ABSTRACT
Milk and milk product consumption is positively associated with bone mineral density (BMD). Emerging evidence suggests that fermented milk products (FMPs) may have specific beneficial effects on skeletal health. We conducted a systematic review and meta-analysis to assess the effect of FMPs on bone health indicators in postmenopausal women given their increased risk for osteoporosis and fragility fractures. Electronic databases were searched for randomized controlled trials (RCTs) and prospective cohort and case-control studies that examined the relation between FMPs and bone health outcomes (fracture incidence, BMD, BMD T-score, and percentage change in bone turnover markers) in postmenopausal women. Two reviewers independently conducted abstract and full-text screenings and data extractions. Risk of bias was assessed using the RoB 2.0 tool and the Newcastle–Ottawa scale for interventional and observational studies. Pooled RRs were obtained using a random-effects model by the DerSimonian–Laird method. Three RCTs, 3 prospective cohorts, and 3 case-control studies met the inclusion criteria. Results of the meta-analysis of 3 cohort studies (n = 102,819) suggest that higher yogurt consumption was associated with reduced hip fracture risk (pooled RR: 0.76; 95% CI: 0.63, 0.92, I2 = 29%), but no difference in hip fracture risk was found between higher and lower cheese consumption (pooled RR: 0.89; 95% CI: 0.73, 1.10, I2 = 0%). Case-control studies revealed that cheese intake had either a null or a protective effect against osteoporosis (BMD T-score ≤−2.5). Daily yogurt or cheese intervention (<2 mo) decreased bone resorption marker concentrations, but had no effect on bone formation markers. In postmenopausal women, of the FMPs studied, only greater yogurt consumption was associated with a reduced risk of hip fracture compared with low or no intake. Daily cheese intake may be associated with higher BMD T-scores, but evidence was limited. Additional and longer-term trials examining these relations are warranted.
Keywords: fermented milk products, yogurt, cheese, postmenopausal women, bone mineral density, fractures, bone turnover markers
Introduction
Osteoporosis and osteoporotic fractures are public health concerns, especially in older women. This skeletal disease, characterized by low bone mass and microarchitectural deterioration of bone tissue, affects approximately 200 million women globally (1–3). Although age-related bone loss affects both men and women, the decline of bone mass is accelerated at menopause when bone resorption exceeds bone formation (4). Moreover, fracture rates are higher in elderly women than in men, an increased risk that is not only a result of predisposed genetic differences between the sexes, but is also attributable to other factors affecting the preservation of bone mass in later adulthood.
For instance, reaching maximal peak bone mass by early adulthood, physical activity, and adequate nutrition are major factors that affect the retention of bone mass across the lifespan. Among the bone-building nutrients, calcium, an important component of bone, plays a primary role in osteoporosis prevention as a modifiable factor that helps reduce bone loss. Findings from a systematic review on dietary calcium intake among adults showed that women generally have a lower average calcium intake than men (5). Furthermore, national surveys from North America indicated that >80% of women ≥50 y have a dietary calcium intake that falls below the current RDA of 1200 mg/day (6–8). To reduce the global burden associated with osteoporosis, optimizing calcium intake is necessary. Although calcium can be found in many foods, milk and milk products such as yogurt and cheese are valued as good or excellent sources of calcium (9, 10).
Evidence from observational studies and randomized controlled trials (RCTs) has shown a positive association between milk or total milk product intake and bone mineral density (BMD) (11–13). Reduced fracture risk is the key clinical outcome sought in bone health interventions, and yet the impact of milk and milk product consumption on fracture risk, including risk of hip fracture, remains unclear (14–16). Milk, yogurt, and cheese have similar yet distinct nutrient profiles that vary in part due to the fermentation process. Fermented milk and fermented milk products (FMPs), also known as cultured milk products, are milk products prepared by lactic acid fermentation (17). The bacterial cultures in cheese are less active than those found in some yogurts in which live bacteria remain active postconsumption. Results from a large cohort study reported an inverse association between FMP consumption and the fracture risk in middle-aged and older women and, in contrast, high milk intake was associated with greater fracture risk (18). Michaëlsson et al. (18) proposed that the higher content of d-galactose found in milk compared with FMPs may act as a prooxidant and promote inflammation based on the positive association between milk intake and markers of oxidative stress (urine 8-iso-PGF2α) and inflammation (IL-6), whereas the probiotic content of FMPs may exhibit antioxidant and anti-inflammatory properties that can benefit bone health. Urine 8-iso-PGF2α and serum IL-6 have previously been shown to be negatively associated with BMD and stimulate bone resorption, respectively (19, 20). Moreover, emerging evidence suggests favorable effects of probiotic supplementation on bone health and reduction in proinflammatory cytokines such as TNF-α and IL-1β (21, 22). Although robust data in humans demonstrating these effects are lacking, these findings are of possible public health interest given that dietary guidelines often recommend milk and milk products collectively yet probiotics are only found in FMPs.
Recently, Bian et al. (23) conducted a meta-analysis to examine the association of different types of milk products with hip fracture risk in men and women and found that yogurt and cheese consumption, but not milk consumption, was associated with reduced fracture risk. However, whether the observed associations differed between men and women was not explored in their study. In view of the higher prevalence in osteoporosis and greater fracture incidence in postmenopausal women than in older men (2, 3), it is important to examine the relation between FMP intake and various bone health indicators in postmenopausal women specifically. The purpose of this systematic review is to summarize the evidence on the association of FMP consumption on skeletal outcomes and bone health indicators in postmenopausal women.
Methods
This review was registered on the International Prospective Register of Systematic Reviews in 2018 (PROSPERO) as CRD42018085232, and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (24).
Literature search
A literature search in Embase Classic + Embase (1947–present, OvidSP), MEDLINE (1946–present, OvidSP), PubMed (1946–present, PubMed), CINAHL Plus (1937–present, EBSCOhost), and the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library) was conducted up to 9 January 2019 for studies of FMP consumption and bone health indicators. An expert librarian was consulted to generate a list of keywords and MeSH terms to conduct the search (Supplemental Methods). Searches were not limited by year of publication and no language restrictions were applied. The reference list of all included studies and nutrition research journals were hand searched individually to identify additional studies eligible for this systematic review. Abstracts of conference proceedings and gray literature were excluded.
Study selection
We included RCTs, prospective cohort studies, and case-control studies that examined the relation between FMP consumption and a bone health outcome in postmenopausal women or women ≥55 y old. Studies evaluating the consumption of FMPs were considered in this systematic review regardless of the type, frequency, or dose of FMP, or the method of assessment of FMP intake. All studies that compared the consumption of FMPs to that of non-FMPs, low consumption, no consumption, or placebo, were included. Studies that included a combination of milk product intake were included only if it was possible to quantify the intake of fermented and non-FMP intake. Studies with mixed interventions were excluded.
Studies were included in this systematic review if they reported on 1 of the following outcomes: 1) incidence of vertebral or nonvertebral fractures; 2 ) percentage change from baseline in BMD of the lumbar spine, the total hip, or the femoral neck. Studies that reported a percentage change in bone mineral content of any site, BMD T-score of the lumbar spine, total hip, or femoral neck, and bone turnover markers were also included in this review. The relative percentage change in BMD following the intervention was a primary outcome of interest (25–29). Although forearm BMD has been suggested as an alternative when BMD of central sites cannot be measured, the BMD of this peripheral site was not included, as our scoping search yielded no prospective data. Currently, DXA is the gold standard assessment for measuring BMD and predicting fracture risk in the clinical setting. BMD can also be classified and expressed as a T-score, which is the difference between a patient's BMD and that of a young adult reference population expressed in SD scores from the reference (30). Individuals with T-scores of ≤−2.5 meet the World Health Organization's criterion for diagnosing osteoporosis (31, 32). We included bone turnover markers as secondary outcomes of interest because these predict the rate of bone loss as well as the risk of fragility fractures, independently of BMD (33–38). Changes in bone turnover markers also occur rapidly in response to osteoporosis treatments and are associated with fracture reduction (39). Studies that reported a change in bone formation markers [osteocalcin, bone-specific alkaline phosphatase (BSAP), procollagen type 1 N-terminal propeptide (P1NP), and procollagen type 1 C-terminal propeptide] or bone resorption markers [tartrate-resistant acid phosphatase 5b (TRACP 5b), pyridinoline, deoxypyridinoline, C-terminal telopeptide of type 1 collagen (CTX), and N-terminal telopeptide of type 1 collagen (NTX)] were included.
Data extraction
Study selection, data extraction and quality assessment were performed independently by 2 reviewers (AMO and KK) and disagreement was resolved by consensus or in consultation with a third reviewer. The following information was extracted for each study: name of first author, year of publication, country or region where the study was performed, study design, duration of the study, sample size, recruitment and study completion rates, participant characteristics (age, ethnicity, level of education, smoking status, osteoporosis status, medication use, dietary intake of FMPs and non-FMPs, calcium and/or vitamin D supplementation, physical activity level), effect estimates of outcome measurements, and variables adjusted for in the multivariate models of each study. In observational studies, only the lowest and highest levels of intake were extracted. Authors were contacted to obtain information on missing or unreported data. When no response was received from the author, then the study was excluded from this review.
Risk of bias and quality assessment
Risk of bias in RCTs was assessed using the Cochrane Risk of Bias Tool 2.0 (RoB 2.0) (40). Each component was categorized as “low risk,” “some concerns,” or “high risk.” Methodological quality of observational studies was assessed using the Newcastle–Ottawa scale (41). High-quality items were awarded 1 star, and the highest quality studies were awarded up to 9 stars. Studies with 0–3, 4–6, and 7–9 stars were considered as low, moderate, and high quality, respectively. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to assess the quality of evidence across studies for each outcome (42).
Data synthesis and analysis
RRs from each study were combined using a random-effects model based on the DerSimonian–Laird method in pooling estimates to minimize problems of heterogeneity (43). Heterogeneity was evaluated using both Cochran's Q test and I2 statistics. The I2 statistical test was performed to complement the Cochran's Q test given that the latter test has low power to detect true heterogeneity when the number of studies is small, while the former test does not depend on the number of studies (44, 45). A significant Q value (P-value < 0.05) or an I2 value >50% was considered a considerable level of heterogeneity. When considerable heterogeneity was observed, reasons for heterogeneity were explored in subgroup analyses. Meta-regression analysis can be considered if the number of studies included exceeds 5 to explore the sources of variability. Due to the limited number of studies, meta-regression analysis was not performed. Meta-analyses were performed using the metafor package of the R software (http://r-project.org/, version 3.1.1).
Results
Search results
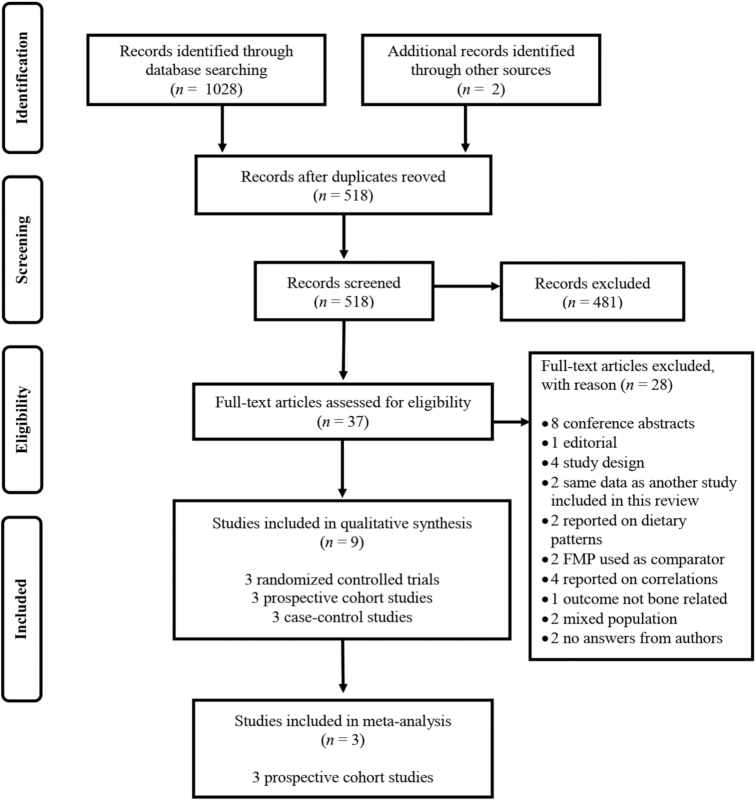
The PRISMA flow diagram illustrating the flow of articles through the search and selection process is shown in Figure 1. The initial search yielded 1028 articles, and after removing 510 duplicates, 518 articles were identified for title and abstract screening. The selection process yielded 37 potentially relevant full-text publications, for which we contacted 7 authors to obtain missing information and 4 responded (46–49). One study with a missing effect estimate for 1 of their subgroups of participants was included in our narrative review (50). Two studies were excluded due to no response (51) and unsuccessful contact with the corresponding authors (52). Following full-text review, we identified 9 studies of FMP intake in postmenopausal women that reported on hip fractures (n = 4), BMD T-scores (n = 2), and bone turnover markers (n = 3). There was no study with vertebral fracture as an outcome.
FIGURE 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram of studies through the review process for the selection of studies for the systematic review and meta-analysis of studies of fermented milk product consumption and bone health in postmenopausal women.
Hip fractures
Yogurt consumption
No RCTs examined hip fracture, or any type of fragility fractures, as an outcome. Evidence from 3 prospective cohort studies indicate that the highest level of yogurt consumption compared with the lowest intake category was associated with a reduced risk of hip fracture (Table 1). Data on hip fractures in women ≥55 y old from the Framingham Original Cohort (47) and the Swedish Mammography Cohort (46) were obtained from the authors to meet the inclusion criteria of this review. A total of 469 women (mean age 77 ± 5 y) from the Framingham Original Cohort were included in this review, with 76 women sustaining an incident hip fracture during a mean follow-up of 11.6 (range 0.04–21.9) y. Intake was assessed at baseline with a semiquantitative food frequency questionnaire. The association of any yogurt intake (>0 servings/wk; 1 serving = 240 mL) on hip fracture risk compared with no yogurt intake was not significant (RR: 1.12; 95% CI: 0.66–1.95) (47). In the Nurses’ Health Study, 80,600 postmenopausal women [mean age 54 (range 34–60) y] were followed for a mean duration of 20.8 y, during which a semiquantitative food frequency questionnaire was administered 9 times during the follow-up period. No significant association between yogurt consumption and hip fracture risk in postmenopausal women was observed (53). Michaëlsson et al. (46) found a 29% reduced risk of hip fracture in a subcohort of 21,750 Swedish women (mean age 63 ± 5 y) who reported a higher consumption of FMP (≥2 servings/d of yogurt and soured milk; 1 serving = 200 mL) than those who were nonconsumers at baseline (RR: 0.71; 95% CI: 0.63–0.79). The meta-analysis of 3 prospective cohort studies resulted in a RR of incident hip fractures of 0.76 (95% CI: 0.63–0.92; P-heterogeneity = 0.25, I2 = 29%) (Figure 2).
TABLE 1.
Characteristics of studies that examined the association between yogurt consumption and hip fractures in postmenopausal women1
| Cases, n | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (reference) | Design (cohort name) | n | Total | Highest-intake group | Lowest-intake group | Age, y | Duration of follow-up, y | Intake categories2 | RR (95% CI) | Adjustments |
| Sahni et al. (47)3 | Prospective cohort (Framingham Original Cohort) | 469 | 76 | 19/113 | 57/356 | 77 ± 4.8 | 11.6 | None vs. >0 serving/wk | 1.12 (0.66–1.91) | Age, BMI, height, total energy intake, current smoking, calcium supplements, vitamin D supplements |
| Feskanich et al. (53) | Prospective cohort (Nurses’ Health Study) | 80,600 | 2138 | 32/49 p-y | 668/560 p-y | 54 (range: 34–60) | 20.8 | None vs. ≥5 servings/wk | 0.77 (0.53–1.12) | Age, follow-up cycle, total energy intake, calcium and vitamin D from nondairy foods plus supplements, protein from nondairy foods, retinol from supplements, vitamin D, caffeine, alcohol, milk during teenage years, BMI, height, physical activity, smoking, use of postmenopausal hormones, use of thiazide diuretics, furosemide-type diuretics and oral steroids, and diagnoses of cancer, diabetes, and cardiovascular disease, milk and cheese intakes |
| Michaëlsson et al. (46)3 | Prospective cohort (Swedish Mammography Cohort) | 27,150 | 4777 | 451/41,108 p-y | 1446/136,145 p-y | 63 ± 5.2 | 22 | None vs. ≥2 servings/d | 0.71 (0.63–0.79) | Age, BMI, height, energy intake, alcohol intake, milk and cheese intake, fruit and vegetable intake, red and processed meat intake, education, cohabitating status, physical activity, smoking habits, ever use of antioxidant-containing supplements, Charlson's weighted comorbidity index. |
p-y, person-years (in thousands).
Intake categories: Sahni et al. (47), 1 serving = 1 cup or 240 mL. Feskanich et al. (53), 1 serving = 1 cup or 240 mL. Michaëlsson et al. (46), yogurt and sour milk were assessed together, 1 serving = 200 mL.
Data for postmenopausal or women ≥55 y old only were obtained from the authors.
FIGURE 2.
Random-effects (RE) model meta-analysis of prospective studies on yogurt consumption (highest compared with lowest intake levels) and risk of hip fractures in postmenopausal women.
Cheese consumption
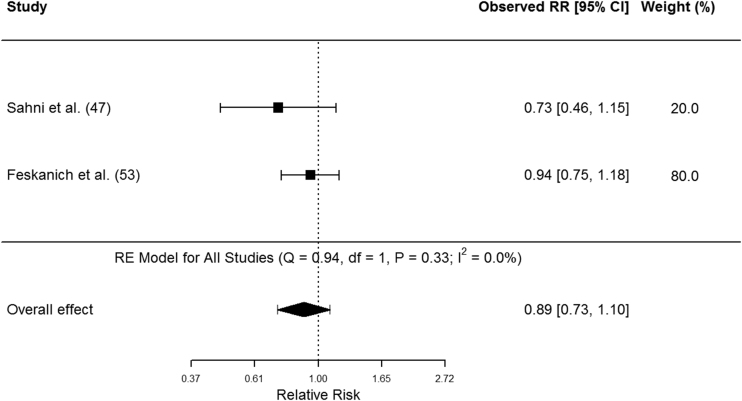
Two prospective cohort studies and 1 case-control study examining the association between cheese intake and hip fractures were identified (Table 2). Cheese intake was not associated with hip fracture risk in postmenopausal women. In the case-control study, which included 241 cases of women [median age 64 (range 45–74) y] hospitalized for a hip fracture and 719 controls, there was no association (OR: 1.0; 95% CI: 0.7–1.5) of hip fracture in women with lower cheese intake (<4 portions/wk) compared with women with higher cheese intake (>6 portions/wk) (54). The amount of cheese per portion was not specified. Evidence from the Framingham Original Cohort showed no association between cheese intake (>1 serving/wk) and hip fracture risk (RR: 0.73; 95% CI: 0.46–1.15) (47). In the Nurses’ Health Study, cumulative consumption of 1 serving/d (28 g of hard cheese or cream cheese, or 120 ml of cottage or ricotta cheese) over the study period was not associated with hip fracture risk as compared with <1 serving/wk of cheese (RR: 0.94; 95% CI: 0.74–1.17) (53). The meta-analysis of the combined findings from the 2 prospective studies yielded a pooled RR of incident hip fractures of 0.89 (95% CI: 0.73–1.10) for the highest cheese intake category compared with the lowest cheese intake category (Figure 3), with no evidence for heterogeneity (P-heterogeneity = 0.33, I2 = 0%).
TABLE 2.
Characteristics of studies that examined the association between cheese consumption and hip fractures in postmenopausal women1
| Design (participant characteristics) | Cases, n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (reference) | n | Total | Highest-intake group | Lowest-intake group | Age, y | Duration of follow-up, y | Intake categories2 | OR/RR (95% CI) | Adjustments | |
| Tavani et al. (54) | Case-control (hospitalized patients) | 960 | 241 | — | — | 64 (range: 45–74) | — | <4 portions/wk vs. >6 portions/wk | OR: 1.0 (0.7–1.5) | Age, education, BMI, smoking status, total alcohol consumption, and estrogen therapy |
| Sahni et al. (47)3 | Prospective cohort (Framingham Original Cohort) | 469 | 76 | 36/259 | 40/210 | 77 ± 4.8 | 11.6 | ≤1 serving/wk vs. >1 serving/wk | RR: 0.73 (0.46–1.15) | Age, BMI, height, total energy intake, current smoking, calcium supplements, vitamin D supplements |
| Feskanich et al. (53) | Prospective cohort (Nurses’ Health Study) | 80,600 | 2138 | 279/261 p-y | 126/112 p-y | 54 (range: 34–60) | 20.8 | <1 serving/wk vs. ≥1 serving/d | RR: 0.94 (0.74–1.17) | Age, follow-up cycle, total energy intake, calcium and vitamin D from nondairy foods plus supplements, protein from nondairy foods, retinol from supplements, vitamin D, caffeine, alcohol, milk during teenage years, BMI, height, physical activity, smoking, use of postmenopausal hormones, use of thiazide diuretics, furosemide-type diuretics and oral steroids, and diagnoses of cancer, diabetes and cardiovascular disease, milk and cheese intakes |
p-y, person-years (in thousands).
Intake categories: Tavani et al. (54), portion size was not described by the authors. Sahni et al. (47), cheese intake was calculated as the combined intake of cottage/ricotta cheese (1 serving = 0.5 cup or 120 mL) and American cheese (1 slice or 1 oz or 28 g) and other cheeses. Feskanich et al. (53), cheese intake was calculated as the combined intake of hard and soft cheeses (1 serving = 1 oz or 28 g), and cottage/ricotta cheese (1 serving = 0.5 cup or 120 mL).
Data for postmenopausal or women ≥55 y old only were obtained from the authors.
FIGURE 3.
Random-effects (RE) model meta-analysis of prospective studies on cheese consumption (highest compared with lowest intake levels) and risk of hip fractures in postmenopausal women.
Osteoporosis as defined by BMD T-score of ≤−2.5
Two case-control studies examined the relation between yogurt/sour cream and cheese intake and BMD T-score ≤−2.5 in postmenopausal women (Table 3). Grgurevic et al. (55) investigated factors related to osteoporosis in postmenopausal women in Serbia (55). Cases included postmenopausal osteoporotic women with a BMD T-score ≤−2.5 at the lumbar spine and controls were age-matched (±2 y) postmenopausal women (mean age 64 ± 9 y) with a normal BMD (lumbar spine T-score >−1.0). Yogurt and sour cream were surveyed as 1 category and there were no associations of daily consumption of yogurt and sour cream with the diagnosis of osteoporosis (55). In the same study, daily cheese consumption was associated with lower odds of osteoporosis than no daily cheese consumption (OR: 0.36; 95% CI: 0.15–0.89) (55). In the other study, Keramat et al. (50) assessed the risk factors for osteoporosis in postmenopausal women from Iran (mean age 56 ± 6 y) and India (mean age 56 ± 8 y). Cases, who were postmenopausal women with a BMD T-score ≤−2.5 at the lumbar spine and/or the total hip, were matched with controls from the same countries by age in 10-y age groups (50). Daily cheese consumption of ≥30 g/d was associated with lower odds of osteoporosis when compared with consumption of <30 g/d in Iranian women (OR: 0.5; 95% CI: 0.3–0.9), but not in Indian women (no effect estimate was provided by the authors). None of the studies included in this review reported on the association of FMP consumption and change in BMD over time.
TABLE 3.
Characteristics of studies that examined the association of fermented milk product consumption (cheese or yogurt) with BMD T-score1
| Author (reference) | Study population | Age, mean ± SD, y | Study period | Bone health outcome | Cases, n | Controls, n | FMP | Intake categories | OR (95% CI) | Matched/adjusted variables |
|---|---|---|---|---|---|---|---|---|---|---|
| Grgurevic et al. (55) | Outpatients | 64 ± 9.0 | 2006–2007 | BMD T-score ≤−2.5 (lumbar spine) | 100 | 100 | Yogurt, sour cream | No daily consumption vs. daily consumption | NR2 | NR |
| Grgurevic et al. (55) | Outpatients | 64 ± 9.0 | 2006–2007 | BMD T-score ≤−2.5 (lumbar spine) | 100 | 100 | Cheese | No daily consumption vs. daily consumption | 0.36 (0.15–0.89) | Body weight <65 kg, thin constitution in childhood, history of previous fracture, family history of fracture, age at menopause <47 y, fish consumption |
| Keramat et al. (50) | Outpatients (Iran) | 57 ± 6.6 | 2002–2005 | BMD T-score ≤−2.5 (lumbar spine and/or total hip) | 178 | 185 | Cheese | <30 g/d vs. ≥30 g/d | 0.5 (0.3–0.9) | Age, height, and weight |
| Keramat et al. (50) | Outpatients (India) | 58 ± 7.8 | 2002–2005 | BMD T-score ≤−2.5 (lumbar spine and/or total hip) | 203 | 151 | Cheese | <30 g/d vs. ≥30 g/d | NR3 | Age, height, and weight |
BMD, bone mineral density; FMP, fermented milk product; NR, not reported.
No data were available for the effect estimate. Grgurevic et al. (55) reported that daily consumption of yogurt/sour cream was no longer significantly (P > 0.05) associated with osteoporosis in multiple forward conditional logistic regression. The unadjusted OR for daily yogurt/sour cream consumption was 0.45 (0.25–0.82).
No data were available for the effect estimate. Keramat et al. (50) reported the OR as nonsignificant (P > 0.05).
Bone turnover markers
All 3 RCTs reported the effect of either yogurt or cheese interventions on different bone turnover markers (Table 4). Heaney et al. (56) conducted a cross-over randomized trial to examine the effect of yogurt compared with a nonnutritious snack on urinary NTX. The authors reported a significant reduction in urinary NTX (−8.2 nmol bone collagen equivalents/g creatinine, P < 0.03) following 7–11 days of intervention of 3 servings of yogurt daily compared with the consumption of a jelled fruit-flavored snack. The amount of yogurt per serving was not specified. Bonjour et al. (57) investigated the effect of 2 servings/d of 100 g of plain cheese made from skimmed milk and fortified with vitamin D and calcium compared with no intervention over 6 wk in 71 postmenopausal women (mean age 57 ± 4 y). There was no significant change from baseline or difference at end of the study between the intervention and control groups in serum osteocalcin, P1NP, BSAP, or CTX. However, there was a significantly greater decrease in TRACP 5b in the intervention group than the control group (−0.64 U/L compared with −0.34 U/L, P = 0.011). Johnson et al. (49) investigated the effect of 85 g of processed cheese compared with no processed cheese on osteocalcin concentrations in older women (mean age 73 ± 7 y). Following a 2-mo intervention, there were decreases in serum osteocalcin concentrations in both groups (−3.7 ng/L compared with −1.8 ng/L), but there were no differences in changes between groups (P = 0.52).
TABLE 4.
Characteristics of randomized controlled trials that assessed the impact of fermented milk product (cheese or yogurt) consumption on bone turnover markers1
| Author (reference) | n | Participant characteristics | Age, mean ± SD, y | Design | Intervention2 | Control | Duration | Bone formation markers | Bone resorption marker | Reported results |
|---|---|---|---|---|---|---|---|---|---|---|
| Heaney et al. (56) | 29 | All white; usual calcium intake <600 mg; BMI: 27.3 ± 3.9 kg/m2 | 61 ± 4.3 | Cross-over | 3 servings/d of fruit-flavored yogurt (n = 29) | 3 servings/d of jelled fruit-flavored snack (n = 29) | 7–11 d | N/A | NTX | −8.2 nmol BCE/g creatinine (22% lower than control) |
| Bonjour et al. (57) | 71 | Postmenopausal ≥3 y; usual calcium intake <600 mg; BMI: 22.9 ± 2.5 and 23.1 ± 2.2 kg/m2 | 57 ± 3.9 | Parallel | 200 g of skimmed-milk, soft, plain cheese (n = 36) | Usual diet (n = 35) | 6 wk | BAP, OC, PINP | CTX, TRACP 5b | Significant decrease in TRACP 5b in both groups and the decline was greater in the treated group vs. the control group (−0.64 vs. −0.34; P = 0.011) |
| Johnson et al. (49)3 | 46 | Usual calcium intake >1000 mg/d | 73 ± 7.0 | Parallel | 85 g of processed cheese (n = 23) | No processed cheese (n = 23) | 2 mo | OC | N/A | No difference in change of OC concentrations between groups |
BAP, bone alkaline phosphatase; BCE, bone collagen equivalents; CTX, C-terminal telopeptide of type I collagen; FMP, fermented milk product; N/A, not available; NTX, N-telopeptide of type I collagen; OC, osteocalcin; PINP, procollagen type I propeptides; TRACP 5b, tartrate-resistant acid phosphatase 5b.
Intervention: Heaney et al. (56), serving size of yogurt was not specified by the authors. Bonjour et al. (57), 2 × 100 g of skimmed milk, soft, plain cheese fortified with 1.25 μg vitamin D and total calcium content of 200 mg per 100 g. Johnson et al. (49), as shown in the table.
Data for female participants were obtained from authors.
Risk of bias and quality assessment
One of 3 trials was considered to be at low risk of bias (56). The other 2 trials had some concerns for bias arising from the lack of information on the allocation sequence and concealment of allocation (49, 57) (Supplemental Table 1). The methodological quality of the prospective cohort studies was rated as high quality (Supplemental Table 2). One case-control study was identified as of high methodological quality (55), whereas the 2 other case-control studies were identified as of moderate quality (50, 54) (Supplemental Table 3). The overall quality of evidence according to the GRADE approach for all outcomes was rated as “very low” (Table 5). The main reasons for downgrading the evidence on hip fracture risk and BMD T-score were inconsistency in FMP exposure in observational studies and high risk of bias in case-control studies related to the selection of controls not representative of the general population of postmenopausal women. Evidence on bone turnover markers was downgraded due to high risk of bias arising from unclear allocation concealment and inconsistency in the reported markers in RCTs.
TABLE 5.
GRADE assessment of the quality of evidence for fermented milk product consumption on bone health outcomes in postmenopausal women1
| FMP | Outcome | Study design, n | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Quality |
|---|---|---|---|---|---|---|---|---|
| Yogurt | Hip fracture | 3 prospective cohort studies | Not serious | Serious inconsistency2 | No serious indirectness | No serious imprecision | Undetected3 | Very low |
| BMD T-score ≤−2.5 | 1 case-control study | Serious4 | Not relevant5 | No serious indirectness | No serious imprecision | Undetected3 | Very low | |
| Bone turnover markers | 1 RCT | Not serious | Not relevant5 | Serious indirectness6 | Serious imprecision7 | Undetected3 | Very low | |
| Cheese | Hip fracture | 2 prospective cohort studies | Not serious | Serious inconsistency8 | No serious indirectness | Serious imprecision9 | Undetected3 | Very low |
| BMD T-score ≤−2.5 | 2 case-control studies | Serious10 | Serious inconsistency11 | No serious indirectness | Serious imprecision12 | Undetected3 | Very low | |
| Bone turnover markers | 2 RCTs | Serious13 | Serious inconsistency14 | Serious indirectness15 | Serious imprecision16 | Undetected3 | Very low |
BMD, bone mineral density; FMP, fermented milk product, RCT, randomized controlled trial.
Serious inconsistency: Although statistical tests suggested no heterogeneity across studies, there was inconsistency in exposure comparisons in the highest intake categories across all 3 studies (i.e., >0 serving/wk compared with ≥5 servings/wk and ≥2 servings/d).
Publication bias: There were >10 studies for each outcome.
Serious risk of bias: The controls were hospital patients which are not considered representative of the general population of postmenopausal women.
Inconsistency category not relevant: Only 1 study reported on this outcome.
Serious indirectness: The comparison used in this study was not a milk product and had a very different nutrient profile than the intervention, which would have influenced the outcome.
Serious imprecision: Small sample size.
Serious inconsistency: Although statistical tests suggest no heterogeneity across studies, there was inconsistency in exposure comparison in the highest intake categories (i.e., >1 serving/wk compared with ≥1 serving/d).
Serious imprecision: The optimal information size is met, but CI overlaps no effect and the CI did not exclude important benefit (i.e., lower bound of CI = 0.73, so >25% for largest plausible effect).
Serious risk of bias: Missing data and potential confounders not adjusted for in the study by Keramat et al. (50), and the controls were hospital patients in the study by Grgurevic et al. (55), which are not considered representative of the general population of postmenopausal women.
Serious inconsistency: The effect size is missing for 1 of the subpopulations in the study by Keramat et al. (50) and we deduced that the CIs would have less overlapping.
Serious imprecision: Large CI.
Serious risk of bias: Unclear allocation concealment in the 2 studies and imbalance in vitamin D status at baseline between study groups in 1 study.
Serious inconsistency: The 2 studies reported on different bone turnover markers and the interventions were different across studies.
Serious indirectness: The comparison used in the 2 studies was a usual diet, which did not allow a direct comparison to the intervention and would have influenced the outcome.
Serious imprecision: Small sample size.
Discussion
In this systematic review and meta-analysis of prospective cohort studies, we found that a higher yogurt intake was associated with a 24% reduction in the risk of hip fracture in postmenopausal women when compared with no yogurt intake. Higher cheese consumption may be associated with reduced risk of hip fractures. However, we did not observe a significant association as our analysis was restricted to 2 studies. Similarly, limited evidence is available to confirm whether FMP intake is beneficial for other skeletal outcomes such as BMD, BMD T-score, and bone turnover markers in postmenopausal women. In a recent meta-analysis of 4 cohorts of men and women, yogurt (RR: 0.75; 95% CI: 0.66–0.86) and cheese consumption (RR: 0.68; 95% CI: 0.61–0.77) associated with a reduced risk of hip fracture (23). In contrast to the authors of this study, we did not include the same studies because 1 study did not stratify their results by sex (58) and results from another were not stratified by age (18), and we identified an additional study that examined the relation between FMP intake and hip fracture risk in postmenopausal women (53). Results from our analysis suggest a protective association of yogurt, but not cheese, with hip fracture risk specifically in postmenopausal women. Our study therefore adds to the literature on the potential benefits of yogurt consumption on bone health in postmenopausal women, although the quality of evidence is low.
Findings from a case-control study that we identified during the selection process suggest that paneer, a form of cottage cheese, was associated with a reduced risk of hip fracture in Indian men and women (OR: 0.152; 95% CI: 0.031–0.741) (52). The lack of association observed in our analysis for cheese intake and hip fracture risk may possibly be explained by the relatively small number of studies (n = 2) and incident fracture events to detect an association specifically in postmenopausal women. Although the heterogeneity of the studies was low, the distributions of yogurt and cheese intake levels in each cohort were dissimilar. For instance, women from the Swedish cohort (46) had higher intakes of FMP than women from the Framingham Original Cohort (47) and the Nurses’ Health Study (53), as demonstrated by the reported highest intake levels (≥2 servings/d of soured milk or yogurt compared with >0 serving/wk and ≥5 servings/wk of yogurt, respectively). There may be a threshold effect of FMP intake on hip fracture risk, but this remains to be confirmed. Future studies must consider a standardized approach to the allocation and assessment of FMP serving sizes.
Higher yogurt consumption may also be a marker of a healthy lifestyle as it has been shown to be a reflection of long-term healthy lifestyles and dietary patterns which are positively associated with bone health (1, 59). Findings from prospective cohort studies suggest that yogurt consumers are generally more physically active, smoke less, and consume less alcohol (60, 61). Frequent yogurt consumers have also been suggested to have overall healthier eating behaviors and diet quality than infrequent consumers (59). Since such lifestyle characteristics have been shown to be protective against osteoporosis (62), it is uncertain whether the observed reduction in risk of hip fracture from the cohort studies is the result of the metabolic effects of yogurt or that of a healthier lifestyle.
The association of yogurt intakes and BMD was investigated in 4310 Irish men and women >60 y old (63). Laird et al. (63) found that BMD at the total hip and femoral neck in women were higher among those with the highest yogurt intake (>1 serving/d) compared with the lowest intake (<1 serving/wk). This study was not included in our review as a result of its cross-sectional design. We identified 1 case-control study in relation to daily yogurt consumption and osteoporosis (BMD T-score ≤−2.5), which reported no association (55). Biver et al. (64) investigated the association of FMP (included yogurts, fresh cheese, “petit-suisse” cheese, quark, and kefir) consumption on bone microstructure and BMD in 482 healthy postmenopausal women followed over 3 ± 0.5 y. This study was not included in our analysis because the associations between FMP intake and BMD were reported as correlations and we were unable to compare extreme FMP intake levels. Nonetheless, similar to the previously mentioned study by Laird et al. (63), Biver et al. (64) found that regular (≥1 serving/d) and occasional consumers of FMPs (1–6 servings/wk) had higher BMD T-scores at the lumbar spine and the total hip than nonconsumers (<1 serving/wk) at baseline. They observed an attenuated age-related cortical bone loss in FMP consumers, independently of total energy, calcium, or protein intake, and found no association in milk or ripened cheese consumers. However, the authors found no relation between FMP intake and the percentage annual change in BMD at the spine or total hip. Although we were unable to include studies that examined the association between kefir consumption and bone health indicators, we identified an RCT that investigated the effect of kefir-fermented milk on BMD during the full-text screening stage of the study selection process. Tu et al. (51) compared the short-term effect of kefir-fermented milk to unfermented raw milk on BMD of the spine, femoral neck, and total hip in 40 osteoporotic men and women. The average BMD increased in both groups at the end of the 6-mo intervention but the changes were not significantly different between the 2 groups. Biver et al. (64) speculate that the benefits of FMP may be involved in the cortical microstructure instead of the mineralization process, but this remains to be investigated. Their study is the first to investigate the association of FMP consumption on changes of bone microstructure in postmenopausal women and provides data to support the hypothesis that FMP may have specific metabolic effects linked to bone health compared with non-FMPs.
The association of cheese intake and BMD or BMD T-scores is less clear. In the Trinity Ulster Department of Agriculture Ageing Cohort Study, cheese intake was not associated with BMD in older women (63). Case-control studies included in the present review showed that cheese intake had either a null or protective effect against osteoporosis. The mixed findings may be explained by the difference in level of cheese intake and dietary patterns between the population groups, or the possibility of no effect to detect. The classification of all cheeses as 1 category in these studies may have also influenced the results, considering the large variety of types of cheese that differ in their fermentation process as well as their nutrient profiles.
Few RCTs were included in our systematic review and consisted of short-term interventions that examined the effect of an FMP on selected bone turnover markers. Daily yogurt intervention decreased the concentration of a bone resorption marker, urinary NTX, but the observed effect may be a result of a higher intake of calcium and protein from consuming a fruit-flavored yogurt during the intervention phase compared with the jelled fruit-flavored snack, which mainly provided carbohydrates, during the control phase of the trial (56). Nonetheless, the inverse association between FMP consumption and bone resorption markers have previously been reported in large cross-sectional studies (63, 64). In our review, cheese interventions did not have an effect on bone formation marker concentrations (49, 57). One study demonstrated an effect of cheese on reducing bone resorption (57). Although TRACP 5b was significantly lower following a daily intervention of 200 g of soft cheese, it is challenging to differentiate whether the observed decrease in concentration of the bone resorption marker was the effect of cheese itself or the effect of the added calcium and vitamin D in the cheese. Moreover, whether the observed reduction in bone resorption markers in the RCTs is clinically important or would be sustained over longer periods is unknown.
Emerging research on the cross-talk between gut microbiota and bone indicates that the gut microbiota has a major influence on bone mass and bone health. Estrogen deficiency and intestinal dysbiosis increase gut permeability, which leads to an increased production of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β by immune cells in the subepithelial compartments of the intestine (65). Inflammation has well been documented to accelerate bone loss as a result of the stimulation of osteoclast formation and increased bone resorption (66). Experimental models in germ-free mice indicated that modulation of the gut microbiota with probiotics can alter intestinal permeability, influence proinflammatory cytokines and receptor activator of NF-κB ligand (RANKL) activity in the intestine and bone, leading to a decrease in osteoclast activity (64, 67). Preclinical investigations have also shown that the probiotic Lactobacillus reuteri ATCCPTA 6475 (L. reuteri 6475), although not usually found in FMPs, prevented femur and vertebral trabecular bone volume loss and increased femoral bone density in ovariectomized mice (68, 69). Findings from a 12-mo double-blind, placebo-controlled study of the probiotic L. reuteri 6475 in 90 women 75–80 y old with low BMD demonstrated reduced loss of tibia total volumetric BMD in the intervention group (70), whereas there was no difference in the markers of inflammation (C-reactive protein and TNF-α). Given that FMPs are predominant sources of different strains of probiotics in the diet, investigation into the effect FMP on a wider panel of proinflammatory cytokines including TNF-α, IL-6, IL-1β, and RANKL may provide a better understanding of the mechanism of action of FMP on skeletal health in postmenopausal women.
The present study has some limitations. For example, we were unable to compare the effect of FMP and non-FMP on bone health indicators in postmenopausal women. The question regarding whether FMPs exert more beneficial effects than non-FMPs on bone health remains unclear. Moreover, cheeses and yogurts were surveyed as 2 generalized groups in the included observational studies. Given that cheeses are produced from a variety of fermentation processes and that live cultures are not found in all cheeses, there is a possibility that each variety may contribute to bone health differently. Different types of cheese were used in the included RCTs, such as soft plain cheese (57) and processed cheese (49), resulting in a challenge to compare outcomes across studies considering the dissimilarities in the preparation and processing of the cheeses as well as their different nutrient profiles. Similarly, none of the identified studies in our review specifically examined the strains of probiotics found in yogurts, or other types of FMP such as Greek-style yogurts or kefir, which have higher protein content than yogurt. Hence, comprehension of the beneficial contribution of live bacteria and that of the food matrix in FMPs on bone health, in combination or separately, requires further exploration. Our study was also limited by the difference in categorization of intakes and lack of detail on serving sizes from some studies, which made it difficult to compare the results across studies. Hence, our analysis primarily considered the highest compared with the lowest exposure category of FMP. Another limitation would be recall bias related to the differential reporting of FMP intake between cases and controls in case-control studies. For example, cases may recall lower intake of FMP than controls when reporting their past food intake and hence introducing bias to the effect estimate. Finally, we did not include studies that reported data on volumetric BMD by peripheral quantitative computed tomography because of the different parameters of bone and use of appendicular sites and we wished to consider measurements used in clinical practice. In addition, most of the studies included in this systematic review are observational studies and causal relations cannot be inferred. Further research is required to confirm our findings and to provide more robust evidence on the potential role of each type of FMP on bone health in postmenopausal women.
Conclusions
Evidence from prospective cohort studies suggest that greater consumption of FMP in the form of yogurt is associated with a reduced risk of hip fracture in postmenopausal women compared with low or no intake, albeit the quality of evidence is very low. Daily cheese consumption may be protective against osteoporosis, but more studies are required to confirm this association. From a public health perspective, more rigorously designed RCTs are required to guide dietary guidelines regarding whether to promote FMPs over milk products overall for bone health in postmenopausal women.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AMO, SNM, and HAW: designed the research; AMO and KK: conducted the research; AMO, SNM, and HAW: analyzed and interpreted the data; AMO, SNM, and HAW: wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
There was no direct funding source for this work. HAW received a salary award from the Canada Research Chairs Program and KK received a training support from the Key Project of the Fourth-Round of Three-Year Public Health Action Plan of Shanghai, China, all unrelated to this project. SNM is scholar of the Fonds de Recherche du Québec en Santé.
Author disclosures: AMO and KK, no conflicts of interest. SNM has received grant funding from the Dairy Farmers of Canada. HAW has received research grants from the Dairy Research Cluster Initiative (Agriculture and Agri-Food Canada, Dairy Farmers of Canada and the Canadian Dairy Commission).
Supplemental Methods and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BMD, bone mineral density; BSAP, bone-specific alkaline phosphatase; CTX, C-terminal telopeptide of type 1 collagen; FMP, fermented milk product; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; L. reuteri 6475, Lactobacillus reuteri ATCCPTA 6475; NTX, N-terminal telopeptide of type 1 collagen; P1NP, procollagen type 1 N-terminal propeptide; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RANKL, receptor activator of NF-κB ligand; RCT, randomized controlled trial; TRACP 5b, tartrate-resistant acid phosphatase 5b.
References
- 1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–95. [DOI] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. [DOI] [PubMed] [Google Scholar]
- 3. International Osteoporosis Foundation. Facts and statistics[Internet]. Nyon (Switzerland): International Osteoporosis Foundation; [updated 2017; cited 2017 Nov 20]. Available from https://www.iofbonehealth.org/facts-statistics. [Google Scholar]
- 4. Reid IR. Menopause. In: Rosen CJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Iowa: Wiley-Blackwell; 2013. pp. 165–70. [Google Scholar]
- 5. Balk EM, Adam GP, Langberg VN, Earley A, Clark P, Ebeling PR, Mithal A, Rizzoli R, Zerbini CAF, Pierroz DD et al.. Global dietary calcium intake among adults: a systematic review. Osteoporos Int. 2017;28(12):3315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for calcium and vitamin D. Ross AC, , Taylor CL, Yaktine AL, Del Valle HB, editors. Washington: National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 7. Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garriguet D. Bone health: osteoporosis, calcium and vitamin D. Health Rep. 2011;3:7–14. [PubMed] [Google Scholar]
- 9. Health Canada. Canadian Nutrient File[Internet]. Health Canada; [updated 2015; cited 2018 Oct 18]. Available from https://food-nutrition.canada.ca/cnf-fce/index-eng.jsp. [Google Scholar]
- 10. Canadian Food Inspection Agency. Specific nutrient content claim requirements—vitamin and mineral nutrient claims [Internet]. [updated 2018; cited 2019 Jan 8]. Available from http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrient-content/specific-claim-requirements/eng/1389907770176/1389907817577?chap=13. [Google Scholar]
- 11. Moschonis G, Manios Y. Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. Br J Nutr. 2006;96(06):1140–8. [DOI] [PubMed] [Google Scholar]
- 12. Sahni S, Tucker KL, Kiel DP, Quach L, Casey VA, Hannan MT. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: the Framingham Offspring Study. Arch Osteoporos. 2013;8(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heaney RP. Dairy and bone health. J Am Coll Nutr. 2009;28(Suppl 1):82S–90S. [DOI] [PubMed] [Google Scholar]
- 14. Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Kanis JA, Orav EJ, Staehelin HB, Kiel DP, Burckhardt P, Henschkowski J, Spiegelman D et al.. Milk intake and risk of hip fracture in men and women: a meta-analysis of prospective cohort studies. J Bone Miner Res. 2011;26(4):833–9. [DOI] [PubMed] [Google Scholar]
- 15. Kanis JA, Johansson H, Oden A, De Laet C, Johnell O, Eisman JA, McCloskey E, Mellstrom D, Pols H, Reeve J et al.. A meta-analysis of milk intake and fracture risk: low utility for case finding. Osteoporos Int. 2005;16(7):799–804. [DOI] [PubMed] [Google Scholar]
- 16. Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A, Reid IR. Calcium intake and risk of fracture: systematic review. BMJ. 2015;351:h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heller KJ. Probiotic bacteria in fermented foods: product characteristics and starter organisms. Am J Clin Nutr. 2001;73(2):374s–9s. [DOI] [PubMed] [Google Scholar]
- 18. Michaëlsson K, Wolk A, Langenskiold S, Basu S, Warensjo Lemming E, Melhus H, Byberg L. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349:g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Östman B, Michaëlsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, Basu S. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radical Biol Med. 2009;47(5):668–73. [DOI] [PubMed] [Google Scholar]
- 20. Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208(1):207–27. [DOI] [PubMed] [Google Scholar]
- 21. Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr. 2017;36(7):497–506. [DOI] [PubMed] [Google Scholar]
- 22. Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9(3):e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bian S, Hu J, Zhang K, Wang Y, Yu M, Ma J. Dairy product consumption and risk of hip fracture: a systematic review and meta-analysis. BMC Public Health. 2018;18(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporos Int. 2005;16(6):581–9. [DOI] [PubMed] [Google Scholar]
- 26. Stone KL, Seeley DG, Lui L-Y, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res. 2003;18(11):1947–54. [DOI] [PubMed] [Google Scholar]
- 27. Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D et al.. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–94. [DOI] [PubMed] [Google Scholar]
- 28. Leslie WD, Tsang JF, Caetano PA, Lix LM. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab. 2007;92(1):77–81. [DOI] [PubMed] [Google Scholar]
- 29. Cummings SR, Browner W, Cummings SR, Black DM, Nevitt MC, Browner W, Genant HK, Cauley J, Ensrud K, Scott J et al.. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341(8837):72–5. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: technical report series 843. Geneva: World Health Organization; 1994. [PubMed] [Google Scholar]
- 31. Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–41. [DOI] [PubMed] [Google Scholar]
- 32. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization Technical Report Series. 1994;843:1–129. [PubMed] [Google Scholar]
- 33. Bruyere O, Collette J, Delmas P, Rouillon A, Roux C, Seidel L, Richy F, Reginster J-Y. Interest of biochemical markers of bone turnover for long-term prediction of new vertebral fracture in postmenopausal osteoporotic women. Maturitas. 2003;44(4):259–65. [DOI] [PubMed] [Google Scholar]
- 34. Chapurlat RD, Garnero P, Brárt G, Meunier PJ, Delmas PD. Serum type I collagen breakdown product (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS study. Bone. 2000;27(2):283–6. [DOI] [PubMed] [Google Scholar]
- 35. Garnero P, Delmas PD. Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuron Interact. 2004;4(1):50–63. [PubMed] [Google Scholar]
- 36. Grados F, Brazier M, Kamel S, Mathieu M, Hurtebize N, Maamer M, Garabédian Ml, Sebert J-L, Fardellone P. Prediction of bone mass density variation by bone remodeling markers in postmenopausal women with vitamin D insufficiency treated with calcium and vitamin D supplementation. J Clin Endocrinol Metab. 2003;88(11):5175–9. [DOI] [PubMed] [Google Scholar]
- 37. Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15(8):1526–36. [DOI] [PubMed] [Google Scholar]
- 38. Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 2000;11:2–17. [DOI] [PubMed] [Google Scholar]
- 39. Hlaing TT, Compston JE. Biochemical markers of bone turnover—uses and limitations. Ann Clin Biochem. 2014;51(2):189–202. [DOI] [PubMed] [Google Scholar]
- 40. Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1):29–31. [Google Scholar]
- 41. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis[Internet]. [updated 2000; cited 2018 Aug 21]. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 42. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, deBeer H et al.. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 43. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 44. Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17(8):841–56. [DOI] [PubMed] [Google Scholar]
- 45. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 46. Michaëlsson K, Wolk A, Lemming EW, Melhus H, Byberg L. Intake of milk or fermented milk combined with fruit and vegetable consumption in relation to hip fracture rates: a cohort study of Swedish women. J Bone Miner Res. 2018;33(3):449–57. [DOI] [PubMed] [Google Scholar]
- 47. Sahni S, Mangano KM, Tucker KL, Kiel DP, Casey VA, Hannan MT. Protective association of milk intake on the risk of hip fracture: results from the Framingham Original Cohort. J Bone Miner Res. 2014;29(8):1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sahni S, Mangano KM, Kiel DP, Tucker KL, Hannan MT. Dairy intake is protective against bone loss in older vitamin D supplement users: the Framingham Study. J Nutr. 2017;147(4):645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson J, Mistry V, Vukovich M, Hogie-Lorenzen T, Hollis B, Specker B. Bioavailability of vitamin D from fortified process cheese and effects on vitamin D status in the elderly. J Dairy Sci. 2005;88(7):2295–301. [DOI] [PubMed] [Google Scholar]
- 50. Keramat A, Patwardhan B, Larijani B, Chopra A, Mithal A, Chakravarty D, Adibi H, Khosravi A. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord. 2008;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tu MY, Chen HL, Tung YT, Kao CC, Hu FC, Chen CM. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS One. 2015;10(12):e0144231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jha RM, Mithal A, Malhotra N, Brown EM. Pilot case-control investigation of risk factors for hip fractures in the urban Indian population. BMC Musculoskelet Disord. 2010;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feskanich D, Meyer HE, Fung TT, Bischoff-Ferrari HA, Willett WC. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporos Int. 2018;29(2):385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tavani A, Negri E, La Vecchia C. Calcium, dairy products, and the risk of hip fracture in women in northern Italy. Epidemiology. 1995;6(5):554–7. [DOI] [PubMed] [Google Scholar]
- 55. Grgurevic A, Gledovic Z, Vujasinovic-Stupar N. Factors associated with postmenopausal osteoporosis: a case-control study of Belgrade women. Women Health. 2010;50(5):475–90. [DOI] [PubMed] [Google Scholar]
- 56. Heaney R, Rafferty K, Dowell M. Effect of yogurt on a urinary marker of bone resorption in postmenopausal women. J Am Diet Assoc. 2002;102(11):1672–4. [DOI] [PubMed] [Google Scholar]
- 57. Bonjour JP, Benoit V, Rousseau B, Souberbielle JC. Consumption of vitamin D- and calcium-fortified soft white cheese lowers the biochemical marker of bone resorption TRAP 5b in postmenopausal women at moderate risk of osteoporosis fracture. J Nutr. 2012;142(4):698–703. [DOI] [PubMed] [Google Scholar]
- 58. Feart C, Lorrain S, Ginder Coupez V, Samieri C, Letenneur L, Paineau D, Barberger-Gateau P. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos Int. 2013;24(12):3031–41. [DOI] [PubMed] [Google Scholar]
- 59. Panahi S, Fernandez MA, Marette A, Tremblay A. Yogurt, diet quality and lifestyle factors. Eur J Clin Nutr. 2016;71:573. [DOI] [PubMed] [Google Scholar]
- 60. Sayón-Orea C, Bes-Rastrollo M, Martí A, Pimenta AM, Martín-Calvo N, Martínez-González MA. Association between yogurt consumption and the risk of metabolic syndrome over 6 years in the SUN study. BMC Public Health. 2015;15(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martinez-Gonzalez MA, Sayon-Orea C, Ruiz-Canela M, de la Fuente C, Gea A, Bes-Rastrollo M. Yogurt consumption, weight change and risk of overweight/obesity: the SUN cohort study. Nutr Metab Cardiovasc Dis. 2014;24(11):1189–96. [DOI] [PubMed] [Google Scholar]
- 62. Zhu K, Prince RL. Lifestyle and osteoporosis. Curr Osteoporos Rep. 2015;13(1):52–9. [DOI] [PubMed] [Google Scholar]
- 63. Laird E, Molloy AM, McNulty H, Ward M, McCarroll K, Hoey L, Hughes CF, Cunningham C, Strain JJ, Casey MC. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos Int. 2017;28(8):2409–19. [DOI] [PubMed] [Google Scholar]
- 64. Biver E, Durosier-Izart C, Merminod F, Chevalley T, van Rietbergen B, Ferrari SL, Rizzoli R. Fermented dairy products consumption is associated with attenuated cortical bone loss independently of total calcium, protein, and energy intakes in healthy postmenopausal women. Osteoporos Int. 2018;29(8):1771–82. [DOI] [PubMed] [Google Scholar]
- 65. Hsu E, Pacifici R. From osteoimmunology to osteomicrobiology: how the microbiota and the immune system regulate bone. Calcif Tissue Int. 2018;102(5):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boyce BF, Schwarz EM, Xing L. Osteoclast precursors: cytokine-stimulated immunomodulators of inflammatory bone disease. Curr Opin Rheumatol. 2006;18(4):427–32. [DOI] [PubMed] [Google Scholar]
- 67. Rizzoli R, Biver E. Effects of fermented milk products on bone. Calcif Tissue Int. 2018;102(4):489–500. [DOI] [PubMed] [Google Scholar]
- 68. Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, McCabe LR, van Wijnen AE. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One. 2016;11(4):e0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.