Abstract
Background
Although surgery plays a crucial diagnostic role in World Health Organization (WHO) grade II 1p/19q-codeleted oligodendrogliomas, the role of maximal tumor surgical resection remains unclear, with early retrospective series limited by lack of molecular classification or appropriate control groups.
Methods
The characteristics, management, and overall survival (OS) of patients ≥20 years old presenting with histology-proven WHO grade II 1p/19q-codeleted oligodendrogliomas during 2010–2016 were evaluated using the National Cancer Database and validated using multi-institutional data. Patients were stratified by watchful waiting (biopsy only) versus surgical resection. OS was analyzed using Kaplan–Meier methods and risk-adjusted proportional hazards.
Results
Five hundred ninety adults met inclusion criteria, of whom 79.0% (n = 466) underwent surgical resection. Of patient and tumor characteristics, younger patients were more likely to be resected. Achieving gross total resection (GTR; n = 320) was significantly associated with smaller tumors, management at integrated network cancer programs (vs community cancer programs), and Medicare insurance (as compared with no, private, or Medicaid/other government insurance) and independent of other patient or tumor characteristics. In risk-adjusted analyses, GTR, but not subtotal resection (STR), demonstrated improved OS (vs biopsy only: hazard ratio 0.28, 95% CI: 0.09–0.85, P = 0.02).
Conclusions
WHO grade II 1p/19q-codeleted oligodendrogliomas amenable to resection demonstrated improved OS with GTR, but not STR, compared with biopsy-only watchful waiting. The OS benefits of GTR were independent of age, tumor size, or tumor location. Medicare-insured and integrated network cancer program patients were significantly more likely to have GTR than other patients, suggesting that insurance status and care setting may play important roles in access to timely diagnosis or innovations that improve maximal resection.
Keywords: diffuse oligodendroglioma, WHO grade II, extent of resection, surgery, watchful waiting
Key Points.
1. GTR improved overall survival versus biopsy-only in WHO grade II 1p/19q-codeleted oligodendroglioma.
2. Maximal extent of resection was significantly associated with insurance status and hospital type.
Importance of the Study.
Early retrospective series assessing the role of extent of resection in the management of WHO grade II 1p/19q-codeleted oligodendrogliomas were limited by the rarity of these tumors, lack of molecular classification, pooling of different types of diffuse low-grade gliomas, difficulty in establishing appropriate control groups, and substandard assessments. Herein, we examine predictors and outcomes of maximal resection in a national cohort of WHO grade II 1p/19q-codeleted oligodendroglioma patients and find an OS benefit for GTR, but not STR, compared with biopsy alone. Additionally, we identify a novel and significant role of insurance status and hospital type in access to maximal resection for low-grade oligodendrogliomas.
World Health Organization (WHO) grade II diffuse infiltrating low-grade gliomas (LGGs) represent a heterogeneous spectrum of tumors that comprise about 4.5% of all primary brain tumors.1,2 The diagnosis of grade II diffuse LGG has recently been updated to incorporate molecular classification in the 2016 WHO guidelines with prognostic significance, to include isocitrate dehydrogenase (IDH) mutations and whole-arm chromosomal 1p/19q codeletion, thereby subdividing LGGs into grade II IDH-mutant and 1p/19q-codeleted oligodendroglioma, grade II IDH-mutant diffuse astrocytoma, and, rarely, grade II IDH-wildtype diffuse astrocytoma. These tumors are characterized by slower growth rates, younger age of presentation, less focal neurologic deficits, and longer survival than their higher-grade (ie, grades III and IV) glioma counterparts—all of which are important factors in deciding the individual risk-benefit balances of resection, radiotherapy, and chemotherapy for each patient. LGG patients are often further stratified by age and extent of resection (EOR) into low risk (ie, younger than 40 years, with gross total resection [GTR]) and high risk (older than 40 years or with subtotal resection [STR]).
The management of LGGs has been evolving in recent years, with many of the key randomized controlled trials of LGGs largely predicated on older histologic classification.3–6 Early studies have been limited by the relative rarity of these tumors, variable assessments of EOR, and importantly, the lack of molecular classification contributing to the pooling of different types of LGGs.7–9 Although surgery plays a crucial diagnostic role in LGGs, the benefits of maximal tumor resection remain unclear. The decision is particularly complex for asymptomatic LGGs, which are frequently diagnosed incidentally during the radiographic workup of patients with head trauma, headache, or other neurologic symptoms. The incorporation of 1p/19q status in US registry data as of 2010 permits a robust evaluation of the predictors and roles of maximal tumor resection in WHO grade II 1p/19q-codeleted oligodendrogliomas nationally.
Materials and Methods
Data Source and Study Design
The National Cancer Database (NCDB) is hospital based, curated by the Commission on Cancer (CoC), and comprises more than 70% of newly diagnosed cancers in the United States. Patients presenting with histologically diagnosed diffuse gliomas between 2010 and 2016 were identified and defined by the histological codes of the International Classification of Diseases for Oncology, third revision (ICD-O-3, v3.1) (ie, codes 9380/3, 9382/3, 9400/3, 9401/3, 9450/3, and 9451/3), involving all brain site codes (ie, 71.0–71.9), as previously described.10 Diffuse oligodendrogliomas were identified using primary brain-specific factors, including loss of heterozygosity/deletion of both chromosome arms 1p and 19q and WHO grade II. Data on 1p/19q and brain cancer site-specific EOR were available in the US registry as of 2010, from which biopsy-only was defined as local excision (biopsy) of tumor, lesion, or mass, with specimen sent to pathology; STR as subtotal resection of tumor, lesion, or mass of brain; and GTR as radical, total, gross resection of tumor, lesion, or mass in brain. In the case of multiple debulking surgeries for the same nonprogression nonrecurrent primary, EOR was encoded as the cumulative result of those surgeries. Exclusion criteria included a previous diagnosis of cancer, age at diagnosis <20 years, and diagnosis at an index institution and treatment entirely elsewhere. Patients who received chemotherapy or radiotherapy within a year of diagnosis were excluded in order to prevent confounding of the benefits of surgical resection. In order to validate the findings from these national data, grade II IDH-mutant and 1p/19q-codeleted oligodendrogliomas diagnosed from 2005 to 2016 were evaluated from 3 tertiary care institutions (Brigham and Women's Hospital, Dana-Farber Cancer Center, and Massachusetts General Hospital).
Variables and Statistical Analyses
The clinicopathologic factors, including age at diagnosis, sex, race/ethnicity, Charlson-Deyo comorbidity index, insurance status, hospital type, tumor location and size, and Karnofsky performance scale (KPS) score were summarized and compared by χ 2 test and t-test as appropriate. Definitive surgical treatment was stratified as biopsy only (ie, watchful waiting), STR, or GTR. Whether a patient underwent watchful waiting (ie, biopsy only, no surgical resection) or surgical resection and whether EOR was STR or GTR were assessed by multivariable logistic regression, which was risk-adjusted similar to previously described methodology.11
Overall survival (OS) was measured from the date of diagnosis to the date of death, with patients censored at the date of most recent follow-up. For multi-institutional data, progression-free survival (PFS) was measured from the date of diagnosis to the date of radiographic progression, with patients censored at the date of most recent follow-up. Unadjusted differences in OS were estimated by Kaplan–Meier methods and compared by log-rank tests. Multivariable Cox proportional hazards were used to assess the association between surgery and OS, risk-adjusted for clinicopathologic variables. The NCDB excludes survival data for patients diagnosed in the final year of the dataset—which for this dataset was 2016—due to limited follow-up. Statistical analyses were conducted using Stata v14.2, with 2-sided P-values <0.05 designated as significant. This study was approved by the Partners HealthCare institutional review board (#2015P002352).
Results
Characteristics of WHO Grade II 1p/19q-Codeleted Oligodendrogliomas Managed with Biopsy versus Surgery
A total of 590 adults were diagnosed with WHO grade II 1p/19q-codeleted oligodendroglioma from 2010–2016 and met inclusion criteria, of whom 21.0% (n = 124) underwent biopsy only and 79.0% (n = 466) were managed with surgical resection (Table 1). In multivariable logistic analysis, only younger age at diagnosis (50–59 y vs 40–49 y: odds ratio [OR] 0.44, 95% CI: 0.23–0.87, P = 0.02) and more recent diagnosis (2016 vs 2010: OR 2.97, 95% CI: 1.16–7.63, P = 0.02) were associated with resection (Table 1). Sex, insurance status, comorbidity index, race/ethnicity, tumor location, tumor size, and facility type were not significant predictors of undergoing resection versus biopsy only (all P > 0.05; Table 1). There were also no significant differences between KPS score (encoded for only 15% of cases, χ 2P = 0.99), postoperative length of stay (median 3 days, interquartile range [IQR]: 2–5, vs median 3 days, IQR 2–4; t-test P = 0.49), or 30-day readmission rates (4.1% vs 6.9%; χ 2P = 0.26) between biopsy-only and resected cases, respectively.
Table 1.
National characteristics of WHO grade II oligodendrogliomas, stratified by resection
| Biopsy only, n | Resection, n | Univariable χ2 P-value | |||
|---|---|---|---|---|---|
| % | % | Multivariable Logistic Regression | |||
| OR | 95% CI | P-value | |||
| Sex | 124 | 466 | 0.80 | ||
| Female (ref male) | 42.7 | 44.0 | 1.00 | (0.65–1.53) | 0.99 |
| Age, y | 124 | 466 | 0.08 | ||
| median, y (IQR) | 38 (29–52) | 39 (31–49) | n/a | ||
| <40 | 53.2 | 50.6 | 1.22 | (0.57–2.61) | 0.61 |
| 40–49 | 17.7 | 26.6 | Reference | ||
| 50–59 | 21.0 | 13.7 | 0.44 | (0.23–0.87) | 0.02 |
| >60 | 8.1 | 9.0 | 1.15 | (0.41–3.21) | 0.80 |
| Year of diagnosis | 124 | 466 | 0.41 | ||
| 2010 | 12.9 | 9.4 | Reference | ||
| 2011 | 12.1 | 14.4 | 1.79 | (0.77–4.12) | 0.17 |
| 2012 | 21.8 | 15.7 | 1.20 | (0.56–2.60) | 0.64 |
| 2013 | 16.1 | 16.1 | 1.38 | (0.62–3.06) | 0.43 |
| 2014 | 18.6 | 18.7 | 1.72 | (0.78–3.77) | 0.18 |
| 2015 | 9.7 | 11.6 | 2.00 | (0.82–4.86) | 0.13 |
| 2016 | 8.9 | 14.2 | 2.97 | (1.16–7.63) | 0.02 |
| Comorbidity index | 124 | 466 | 0.73 | ||
| 0 | 83.1 | 85.4 | Reference | ||
| 1 | 12.1 | 9.7 | 0.80 | (0.40–1.58) | 0.52 |
| 2 | 4.8 | 4.9 | 0.83 | (0.30–2.30) | 0.72 |
| Race/ethnicity | 123 | 464 | 0.52 | ||
| White, non-Hispanic | 82.1 | 84.5 | Reference | ||
| Nonwhite | 17.9 | 15.5 | 0.96 | (0.54–1.7) | 0.88 |
| Primary payor | 121 | 462 | 0.03 | ||
| Not insured | 11.6 | 7.6 | Reference | ||
| Private insurance | 62.8 | 75.6 | 1.77 | (0.87–3.62) | 0.12 |
| Medicare | 9.9 | 7.6 | 0.94 | (0.32–2.78) | 0.91 |
| Medicaid/other government | 15.7 | 8.9 | 0.72 | (0.30–1.72) | 0.45 |
| Tumor location | 124 | 466 | 0.78 | ||
| Frontal lobe | 65.3 | 68.5 | Reference | ||
| Temporal lobe | 11.3 | 8.4 | 0.64 | (0.32–1.28) | 0.21 |
| Parietal lobe | 11.3 | 11.6 | 1.05 | (0.53–2.06) | 0.89 |
| Other/overlapping | 12.1 | 11.6 | 0.96 | (0.49–1.88) | 0.90 |
| Tumor size, cm | 124 | 466 | 0.17 | ||
| median (IQR) | 4 (3–5) | 4 (4–6) | n/a | ||
| ≤2.0 | 8.1 | 5.6 | 0.85 | (0.35–2.05) | 0.72 |
| 2.1–4.0 | 33.9 | 34.8 | Reference | ||
| 4.1–6.0 | 32.3 | 24.0 | 0.77 | (0.46–1.31) | 0.34 |
| >6.0 | 7.3 | 11.2 | 1.78 | (0.78–4.03) | 0.17 |
| n/a | 18.6 | 24.5 | 1.38 | (0.75–2.52) | 0.30 |
| Facility type | 124 | 466 | 0.21 | ||
| Community cancer center | 14.5 | 9.4 | Reference | ||
| Academic/NCI-designated | 25.8 | 33.9 | 1.93 | (0.94–3.95) | 0.07 |
| Integrated network care program | 6.5 | 6.0 | 1.46 | (0.52–4.12) | 0.48 |
| Suppressed due to age <40 y* | 53.2 | 50.6 | Omitted due to collinearity | ||
| KPS** | 13 | 76 | 0.99 | ||
| <70 | 7.7 | 6.6 | n/a | ||
| 70–80 | 23.1 | 22.4 | |||
| 90–100 | 69.2 | 71.1 | |||
NCI = National Cancer Institute.
*NCDB suppresses facility data for patients younger than 40 y to help ensure de-identification; as a result, in multivariable analysis, this factor is collinear with the age <40 factor.
**KPS was encoded for only 15% of cases and so was excluded from multivariable analyses.
A multi-institutional cohort of WHO grade II 1p/19q-codeleted oligodendrogliomas was included (Table 2) in order to further investigate differences between biopsy only (n = 4; 7.1%) and surgical resection (n = 52; 92.9%) and to help validate the national findings. In this cohort, only tumor location (frontal lobe situated: 25.0% vs 71.2%, χ 2P = 0.01) varied significantly between biopsy-only and resection cases, respectively. Sex, age at diagnosis, insurance status, tumor size, involvement of eloquent neuroanatomy, KPS, and incidental presentation (biopsy-only 25.0% vs resection 19.2%, χ 2P = 0.78) were not substantially different (all χ 2P > 0.05). Forty of the cases were encoded into the registry, of which the positive predictive value of EOR coding by brain cancer site-specific EOR variable was 87.5% (two STR cases were encoded as GTRs, two GTR cases were encoded as local excision, and one STR case only had the initial biopsy encoded). All 4 biopsy-only cases, including the 2 registry-encoded cases, were designated by the corresponding neuro-oncology/neurosurgery teams as watchful-waiting management.
Table 2.
Characteristics of multi-institutional WHO grade II oligodendrogliomas, stratified by EOR
| Biopsy Only | STR | GTR | P-value | |
|---|---|---|---|---|
| % | % | % | ||
| Total (n) | 4 | 30 | 22 | |
| Sex | 0.57 | |||
| Female (ref male) | 75.0 | 46.7 | 50.0 | |
| Age, y | 0.47 | |||
| median (IQR) | 42 (33–50) | 38 (32–52) | 40 (31–47) | |
| <40 | 25.0 | 56.7 | 45.5 | |
| 40–49 | 25.0 | 13.3 | 31.8 | |
| 50–59 | 50.0 | 20.0 | 13.6 | |
| >60 | 0 | 10.0 | 9.1 | |
| Primary payor | 0.83 | |||
| Not insured | 0 | 3.3 | 0 | |
| Private insurance | 100.0 | 73.3 | 86.4 | |
| Medicare | 0 | 10.0 | 4.6 | |
| Medicaid | 0 | 13.3 | 9.1 | |
| Tumor location | 0.08 | |||
| Frontal lobe | 25.0 | 73.3 | 68.1 | |
| Temporal lobe | 25.0 | 10.0 | 9.1 | |
| Parietal lobe | 0 | 10.0 | 18.2 | |
| Other/overlapping | 50.0 | 6.7 | 4.6 | |
| Tumor size, cm | 0.16 | |||
| median (IQR) | 7.0 (3.7–7.3) | 5.6 (4.0–7.1) | 5.0 (3.6–5.8) | |
| ≤2.0 | 0 | 0 | 4.6 | |
| 2.1–4.0 | 25.0 | 23.3 | 18.2 | |
| 4.1–6.0 | 0 | 26.7 | 59.1 | |
| >6.0 | 50.0 | 33.3 | 9.1 | |
| n/a | 25.0 | 16.7 | 9.1 | |
| Eloquent location | 0.83 | |||
| Yes (ref no) | 25.0 | 20.0 | 27.3 | |
| Incidental | 0.83 | |||
| Yes (ref no) | 25.0 | 16.7 | 22.7 | |
| KPS | 0.15 | |||
| <70 | 0 | 0 | 4.8 | |
| 70–80 | 66.7 | 22.7 | 9.5 | |
| 90–100 | 33.3 | 77.3 | 85.7 |
Predictors of Extent of Resection in WHO Grade II 1p/19q-Codeleted Oligodendrogliomas
Of the 466 patients managed with surgical resection, GTR was reported in 68.7% (n = 320). Time from initial clinical or radiographic diagnosis to definitive surgery (STR median 6.5 days, IQR 0–36.5; GTR median 14 days, IQR 0–42; t-test P = 0.40) and postoperative length of stay (STR median 3 days, IQR 2–4; GTR median 3 days, IQR 2–4; t-test P = 0.20) did not significantly vary by EOR. In unilateral oligodendrogliomas, the lesion was located on the left in 51.2% of STR and 45.6% of GTR cases (χ 2P = 0.29). Characteristics of patients achieving GTR versus STR are reported in Table 3, with no significant differences between sex, race/ethnicity, age at diagnosis, year of diagnosis, comorbidity index, or tumor location (all P > 0.05) in multivariable logistic analyses.
Table 3.
National characteristics of resected WHO grade II oligodendrogliomas, stratified by EOR
| STR, n | GTR, n | Univariable χ2 P-value | |||
|---|---|---|---|---|---|
| % | % | Multivariable Logistic Regression | |||
| OR | 95% CI | P-value | |||
| Sex | 146 | 320 | 0.72 | ||
| Female (ref male) | 45.2 | 43.4 | 0.84 | (0.55–1.30) | 0.43 |
| Age, y | 146 | 320 | 0.57 | ||
| median (IQR) | 39 (31–50) | 40 (32–48) | n/a | ||
| <40 | 52.1 | 50.0 | 1.34 | (0.59–3.03) | 0.49 |
| 40–49 | 22.6 | 28.4 | Reference | ||
| 50–59 | 15.1 | 13.1 | 0.75 | (0.37–1.51) | 0.42 |
| ≥60 | 10.3 | 8.4 | 0.42 | (0.16–1.13) | 0.09 |
| Year of diagnosis | 146 | 320 | 0.48 | ||
| 2010 | 11.0 | 8.8 | Reference | ||
| 2011 | 13.0 | 15.0 | 1.42 | (0.60–3.41) | 0.43 |
| 2012 | 14.4 | 16.3 | 1.60 | (0.68–3.75) | 0.28 |
| 2013 | 18.5 | 15.0 | 1.08 | (0.47–2.49) | 0.86 |
| 2014 | 18.5 | 18.8 | 1.18 | (0.52–2.71) | 0.69 |
| 2015 | 14.4 | 10.3 | 1.00 | (0.42–2.42) | 1.00 |
| 2016 | 10.3 | 15.9 | 2.06 | (0.84–5.06) | 0.11 |
| Comorbidity index | 146 | 320 | 0.68 | ||
| 0 | 84.9 | 85.6 | Reference | ||
| 1 | 8.9 | 10.0 | 1.11 | (0.54–2.29) | 0.78 |
| 2 | 6.2 | 4.4 | 0.92 | (0.33–2.54) | 0.87 |
| Race/ethnicity | 146 | 320 | 0.38 | ||
| White, non-Hispanic | 86.3 | 83.1 | Reference | ||
| Nonwhite | 13.7 | 16.9 | 1.42 | (0.77–2.63) | 0.26 |
| Primary payor | 146 | 320 | 0.34 | ||
| Not insured | 9.6 | 6.6 | Reference | ||
| Private insurance | 75.3 | 75.3 | 1.60 | (0.74–3.47) | 0.23 |
| Medicare | 4.8 | 8.8 | 5.13 | (1.40–18.84) | 0.01 |
| Medicaid/other government | 10.3 | 9.4 | 1.37 | (0.49–3.81) | 0.55 |
| Tumor location | 146 | 320 | 0.26 | ||
| Frontal lobe | 63.0 | 70.9 | Reference | ||
| Temporal lobe | 11.0 | 7.2 | 0.67 | (0.32–1.40) | 0.29 |
| Parietal lobe | 11.6 | 11.6 | 0.88 | (0.45–1.71) | 0.70 |
| Other/overlapping | 14.4 | 10.3 | 0.69 | (0.37–1.31) | 0.26 |
| Tumor size, cm | 146 | 320 | 0.002 | ||
| median (IQR) | 5 (4–6) | 4 3-5) | n/a | ||
| ≤2.0 | 2.1 | 7.2 | 2.02 | (0.56–7.32) | 0.29 |
| 2.1–4.0 | 26.0 | 38.8 | Reference | ||
| 4.1–6.0 | 36.0 | 23.1 | 0.51 | (0.29–0.91) | 0.02 |
| >6.0 | 16.4 | 8.8 | 0.30 | (0.15–0.60) | 0.001 |
| n/a | 29.5 | 22.2 | 0.45 | (0.26–0.78) | 0.005 |
| Facility type | 146 | 320 | 0.17 | ||
| Community cancer center | 11.6 | 8.4 | Reference | ||
| Academic/NCI-designated | 33.6 | 34.1 | 1.46 | (0.67–3.18) | 0.34 |
| Integrated network care program | 2.7 | 7.5 | 4.35 | (1.18–16.07) | 0.03 |
| Suppressed due to age <40 y* | 52.1 | 50.0 | Omitted due to collinearity | ||
| KPS** | 24 | 52 | 0.18 | ||
| <70 | 12.5 | 3.9 | |||
| 70–80 | 29.2 | 19.2 | |||
| 90–100 | 58.3 | 76.9 | |||
NCI = National Cancer Institute.
*NCDB suppresses facility data for patients younger than 40 y to help ensure de-identification; as a result, in multivariable analysis, this factor is collinear with the age <40 y factor.
**KPS was encoded for only 15% of cases and so was excluded from multivariable analyses.
However, Medicare-insured patients who underwent resection (80.0% had GTR), were significantly more likely to achieve GTR than patients who were either privately insured (68.7% had GTR; OR 3.20, 95% CI: 1.08–9.46, P = 0.04), insured by Medicaid/other government insurance (66.7% had GTR; OR 3.75, 95% CI: 1.05–13.35, P = 0.04), or uninsured (60.0% had GTR; OR 5.13, 95% CI: 1.40–18.84, P = 0.01) (Table 3). Tumor size also impacted success of EOR—larger tumors were less likely to achieve GTR: 76.5% of 2.1–4.0 cm tumors (reference) and 88.5% of ≤2.0 cm (OR 2.02, 95% CI: 0.56–7.32, P = 0.29) achieved GTR compared with 66.1% of 4.1–6.0 cm (OR 0.51, 95% CI: 0.29–0.91, P = 0.02) and 53.9% of >6.0 cm tumors (OR 0.30, 95% CI: 0.15–0.60, P = 0.001). GTR was also more likely at CoC-designated integrated network cancer program hospitals (85.7% of resected cases achieved GTR) compared with CoC-designated community cancer programs (61.3% had GTR; OR 4.35, 95% CI: 1.18–16.07, P = 0.03). In the multi-institutional cohort, there was no difference in sex, age at diagnosis, insurance status, tumor location or size, eloquent neuroanatomy involvement, KPS, or incidental presentation between cases that achieved GTR versus STR (all χ 2P > 0.05).
Overall Survival in Patients by Extent of Resection
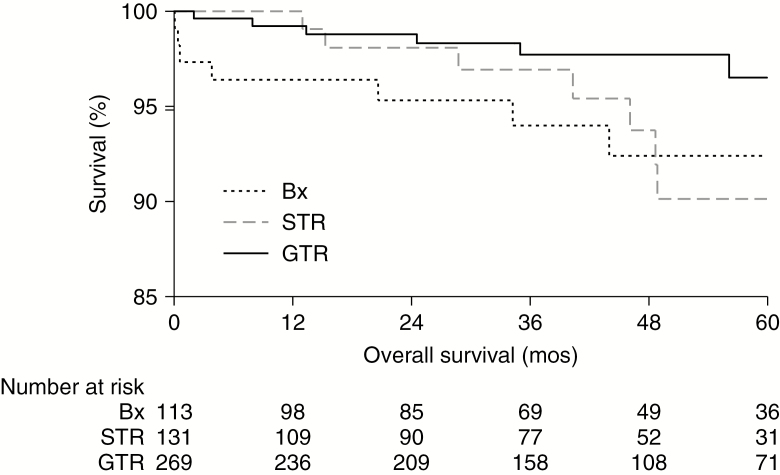
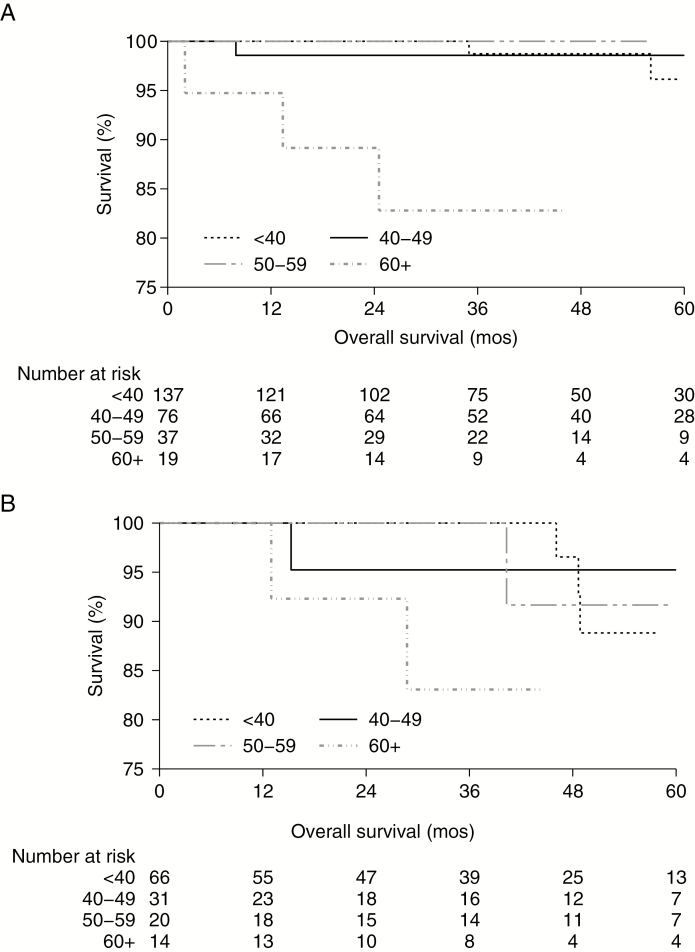
For OS analyses, the median follow-up was 41.5 months (IQR: 23.8–61.6), with 4.7% (n = 24) reaching endpoint. The unadjusted 5-year OS rates were: biopsy-only 92.4% (95% CI: 84.4–96.4), STR 90.1% (95% CI: 79.9–95.3), and GTR 96.5% (95% CI: 91.7–98.6; P = 0.03) (Fig. 1). The subset of patients who died differed substantially from their censored compatriots: 29.2% were 60+ years old (vs 7.2%, P < 0.001); 62.5% had tumors ≥4.1 cm (vs 45.2%, P = 0.04); and 16.7% were uninsured (vs 8.8%, P = 0.003). In resected patients, the unadjusted 5-year OS rates only varied significantly by age for GTR (ie, considered high-risk cases if 40+ years old, P = 0.0003; Figure 2A), but not STR (ie, high-risk, P = 0.37; Fig. 2B).
Fig. 1.
Improved unadjusted OS estimates with GTR. GTR (solid line; n = 269) of WHO grade II 1p/19q-codeleted oligodendrogliomas demonstrated significantly improved unadjusted OS compared with biopsy-only (dotted line; n = 113) or STR (dashed line; n = 131) in Kaplan–Meier estimates (log-rank test P = 0.03; with the corresponding number-at-risk table).
Fig. 2.
OS estimates stratified by age in STR and GTR. The unadjusted OS rates only varied significantly by age for (A) GTR, with an 82.8% 5-year OS for 60+ year olds (dash-dotted line, 95% CI: 55.4–94.2, P = 0.0003), compared with <40 year olds 96.1% (dotted line, 95% CI: 84.3–99.1), 40–49 year olds 98.6% (solid line, 95% CI: 90.3–99.8), and 50–59 year olds 100.0% (gray dashed line, no endpoints reached after a median of 40.5 mo of follow-up), but not for (B) STR (P = 0.37).
In risk-adjusted multivariable Cox regression, being privately insured was associated with an OS benefit (reference uninsured; hazard ratio [HR] 0.24, 95% CI: 0.06–0.92, P = 0.04), whereas sex, age at diagnosis, race/ethnicity, comorbidity index, year of diagnosis, tumor location, and facility type had no impact (all P < 0.05; Table 4). Tumors >6.0 cm displayed worse risk-adjusted OS (reference 2.1–4.0 cm: HR 4.56, 95% CI: 1.27–16.40, P = 0.02). Notably, GTR, but not STR, conferred a risk-adjusted OS benefit in comparison to biopsy only (HR 0.28, 95% CI: 0.09–0.85, P = 0.02) and was independent of age at diagnosis. In the multi-institutional cohort, there were no deaths and no significant difference in progression-free survival by EOR (log-rank P = 0.90) during a median follow-up of 55.3 months (IQR: 33.0–79.1).
Table 4.
Risk-adjusted predictors of OS in WHO grade II oligodendroglioma patients
| Multivariate Cox Regression | |||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Extent of Resection | |||
| Biopsy only | Reference | ||
| STR | 0.67 | (0.23–1.95) | 0.46 |
| GTR | 0.28 | (0.09–0.85) | 0.02 |
| Sex | |||
| Female (ref male) | 0.47 | (0.18–1.22) | 0.12 |
| Age, y | |||
| <40 | 0.54 | (0.11–2.65) | 0.45 |
| 40–49 | Reference | ||
| 50–59 | 1.76 | (0.49–6.37) | 0.39 |
| >60 | 2.89 | (0.59–14.17) | 0.19 |
| Year of diagnosis | |||
| 2010 | Reference | ||
| 2011 | 0.20 | (0.04–1.19) | 0.08 |
| 2012 | 0.72 | (0.16–3.27) | 0.67 |
| 2013 | 2.94 | (0.73–11.9) | 0.13 |
| 2014 | 1.42 | (0.26–7.86) | 0.69 |
| 2015 | 1.01 | (0.10–10.43) | 0.99 |
| Comorbidity index | |||
| 0 | Reference | ||
| 1 | 2.40 | (0.72–7.97) | 0.15 |
| 2 | 3.01 | (0.57–16.01) | 0.20 |
| Race/ethnicity | |||
| White, non-Hispanic | Reference | ||
| Nonwhite | 0.80 | (0.22–2.92) | 0.74 |
| Primary payor | |||
| Not insured | Reference | ||
| Private insurance | 0.24 | (0.06–0.92) | 0.04 |
| Medicare | 0.53 | (0.09–2.95) | 0.47 |
| Medicaid/other government | 0.22 | (0.02–2.23) | 0.20 |
| Tumor location | |||
| Frontal lobe | Reference | ||
| Temporal lobe | 0.64 | (0.32–1.28) | 0.21 |
| Parietal lobe | 1.05 | (0.53–2.06) | 0.89 |
| Other/Overlapping | 0.64 | (0.32–1.28) | 0.21 |
| Tumor size, cm | |||
| ≤2.0 | 0.97 | (0.10–9.03) | 0.98 |
| 2.1–4.0 | Reference | ||
| 4.1–6.0 | 0.91 | (0.23–3.65) | 0.90 |
| >6.0 | 4.56 | (1.27–16.4) | 0.02 |
| n/a | 2.15 | (0.62–7.47) | 0.23 |
| Facility type | |||
| Community cancer center | Reference | ||
| Academic/ NCI-designated | 1.13 | (0.36–3.55) | 0.84 |
| Integrated network care program | 0.65 | (0.06–6.62) | 0.72 |
| Suppressed due to age <40 y* | omitted due to collinearity | ||
NCI = National Cancer Institute.
*NCDB suppresses facility data for patients younger than 40 y to help ensure de-identification; as a result, in multivariable analysis, this factor is collinear with the age <40 y factor.
Discussion
Although WHO grade II diffuse gliomas demonstrate markedly slower growth and improved survival compared with their high-grade counterparts, they nevertheless represent malignant disease that eventually leads to disease relapse, malignant transformation to a higher-grade pathology, and ultimately mortality.1,12 The estimated OS for diffuse LGG is 5–10 years from initial diagnosis.13 Diffuse LGGs represent a heterogeneous set of tumors that are now subdivided into grade II IDH-mutant and 1p/19q-codeleted oligodendroglioma, grade II IDH-mutant diffuse astrocytoma, and, rarely, grade II IDH-wildtype diffuse astrocytoma. Oligodendroglioma, WHO grade II, is characterized by IDH mutation, alpha thalassemia/mental retardation syndrome X-linked (ATRX) wildtype, and whole-arm codeletion of 1p and 19q. Prior studies of management in LGG patients were limited by the use of older histology-only classification schema, which resulted in heterogeneous and inaccurate LGG study populations.10 This pooling of diffuse LGG subtypes may have obscured the effects of surgical management in these prior studies. Despite many recent advances in classification and management, the benefit of maximal resection, particularly for incidental or asymptomatic LGGs, remains unclear.12,13 In this analysis, we examined the role of early surgical resection and EOR in a large national cohort of patients with WHO grade II 1p/19q-codeleted oligodendrogliomas, augmented by a multi-institutional cohort for validation.
Surgery versus Biopsy for Initial Management of WHO Grade II 1p/19q-Codeleted Oligodendrogliomas
Early studies of surgery versus biopsy have been limited by the rarity of diffuse LGGs, lack of molecular classification, pooling of different diffuse LGG subtypes, difficulty in establishing appropriate control groups, and variable assessments of EOR.7–9 Although surgery has a critical diagnostic role in diffuse LGGs, the benefits of substantive tumor resection remain uncertain. The decision is particularly challenging for young patients with asymptomatic diffuse LGGs, which are often diagnosed incidentally during the radiographic workup of patients with headache, head trauma, or other neurological complaints.14 Many of the biopsy-only cases in our cohort likely represented incidentally diagnosed oligodendrogliomas; although there was no association between incidental presentation and EOR in our multi-institutional cohort. In our multivariable analyses, only younger age and more recent diagnosis were associated with a higher rate of surgical resection in the national cohort.
There has yet to be a randomized trial specifically comparing biopsy and surgery for the management of newly diagnosed WHO grade II 1p/19q-codeleted oligodendrogliomas.12,13,15 Due to tumor heterogeneity, biopsy alone may undersample diffuse gliomas, leading to inappropriate classification and management, with prior studies suggesting that such discordance is more prevalent with contrast-enhancing tumors.16,17 Despite the absence of level I evidence, upfront maximal resection for LGG—particularly in the setting of symptomatic lesions—has become largely accepted on the basis of prior retrospective studies.7,8 There have been 2 “near-randomized” studies comparing biopsy and surgery for diffuse LGG where researchers compared OS between patients treated in centers that prefer biopsy versus those that preferred resection.15,18 In the Norwegian study, biopsy was independently associated with worse OS (HR 1.8, 95% CI: 1.1–2.9, P = 0.03), when adjusting for the patient's Pignatti score—a composite LGG prognosis score incorporating age, tumor location and size, histology, and presence of neurologic deficit.18 In the German study, resection was a predictor of improved OS on univariable but not multivariable analysis.15 However, stratification by volume of residual tumor demonstrated that a residual tumor volume of >15 cm3 was associated with worse OS (HR 3.8, 95% CI: 1.1–13.0, P = 0.03), when adjusting for age and histology. When taking into account molecular classification, reassessment of a prior retrospective cohort also demonstrated improved OS in patients managed with surgical resection in IDH-mutated, 1p/19q-codeleted oligodendrogliomas, compared with biopsy.18,19 We find that GTR, but not STR, independently demonstrated improved OS relative to biopsy-only watchful waiting.
Maximal Resection for WHO Grade II 1p/19q-Codeleted Oligodendrogliomas
Given the progressive nature of diffuse LGGs, most patients managed with biopsy alone will eventually require surgical resection, either for tumor progression (estimated growth rate of 4 mm/y) or for malignant transformation.20 The impact of EOR on PFS has been inconsistent in the literature, with some studies showing an association and others a lack thereof.21–27 However, multiple studies have found OS benefits of GTR in diffuse LGG.13,15,21,23,26–30 These OS benefits are posited to be from, in part, GTR's reduction of the number of neoplastic cells able to transform, and thereby delaying malignant transformation of diffuse LGGs.18 In a pooled analysis of diffuse LGGs, both larger tumor size and STR were independent predictors of malignant transformation.26 In a prospective case series of 111 patients with diffuse LGG, smaller tumors and resection achieving <1 cm of residual tumor were independently associated with improved OS.23 Similarly, in a series of 216 diffuse LGG patients, both GTR and smaller tumors were independently associated with OS and malignant-progression free survival benefits.27 These results may also reflect the wide variability in methods for assessing EOR.13 There have notably been few volumetric analyses of the impact of EOR on OS in LGG patients: in one study, diffuse LGG patients with ≥90% EOR had increased OS relative to those with <90% EOR (5-y OS: 97% vs 76%).15,21,27 We have previously shown that increased postoperative residual tumor volume is associated with worse OS in WHO grade II 1p/19q-codeleted oligodendrogliomas (HR 1.05/cm3, 95% CI: 1.00–1.09, P = 0.03).31 The integration of non–fluid attenuated inversion recovery T2-weighted imaging, volumetric segmentation, and standardized definitions could help improve the accuracy of EOR evaluation in diffuse LGG undergoing surgery.32
Postoperative watchful-waiting approaches for adjuvant therapies are poorly defined in diffuse LGGs. In order to prevent confounding by adjuvant therapies, our analyses included only patients who underwent biopsy or surgery without adjuvant radio/chemotherapy within a year of initial diagnosis, and so likely represent patients who were managed with postoperative watchful waiting. In 7% and 11% of our national cohort of WHO grade II oligodendrogliomas, adjuvant radiotherapy and chemotherapy, respectively, were initially part of the treatment plan but either not administered or refused by the patient. Early intervention with radiotherapy has been found to improve PFS and seizure control rate, but with minimal OS benefit in the European Organisation for Research and Treatment of Cancer (EORTC) 22845 trial.6 Focusing on high-risk diffuse LGGs, clinical trials (including Radiation Therapy Oncology Group [RTOG] 9802, EORTC 22033–26033, and RTOG 0424) have shown survival benefits with chemotherapy and radiotherapy.3–5 Given the impact of age on 5-year OS rates in resected patients, our analyses suggest that older WHO grade II 1p/19q-codeleted oligodendroglioma patients who undergo resection may especially benefit from the early addition of adjuvant therapy and, as high-risk patients, may have been suboptimally managed, whereas younger patients demonstrated favorable short-term OS rates with surgical resection alone. Our data confirm the high-risk nature of low-grade oligodendrogliomas that undergo only STR, regardless of age, and suggest that in the early setting, GTR patients aged 41–60 years behave similarly to canonical low-risk cases.
The Relationships of Tumor and Patient Characteristics with Maximal Resection
Diffuse LGG size has often been correlated with outcomes in the literature, which we confirmed in our analyses: larger tumors were less likely to achieve GTR and were associated with worse OS in multivariable analyses. Using tumor sidedness as a surrogate for dominance, left-sided tumors were equally likely to be totally resected as right-sided tumors, and there was no difference in EOR by cerebral lobar location of the oligodendroglioma. The lack of granularity about tumor location and involvement of eloquent structures in the NCDB limited detailed evaluation of the relationship between oligodendroglioma location and EOR in our analyses. Studies have consistently shown that diffuse LGGs located in eloquent or near-eloquent areas are associated with decreased likelihood of achieving GTR and that diffuse LGGs in non-eloquent locations are associated with improved OS.21,22,27 In those studies, when controlling for involvement of eloquent structures, GTR continued to be associated with longer OS.22 In our multi-institutional cohort, eloquent location was not associated with EOR. Increasingly, the incorporation of intraoperative mapping and/or imaging has enhanced the EOR for diffuse LGGs located in eloquent, as well as non-eloquent, areas.24,30
Notably, our findings suggest that nationally, insurance status is an independent predictor of both EOR and OS in WHO grade II 1p/19q-codeleted oligodendrogliomas treated in the US, with Medicare-insured patients more likely to undergo GTR, but privately insured patients to be independently associated with improved OS. Although insurance status had no impact on the biopsy-only versus surgical resection decision, resected oligodendroglioma Medicare-insured patients were significantly more likely than all other patients to have GTR over STR. Insurance status was not associated with EOR in the multi-institutional cohort, but because Massachusetts has mandated minimum health insurance coverage for all residents (only one patient in the cohort was uninsured), our cohort represents a skewed sample set. Additionally, although most WHO grade II 1p/19q-codeleted oligodendrogliomas were managed in academic medical centers, only CoC-designated integrated network cancer programs were associated with higher rates of GTR compared with community cancer programs. OS was not associated with hospital type.
There has been limited research into the relationships between socioeconomic status, management strategies, and outcomes for diffuse LGGs. One study, analyzing data from a national administrative claims database, suggested that Medicare patients with malignant primary brain tumors (of all histologic subtypes) were more likely to have surgery at low-volume hospitals, whereas privately insured patients were more likely to have surgery at high-volume hospitals; but the study was unable to assess the impact of insurance status on EOR.33 The study's data also suggested that insurance status was a significant predictor of inpatient mortality for patients undergoing craniotomy for brain tumors, with private insurance reducing the risk of mortality. Our results suggest that, for patients with WHO grade II 1p/19q-codeleted oligodendrogliomas, insurance status may play an important role in timely diagnosis and access to newer technologies that may increase GTR rates, such as intraoperative mapping and/or imaging and improved volumetric imaging. Although access to care has been associated with outcome disparities for many cancer and brain tumor types, we demonstrate the first such data that access may contribute to the management and outcomes of low-grade oligodendrogliomas—indicating that further research in this field is needed.34,35
Limitations
The breadth of national registry-based cancer databases, like the NCDB, is especially suited for examining rarer tumor types, where single and even multi-institutional studies are underpowered. Nevertheless, the NCDB is constrained by several important limitations. Survival outcome data are restricted to OS, which precludes the analysis of diffuse LGG PFS or malignant transformation rates. Likewise, information about tumor management is restricted to the initial treatment courses, and the effects of subsequent treatments cannot be accounted for. Details about symptomatology, incidental presentation, involvement of eloquent structures, operative considerations, and methods of EOR determination are notably lacking in the NCDB. In this national cohort, 5% of STR and 2% of GTR had an initial surgical procedure/diagnostic that preceded the definitive resection (by a median of 49 and 20 days, respectively). In the remaining cases, the definitive resection was encoded as the initial surgical procedure. Although it is possible that these few patients represent crossover from a watchful-waiting or biopsy-only group to maximal resection, the median time from initial to definitive surgery would suggest that a majority of cases perhaps represented a diagnostic biopsy followed by definitive resection.
To help address these limitations, we examined a multi-institutional cohort of WHO grade II 1p/19q-codeleted oligodendrogliomas. Many of the biopsy-only cases in the national cohort likely represent oligodendrogliomas that were incidentally diagnosed—indeed, 25% of our multi-institutional biopsy-only cases were incidentally diagnosed, whereas the other 75% presented with seizures. In all 4 biopsy-only multi-institutional cases, the treating neuro-oncologist or neurosurgeon designated the management as watchful waiting. Nationally, the KPS, 30-day readmission rate, and postoperative length of stay did not vary significantly between biopsy-only and resected cases, suggesting that the resected oligodendrogliomas did not present with markedly worse clinical features that would necessitate surgery. Additionally, in our multi-institutional cohort, there were no significant differences in the incidental diagnosis rate, KPS, or involvement of eloquent neuroanatomy between biopsy-only and surgical resection. In our multi-institutional cases that were encoded for inclusion into national cancer registries including the NCDB, registrars based the EOR determination on the neuro-oncologist's, neurosurgeon's, and/or neuroradiologist's postoperative interpretation, as detailed in the operative, clinical, or radiology notes. Due to the complexities of determining EOR, registry encoding of such data can be suboptimal: 13% of our registry-encoded multi-institutional cases were erroneously encoded for EOR. Additionally, the NCDB also does not yet incorporate IDH status for diffuse gliomas, so oligodendrogliomas are defined herein solely by their 1p/19q-codeleted status.36 Another limitation is that our findings relate to short-term all-cause OS. Given the relatively short follow-up in our study, it is possible that factors associated with long-term cancer-specific survival may change.
Conclusions
Herein we investigate the predictors and outcomes of maximal resection in a national cohort of patients with WHO grade II 1p/19q-codeleted oligodendrogliomas. Compared with biopsied-only cases, GTR, but not STR, was associated with an OS benefit, and was independent of patient age, tumor size, tumor location, or treating hospital type. Older patients in particular may benefit from the early addition of adjuvant chemoradiotherapy. Patients insured through Medicare and managed at an integrated network cancer program were significantly more likely to have a GTR, whereas private insurance demonstrated an independent OS benefit, suggesting that insurance status and care setting may play important roles in access to timely diagnosis or innovations that improve maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas.
Funding
JBI is supported by an NIH 5T32HL007627 award.
Conflict of interest statement. The authors report no conflicts of interest.
Authorship statement. Conception and study design: JBI. Data analysis and interpretation: MH and JBI. Manuscript writing: All authors. Critical review and revisions: All authors.
References
- 1. Cavaliere R, Lopes MB, Schiff D. Low-grade gliomas: an update on pathology and therapy. Lancet Neurol. 2005;4(11):760–770. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher BJ, Hu C, Macdonald DR, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015;91(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Bent MJ, Afra D, de Witte O, et al. ; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 7. Central Nervous System Cancers (Version 1.2017). National Comprehensive Cancer Network https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed June 10, 2018. [DOI] [PubMed]
- 8. van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017;35(21):2394–2401. [DOI] [PubMed] [Google Scholar]
- 9. Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125(3):503–530. [DOI] [PubMed] [Google Scholar]
- 10.Iorgulescu JB, Torre M, Harary M, et al. The misclassification of diffuse gliomas: rates and outcomes. Clin Cancer Res. 2019;25(8):2656–2663. [DOI] [PMC free article] [PubMed]
- 11. Iorgulescu JB, Harary M, Zogg CK, et al. Improved risk-adjusted survival for melanoma brain metastases in the era of checkpoint blockade immunotherapies: results from a national cohort. Cancer Immunol Res. 2018;6(9):1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang B, Chaichana K, Veeravagu A, Chang SD, Black KL, Patil CG. Biopsy versus resection for the management of low-grade gliomas. Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group, ed. Cochrane Database Syst Rev. 2017. doi: 10.1002/14651858.CD009319.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95(5):735–745. [DOI] [PubMed] [Google Scholar]
- 14. Shah AH, Madhavan K, Heros D, et al. The management of incidental low-grade gliomas using magnetic resonance imaging: systematic review and optimal treatment paradigm. Neurosurg Focus. 2011;31(6):E12. [DOI] [PubMed] [Google Scholar]
- 15. Roelz R, Strohmaier D, Jabbarli R, et al. Residual tumor volume as best outcome predictor in low grade glioma—a nine-years near-randomized survey of surgery vs. biopsy. Sci Rep. 2016;30(6):32286. doi: 10.1038/srep32286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGirt MJ, Villavicencio AT, Bulsara KR, Friedman AH. MRI-guided stereotactic biopsy in the diagnosis of glioma: comparison of biopsy and surgical resection specimen. Surg Neurol. 2003;59(4):277–281; discussion 281. [DOI] [PubMed] [Google Scholar]
- 17. Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3(3):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 19. Jakola AS, Skjulsvik AJ, Myrmel KS, et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28(8):1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524–528. [DOI] [PubMed] [Google Scholar]
- 21. Majchrzak K, Kaspera W, Bobek-Billewicz B, et al. The assessment of prognostic factors in surgical treatment of low-grade gliomas: a prospective study. Clin Neurol Neurosurg. 2012;114(8):1135–1144. [DOI] [PubMed] [Google Scholar]
- 22. Jung TY, Jung S, Moon JH, Kim IY, Moon KS, Jang WY. Early prognostic factors related to progression and malignant transformation of low-grade gliomas. Clin Neurol Neurosurg. 2011;113(9):752–757. [DOI] [PubMed] [Google Scholar]
- 23. Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109(5):835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–1233. [DOI] [PubMed] [Google Scholar]
- 25. Kılıç T, Özduman K, Elmacı İ, Sav A, Necmettin Pamir M. Effect of surgery on tumor progression and malignant degeneration in hemispheric diffuse low-grade astrocytomas. J Clin Neurosci. 2002;9(5):549–552. [DOI] [PubMed] [Google Scholar]
- 26. Chaichana KL, McGirt MJ, Laterra J, Olivi A, Quiñones-Hinojosa A. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg. 2010;112(1):10–17. [DOI] [PubMed] [Google Scholar]
- 27. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 28. Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. [DOI] [PubMed] [Google Scholar]
- 29. McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–707; author reply 707. [DOI] [PubMed] [Google Scholar]
- 30. Duffau H. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76(6):845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kavouridis VK, Boaro A, Dorr J, et al. Contemporary assessment of extent of resection in molecularly-1 defined categories of diffuse low-grade glioma—a volumetric analysis. J Neurosurg. 2019; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verburg N, Hoefnagels FWA, Barkhof F, et al. Diagnostic accuracy of neuroimaging to delineate diffuse gliomas within the brain: a meta-analysis. AJNR Am J Neuroradiol. 2017;38(10):1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Curry WT, Carter BS, Barker FG. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988–2004. Neurosurgery. 2010;66(3):427–438. [DOI] [PubMed] [Google Scholar]
- 34. Curry WT Jr, Barker FG 2nd. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93(1):25–39. [DOI] [PubMed] [Google Scholar]
- 35. Deb S, Pendharkar AV, Schoen MK, Altekruse S, Ratliff J, Desai A. The effect of socioeconomic status on gross total resection, radiation therapy and overall survival in patients with gliomas. J Neurooncol. 2017;132(3):447–453. [DOI] [PubMed] [Google Scholar]
- 36. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]