that rely on the T cell to exert an antitumor effector response. Patients who have low levels of GAM infiltration may be more responsive to immunotherapy, but a companion biomarker would need to be developed that incorporates features of immune function.
The profound influence of the GAM in the TME also provides compelling rationale for the therapeutic modulation of the macrophage population for optimizing T-cell effector responses. To date, most clinical trials of immune checkpoint inhibitors are focused on analysis of T-cell responses; however, other immune cells such as NK and macrophages could mediate antitumor immune responses and probably need to be further considered. Future studies are being directed at determining if the presence of macrophages, including their distinct immune phenotypes, would render gliomas more sensitive or resistant to treatment with immune checkpoint inhibitors. It is possible that anti–PD-1 therapy may be triggering a proinflammatory macrophage phenotype in TME and therefore may be contributing to the therapeutic effect in GBM patients.154 Clarification will require comprehensive immune profiling to ascertain their specific role in this context.
Microglia-Directed Therapeutics
In contrast to targeting the macrophage population that originates from the peripherally derived monocyte, targeting the microglia population needs to be considered by manipulating the BBB with permeable druggable agents. Therapeutics directed specifically at microglia are not as clinically advanced as those for macrophages. Minocycline, a semisynthetic broad-spectrum tetracycline antibiotic has the capacity to counteract microglial activation and can reduce tumor growth by inhibiting microglial membrane type 1–matrix metalloprotease (MT1-MMP) expression.155 Propentofylline, an atypical synthetic methylxanthine, is capable of targeting microglial cells via inhibition of TNFRSF19 (tumor necrosis factor receptor superfamily member 19).156 Amphotericin B (a polyene antifungal drug) stimulates glioma-associated microglia through TLR signaling, and daily treatment of glioma-bearing mice with this drug substantially prolongs their survival.14,15,86 At this junction, it is unclear relative to the macrophage population how impactful specific immune modulation of microglia would be relative to the macrophage population.
Perspectives
Despite the abundance of preclinical trials conducted to identify novel, effective therapies for gliomas inclusive of GBM, translation into actual clinical benefit has been rare. In vitro and in vivo investigations have contributed substantially to our understanding of GAM biology, but it is still unclear whether GAM-directed therapies would yield desirable therapeutic effects in glioma patients in the purview of limited understanding of diverse fluidic and functional states linked to bona fide brain-resident and infiltrating macrophages. Hence, dissecting the conserved and differential functions of remarkably heterogeneous resident microglial and peripherally recruited macrophage subpopulations in the context of compartmentalized anatomic niches of the normal and malignant brain would be critical before embarking onto tailored macrophage-centric therapeutics development. In addition, a deeper insight to uncover spatiotemporal dynamics that imprint pro- and antitumoral macrophage phenotypes is required to enable rationale design for GAM-directed therapies and their potential for incorporation into current treatment regimens. Furthermore, deep immunophenotyping of immune cell infiltrates from molecularly stratified patients to define the phenotypic, spatial, and functional contexts of pro- and antitumoral resident and recruited cell populations in the glioma microenvironment and circulation would facilitate our understanding of the coexistence of molecular and immune correlates as indicators of prognosis and as informed proxies for therapeutically aligned clinical trials. Concomitantly, glioma animal models need to be improvised and validated to mimic human glioma immunopathology for head-to-head comparison and alignment of tumor immune repertoire in gliomas for an immediate translation from in vivo findings to investigative clinical trial studies. Ultimately, efforts to reinvigorate compromised adaptive effector responses will need to be used in combination with strategies that modulate innate immunity to control brain tumor growth but will require extensive further development of the next generation of immune therapeutics.
Funding
This research was supported by the Brockman Foundation, Dr Marnie Rose Foundation, the GBM Moonshot, and the National Institutes of Health (grant no. CA 1208113).
Conflict of interest statement: None.
References
- 1. Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife. 2017;6:e29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hussain SF, Yang D, Suki D, Grimm E, Heimberger AB. Innate immune functions of microglia isolated from human glioma patients. J Transl Med. 2006;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rock RB, Gekker G, Hu S, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17(4):942–964, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB.. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gutmann DH, McLellan MD, Hussain I, et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013;23(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 1: studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. J Neurosurg. 1979;50(3):298–304. [DOI] [PubMed] [Google Scholar]
- 9. Kierdorf K, Masuda T, Jordão MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20(9):547–562. [DOI] [PubMed] [Google Scholar]
- 10. Masuda T, Sankowski R, Staszewski O, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388–392. [DOI] [PubMed] [Google Scholar]
- 11. Van Hove H, Martens L, Scheyltjens I, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021–1035. [DOI] [PubMed] [Google Scholar]
- 12. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- 13. Smolders J, Remmerswaal EB, Schuurman KG, et al. Characteristics of differentiated CD8(+) and CD4 (+) T cells present in the human brain. Acta Neuropathol. 2013;126(4):525–535. [DOI] [PubMed] [Google Scholar]
- 14. Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179(2):845–853. [DOI] [PubMed] [Google Scholar]
- 15. Waziri A, Killory B, Ogden AT 3rd, et al. Preferential in situ CD4+CD56+ T cell activation and expansion within human glioblastoma. J Immunol. 2008;180(11):7673–7680. [DOI] [PubMed] [Google Scholar]
- 16. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Groot J, Penas-Prado M, Alfaro-Munoz KD, et al. Window-of-opportunity clinical trial of pembrolizumab in patients with recurrent glioblastoma reveals predominance of immune-suppressive macrophages. Neuro Oncol. Neuro Oncol. 2019;pii:noz185. doi: 10.1093/neurono/noz185. [Epub ahead of print] PMID:31755915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garber ST, Hashimoto Y, Weathers SP, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro Oncol. 2016;18(10):1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma J, Xu H, Wang S. Immunosuppressive role of yeloid-derived suppressor cells and therapeutic targeting in lung cancer. J Immunol Res. 2018;2018:6319649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi J, Gyamfi J, Jang H, Koo JS. The role of tumor-associated macrophage in breast cancer biology. Histol Histopathol. 2018;33(2):133–145. [DOI] [PubMed] [Google Scholar]
- 23. Pergamo M, Miller G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther. 2017;24(3):100–105. [DOI] [PubMed] [Google Scholar]
- 24. Won WJ, Deshane JS, Leavenworth JW, Oliva CR, Griguer CE. Metabolic and functional reprogramming of myeloid-derived suppressor cells and their therapeutic control in glioblastoma. Cell Stress. 2019;3(2):47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pereira MB, Barros LRC, Bracco PA, et al. Transcriptional characterization of immunological infiltrates and their relation with glioblastoma patients overall survival. Oncoimmunology. 2018;7(6):e1431083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. [DOI] [PubMed] [Google Scholar]
- 27. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Müller S, Kohanbash G, Liu SJ, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carmona-Fontaine C, Bucci V, Akkari L, Deforet M, Joyce JA, Xavier JB. Emergence of spatial structure in the tumor microenvironment due to the Warburg effect. Proc Natl Acad Sci U S A. 2013;110(48):19402–19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carmona-Fontaine C, Deforet M, Akkari L, Thompson CB, Joyce JA, Xavier JB. Metabolic origins of spatial organization in the tumor microenvironment. Proc Natl Acad Sci U S A. 2017;114(11):2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113(12):E1738–E1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bowman RL, Klemm F, Akkari L, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17(9):2445–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buttgereit A, Lelios I, Yu X, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016;17(12):1397–1406. [DOI] [PubMed] [Google Scholar]
- 35. Liu Z, Gu Y, Chakarov S, et al. Fate map ping via Ms4a3 expression history traces monocyte-derived cells. 2019;178:1509–1525. [DOI] [PubMed] [Google Scholar]
- 36. Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46(4):957–961;discussion 961–962. [DOI] [PubMed] [Google Scholar]
- 37. Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009;110(3):572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Müller A, Brandenburg S, Turkowski K, Müller S, Vajkoczy P. Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer. 2015;137(2):278–288. [DOI] [PubMed] [Google Scholar]
- 39. Chen Z, Feng X, Herting CJ, et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77(9):2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chakarov S, Lim HY, Tan L, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363(6432):1154–1166. [DOI] [PubMed] [Google Scholar]
- 41. Helmke A, Nordlohne J, Balzer MS, et al. CX3CL1-CX3CR1 interaction mediates macrophage-mesothelial cross talk and promotes peritoneal fibrosis. Kidney Int. 2019;95(6):1405–1417. [DOI] [PubMed] [Google Scholar]
- 42. Panek CA, Bruballa AC, Pineda GE, et al. Cytokines use different intracellular mechanisms to upregulate the membrane expression of CX3CR1 in human monocytes. Mol Immunol. 2019;108:23–33. [DOI] [PubMed] [Google Scholar]
- 43. Boddeke EW, Meigel I, Frentzel S, et al. Cultured rat microglia express functional beta-chemokine receptors. J Neuroimmunol. 1999;98(2):176–184. [DOI] [PubMed] [Google Scholar]
- 44. Jiang Y, Salafranca MN, Adhikari S, et al. Chemokine receptor expression in cultured glia and rat experimental allergic encephalomyelitis. J Neuroimmunol. 1998;86(1):1–12. [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Sarkar S, Cua R, Zhou Y, Hader W, Yong VW. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012;33(2):312–319. [DOI] [PubMed] [Google Scholar]
- 46. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. [DOI] [PubMed] [Google Scholar]
- 47. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19(8):987–991. [DOI] [PubMed] [Google Scholar]
- 48. Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6(8):e23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gabrusiewicz K, Rodriguez B, Wei J, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI insight. 2016;1(2):e85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14(8):958–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83(5):1136–1144. [DOI] [PubMed] [Google Scholar]
- 52. Hattermann K, Sebens S, Helm O, et al. Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol Rep. 2014;32(1):270–276. [DOI] [PubMed] [Google Scholar]
- 53. Szulzewsky F, Pelz A, Feng X, et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One. 2015;10(2):e0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Franco R, Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. [DOI] [PubMed] [Google Scholar]
- 55. Platten M, Kretz A, Naumann U, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54(3):388–392. [DOI] [PubMed] [Google Scholar]
- 56. Okada M, Saio M, Kito Y, et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34(6):1621–1627. [DOI] [PubMed] [Google Scholar]
- 57. Wang SC, Hong JH, Hsueh C, Chiang CS. Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab Invest. 2012;92(1):151–162. [DOI] [PubMed] [Google Scholar]
- 58. Feng X, Szulzewsky F, Yerevanian A, et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6(17):15077–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coniglio SJ, Eugenin E, Dobrenis K, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen P, Zhao D, Li J, et al. Symbiotic macrophage-glioma cell interactions reveal synthetic lethality in PTEN-null glioma. Cancer Cell. 2019;35(6):868–884 e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ku MC, Wolf SA, Respondek D, et al. GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol. 2013;125(4):609–620. [DOI] [PubMed] [Google Scholar]
- 62. Badie B, Schartner J, Klaver J, Vorpahl J. In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth factor/scatter factor. Neurosurgery. 1999;44(5):1077–1082;discussion 1082–1083. [DOI] [PubMed] [Google Scholar]
- 63. Sielska M, Przanowski P, Wylot B, et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230(3):310–321. [DOI] [PubMed] [Google Scholar]
- 64. Liu C, Luo D, Streit WJ, Harrison JK. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol. 2008;198(1-2):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. [DOI] [PubMed] [Google Scholar]
- 66. Bhat KPL, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. An Z, Knobbe-Thomsen CB, Wan X, Fan QW, Reifenberger G, Weiss WA. EGFR cooperates with EGFRvIII to recruit macrophages in glioblastoma. Cancer Res. 2018;78(24):6785–6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yi L, Xiao H, Xu M, et al. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232(1-2):75–82. [DOI] [PubMed] [Google Scholar]
- 70. Zhou W, Ke SQ, Huang Z, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wei J, Marisetty A, Schrand B, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68(24):10358–10366. [DOI] [PubMed] [Google Scholar]
- 73. Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. [DOI] [PubMed] [Google Scholar]
- 74. Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bettinger I, Thanos S, Paulus W. Microglia promote glioma migration. Acta Neuropathol. 2002;103(4):351–355. [DOI] [PubMed] [Google Scholar]
- 76. Markovic DS, Glass R, Synowitz M, Rooijen Nv, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64(9):754–762. [DOI] [PubMed] [Google Scholar]
- 77. Markovic DS, Vinnakota K, Chirasani S, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A. 2009;106(30):12530–12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Carvalho da Fonseca AC, Wang H, Fan H, et al. Increased expression of stress inducible protein 1 in glioma-associated microglia/macrophages. J Neuroimmunol. 2014;274(1–2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wesolowska A, Kwiatkowska A, Slomnicki L, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion–an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27(7):918–930. [DOI] [PubMed] [Google Scholar]
- 80. Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001;53(2):177–185. [DOI] [PubMed] [Google Scholar]
- 81. Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58(3):253–263. [DOI] [PubMed] [Google Scholar]
- 82. Vinnakota K, Hu F, Ku MC, et al. Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neuro Oncol. 2013;15(11):1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hu F, Dzaye O, Hahn A, et al. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages Toll-like receptor 2 signaling. Neuro Oncol. 2015;17(2):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hu F, Ku MC, Markovic D, et al. Glioma-associated microglial MMP9 expression is upregulated by TLR2 signaling and sensitive to minocycline. Int J Cancer. 2014;135(11):2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27(10):2383–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sarkar S, Döring A, Zemp FJ, et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci. 2014;17(1):46–55. [DOI] [PubMed] [Google Scholar]
- 87. Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen X, Zhang L, Zhang IY, et al. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014;74(24):7285–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kane RJ, Zhao J, Tsujiuchi T, et al. CD8+ T-cell-mediated immunoediting influences genomic evolution and immune evasion in murine gliomas. In press. [DOI] [PMC free article] [PubMed]
- 90. Rao G, Ling X, Doucette T, et al. Innate immunological compensatory control of gliomagenesis and malignant transformation in murine CD8α knockout models. Paper presented at: 20 th Annual Scientific Meeting of the Society for Neuro-Oncology 2015; November 19–22; 2015; San Antonio, TX. [Google Scholar]
- 91. Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg. 2011;115(3):505–511. [DOI] [PubMed] [Google Scholar]
- 92. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. [DOI] [PubMed] [Google Scholar]
- 93. Dorward IG, Luo J, Perry A, et al. Postoperative imaging surveillance in pediatric pilocytic astrocytomas. J Neurosurg Pediatr. 2010;6(4):346–352. [DOI] [PubMed] [Google Scholar]
- 94. Bowman RL, Wang Q, Carro A, Verhaak RG, Squatrito M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro Oncol. 2017;19(1):139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yan J, Zhao Q, Gabrusiewicz K, et al. FGL2 promotes tumor progression in the CNS by suppressing CD103+ dendritic cell differentiation. Nat Commun. 2019;10(1):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wlodarczyk A, Løbner M, Cédile O, Owens T. Comparison of microglia and infiltrating CD11c⁺ cells as antigen presenting cells for T cell proliferation and cytokine response. J Neuroinflammation. 2014;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Asano K, Nabeyama A, Miyake Y, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34(1):85–95. [DOI] [PubMed] [Google Scholar]
- 98. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Poli A, Wang J, Domingues O, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4(9):1527–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Etzerodt A, Tsalkitzi K, Maniecki M, et al. Specific targeting of CD163+ TAMs mobilizes inflammatory monocytes and promotes T cell-mediated tumor regression. J Exp Med. 2019;216(10):2394–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. [DOI] [PubMed] [Google Scholar]
- 102. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Butowski N, Colman H, De Groot JF, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Quail DF, Bowman RL, Akkari L, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288):aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kees T, Lohr J, Noack J, et al. Microglia isolated from patients with glioma gain antitumor activities on poly (I:C) stimulation. Neuro Oncol. 2012;14(1):64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chiu TL, Peng CW, Wang MJ. Enhanced anti-glioblastoma activity of microglia by AAV2-mediated IL-12 through TRAIL and phagocytosis in vitro. Oncol Rep. 2011;25(5):1373–1380. [DOI] [PubMed] [Google Scholar]
- 107. Lisi L, Laudati E, Navarra P, Dello Russo C. The mTOR kinase inhibitors polarize glioma-activated microglia to express a M1 phenotype. J Neuroinflammation. 2014;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xu S, Wei J, Wang F, et al. Effect of miR-142-3p on the M2 macrophage and therapeutic efficacy against murine glioblastoma. J Natl Cancer Inst. 2014;106(8):dju162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Downey CM, Aghaei M, Schwendener RA, Jirik FR. DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2′3′-cGAMP, induces M2 macrophage repolarization. PLoS One. 2014;9(6):e99988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ohkuri T, Ghosh A, Kosaka A, et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2(12):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ager CR, Reilley MJ, Nicholas C, Bartkowiak T, Jaiswal AR, Curran MA. Intratumoral STING Activation with T-cell Checkpoint Modulation Generates Systemic Antitumor Immunity. Cancer Immunol Res. 2017;5(8):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gholamin S, Mitra SS, Feroze AH, et al. Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med. 2017;9(381):eaaf2968. [DOI] [PubMed] [Google Scholar]
- 114. Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hutter G, Theruvath J, Graef CM, et al. Microglia are effector cells of CD47-SIRPα antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci U S A. 2019;116(3):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang X, Chen W, Fan J, et al. Disrupting CD47-SIRPα axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis. 2018;39(5):689–699. [DOI] [PubMed] [Google Scholar]
- 117. Liu M, O’Connor RS, Trefely S, Graham K, Snyder NW, Beatty GL. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat Immunol. 2019;20(3):265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Morrissey MA, Williamson AP, Steinbach AM, et al. Chimeric antigen receptors that trigger phagocytosis. eLife. 2018;7:e36688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhang M, Hutter G, Kahn SA, et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes m1 polarized macrophages in vivo. PLoS One. 2016;11(4):e0153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shingu T, Ho AL, Yuan L, et al. Qki deficiency maintains stemness of glioma stem cells in suboptimal environment by downregulating endolysosomal degradation. Nat Genet. 2017;49(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. de Bruin RG, Shiue L, Prins J, et al. Quaking promotes monocyte differentiation into pro-atherogenic macrophages by controlling pre-mRNA splicing and gene expression. Nat Commun. 2016;7:10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Doucette TA, Kong LY, Yang Y, et al. Signal transducer and activator of transcription 3 promotes angiogenesis and drives malignant progression in glioma. Neuro Oncol. 2012;14(9):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hussain SF, Kong LY, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–9636. [DOI] [PubMed] [Google Scholar]
- 124. Wei J, Barr J, Kong LY, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wei J, Wang F, Kong LY, et al. miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res. 2013;73(13):3913–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yu H, Jove R. The STATs of cancer–new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105. [DOI] [PubMed] [Google Scholar]
- 127. Doucette T, Rao G, Rao A, et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol Res. 2013;1(2):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11(12):1314–1321. [DOI] [PubMed] [Google Scholar]
- 129. Kortylewski M, Yu H. Stat3 as a potential target for cancer immunotherapy. J Immunother. 2007;30(2):131–139. [DOI] [PubMed] [Google Scholar]
- 130. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51. [DOI] [PubMed] [Google Scholar]
- 131. Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169(5):2253–2263. [DOI] [PubMed] [Google Scholar]
- 132. O’Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17(4):1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10(1):39–49. [DOI] [PubMed] [Google Scholar]
- 134. Sumpter TL, Thomson AW. The STATus of PD-L1 (B7-H1) on tolerogenic APCs. Eur J Immunol. 2011;41(2):286–290. [DOI] [PubMed] [Google Scholar]
- 135. Kang FB, Wang L, Li D, Zhang YG, Sun DX. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7-H3 expression. Oncol Rep. 2015;33(1):274–282. [DOI] [PubMed] [Google Scholar]
- 136. Yao Y, Ye H, Qi Z, et al. B7-H4(B7x)-mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients. Clin Cancer Res. 2016;22(11):2778–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57(13):1458–1467. [DOI] [PubMed] [Google Scholar]
- 140. Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med. 2011;17(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Oweida AJ, Darragh L, Phan A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer [published online ahead of print March 13, 2019]. J Natl Cancer Inst. doi: 10.1093/jnci/djz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB. Arginase: a multifaceted enzyme important in health and disease. Physiol Rev. 2018;98(2):641–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pham TN, Liagre B, Girard-Thernier C, Demougeot C. Research of novel anticancer agents targeting arginase inhibition. Drug Discov Today. 2018;23(4):871–878. [DOI] [PubMed] [Google Scholar]
- 144. Pudlo M, Demougeot C, Girard-Thernier C. Arginase inhibitors: a rational approach over one century. Med Res Rev. 2017;37(3):475–513. [DOI] [PubMed] [Google Scholar]
- 145. Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75(6):639–653. [DOI] [PubMed] [Google Scholar]
- 146. West AP, Brodsky IE, Rahner C, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rodríguez-Prados JC, Través PG, Cuenca J, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605–614. [DOI] [PubMed] [Google Scholar]
- 148. Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wen ZF, Liu H, Gao R, et al. Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1. J Immunother Cancer. 2018;6(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gabrusiewicz K, Li X, Wei J, et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology. 2018;7(4):e1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Dhupkar P, Gordon N, Stewart J, Kleinerman ES. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7(6):2654–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. de Groot JF, Penas-Prado M, Mandel JJ. Window-of-opportunity clinical trial of a PD-1 inhibitor in patients with recurrent glioblastoma. Oncology JoC: American Society of Clinical Oncology. 2018:2008-. [Google Scholar]
- 155. Markovic DS, Vinnakota K, van Rooijen N, et al. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011;25(4):624–628. [DOI] [PubMed] [Google Scholar]
- 156. Jacobs VL, Landry RP, Liu Y, Romero-Sandoval EA, De Leo JA. Propentofylline decreases tumor growth in a rodent model of glioblastoma multiforme by a direct mechanism on microglia. Neuro Oncol. 2012;14(2):119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]