Abstract
Background
Resting-state functional neuroimaging captures large-scale network organization; whether this organization is intact or disrupted during adolescent development across the psychosis spectrum is unresolved. We investigated the integrity of network organization in psychosis spectrum youth and those with first episode psychosis (FEP) from late childhood through adulthood.
Methods
We analyzed data from Philadelphia Neurodevelopmental Cohort (PNC; typically developing = 450, psychosis spectrum = 273, 8–22 years), a longitudinal cohort of typically developing youth (LUNA; N = 208, 1–3 visits, 10–25 years), and a sample of FEP (N = 39) and matched controls (N = 34). We extracted individual time series and calculated correlations from brain regions and averaged them for 4 age groups: late childhood, early adolescence, late adolescence, adulthood. Using multiple analytic approaches, we assessed network stability across 4 age groups, compared stability between controls and psychosis spectrum youth, and compared group-level network organization of FEP to controls. We explored whether variability in cognition or clinical symptomatology was related to network organization.
Results
Network organization was stable across the 4 age groups in the PNC and LUNA typically developing youth and PNC psychosis spectrum youth. Psychosis spectrum and typically developing youth had similar functional network organization during all age ranges. Network organization was intact in PNC youth who met full criteria for psychosis and in FEP. Variability in cognitive functioning or clinical symptomatology was not related to network organization in psychosis spectrum youth or FEP.
Discussion
These findings provide rigorous evidence supporting intact functional network organization in psychosis risk and psychosis from late childhood through adulthood.
Keywords: resting-state functional neuroimaging, longitudinal, schizophrenia, community detection
Introduction
The first symptoms of psychosis often emerge during adolescence, when the incidence of psychotic disorders increases.1–3 Many brain systems affected in psychosis also show a protracted developmental course normatively, with changes occurring throughout adolescence and into adulthood,1,4 suggesting that adolescence is a time when neural circuitry relevant to emergence of psychosis is vulnerable. Still, the specific role that adolescent neurodevelopment plays in the emergence of psychosis is not fully known.
Many hypothesize psychotic symptoms result from aberrant integration of information processing between networks, resulting in “dysconnectivity” between brain regions.5–7 We define dysconnectivity as disrupted synaptic communication that influences intrinsic functional brain connectivity.7 However, at what point in the developmental course of psychosis this dysconnectivity emerges is not known. Aberrant, large-scale functional reorganization of the brain from late adolescence and early adulthood could contribute to the development of psychotic symptoms8 and reflect the dysconnectivity believed to occur in psychosis.
Ideally, brain network organization, as measured by resting-state functional connectivity (rsfMRI), could detect cumulative effects of neuronal circuit “dysconnectivity.” The brain is composed of individual regions that cooperate in networks to perform multiple cognitive operations. Networks are identified by dense connectivity within regions that “work together,” and weaker connectivity with other regions.9 Brain network organization is defined by how specific regions of the brain group together to form clusters (ie, networks or communities,10,11). For our purposes, we consider “canonical network organization” to be the labeled networks in a widely used, published parcellation.12
A large body of research has shown that network organization measurements provide a framework to assess large-scale, canonical coordination of the functional brain.11,13–19 In healthy adults, approximately 10–20 functional networks have been repeatedly identified and linked to sensory and cognitive functions.11,12,20,21 There is high correspondence between these networks and task-based functional magnetic resonance imaging networks22; furthermore, rsfMRI connectivity predicts task-based MRI connectivity.23–25 It is likely that rsfMRI network organization provides a framework for the engagement of task-based brain activity.
rsfMRI networks are already present in the fetal,26 preterm,27,28 and infant phases of development,29–31 providing evidence that network organization is established early in development. Research in typical development supports this hypothesis, with stable network organization from late childhood through adulthood.10,32 However, there are reports of considerable functional network reorganization during adolescence.13,33 Nevertheless, all extant developmental examinations of network organization assess the community structure as a single snapshot in time, when development is a dynamic process.34,35
Organization of these canonical networks, or how regions of the brain affiliate with one another, may develop abnormally in psychosis, particularly if there is reorganization taking place during adolescence. Using measures that compare the similarity of network clustering in 2 groups,36,37 we can determine to what extent and how network organization becomes disrupted in psychosis. Characterizing age-associated deviations in network organization in psychosis can tell us when the alteration occurs. Understanding how and when age-associated network organization is altered could identify developmentally sensitive periods for intervention in youth at high risk for developing psychosis.38
Although many studies of psychosis report that connectivity between and within rsfMRI communities is altered in psychosis,39–41 the extent to which canonical network organization is disrupted is not fully understood. One study found that, at the group level, network organization in adult schizophrenia patients was visually similar to network organization of healthy adults42; however, they found subtle changes in network structure when they examined individual-level network organization. Another study of first episode psychosis (FEP) found that network organization was intact at baseline and remained stable over 12 months.43 Finally, a recent study found that youth at clinical high risk for psychosis who converted to a psychotic disorder exhibited modular network organization differences in comparison to typically developing youth and high-risk youth who did not develop a psychotic disorder.44 We build on these approaches by probing the age-associated differences in psychosis spectrum youth (8–22 years old) in comparison to typically developing youth, to assess if and when there are distinct alterations in rsfMRI network organization.
A limitation in developmental network organization studies is age-related differences in sources of artifact, namely head motion artifact, which decreases with age. Although initial studies characterizing age-related rsfMRI changes found that network organization continued to change into adulthood,13 research has shown that age-related differences in head motion confounded these connectivity distance findings.45–47 Variations in magnetic resonance imaging (MRI) processing methods also influence the results of network organization studies,45,48,49 and different community detection algorithms or clustering metrics likely provide different results. Given the lack of consistency across graph theory publications in both psychiatry and developmental neuroscience, it is difficult to make comparison across studies.50 To ensure robust and replicable results, we used multiple methods, in this study. In this regard, a new community detection method, persistent communities by eigenvector smoothing (PisCES), was developed to detect time-varying changes in network organization.36 By combining information from multiple time periods longitudinally, inference is strengthened for each individual time period.36 We use PisCES and other more established tools to assess network organization.
We leveraged 2 large cohorts with rsfMRI data to (1) extend upon work showing that network organization is stable across development10 in a longitudinal sample, (2) replicate network organization stability in a cross-sectional typically developing independent cohort, and (3) examine the extent to which this network organization is intact or disrupted across the psychosis spectrum from late childhood through adulthood. To ensure our results generalized to help-seeking individuals meeting full criteria for a psychotic disorder, we compared network organization between FEP and matched controls. Finally, we explored whether clinical symptomatology or cognitive functioning is related to disruption of network organization across the psychosis spectrum.
Participants
Neuroimaging data consisted of participants from 3 samples: one longitudinal dataset acquired at the University of Pittsburgh (LUNA, including a portion of previously-published cross-sectional data) and 2 cross-sectional data sets acquired at the University of Pennsylvania (Philadelphia Neurodevelopmental Cohort [PNC]51,52) and the University of Pittsburgh (Pitt).
LUNA
The LUNA cohort was a longitudinal sample of typically developing youth (1–3 visits, N = 208, 10–25 years old). Participants did not have a psychiatric disorder, as determined by phone screen and a clinical questionnaire.53 Exclusion criteria for all participants included medical illness affecting the central nervous system function, IQ lower than 80,54 a first-degree relative with a major psychiatric disorder, or MRI contraindications.
Philadelphia Neurodevelopmental Cohort.
PNC data were obtained through the Database of Genotypes and Phenotypes platform (B.L., no. 43787). The PNC is a population sample consisting of 9498 youth who participated in neurocognitive and genetic assessment. A subset of this cohort also underwent neuroimaging.55 The final PNC rsfMRI sample size was 723 (N typical development = 450, N psychosis spectrum = 273, 8–22 years old; supplementary figure S1 for sample exclusions).
Psychopathology was assessed using a computerized, structured interview (GOASSESS51). We created categorical and dimensional measures of psychosis from clinical symptom responses to GOASSESS, the Structured Interview for Prodromal Syndromes (SIPS56), and a 12-item PRIME Screen–Revised questionnaire (PS-R57). Categorical psychosis spectrum group was defined as (1) a score that is 2 standard deviations (SDs) or greater than age-matched peers on the SIPS or PS-R, (2) definite or possible hallucinations or delusions endorsed in responses to GOASSESS psychosis items, or (3) a minimum of 1 PS-R item rated 6 (definitely agree) or at least 3 items rated 5 (somewhat agree). This definition is consistent with previous publications.58–61 We also identified a subset of psychosis spectrum youth who met full criteria for a psychotic disorder (supplementary materials).
The PNC typically developing group consisted of youth who denied clinically significant symptoms of psychopathology, based on GOASSESS responses. An Axis I disorder was assigned if (1) symptoms endorsed were consistent with the frequency and duration of symptoms expected from a Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) psychiatric disorder, and (2) accompanied by significant distress or impairment (a rating of ≥5 on a scale of 0–1051). Any individual from the PNC that met criteria for an Axis I disorder (other than a psychotic disorder) was removed from all analyses.
PNC Clinical Symptomatology and Cognitive Functioning
Positive and negative symptoms were measured dimensionally by summing the relevant SIPS/PS-R responses (supplementary table S1). The Wide Range Achievement Test–Fourth Edition reading subtest62 was used in the PNC to assess basic academic ability.
University of Pittsburgh
Inclusion criteria for FEP in the Pitt sample were experiencing one’s first psychotic episode and seeking help for psychotic symptoms for the first time and antipsychotic naive or prescribed antipsychotic treatment for less than 2 months. Diagnoses were determined using information gathered from a Structured Clinical Interview for DSM-IV (SCID63). Inclusion criteria for controls were no lifetime history of a major psychiatric disorder or antipsychotic treatment, and no first-degree family member with a psychotic disorder. Exclusion criteria for all participants included DSM-IV substance abuse disorder currently or within the previous 6 months, significant neurological disorder or head injury or mental retardation as defined by the DSM-IV (IQ < 75), medical illness affecting the central nervous system function, or MRI contraindications.
MR Data Acquisition
Data were acquired using Siemens 3 Tesla Tim Trios. rsfMRI data were collected using an echo-planar sequence sensitive to blood-oxygen-level-dependent contrast (T2*). A magnetization-prepared rapid gradient-echo sequence was acquired to measure brain structure and for alignment of the rsfMRI images. Supplementary table S2 includes scan instructions and parameters for each sample.
rsfMRI Processing
We used resting-state processing methods that are consistent with our previous publications.10,45,61 Briefly, these steps included warping to Montreal Neurological Instituite standardized space, spatial smoothing, wavelet despiking, and simultaneous nuisance regression and bandpass filtering. Details are in the supplementary materials.
Functional Network Parcellation
We applied a previously defined functional connectome parcellation of 333 functional regions of interest (ROIs) across cortical structures (ie, the “reference parcellation”12) to each participant’s rsfMRI data. We chose this parcellation because the identified networks were well validated and replicated in healthy adults and it is widely used as a standard rsfMRI parcellation in pediatric and adult samples.12,23,64–68 For each participant, we computed Pearson correlations of each ROI’s time series with every other ROI (producing a 333 × 333 correlation matrix). Pearson correlations were transformed to z-values using Fisher z transformation.
Statistical Analysis
Group Network Organization
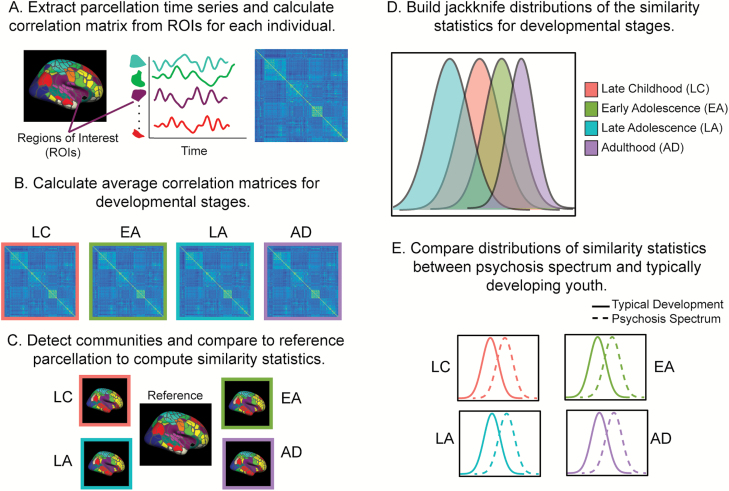
Participants were grouped into 1 of 4 age groups: late childhood (LUNA: 10–12 years, PNC: 8–12 years), early adolescence (13–15 years), late adolescence (16–19 years), and adults (20+ years). These groups map onto distinct developmental phases that are demarked by physical, social, and psychological changes69,70 and are consistent with relevant literature.10 Individual correlation matrices were averaged within each age group (figure 1A and B). We applied 2 community detection techniques: Louvain and PisCES. Within each age group, the Louvain method divides the nodes (ROIs) into different communities by grouping together nodes that are densely connected with each other, and separating weakly connected nodes into other communities.37 For the Louvain method, network resolution parameter γ was set to 1.3; when this parameter was applied to all data, the Louvain method identified a similar number of communities14 to the reference parcellation.12 PisCES performs dynamic community detection by combining all age groups into a series and using an eigenvector smoothing algorithm to estimate time-varying community structure in the data.36
Fig. 1.
(A) After resting-state fMRI data were processed, we extracted out the time series from an established parcellation12 and calculated a correlation matrix for each individual. (B) We grouped individuals by developmental stages (late childhood, early adolescence late adolescence, adulthood) and calculated an average correlation matrix for each stage. (C) We detected the communities (using Louvain and persistent communities by eigenvector smoothing [PisCES]) for each cohort at each developmental stage. We compared the community structure to the reference parcellation12 and calculated multiple similarity metrics (Normalized Mutual Information, Adjusted Rand Index). (D) We built a jackknife distribution for the mean correlation for each developmental stage by leave-one-out resampling to obtain a distribution of the mean, compare means for different developmental stages, and produce P values for the significance of the differences in mean. (E) To compare network organization of psychosis spectrum youth to typical development, we compared the jackknife distributions at each developmental stage.
To compare the similarity of the identified network organization to the reference parcellation18 (figure 1C), we calculated 2 measures used to compare clusters of variables, Normalized Mutual Information (NMI) and Adjusted Rand Index (ARI). Both have been used to compare sets of network assignments in rsfMRI data.10,33 ARI and NMI values range from 0 to 1, with 0 values referring to complete dissimilarity of the 2 networks and a value of 1 referring to identical community assignment.
Assessment of Stability Across Development
Within each cohort (LUNA, PNC) and each group (patient, typically developing), we used a jackknife procedure to create subpopulations for each age group and produce NMI and ARI distributions. We chose the jackknife procedure because it is an assumption-free analytic approach that maintains good power.71,72 For each age group with n subjects, we applied the jackknife procedure with leave-one-out resampling. Community detection was performed on the average correlation matrix of n − 1 subjects. We calculated NMI and ARI values between our community detection results and the reference labels and estimated distributions by repeating this process n times. Jackknife mean, variance, and confidence intervals of NMI and ARI values were created for each age group (figure 1D). To compare each age group to the other, we then subtracted the jackknife mean similarity metric (NMI, ARI) in age group i vs another group j to obtain δij.
The prior calculations treat each age group separately, assuming there are age-specific differences in network organization. What if networks were similar over this range of development? Under this scenario, age is not meaningful for organization. We generated a null distribution by shuffling subjects and randomly assigning them to age group, conditional on achieving an appropriate n. Community detection was performed on each age group. We repeated the procedure 10 000 times, computing the distribution of mean value for each age group, as well as the distribution of NMI and ARI differences between each pair of groups. We compared the original difference δij with null distribution to generate a P value for the significance level of the difference.
Comparison of Typical Development to Patient Samples.
We used jackknifing resampling and permutation testing, as described earlier, to compare distributions of PNC typically developing youth to PNC psychosis spectrum youth in each age group (figure 1E). We also compared the distributions of PNC typically developing youth to PNC youth that met criteria for a psychotic disorder (N = 99) in each age group. To ensure that the PNC results generalized to a more typically diagnosed clinical group, we performed a group-level comparison of the distributions of FEP to matched controls (Pitt sample).
Effects of Possible Confounds
Global signal regression and censoring of high motion subjects significantly affect graph theory metrics73–75; thus, we ran all analyses with and without applying global signal regression and high-motion censoring (framewise displacement [FD] > 0.3 mm). Because the reference parcellation included only cortical regions, we reran all analyses including subcortical regions from the Harvard-Oxford atlas.76 We reran all analyses using 2 additional rsfMRI reference parcellations11,21 because calculating community structure across a defined set of nodes (ie, 333) could limit generalization of findings. We also created a youth-derived reference parcellation from the LUNA sample (supplementary materials) and repeated PNC-related analyses to ensure that our parcellation was suitable for the age ranges examined.
Previous studies of network organization in psychosis have conducted statistical analyses on similarity metrics calculated at the individual level (eg, Lerman-Sinkoff and Barch42). To ensure consistency with previous studies, we performed comparable analyses (supplementary materials).
Use of Repeated Measures
We had repeated measures in the analyses of network organization stability in the LUNA cohort (1 visit = 126, 2 visits = 48, 3 visits = 34; supplementary figure S2). If we explicitly modeled the effects of repeated measures, the correlation due to observations on the same subject will increase the variance of the mean and is expected to decrease the test statistic for mean differences. However, because we did not model them and take a less conservative approach, we increased the likelihood that we detect a significant difference. If we observed a significant difference, we would have reason to assess this dependence. However, we did not detect significant differences. In addition, the community detection procedures used do not require the samples to be independent. As a final robustness evaluation, we also reanalyzed the age-associated LUNA analyses with cross-sectional data only.
Power Calculations.
We estimated the effect sizes we were powered to detect (R package pwr77). Results are reported in supplementary tables S3 and S4.
Influence of Clinical Symptomatology and Cognitive Functioning on Network Organization in Psychosis Spectrum Youth.
We separated the PNC psychosis spectrum sample into 4 quantile groups based on each selected measure (ie, positive symptoms, negative symptoms, cognitive functioning). Supplementary figure S3 presents distributions of measures and respective quantile groups. We used jackknifing resampling and permutation testing to assess network organization at the different quantiles. We also examined relationships between network organization and these measures using a continuous approach (supplementary materials).
Results
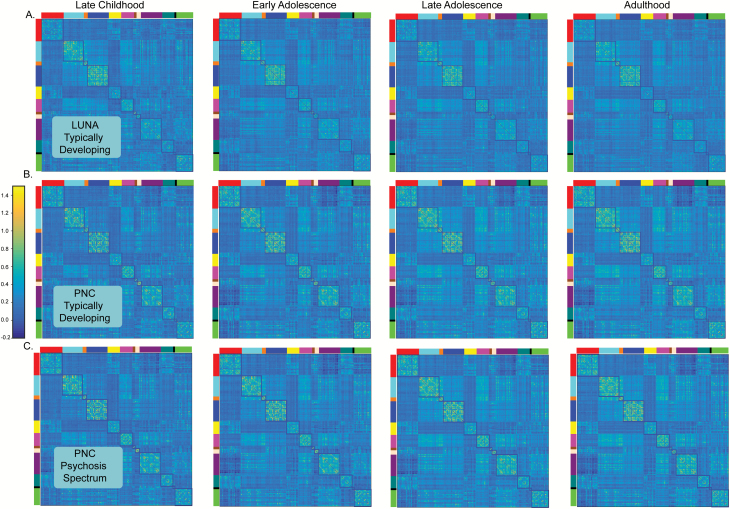
Participant information is presented in table 1. Supplementary table S5 breaks down participant samples by age group. Correlation matrices for LUNA and PNC in each age group are presented in figure 2 and community structure is shown in supplementary figure S4. Notably, the correlation matrices of all 3 cohorts in each age group are quite similar, as is the community structure.
Table 1.
Participant Information for Each Sample
| LUNA Typically Developing (N = 208) | PNC Typically Developing (N = 450) | PNC Psychosis Spectrum (N = 273) | Pitt Controls (N = 34) | Pitt First Episode Psychosis (N = 39) | |
|---|---|---|---|---|---|
| Mean age [+/− SD] | 18.5 [4.3] | 16.1 [3.6] | 16.1 [2.9] | 21.5 [3.3] | 22.0 [3.9] |
| Age range | 10.1–25.9 years | 8–22 years | 8–22 years | 14.7–29 years | 13.0–30.5 years |
| F/M | 156/168 | 229/221 | 151/122 | 12/22 | 14/15 |
| Total positive symptom scorea [+/− SD] | NA | 2.7 [4.6] | 21.3 [13.9] | NA | 12.9 [3.8] |
| Total negative symptom scorea [+/− SD] | NA | 1.5 [1.1] | 4.1 [4.4] | NA | 6.8 [2.5] |
| Mean cognitive functioning scoreb [+/− SD] | 115.6 [11.3] | 104.8 [16.3] | 98.1 [16.9] | 106.9 [10.2] | 107.2 [13.2] |
| Mean framewise displacement [+/− SD] | 0.15 [0.05] | 0.11 [0.06] | 0.12 [0.06] | 0.14 [0.06] | 0.15 [0.05] |
Note: For the Philadelphia Neurodevelopmental Cohort (PNC) youth, positive symptoms (range 0–72) and negative symptoms (range 0–30) scores were derived from summed relevant responses to the Structured Interview for Prodromal Syndromes (SIPS,56), and a 12-item PRIME Screen–Revised questionnaire (PS-R57). Exact items used to calculate these scores can be found in supplementary table S1. Positive and negative symptoms for the University of Pittsburgh (Pitt) first episode psychosis participants were summed from positive and negative symptom responses to the Brief Psychiatric Rating Scale78 ( negative symptom range: 3–21; positive symptom range: 4–28).
aCognitive functioning for the LUNA typically developing youth was determined through the Reynolds Intellectual Assessment Scale.54 Cognitive functioning for the PNC youth was determined from the standardized Wide Range Achievement Test–Fourth Edition reading subtest.62 Cognitive functioning for the Pitt sample was determined using the Wechsler Abbreviated Scale of Intelligence.79
Fig. 2.
Correlation matrices at each developmental stage (late childhood, early adolescence, late adolescence, adulthood) for (A) LUNA typically developing, (B) Philadelphia Neurodevelopmental Cohort (PNC) typically developing, and (C) PNC psychosis spectrum. The correlation structure for all cohorts at each developmental stage is remarkably similar. The blocked colors around the edges of the correlation matrices refer to the resting-state fMRI networks identified from the reference parcellation.12 Networks are defined as follows : red (Default), light blue (somatomotor-hand); orange (somatomotor-hand), dark blue (visual); yellow (Frontoparietal); pink (Auditory); brown (Cingulo-parietal); peach (retrosplenial temporal); purple (Cingulo-opercular); dark green (Ventral Attention) black (Salience); bright green (Dorsal Attention). The color bar reflects the strength of the FIsher-Z transformed correlation.
Network Organization Is Stable From Late Childhood Through Adulthood in Typically Developing Youth and Psychosis Spectrum Youth
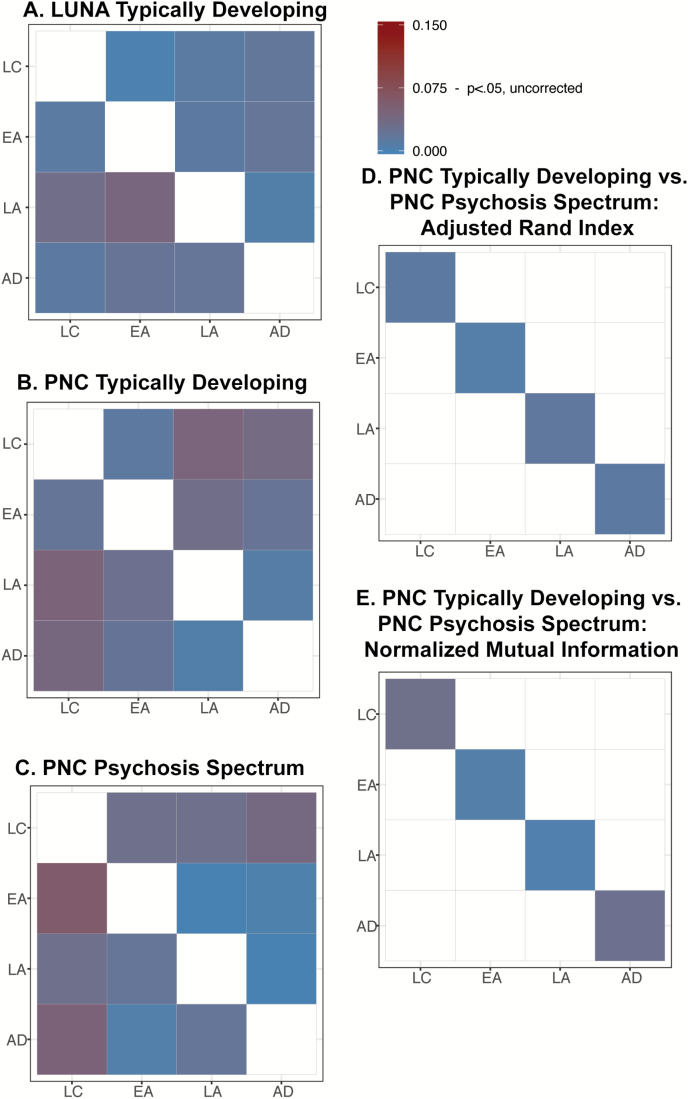
Typically developing youth exhibited stable network organization from 8 to 25 years in LUNA and PNC (figure 3A and B, supplementary table S6, P > .28). LUNA results remained unchanged when we analyzed the only cross-sectional data. PNC psychosis spectrum youth also exhibited stable network organization across this age range (figure 3C, supplementary table S7A, P > .26). Results remained consistent when we analyzed a subset of PNC youth who met criteria for a full psychotic disorder (figure 4A, supplementary table S7B, P > .6). Supplementary figure S5 shows jackknife means and confidence intervals of the similarity metrics.
Fig. 3.
Dissimilarity statistics (δij) for the within cohort developmental comparison in (A) LUNA typically developing, (B) Philadelphia Neurodevelopmental Cohort (PNC) typically developing, and (C) PNC psychosis spectrum. Cool colors indicate that the cohorts are similar to each other, whereas warm colors reflect greater dissimilarity. For these comparisons, the upper right off-diagonals report test statistics for Adjusted Rand Index (ARI) and the lower left off-diagonals report test statistics for Normalized Mutual Information (NMI). Dissimilarity statistics (δij) for the comparison between PNC typically developing and PNC psychosis spectrum youth. (D) Bars reflect the test statistics for NMI. (E) Bars reflect the test statistic for ARI. All test statistics were much smaller than the approximate value needed to reach significance (0.075), uncorrected for multiple comparisons.
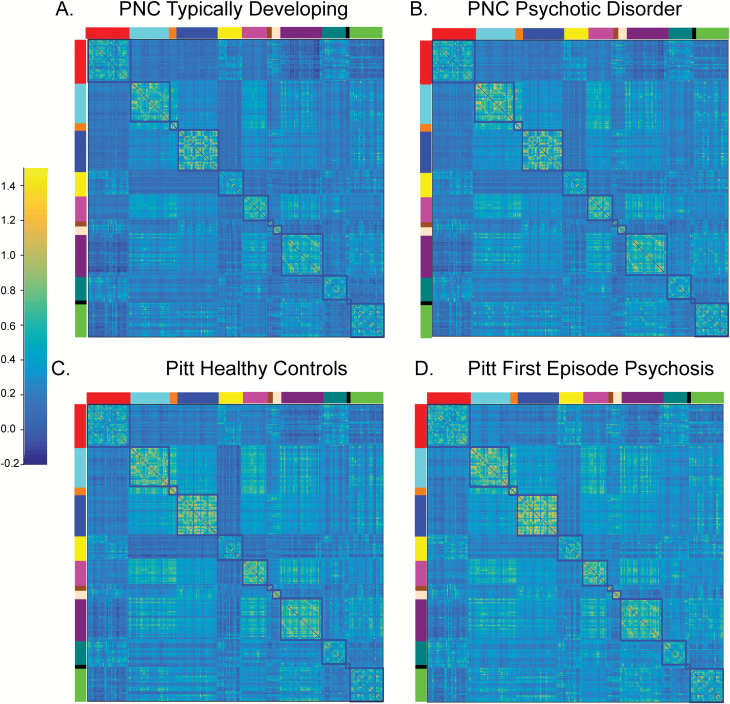
Fig. 4.
Correlation matrices at the group level for (A) Philadelphia Neurodevelopmental Cohort (PNC) typically developing, (B) PNC youth meeting full criteria for a psychotic disorder, (C) University of Pittsburgh (Pitt) healthy controls and (D) Pitt first episode psychosis. The correlation structure for all groups and cohorts is remarkably similar. The blocked colors around the edges of the correlation matrices refer to the resting-state fMRI networks identified from the reference parcellation.12 Networks are defined as follows: red (Default), light blue (somatomotor-hand); orange (somatomotor-hand), dark blue (visual); yellow (Frontoparietal); pink (Auditory); brown (Cingulo-parietal); peach (Retrosplenial Temporal); purple (Cingulo-opercular); dark green (Ventral Attention) black (Salience); bright green (Dorsal Attention). The color bar reflects the strength of the FIsher-Z transformed correlation.
Network Organization Is Intact in Psychosis in Comparison to Typically Developing Youth
In comparison to PNC typically developing youth, psychosis spectrum youth exhibited similar functional network organization in late childhood through adulthood (figure 3B, supplementary table S8, P > .5). Results remained consistent when we examined a subset of PNC youth who met full criteria for a psychotic disorder (figure 4B, supplementary tables S8 and S9, P > .5). In the Pitt sample, in comparison to controls, FEP exhibited intact network organization (supplementary Table S9, P > .16).
Changes to rsfMRI Processing Pipeline, Community Detection Parameters, and Parcellation Do Not Alter Results
We assessed whether all developmental/age-associated and group comparisons remained consistent with methodological changes. Results (supplementary tables S10–S18) remained unchanged when we (1) implemented global signal regression and (2) excluded high-motion repetition times (FD > 0.3 mm) during rsfMRI preprocessing, (3) applied different γ parameters to the Louvain method, (4) added subcortical regions to the reference parcellation, (5) used other reference rsfMRI parcellations,11,21 (6) derived a parcellation from the LUNA typically developing youth and used it as the reference, and (7) conducted analyses on similarity metrics calculated at the individual level.
Clinical Symptom Severity and Cognitive Functioning Are Not Related to Network Organization in Psychosis Spectrum Youth
In PNC psychosis spectrum youth, network organization was not different in quantile levels of cognitive function, or positive or negative symptom severity (P > .68, supplementary table S19). Results remained consistent when we used individual similarity metrics and dimensional measures (Padjusted > 0.36, supplementary table S20).
Discussion
Across adolescent development, network organization is stable in typically developing youth and strikingly similar across independent data sets, as evidenced in other studies.10 Psychosis spectrum youth also exhibit stable network organization during these age ranges. Compared to typically developing youth, psychosis spectrum youth and individuals experiencing their FEP exhibit intact functional network architecture. Our results are consistent with the existing evidence that adolescence is a time of refinement and strengthening of already established networks10,32 and suggest that network organization does not contribute to the development of psychotic symptoms during adolescence. Given that network organization was not different as a function of symptom severity or cognitive function across the psychosis spectrum, our results suggest that psychosis symptom development and cognitive problems in this group are due to other disruptions. Finally, we show that our results remain robust across multiple methods.
To the best of our knowledge, this is the first assessment of network stability in adolescent development using a longitudinal sample and implementing a community detection method (PisCES) that takes advantage of this information.36 PisCES performed similarly to the more commonly used Louvain technique. This similar level of performance is likely due to the fact that PisCES was developed to detect dynamic change, which is not present in our data. Our findings of stable, overall network organization across adolescent development are consistent with previous, cross-sectional reports.10,32 The foundational structure of the brain is laid out quite early in neurodevelopment80 and this “backbone” of the functional architecture remains stable over time. Similarly, resting-state networks are already present in the fetal,26 preterm,27,28 and infant phases of development.29–31
Several previous studies have examined group differences in rsfMRI network organization in adults with psychosis. One study found that, though adults with schizophrenia exhibited visually similar network organization in comparison to healthy controls, there were network organization alterations at the individual level.42 When we implemented a comparable analysis, we did not identify network organization disruptions across the psychosis spectrum (supplementary table S17). These differences may be due to the fact that the sample in Lerman-Sinkoff and Barch42 was older, the majority of the participants had chronic schizophrenia, and all were prescribed antipsychotic medications.
Though our findings support intact network organization in psychosis, they conflict with a recent study that identified disrupted network modularity in clinical high-risk individuals who converted to a psychotic disorder.44 The discrepant results could be due to methodological differences, as Collin et al44 used surface-based rsMRI processing. This study also derived the reference parcellation from controls in their study. Our study tested 3 standard reference parcellations and parcellation derived from same-aged youth in an independent sample, maintaining assumptions of independence. Furthermore, this study44 altered parameters that determine the number of networks identified in the community detection method, group differences were no longer present. However, when we tested multiple parameters, our results remained consistent.
Consistent with our findings, however, a recent report found that resting-state network organization was intact in FEP relative to healthy controls and remained unchanged after a 12-month follow-up.43 Similarly, in another study, when FEP individuals engaged in a cognitive control task, they exhibited comparable functional network architecture to controls.81 We add to this literature, showing that differences do not occur in different phases of adolescent development. We were well powered to detect small effect sizes in our analyses (supplementary tables S4 and S5), and because we examined individuals both at risk for and with a psychotic disorder in separate samples, we can be confident that overall network organization is either wholly intact in psychosis or differs only slightly from typical development. Taken together, these results suggest that brain-wide functional efficiency, as measured by network organization, is not impaired across the psychosis spectrum youth in comparison to typical controls across adolescent development.
We must present our null findings within the context of the existing rsfMRI literature. Many studies report alterations in rsfMRI networks in psychosis, finding altered connectivity with and between regions in the frontal parietal, cingulo-opercular/salience and default mode networks.39–41,82–86 Critically, connectivity between these networks increases through adolescence, whereas connectivity within networks decreases through adolescence.10 We hypothesize that these more subtle between- and within-network changes observed during adolescence, not the network organization metrics measured in this study, underlie the developmental disruptions that contribute to psychosis onset.
Network organization similarity metrics could be insensitive to regionally, targeted effects of pathology or neurodevelopment. Global metrics used in this study were not designed to assess group differences in regional connectivity; thus, we cannot refute studies that found group differences in small-world topology87–90 or changes in seed-based approaches focusing on specific brain regions.91–98 In the future, it is important to incorporate a developmental perspective when assessing these measures in psychosis spectrum youth. For example, we recently found that amygdala connectivity is altered during distinct phases of adolescent development across the psychosis spectrum.61
Limitations
Clinical samples were assessed at 1 timepoint and these cross-sectional data are a limitation. Longitudinal studies of psychosis spectrum youths could be of great value for our understanding of psychosis.99–102 Nonetheless, based on our results, we do not expect such studies to reveal markedly different organization of canonical networks. Though we failed to detect network organization differences, it is possible that the scan length (3–6 min) did not allow for meaningful community detection of the individual connectome. A recent study reported that individual-level network organization could be quantified using >1 hour of data.14 In addition, our analyses were performed in volume space. Surfaced-based analyses can be methodologically superior (eg, improved registration, higher signal preservation) in comparison to volume-based analyses.103–105 Also, the Pitt sample was relatively underpowered in comparison to the PNC, and findings need to be replicated in another FEP sample. Finally, there are alternative community detection techniques that do not require the node to be assigned to only 1 network.106 Some brain regions play important roles in multiple cognitive functions and likely can shift membership to different communities. Such information is neglected by assigning each node to one specific community. Individuals across the psychosis spectrum may lack the flexibility for specific nodes to shift communities.
Conclusions & Future Directions
Our results provide compelling evidence that network organization is stable and intact from late childhood through adulthood in psychosis spectrum youth and FEP. We provide additional evidence of widely distributed, stable networks in 2 independent cohorts of typically developing youth. Future work will examine if and how age-associated integration and segregation of specialized networks are disrupted in psychosis spectrum youth.
Funding
National Institute of Mental Health (R01 MH080243 to B.L., R37 MH057881 to B.D., K01 MH112774 to M.J.).
Conflicts of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
References
- 1. Cannon TD, Chung Y, He G, et al. ; North American Prodrome Longitudinal Study Consortium. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cannon TD, Cadenhead K, Cornblatt B, et al. . Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Häfner H, Maurer K, Löffler W, an der Heiden W, Hambrecht M, Schultze-Lutter F. Modeling the early course of schizophrenia. Schizophr Bull. 2003;29(2):325–340. [DOI] [PubMed] [Google Scholar]
- 4. Sun D, Phillips L, Velakoulis D, et al. . Progressive brain structural changes mapped as psychosis develops in “at risk” individuals. Schizophr Res. 2009;108(1–3):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35(5):1110–1124. [DOI] [PubMed] [Google Scholar]
- 6. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35(3):509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2–3):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collin G, Keshavan MS. Connectome development and a novel extension to the neurodevelopmental model of schizophrenia. Dialogues Clin Neurosci. 2018;20(2):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103(23):8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13(12):e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Power JD, Cohen AL, Nelson SM, et al. . Functional network organization of the human brain. Neuron. 2011;72(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fair DA, Cohen AL, Power JD, et al. . Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gratton C, Laumann TO, Nielsen AN, et al. . Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron. 2018;98(2):439–452.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damoiseaux JS, Rombouts SA, Barkhof F, et al. . Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103(37):13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp. 2013;34(9):2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolt T, Nomi JS, Yeo BTT, Uddin LQ. Data-driven extraction of a nested model of human brain function. J Neurosci. 2017;37(30):7263–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeo BT, Krienen FM, Sepulcre J, et al. . The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glasser MF, Coalson TS, Robinson EC, et al. . A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith SM, Fox PT, Miller KL, et al. . Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106(31):13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cole MW, Ito T, Bassett DS, Schultz DH. Activity flow over resting-state networks shapes cognitive task activations. Nat Neurosci. 2016;19(12):1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krienen FM, Yeo BTT, Buckner RL. Reconfigurable task- dependent functional coupling modes cluster around a core functional architecture. Phil Trans R Soc B Sci 2014;369:20130526. doi:10.1098/rstb.2013.0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mennes M, Kelly C, Zuo XN, et al. . Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50(4):1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomason ME, Brown JA, Dassanayake MT, et al. . Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One. 2014;9(5):e94423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He L, Parikh NA. Brain functional network connectivity development in very preterm infants: the first six months. Early Hum Dev. 2016;98:29–35. [DOI] [PubMed] [Google Scholar]
- 28. Doria V, Beckmann CF, Arichi T, et al. . Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. 2010;107(46):20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. Development of human brain cortical network architecture during infancy. Brain Struct Funct. 2015;220(2):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fransson P, Skiöld B, Horsch S, et al. . Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104(39):15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21(1):145–154. [DOI] [PubMed] [Google Scholar]
- 32. Hwang K, Hallquist MN, Luna B. The development of hub architecture in the human functional brain network. Cereb Cortex. 2013;23(10):2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu S, Satterthwaite TD, Medaglia JD, et al. . Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci USA. 2015;112(44):13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annu Rev Neurosci. 2015;38:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Di Martino A, Fair DA, Kelly C, et al. . Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83(6):1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu F, Choi D, Xie L, Roeder K. Global spectral clustering in dynamic networks. Proc Natl Acad Sci USA. 2018;115(5):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blondel V, Guillaume J, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech. 2008;P10008. [Google Scholar]
- 38. Satterthwaite TD, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol. 2015;30:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baker JT, Holmes AJ, Masters GA, et al. . Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alonso-Solís A, Corripio I, de Castro-Manglano P, et al. . Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr Res. 2012;139(1-3):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Satterthwaite TD, Vandekar SN, Wolf DH, et al. . Connectome-wide network analysis of youth with psychosis-spectrum symptoms. Mol Psychiatry. 2015;20(12):1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lerman-Sinkoff DB, Barch DM. Network community structure alterations in adult schizophrenia: identification and localization of alterations. Neuroimage Clin. 2016;10:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ganella EP, Seguin C, Pantelis C, et al. . Resting-state functional brain networks in first-episode psychosis: a 12-month follow-up study. Aust N Z J Psychiatry. 2018;52(9):864–875. [DOI] [PubMed] [Google Scholar]
- 44. Collin G, Seidman LJ, Keshavan MS, et al. . Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol Psychiatry. 2018. https://www.ncbi.nlm.nih.gov/pubmed/30410064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ciric R, Wolf DH, Power JD, et al. . Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shirer WR, Jiang H, Price CM, Ng B, Greicius MD. Optimization of rs-fMRI pre-processing for enhanced signal-noise separation, test-retest reliability, and group discrimination. Neuroimage. 2015;117:67–79. [DOI] [PubMed] [Google Scholar]
- 50. Hallquist MN, Hillary FG. Graph theory approaches to functional network organization in brain disorders: a critique for a brave new small-world. Netw Neurosci. 2019;3(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calkins ME, Merikangas KR, Moore TM, et al. . The Philadelphia neurodevelopmental cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56(12):1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Satterthwaite TD, Elliott MA, Ruparel K, et al. . Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 2014;86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Achenbach TM, Rescorla LA.. The Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 54. Reynolds CR, Kmaphaus RW.. Reynolds Intellectual Assessment Scales. Lutz, FL: Psychological Assessment Resources Inc.; 2003. [Google Scholar]
- 55. Satterthwaite TD, Connolly JJ, Ruparel K, et al. . The Philadelphia neurodevelopmental cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124(pt B):1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miller TJ, McGlashan TH, Rosen JL, et al. . Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 57. Kobayashi H, Nemoto T, Koshikawa H, et al. . A self-reported instrument for prodromal symptoms of psychosis: testing the clinical validity of the PRIME Screen-Revised (PS-R) in a Japanese population. Schizophr Res. 2008;106(2–3):356–362. [DOI] [PubMed] [Google Scholar]
- 58. Wolf DH, Satterthwaite TD, Calkins ME, et al. . Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2015;72(5):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Calkins ME, Moore TM, Merikangas KR, et al. . The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia neurodevelopmental cohort. World Psychiatry. 2014;13(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Satterthwaite TD, Wolf DH, Calkins ME, et al. . Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2016;73(5):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jalbrzikowski M, Murty VP, Tervo-Clemmens B, Foran W, Luna B. Age-associated deviations of amygdala functional connectivity in youths with psychosis spectrum disorders: relevance to psychotic symptoms. Am J Psychiatry. 2019;176(3):196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. SCID-I/P. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 63. Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 2017;82(7):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miranda-Dominguez O, Feczko E, Grayson DS, Walum H, Nigg JT, Fair DA. Heritability of the human connectome: a connectotyping study. Netw Neurosci. 2018;2(2):175–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shine JM, Bissett PG, Bell PT, et al. . The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron. 2016;92(2):544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Poldrack RA, Laumann TO, Koyejo O, et al. . Long-term neural and physiological phenotyping of a single human. Nat Commun. 2015;6:8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laumann TO, Gordon EM, Adeyemo B, et al. . Functional system and areal organization of a highly sampled individual human brain. Neuron. 2015;87(3):657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hearne LJ, Mattingley JB, Cocchi L. Functional brain networks related to individual differences in human intelligence at rest. Sci Rep. 2016;6:32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lerner RM, Steinberg L, eds. The scientific study of adolescent development. In: Handbook of Adolescent Psychology. Vol 2 Hoboken, NJ: John Wiley & Sons; 2004:1–12. [Google Scholar]
- 70. Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. [DOI] [PubMed] [Google Scholar]
- 71.Efron, B. (1981). Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika, 68(3):589–599. [Google Scholar]
- 72.Efron B & Stein C. (1981). The jackknife estimation of variance. The Annals of Statistics. Vol 9, No 3, 586–596. [Google Scholar]
- 73. Guo CC, Kurth F, Zhou J, et al. . One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage. 2012;61(4):1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Braun U, Plichta MM, Esslinger C, et al. . Test-retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage. 2012;59(2):1404–1412. [DOI] [PubMed] [Google Scholar]
- 75. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. [DOI] [PubMed] [Google Scholar]
- 77. Champely S. pwr: Basic Functions for Power Analysis. n.d. http://CRAN.R-project.org/package=pwr. Accessed April 20, 2019. [Google Scholar]
- 78. Bell M, Milstein R, Beam-Goulet J, Lysaker P, Cicchetti D. The positive and negative syndrome scale and the brief psychiatric rating scale. Reliability, comparability, and predictive validity. J Nerv Ment Dis. 1992;180(11):723–728. [DOI] [PubMed] [Google Scholar]
- 79. Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- 80. Schwartz ML, Goldman-Rakic P. Development and plasticity of the primate cerebral cortex. Clin Perinatol. 1990;17(1):83–102. [PubMed] [Google Scholar]
- 81. Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130(1–3):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Guo W, Liu F, Chen J, et al. . Hyperactivity of the default-mode network in first-episode, drug-naive schizophrenia at rest revealed by family-based case-control and traditional case-control designs. Medicine (Baltimore). 2017;96(13):e6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. [DOI] [PubMed] [Google Scholar]
- 85. Kim DI, Manoach DS, Mathalon DH, et al. . Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009;30(11):3795–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ongür D, Lundy M, Greenhouse I, et al. . Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu Y, Liang M, Zhou Y, et al. . Disrupted small-world networks in schizophrenia. Brain. 2008;131(Pt 4):945–961. [DOI] [PubMed] [Google Scholar]
- 88. Rubinov M, Knock SA, Stam CJ, et al. . Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30(2):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lynall ME, Bassett DS, Kerwin R, et al. . Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30(28):9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Micheloyannis S, Pachou E, Stam CJ, et al. . Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87(1–3):60–66. [DOI] [PubMed] [Google Scholar]
- 91. Anticevic A, Haut K, Murray JD, et al. . Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72(9):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Anticevic A, Cole MW, Repovs G, et al. . Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24(12):3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anticevic A, Brumbaugh MS, Winkler AM, et al. . Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73(6):565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Samudra N, Ivleva EI, Hubbard NA, et al. . Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Res. 2015;233(2):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Woodward ND, Cascio CJ. Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry. 2015;72(8):743–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. . Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Penner J, Ford KA, Taylor R, et al. . Medial prefrontal and anterior insular connectivity in early schizophrenia and major depressive disorder: a resting functional MRI evaluation of large-scale brain network models. Front Hum Neurosci. 2016;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 2013;33(46): 18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Simmonds DJ, Hallquist MN, Luna B. Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: a longitudinal fMRI study. Neuroimage. 2017;157:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Grimm KJ, Ram N, Hamagami F. Nonlinear growth curves in developmental research. Child Dev. 2011;82(5): 1357–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Anticevic A, Dierker DL, Gillespie SK, et al. . Comparing surface-based and volume-based analyses of functional neuroimaging data in patients with schizophrenia. NeuroImage. 2008;41:835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Turner R, Geyer S. Comparing like with like: the power of knowing where you are. Brain Connect. 2014;4(7):547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Glasser MF, Smith SM, Marcus DS, et al. . The Human Connectome Project’s neuroimaging approach. Nat Neurosci. 2016;19(9):1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li W, Jiang S, Jin Q. Overlap community detection using spectral algorithm based on node convergence degree. Future Gener Comput Syst. 2018;79:408–416. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.