ABSTRACT
Findings on the effect of whole-grain consumption on inflammatory biomarkers are conflicting. This study aimed to summarize available studies on the effects of whole-grain consumption on inflammatory biomarkers in adults. Online databases including PubMed, Scopus, ISI Web of Science, and Google Scholar were searched for relevant studies published up to January 2018, using relevant keywords. We included randomized controlled trials (RCTs) investigating the effect of whole-grain foods or diets high in whole-grain foods on markers of inflammation. Studies were selected if they had a control diet low in whole grains or diets without whole grains, whether calorie restricted or not. We did not include studies that examined the effect of individual grain components, including bran or germ, or fiber-based diets. Overall, 14 RCTs, with 1238 individuals aged ≥18 y, were included. Pooling 13 effect sizes from 11 RCTs on serum C-reactive protein (CRP) concentrations, we found no significant effect of whole-grain consumption on serum CRP concentrations [weighted mean difference (WMD): −0.29 mg/L; 95% CI: −1.10, 0.52 mg/L]. However, the beneficial effects of whole-grain intake on serum CRP concentrations were observed in studies in individuals with elevated serum concentrations of CRP and studies with isocaloric diets. Combining 11 effect sizes from 10 RCTs, we found no significant effect of whole-grain consumption on serum IL-6 concentrations (WMD: −0.08 pg/mL; 95% CI: −0.27, 0.11 pg/mL). Nevertheless, we observed a significant effect of whole-grain consumption on serum IL-6 concentrations in studies in unhealthy individuals. A nonsignificant effect of whole-grain intake on circulating serum TNF-α concentrations was also seen when we summarized effect sizes from 7 RCTs (WMD: −0.06 pg/mL; 95% CI: −0.25, 0.14 pg/mL). Such a nonsignificant effect was observed for serum concentrations of plasminogen activator inhibitor-1 (PAI-1) (WMD: −3.59; 95% CI: −1.25, 8.44 kU/L). Unlike observational studies, we found no significant effect of whole-grain consumption on serum concentrations of inflammatory cytokines, including serum concentrations of CRP, IL-6, TNF-α, and PAI-1. However, beneficial effects of whole grains were found in some subgroups. Given the high between-study heterogeneity, deriving firm conclusions is difficult.
Keywords: whole grains, diet, inflammation, meta-analysis, clinical trials
Introduction
Whole grains contain high amounts of bioactive compounds including fiber, vitamins B and E, magnesium, antioxidants, and phytoestrogens (1). Greater consumption of whole grains is associated with reduced risk of mortality and morbidity (2). However, it is not clear if the effect of whole-grain intake on reduced risk of chronic diseases is mediated through the effects of whole grains on inflammation (3). The anti-inflammatory properties of components of whole grains—through influencing gene regulation and cell signaling—have been shown (4). Earlier observational studies showed an inverse association between whole-grain consumption and serum concentrations of proinflammatory cytokines (5–7); however, this relation was attenuated after controlling for other lifestyle factors (7). In contrast to overall favorable links between whole-grain intake and subclinical inflammation found in observational studies, discrepant findings were reported from clinical trials (8–16). Whereas some studies reported a beneficial effect of whole-grain consumption on inflammatory cytokines (17, 18), others failed to demonstrate a significant effect (19–21). In 2 recently published meta-analyses summarizing previous clinical trials (22, 23), a favorable effect of whole-grain consumption on systemic inflammation was observed; however, several limitations of these meta-analyses make their findings misleading (24). For instance, despite the different nature of inflammation in children and adults (25), both studies combined investigations in children and adults (22, 23). In addition, several relevant studies (11, 18, 26–28) were not included in their analyses.
Therefore, a comprehensive meta-analysis examining this issue by summarizing all available studies was lacking. Hence, the current comprehensive systematic review and meta-analysis of published randomized controlled trials (RCTs) was conducted to examine whether consumption of whole grains can ameliorate inflammation in adults.
Methods
Search strategy
We performed a systematic review and meta-analysis of RCTs that assessed the effects of whole-grain consumption on inflammatory markers. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines were followed in reporting this meta-analysis (29). We searched PubMed, the Cochrane Library, Scopus, Clarivate-Web of Science, and Google Scholar databases up to January 2019. Detailed information about specific search strategy is provided in Supplemental Table 1. In addition, a manual search was performed to complete the electronic search. No language or time restriction was applied. Two reviewers screened each report independently, and a third author was consulted in case of disagreements (provided below).
Inclusion criteria
We included RCTs investigating the effect of whole-grain foods or diets high in whole-grain foods on markers of inflammation. Studies were selected if they had a control diet low in whole grains or diets without whole grains, whether they were calorie restricted or not. If in a given study several interventions had been performed, we included that study only when the effect of whole grains was separately reported. Moreover, we excluded studies that examined the effect of individual grain components, including bran or germ, or fiber-based diets. Studies with 3 eligible arms were considered as 2 separate studies.
Exclusion criteria
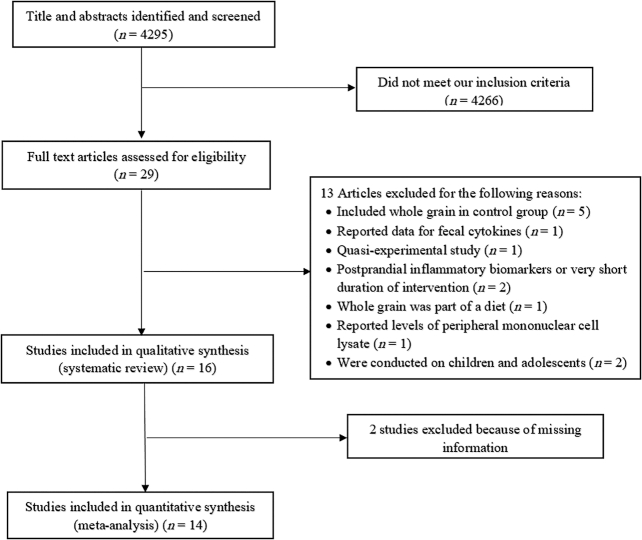
We excluded letters, comments, short communications, reviews, meta-analyses, ecological studies, and animal studies. We found 4295 publications in our initial search, from which 4238 studies were identified as unrelated after reviewing for titles and abstracts. After excluding cohort (n = 7) and cross-sectional (n = 6) studies, 30 relevant clinical trials remained for further investigation. Of these 30 RCTs, 5 studies, in which individuals in the control group had also taken whole grains, were excluded (28, 30–33). The study of Vanegas et al. (34) was excluded because of reporting data for fecal cytokines instead of blood cytokines. One quasiexperimental study that had no control group was also excluded (35). Two studies were excluded because they had assessed the effects of whole-grain consumption on postprandial inflammatory biomarkers or the duration of intervention was very short (<3 d) (5, 36). One study, in which whole-grain foods were only part of a Mediterranean-style diet, was not included (37). The study of Giacco et al. (6) was excluded because only the final values of inflammatory cytokines were reported. Due to lack of reporting the baseline values, we were not able to compute mean differences of cytokines. We also did not include the study of Meydani et al. (16) because it was published only as an abstract in a journal supplement, without reporting required information. Both Giacco et al. (6) and Meydani et al. (16) were contacted several times to obtain required information, but we got no response. We also excluded the study of Wang et al. (27) due to reporting concentrations of peripheral mononuclear cell lysate rather than serum concentrations of TNF-α. Two clinical trials in children and adolescents were also excluded (38, 39). Finally, 16 studies remained for inclusion in the systematic review, of which 14 were included in the meta-analysis. The flow diagram of the study selection is provided in Figure 1. Among these 14 RCTs, 13 studies had provided data for serum concentrations of CRP (8, 10–15, 17–21, 26), 10 for serum concentrations of IL-6 (8–12, 14, 15, 17, 19, 20), 7 for serum concentrations of TNF-α (9–12, 15, 17, 19), and 3 for serum concentrations of plasminogen activator inhibitor-1 (PAI-1) (10, 20, 21). Data on other inflammatory biomarkers—IL-1β (n = 2), IL-10 (n = 2), IL-8, (n = 1), and IL-1RA (n = 2)—were insufficient for a meta-analysis.
FIGURE 1.
Flow diagram of study selection process.
Data extraction
We collected data on first author's name, year of publication, mean age ± SD of participants in each group, health status of study subjects, sample size, number and gender of participants in each group, length of intervention (weeks), study design (parallel, crossover), types and amounts of whole grains, control diet, and feeding status (feeding or free-living) (Table 1). When a cytokine concentration was reported in different units, we converted them to the most frequently used unit.
TABLE 1.
The effect of whole-grain intake on inflammatory biomarkers in adults aged ≥18 y1
| Diet type | Outcome2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year (ref) | Participants, n | Age,3 y | Health condition | Feeding status | Design | Intervention | Control | Duration, wk | Intervention (mean ± SD) | Control (mean ± SD) | Adjust/matching4 | Risk of bias5 |
| Giacco et al. 2010 (6) | F: 12, M: 3 | 54.5 ± 7.6 | Overweight and obesity | Feeding | RCT, crossver | Whole-wheat products | Refined wheat products | 3 | CRP; post: 1.8 ± 2.3 | CRP; post: 2.9 ± 4.1 | — | L/U/L/U |
| Kristensen et al. 2012 (8) | F: 72 WG: 38, RG: 34 | WG: 59.1 ± 5.6; RG: 60.3 ± 5.3 | Overweight and obesity | Feeding | RCT, parallel | Whole-wheat; WG = 105 g/d, hypocaloric diet | Refined wheat; WG = 0, hypocaloric diet | 12 | CRP; pre: 0.95 ± 0.30, post: 0.85 ± 0.30 | CRP; pre: 1.00 ± 0.29, post: 1.07 ± 0.29 | — | L/U/H/L |
| IL-6: pre: 2.45 ± 0.16, post: 2.65 ± 1.57 | IL-6; pre: 1.70 ± 0.17, post: 1.83 ± 0.16 | |||||||||||
| Vitaglione et al. 2015 (9) | F: 68 WG: 36, RG: 32 | WG: 40 ± 2.0; RG: 37 ± 2.0 | Overweight and obesity | Feeding | RCT, parallel | 100% WG wheat product; WG = 70 g/d | Refined wheat products | 8 | IL-6; pre: 57.50 ± 7.50, post: 46.9 ± 4.00 | IL-6; pre: 65.50 ± 7.50, post: 60.20 ± 7.20 | — | L/L/L/L |
| TNF-α; pre: 341.90 ± 25.50, post: 243.0 ± 26.0 | TNF-α; pre: 321.90 ± 52.10, post: 329.80 ± 50.60 | |||||||||||
| Katcher et al. 2008 (10) | F: 25, M: 25 WG: 25, CON: 25 | WG: 45.4 ± 8; CON: 46.6 ± 9.7 | Obesity with metabolic syndrome | Nonfeeding | RCT, parallel | Different WGs; WGs = 4, 5, 6, or 7 servings/d; hypocaloric diet | Nonconsumption of WG foods based on a list of WG foods; hypocaloric diet | 12 | CRP; change: −2.4 ± 5.10 | CRP; change: 0.2 ± 2.9 | 2,3 | L/U/L/L |
| TNF-α; change: −0.04 ± 0.30 | TNF-α; change: 0.10 ± 0.20 | |||||||||||
| IL-6; change: −0.90 ± 3.60 | IL-6; change: −0.1 ± 0.4 | |||||||||||
| Harris Jackson et al. 2014 (11) | F: 25, M: 25 WG: 25, RG: 25 | WG: 46.4 ± 5.9; RG: 45.8 ± 6 | Overweight and obesity with metabolic syndrome | Feeding | RCT, parallel | Different WG foods; isocaloric diet for the first 6 wk, followed by a hypocaloric diet for the second 6 wk; WG = 187 g/d | RG foods; isocaloric diet for the first 6 wk, followed by a hypocaloric diet for the second 6 wk; WG = 0 g/d | 12 | CRP; pre: 3.0 ± 1.93, post: 0.60 ± 0.50 | CRP; pre: 2.10 ± 1.26, post: 0.60 ± 0.40 | 1,2,3 | L/U/L/U |
| IL-6; pre: 1.70 ± 1.26, post: 0.10 ± 0.20 | IL-6; pre: 1.70 ± 0.74, post: 0.10 ± 0.20 | |||||||||||
| TNF-α; pre: 1.20 ± 0.22, post: 0.00 ± 0.10 | TNF-α; pre: 1.40 ± 0.37, post: −0.10 ± 0.10 | |||||||||||
| Roager et al. 2019 (12) | F: 32, M: 18 WG: 50, RG: 50 | 20–65 | At risk of metabolic syndrome | Feeding | RCT, crossover | WG products; WG = 157.9 ± 35.0 | RG products; WG = 6.0 ± 4.8 | 8 | CRP; pre: 6.30 ± 14, post: 4.20 ± 6.80 | CRP; pre: 3.10 ± 2.60, post: 5.0 ± 5.80 | 1,2,4 | L/L/L/L |
| TNF-α; pre: 1.70 ± 0.90, post: 1.70 ± 0.90IL-6; pre: 1.60 ± 1.02, post: 1.40 ± 1.10 | TNF-α; pre: 1.70 ± 0.80, post: 1.70 ± 0.08 | |||||||||||
| IL-6; pre: 1.20 ± 0.70, post: 2.0 ± 2.0 | ||||||||||||
| Ross et al. 2011 (13) | F: 11, M: 6 WG: 17, RG: 17 | WG: 36.5 ± 4.2; CON: 36.5 ± 4.2 | Healthy | Feeding | RCT, crossover | Different WG foods; WG = 151 g/d | Different RG foods | 2 | CRP; pre, females: 3.0 ± 1.0, males: 2.20 ± 0.90 | CRP; pre, females: 5.60 ± 2.0, males: 1.70 ± 0.20 | 1,2,3,5,7 | L/L/L/L |
| Post: both sexes: 3.38 ± 0.92 | Post: both sexes: 3.01 ± 0.90 | |||||||||||
| Tighe et al. 2010 (14) | F: 102, M: 104 WG: 73, RG: 63 | WG: 52.1 ± 0.9; RG: 51.8 ± 0.8 | Healthy | Feeding | RCT, parallel | Group 1: 3 servings of whole-wheat foods (70–80 g WG bread + 30–40 g WG cereals) | Refined cereals and white bread | 12 | Group 1: CRP; pre: 3.30 ± 1.03, post: 0.90 ± 0.88Group 1: IL-6; pre: 1.20 ± 1.03, post: 1.40 ± 1.07 | CRP; pre: 1.40 ± 1.25, post: 1.10 ± 1.33IL-6; pre: 1.30 ± 1.14, post: 1.40 ± 1.25 | 1,2,3,5 | L/L/L/L |
| Group 2: 1 serving of whole-wheat foods and 2 servings of oats | ||||||||||||
| Group 2: CRP; pre: 1.0 ± 0.74, post: 1.0 ± 1.07 | ||||||||||||
| Group 2: IL-6; pre: 1.10 ± 0.92, post: 1.10 ± 0.88 | ||||||||||||
| Vetrani et al. 2016 (15) | F: 24, M: 16 WG: 21, RG: 19 | WG: 57.2 ± 1.9; RG: 58.4 ± 1.6 | Metabolic syndrome | Feeding | RCT, parallel | WG products plus a small portion of endosperm rye bread | Commercial products based on refined cereals | 12 | CRP; pre: 2.52 ± 0.50, post: 2.44 ± 0.50 | CRP; pre: 2.27 ± 0.40, post: 2.39 ± 0.40 | — | L/U/H/L |
| TNF-α; pre: 1.71 ± 0.60, post: 1.50 ± 0.60 | TNF-α; pre: 1.07 ± 0.40, post: 1.31 ± 0.50 | |||||||||||
| IL-6: pre: 1.84 ± 0.20, post: 2.23 ± 0.30 | IL-6; pre: 1.69 ± 0.30, post: 1.70 ± 0.30 | |||||||||||
| Meydani et al. 2016 (16) | F: 49, M: 32 WG: 41, RG: 40 | 40–65 | Healthy | Feeding | RCT, parallel | Different WGs: WG = 207 ± 39 | Different RGs: WG = 0 | 6 | CRP | CRP | — | — |
| de Mello et al. 2011 (17) | F: 34, M: 34 WG: 34, RG: 34 | WG: 58 ± 8; RG: 59 ± 7 | Overweight and obesity | Nonfeeding | RCT, parallel | Consumption of usual cereal products with ≥50% of their composition from a WG source plus WG oat snack bars once per day | Were asked to replace the breads with refined wheat breads, and other cereal products with low-fiber products | 12 | CRP; pre: 1.50 ± 1.70, post: 1.20 ± 0.92 | CRP; pre: 2.86 ± 2.96, post: 2.34 ± 1.57 | 1,2,3,6,5 | L/L/H/L |
| TNF-α; pre: 0.70 ± 0.51, post: 0.60 ± 0.48 | TNF-α; pre: 0.6.0 ± 0.40, post: 0.50 ± 0.44 | |||||||||||
| IL-6; pre: 1.40 ± 1.22, post: 1.50 ± 1.11 | IL-6; pre: 1.30 ± 1.03, post: 1.40 ± 1.07 | |||||||||||
| Kondo et al. 2017 (18) | F: 10, M: 18 WG: 14, RG: 14 | 40–80 | Type 2 diabetes | Feeding | RCT, parallel | Brown rice; 10 of 21 meals/wk | White rice; 10 of 21 meals/wk | 8 | CRP; pre: 0.09 ± 0.12, post: 0.05 ± 0.05 | CRP; pre: 0.04 ± 0.03, post: 0.05 ± 0.06 | 1,2,3,5 | L/L/L/U |
| Ampatzoglou et al. 2016 (19) | F: 21, M: 12 WG: 33, CON: 33 | 48.8 ± 1.1 | Healthy | Feeding | RCT, crossover | Diet high in WG (>80 g/d) | Diet low in WG (<16 g/d, RG diet) | 6 | CRP; pre: 2.20 ± 0.50, post: 1.60 ± 0.40 | CRP; pre: 1.70 ± 0.30, post: 1.80 ± 0.30 | — | L/U/L/U |
| TNF-α; pre: 10.80 ± 0.40, post: 10.80 ± 0.60 | TNF-α; pre: 10.50 ± 0.50, post: 10.70 ± 0.50 | |||||||||||
| IL-6; pre: 1.20 ± 0.20, post: 1.20 ± 0.10 | IL-6; pre: 1.30 ± 0.20, post: 1.40 ± 0.20 | |||||||||||
| Andersson et al. 2007 (20) | F: 22, M: 8 WG: 30, RG: 30 | 59 ± 5 | Overweight | Feeding | RCT, crossover | Different WGs; WG = 112 g/d | Different RGs; RG = 111 g | 6 | CRP; pre: 2.03 ± 1.62, post: 2.38 ± 2.29 | CRP; pre: 2.86 ± 2.96, post: 2.34 ± 1.57 | — | L/L/L/L |
| IL-6; pre: 14.80 ± 32.20, post: 15.20 ± 33.20 | IL-6; pre: 15.90 ± 32.40, post: 15.80 ± 30.90 | |||||||||||
| Brownlee et al.2010 (21) | F: 133, M: 133 Group 1: 85, Group 2: 81, CON: 100 | Int 1: 45.9 ± 10.1; Int 2: 45.7 ± 9.9; CON: 45.6 ± 1.0 | Overweight | Feeding | RCT, parallel | Group 1: 60 g WG/d for 16 wk, Group 2: 60 g WG/d for 8 wk followed by 120 g WG/d for 8 wk | No dietary changes; habitual diet (WG ≤30 g/d) | 16 | Group 1; CRP; pre: 2.40 ± 9.90, 16 wk: 3.10 ± 4.30 | CRP; pre: 2.40 ± 2.30, 8 wk: 2.70 ± 2.80, 16 wk: 2.90 ± 3.50 | 1,2,3 | L/U/L/U |
| Group 2; CRP; pre: 3.20 ± 4.60, 8 wk: 3.50 ± 7.20, 16 wk: 3.2 ± 5.90 | ||||||||||||
| Kirwan et al. 2016 (26) | F: 27, M: 6, WG: 33, RG: 33 | 39 ± 7.0 | Overweight and obesity | Feeding | RCT, crossover | WG diet; WG = 93 ± 19 | RG diet, WG = 0 | 8 | CRP; change: 0.80 ± 2.74 | CRP; change: −2.30 ± 3.56 | 1,2,4,5,9 | L/L/L/H |
| TNF-α; pre: 71.0 ± 36.0, post: 55.0 ± 24.0 | TNF-α; pre: 62.0 ± 38.0, post: 51.0 ± 19.0 | |||||||||||
CON, control; CRP, C-reactive protein; F, female; H, high risk; Int, intervention; L, low risk; RCT, randomized controlled trial; ref, reference; RG, refined grain; U, unclear risk; WG, whole grain.
CRP reported as mg/L, IL-6 as pg/mL, TNF-α as pg/mL.
Mean ± SD (all such values).
Age (1), sex (2), BMI (3), body weight (4), baseline measurements (5), fasting plasma glucose (6), treatment order (7), change in body fat (8), fiber intake (9).
Risk of bias was assessed as: 1) Sequence generation and allocation concealed? 2) All subjects received the same attention? 3) Was analysis in an intent-to-treat population? and 4) Was the article selective in outcome reporting? Studies were considered as “high risk” if they contained methodological flaws that could have affected the results, “low risk” if the flaw was deemed inconsequential, and “unclear risk” if information was insufficient to determine. In the current meta-analysis, studies that were “low risk” for all domains were considered as high quality or having low risk of bias.
Risk-of-bias assessment
Each study was assessed for risk of bias by 2 independent authors using the Cochrane Risk of Bias Assessment tool. Domains of assessment included: 1) described the method used to generate the allocation sequence?, 2) described the method used to conceal the allocation sequence?, 3) was the analysis in an intent-to-treat population?, and 4) was the article selective in its reporting of the outcome? (40). Studies were considered as “high risk” if they contained methodological flaws that could have affected the results, “low risk” if the flaw was deemed inconsequential, and “unclear risk” if information was insufficient to determine. In the current meta-analysis, studies that were “low risk” for all domains were considered as high quality or having a low risk of bias (Table 1). Disagreements were resolved by consensus.
Statistical analysis
The mean differences in changes of cytokine concentrations, comparing whole-grain and control groups, were used to calculate the overall effect sizes. When mean differences were not reported, we calculated them by considering changes in each cytokine concentration throughout the study. We converted reported SEs, 95% CIs, and IQRs to SDs. For 2 studies that had 2 different arms of intervention, we considered each arm of intervention as a separate study. In the study of Brownlee et al. (21), there were 2 intervention arms with different doses of whole grains: in 1 arm the intervention was 60 g/d of whole grains for 16 wk, whereas in the other arm it was 120 g/d of whole grains for 8 wk. After consultation, we decided to include this study in the meta-analysis as 2 separate studies. Similarly, the study of Tighe et al. (14) had 2 arms of intervention with different types of whole grains. When we extracted data from the study of Kondo et al. (18), we found that the values reported for serum CRP concentrations were 10 times lower than those in other studies. Because we got no answer after communicating with the authors via e-mail, we resolved this disagreement by deciding to multiply this value by 10 and then included the findings in our meta-analysis.
The overall effect size was calculated using a random-effects model, which takes between-study variation into account. Cochran Q test and I2 statistic were used to assess between-study heterogeneity. In addition, we used subgroup analysis to detect probable sources of heterogeneity with the use of a fixed-effects model. These subgroups included mean baseline serum concentrations of CRP (<3 mg/L compared with ≥3 mg/L), participants’ health condition (healthy compared with unhealthy individuals), duration of intervention (≥8 wk compared with <8 wk), study design (parallel compared with crossover), participants’ compliance, hypocaloric compared with isocaloric diets, adjustment for baseline levels of the outcome variable, type of intervention (whole-grain–containing diet compared with specific types of whole-grain foods), and risk of bias (low risk compared with others) (Table 2). In these analyses, studies conducted in healthy individuals were combined with those in overweight or obese people, and the term “healthy subgroup” was used for these studies. Other remaining studies were defined as the “unhealthy subgroup.” When the intervention was based on a mix of different types of whole-grain foods, we called it “whole-grain–containing diets.” When specific types of whole-grain foods like brown rice, whole wheat, and so forth were used as intervention, we defined these in the “specific types of whole-grain foods” category. Furthermore, we applied metaregression to determine the contribution of whole-grain doses to between-study heterogeneity (Table 3). Sensitivity analysis was used to explore the extent to which inferences might depend on a particular study or group of studies. Publication bias was examined by visual inspection of funnel plots and the application of the Egger and Begg tests. All statistical analyses were conducted using Stata, version 11.2 (StataCorp). P values <0.05 were considered statistically significant.
TABLE 2.
Subgroup analysis based on fixed-effects models for the effects of whole-grain consumption on inflammatory biomarkers in adults aged ≥18 y
| Effect size, n | Mean (95% CI) | P-within | I 2, % | P-between | |
|---|---|---|---|---|---|
| Whole-grain consumption on serum concentrations of C-reactive protein | |||||
| Overall | 15 | −0.29 (−1.10, 0.52) | 0.48 | 97.0 | |
| Baseline concentrations | <0.001 | ||||
| Normal (<3 mg/L) | 8 | 0.04 (−0.12, 0.20) | 0.619 | 84.2 | |
| High (≥3 mg/L) | 7 | −1.10 (−1.30, −0.90) | <0.001 | 98.2 | |
| Health condition | 0.006 | ||||
| Healthy | 10 | −0.35 (−0.48, −0.22) | <0.001 | 97.9 | |
| Unhealthy | 5 | −0.97 (−1.39, −0.55) | <0.001 | 77.2 | |
| Duration of intervention | <0.001 | ||||
| <8 wk | 6 | 0.94 (0.67, 1.22) | <0.001 | 95.9 | |
| ≥8 wk | 9 | −0.76 (−0.90, −0.62) | <0.001 | 96.5 | |
| Study design | <0.001 | ||||
| Parallel | 10 | −0.74 (−0.88, −0.60) | <0.001 | 96.0 | |
| Crossover | 5 | 1.47 (1.15, 1.80) | <0.001 | 95.2 | |
| Calorie restriction | 0.265 | ||||
| Hypocaloric diet | 3 | −0.22 (−0.57, 0.14) | 0.232 | 53.3 | |
| Isocaloric diet | 12 | −0.43 (−0.57, −0.30) | <0.001 | 97.6 | |
| Type of intervention | 0.48 | ||||
| Whole-grain–containing diet1 | 12 | −0.33 (−1.36, 0.70) | 0.524 | 97.6 | |
| Specif ic whole-grain product foods2 | 3 | −0.29 (−1.10, 0.52) | 0.083 | 0.0 | |
| Adjustment for baseline values | 0.013 | ||||
| Nonadjusted | 9 | −0.10 (−0.37, 0.17) | 0.462 | 91.2 | |
| Adjusted | 6 | −0.49 (−0.63, −0.35) | <0.001 | 97.6 | |
| Risk of bias | <0.001 | ||||
| Others | 10 | 0.11 (−0.10, 0.31) | 0.316 | 94.5 | |
| Low | 5 | −0.70 (−0.86, −0.54) | <0.001 | 98.5 | |
| Whole-grain consumption on serum concentrations of IL-6 | |||||
| Overall | 11 | −0.08 (−0.27, 0.11) | 0.399 | 67.5 | |
| Baseline concentrations | 0.84 | ||||
| Normal (<4.4 pg/mL) | 10 | −0.03 (−0.11, 0.05) | 0.445 | 70.7 | |
| High (≥4.4 pg/mL) | 1 | 0.50 (−4.67, 5.67) | 0.850 | 0.0 | |
| Health condition | 0.026 | ||||
| Healthy | 7 | −0.00 (−0.09, 0.08) | 0.935 | 0.0 | |
| Unhealthy | 4 | −0.33 (−0.61, −0.06) | 0.019 | 85.2 | |
| Duration of intervention | <0.001 | ||||
| <8 wk | 4 | −0.62 (−0.92, −0.32) | <0.001 | 59.2 | |
| ≥8 wk | 7 | 0.01 (−0.07, 0.10) | 0.733 | 21.2 | |
| Study design | <0.001 | ||||
| Parallel | 8 | 0.01 (−0.07, 0.10) | 0.736 | 14.2 | |
| Crossover | 3 | −0.62 (−0.92, −0.32) | <0.001 | 67.5 | |
| Calorie restriction | 0.413 | ||||
| Hypocaloric diet | 3 | 0.07 (−0.19, 0.32) | 0.594 | 0.0 | |
| Isocaloric diet | 8 | −0.04 (−0.13, 0.04) | 0.326 | 75.4 | |
| Type of intervention | 0.399 | ||||
| Whole-grain–containing diet | 8 | −0.14 (−0.37, 0.09) | 0.243 | 75.9 | |
| Specific whole-grain product foods | 3 | 0.10 (−0.16, 0.36) | 0.465 | 67.5 | |
| Adjustment for baseline values | 0.44 | ||||
| Nonadjusted | 8 | −0.05 (−0.17, 0.27) | 0.659 | 0.0 | |
| Adjusted | 3 | −0.04 (−0.13, 0.04) | 0.322 | 81.5 | |
| Risk of bias | 0.355 | ||||
| Others | 6 | 0.03 (−0.12, 0.18) | 0.70 | 0.0 | |
| Low | 5 | −0.06 (−0.15, 0.04) | 0.25 | 84.3 | |
| Whole-grain consumption on serum concentrations of TNF-α | |||||
| Overall | 7 | −0.06 (−0.25, 0.14) | 0.56 | 73.7 | |
| Baseline concentrations | 0.67 | ||||
| Normal (<2.3 pg/mL) | 5 | −0.03 (−0.10, 0.04) | 0.35 | 26.8 | |
| High (≥2.3 pg/mL) | 2 | −0.24 (−1.22, 0.74) | 0.63 | 94.2 | |
| Health condition | 0.26 | ||||
| Healthy | 3 | −0.00 (−0.09, 0.09) | 0.96 | 88.5 | |
| Unhealthy | 4 | −0.08 (−0.19, 0.03) | 0.14 | 27.8 | |
| Duration of intervention | 0.853 | ||||
| <8 wk | 3 | −0.01 (−0.24, 0.21) | 0.91 | 88.5 | |
| ≥8 wk | 4 | −0.03 (−0.11, 0.04) | 0.33 | 44.3 | |
| Study design | 0.83 | ||||
| Parallel | 5 | −0.04 (−0.11, 0.04) | 0.33 | 82.3 | |
| Crossover | 2 | −0.01 (−0.24, 0.21) | 0.92 | 0.0 | |
| Calorie restriction | 0.28 | ||||
| Hypocaloric diet | 2 | −0.09 (−0.22, 0.04) | 0.15 | 56.3 | |
| Isocaloric diet | 5 | −0.01 (−0.09, 0.07) | 0.82 | 79.4 | |
| Type of intervention | 0.56 | ||||
| Whole-grain–containing diet | 5 | −0.05 (−0.14, 0.04) | 0.25 | 13.8 | |
| Specific whole-grain product foods | 2 | −50.25 (−154.84, 54.33) | 0.346 | 94.2 | |
| Adjustment for baseline values | 0.176 | ||||
| Nonadjusted | 6 | −0.39 (−0.91, 0.13) | 0.143 | 76.6 | |
| Adjusted | 1 | 0.03 (−0.10, 0.04) | 0.444 | 18.5 | |
| Risk of bias | 0.78 | ||||
| Others | 5 | −0.04 (−0.11, 0.04) | 0.32 | 27.2 | |
| Low | 2 | −0.00 (−0.23, 0.23) | 0.98 | 94.2 | |
Whole-grain–containing diet: contained a mix of different types of whole-grain foods.
Specific types of whole-grain foods: limited to specific foods like brown rice, whole wheat, etc.
TABLE 3.
Findings from metaregression on the effects of whole-grain consumption on serum inflammatory biomarkers by considering dose of whole grains in adults aged ≥18 y
| Effect size, n | β (95% CI) | I 2 residual | P value | |
|---|---|---|---|---|
| C-reactive protein | 11 | −0.002 (−0.044, 0.040) | 34.29 | 0.90 |
| IL-6 | 8 | 0.0008 (−0.015, 0.015) | 0 | 0.99 |
| TNF-α | 4 | 0.0001 (−0.034, 0.035) | 2.19 | 0.98 |
Results
Findings from the systematic review
Characteristics of the included studies are shown in Table 1. These trials were published between 2002 and 2018. A total of 1334 participants, aged ≥18 y, were included in these studies. Most studies were conducted on both sexes, but 2 studies were performed on women only (8, 9). Three studies were performed in the United States (10, 11, 16), 1 in an Asian population (18), and the remainder in Europeans (6, 8, 9, 12–15, 17, 19–21, 26). Of 16 clinical trials, 6 had a crossover design (6, 12, 15, 19, 20, 26) and the others were parallel (8–11, 14, 16–18, 21).
Seven studies had enrolled individuals with overweight or obesity (6, 8, 9, 17, 20, 21, 26), 4 had recruited healthy individuals (13, 14, 16, 19), 4 studies were in persons with the metabolic syndrome (10–12, 15), and 1 was in patients with type 2 diabetes (18). Three studies were hypocaloric (8, 10, 11), and the remainder were isocaloric with energy requirements (9, 12–15, 17–21, 26). Ten studies provided whole-grain–containing diets (10, 12–15, 17, 19–21, 26), whereas the other studies were limited to specific types of whole-grain foods (8, 9, 18). All included studies were conducted in a free-living environment. Fourteen studies were feeding trials, in which whole grains were provided to study participants (6, 8, 9, 11–16, 18–21, 26). Two studies were behavioral counseling studies in which participants received dietary advice on whole-grain intake (10, 17). Except for 2 studies (14, 18), all reported good compliance of subjects with the treatment. The duration of intervention in included clinical trials varied from 2 to 16 wk. Five studies had controlled their analysis for baseline concentrations of inflammatory biomarkers (13, 14, 17, 18, 26). Five studies had a low risk of bias in all aspects of the Cochrane method (9, 12–14, 21), whereas others had a high risk of bias or unclear risk in at least 1 aspect of this method (6, 8, 10, 11, 15–19, 21, 26).
Among 15 studies of serum CRP concentrations, 4 studies reported a significant reduction in serum CRP concentrations following whole-grain intake (10, 12, 17, 18), whereas others revealed no significant change (6, 8, 11, 13–16, 19–21, 26). In terms of serum IL-6 concentrations, only 2 clinical trials reported a significant reduction in the whole-grain group compared with the control group (9, 19), whereas others failed to find any significant effect (8, 10–12, 15, 17, 19, 20). Of 7 studies that examined the effects of whole-grain consumption on serum TNF-α, only 1 study reported a beneficial effect (9) whereas the remaining 6 studies revealed no significant change in serum TNF-α concentrations after whole-grain consumption, compared with the control group (10–12, 15, 17, 19). With regard to serum concentrations of PAI-1, 2 studies reported a lowering effect in the whole-grain group compared with the control group (10, 21), whereas 1 study demonstrated a positive effect (20).
Findings from the meta-analysis
Of the 16 studies in our systematic review, 14 clinical trials were included in the current meta-analysis (8–15, 17–21, 26). We considered studies that administered whole grains in the framework of a diet as well as those that administered whole-grain products individually. In addition, studies that compared brown rice with white rice, and whole-wheat products with refined-wheat products, were considered as specific whole-grain foods in the analysis. These studies included 1238 individuals aged ≥18 y.
The effect of whole grains on serum CRP concentrations
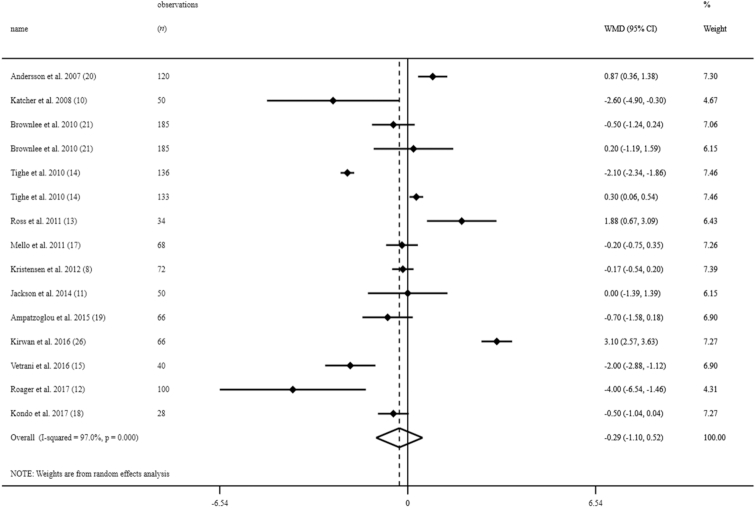
Overall, 15 effect sizes from 13 clinical trials in the overall population of 1170 individuals were included in this analysis (8, 10–15, 17–21, 26). Two clinical trials had a third arm with a different dose of whole grains (21, 14). We considered each arm as a separate study. Combining effect sizes from 13 studies, we found no significant effect of whole-grain consumption on serum CRP concentrations [weighted mean difference (WMD): −0.29 mg/L; 95% CI: −1.10, 0.52 mg/L] (Figure 2). However, between-study heterogeneity was significant (I2: 97.0; P < 0.001). When we excluded the study of Kondo et al. (18), which had a very different mean for serum CRP concentrations compared with other publications, the findings did not change (WMD: −0.28 mg/L; 95% CI: −1.15, 0.59 mg/L).
FIGURE 2.
Forest plots for the effect of whole-grain consumption on serum CRP concentrations in adults aged ≥18 y, expressed as mean differences between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. CRP, C-reactive protein; WMD, weighted mean difference.
In the subgroup analysis, we found that mean baseline serum concentrations of CRP, duration of intervention, study design, hypocaloric compared with isocaloric diets, adjustment for baseline concentrations, and risk of bias could explain between-study heterogeneity. Whole-grain intake resulted in a significant reduction in serum CRP concentrations in studies in individuals with elevated serum concentrations of CRP (WMD: −1.10 mg/L; 95% CI: −1.30, −0.90 mg/L), in studies performed in healthy (WMD: −0.35 mg/L; 95% CI: −0.48, −0.22 mg/L) and unhealthy individuals (WMD: −0.97 mg/L; 95% CI: −1.39, −0.59 mg/L), as well as those that had a duration of intervention eight weeks or more (WMD: −0.76 mg/L; 95% CI: −0.90, −0.52 mg/L). In addition, the beneficial effects of whole-grain intake on serum CRP concentrations were observed in parallel RCTs (WMD: −0.74 mg/L; 95% CI: −0.88, −0.60 mg/L), studies with isocaloric diets (WMD: −0.43 mg/L; 95% CI: −0.57, −0.30 mg/L), those that adjusted for baseline serum concentrations of CRP (WMD: −0.48 mg/L; 95% CI: −0.63, −0.34 mg/L), and studies with a low risk of bias (WMD: −0.70 mg/L; 95% CI: −0.86, −0.54 mg/L). However, we did not observe any significant effect in studies with hypocaloric diets (WMD: −0.22 mg/L; 95% CI: −0.57, 0.14 mg/L). Combining effect sizes from crossover studies (WMD: 1.47 mg/L; 95% CI: 1.15, 1.80 mg/L) as well as those with a duration of intervention <8 wk (WMD: 0.94 mg/L; 95% CI: 0.67, 1.22 mg/L) revealed a significant increase in serum CRP concentrations following whole-grain intake.
The sensitivity analysis revealed that exclusion of any single study did not alter the overall effect of whole-grain consumption on serum CRP concentrations (range of summary 95% CI: −1.29, 0.69). In addition, visual inspection of funnel plots revealed no evidence of substantial publication bias (Supplemental Figure 1A). When we excluded 2 studies in which participants received only dietary advice on whole-grain intake (10, 17), no change in findings was seen (WMD: −0.18 mg/L; 95% CI: −1.07, 0.71 mg/L). Moreover, after excluding 2 studies with poor compliance (14, 18), the results did not significantly change (WMD: −0.15 mg/L; 95% CI: −1.05, 0.75 mg/L).
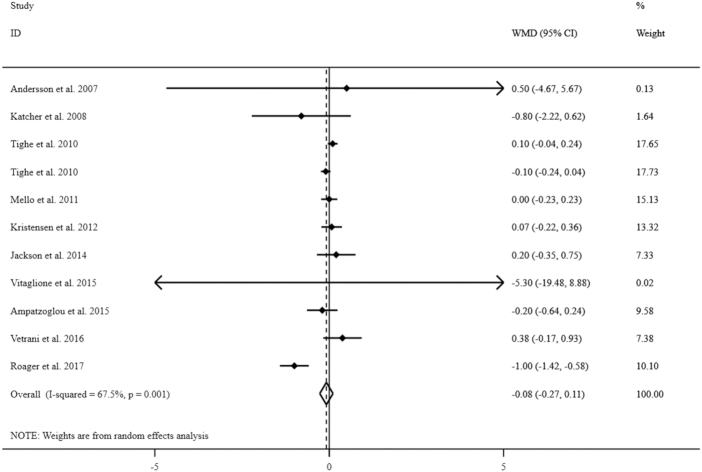
The effect of whole grains on serum IL-6 concentrations
Ten clinical trials (8–12, 14, 15, 17, 19, 20), including 903 people, were included in this meta-analysis. However, due to the study of Tighe et al. (14) having 2 intervention arms we had 11 effect sizes. Combining these effect sizes, we found no significant effect of whole-grain consumption on serum IL-6 concentrations (WMD: −0.08 pg/mL; 95% CI: −0.27, 0.11 pg/mL) (Figure 3). Significant between-study heterogeneity was found (I2: 67.5; P = 0.001). When we excluded the studies on specific whole-grain foods from the analysis, the findings did not change (WMD: −0.08 pg/mL; 95% CI: −0.27, 0.11 pg/mL).
FIGURE 3.
Forest plots for the effect of whole-grain consumption on serum IL-6 concentrations in adults aged ≥18 y, expressed as mean differences between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference.
We performed subgroup analysis based on mean baseline serum concentrations of IL-6 (<4.4 pg/mL compared with ≥4.4 pg/mL), participants’ health condition (healthy compared with unhealthy individuals), duration of intervention (≥8 wk compared with <8 wk), study design (parallel compared with crossover), hypocaloric compared with isocaloric diets, type of intervention (whole-grain–containing diet compared with specific types of whole-grain foods), adjustment for baseline concentrations, and risk of bias (low-risk compared with others) to find the possible sources of between-study heterogeneity. We found that participants’ health conditions, duration of intervention, and study design explained between-study heterogeneity. We observed a significant effect of whole-grain consumption on serum IL-6 concentrations in studies in unhealthy individuals (WMD: −0.33 pg/mL; 95% CI: −0.61, −0.03 pg/mL), those with <8 wk duration of intervention (WMD: −0.62 pg/mL; 95% CI: −0.92, −0.32 pg/mL), and studies with crossover design (WMD: −0.62 pg/mL; 95% CI: −0.92, −0.32 pg/mL). However, we did not observe any significant effect in studies with duration ≥8 wk (WMD: 0.01 pg/mL; 95% CI: −0.07, 0.10 pg/mL).
Based on findings from sensitivity analysis, no single study influenced the final findings on the effect of whole-grain consumption on serum IL-6 concentrations (range of summary 95% CI: −0.35, 0.16). Although a moderate asymmetry was visually seen in the funnel plot (Supplemental Figure 1B), the Egger regression test (P = 0.74) rejected our hypothesis about the presence of substantial publication bias. When we excluded 2 studies in which participants received only dietary advice on whole-grain intake (10, 17), no significant change in findings was observed (WMD: −0.08 pg/mL; 95% CI: −0.31, 0.14 pg/mL). Without 2 studies with poor compliance, the results did not alter much (WMD: −0.13 pg/mL; 95% CI: −0.46, 0.19 pg/mL).
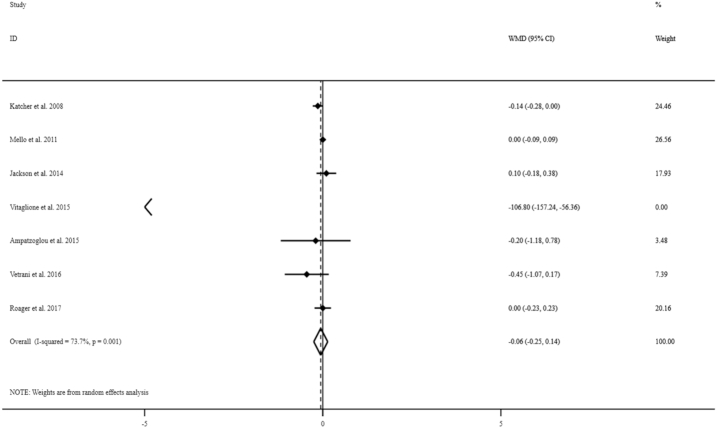
The effect of whole grains on serum TNF-α concentrations
Overall, 7 studies with a total sample of 442 people were considered to examine the effect of whole-grain consumption on serum TNF-α concentrations (9–12, 15, 17, 19). Combining estimates of these studies, we found no significant effect of whole-grain consumption on serum TNF-α concentrations (WMD: −0.06 pg/mL; 95% CI: −0.25, 0.14 pg/mL) (Figure 4). There was evidence of high between-study heterogeneity (I2: 73.7; P = 0.001). When we excluded the studies on specific whole-grain foods, our findings did not alter (WMD: −0.04 pg/mL; 95% CI: −0.12, 0.04 pg/mL); however, between-study heterogeneity was reduced (I2: 10.4, P = 0.34).
FIGURE 4.
Forest plots for the effect of whole-grain consumption on serum TNF-α concentrations in adults aged ≥18 y, expressed as mean differences between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference.
We conducted subgroup analysis based on mean baseline serum concentrations of TNF-α (<2.3 pg/mL compared with ≥2.3 pg/mL), participants’ health condition (healthy compared with unhealthy individuals), duration of intervention (≥8 wk compared with <8 wk), study design (parallel compared with crossover), hypocaloric compared with isocaloric diets, type of intervention (whole-grain–containing diet compared with specific types of whole-grain foods), adjustment for baseline concentrations, and risk of bias (low risk compared with others). We did not observe any significant effect in studies performed on healthy (WMD: −0.00 pg/mL; 95% CI: −0.09, 0.09 pg/mL) and unhealthy individuals (WMD: −0.08 pg/mL; 95% CI: −0.19, 0.03). None of these variables explained between-study heterogeneity.
We observed that the overall effect of whole-grain consumption on serum TNF-α concentrations did not depend on a single study based on our sensitivity analysis (range of summary 95% CI: −0.41, 0.23). No evidence of publication bias was seen (P = 0.12) (Supplemental Figure 1C). When the analyses were repeated after exclusion of 2 studies in which participants received only dietary advice on whole-grain intake (10, 17), no significant change in findings was observed (WMD: −0.11 pg/mL; 95% CI: −0.62, 0.40 pg/mL). Moreover, after exclusion of 2 studies with poor compliance, we did not observe any significant change (WMD: −0.06 pg/mL; 95% CI: −0.25, 0.14 pg/mL).
The effect of whole grains on serum PAI-1 concentrations
Overall, 4 effect sizes from 3 clinical trials in 540 individuals were included in this analysis (10, 20, 21). The study by Brownlee et al. (21) had a third arm with a different dose of whole grains. We considered each arm as a separate study. Combining these effect sizes, we found no significant effect of whole grain consumption on serum PAI-1 concentrations (WMD: −3.59 ku/L; 95% CI: −1.25, 8.44 ku/L).
Dose–response effect of whole grains on inflammation
We used metaregression to assess the dose–response effect of whole grains on inflammatory biomarkers. With regards to serum CRP concentrations, pooling the information from 9 studies (8, 11–14, 19–21, 26), we found no significant dose–response effect of whole-grain consumption on serum CRP concentrations (β = −0.002; 95% CI: −0.044, 0.040; P = 0.90). This was also the case for circulating serum IL-6 concentrations. Combining information from 6 studies in the meta-regression (8, 9, 11, 12, 14, 19), no significant dose–response effect was observed (β = 0.0008; 95% CI: −0.015; 0.015, P = 0.99). With regards to serum TNF-α concentration, 4 studies that provided required information on whole-grain doses were included in the metaregression (9, 11, 12, 19). This analysis revealed no dose–response effect of whole-grain intake on serum TNF-α concentrations as well (β = 0.0001; 95% CI: −0.034, 0.035; P = 0.98).
Discussion
Overall findings of the current study do not show beneficial effects of whole-grain intake on serum concentrations of CRP, IL-6, and TNF-α. However, we found a significant reduction in serum concentrations of CRP among studies with ≥8 wk of intervention, those that adjusted for baseline outcome variable, studies with low risk of bias, those that administered isocaloric diets, and those that enrolled participants with elevated inflammation. Moreover, following whole-grain intake, serum IL-6 concentrations were significantly reduced in studies with <8 wk of intervention, studies with a crossover design, and those that included unhealthy participants. In addition, no dose–response effect of whole-grain intake on serum concentrations of CRP, IL-6, and TNF-α was seen. To the best of our knowledge, this study is the first comprehensive meta-analysis that summarizes prior publications on the effects of whole-grain consumption on systemic inflammation. Moreover, 2 earlier meta-analyses in this field (22, 23) have several limitations that make their findings misleading.
Observational studies indicated an inverse relation between whole-grain intake and inflammatory markers (5–7). However, findings from the epidemiological studies should be interpreted with caution, because different definitions of “whole-grain foods” have been proposed across studies. Besides, they might include some products that do not meet the definition of whole grains (e.g., bran cereals), or exclude potentially important sources of whole grains (e.g., brown rice) (41). Despite significant effects in some subgroups, we did not find any beneficial effect of whole-grain consumption on serum CRP concentrations. Based on a review of 13 epidemiological and 5 interventional studies, Lefevre and Jonnalagadda (41) reported that consumption of each serving of whole grains might reduce serum CRP by ∼7%. However, they did not demonstrate a clear effect of increased whole-grain consumption on markers of inflammation. In a meta-analysis of studies investigating the effect of a whole-grain–rich Mediterranean-style diet on inflammatory cytokines, no overall significant effect on serum CRP concentrations was found (42). Unlike our findings, 2 recent meta-analyses reported an overall significant reduction in serum CRP concentrations in a heterogeneous group of participants allocated to receive whole-grain intake (22, 23). Some methodological limitations in these studies might explain the discrepant findings. In the study of Hajihashemi and Haghighatdoost (22), 6 relevant RCTs were not included (11, 14, 17–19, 26); of these 6 studies 4 publications failed to find any significant effect of whole-grain consumption on serum CRP concentrations (11, 14, 21, 26). In addition, the authors included a quasiexperimental study without any control group (35). Xu et al. (23) did not include the study of Kirwan et al. (26) despite its meeting all required criteria for inclusion. Furthermore, in both above-mentioned meta-analyses, the investigators included an RCT performed in children, in which whole-grain consumption significantly reduced serum CRP concentrations (43). Combining the effect sizes from studies performed in children with those in adults can distort the findings, because the nature of inflammation in children and adults might have different origins: in particular, in children it might be due to growth and development and changes in concentrations of growth hormone and insulin-like growth factor I (25).
In the current meta-analysis, we found a significant reduction in serum concentrations of CRP in studies with a duration ≥8 wk. This is in line with the findings of a previous meta-analysis by Hajihashemi and Haghighatdoost (22) and in contrast to the findings of Xu et al. (23) in another meta-analysis. When we confined the analysis to studies with elevated baseline serum CRP concentrations, a significant reduction was seen following whole-grain intake. Similar findings were also achieved in studies with a duration of intervention ≥8 wk. In crossover studies, whole-grain intake resulted in a significant increase in serum CRP concentrations. Earlier publications suggested that initial serum concentrations of CRP could be a predictor of response to dietary interventions including whole-grain foods (7). Because each mentioned category consisted of studies with different baseline serum concentrations of CRP, interpretation of the results was complicated. However, we found a significant reduction in serum CRP concentrations in studies that adjusted for baseline values of serum CRP. Surprisingly, despite a significant decline in serum CRP concentrations in studies with isocaloric diets, we did not find any significant decrease in serum CRP concentrations in studies with hypocaloric diets (WMD: −0.22 mg/L; 95% CI: −0.57, 0.14 mg/L). However, in such studies, both whole-grain and control groups had received calorie-restricted diets (8, 10, 11). When we stratified studies by the health status of the studied population, we observed a significant reduction in serum concentrations of CRP following whole-grain intake in both subgroups of healthy and nonhealthy participants. This could be attributed to the high frequency of overweight and obese individuals among apparently healthy participants, in which slightly elevated levels of inflammation are evident.
We did not find any significant effect of whole-grain intake on serum concentrations of IL-6 (WMD: −0.08 pg/mL; 95% CI: −0.27, 0.11 pg/mL). This finding was not consistent with the results of 2 recently published meta-analyses (22, 23), in which a significant reduction in serum IL-6 concentrations occurred following whole-grain intake. However, their findings might be misleading because of several limitations. For instance, in the study of Hajihashemi and Haghighatdoost (22) 2 RCTs that reported no significant effect of whole-grain consumption on serum IL-6 concentrations were not included (11, 17). Moreover, Xu et al. (23) excluded 2 eligible studies in which nonsignificant effects of whole-grain intake on serum IL-6 were reported (8, 12). In a meta-analysis of studies of whole-grain–rich Mediterranean diets, significant reductions in serum IL-6 concentrations were seen (43). However, these reductions might also be attributed to the high content of other components in the Mediterranean diet such as fruits and vegetables. Despite the lack of any significant effect of whole-grain intake on serum IL-6 concentrations in this study (WMD: −0.08 pg/mL; 95% CI: −0.27, 0.11 pg/mL), our stratified analysis revealed a significant decline in this biomarker in studies conducted on unhealthy individuals. In addition, we found a significant reduction in serum IL-6 concentrations in studies with a short duration of intervention (<8 wk). This would seem to contradict our findings about CRP, in which the effect was seen for studies with a long duration of intervention. The discrepant findings about CRP and IL-6 might be explained by the fact that most studies examining the effect on serum IL-6 did not control for baseline concentrations of this biomarker, whereas studies on CRP mostly adjusted for the baseline concentrations (12, 13, 18, 26). Moreover, lack of a significant effect for IL-6 in studies with a long duration of intervention (≥8 wk) might be due to changes in weight between intervention and control groups (WMD: 0.01; 95% CI: −0.07, 0.10 pg/mL). For instance, in 2 of 6 RCTs with an intervention duration ≥8 wk, participants in the control group had greater weight loss than those in the intervention group (10, 11).
In the current study, we found no significant effect of whole-grain consumption on serum TNF-α concentration (WMD: −0.06 pg/mL; 95% CI: −0.25, 0.14 pg/mL). Such a finding was also seen in our subgroup analyses. Recent meta-analyses showed no significant effect of whole-grain consumption on serum TNF-α concentration (22, 23). In addition to missing some eligible RCTs in both meta-analyses, Hajihashemi and Haghighatdoost (22) included a quasiexperimental study in the analysis of serum concentrations of TNF-α. Furthermore, Xu et al. (23) included a clinical trial that examined the influence of whole-grain consumption on immune cell TNF-α production, not serum concentrations. Lack of significant effects of whole-grain intake on serum TNF-α concentration was also reported in another meta-analysis in healthy individuals who received a whole-grain–rich diet (42). Lack of a significant effect of whole-grain consumption on serum TNF-α concentration might be explained by the limited number of RCTs with a low risk of bias. Overall, further studies are needed to reveal the true effect of whole-grain consumption on serum TNF-α concentration.
Although we found no significant effect of whole-grain consumption on inflammatory biomarkers, the anti-inflammatory properties of this food group have been shown in previous studies (10, 17, 18). Whole-grain intake can decrease insulin resistance through which it can reduce elevated concentrations of inflammatory biomarkers (44). Furthermore, whole-grain intake can decrease inflammation through weight loss (12). Whole grains contain high amounts of fiber, polyphenols, and omega-3 fatty acids, all of which have anti-inflammatory properties (1). In addition, whole grains might influence inflammation through their effects on the gut microbiome population (12).
As strengths of the current study, it must be noted that we considered all clinical trials conducted on the effect of whole-grain intake, whether as a whole-grain–rich diet or specific whole-grain foods, on inflammatory biomarkers. However, some limitations should be considered. For instance, different methods of whole-grain prescription, calorie restriction in a number of studies, different methods used for measuring inflammatory biomarkers, lack of controlling for baseline measures in some studies, and different study designs should be taken into account.
Altogether, unlike observational studies, we found no evidence of the favorable effect of whole-grain intake on selected markers of subclinical inflammation when combining published data from interventional studies. However, a significant reduction in serum concentrations of CRP was seen for studies with ≥8 wk of intervention, those that adjusted for baseline levels of inflammation, studies with low risk of bias, those that administered isocaloric diets, and those that enrolled participants with elevated inflammation. Moreover, high intakes of whole grains resulted in reduced serum IL-6 concentrations in studies with <8 wk of intervention, studies with crossover designs, and those that included unhealthy participants. It must be noted that the current meta-analysis reveals significant heterogeneity among the studies, overall and in subgroups, making it inappropriate to derive firm conclusions (for or against) on the effect of whole grains on systemic inflammation. Future interventional studies recruiting homogeneous groups of participants with a long period of intervention are required in this area. Moreover, conducting future studies with higher doses might not add much because we did not find any dose–response effect in spite of the wide range of whole-grain doses in the analyzed studies (60–160 g/d), which ranged higher than the recommended amount of 75 g/d.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SR, NS: conducted the research; OS: analyzed the data; MS, AE: wrote the manuscript; AE: had primary responsibility for the final content; and all authors: designed the research, and read and approved the final manuscript.
Notes
The study was supported by an Elites Grant (grant no. 982674) from the National Institute for Medical Research Development, Tehran, Iran, and by the Students' Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran.
Author disclosures: SR, OS, MS, NS, BL, and AE, no conflicts of interest. The Students' Scientific Research Center had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
SR and OS were equally involved in the current study.
Abbreviations used: CRP, C-reactive protein; PAI-1, plasminogen activator inhibitor-1; RCT, randomized controlled trial; WMD, weighted mean difference.
References
- 1. Bartlomiej S, Justyna RK, Ewa N. Bioactive compounds in cereal grains – occurrence, structure, technological significance and nutritional benefits – a review. Food Sci Technol Int. 2012;18(6):559–68. [DOI] [PubMed] [Google Scholar]
- 2. Zong G, Gao A, Hu FB, Sun Q. Whole grain intake and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Circulation. 2016;133(24):2370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seal CJ, Brownlee IA. Whole-grain foods and chronic disease: evidence from epidemiological and intervention studies. Proc Nutr Soc. 2015;74(3):313–9. [DOI] [PubMed] [Google Scholar]
- 4. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre?. Nutr Res Rev. 2010;23(1):65–134. [DOI] [PubMed] [Google Scholar]
- 5. Sandberg JC, Bjorck IME, Nilsson AC. Effects of whole grain rye, with and without resistant starch type 2 supplementation, on glucose tolerance, gut hormones, inflammation and appetite regulation in an 11–14.5 hour perspective; a randomized controlled study in healthy subjects. Nutr J. 2017;16(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giacco R, Clemente G, Cipriano D, Luongo D, Viscovo D, Patti L, Di Marino L, Giacco A, Naviglio D, Bianchi MA et al.. Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutr Metab Cardiovasc Dis. 2010;20(3):186–94. [DOI] [PubMed] [Google Scholar]
- 7. Sutliffe JT, Wilson LD, de Heer HD, Foster RL, Carnot MJ. C-reactive protein response to a vegan lifestyle intervention. Complement Ther Med. 2015;23(1):32–7. [DOI] [PubMed] [Google Scholar]
- 8. Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr. 2012;142(4):710–6. [DOI] [PubMed] [Google Scholar]
- 9. Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, Gibbons SM, La Storia A, Gilbert JA, Jonnalagadda S et al.. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr. 2015;101(2):251–61. [DOI] [PubMed] [Google Scholar]
- 10. Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87(1):79–90. [DOI] [PubMed] [Google Scholar]
- 11. Harris Jackson K, West SG, Vanden Heuvel JP, Jonnalagadda SS, Ross AB, Hill AM, Grieger JA, Lemieux SK, Kris-Etherton PM. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr. 2014;100(2):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roager HM, Vogt JK, Kristensen M, Hansen LBS, Ibrugger S, Maerkedahl RB, Vidgen E, Parker T, Lau H, Connelly PW et al.. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ross AB, Bruce SJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Bourgeois A, Nielsen-Moennoz C, Vigo M, Fay LB, Kochhar S et al.. A whole-grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a refined-grain diet in healthy subjects. Br J Nutr. 2011;105(10):1492–502. [DOI] [PubMed] [Google Scholar]
- 14. Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr. 2010;92(4):733–40. [DOI] [PubMed] [Google Scholar]
- 15. Vetrani C, Costabile G, Luongo D, Naviglio D, Rivellese AA, Riccardi G, Giacco R. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition. 2016;32(2):217–21. [DOI] [PubMed] [Google Scholar]
- 16. Meydani M, Thomas M, Barnett JB, Chen O, Dplinkowski G, Jonnalagadda S, Saltzman E, Roberts S, Meydani SN.. Short term consumption of whole grain foods independent of weight loss does not affect surrogate markers of CVD. FASEB J. 2016;30:(1 Suppl). [Google Scholar]
- 17. de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, Mykkanen H, Uusitupa M. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia. 2011;54(11):2755–67. [DOI] [PubMed] [Google Scholar]
- 18. Kondo K, Morino K, Nishio Y, Ishikado A, Arima H, Nakao K, Nakagawa F, Nikami F, Sekine O, Nemoto KI et al.. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: a randomized controlled trial. PLoS One. 2017;12(6):e0179869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ampatzoglou A, Williams CL, Atwal KK, Maidens CM, Ross AB, Thielecke F, Jonnalagadda SS, Kennedy OB, Yaqoob P. Effects of increased wholegrain consumption on immune and inflammatory markers in healthy low habitual wholegrain consumers. Eur J Nutr. 2016;55(1):183–95. [DOI] [PubMed] [Google Scholar]
- 20. Andersson A, Tengblad S, Karlstrom B, Kamal-Eldin A, Landberg R, Basu S, Aman P, Vessby B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr. 2007;137(6):1401–7. [DOI] [PubMed] [Google Scholar]
- 21. Brownlee IA, Moore C, Chatfield M, Richardson DP, Ashby P, Kuznesof SA, Jebb SA, Seal CJ. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br J Nutr. 2010;104(1):125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hajihashemi P, Haghighatdoost F.. Effects of whole-grain consumption on selected biomarkers of systematic inflammation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr. 2019;38(3):275–85. [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Wan Q, Feng J, Du L, Li K, Zhou Y. Whole grain diet reduces systemic inflammation: a meta-analysis of 9 randomized trials. Medicine. 2018;97(43):e12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadeghian M, Sadeghi O, Esmaillzadeh A. Findings from the meta-analysis on whole-grain consumption and biomarkers of systemic inflammation are misleading. J Am Coll Nutr. 2019:1–2.. doi:10.1080/07315724.2019.1579117. [DOI] [PubMed] [Google Scholar]
- 25. Wong SC, Dobie R, Altowati MA, Werther GA, Farquharson C, Ahmed SF. Growth and the growth hormone-insulin like growth factor 1 axis in children with chronic inflammation: current evidence, gaps in knowledge, and future directions. Endocr Rev. 2016;37(1):62–110. [DOI] [PubMed] [Google Scholar]
- 26. Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, Haus JM, Filion J, Godin JP, Kochhar S et al.. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr. 2016;146(11):2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang B, Medapalli R, Xu J, Cai W, Chen X, He JC, Uribarri J. Effects of a whole rice diet on metabolic parameters and inflammatory markers in prediabetes. ESPEN J. 2013;8(1):e15–20. [Google Scholar]
- 28. Dinu M, Whittaker A, Pagliai G, Giangrandi I, Colombini B, Gori AM, Fiorillo C, Becatti M, Casini A, Benedettelli S et al.. A Khorasan wheat-based replacement diet improves risk profile of patients with nonalcoholic fatty liver disease (NAFLD): a randomized clinical trial. J Am Coll Nutr. 2018;37(6):508–14. [DOI] [PubMed] [Google Scholar]
- 29. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson K, Mathai ML, Ashton JF, Donkor ON, Vasiljevic T, Mamilla R, Stojanovska L. Effects of malted and non-malted whole-grain wheat on metabolic and inflammatory biomarkers in overweight/obese adults: a randomised crossover pilot study. Food Chem. 2016;194:495–502. [DOI] [PubMed] [Google Scholar]
- 31. Martinez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA et al.. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Price RK, Wallace JM, Hamill LL, Keaveney EM, Strain JJ, Parker MJ, Welch RW. Evaluation of the effect of wheat aleurone-rich foods on markers of antioxidant status, inflammation and endothelial function in apparently healthy men and women. Br J Nutr. 2012;108(9):1644–51. [DOI] [PubMed] [Google Scholar]
- 33. Stefoska-Needham A, Beck EJ, Johnson SK, Batterham MJ, Grant R, Ashton J, Tapsell LC. A diet enriched with red sorghum flaked biscuits, compared to a diet containing white wheat flaked biscuits, does not enhance the effectiveness of an energy-restricted meal plan in overweight and mildly obese adults. J Am Coll Nutr. 2017;36(3):184–92. [DOI] [PubMed] [Google Scholar]
- 34. Vanegas SM, Meydani M, Barnett JB, Goldin B, Kane A, Rasmussen H, Brown C, Vangay P, Knights D, Jonnalagadda S et al.. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr. 2017;105(3):635–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durazzo A, Carcea M, Adlercreutz H, Azzini E, Polito A, Olivieri L, Zaccaria M, Meneghini C, Maiani F, Bausano G et al.. Effects of consumption of whole grain foods rich in lignans in healthy postmenopausal women with moderate serum cholesterol: a pilot study. Int J Food Sci Nutr. 2014;65(5):637–45. [DOI] [PubMed] [Google Scholar]
- 36. Sandberg JC, Björck IME, Nilsson AC. Rye-based evening meals favorably affected glucose regulation and appetite variables at the following breakfast; a randomized controlled study in healthy subjects. PLoS One. 2016;11(3):e0151985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Esposito KMD, Marfella RMDP, Ciotola MMD, Di Palo CMD, Giugliano FMD, Giugliano GMD, D'Armiento M, D'Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–6. [DOI] [PubMed] [Google Scholar]
- 38. Hajihashemi P, Azadbakht L, Hashemipor M, Kelishadi R, Esmaillzadeh A. Whole-grain intake favorably affects markers of systemic inflammation in obese children: a randomized controlled crossover clinical trial. Mol Nutr Food Res. 2014;58(6):1301–8. [DOI] [PubMed] [Google Scholar]
- 39. Langkamp-Henken B, Nieves C Jr, Culpepper T, Radford A, Girard SA, Hughes C, Christman MC, Mai V, Dahl WJ, Boileau T et al.. Fecal lactic acid bacteria increased in adolescents randomized to whole-grain but not refined-grain foods, whereas inflammatory cytokine production decreased equally with both interventions. J Nutr. 2012;142(11):2025–32. [DOI] [PubMed] [Google Scholar]
- 40. Higgins JPT, Green S, Cochrane handbook for systematic reviews of interventions, version 5.1.0, London: The Cochrane Collaboration, Updated March 2011. Available from: http://www.cochrane.org/training/cochrane-handbook. [Accessed Jan 20, 2019]. [Google Scholar]
- 41. Lefevre M, Jonnalagadda S.. Effect of whole grains on markers of subclinical inflammation. Nutr Rev. 2012;70(7):387–96. [DOI] [PubMed] [Google Scholar]
- 42. Mayr HL, Tierney AC, Thomas CJ, Ruiz-Canela M, Radcliffe J, Itsiopoulos C. Mediterranean-type diets and inflammatory markers in patients with coronary heart disease: a systematic review and meta-analysis. Nutr Res. 2018;50:10–24. [DOI] [PubMed] [Google Scholar]
- 43. Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–39. [DOI] [PubMed] [Google Scholar]
- 44. Liese AD, Roach AK, Sparks KC, Marquart L, D'Agostino RB Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. 2003;78(5):965–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.