Abstract
Background
Cerebellar mutism syndrome (CMS) is a common complication following resection of posterior fossa tumors, most commonly after surgery for medulloblastoma. Medulloblastoma subgroups have historically been treated as a single entity when assessing CMS risk; however, recent studies highlighting their clinical heterogeneity suggest the need for subgroup-specific analysis. Here, we examine a large international multicenter cohort of molecularly characterized medulloblastoma patients to assess predictors of CMS.
Methods
We assembled a cohort of 370 molecularly characterized medulloblastoma subjects with available neuroimaging from 5 sites globally, including Great Ormond Street Hospital, Christian Medical College and Hospital, the Hospital for Sick Children, King Hussein Cancer Center, and Lucile Packard Children’s Hospital. Age at diagnosis, sex, tumor volume, and CMS development were assessed in addition to molecular subgroup.
Results
Overall, 23.8% of patients developed CMS. CMS patients were younger (mean difference −2.05 years ± 0.50, P = 0.0218) and had larger tumors (mean difference 10.25 cm3 ± 4.60, P = 0.0010) that were more often midline (odds ratio [OR] = 5.72, P < 0.0001). In a multivariable analysis adjusting for age, sex, midline location, and tumor volume, Wingless (adjusted OR = 4.91, P = 0.0063), Group 3 (adjusted OR = 5.56, P = 0.0022), and Group 4 (adjusted OR = 8.57 P = 9.1 × 10−5) tumors were found to be independently associated with higher risk of CMS compared with sonic hedgehog tumors.
Conclusions
Medulloblastoma subgroup is a very strong predictor of CMS development, independent of tumor volume and midline location. These findings have significant implications for management of both the tumor and CMS.
Keywords: cerebellar affective disorder, cerebellar mutism, medulloblastoma, posterior fossa syndrome, postoperative cerebellar mutism
Key Points.
1. Molecular subgroup is a powerful predictor of developing cerebellar mutism syndrome after resection for medulloblastoma.
2. Large Groups 3 and 4 tumors are at highest risk of developing cerebellar mutism syndrome.
Importance of the Study.
Medulloblastoma is now clearly recognized as being 4 distinct molecular subgroups with clear clinical differences. Cerebellar mutism syndrome is a common occurrence after surgery for medulloblastoma; however, accurate risk prediction remains a challenge. We show that by incorporating molecular subgroup into risk modeling, we can more accurately predict the occurrence of cerebellar mutism. The observation that larger Group 3 and Group 4 medulloblastoma tumors increase the risk of CMS further supports the hypothesis that debulking large midline tumors more frequently perturbs proximal cerebellar output tracts. Our study provides further evidence of the clear clinical differences between the 4 medulloblastoma subgroups, and provides a potential framework for preoperative prediction of the development of cerebellar mutism. Applying radiogenomic preoperative prediction of subgroup can potentially allow the consideration of neoadjuvant chemotherapy approaches or other new preventative measures for medulloblastoma patients at highest risk for the development of cerebellar mutism.
Cerebellar mutism syndrome (CMS), also known as posterior fossa syndrome, is a common condition which develops after surgery for cerebellar tumors in children.1–3 CMS is a complex constellation of neurological symptoms, but there is a consensus that postoperative pediatric CMS is characterized by delayed-onset mutism and reduced speech and emotional lability.1 Other symptoms can occur, including hypotonia, oropharyngeal dysfunction/dysphagia, and brainstem dysfunction. The mutism is transient but speech and language dysfunction often persist, with frequent long-term neurocognitive impairment.4–6 In several series, CMS is a significant predictor of reduced IQ. The cause of CMS is likely secondary to disruption of cerebellar outflow tracts, and previously it has been suggested that the splay of the superior cerebellar peduncle pre-surgically has a role in the pathogenesis of CMS.7,8 Other studies of clinical and neuroanatomic predictors have suggested larger tumor size, left-handedness, and white matter changes in the cerebello-thalamo-cortical pathways as significant predictors of CMS.4 Across several studies, surgical factors have been shown not to modify the risk of CMS, suggesting that different approaches are needed in those patients potentially at risk.8
Genomic advances in the past decade have revealed significant heterogeneity across medulloblastoma, and it is now clear that medulloblastoma consists of at least 4 distinct molecular subgroups with highly disparate demographics, genetics, cell of origin, and outcomes.9–13 Indeed, the 4 subgroups—Wingless (WNT), sonic hedgehog (SHH), Group 3, and Group 4—represent distinct disease entities, and can even be distinguished using conventional MRI, based partly on their distinct locations.14–18 SHH tumors are almost always within the cerebellum, and frequently located laterally in the cerebellar hemispheres; WNT tumors frequently arise out of the lateral recess; and Groups 3 and 4 occupy the fourth ventricle.13,14,19,20 Previously it has been shown that Group 4 tumors have worse neurocognitive outcomes, and it has been suggested that CMS is less common in SHH patients.5 However, no comprehensive study has evaluated whether subgroup can be used as a predictor of CMS. In order to examine the association between medulloblastoma subgroup and the development of CMS, we assembled a large cohort of 370 patients, and interrogated whether there is a subgroup specificity of CMS.
Materials and Methods
Study Cohort
This international, multicenter study was approved by the institutional review board or research ethics board of all participating institutions, including Christian Medical College and Hospital (CMCH), Great Ormond Street Hospital (GOSH), the Hospital for Sick Children (HSC), King Hussein Cancer Center (KHCC), and the Lucile Packard Children’s Hospital (LPCH). All medulloblastoma patients at each institution from 2000–2018 were included. Patients were screened for inclusion based on the availability of preoperative MRI allowing for measurement of tumor volume, molecular subgrouping, and the availability of perioperative clinical notes allowing for determination of CMS development. Patient age at diagnosis and sex were additionally gathered on review of patient charts. In total, we assembled a cohort of 370 medulloblastoma patients from our 5 sites as follows: CMCH (n = 89), GOSH (n = 25), HSC (n = 111), KHCC (n = 98), and LPCH (n = 47). A summary of patient demographics and clinical characteristics by molecular subgroup can be found in Table 1.
Table 1.
Clinical characteristics by medulloblastoma subgroup
| WNT (n = 77) | SHH (n = 85) | Group 3 (n = 64) | Group 4 (n = 102) | Non-WNT/SHH (n = 42) | P-value | |
|---|---|---|---|---|---|---|
| Age | 10.05 a (7.50–14.00) | 5.30 (2.65–11.90) | 4.90 (3.09–7.48) | 8.55 (6.00–10.60) | 9.00 (5.00–12.00) | 2.07x10 −11 |
| Male sex | 0.49a | 0.58 | 0.70 | 0.74 | 0.71 | 0.0040 |
| Tumor volume (cm3) | 62.32b (39.20–84.08) | 74.18 b (56.84–117.40) | 60.70b (40.95–95.00) | 69.50 b (45.57–100.00) | 68.15 b (48.45–99.81) | 0.0317 |
| Midline location | 0.77 | 0.42 | 0.91 | 0.92 | 0.90 | 8.2 × 10−16 |
| CMS | 0.21 | 0.07 | 0.31 | 0.35 | 0.24 | 4.2 × 10− 5 |
a The age and sex of one patient with a WNT tumor was unknown and was not included in the analysis.
b Tumor volume was determined for WNT (n = 71), SHH (n = 74), Group 3 (n = 55), Group 4 (n = 91), and non-WNT/SHH patients (n = 40).
Continuous variables are presented as medians with interquartile range provided in parentheses and were compared using the Kruskal–Wallis test. Categorical variables are presented as fractions and were compared using the chi-square test. Significant P-values <0.05 are in bold. Non-WNT/SHH represent unassigned Group 3 or Group 4 cases.
CMS Status
Patients were identified as having developed CMS based on review of perioperative neuro-oncology and/or neurosurgery notes stating that the patient had CMS and/or by documented neurologic exams noting mutism and at least one other symptom of CMS. CMS was defined as per the recent Delphi consensus conference.1
Tumor Location and Volume
Tumor location was classified as either midline or lateral on review of axial T2-weighted MRI. Tumor volume was calculated after measuring largest craniocaudal, anteroposterior, and transverse diameters. Craniocaudal diameter was measured on sagittal T1-weighted MRI, and anteroposterior and transverse diameters were measured on axial T2-weighted MRI. All patients from CMCH, GOSH, HSC, KHCC, and LPCH were imaged on a 1.5T or 3T scanner. (CMCH: 1.5T Siemens Avanto, 1.5T and 3T Philips Intera Achieva; GOSH: 1.5T Siemens Avanto, 3T Siemens Prisma; HSC: 1.5T GE Signa, 3T Philips Intera Achieva, 1.5T Siemens Avanto; KHCC: 1.5T Siemens Avanto, 3T Philips Ingenia; LPCH: 1.5T GE Signa or 3T GE Discovery).
Molecular Subgrouping
Medulloblastoma subgroup determination of HSC, GOSH, KHCC, and LPCH patients was performed as previously described using nanoString limited gene expression profiling and/or Illumina genome-wide methylation arrays.21–23 At CMCH, SHH, WNT and non-SHH/WNT subgroups were ascertained using immunohistochemical markers including the antibodies B-catenin, growth factor receptor bound protein 2–associated-binding protein 1, filamin A, and Yes-associated protein 1.
Statistical Methods
Statistical analyses were performed using R statistical software (v3.6.1) with an a priori significance level of P < 0.01 to correct for multiple analyses within each subgroup. Categorical variables (midline location, sex, CMS) were compared across subgroups and within subgroups stratified by CMS status using the chi-square test. Continuous variables (age and volume) were compared across subgroups using the Kruskal–Wallis test. The Mann–Whitney test was used to compare differences in continuous variables stratified by CMS status. Welch’s t-test was used to calculate mean difference for continuous variables stratified by CMS status. On multivariable analysis of predictors of CMS we sequentially isolated the risk of relevant variables using a generalized linear model. For assessment of subgroup-specific risk, we specifically adjusted for age, sex, midline location, and tumor volume.
Results
Patient Characteristics by CMS Status
Overall, we found that 23.8% of our patients developed CMS (Table 2). Patients who developed CMS were diagnosed with medulloblastoma at a younger age (mean difference −2.05 years ± 0.50, P = 0.0218). There was no difference in sex between patients who developed CMS and those who did not (P = 0.4037). CMS patients had tumors with greater volumes (mean difference 10.25 cm3 ± 4.60, P = 0.0010), which were more often located in the midline (odds ratio [OR] = 5.72, P = 6.6 × 10−5).
Table 2.
Clinical characteristics of medulloblastoma patients by CMS status
| No CMS (n = 282) | CMS (n = 88) | Unadjusted Odds Ratio/ Difference Between Means | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|
| Age | 8.00a (4.80–12.00) | 7.00 (4.54–9.28) | 2.05 ± 0.50 | 1.07–3.04 | 0.0218 |
| Male sex | 0.60 a | 0.65 | 1.23 | 0.75–2.02 | 0.4037 |
| Tumor volume (cm3) | 63.80 (40.39–95.00) | 80.90 (60.40–101.60) | −10.25 ± 4.60 | −19.33–1.17 | 0.0010 |
| Midline location | 0.70 | 0.93 | 5.72 | 2.55–12.60 | 6.6 × 10−5 |
a The age and sex of one patient who did not develop CMS was unknown and was not included in the analysis.
b Tumor volume was determined for CMS patients (n = 80) and non-CMS patients (n = 251). Continuous variables are presented as medians with interquartile range provided in parentheses and compared using the Mann–Whitney test. Categorical variables are presented as fractions and were compared using the chi-square test. Welch’s t-test was used to calculate mean difference for continuous variables stratified by CMS status. Significant P-values <0.05 are in bold.
Patient Characteristics by Medulloblastoma Subgroup
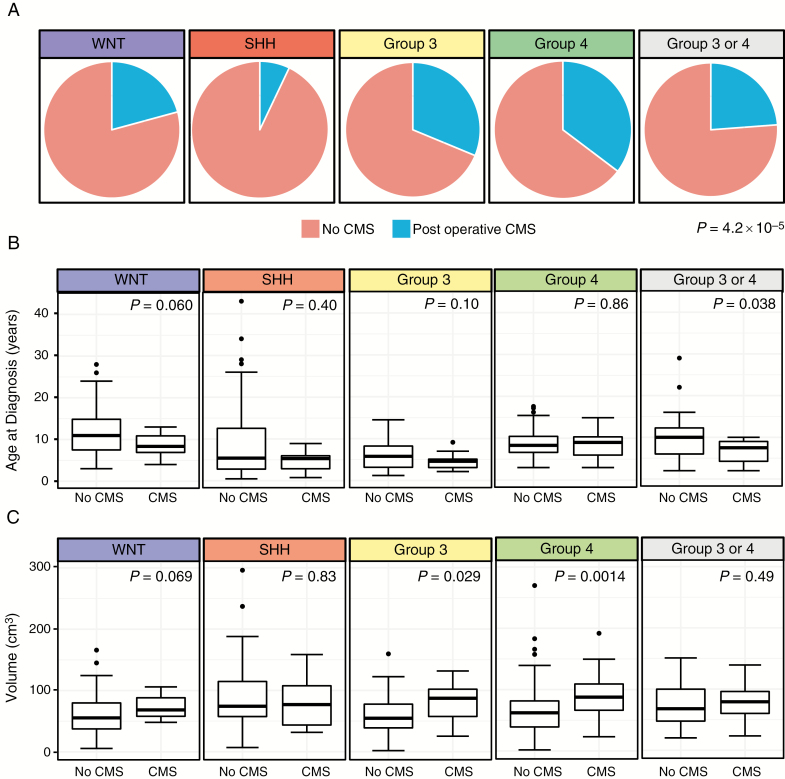
CMS incidence varies significantly by subgroup (P = 4.2 × 10−5) (Fig. 1A). Group 4 patients had the highest rate of CMS, with 35% of patients developing the postoperative complication. Group 3 patients followed closely behind at 31%. SHH patients had the lowest rate of CMS at 7%. WNT patients had an intermediate rate of CMS standing at 21% (Table 1). Age at diagnosis (P = 2.07 × 10−11), sex distribution (P = 0.0040), and tumor volume (P = 0.0317) varied significantly among the different subgroups. Within subgroups, age was not significantly associated with an increased risk for CMS, although there was a trend within non-SHH groups toward younger age being a predisposing factor (Fig. 1B). Within subgroups, greater tumor volume significantly increased risk of CMS within Group 4 (P = 0.0012) patients, with a trend toward increased risk within WNT (P = 0.0697) and Group 3 (P = 0.0281) (Fig. 1C).
Fig. 1.
(A) Proportion of cases developing CMS by medulloblastoma subgroup (P = 0.0001, chi-square test). (B) Age at diagnosis in years stratified by medulloblastoma subgroup and CMS status. Boxes represent median and interquartile range and whiskers represent 10–90% confidence intervals (Mann–Whitney test). (C) Tumor volume in cm3 stratified by medulloblastoma subgroup and CMS status. Boxes represent median and interquartile range and whiskers represent 10–90% confidence intervals (Mann–Whitney test).
Multivariable Analysis of Risk Factors
On multivariable analysis we found that WNT (adjusted OR = 4.91, P = 0.006), Group 3 (adjusted OR = 5.56, P = 0.002), and Group 4 (adjusted OR = 8.57 P = 9.1 × 10−5) tumor subgroups independently increase the risk of CMS compared with the SHH subgroup (Table 3). Tumor volume increased risk of CMS in a linear fashion, with tumors between 50 and 100 cm3 having an adjusted OR of 2.18 (P = 0.025) and tumors greater than 100 cm3 having an adjusted OR of 2.78 (P = 0.010), compared with tumors less than 50 cm3. Older age decreased the risk of CMS (adjusted OR = 0.90, P = 0.015). Midline location (adjusted OR = 1.92, P = 0.196) and sex (adjusted OR = 0.77, P = 0.347) were not independent predictors of developing CMS.
Table 3.
Multivariable analysis of predictors of CMS (N = 328)
| Adjusted Odds Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|
| Age | 0.90 | 0.83–0.98 | 0.015 |
| Male sex | 0.60 | 0.33–1.08 | 0.090 |
| Midline location | 1.92 | 0.75–5.57 | 0.196 |
| Tumor volume (cm3) | |||
| <50 | Reference | Reference | |
| 50–100 | 2.18 | 1.12–4.36 | 0.025 |
| >100 | 2.78 | 1.28–6.15 | 0.010 |
| SHH | Reference | Reference | |
| WNT | 4.91 | 1.64–16.52 | 0.0063 |
| Group 3 | 5.56 | 1.95–17.94 | 0.0022 |
| Group 4 | 8.57 | 3.11–27.33 | 9.1 × 10−5 |
Significant P-values <0.05 are in bold.
Discussion
Herein we show in the largest molecularly characterized medulloblastoma cohort to date that molecular subgroup is a strong predictor of CMS. Our study suggests that many of the previous risk factors identified for CMS, specifically midline location, younger age, and increased tumor volume are likely reflections of underlying subgroup. Moreover, by using molecular subgroup we have been able to develop a more robust model of prediction of CMS.
Our finding that SHH medulloblastoma are at low risk for the development of CMS is consistent with previous observations that midline tumors are at much higher risk for development of CMS.4,7,24 Lateral location is strongly enriched in SHH medulloblastoma; however, CMS was very rare in the 40% of SHH with a midline location, which accounts for why in our multivariable model incorporating subgroup, midline location is not an independent predictor of CMS. Indeed, we and others have previously shown that almost all SHH medulloblastoma are not in the fourth ventricle but rather within the cerebellum itself, pertaining to the disparate cells of origin between the 4 groups. Specifically, SHH arises from the external granule layer, while WNT is thought to arise from the lower rhombic lip, Group 3 from Nestin+ cells, and Group 4 from the unipolar brush cells.13 This is supported by the 4 cases of CMS in SHH that we observed where tumor was arising from the vermis.16,19 This is consistent with the previous hypotheses that the putative cause of CMS is an increased distance or splay between the superior cerebellar peduncles, resulting in postsurgical swelling and disruption of the proximal dentatothalamocortical pathway.7 Indeed, our findings of large volume midline WNT, Group 3, and Group 4 tumors being at highest risk for CMS are consistent with this finding, whereas cerebellar SHH tumors do not disrupt these cerebellar outflow tracts to the same degree (Fig. 2). This is supported by our observation that although 20% of WNT tumors have a lateral location, none developed CMS. Alternatively, it is possible that the subgroup-specific differences pertain more to the 4 subgroups having distinct regional neuronal circuitry pertaining to their cell of origin.22,25 Specifically, it is possible that the unipolar brush cells that Group 4 tumors arise from disrupt different neuronal circuits than the external granule cell derived SHH tumors, due to subgroup-specific interactions with their neuronal microenvironment. Further studies, such as quantitative diffusion tensor MRI methods that probe tissue microarchitecture, may provide further insight into the relative contribution of global anatomic location and remodeling of the microenvironment postoperatively.
Fig. 2.
(A) Nine-year-old female who presented with a Group 4 medulloblastoma and subsequently developed CMS. Postoperative MRI shows mild T2 signal abnormality and subtle irregular contour of the superior cerebellar peduncles (SCPs) (arrows). (B) Corresponding color-encoded fractional anisotropy (FA) map of diffusion tensor MRI shows attenuated directionality of the SCPs (arrows). (C) Color FA map of a 9-year-old boy who presented with a Group 3 medulloblastoma and did not develop CMS. The SCP projections appear intact (arrows) with more robust color-encoded directionality compared with B. (D) Color FA map of a 21-month-old boy who presented with an SHH medulloblastoma and did not develop CMS. The SCP projections are slightly displaced, but the color-encoded directionality is overall preserved (arrows) compared with B.
Our multivariable regression model shows that even when accounting for age, volume and midline location, WNT, Group 3, and Group 4 tumors have a significantly increased risk of developing CMS compared with SHH tumors. Previously we have shown that radio-genomics can be applied to predict subgroup preoperatively; specifically, SHH tumors are almost always cerebellar and Group 3 and Group 4 tumors are fourth ventricular tumors which do not enhance.14,16,26 WNT tumors frequently arise from the lateral recess. Alternatively, previous reports that surgical approach is not a statistically significant predictor of the development of CMS were not subgroup specific, and as such, it is possible that alternate perioperative strategies may be effective when enriching for the highest risk groups.3,8,27,28 The emergence of pharmacological interventions such as zolpidem and bromocriptine may possibly play a role preoperatively or in the immediate postoperative period in the highest risk patients, although these interventions have not shown robust data in properly controlled prospective trials.29–33 Indeed, we have recently shown that machine learning can be applied to predict subgroup on preoperative imaging, suggesting that incorporation of clinical variables such as risk of mutism could be done remotely, and consistently allowing for early intervention.26 A provocative strategy could be consideration of neoadjuvant chemotherapy for patients considered to be at highest risk for the development of mutism. Indeed, this approach has been evaluated in a pilot study in France without compromising survival, suggesting that it is at least worthy of consideration.34
Our study has the limitations of a retrospectively collected cohort. However, we compiled a large sample of all consecutive cases across 5 diverse hospitals in an attempt to mitigate bias. There are unfortunately no robust prospective studies of predictors of CMS development, which has been a major limitation of all previous studies of CMS. Prospective evaluation of the development of CMS by cooperative groups with rigid inclusion criteria is required to advance our understanding of this condition, with pre- and postoperative speech-language pathology assessments. Our results provide a framework for the prediction of the highest risk patients, and suggest that preoperative or emerging intraoperative methods to determine molecular subgroup can allow for early identification of the highest risk patients.
Taken together, this study highlights another significant clinical difference between the 4 core medulloblastoma subgroups. Our work provides further insights into risk factors for CMS, and support potential mechanisms of its development, specifically perturbation of the cerebellar outflow tracts. These results are in line with our previous work suggesting that long-term outcomes have a subgroup specificity, and suggest that supportive care studies in medulloblastoma should also be conducted in a subgroup-specific manner. Future studies of CMS risk in medulloblastoma should incorporate molecular subgroup, opening up a potential new avenue of robust preoperative prediction.
Supplementary Material
Conflict of interest statement. The authors declare no conflicts of interest.
Authorship statement. Conceptualization: NA, KY, VR. Methodology: RJ, NA, KM, KWY, VR. Investigation: RJ, NA, DY. Data curation: RJ, NA, DY, LN, SVS, PM, GC, LGM. Formal analysis: RJ, KWY, VR. Writing, review, and editing: RJ, KWY, VR. Resources: YH, ST, SVS, PM, SL, GC, LGM, PGF, DH, EB, UB, UT, MDT, SMP, KA, GAG, KM, KWY, VR. Project administration: KWY, VR. Supervision: KWY, VR. Funding acquisition: KWY, VR.
Funding
Funding for this work was provided by operating funds from the Canadian Institutes of Health Research, American Brain Tumor Association, the Garron Family Cancer Center, Meagan’s Walk, b.r.a.i.n.child, Nelina’s Hope, the C.R. Younger Foundation, and the Brain Tumor Foundation of Canada to VR. NA was funded by a Conquer Cancer Foundation of ASCO Long-term International Fellowship. Any opinion, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer Foundation. NA was also supported by funds from the Intramural Research Grant Program of the King Hussein Cancer Center. Funds from the Lucile Packard Children’s Foundation Brain & Behavior Center Award in Pediatric Neurosciences went to KY. Funds from the Pediatric Brain Tumor Foundation, Canadian Institutes for Health Research, and National Institutes of Health (R01CA148699 and R01CA159859) went to MDT. All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health was made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
References
- 1. Gudrunardottir T, Morgan AT, Lux AL, et al. ; Iceland Delphi Group Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–1203. [DOI] [PubMed] [Google Scholar]
- 2. Robertson PL, Muraszko KM, Holmes EJ, et al. ; Children’s Oncology Group Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg. 2006;105(6 Suppl):444–451. [DOI] [PubMed] [Google Scholar]
- 3. Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37(5):885–893. [DOI] [PubMed] [Google Scholar]
- 4. Law N, Greenberg M, Bouffet E, et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol. 2012;14(10):1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moxon-Emre I, Taylor MD, Bouffet E, et al. Intellectual outcome in molecular subgroups of medulloblastoma. J Clin Oncol. 2016;34(34):4161–4170. [DOI] [PubMed] [Google Scholar]
- 6. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
- 7. Morris EB, Phillips NS, Laningham FH, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132(Pt 11):3087–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Renne B, Radic J, Agrawal D, et al. Cerebellar mutism after posterior fossa tumor resection in children: a multicenter international retrospective study to determine possible modifiable factors [published online ahead of print January 18, 2019]. Childs Nerv Syst. 2019. doi:10.1007/s00381-019-04058-7. [DOI] [PubMed] [Google Scholar]
- 9. Nör C, Ramaswamy V. Clinical and pre-clinical utility of genomics in medulloblastoma. Expert Rev Neurother. 2018;18(8):633–647. [DOI] [PubMed] [Google Scholar]
- 10. Ramaswamy V, Taylor MD. Medulloblastoma: from myth to molecular. J Clin Oncol. 2017;35(21):2355–2363. [DOI] [PubMed] [Google Scholar]
- 11. Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramaswamy V, Remke M, Adamski J, et al. Medulloblastoma subgroup-specific outcomes in irradiated children: who are the true high-risk patients? Neuro Oncol. 2016;18(2):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vladoiu MC, El-Hamamy I, Donovan LK, et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature. 2019;572(7767):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perreault S, Ramaswamy V, Achrol AS, et al. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35(7):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wefers AK, Warmuth-Metz M, Pöschl J, et al. Subgroup-specific localization of human medulloblastoma based on pre-operative MRI. Acta Neuropathol. 2014;127(6):931–933. [DOI] [PubMed] [Google Scholar]
- 16. Mata-Mbemba D, Zapotocky M, Laughlin S, Taylor MD, Ramaswamy V, Raybaud C. MRI characteristics of primary tumors and metastatic lesions in molecular subgroups of pediatric medulloblastoma: a single-center study. AJNR Am J Neuroradiol. 2018;39(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zapotocky M, Mata-Mbemba D, Sumerauer D, et al. Differential patterns of metastatic dissemination across medulloblastoma subgroups. J Neurosurg Pediatr. 2018;21(2):145–152. [DOI] [PubMed] [Google Scholar]
- 18. Patay Z, DeSain LA, Hwang SN, Coan A, Li Y, Ellison DW. MR imaging characteristics of wingless-type-subgroup pediatric medulloblastoma. AJNR Am J Neuroradiol. 2015;36(12):2386–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramaswamy V, Remke M, Shih D, et al. Duration of the pre-diagnostic interval in medulloblastoma is subgroup dependent. Pediatr Blood Cancer. 2014;61(7):1190–1194. [DOI] [PubMed] [Google Scholar]
- 20. Raybaud C, Ramaswamy V, Taylor MD, Laughlin S. Posterior fossa tumors in children: developmental anatomy and diagnostic imaging. Childs Nerv Syst. 2015;31(10):1661–1676. [DOI] [PubMed] [Google Scholar]
- 21. Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hovestadt V, Smith KS, Bihannic L, et al. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature. 2019;572(7767):74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31(6):737–754.e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu JF, Dineen RA, Avula S, et al. Development of a pre-operative scoring system for predicting risk of post-operative paediatric cerebellar mutism syndrome. Br J Neurosurg. 2018;32(1):18–27. [DOI] [PubMed] [Google Scholar]
- 25. Vladoiu MC, El-Hamamy I, Donovan LK, et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature. 2019;572(7767):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iv M, Zhou M, Shpanskaya K, et al. MR imaging-based radiomic signatures of distinct molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2019;40(1):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson EM, Bramall A, Herndon JE 2nd, Taylor MD, Ramaswamy V. The clinical importance of medulloblastoma extent of resection: a systematic review. J Neurooncol. 2018;139(3):523–539. [DOI] [PubMed] [Google Scholar]
- 28. Thompson EM, Hielscher T, Bouffet E, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17(4):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shyu C, Burke K, Souweidane MM, et al. Novel use of zolpidem in cerebellar mutism syndrome. J Pediatr Hematol Oncol. 2011;33(2):148–149. [DOI] [PubMed] [Google Scholar]
- 30. Brefel-Courbon C, Payoux P, Ory F, et al. Clinical and imaging evidence of zolpidem effect in hypoxic encephalopathy. Ann Neurol. 2007;62(1):102–105. [DOI] [PubMed] [Google Scholar]
- 31. Clauss R, Nel W. Drug induced arousal from the permanent vegetative state. NeuroRehabilitation. 2006;21(1):23–28. [PubMed] [Google Scholar]
- 32. Adachi J, Nishikawa R, Hirose T, Matsutani M. Mixed neuronal-glial tumor of the fourth ventricle and successful treatment of postoperative mutism with bromocriptine: case report. Surg Neurol. 2005;63(4):375–379. [DOI] [PubMed] [Google Scholar]
- 33. Caner H, Altinörs N, Benli S, Calişaneller T, Albayrak A. Akinetic mutism after fourth ventricle choroid plexus papilloma: treatment with a dopamine agonist. Surg Neurol. 1999;51(2):181–184. [DOI] [PubMed] [Google Scholar]
- 34. Grill J, Lellouch-Tubiana A, Elouahdani S, et al. Preoperative chemotherapy in children with high-risk medulloblastomas: a feasibility study. J Neurosurg. 2005;103(4 Suppl):312–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.