Abstract
Background and Aims
Ecosystem-based flood defence including salt-marsh as a key component is increasingly applied worldwide due to its multifunctionality and cost-effectiveness. While numerous experiments have explored the wave-attenuation effects of salt-marsh plants critical to flood protection, little is known about the physiological and biochemical responses of these species to continuous wave exposure.
Methods
To address this knowledge gap, we developed a shallow-water wave simulator to expose individual Spartina alterniflora plants to waves in a greenhouse for 8 weeks. S. alterniflora individuals were partially submerged and experienced horizontal sinusoidal motion to mimic plant exposure to shallow water waves. A factorial experiment was used to test the effects of three wave heights (4.1 cm, 5.5 cm and a no-wave control) and two wave periods (2 s and 3 s) on the following key physiological and biochemical plant parameters: plant growth, antioxidant defence and photosynthetic capacity.
Key Results
Comparison of wave treatment and control groups supported our hypotheses that wave exposure leads to oxidative stress in plants and suppresses plant photosynthetic capacity and thereby growth. In response, the wave-exposed plants exhibited activated antioxidant enzymes. Comparison between the different wave treatment groups suggested the wave effects to be generally correlated positively with wave height and negatively with wave period, i.e. waves with greater height and frequency imposed more stress on plants. In addition, wave-exposed plants tended to allocate more biomass to their roots. Such allocation is favourable because it enhances root anchorage against the wave impact.
Conclusions
Simulated wave exposure systems such as the one used here are an effective tool for studying the response of salt-marsh plants to long-term wave exposure, and so help inform ecosystem-based flood defence projects in terms of plant selection, suitable transplantation locations and timing, etc. Given the projected variability of the global wave environment due to climate change, understanding plant response to long-term wave exposure has important implications for salt-marsh conservation and its central role in natural flood defence.
Keywords: Ecosystem-based flood defence, salt-marsh, wave stress, response, Spartina alterniflora
Introduction
Salt-marshes are an important pathway for mass and energy transfer between land and ocean and provide numerous valuable ecosystem services, including carbon sequestration, food production, habitat provisioning, flood protection and water purification (Barbier et al., 2011). Due to sea level rise and increasing extreme weather events caused by climate change (IPCC, 2014), coastal communities worldwide are facing an increasing threat from flooding, and the function of salt-marshes as natural buffer zones protecting coastal areas from waves, tides and storms has drawn increasing attention (Gedan et al., 2011; Shepard et al., 2011). At the same time, purpose-built ecosystem-based flood defences, i.e. eco-shorelines or living shorelines, that are more cost-effective than conventional engineering infrastructures such as sea walls and levees and concurrently provide crucial habitat in intertidal zones, are now implemented on a large scale (Temmerman et al., 2013). In both natural and man-made systems, salt-marsh vegetation plays a key role in flood and wave attenuation (Moller, 2006; Temmerman et al., 2012), even under extreme conditions, such as storm surges (Moller et al., 2014). Importantly, the establishment, survival and growth of salt-marsh plants are subject to hydrodynamic forcing, including wave conditions (Bouma et al., 2009, 2016; Callaghan et al., 2010; Friess et al., 2012) and consequently, understanding the response of salt-marsh plants to wave stress is thus imperative within the context of ecosystem-based coastal management (Barbier et al., 2008).
Numerous short-term experimental studies have quantified the wave-attenuation effects of plants (Augustin et al., 2009; Anderson and Smith, 2014; Ozeren et al., 2014). The specific effect was found to depend on hydrodynamic parameters, such as wave height (Anderson and Smith, 2014), wave period (Jadhav et al., 2013) and water depth (Paquier et al., 2017), as well as plant traits, such as stem height, diameter, density and flexibility (Bouma et al., 2005, 2010; Marsooli and Wu, 2014; Paul et al., 2016). Furthermore, a number of short-term studies have explored the response of salt-marsh plants to waves. Such flume studies identified drag forces and scouring of sediment around plant stems as the two primary mechanisms that limit seedling establishment and may cause plant failure (Bouma et al., 2009; Henry and Myrhaug, 2013). In another flume study, Silinski et al. (2015) explored the effects of wind (short) and ship (long) waves on seedlings and adult shoots of Scirpus maritimus, reporting that plants were well adapted to ‘wind’ waves during both life stages, whereas ‘ship’ waves tended to strongly bend their stems and potentially limited their survival. Short-term flume studies have also shown severe stem breakage and loss of above-ground biomass in response to extreme wave conditions under storm conditions (Rupprecht et al., 2017).
Long-term studies focusing on the impact of waves on vegetation are, however, much rarer than short-term experiments. Coops and Van der Velde (1996) studied the effects of wave exposure on the mean stem height and density of Phragmites australis and Scirpus lacustris stands. By measuring the mechanical properties of both species, they demonstrated that P. australis stands withstand wave exposure better than S. lacustris due to the higher stem stiffness and lower susceptibility to breakage. Using wave mesocosms, La Nafie et al. (2012) showed that the survival of seagrasses was reduced by a combination of waves and nutrients, whereas waves reduced growth and nutrient loading reduced leaf strength. By comparing S. maritimus stands from areas with different wave exposures in the Scheldt Estuary (south-west Netherlands), Silinski et al. (2018) reported that shoots from more wave-exposed conditions developed significantly shorter, more flexible and thicker stems than those from more sheltered conditions, indicating plasticity in response to wave exposure following a stress-avoidance strategy. So far, however, the very few existing long-term studies on the impacts of waves have mainly focused on the growth, morphological and biomechanical properties of salt-marsh plants, whereas plant physiological and biochemical responses to long-term wave exposure remain elusive. Given that hydrodynamic forces such as flow and turbulence cause oxidative stress in plants and suppress photosynthetic capacity and growth (Kankanamge et al., 2011; Ellawala et al., 2013), long-term wave exposure may cause additional negative effects.
Under abiotic stress, plants typically exhibit oxidative stress (Mittler, 2002), which typically manifests as an elevated tissue concentration of reactive oxygen species (ROS), such as the superoxide anion (O2−), H2O2 and the hydroxyl radical (OH−) (Navrot et al., 2007). The over-accumulation of ROS induces oxidative processes such as membrane lipid peroxidation, protein oxidation, enzyme inhibition and DNA and RNA damage, resulting in cell injury and even cell death (Moller et al., 2007). The content of malondialdehyde (MDA), as a secondary breakdown product of lipid peroxidation, has often been used as a tool to assess the severity of oxidative damage in plants (Canalejo et al., 2014). As protection from oxidative damage, plants have evolved defence mechanisms that comprise the activation of ROS-scavenging antioxidant enzymes such as ascorbate peroxidase (APX), superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POD) and glutathione peroxidase (GPX), as well as the synthesis of non-enzymatic antioxidants such as ascorbate and glutathione (GSH) (Arora et al., 2002; Gill and Tuteja, 2010). Hence, the activities of antioxidant enzymes such as APX, SOD, CAT, POD and GPX are commonly measured to examine the stress responses of plants (Duarte et al., 2016; Asaeda et al., 2017). In addition, stress often adversely affects the photosynthetic machinery, which is typically reflected by reduced photochemical quenching (qP), indicating reduction in the proportion of open photosystem II reaction centres, as well as increased non-photochemical quenching (NPQ), indicating reduced demand for products of electron transport and hence increased energy dissipation as heat (Moradi and Ismail, 2007). The damaged plant photosynthetic machinery would result in reduced photosynthetic capacity (typically indicated by the maximum quantum efficiency of photosystem II (Fv/Fm)) and further suppress plant growth (Ellawala et al., 2013; Atapaththu and Asaeda, 2015).
Spartina alterniflora is a perennial grass native to the east coast of North America and is spreading rapidly to estuaries and coastal salt-marshes in other parts of the world, including the Pacific coast of North America, Europe, New Zealand and China. In addition to its wide geographical distribution, S. alterniflora is often employed in eco-shoreline projects due to its tolerance of seawater inundation, ability to colonize and grow in a variety of wave environments, and effective erosion-mitigation and wave-attenuation abilities (Manis et al., 2015). In order to better understand the suitability of S. alterniflora for use in eco-shoreline projects, we investigate the hypotheses that (1) long-term wave exposure causes oxidative stress in the salt-marsh plant S. alterniflora and suppresses its photosynthetic capacity and thereby growth; (2) wave-exposed S. alfterniflora activates antioxidant enzymes in response to wave-induced oxidative stress; and (3) the wave effects depend on wave parameters such as height and period. These questions were addressed by quantifying the effects of oscillatory currents induced by shallow-water waves on Spartina growth and ecophysiology, specifically (a) antioxidant defence via enzyme activities as indicators of plant response to oxidative stress induced by wave exposure, (b) lipid peroxidation as an indicator of oxidative damage to the plant, and (c) chlorophyll fluorescence as an indicator of the photosynthetic capacity of the plant. To translate current findings into what it means with respect to predicted shifts in wave environments experienced by natural salt-marshes (Hemer et al., 2013; IPCC, 2014), we discuss the potential impacts of projected variability of the global wave environment due to climate change on the S. alterniflora communities in its primary geographical distribution areas (i.e. including its native and invasive ranges across the globe) and the relevant management implications.
MATERIALS AND METHODS
Shallow-water wave simulator
The remarkable lack of long-term wave-exposure studies is most likely attributable to technical constraints. Traditional wave flumes are not designed for long-term plant exposure experiments, as they do not allow the continuous operation for extended periods necessary for physiological experiments, not to mention their additional requirement of simultaneous replications (La Nafie et al., 2012). To resolve this issue, we adapted a slider-crank mechanism that has been previously used to study the discharge of jets into an oscillatory cross-flow (Lam and Xia, 2001) to create a system by which plants can be exposed under controlled conditions to contrasting wave regimes. In essence, the plants were grown on a swing system that moves the plants continuously back and forth through the water (Fig. 1) to mimic exposure to long-term shallow-water waves (for a full description of wave simulator design, see Supplementary Data Appendix A). The system was placed in a 45-m2 purpose-built greenhouse located at the State Key Laboratory of Earth Surface Processes and Resource Ecology, Beijing Normal University.
Fig. 1.
Schematic diagram of the experimental setup. (A) Front view. (B) Side view. (C) Top view. (D) Streamlined container.
The plants were exposed to four field-relevant types of shallow-water waves: two wave heights (4.1 and 5.5 cm) and two wave periods (2 and 3 s) were tested in a full factorial experiment, with the treatments coded as T2H4, T2H5, T3H4 and T3H5, respectively. A no-wave condition was also tested as a control (coded T0H0). Notably, the wave force (stress) acting directly on the plant is correlated positively with wave height H and negatively with wave period T. Therefore, T2H5 corresponded to the condition with the strongest wave stress and T3H4 the weakest. For full details of the selection of wave parameters and a basic explanation of the functional relationship between wave stress, wave height and wave period, see Supplementary Data Appendix B.
Four treatment tanks were installed, one for each wave treatment, plus another control tank. The control tank had a similar setup and management except for the absence of any water motion inside the tank. The tanks were all filled with a 31-cm water column, and the submergence depth above the containers was maintained at 10 cm. To reduce the effect of wave reflection, 10-cm-thick coil mats were placed inside the tank to absorb the waves, and the wave parameters were carefully checked to avoid creating standing waves, i.e. the generated wave length was equal to twice the length of the tank.
Plant material and growth conditions
A total of 100 soil cores (length 15 cm, width 15 cm, height 25 cm), each containing ten intact S. alterniflora individuals, were collected from salt-marsh in the Yellow River Estuary, Shandong Province, China, in October 2016 and transported to the laboratory. The soil cores were transplanted in pots (diameter 20 cm, height 30 cm) in the greenhouse and irrigated with fresh water in the first week and 5 % Hoagland nutrient solution prepared using 5 ‰ artificial seawater from the second week onward. At the end of the growing season, when the plants had senesced, the above-ground parts were harvested and the below-ground parts were continuously cultured. In April 2017, soil cores with healthy individuals, ~35 cm in height, were randomly selected and fitted into the plant culture zone of the containers. The soil surface was flush with the edge of the containers, and excess stems were trimmed to leave approximately nine individuals per container, i.e. a shoot density equivalent to 400 stems m−2, representative of natural S. alterniflora marshes (Knutson et al., 1982). The same culture solution was used to fill the water tank and was renewed weekly. The temperature was controlled between 24 and 30 °C throughout the experiments in an unheated, naturally lit greenhouse. After being acclimatized for 1 week, plants were exposed to the four wave treatments and the no-wave control for 8 weeks. For each treatment, the three containers served as triplicates.
Growth measurements
At the end of each experiment, plant growth (individual stem height) was measured and plants were harvested and rinsed to remove soil and inorganic material. The above- and below-ground live materials were separated and oven-dried at 60 °C to a constant weight to estimate biomasses.
Antioxidant enzyme activities as markers of stress
Leaf samples were randomly extracted on the first day of every week. Plant tissue was homogenized with phosphate buffer solution (0.1 m, pH 7.4) at a 1:9 ratio (g FW:mL), and the homogenate was centrifuged at 3500 rpm for 15 min. Antioxidant enzyme activities were assayed using commercial kits (Nanjing Jiancheng Bioengineering Institute, China). Specifically, SOD activity was assayed using the nitro blue tetrazolium (NBT) method, whereby one unit of SOD activity was defined as the amount of SOD required to inhibit 50 % of the reduction in NBT dye per millilitre of reaction solution per milligram of tissue protein. CAT activity was assayed using the hydrogen peroxide (H2O2) oxidation method, whereby one unit of CAT activity was defined as the amount of 1 µm H2O2 decomposed per milligram of tissue protein per second. GPX activity was assayed using 5,5-dithiobis-2-nitrobenzoic acid (DTNB) spectrophotometry, whereby one unit of GPX activity was defined as the amount of enzyme in 1 mL of reaction solution that catalyses the oxidation of 1 μm of GSH per minute at 37 °C (pH 7.4). POD activity was assayed using the guaiacol method, whereby one unit of POD activity was defined as the amount of enzyme that catalyses the production of 1 μg of substrate per milligram of tissue protein per minute at 37 °C.
Lipid peroxidation as a marker of cell damage
The MDA content of the plant samples was used as a marker of lipid peroxidation using a commercial kit (Nanjing Jiancheng Bioengineering Institute, China). The MDA content was measured through the absorbance of thiobarbituric acid-reactive substances (TBARS) at 532 nm and expressed as nanomoles of MDA per milligram of tissue protein.
Chlorophyll fluorescence as a marker of photosynthetic capacity
Chlorophyll fluorescence measurement was conducted using a FluorPen (FP 100, Photon Systems Instruments, Czech Republic) on the first day of every week. The third and fourth completely unfolded and undamaged leaves of randomly selected healthy S. alterniflora individuals were dark-adapted for 20 min before detection, and the saturation pulse of the FluorPen was set to 6000 μm m−2 to detect the chlorophyll fluorescence. Key chlorophyll fluorescence parameters, such as Fv/Fm, qP, fluorescence decay rate (Rfd) and NPQ, were recorded.
Statistical analysis
The data were tested for normality and homogeneity of variance using a Shapiro–Wilk test and Levene’s test, respectively, before statistical analyses. The results are presented as mean ± s.d. (n = 27 for stem height and biomass, n = 6 for enzyme assays and n = 5 for chlorophyll fluorescence assays). Differences in the temporal variation in parameters measured during the experimental period among the different wave treatments were analysed by repeated measures ANOVA (RM-ANOVA) to detect significant differences (P < 0.05), with Bonferroni correction for pairwise comparisons. When the assumption of sphericity was violated, Greenhouse–Geisser correction was applied. The parameters measured only at the end of the experimental period were analysed using one-way ANOVA followed by a post hoc Tukey test. Statistical analysis was performed using SPSS (v22.0) software.
RESULTS
Plant growth and biomass
Spartina stem height in the wave treatment groups was generally lower than in the control group (Table 1), indicating that waves had negative effects on plant growth. For the comparison among the different treatments, the 2-s wave treatments (T2 treatments; Table 1), which presumably corresponded to more stressful conditions for identical wave heights, yielded lower stem heights than the control (P < 0.05). Stem heights within the different treatment groups did not differ. The same was true of the 3-s wave treatments (T3 treatments) and the control group.
Table 1.
Plant height and biomass
| Responses | Treatment | F (4,130) | P | ||||
|---|---|---|---|---|---|---|---|
| Control | T3H4 | T3H5 | T2H4 | T2H5 | |||
| Plant height (cm) | 79.31 ± 8.55a | 76.89 ± 6.76ab | 72.06 ± 3.55ab | 59.76 ± 2.33b | 53.81 ± 2.83b | 4.67 | <0.01 |
| Total biomass (g) | 2.42 ± 0.12a | 2.29 ± 0.12ab | 2.16 ± 0.08bc | 2.02 ± 0.06c | 1.83 ± 0.05d | 46.29 | <0.01 |
| Above-ground biomass (g) | 1.65 ± 0.04a | 1.42 ± 0.02b | 1.27 ± 0.02c | 1.21 ± 0.02c | 1.06 ± 0.03d | 230.57 | <0.01 |
| Below-ground biomass (g) | 0.77 ± 0.04a | 0.88 ± 0.02b | 0.88 ± 0.02b | 0.81 ± 0.02a | 0.77 ± 0.03a | 14.19 | <0.01 |
| Above-ground/below-ground biomass ratio | 2.15 ± 0.16a | 1.62 ± 0.06b | 1.44 ± 0.05b | 1.51 ± 0.05b | 1.37 ± 0.08b | 33.28 | <0.01 |
Data are mean ± s.d., n = 27.
Different superscripts in the same row indicate a significant difference between different wave treatment groups (P < 0.05).
Consistent with the negative effects of wave treatment on plant elongation, total biomass was reduced by wave exposure (Table 1). Although no significant difference in the total biomass was evident between the T3H4 treatment (weakest wave stress among all wave treatment groups) and the control group, total biomass was reduced in other wave treatment groups with stronger wave stress. Significant decreases in above-ground biomass and the above-ground/below-ground biomass ratio were also apparent between the individual wave treatment and control groups. Notably, significant difference were consistently found between the T3H4 (weakest wave stress) and T2H5 (strongest wave stress) treatments, except for the above-ground/below-ground biomass ratio.
Antioxidant enzyme activities as markers of stress
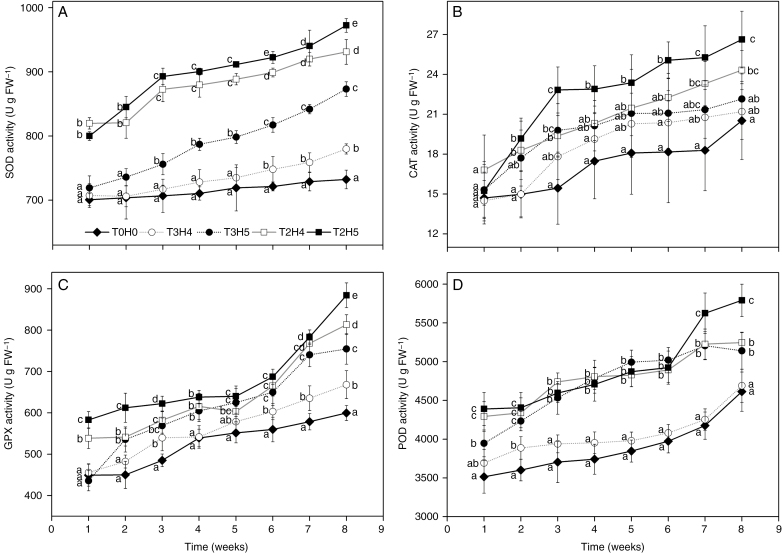
Wave treatment stimulated elevated SOD (RM-ANOVA, F28,175 = 10.116, P < 0.01), CAT (RM-ANOVA, F18.596,116.222 = 1.739, P < 0.05), GPX (RM-ANOVA, F28,175 = 9.342, P < 0.01) and POD (RM-ANOVA, F28,175 = 4.772, P < 0.01) activities compared with the control throughout the experimental period (Fig. 2). Among the different treatments (Fig. 2), the highest SOD activity was found in the plants subjected to the T2 treatments (i.e. stronger wave stress), whereas the plants grown in the T3H4 treatment (weakest wave stress among all wave treatment groups) and control exhibited the lowest SOD activities. CAT activities did not vary throughout most of the experiment, except in the T2H5 treatment (strongest wave stress among all wave treatment groups), which from the second week onward exhibited higher CAT activity than the control group. GPX activities in the wave treatment groups were higher than in the control group throughout most of the experiment, and higher activities were observed in treatments with more stress. POD activities in the T2 and T3H5 treatments were significantly higher than those in the T3H4 treatment and the control group throughout.
Fig. 2.
Temporal variation in antioxidant enzyme activities in leaves of S. alterniflora during the experimental period when exposed to different wave treatments. Data are mean ± s.d., n = 6. Different letters at the same time point indicate a significant difference between different wave treatment groups (P < 0.05).
Lipid peroxidation as a marker of cell damage
The MDA content (Fig. 3) increased in the wave treatment groups compared with the control throughout the experiment (RM-ANOVA, F12.838,80.238 = 2.604, P < 0.01), regardless of the high POD activities. Among the different treatments, the MDA contents were similar among all groups throughout most of the experiment, except in the T2H5 treatment, which had a significantly higher MDA content than all other groups.
Fig. 3.
Temporal variation in MDA contents in leaves of S. alterniflora during the experimental period when exposed to different wave treatments. Data are mean ± s.d., n = 6. Different letters at the same time point indicate a significant difference between different wave treatment groups (P < 0.05).
Chlorophyll fluorescence as a marker of photosynthetic capacity
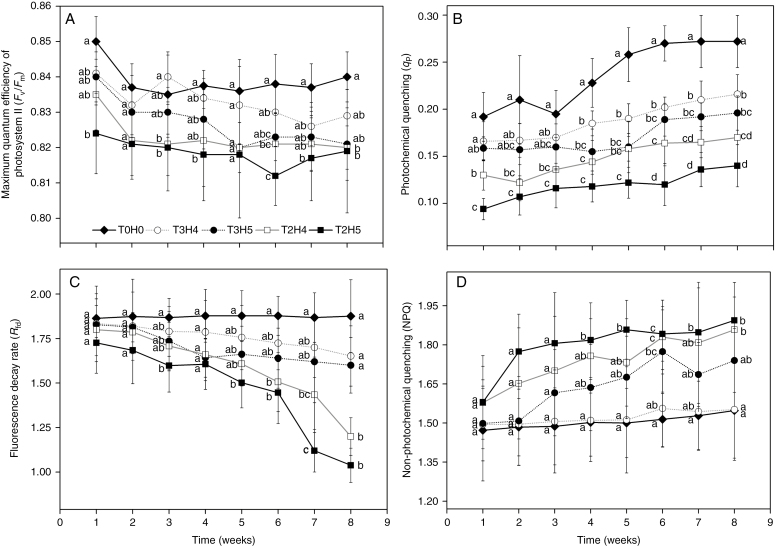
Chlorophyll fluorescence parameters indicative of the plant’s ability to produce carbohydrates, such as Fv/Fm (RM-ANOVA, F28,140 = 2.539, P < 0.01),qP (RM-ANOVA, F28,140 = 1.561, P < 0.05) and Rfd (RM-ANOVA, F28,140 = 2.567, P < 0.01), were generally lower in the wave treatment groups than in the control (Fig. 4). At the same time, NPQ (RM-ANOVA, F12.572,62.859 = 2.162, P < 0.05) increased in the wave treatment groups. Among the different treatments, the qP value was more sensitive to wave stress than the other chlorophyll fluorescence parameters, and all wave treatment groups exhibited significantly lower qP values than the control group throughout most of the experiment.
Fig. 4.
Temporal variation in chlorophyll fluorescence parameters in leaves of S. alterniflora during the experimental period when exposed to different wave treatments. Data are mean ± s.d., n = 5. Different letters at the same time point indicate a significant difference between different wave treatment groups (P < 0.05).
Discussion
In accordance with our hypotheses, we observed that wave exposure subjected salt-marsh plants to oxidative stress with concomitant negative effects on photosynthetic capacity and growth. In response, wave-exposed Spartina exhibited activated antioxidant enzymes and tended to allocate more biomass to roots to enhance anchorage. Also, the wave effects were found to depend on wave height and frequency such that waves with greater height and frequency generally imposed more stress on plants. Overall, the results of this study indicate that long-term wave exposure has a major influence on the performance of this key temperate salt-marsh species, with possible repercussions for the response of the habitat to storm events.
Growth and ecophysiological responses
The reduction in S. alterniflora stem height caused by wave stress supports the hypothesis that long-term wave exposure suppresses its growth, which is consistent with previous laboratory and field investigations on wave exposure of the salt-marsh plants S. lacustris (Coops and Van der Velde, 1996) and S. maritimus (Silinski et al., 2018). In previous studies on the effects of mechanical stresses induced by flow and turbulence on submerged freshwater macrophytes (Ellawala et al., 2013; Atapaththu and Asaeda, 2015), reduced shoot height was attributed to the retardation of tissue elongation, i.e. thigmomorphogenesis (Biddington, 1986). Physiologically, this effect is also related to reduced photosynthetic capacity under wave stress. This response allows the plant to conserve energy and initiate the appropriate defence mechanisms necessary for survival (Van Breusegem et al., 2001).
The decreased above-ground/below-ground biomass ratio indicated that plants allocated more biomass to their roots to enhance their anchorage against wave impact. Similar shifts in biomass allocation have been observed in seagrasses (Peralta et al., 2006) and submerged freshwater macrophytes (Ellawala et al., 2013). The gradual scouring of the surface substrate and hence the exposure of the top roots due to wave impacts make enhanced anchorage imperative for plant survival. Notably, however, previous studies on freshwater plant responses to increased flow regimes (Puijalon et al., 2007) and seagrass responses to waves (La Nafie et al., 2012) also reported contrasting effects of reduced below-ground growth. It is well established that moderate water stirring reduces the thickness of the diffusive boundary layer and leads to enhanced nutrient and gas fluxes, and hence increased photosynthesis and plant growth (Bornette and Puijalon, 2011; Asaeda et al., 2017). Therefore, the above-ground/below-ground biomass ratio may depend on the trade-off between the positive effects of wave exposure in the reduced diffusive boundary layer (especially for leafy plants) and negative effects, including scouring, which is presumably dependent on wave height and the frequency of wave exposure, with greater wave height and frequency likely leading to more negative effects and vice versa. Thus, the contrasting effects of reduced below-ground growth reported in La Nafie et al. (2012) may be attributed to the longer wave period (~50 s) and hence lower frequency in their experiment.
The elevated antioxidant enzyme activities in wave-exposed plants support the hypothesis that the ROS-scavenging system is activated when salt-marsh plants are exposed to waves. ROS levels in plants increase in response to hydrodynamic stresses and consequently trigger increases in antioxidant enzyme activities. For instance, previous studies reported the accumulation of H2O2 and elevated activity levels of antioxidant enzymes, including CAT, APX, POD and GPX, in some aquatic macrophytes subjected to turbulence (Ellawala et al., 2011b; Atapaththu and Asaeda, 2015). Indole acetic acid (IAA) is the principal form of auxin involved in the stimulation of plant elongation by inducing cell division. High POD activities are known to catabolize IAA and reduce auxin-regulated growth (Siegel, 1993), which may lead to reduced plant growth. Notably, POD is one of the ROS-scavenging enzymes with a high affinity and is activated in short-term responses (Dominguez et al., 2010). Therefore, a decrease in POD activity may follow long-term exposure to mechanical stress (Kankanamge et al., 2011; Ellawala et al., 2013). However, POD activity measured in our study remained in the range of steady increase, albeit at varying rate.
Increased MDA content supports the hypothesis that long-term wave exposure causes oxidative damage to S. alterniflora. Previous studies regarding the effect of flow turbulence on aquatic plants also showed that with increasing turbulence the MDA content increased (Asaeda and Rashid, 2017). Observation of chlorophyll fluorescence parameters supports the hypothesis that the photosynthetic capacity of wave-stressed plants is reduced. This finding corroborates Ellawala et al. (2011a), who reported a reduction in photosynthetic capacity (reflected in reduced qP and increased NPQ values) in plants exposed to turbulence. Trends similar to the reduced Fv/Fm ratio detected in this study were observed by Ellawala et al. (2013) and Atapaththu and Asaeda (2015).
As mentioned above, plant response to water motion depends on the trade-off between positive and negative effects and thus may vary with the magnitude of the motion (Bornette and Puijalon, 2011; Asaeda et al., 2017). Indeed, comparison of the present findings with the results of previous studies indicates that the biomass allocation of wave-exposed plants depends on the trade-off and thus wave height and frequency, with greater wave height and frequency likely leading to increased below-ground biomass. In principle, this trade-off between positive and negative wave effects should apply equally to all the growth and ecophysiological responses we examined here. However, the results obtained in our study suggested a general positive correlation between wave effects and wave force (stress), which is further correlated positively with wave height and negatively with wave period. This suggests that the wave parameters adopted in our study fell mostly within the more stressful range, and thus the negative wave effects dominated.
Projected changes in wave climate: impacts on Spartina-dominated marshes
Our experimental findings imply that the successful adaptation of the salt-marsh plant S. alterniflora to long-term wave exposure may be largely attributed to its response mechanisms, such as its antioxidant defence system and shifted biomass allocation. Long-term wave exposure also caused notable negative effects on the plants, as indicated by lipid peroxidation and reduced photosynthetic capacity and plant growth. Given the dependence of the wave effects on wave parameters, it is of interest to assess how the projected changes in global wave environment due to climate change would impact the stress on S. alterniflora communities and hence their resilience.
According to a recent study on the projected changes in wave environment due to climate change (Hemer et al., 2013; note that these wave–climate projections are used throughout the discussion), we would foresee more wave-induced stressful conditions for the species to grow along the southern part of the east coast of North America and the Gulf coast, as a result of projected increase in wave height plus decrease in wave period (Supplementary Data Fig. S1b, c). In contrast, along the Pacific coast of North America the wave climate is predicted to be less stressful due to projected decrease in wave height plus increase in wave period, whereas for the rest of the primary geographical distribution areas of the species (e.g. the northern part of the east coast of North America, the coast of China and the European Atlantic coast; Supplementary Data Fig. S1a), the net effects are hard to assess due to the counteracting effects of concurrently decreasing wave height and period. Current findings imply that extra care should be taken to preserve S. alterniflora marshes in those areas of its native range where wave conditions are expected to become more stressful, i.e. the southern part of the east coast of North America and the Gulf coast, as plant growth performance will suffer. This may imply that extra management measures are needed in these areas to preserve the extant marshes and to be able to use S. alterniflora in eco-shoreline projects. Wave-attenuating reefs fronting the marshes may offer solutions (e.g. Bouma et al., 2014). Whereas for the invasive range of the Pacific coast of North America the foreseeable less stressful conditions would mean more prevention and mitigation efforts. Finally, it should be noted that the above discussion is restricted to wave stress. Further studies should hence focus on the interaction between wave stress and other climate-change and anthropogenic stress factors, such as sea-level rise, increasing temperature and eutrophication.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix A: shallow-water wave simulator. Appendix B: selection of wave parameters and functional relationship between wave stress, wave height and wave period. Figure S1: (a) global distribution of Spartina alterniflora; (b) (left panel) averaged multi-model annual significant wave height for the time-slice representing the present climate (∼1979–2009); (right panel) projected future changes in multi-model averaged annual significant wave height for the future time-slice (∼2070–2100) relative to the present climate time-slice (∼1979–2009); (c) (left panel) averaged multi-model annual mean wave period for the time-slice representing the present climate (∼1979–2009); (right panel) projected future changes in multi-model averaged annual mean wave period for the future time-slice (∼2070–2100) relative to the present climate time-slice (∼1979–2009).
FUNDING
This work was supported by the Key Project of the National Natural Science Foundation of China (grant 51639001), the National Key Research and Development Program of China (grant 2016YFA0602304) and Interdisciplinary Research Funds of Beijing Normal University.
LITERATURE CITED
- Anderson ME, Smith JM. 2014. Wave attenuation by flexible, idealized salt marsh vegetation. Coastal Engineering 83: 82–92. [Google Scholar]
- Arora A, Sairam RK, Srivastava GC. 2002. Oxidative stress and antioxidative system in plants. Current Science 82: 1227–1238. [Google Scholar]
- Asaeda T, Rashid MH. 2017. Effects of turbulence motion on the growth and physiology of aquatic plants. Limnologica 62: 181–187. [Google Scholar]
- Asaeda T, Sanjaya K, Kaneko Y. 2017. Effects of mechanical stressors caused by mean flow and turbulence on aquatic plants with different morphologies. Ecohydrology 10: e1873. [Google Scholar]
- Atapaththu KSS, Asaeda T. 2015. Growth and stress responses of Nuttall's waterweed Elodea nuttallii (Planch) St. John to water movements. Hydrobiologia 747: 217–233. [Google Scholar]
- Augustin LN, Irish JL, Lynett P. 2009. Laboratory and numerical studies of wave damping by emergent and near-emergent wetland vegetation. Coastal Engineering 56: 332–340. [Google Scholar]
- Barbier EB, Koch EW, Silliman BR, et al. 2008. Coastal ecosystem-based management with nonlinear ecological functions and values. Science 319: 321–323. [DOI] [PubMed] [Google Scholar]
- Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81: 169–193. [Google Scholar]
- Biddington NL. 1986. The effects of mechanically-induced stress in plants – a review. Plant Growth Regulation 4: 103–123. [Google Scholar]
- Bornette G, Puijalon S. 2011. Response of aquatic plants to abiotic factors: a review. Aquatic Sciences 73: 1–14. [Google Scholar]
- Bouma TJ, De Vries MB, Low E, et al. 2005. Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. Ecology 86: 2187–2199. [Google Scholar]
- Bouma TJ, Friedrichs M, Klaassen P, et al. 2009. Effects of shoot stiffness, shoot size and current velocity on scouring sediment from around seedlings and propagules. Marine Ecology Progress Series 388: 293–297. [Google Scholar]
- Bouma TJ, De Vries MB, Herman PMJ. 2010. Comparing ecosystem engineering efficiency of two plant species with contrasting growth strategies. Ecology 91: 2696–2704. [DOI] [PubMed] [Google Scholar]
- Bouma TJ, van Belzen J, Balke T, et al. 2014. Identifying knowledge gaps hampering application of intertidal habitats in coastal protection: opportunities & steps to take. Coastal Engineering 87: 147–157. [Google Scholar]
- Bouma TJ, van Belzen J, Balke T, et al. 2016. Short-term mudflat dynamics drive long-term cyclic salt marsh dynamics. Limnology and Oceanography 61: 2261–2275. [Google Scholar]
- Van Breusegem F, Vranova E, Dat JF, Inze D. 2001. The role of active oxygen species in plant signal transduction. Plant Science 161: 405–414. [Google Scholar]
- Callaghan DP, Bouma TJ, Klaassen P, van der Wal D, Stive MJF, Herman PMJ. 2010. Hydrodynamic forcing on salt-marsh development: distinguishing the relative importance of waves and tidal flows. Estuarine Coastal and Shelf Science 89: 73–88. [Google Scholar]
- Canalejo A, Martinez-Dominguez D, Cordoba F, Torronteras R. 2014. Salt tolerance is related to a specific antioxidant response in the halophyte cordgrass, Spartina densiflora. Estuarine Coastal and Shelf Science 146: 68–75. [Google Scholar]
- Coops H, Van der Velde G. 1996. Effects of waves on helophyte stands: mechanical characteristics of stems of Phragmites australis and Scirpus lacustris. Aquatic Botany 53: 175–185. [Google Scholar]
- Dominguez DM, Garcia FC, Raya AC, Santiago RT. 2010. Cadmium-induced oxidative stress and the response of the antioxidative defense system in Spartina densiflora. Physiologia Plantarum 139: 289–302. [DOI] [PubMed] [Google Scholar]
- Duarte B, Marques JC, Cacador I. 2016. Ecophysiological response of native and invasive Spartina species to extreme temperature events in Mediterranean marshes. Biological Invasions 18: 2189–2205. [Google Scholar]
- Ellawala C, Asaeda T, Kawamura K. 2011a Influence of flow turbulence on Chara fibrosa: growth, stress, and tissue carbon content. Journal of Freshwater Ecology 26: 507–515. [Google Scholar]
- Ellawala C, Asaeda T, Kawamura K. 2011b Influence of flow turbulence on growth and indole acetic acid and H2O2 metabolism of three aquatic macrophyte species. Aquatic Ecology 45: 417–426. [Google Scholar]
- Ellawala C, Asaeda T, Kawamura K. 2013. Water movement induced variations in growth regulation and metabolism of freshwater macrophyte Vallisneria spiralis L. in early growth stages. Hydrobiologia 709: 173–182. [Google Scholar]
- Friess DA, Krauss KW, Horstman EM, et al. 2012. Are all intertidal wetlands naturally created equal? Bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems. Biological Reviews 87: 346–366. [DOI] [PubMed] [Google Scholar]
- Gedan KB, Kirwan ML, Wolanski E, Barbier EB, Silliman BR. 2011. The present and future role of coastal wetland vegetation in protecting shorelines: answering recent challenges to the paradigm. Climatic Change 106: 7–29. [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930. [DOI] [PubMed] [Google Scholar]
- Hemer MA, Fan Y, Mori N, Semedo A, Wang XL. 2013. Projected changes in wave climate from a multi-model ensemble. Nature Climate Change 3: 471. [Google Scholar]
- Henry PY, Myrhaug D. 2013. Wave-induced drag force on vegetation under shoaling random waves. Coastal Engineering 78: 13–20. [Google Scholar]
- IPCC. 2014. Core Writing Team, Pachauri RK, Meyer LA, eds. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC. [Google Scholar]
- Jadhav RS, Chen Q, Smith JM. 2013. Spectral distribution of wave energy dissipation by salt marsh vegetation. Coastal Engineering 77: 99–107. [Google Scholar]
- Kankanamge CE, Asaeda T, Kawamura K. 2011. The effect of flow turbulence on plant growth and several growth regulators in Egeria densa Planchon. Flora 206: 1085–1091. [Google Scholar]
- Knutson PL, Brochu RA, Seelig WN, Inskeep M. 1982. Wave damping in Spartina alterniflora marshes. Wetlands 2: 87–104. [Google Scholar]
- Lam KM, Xia LP. 2001. Experimental simulation of a vertical round jet issuing into an unsteady cross-flow. Journal of Hydraulic Engineering 127: 369–379. [Google Scholar]
- Manis JE, Garvis SK, Jachec SM, Walters LJ. 2015. Wave attenuation experiments over living shorelines over time: a wave tank study to assess recreational boating pressures. Journal of Coastal Conservation 19: 1–11. [Google Scholar]
- Marsooli R, Wu WM. 2014. Numerical investigation of wave attenuation by vegetation using a 3D RANS model. Advances in Water Resources 74: 245–257. [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7: 405–410. [DOI] [PubMed] [Google Scholar]
- Moller I. 2006. Quantifying saltmarsh vegetation and its effect on wave height dissipation: results from a UK East coast saltmarsh. Estuarine Coastal and Shelf Science 69: 337–351. [Google Scholar]
- Moller I, Kudella M, Rupprecht F, et al. 2014. Wave attenuation over coastal salt marshes under storm surge conditions. Nature Geoscience 7: 727–731. [Google Scholar]
- Moller IM, Jensen PE, Hansson A. 2007. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 58: 459–481. [DOI] [PubMed] [Google Scholar]
- Moradi F, Ismail AM. 2007. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of Botany 99: 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Nafie YA, de los Santos CB, Brun FG, van Katwijk MM, Bouma TJ. 2012. Waves and high nutrient loads jointly decrease survival and separately affect morphological and biomechanical properties in the seagrass Zostera noltii. Limnology and Oceanography 57: 1664–1672. [Google Scholar]
- Navrot N, Rouhier N, Gelhaye E, Jacquot JP. 2007. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiologia Plantarum 129: 185–195. [Google Scholar]
- Ozeren Y, Wren DG, Wu W. 2014. Experimental investigation of wave attenuation through model and live vegetation. Journal of Waterway Port Coastal and Ocean Engineering 140: 04014019. [Google Scholar]
- Paquier AE, Haddad J, Lawler S, Ferreira C. 2017. Quantification of the attenuation of storm surge components by a coastal wetland of the US mid Atlantic. Estuaries and Coasts 40: 930–946. [Google Scholar]
- Paul M, Rupprecht F, Moller I, et al. 2016. Plant stiffness and biomass as drivers for drag forces under extreme wave loading: a flume study on mimics. Coastal Engineering 117: 70–78. [Google Scholar]
- Peralta G, Brun FG, Perez-Llorens JL, Bouma TJ. 2006. Direct effects of current velocity on the growth, morphometry and architecture of seagrasses: a case study on Zostera noltii. Marine Ecology Progress Series 327: 135–142. [Google Scholar]
- Puijalon S, Lena JP, Bornette G. 2007. Interactive effects of nutrient and mechanical stresses on plant morphology. Annals of Botany 100: 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht F, Moller I, Paul M, et al. 2017. Vegetation-wave interactions in salt marshes under storm surge conditions. Ecological Engineering 100: 301–315. [Google Scholar]
- Shepard CC, Crain CM, Beck MW. 2011. The protective role of coastal marshes: a systematic review and meta-analysis. PLoS ONE 6: e27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel BZ. 1993. Plant peroxidases – an organismic perspective. Plant Growth Regulation 12: 303–312. [Google Scholar]
- Silinski A, Heuner M, Schoelynck J, et al. 2015. Effects of wind waves versus ship waves on tidal marsh plants: a flume study on different life stages of Scirpus maritimus. PLoS ONE 10: e0118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinski A, Schoutens K, Puijalon S, et al. 2018. Coping with waves: plasticity in tidal marsh plants as self-adapting coastal ecosystem engineers. Limnology and Oceanography 63: 799–815. [Google Scholar]
- Temmerman S, De Vries MB, Bouma TJ. 2012. Coastal marsh die-off and reduced attenuation of coastal floods: a model analysis. Global and Planetary Change 92–93: 267–274. [Google Scholar]
- Temmerman S, Meire P, Bouma TJ, Herman PMJ, Ysebaert T, De Vriend HJ. 2013. Ecosystem-based coastal defence in the face of global change. Nature 504: 79–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.