ABSTRACT
Insufficient intake of total fruits and vegetables is linked to an increased cancer risk, but the relation is not understood for dried fruits. Dried fruits are generally perceived, by both consumers and researchers, as a less attractive but shelf-stable equivalent to fresh fruits and constitute a small but significant proportion of modern diets. Chemical compositions of raw and dried fruits, however, may differ substantially. Several clinical and laboratory intervention studies have reported the protective effects of dehydrated fruits against the progression of some cancers and the modulating effects of dried fruits on common cancer risk factors. In this systematic review, we identified, summarized, and critically evaluated 9 prospective cohort and 7 case-control studies that examined the relations between traditional dried fruit (raisins, prunes, dates) consumption and cancer risk in humans. Prospective cohort studies determined that significant reductions in relative risk of precancerous colorectal polyps, incidence of prostate cancer, or mortality from pancreatic cancer, by, respectively, 24%, 49%, and 65%, were associated with 3–5 or more servings of dried fruits per week. Selected case-control studies revealed inverse associations between dried fruit intake and risk of cancer as well. The reported associations were comparable to or stronger than those observed for total or raw fruits. Although the small number and high heterogeneity impede meta-analysis of these studies, we conclude that currently available data provide some initial evidence that consumption of dried fruits may be associated with a lower cancer incidence or mortality in populations. The data suggest that higher intake of raisins and other dried fruits may be important in the prevention of cancers of the digestive system. Because only a limited number of health outcome and dried fruit intake relations have been evaluated in prospective studies to date, reanalyzing existing high-quality epidemiological data may expand the knowledge base.
Keywords: cancer risk, nutrition, epidemiology, raisins, prunes, date palm fruits
Introduction
Cancer is the second leading cause of morbidity and mortality worldwide, with an estimated 18.1 million new cases and 9.6 million cancer deaths in 2018 alone (1). However, evidence suggests that over 40% of cancer deaths could be prevented through changes in lifestyles, including diet (2). Higher intake of some foods, and dietary factors such as alcohol, processed and red meat, or saturated fat, may increase cancer risk, whereas calcium, dietary fiber, tea, or coffee were associated with lower risk of colorectal and other cancers (3, 4). During past decades, numerous studies have observed an inverse relation between consumption of produce and death from major chronic diseases, including cancer (5), and thus placed insufficient fruit and vegetable intake into the top 10 risk factors of global mortality (6). Certain subgroups of processed fruits and vegetables may have specific impacts on cancer risk, however. For instance, consumption of salted and pickled fruits and vegetables was associated with increased risk of esophageal and gastric cancers (7, 8), whereas intake of cooked, rather than raw, tomatoes was attributed to decreased risk of prostate cancer (PCa) (9). It was argued (10) that consideration of food processing in epidemiological studies may assist better understanding of links between food and health.
Drying is one of the most ancient technologies of food preservation. For example, the Bible mentions raisins, dates, and figs, thus providing a testament to their ancient production and use in the Mediterranean region (11). In modern populations, dried fruits represent a relatively small proportion of human diets, which is more significant in hot, dry climates. Both the general public and research community in Western countries generally perceive dried fruits as a less attractive, albeit shelf-stable, equivalent to fresh fruits (12). High sugar content, loss of vitamins and other unstable nutrients, as well as potential presence of contaminants, may be viewed as detrimental, unhealthy attributes of traditional dried fruits, when compared to their raw counterparts. For instance, mycotoxins have been implicated in a few adverse health effects, including carcinogenesis (13, 14). On the other hand, several clinical (15, 16) and laboratory (17–19) intervention studies reported the protective effects of dehydrated fruits against progression of some cancers. In addition, a number of human intervention studies established that intake of dried fruits can decrease excessive weight (20), inflammation (21), or hyperglycemia (22), which are recognized risk factors of cancer.
The overwhelming majority of observational studies establishing health effects of diets customarily place dried fruits into the same category with raw fruits or do not mention dried fruits at all. No studies explicitly focused on fruit dehydration as a potential determinant of cancer risk in consumers. Furthermore, in a significant number of publications that consider importance of dietary dried fruits to human health [see, for example, the most recent collection of reviews on biological effects of dried fruits (23)], discussions of the potential dried fruit–cancer relations are based on laboratory data obtained in animal models or cell culture only, and any appraisal of available data from epidemiological studies is lacking. Given continuously growing attention of both the scientific community and the general public to nutritional aspects of health, there is clearly a need for an up-to-date summary of cancer risk effects of a popular snack: dried fruits.
To address the knowledge gap, we performed a systematic review, with the objectives being 1) to conduct an exhaustive search of available published studies that linked dehydrated fruit intake and cancer-related outcomes in humans and to map out the characteristics and findings of the identified studies; 2) to ascertain knowledge gaps and limitations of the existing literature and to propose recommendations for advancing the field to make it informative to health practitioners and consumers.
Dried fruits in human diets
Traditional and modern food technologies offer a broad variety of products made with the use of fruit dehydration processes. There are several commonly recognized categories of such products:
traditional dried fruits (raisins, prunes, dates, dried figs)
sweetened dried fruits (dried cranberries, candied pineapple)
desiccated fruits (freeze-dried berries)
fruit/vegetable powders (tomato powder, chili pepper powder)
evaporated juices and pulps (juice concentrates, fruit leather, tomato paste)
sweetened evaporated juices and pulps (fruit preserves, jams)
deep-fried fruits (banana chips)
Food items that are commonly recognized as traditional dried fruits are chiefly defined as whole or cut fruits that were dehydrated following a heat-assisted process, such as by sun drying or rack drying with heated air. These include raisins, prunes, and figs. Dates are also included, even though some varieties are not dried but still have naturally low moisture content. Less commonly recognized forms of dried fruit include dehydrated vegetables (dried peppers or tomatoes), whereas sugar-sweetened dehydrated fruits and evaporated fruit juices, dehydrated products with very low moisture content, are not considered traditional dried fruits. These, as well as rehydrated products, such as “prune juice” or reconstituted tomato paste, escape being categorized as “dried fruit” in dietary questionnaires and trade statistics, even though they share many unique chemical signatures with traditionally dried fruits.
The most commonly consumed types of traditional dried fruit are listed in Table 1. The production values in Table 1 are based on traceable data from official trade reports and thus may significantly underestimate the actual production volumes. For example, the world date palm crop estimate is over 8 million metric tons (24), providing for the largest dried fruit production by far. However, only about 12% of harvested dates are conditioned for trade and are included in official statistics, whereas the rest are used for making syrup, stock feed, protective coatings, or local consumption. Naturally, the highest per capita consumption of dried fruits is found in the North Africa/Middle East region, the chief date palm growing area. Thus, per capita consumption of dates in some Arabic countries may exceed 30 kg/y (25). For comparison, in the United States, only about 7% of general population reported daily consumption of traditional dried fruits (26). Europeans consume dried fruit only occasionally as well, with over half of respondents in some countries, such as the Netherlands, claiming they never eat dried fruit, although they might regularly consume products containing dried fruits, such as cereals or cookies (27). Vegetarians, as might be expected, consume dried fruits more often than nonvegetarians (28). In Western countries, the variety of consumed dried fruits is diversified, with raisins keeping the lead (Table 1). Interestingly, some fruits, such as dates and figs, are eaten almost entirely in dried form in the nonproducing countries, and even more traditional for Westerners, plums and apricots are consumed preferentially as dried fruit. Not listed in Table 1 are a large variety of other, less popular, dried fruits and berries (such as dried bananas, pears, mangoes, pineapples, strawberries, and so on), as well as sun-dried tomatoes, bell and chili peppers, which usually fall into the vegetable category. Although in Western tradition a tomato is considered a vegetable, botanically it is a fruit, with sugar content (over 50% glucose plus fructose, per dry weight in ripe tomatoes) comparable to most conventional fruits. The global production of tomato spray-dried powder is 50,000 metric tons/y (31); however, it is used alternatingly with the more abundant tomato paste (global production is over 30 million metric tons/y) in preparation of tomato sauces, soups, or seasonings, and its contribution to specific tomato products is difficult to trace, with a few exceptions.
TABLE 1.
Market and consumption of major traditional dried fruits and their fresh counterparts1
| Dried fruit conditioned for sale | Typical foods containing dry fruits | Annual world trade of dried fruits in 2017/18, metric tons | Annual US consumption of dried fruit in 2017, kg per capita 2 | Annual US consumption of fresh fruit in 2017, kg per capita |
|---|---|---|---|---|
| Raisins, sultanas, currants | Bakery, chocolates, snack bars and mixtures | 1196,500 | 0.60 (2.8) | 3.7 3 |
| Dates | Bakery | 1025,000 | 0.15 (0.2) | — |
| Dried plums (prunes) | Dishes, beverages | 242,670 | 0.20 (1.1) | 0.29 |
| Dried apricots | Leather, mixtures | 226,760 | 0.04 (0.2) | 0.05 |
| Dried figs | Pastries, snack bars | 135,400 | 0.04 (0.1) | ≤0.01 |
| Dried apples | Cereal and snack mixtures, beverages | ≤2% of total apple production | 0.07 (0.5) | 8.0 |
| Dried peaches | Bakery, snacks | ∼1% of total peach production | 0.015 (0.1) | 1.3 |
| Total4 | — | 2826,000 | 1.12 (4.5) | 53.1 |
FFQs may not contain all of the dehydrated fruit categories that are mentioned above. For example, the FFQ employed in the Nurses’ Health Study (NHS) (32) contains items from 4 such categories: a. (raisins, prunes, mixed dried fruit), b. (dried cranberries), e. (tomato sauce, ketchup, red chili sauce), and f. (jams, preserves). Representatives of only 2 categories, a. (traditional dried fruits) and e. (tomato paste and foods prepared with its use), have been specifically examined in epidemiological studies as nutritional factors for cancer risk. Thus, tomato products have been a focus of many observational studies on the relation between consumption of tomato-based foods and PCa risk or mortality, possibly because of the protective effects of tomato lycopene and other chemical species. These studies have been summarized in a number of conceptual and systematic reviews (9, 33–39). For this reason, and also because a large proportion of tomato-based products contain cooked, rather than dehydrated, tomatoes, we did not include such studies in the main focus of this review. Finally, representatives of category c. (freeze-dried black raspberries, strawberries, and grapes) have been tested as therapeutic agents against progression of tumorigenesis in clinical trials only (15, 16).
To sum up, dried fruits constitute a relatively small but significant part of the human diet, with considerable variations across cultural traditions and geographical regions of the world. Assessed frequency and amounts of dehydrated fruit intake are generally underestimated, because of restriction of the “consumed dried fruit” definition to the traditional dried fruits that the respondents recall “putting hands on.”
Methods
To select a suitable methodological approach for this review, the following research question was formulated: What is the extent of epidemiological literature reporting on the relations between dried fruit intake and cancer-related outcomes, including development of precancerous lesions, cancer incidence, and mortality in humans? The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework has been developed for systematic reviews to help answer such questions (40). This approach assumes a few key steps after formulation of the research question, including identification and selection of relevant studies, charting data, presenting summarized results, and discussion of the results, their limitations, and future research.
Search strategy and study selection
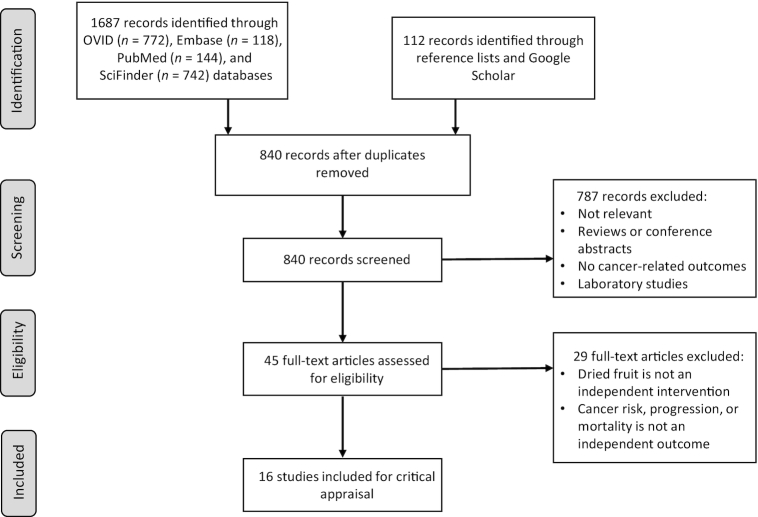
One author (VVM) conducted a systematic literature search in OVID (integrates Biological Abstracts, Cochrane Library, Embase, Medline, Ovid Journals), SciFinder (integrates Chemical Abstracts Plus and Medline), and PubMed databases through January, 2019. The PICOS (Population, Intervention, Comparator, Outcome, Study design) approach (40) was employed to formulate the search criteria and statements for the disease and outcome using the following keywords: “cancer,” “neoplasia,” “hyperplasia,” “dysplasia”; for the intervention, search items such as “dried fruits,” “dehydrated fruits,” “raisins,” “date palm fruits,” “dried figs,” “dried apricots,” and “prunes” were used; the study design search involved the terms “epidemiology,” “prospective,” “observational,” “cohort,” “case-control,” and their variants. An additional updating search run for papers published between January and April, 2019 was done during the manuscript revision phase. A reference list search from full-text articles (articles cited in the eligible papers and articles citing the eligible papers) was also performed. Google Scholar was employed to search entire article texts; only the most relevant 100 hits were screened. No date, language, or model quality restrictions were imposed. The following criteria were used for inclusion of a study: 1) quantitatively evaluated the association between intake of dried fruit and a specific cancer outcome, including incidence of a precancerous condition and cancer incidence or mortality; 2) observational studies done in human subjects of any age; 3) publication in a journal article accessible in a full-text format. All citations were imported into the bibliographic manager EndNote (Thomson Reuters), and duplicate citations were removed by the software. Separation of records that were not scientific journal articles was performed at this stage. We initially obtained 772 returns from the OVID database search, Embase and PubMed provided 118 and 144 hits, respectively, and 742 returns came from the SciFinder database search. After exclusion of duplicates and references that did not meet the selection criteria, the number of potentially relevant studies narrowed to 45. We next examined the full text of these and excluded 29 papers that did not contain sufficient data on intake of traditional dried fruits separately from raw fruits or nuts, cited foods prepared by pickling dried fruits, or did not specifically relate dried fruit consumption to cancer. The search process is depicted in Figure 1.
FIGURE 1.
PRISMA flowchart of the study selection process.
To assess the quality of each study, we used the Newcastle-Ottawa Quality Assessment Scale (41). The instrument consists of 3 domains indicating the study quality: selection (4 points), comparability (2 points), and outcome (3 points), with a maximal score of 9 points. Studies scoring 9–7, 6–4, and 3–0 points were rated as high-quality, moderate-quality, and low-quality studies, respectively.
Results
An exhaustive search of databases allowed identification and selection of 16 publications (42–57) that assessed relations between dried fruit consumption and risk of cancer or precancerous lesions in prospective cohort or case-control studies. In total, 12,732 cases were reported from 437,298 participants. General characteristics of selected studies are provided in Table 2. All included studies were published between 1985 and 2018, with 5 (31%) papers published within the last decade. One study (57) was published in French and 15 papers were in English. The largest number of studies, 6 (37.5%), were conducted in the United States, followed by the Netherlands (3 publications) and Spain (2 studies). Asia and South America are the major geographic regions that did not contribute to the pool of selected papers.
TABLE 2.
Characteristics of selected studies
| Study characteristics (total n = 16) | Publications, n (%) | Participants per study range, n | Total participants across all studies, n |
|---|---|---|---|
| Year of publication | |||
| Before 1990 | 3 (19) | 1184–34,198 | 35,382 |
| 1990–1999 | 5 (31) | 357–120,852 | 156,115 |
| 2000–2009 | 3 (19) | 144–120,852 | 121,581 |
| 2010–2019 | 5 (31) | 212–182,145 | 185,998 |
| Geographic region | |||
| Mediterranean | 5 (31) | 144–708 | 1887 |
| North Europe | 4 (25) | 585–120,852 | 121,437 |
| North America | 6 (37.5) | 1184–182,145 | 220,345 |
| Australia | 1 (6) | 357 | 357 |
| Study design | |||
| Prospective cohort | 9 (56) | 1184–182,145 | 341,197 |
| Case-control | 7 (44) | 144–708 | 2829 |
| Type of dried fruit | |||
| Combined dried fruit | 13 (81) | 212–120,852 | 161,380 |
| Dried grapes | 2 (12.5) | 144–357 | 501 |
| Prunes | 1 (6) | 182,145 | 182,145 |
| Dried figs | 1 (6) | 144 | 144 |
| Dates | 1 (6) | 501 | 501 |
| Outcome | |||
| Overall cancer mortality | 1 (6) | 1184 | 1184 |
| Bladder cancer incidence | 2 (12.5) | 585–120,852 | 121,437 |
| Breast cancer incidence | 1 (6) | 182,145 | 182,145 |
| Colorectal cancer incidence | 1 (6) | 501 | 501 |
| Colorectal polyps incidence | 1 (6) | 2818 | 2818 |
| Lung cancer incidence | 1 (6) | 34,198 | 34,198 |
| Nasopharyngeal cancer incidence | 1 (6) | 144 | 144 |
| Prostate cancer incidence | 3 (19) | 322–58,279 | 72,601 |
| Pancreatic cancer incidence or mortality | 2 (12.5) | 357–34,198 | 34,555 |
| Stomach cancer incidence | 3 (19) | 212–120,852 | 121,772 |
The publications were comparably distributed between 2 study designs: prospective cohort and case-control. The total of traditional dried fruits was the most common intervention factor in observational studies (13 reports, 81%). Types of dried fruit reported as a separate category include raisins (2 studies), prunes, dates, and dried figs (1 study each).
There was a relatively broad variety of outcomes (10 in total) reported as endpoints in the studies. These included incidence of precancerous lesions (1 study), cancer incidence (14 studies, 88%), and overall cancer mortality (1 study). Most studies focused on cancers of the digestive system (9 studies, 56%), including stomach (3 studies), colon and rectum, pancreas, and bladder (2 studies each). Other sites included prostate, nasopharynx, lung, and breast.
Prospective studies
Nine publications examined data from only 5 prospective studies: the Massachusetts Health Care Panel Study (1976–1980) (42), the Adventist Health Study-1 (AHS-1, 1974–1982) (43–46), the Adventist Health Study-2 (AHS-2, 2002–ongoing) (46), the Netherlands Cohort Study (NLCS, 1986–1992) (47–49), and the NHS (NHS-1 and NHS-2, 1976–ongoing) (50), which included dried fruit as a separate intake category (Table 3). The number of participants in these studies varied broadly, ranging from 1184 subjects in the earliest study (42) to 182,145 in the latest (50). Three publications considered only men or women, whereas 6 papers included both men and women. Only 1 study (50) considered intake of a specific dried fruit (prunes); the rest listed total dried fruit as a separate food variable. Seven (43, 44, 46–50) out of 9 publications provided data for both dried and raw or total fruit in the same study. All but 1 (42) publication were concerned with organ-specific cancers, including cancers of the prostate, stomach, pancreas, colon, breast, and lung, as well as urothelial cancers. Seven publications documented incidence of cancer or precancerous polyps as the outcome; the cancer mortality was reported in 2 works (42, 43). Analyses of the NLCS and NHS-1/2 data (47–50) were performed by treating dried fruit intake as continuous variables, whereas the AHS-1/2 data (43–46) were analyzed by categorizing subjects into quantile levels of dried fruit consumption. Such a limited number and high heterogeneity of the studies' outcomes thus precluded their meta-analysis.
TABLE 3.
Summary of prospective cohort studies reporting on associations between dried fruit intake and cancer risk1
| Author, year (ref), study | Outcome | Cohort/cases | Fruit type | Intake frequency | Adjusted relative risk (95% CI) | Adjusted factors |
|---|---|---|---|---|---|---|
| Botterweck, 2001 (49), NLCS | Stomach cancer incidence | 120,852/282 | Dried fruitTotal fruit | Continuous variables, per 25 g/d | 0.54 (0.13, 2.23)0.98 (0.96, 1.01) | Age, sex, smoking, education, stomach disorders, history of stomach cancer, total vegetable consumption |
| Mills, 1988 (43), AHS-1 | Pancreatic cancer deaths | 34,198/40 | Raisins, dates, other dried fruit | < 1/wk1–2/wk | 10.47 (0.19, 1.15) | Age, sex |
| ≥ 3/wk | 0.35 (0.17, 0.73) | |||||
| P-trend = 0.009 | ||||||
| Tantamango, 2011 (46), AHS-1 and AHS-2 | Colorectal polyps incidence | 2818/441 | Dried fruit | < 1/wk1–2/wk | 10.77 (0.59, 1.01) | Age, sex, BMI |
| ≥ 3/wk | 0.76 (0.58, 0.98) | |||||
| P-trend = 0.03 | ||||||
| Fresh noncitrus fruit | < 1/wk | 1 | ||||
| 1–2/wk | 0.85 (0.63, 1.17) | |||||
| ≥ 3/wk | 0.82 (0.62, 1.08) | |||||
| P-trend = 0.18 | ||||||
| Zeegers, 2001 (48), NLCS | Urothelial cancer incidence | 120,852/569 | Raisins, other dried fruit | Continuous variables, per 25 g/d | 0.37 (0.10, 1.43) | Age, sex, number of cigarettes per day, years of smoking, total vegetable consumption |
| Total fruit | 0.99 (0.96, 1.01) | |||||
| Mills, 1989 (44), AHS-1 | Prostate cancer incidence | 14,000/180 | Raisins, dates, other dried fruit | < 1/wk1–4/wk | 10.96 (0.68, 1.36) | Age |
| ≥ 5/wk | 0.51 (0.31, 0.85) | |||||
| P-trend = 0.01 | ||||||
| Fresh noncitrus fruit | < 1/wk | 1 | ||||
| 1–4/wk | 0.79 (0.52, 1.19) | |||||
| ≥ 5/wk | 0.78 (0.50, 1.20) | |||||
| P-trend = 0.45 | ||||||
| Schuurman, 1998 (47), NLCS | PCa incidence | 58,279/642 | Raisins, other dried fruit | Continuous variables, per 25 g/d | 0.52 (0.20, 1.39) | Age, family history of PCa, socioeconomic status, total vegetable consumption |
| Total fruit | 1.01 (0.99, 1.04) | |||||
| Fraser, 1991 (45), AHS-1 | Lung cancer incidence | 34,198/61 | Raisins, dates, other dried fruit | < 3/wk≥ 3/wk | 10.89 (0.50, 1.60) | Age, sex, smoking |
| Fresh noncitrus fruit | < 3/wk | 1 | ||||
| ≥ 3/wk | 0.73 (0.43, 1.26) | |||||
| Farvid, 2019 (50), NHS-1 and NHS-2 | Breast cancer incidence | 182,145/7,4642/1,7943 | Prunes | Continuous variables, per 2 servings/wk or per 2 servings/d | 1.02 (0.97, 1.08) 20.94 (0.81, 1.08) 3 | Age, family history of breast cancer, height, smoking, BMI, physical activity, alcohol, total energy, fiber consumption, and more |
| Total fruit | 0.94 (0.88, 1.01) 2 | |||||
| 0.90 (0.79, 1.03) 3 | ||||||
| Colditz, 1985 (42), Massachusetts Health Care Panel Study | Cancer deaths | 1184/42 | Dried fruits | < 1/wk≥ 1/wk | 10.6 (0.3, 1.4) | Age |
1AHS, Adventist Health Study; NHS, Nurses’ Health Study; NLCS, Netherlands Cohort Study; NPC, nasopharyngeal cancer; PCa, prostate cancer; ref, reference.
2Estrogen receptor-positive breast cancer.
3Estrogen receptor-negative breast cancer.
The key findings from the prospective studies can be summarized as follows:
Eight (89%) publications reported an inverse relation between total dried fruit consumption and cancer incidence or mortality, but the associations were significant (P-trend < 0.03) in only 3 studies (43, 44, 46). The relative cancer risk reductions associated with dried fruit intake were in the range 24–65%. In 1 study (50), the association was null.
Three publications reported dose-response for associations between dried fruit intake and mortality from pancreatic cancer (43), incidence of PCa (44), or incidence of precancerous colorectal polyps (46).
In 5 (44, 46–49) out of 7 studies that reported data for both dried and raw (or total) fruit consumption, the intake of dried fruits demonstrated stronger protective associations, compared to the associations between raw (or total) fruit intake and cancer, none of which were significant.
Assessment of study quality (Supplemental Table 1) indicates that the selected cohort studies have a rather low risk of bias: all studies scored 7 or 8 on the Newcastle-Ottawa scale, with mean [median] score 7.7 [8]. The lowest contribution to the quality score was from the Representativeness of the Exposed Cohort item; thus the selection of participants was the most common quality risk factor in these studies.
Case-control studies
Seven case-control studies, with a total of 1217 cases of cancer, reported on associations between dried fruit intake and cancer risk. The number of participants in each study varied between 144 and 708. The studies were conducted in Australia (56), Belgium (51), and the Mediterranean region (52–55, 57), between 1984 and 2015. The case-control studies focused on cancers in 6 different cancer sites: stomach (52, 53), colon (55), bladder (51), pancreas (56), prostate (54), and nasopharynx (57), and, in 3 studies, reported on specific dried fruit: raisins (56, 57), dried figs (57), and dates (55). The studies are also summarized in Table 4.
TABLE 4.
Summary of case-control studies evaluating the relations between dried fruit intake and cancer risk
| Author (reference), country | Years of study | Outcome | Cases/controls | Fruit type | Intake frequency | Adjusted OR (95% CI) | Adjusted factors |
|---|---|---|---|---|---|---|---|
| Gonzalez (53), Spain | 1987–1989 | Stomach cancer incidence | 354/354 | Dried fruit | 0.4 (0.2, 0.8) | Age, sex, area of residence, total calories, all food groups | |
| Fresh fruit | 0.7 (0.4, 1.2) | ||||||
| Yassibas (52), Turkey | 2008–2009 | Stomach cancer incidence | 106/106 | Dried fruit | <1/mo1–2 wk>1/d | 10.22 (0.08, 0.64)0.041 (0.007, 0.236) | Sex, residence, education, smoking, alcohol consumption, familial history of cancer or gastric cancer |
| P-trend = 0.002 | |||||||
| Fresh fruit | <1/mo | 1 | |||||
| 1–2/wk | 0.166 (0.012, 2.20) | ||||||
| >1/d | 0.282 (0.027, 2.97) | ||||||
| P-trend = 0.62 | |||||||
| Baghurst (56), Australia | 1984–1987 | Pancreatic cancer | 104/253 | Dried grapes | < 1 | ||
| P < 0.01 | |||||||
| Tayyem (55), Jordan | 2010–2012 | Colorectal cancer incidence | 220/281 | Dates | ≤1/wk3–4/wk1/d | 10.68 (0.30, 1.53)0.482 (0.27, 0.86) P-trend = 0.004 | Age, sex, total energy, metabolic equivalent min/wk, tobacco use, education level, marital status, work, income, family history of colorectal cancer |
| Other dried fruits | ≤1/wk2/wk | 1 4.13 (0.40, 42.9) | |||||
| 3–4/wk | 1.19 (0.21, 6.86) | ||||||
| P-trend = 0.864 | |||||||
| All fruits | ≤1/d | 1 | |||||
| 3/d | 1.00 (0.48, 2.07) | ||||||
| 5/d | 0.97 (0.22, 4.47) | ||||||
| P-trend = 0.23 | |||||||
| Kellen (51), Belgium | 1999–2004 | Bladder cancer incidence | 200/385 | Dried fruit | <1 g/d1–1.9 g/d>2 g/d | 10.64 (0.26, 1.54)0.60 (0.34, 1.04) | Age, sex, smoking status, occupational exposure to polyaromatic hydrocarbons or aromatic amines |
| P-trend = 0.05 | |||||||
| Total fruit | <189 g/d | 1 | |||||
| 189–360 g/d | 0.63 (0.40, 1.00) | ||||||
| >360 g/d | 0.61 (0.37, 0.99) | ||||||
| P-trend = 0.03 | |||||||
| Alvarez-Cubero (54), Spain | 2012–2015 | Prostate cancer incidence | 161/161 | Dried fruit | 0.79 (0.59, 1.06) | ||
| Laouamri (57), Algeria | 1994–1997 | Nasopharyngeal cancer incidence | 72/72 | Raisins | 0.24 (0.08, 0.67) | ||
| Dried figs | 2.66 (1.08, 6.54) |
Key findings from the case-control studies can be summarized as follows:
All 7 studies reported an inverse association between consumption of total dried fruit (51–54), raisins (56, 57), or dates (55) and cancer incidence, with the OR values for the highest compared with the lowest quantiles falling into the range 0.041–0.79, but the associations were significant in only 5 studies (52, 53, 55–57).
One study (57) reported a significant detrimental association between consumption of dried figs and incidence of nasopharyngeal cancer (NPC) (OR = 2.66).
Four studies also determined the inverse associations between consumption of fresh or total fruits and cancer incidence. Of these, 3 (52, 53, 55) reported that the protective associations were stronger for intake of dried fruits, compared to raw fruit consumption; in 1 instance (51), strengths of the associations were similar.
With mean [median] score 5.3 [6] on the Newcastle-Ottawa scale (Supplemental Table 2), the study quality of the selected case-control studies was notably lower than the quality of the cohort studies. One case-control study (57) was judged to have high risk of bias, whereas 2 studies (51, 53) fared well, scoring 7 points each on the scale. The most common sources of potential bias in the case-control studies were lack of information about nonresponse rate and history of cancer disease in controls, as well as lack of blinded ascertainment of dietary exposure.
Stomach cancer
One cohort (49) and 2 case-control studies (52, 53) investigated the associations between dried fruit intake and incidence of stomach cancer in the Netherlands, Spain, and Turkey. All 3 studies reported inverse associations between gastric cancer risk and consumption of dried fruits. Whereas in the NLCS study (49) the association was suggestive (RR for continuous variables, per 25 g: 0.54; 95% CI: 0.13, 2.23), both case-control studies indicated strong and significant protective effects of dried fruits, with OR for the highest compared with lowest quantile 0.4 [95% CI: 0.2, 0.8; no P-trend reported; ref. (53)] and 0.041 [95% CI: 0.007, 0.236; P-trend = 0.002; ref. (52)]. All 3 studies reported data on the relations between total or fresh fruit intake and risk of stomach cancer as well. None of these studies determined that consumption of total/fresh fruit was significantly associated with the cancer risk (Tables 3 and 4), however.
Pancreatic cancer
In the 1980s, 1 cohort study (43) and 1 case-control study (56) examined the associations between consumption of raisins, other dried fruit and lethality from pancreatic cancer. In the California AHS (43), a significant, 65% decrease in risk of dying from cancer of the pancreas was observed in subjects consuming 3 or more servings of dried fruit per week, compared to low-consumers or nonconsumers (RR for the highest compared with the lowest tertiles: 0.35; 95% CI: 0.17, 0.73; P-trend = 0.009). In the Australian case-control study (56), consumers of dried grapes had a significantly lower risk of pancreatic cancer (P < 0.01) as well. No data on total/fresh fruit intake were provided in these studies.
Colorectal cancer
The association between dried fruit intake and incidence of precancerous colorectal polyps was evaluated in a subcohort of the AHS-1 participants who also agreed to participate in the AHS-2 (46). Consumption of only 4 foods/food groups was inversely related to the polyps risk: cooked green vegetables, brown rice, legumes, and dried fruits. The calculated OR (considering the highest compared with the lowest tertiles) for the latter was 0.76 (95% CI: 0.58, 0.99; P-trend = 0.03). This work did not establish any significant associations between raw fruits (citrus, winter, or “other fruit”) and incidence of colorectal polyps. Similar conclusions were drawn in a Jordanian case-control study (55). After multivariate adjustments, no association was found between total fruit consumption and risk of colorectal cancer (OR: 0.97; 95% CI: 0.22, 4.47; P-trend = 0.23). However, there were inverse relations between consumption of dates (OR for the highest compared with the lowest quintile: 0.48; 95% CI: 0.27, 0.86; P-trend = 0.004) or figs (OR: 0.60; 95% CI: 0.34, 1.06; P-trend = 0.003) and the cancer risk.
Bladder cancer
One prospective cohort study (48) and 1 case-control study (51), carried out in the Netherlands and Belgium, reported suggestive, but not significant, inverse associations between dried fruit intake and incidence of bladder cancer [RR for continuous variables: 0.37; 95% CI: 0.10, 1.43; ref. (48) and OR for the highest compared with the lowest tertile 0.60; 95% CI: 0.34, 1.04; P-trend = 0.05; ref. (51)].
Prostate cancer
The relation between consumption of dried fruits and incidence of PCa in men was considered in 2 prospective cohort studies (44, 47) and 1 Spanish case-control study (54). A significant inverse association between dried fruit intake and incidence of PCa was established in the Adventist cohort (44), with RR for the highest compared with the lowest tertile 0.62 (95% CI: 0.36, 1.06; P-trend = 0.06). In contrast, consumption of total fruit was not associated with PCa risk (RR: 1.07; 95% CI: 0.72, 1.58; P-trend = 0.37) in this study. The other 2 studies found suggestive, but not significant, associations between consumption of dried fruit and PCa (Tables 3 and 4).
Other cancers
Three studies investigated the relations between consumption of dried fruits and incidence of other cancers, including lung (45), breast (50), and nasopharynx (57). One cohort study (42) examined the association between dried fruit intake and mortality from any cancer. Significant associations between dried fruit and NPC were reported in a small case-control study held in Algeria (57): raisins displayed an inverse association (adjusted OR: 0.24; 95% CI: 0.08, 0.67; P = 0.01), whereas consumption of dried figs was associated with increased NPC incidence (OR: 2.66; 95% CI: 1.08, 6.54; P = 0.05). In the rest of the studies, the associations between dried fruit intake and cancer risk were not significant (Table 3).
Discussion
This is, to the best of our knowledge, the first review to assess the relation between consumption of traditional dried fruits and risk of cancer based on existing epidemiological data. Previous laboratory and intervention studies suggested that dietary intake of dehydrated fruits could affect tumorigenesis through mechanisms, although not firmly established, likely attributed to changes in chemical composition of fruits during dehydration (15, 18, 33, 58). Two large cross-sectional studies (26, 59) based on the NHANES, established that dried fruit consumption was associated with healthier lifestyles and a variety of health parameters that are recognized as common risk factors in cancer, including adiposity, chronic inflammation, or glycemic control. Taken together, these findings lead to the question of whether dietary dried fruits could affect cancer risk in populations. We have identified 16 observational studies, published between 1985 and 2018, that relate dried fruit intake to cancer risk. However, differences in study designs, including study populations, preferences of dried fruit consumed across different geographical regions and cultures, and a broad variety of outcomes, made comparison of the results across the selected studies difficult. Thus, although this review describes the literature comprehensively, high heterogeneity of the studies prohibited interpretation of the data by meta-analysis.
Principal findings
Overall, data presented in this review indicate that increasing dried fruit consumption to 3–5 servings/wk may have health beneficial effects related to risk of certain cancers, including cancers of the pancreas, prostate, stomach, bladder, and colon. No such effect was found for lung or breast cancers. Another, and rather surprising, finding from the selected studies is that the associations between consumption of total/fresh fruits and cancer risk were generally weaker than the associations determined for dried fruit intake and cancer.
Strength of evidence
One strength of the evidence presented by this review is consistency in directionality of the associations across the studies. Overall, the selected studies assessed 18 dried fruit intake–cancer risk associations, 39% (7 relations) of which were significantly protective and another 39% (7 relations) were suggestively inverse. Strong inverse associations were determined between dried fruit intake and pancreatic cancer [65% decrease in risk of mortality at >3 servings/wk (43)], stomach cancer [60–96% decrease in risk of incidence (52, 53)], or NPC [76% decrease in incidence (57)]. Only 1 relation, between dried fig intake and NPC incidence (57), was appraised as potentially hazardous, but the study quality was rated as low.
Eleven (69% of a total 16) studies presented data for both dried and total/fresh fruit consumption, thus allowing for comparisons of the associations. In 8 studies (73% of the 11), the inverse associations between dried fruit intake and cancer risk were stronger than those found for total/fresh fruit, whereas in 3 studies (27%), the effects were comparable. Interestingly, in a cross-sectional study (60), French researchers reported a significant reduction in risk of high BMI with increasing intake of dried fruit (OR: 0.60; 95% CI: 0.50, 0.83; P-trend < 0.0001), but not total fruit (OR: 0.99; 95% CI: 0.80, 1.21; P-trend = 0.69). Consumption of dried, but not fresh, fruit was also inversely associated with high apoB, triacylglycerol, and fasting glucose concentrations (P-trend = 0.02, 0.01, and < 0.0001, respectively) and positively associated with high lipoprotein(a) (P-trend = 0.02) in this study. On the other hand, the cancer protective effect of consumed dried fruits was established largely for cancers of the digestive system (9 studies, 56%). This observation concurs with results of recent clinical trials (61), which demonstrated the preventive potential of dehydrated berries against progression of several cancers of the digestive tract. This “specificity of outcome” may serve as additional evidence in favor of dried fruit intake being a potentially protective dietary factor against cancer risk.
Six studies assessed dose–response effects of dried fruit consumption with cancer risk. The effect was significant (P-trend ≤ 0.05) in all 6 studies (100%), thus suggesting a biological gradient exists for the exposure. The trend is also in tune with a randomized clinical intervention study (16), which demonstrated the dose-dependent protective effect of dried strawberry intake against progression of esophageal cancer.
Limitations
The main limitation of this systematic review is high heterogeneity of the reviewed studies, especially at the outcome level. There are 10 different outcomes reported in the 16 selected studies, including 8 cancer sites and just 1 study concerned with overall cancer, 3 levels of the disease, including precancerous lesions, incidence of confirmed cancer, and death from cancer. Some major types of cancers, such as hematological cancers, skin cancers, uterine cancers, or cancers of the central nervous system, have not been addressed in the literature. Three studies lack adjustment for any confounders and 3 studies adjust for age and sex only. This review also has methodological limitations: 1) screening of the articles and data extraction were performed by 1 author only, so it is possible that some relevant articles were missed or that errors in data collection were made; 2) there was no comprehensive search of literature other than journal articles, and there was no attempt to contact investigators of the original studies for additional data, so it is possible that there are more data on the associations between dried fruit intake and cancer risk that were omitted from this review; 3) the risk of publication bias was not assessed because of the inability to perform a meta-analysis.
Comparison with other studies
To the best of our knowledge, there have been no other reviews, systematic or narrative, summarizing data from epidemiological studies that examined the relations between dried fruit intake and cancer risk. Some of the papers relevant to the subject of this review are summaries of clinical trials assessing the effects of freeze-dried berries in patients with precancerous lesions (15, 61, 62) and reviews of laboratory data obtained in animal and in vitro models of cancers (18, 63, 64). The summaries from both clinical trials and laboratory models suggest the health beneficial effects of dried fruits in humans, but offer no comparison of the effects provided by dried and fresh fruits. A few reviews compared epidemiological data on the relations between intake of raw tomatoes or tomato products and PCa risk (34, 65, 66). As mentioned earlier in this text, only a small proportion of tomato products are made of dehydrated tomato powder or dried tomatoes; therefore, the effects of dried tomatoes on the risk of PCa could not be characterized.
Knowledge gaps to address
To date, evidence concerning the impact of dried fruit consumption on risk of cancer in populations is sparse. We have identified only 16 publications that reported on associations between dried fruit intake and cancer incidence or mortality. For a comparison, there are over 100 currently recognized types of cancer. The modest number of identified publications reporting on dried fruits is also in stark contrast with hundreds of epidemiological studies and reviews that examined the relation between cancer risk and intake of raw or total fruits and vegetables. It is safe to argue that the data concerning the impact of dietary dried fruits on human health are very limited and, in the case of cancer, rare. Yet, dried fruits are customarily included as part of fruit lists in dietary recommendations concerning healthy lifestyles. What could be done to alleviate the current deficit of knowledge on the relation between dried fruit and health?
Dried fruit characterization
The observation of a trend that the association between dried fruit intake and cancer risk is somewhat stronger than the association between raw fruit and the disease deserves further attention. What are the differences between dehydrated and raw fruits that would justify separation of the former into a distinct dietary category? A number of studies have demonstrated that fruits may undergo significant chemical changes upon their dehydration and storage (13, 33, 67), giving rise to chemical species that may also distinguish dried fruits from their raw counterparts for a variety of biological effects, ranging from immunomodulation (68) to insulin response (59) to changes in gut microbiota composition and activity (69). Analytical data on dehydration-specific products in dried fruits are scarce. More work is needed to accurately characterize dried fruit composition: in addition to traditional basic nutrients, it would be important to assess water-soluble fiber, fructosamine, and reactive carbonyl species content. This information needs to be further systematized, to identify candidate biomarkers of consumed dried fruits, as well as to provide a background for future mechanistic studies. For example, 5-hydroxymethylfurfural is a representative reactive carbonyl species and a candidate for universal biomarkers of the Maillard reaction, which is a hallmark of dehydrated foods. Chromatographic methods for determination of its major metabolites excreted in urine are available and have been applied in both laboratory studies and clinical trials (70). However, a potential pitfall is that, besides dried fruits, there are other sources of dietary 5-hydroxymethylfurfural, including baked goods, honey and confectionery, juice, coffee, beer, wine, condiments, soy sauce, or vinegar (71).
Target populations
Dried fruits have been an essential part of Mediterranean and Mid-Eastern diets for millennia. Thus, they may have constituted an environmental factor, for which respective populations have developed adaptations at genomic level. It would be of interest to assess the impact of diminished dried fruit consumption on cancer risk in individuals of Mediterranean/Mid-Eastern origin. By the same logic, populations whose diets historically lacked dried fruit, may respond differently from populations with traditionally high intakes of these.
Outcomes
The summary of studies’ characteristics presented in Table 2 displays a prohibitively thin distribution of the outcomes across the studies. Unarguably, existing data from a handful of observational studies is suboptimal to that needed to draw any solid conclusions about the impact of dietary dried fruit on specific cancer risk or human health in general. Helpful in closing the gap could be reassessment and analysis of available data from the California Adventist (72), NCLS (73), and NHS (74) prospective cohort studies, and possibly other completed relevant prospective studies that contained dried fruit intake in the FFQ. Perhaps the best approach would be to examine these data by pooling the cohort studies, thus diminishing publication bias. Because each of the aforementioned prospective studies documented multiple health outcomes, databases of these studies could provide for assessing relations between consumption of dried fruits and risk of multiple cancer types, in addition to those few that have been published so far. Incorporation of the dried fruit category in diet questionnaires of already planned or ongoing epidemiological studies would benefit the field without the need for additional resources.
Although the effect of dried fruit intake on cancer risk could be addressed by randomized clinical studies, high cost and follow-up time limitations deem such studies unlikely. As exemplified by results of clinical trials testing the chemopreventive efficacy of dehydrated berries (15, 16), there is potential in conducting intervention studies where the outcome is a measure of cancer progression. Such studies would still be affected by the primary therapies. Another area of interest for future intervention studies would be determination of Dietary Inflammatory Index values (75) for individual dried fruits.
Mechanistic studies
Available comparative data on composition of fresh and dehydrated fruits suggest that dried fruits may contain equal or lower amounts of nutrients, but a higher content of reactive species resulting from thermal degradation and oxidation reactions occurring during dried fruit processing (33, 76), as well as microbial contaminants (13). Some of these chemical species, which are characteristic of dried fruits, such as 5-hydroxymethylfurfural or mycotoxins, have been noted for potentially hazardous outcomes, including carcinogenesis, when tested individually (14, 77). Why then would the overall effect of dietary dried fruits tend toward decreased cancer risk? The dose–response relations for exogenous toxins are generally nonlinear and in most cases include regions of beneficial physiological impact, also known as the hormetic effects (78). In regard to reactive agents from dried fruits, for example, ingestion of these at low doses may elicit mild and transient activation of physiological responses that can counteract chronic inflammation. These ideas need thorough verification in in vitro and preclinical settings to build a substantial knowledge base for future intervention and prospective observational studies.
In conclusion, the majority of prospective cohort and case-control studies that include data on relations between consumption of dried fruits and cancer suggest that increasing intake of dried fruits may lower risk of the disease. This conclusion, however, should be treated with great caution. There are only a limited number of cancer sites for which data are available, whereas an association of increased dried fruit consumption with healthier lifestyles implies that the dried fruit–cancer relation may not be necessarily causal. Yet, our analysis revealed a pattern that persists across the prospective, case-control, and cross-sectional studies and that suggests stronger health beneficial associations provided by dietary dried fruit compared to its raw equivalent. This conclusion is counterintuitive and deserves further attention. To date, a rather ambiguous recognition of dried fruit health benefits by the general population and by clinical and scientific communities may stem from lack of an adequate research base when it is not possible to make clear dietetic recommendations, although the available limited data look promising. Hence, future studies that 1) pool and reanalyze with greater power existing data from different cohorts, to detect the associations between dried fruit intake and risk of cancers at sites other than those reported so far; 2) assess cancer-related outcomes in response to controlled interventions in human subjects; 3) accurately characterize dried fruit composition and identify metabolites and/or biomarkers specific for dried fruit intake; and4) elucidate relevant mechanisms in laboratory and clinical settings, will be helpful to establish whether there is true association between dietary dried fruits and cancer risk. Expanding the knowledge base would guide clinical practice, public health interventions targeting fruit and vegetable consumption for cancer reduction (particularly in populations with limited access to fresh produce), as well as recommendations for reducing the risk of cancer with healthy food choices.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—VVM designed the study, conducted a systematic literature search, analyzed the data, wrote the manuscript, and had primary responsibility for final content; and all authors revised the manuscript critically for important intellectual content, and read and approved the final version of the manuscript.
Notes
VVM's and TPM's research is supported by the University of Missouri Agriculture Experiment Station Chemical Laboratories and by the National Institute of Food and Agriculture (grant no. MO-HABC0002).
Author disclosures: VVM, TPM, and ELG, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AHS, Adventist Health Study; NHS, Nurses’ Health Study; NLCS, Netherlands Cohort Study; NPC, nasopharyngeal cancer; PCa, prostate cancer.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Islami F, Goding SA, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I et al.. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. [DOI] [PubMed] [Google Scholar]
- 3. World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC: AICR, 2007. [Google Scholar]
- 4. McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. 2004;23(38):6349–64. [DOI] [PubMed] [Google Scholar]
- 5. Aune D, Vatten LJ, Norat T, Riboli E, Giovannucci E, Keum N, Boffetta P, Fadnes LT, Greenwood DC, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases. Diet, Nutrition, and the Prevention of Chronic Diseases. WHO Technical Report Series, 2003; 916. [PubMed] [Google Scholar]
- 7. Islami F, Ren JS, Taylor PR, Kamangar F. Pickled vegetables and the risk of oesophageal cancer: a meta-analysis. Br J Cancer. 2009;101(9):1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren J-S, Kamangar F, Forman D, Islami F. Pickled food and risk of gastric cancer-a systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev. 2012;21(6):905–15. [DOI] [PubMed] [Google Scholar]
- 9. Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91(4):317–31. [DOI] [PubMed] [Google Scholar]
- 10. Fardet A, Rock E, Bassama J, Bohuon P, Achir N, Prabhasankar P, Monteiro C, Moubarac J-C. Current food classifications in epidemiological studies do not enable solid nutritional recommendations for preventing diet-related chronic diseases: the impact of food processing. Adv Nutr. 2015;6(6):629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berry EM, Arnoni Y, Aviram M. The Middle Eastern and biblical origins of the Mediterranean diet. Pub Health Nutr. 2011;14(12A):2288–95. [DOI] [PubMed] [Google Scholar]
- 12. Sijtsema SJ, Jesionkowska K, Symoneaux R, Konopacka D, Snoek H. Perceptions of the health and convenience characteristics of fresh and dried fruits. LWT-Food Sci Technol. 2012;49(2):275–81. [Google Scholar]
- 13. Trucksess MW, Scott PM. Mycotoxins in botanicals and dried fruits: a review. Food Addit Contam. 2008;25(2):181–92. [DOI] [PubMed] [Google Scholar]
- 14. Wu F, Groopman JD, Pestka JJ. Public health impacts of foodborne mycotoxins. Annu Rev Food Sci Technol. 2014;5:351–72. [DOI] [PubMed] [Google Scholar]
- 15. Kresty LA, Mallery SR, Stoner GD, Seeram NP, Shukkitt-Hale B. Black raspberries in cancer clinical trials: past, present and future. J Berry Res. 2016;6(2):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen T, Yan F, Qian J, Guo M, Zhang H, Tang X, Chen F, Stoner GD, Wang X. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev Res. 2012;5(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mossine VV, Chopra P, Mawhinney TP. Interaction of tomato lycopene and ketosamine against rat prostate tumorigenesis. Cancer Res. 2008;68(11):4384–91. [DOI] [PubMed] [Google Scholar]
- 18. Stoner GD, Chen T, Kresty LA, Aziz RM, Reinemann T, Nines R. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr Cancer. 2006;54(1):33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuniga KE, Clinton SK, Erdman JW Jr. The interactions of dietary tomato powder and soy germ on prostate carcinogenesis in the TRAMP model. Cancer Prev Res. 2013;6(6):548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chai SC, Hooshmand S, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Daily apple versus dried plum: impact on cardiovascular disease risk factors in postmenopausal women. J Acad Nutr Diet. 2012;112(8):1158–68. [DOI] [PubMed] [Google Scholar]
- 21. Puglisi MJ, Vaishnav U, Shrestha S, Torres-Gonzalez M, Wood RJ, Volek JS, Fernandez ML. Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids Health Dis. 2008;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bays H, Weiter K, Anderson J. A randomized study of raisins versus alternative snacks on glycemic control and other cardiovascular risk factors in patients with type 2 diabetes mellitus. Phys Sportsmed. 2015;43(1):37–43. [DOI] [PubMed] [Google Scholar]
- 23. Alasalvar C, Shahidi F, editors. Dried Fruits: Phytochemicals and Health Effects. Ames (IA): Wiley-Blackwell, 2013. [Google Scholar]
- 24. FAOStat Data. Crops [Internet]. Food and Agriculture Organization of the United Nations; 2018 [accessed 31 January, 2019]. Available from http://www.fao.org/faostat/en/#data/QC. [Google Scholar]
- 25. FAOStat Data. Food Supply – Crops Primary Equivalent [Internet]. Food and Agriculture Organization of the United Nations; 2018 [accessed 31 January, 2019]. Available from http://www.fao.org/faostat/en/#data/CC. [Google Scholar]
- 26. Keast DR, O'Neil CE, Jones JM. Dried fruit consumption is associated with improved diet quality and reduced obesity in US adults: National Health and Nutrition Examination Survey, 1999-2004. Nutr Res. 2011;31(6):460–7. [DOI] [PubMed] [Google Scholar]
- 27. Jesionkowska K, Sijtsema S, Simoneaux R, Konopacka D, Płocharski W. Preferences and consumption of dried fruit and dried fruit products among Dutch, French and Polish consumers. J Fruit Ornam Plant Res. 2008;16:261–74. [Google Scholar]
- 28. Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California seventh-day Adventists. Am J Clin Nutr. 1999;70(3, Suppl.):532S–8S. [DOI] [PubMed] [Google Scholar]
- 31. Branthôme F-X. Spain: the other European giant [Internet]. Tomato News. The World Processing Tomato Council; 2017 [updated 15 March, 2017; accessed 31 January, 2019]. Available from http://www.tomatonews.com/en/spain-the-other-european-giant_2_264.html. [Google Scholar]
- 29. INC Statistics Committee. Nuts and Dried Fruits. Statistical Yearbook 2017/2018. Reus (Spain): International Nut & Dried Fruit Council, 2018. [Google Scholar]
- 30. US Department of Agriculture. Fruit and Tree Nut Yearbook Tables [Internet]. USDA Economic Research Service; 2018 [updated 31 October, 2018; accessed 31 January, 2019]. Available from https://www.ers.usda.gov/data-products/fruit-and-tree-nut-data/fruit-and-tree-nut-yearbook-tables/#All. [Google Scholar]
- 32. Nurses’ Health Study II Questionnaires [Internet]. Boston: Harvard T.H. Chan School of Public Health's Nutrition, 2016; [accessed 31 January, 2019]. Available from http://www.nurseshealthstudy.org/participants/questionnaires. [Google Scholar]
- 33. Mossine VV, Mawhinney TP. Significance of processing for the chemopreventive potential of tomato-based products. In: Watson RR, Preedy VR, eds. Bioactive Foods and Extracts: Cancer Treatment and Prevention. Boca Raton, FL: CRC Press, 2011:279–300. [Google Scholar]
- 34. Rowles JL, Ranard KM, Applegate CC, Jeon S, Erdman JW, An R. Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis. Prostate Cancer Prostatic Dis. 2018;21:319–36. [DOI] [PubMed] [Google Scholar]
- 35. Tan H-L, Thomas-Ahner JM, Grainger EM, Wan L, Francis DM, Schwartz SJ, Erdman JW, Clinton SK. Tomato-based food products for prostate cancer prevention: What have we learned?. Cancer Metastasis Rev. 2010;29(3):553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giovannucci E. Lycopene and prostate cancer risk. Methodological considerations in the epidemiologic literature. Pure Appl Chem. 2002;74(8):1427–34. [Google Scholar]
- 37. Wei MY, Giovannucci EL. Lycopene, tomato products, and prostate cancer incidence: a review and reassessment in the PSA screening era. J Oncol. 2012;2012:271063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu X, Li J, Wang X, Wang S, Meng S, Zhu Y, Liang Z, Zheng X, Xie L. Tomato consumption and prostate cancer risk: a systematic review and meta-analysis. Sci Rep. 2016;6:37091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petimar J, Wu K, Giovannucci EL, Smith-Warner SA, Wilson KM, Wang M, Albanes D, Cook MB, Liao LM, Ziegler RG et al.. A pooled analysis of 15 prospective cohort studies on the association between fruit, vegetable, and mature bean consumption and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liberati A, Altman D G, Tetzlaff J, Mulrow C, Gotzsche P C, Ioannidis J PA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses [Internet]. The Ottawa Hospital Research Institute, 2019; [accessed 2 March, 2019]. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 42. Colditz GA, Branch LG, Lipnick RJ, Willett WC, Rosner B, Posner BM, Hennekens CH. Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am J Clin Nutr. 1985;41(1):32–6. [DOI] [PubMed] [Google Scholar]
- 43. Mills PK, Beeson WL, Abbey DE, Fraser GE, Phillips RL. Dietary habits and past medical history as related to fatal pancreas cancer risk among Adventists. Cancer. 1988;61(12):2578–85. [DOI] [PubMed] [Google Scholar]
- 44. Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64(3):598–604. [DOI] [PubMed] [Google Scholar]
- 45. Fraser GE, Beeson WL, Phillips RL. Diet and lung cancer in California Seventh-day Adventists. Am J Epidemiol. 1991;133(7):683–93. [DOI] [PubMed] [Google Scholar]
- 46. Tantamango YM, Knutsen SF, Beeson WL, Fraser G, Sabate J. Foods and food groups associated with the incidence of colorectal polyps: the Adventist Health Study. Nutr Cancer. 2011;63(4):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Vegetable and fruit consumption and prostate cancer risk: a cohort study in The Netherlands. Cancer Epidemiol Biomark Prev. 1998;7(8):673–80. [PubMed] [Google Scholar]
- 48. Zeegers MPA, Goldbohm RA, van den Brandt PA. Consumption of vegetables and fruits and urothelial cancer incidence: a prospective study. Cancer Epidemiol Biomark Prev. 2001;10(11):1121–8. [PubMed] [Google Scholar]
- 49. Botterweck AAM, van den Brandt PA, Goldbohm RA. A prospective cohort study on vegetable and fruit consumption and stomach cancer risk in The Netherlands. Am J Epidemiol. 1998;148(9):842–53. [DOI] [PubMed] [Google Scholar]
- 50. Farvid MS, Chen WY, Rosner BA, Tamimi RM, Willett WC, Eliassen AH. Fruit and vegetable consumption and breast cancer incidence: repeated measures over 30 years of follow-up. Int J Cancer. 2019;144(7):1496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kellen E, Zeegers M, Paulussen A, Van Dongen M, Buntinx F. Fruit consumption reduces the effect of smoking on bladder cancer risk. The Belgian case control study on bladder cancer. Int J Cancer. 2006;118(10):2572–8. [DOI] [PubMed] [Google Scholar]
- 52. Yassıbaş E, Arslan P, Yalçın Ş. Evaluation of dietary and life-style habits of patients with gastric cancer: a case-control study in Turkey. Asian Pac J Cancer Prev. 2012;13(5):2291–7. [PubMed] [Google Scholar]
- 53. González CA, Sanz JM, Marcos G, Pita S, Brullet E, Saigi E, Badia A, Riboli E. Dietary factors and stomach cancer in Spain: a multi-centre case-control study. Int J Cancer. 1991;49(4):513–9. [DOI] [PubMed] [Google Scholar]
- 54. Alvarez-Cubero MJ, Pascual-Geler M, Martinez-Gonzalez LJ, Ruiz ME, Saiz M, Cozar JM, Lorente JA. Association between RNASEL, MSR1, and ELAC2 single nucleotide polymorphisms and gene expression in prostate cancer risk. Urol Oncol Semin Orig Invest. 2016;34(10):431.e1–e8. [DOI] [PubMed] [Google Scholar]
- 55. Tayyem RF, Shehadah I, Abu-Mweis SS, Bawadi HA, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M, Heath DD. Fruit and vegetable intake among Jordanians: results from a case-control study of colorectal cancer. Cancer Control. 2014;21(4):350–60. [DOI] [PubMed] [Google Scholar]
- 56. Baghurst PA, McMichael AJ, Slavotinek AH, Baghurst KI, Boyle P, Walker AM. A case-control study of diet and cancer of the pancreas. Am J Epidemiol. 1991;134(2):167–79. [DOI] [PubMed] [Google Scholar]
- 57. Laouamri S, Hamdi-Cherif M, Sekfali N, Mokhtari L, Kharchi R. Dietary risk factors of nasopharyngeal carcinoma in the Setif area in Algeria. Rev Epidémiol Santé Publ. 2001;49(2):145–56. [PubMed] [Google Scholar]
- 58. Chang SK, Alasalvar C, Shahidi F. Review of dried fruits: phytochemicals, antioxidant efficacies, and health benefits. J Funct Foods. 2016;21:113–32. [Google Scholar]
- 59. Fulgoni VL, Painter J, Carughi A. Association of raisin consumption with nutrient intake, diet quality, and health risk factors in US adults: National Health and Nutrition Examination Survey 2001-2012. Food Nutr Res. 2017;61(1):1378567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, Boutron-Ruault M-C. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr. 2005;82(6):1185–94. [DOI] [PubMed] [Google Scholar]
- 61. Bishayee A, Haskell Y, Do C, Siveen KS, Mohandas N, Sethi G, Stoner GD. Potential benefits of edible berries in the management of aerodigestive and gastrointestinal tract cancers: preclinical and clinical evidence. Crit Rev Food Sci Nutr. 2016;56(10):1753–75. [DOI] [PubMed] [Google Scholar]
- 62. Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res. 2009;2(3):187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kundu JK, Chun K-S. The promise of dried fruits in cancer chemoprevention. Asian Pac J Cancer Prev. 2014;15(8):3343–52. [DOI] [PubMed] [Google Scholar]
- 64. Schuster MJ, Wang X, Hawkins T, Painter JE. A comprehensive review of raisins and raisin components and their relationship to human health. J Nutr Health. 2017;50(3):203–16. [Google Scholar]
- 65. Chen J, Song Y, Zhang L. Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta-analysis of prospective studies. J Nutr Sci Vitaminol. 2013;59(3):213–23. [DOI] [PubMed] [Google Scholar]
- 66. Giovannucci E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp Biol Med. 2002;227(10):852–9. [DOI] [PubMed] [Google Scholar]
- 67. Göncüoğlu N, Mogol BA, Gökmen V. Phytochemicals and health benefits of dried apricots. In: Alasalvar C, Shahidi F, eds. Dried Fruits: Phytochemicals and Health Effects. Ames (IA): Wiley-Blackwell, 2013:226–42. [Google Scholar]
- 68. Basu A, Kurien BT, Tran H, Byrd BA, Maher J, Schell J, Masek E, Barrett JR, Lyons TJ, Betts NM et al.. Strawberries decrease circulating levels of tumor necrosis factor and lipid peroxides in obese adults with knee osteoarthritis. Food Funct. 2018;9:6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rodriguez-Morato J, Matthan NR, Liu J, de la Torre R, Chen CYO. Cranberries attenuate animal-based diet-induced changes in microbiota composition and functionality: a randomized crossover controlled feeding trial. J Nutr Biochem. 2018;62:76–86. [DOI] [PubMed] [Google Scholar]
- 70. Mousavi R, Alizadeh M, Saleh-Ghadimi S. Consumption of 5-hydroxymethylfurfural-rich dried fruits is associated with reduction in urinary excretion of 8-hydroxy-2'-deoxyguanosine: a randomized clinical trial. Eur Food Res Technol. 2016;242(5):677–84. [Google Scholar]
- 71. Degen J, Hellwig M, Henle T. 1,2-Dicarbonyl compounds in commonly consumed foods. J Agric Food Chem. 2012;60(28):7071–9. [DOI] [PubMed] [Google Scholar]
- 72. Adventist Health Studies [Internet]. Loma Linda (CA): Loma Linda University School of Public Health, 2019; [accessed 31 January, 2019]. Available from https://publichealth.llu.edu/adventist-health-studies. [Google Scholar]
- 73. van den Brandt PA, Goldbohm RA, van ’t Veer P, Volovics A, Hermus RJ, Sturmans F. A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol. 1990;43(3):285–95. [DOI] [PubMed] [Google Scholar]
- 74. Nurses’ Health Study [Internet]. Boston: Harvard T.H. Chan School of Public Health's Nutrition, 2016; [accessed 31 January, 2019]. Available from http://www.nurseshealthstudy.org/. [Google Scholar]
- 75. Tabung FK, Smith-Warner SA, Chavarro JE, Hu FB, Willett WC, Giovannucci EL, Wu K, Fuchs CS, Chan AT. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146(8):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carranza-Concha J, Benlloch M, Camacho MM, Martinez-Navarrete N. Effects of drying and pretreatment on the nutritional and functional quality of raisins. Food Bioprod Process. 2012;90(2):243–8. [Google Scholar]
- 77. Monien BH, Frank H, Seidel A, Glatt H. Conversion of the common food constituent 5-hydroxymethylfurfural into a mutagenic and carcinogenic sulfuric acid ester in the mouse in vivo. Chem Res Toxicol. 2009;22(6):1123–8. [DOI] [PubMed] [Google Scholar]
- 78. Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43(7):580–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.