Abstract
Background
Diffuse intrinsic pontine gliomas (DIPGs) are highly lethal childhood brain tumors. Their unique genetic makeup, pathological heterogeneity, and brainstem location all present challenges to treatment. Developing mouse models that accurately reflect each of these distinct features will be critical to advance our understanding of DIPG development, progression, and therapeutic resistance. The aims of this study were to generate new mouse models of DIPG and characterize the role of specific oncogenic combinations in DIPG pathogenesis.
Methods
We used in utero electroporation (IUE) to transfect neural stem cells in the developing brainstem with PiggyBac DNA transposon plasmids. Combinations of platelet-derived growth factor B (PDGFB), PdgfraD842V, or PdgfraWT, combined with dominant negative Trp53 (DNp53) and H3.3K27M expression, induced fully penetrant brainstem gliomas.
Results
IUE enabled the targeted transfection of brainstem neural stem cells. PDGFB + DNp53 + H3.3K27M induced the rapid development of grade IV gliomas. PdgfraD842V + DNp53 + H3.3K27M produced slower forming grade III gliomas. PdgfraWT + DNp53 + H3.3K27M produced high- and low-grade gliomas with extended latencies. PDGFB, PdgfraD842V, and PdgfraWT DIPG models display unique histopathological and molecular features found in human DIPGs. H3.3K27M induced both overlapping and unique gene expression changes in PDGFB and PdgfraD842V tumors. Paracrine effects of PDGFB promote disruption of pericyte-endothelial interactions and angiogenesis in PDGFB DIPG mouse models.
Conclusion
Brainstem-targeted IUE provides a rapid and flexible system to generate diverse DIPG mouse models. Using IUE to investigate mutation and pathohistological heterogeneity of DIPG will provide a valuable tool for future genetic and preclinical studies.
Keywords: DIPG, PDGFB, Pdgfra, mouse models, in utero electroporation, H3, 3K27M
Key Points.
1. Generation of DIPG mouse models by in utero electroporation is efficient.
2. DIPG mouse models display histopathology and molecular similarities to human tumors.
3. Non-cell-autonomous influence of PDGFB promotes vascular differences between mouse models.
Importance of the Study.
The development of mouse models that accurately reflect the locational, molecular, and histologic features of DIPG will be critical to advance our understanding of tumorigenesis. We have generated multiple mouse models of DIPG by IUE. Targeted co-transfection of brainstem neural stem cells with stably integrating plasmids expressing PDGFB, PdgfraD842V, or PdgfraWT, combined with DNp53 and H3.3K27M, induces fully penetrant gliomas. PDGFB + DNp53 + H3.3K27M induces the rapid formation of brainstem glioblastoma. PdgfraD842V + DNp53 + H3.3K27M generates infiltrative high-grade gliomas with minimal vascular abnormalities. PdgfraWT + DNp53 + H3.3K27M induces high- and low-grade gliomas with extended latencies. The IUE platform provides a flexible system to generate regionally defined gliomas with specific genetic drivers in an immunocompetent setting. We believe it will be a very useful tool for dissecting the contribution of recently identified DIPG mutations and performing preclinical testing of therapeutics.
Diffuse intrinsic pontine glioma (DIPG) is a fatal childhood brain tumor located in the brainstem. Treatment options remain limited; its location and diffuse nature prevent surgical resection, radiation therapy only provides a transient benefit, and no chemotherapy or small molecules have demonstrated a significant improvement in survival beyond what is achieved with standard radiation therapy alone.1 DIPG patients display a median overall survival of 10.5 months and a 2-year overall survival rate of 5.2%.2 Recent comprehensive genomic studies of DIPG patient samples have identified recurrent alterations, patterns of mutation co-segregation, and associated downstream signaling pathways. Recurrent alterations include amplification of platelet-derived growth factor receptor A (~40%) or activating mutations,2–4 K27M mutations in H3F3A or HIST1H3B (H3.3 or H3.1), which are present in approximately 63% and 21% of DIPGs, respectively,22 and TP53 loss of function mutations (~77%).2 In addition to their unique genetic alterations and molecular signatures, DIPGs display vascular differences relative to supratentorial pediatric high-grade glioma and adult glioblastoma. DIPGs commonly display little to no contrast enhancement on MRI, suggesting that they maintain a mostly intact blood–brain barrier.1,5 The failure of drugs to penetrate into DIPG at appreciable levels is a commonly cited reason for therapy failure and resistance.1,5
Limited availability of animal models that faithfully reproduce the genetic and region-specific features of DIPG has restricted progress in the field. Postnatal delivery of platelet-derived growth factor B (PDGFB) and H3.3K27M via the RCAS (replication-competent avian sarcoma) viral system can successfully induce brainstem gliomas that recapitulate some features of DIPG.6 The ability of PDGFB to rapidly induce glioma formation and growth at different ages and locations in the brain makes it a useful tool, but PDGFB amplifications are rarely found in DIPG or pediatric high-grade gliomas.2 Orthotopic implantation of transformed normal human astrocytes or induced pluripotent stem cell–derived human neural stem cells has also been used to model DIPG, demonstrating the ability of PdgfraD842V, TP53, and H3.3K27M mutations to promote gliomagenesis.4,7 A more recent study demonstrated the ability of H3.3K27M and Trp53 mutations to transform neural stem cells in vivo after focal delivery of DNA plasmids into the embryonic cortex using in utero electroporation (IUE). While under technical challenges, limited brainstem-targeted electroporations demonstrated a key role for H3.3K27M mutations in DIPG development.8
Here, we demonstrate the efficient generation of DIPG mouse models by transfecting defined combinations of oncogenic plasmids into the developing embryonic brainstem via IUE. Successfully targeted mice develop fully penetrant brainstem gliomas with different latencies, pathohistologies, and molecular signatures based on the combination of plasmids delivered. Molecular analysis reveals differences in PDGFB, PdgfraD842V, and PdgfraWT driven DIPG models. We show that PdgfraD842V DIPGs display minimal changes in brainstem vasculature structure, while PDGFB DIPGs induce non-cell-autonomous perivascular alterations that likely contribute to their pro-angiogenic phenotype and highly aggressive nature. Overall, our data demonstrate a highly efficient system to create genetically diverse DIPG mouse models, and provide insight into the pathological heterogeneity induced by specific oncogenic drivers.
Methods
DNA Plasmid Construction
PiggyBac donor plasmids (PBCAG-Ires-eGFP and PBCAG-Ires-luciferase) were generated by removing enhanced green fluorescent protein (eGFP) from the PBCAG-eGFP vector (a kind gift from Dr Joseph LoTurco, University of Connecticut) and replacing it with Ires-eGFP or Ires-luciferase by In-Fusion cloning (Clontech). PDGFB (Addgene #17757), PdgfraD842V (Addgene #66789), PdgfraWT (Addgene #66787), DNp53,9 H3.3WT (Addgene #42632), and H3.3K27M (a kind gift from Dr Q. Richard Lu, CCHMC) were PCR amplified from template plasmids and inserted into EcoR1 linearized PBCAG-Ires-eGFP or PBCAG-Ires-luciferase using the In-Fusion cloning kit (Clontech). Plasmids were verified by Sanger sequencing and propagated in Stellar competent bacteria cells (Clontech), and endotoxin-free DNA was prepared from sequence verified plasmids (Machery-Nagel).
Mouse Colony and In Utero Electroporation
All mouse work was done according to institutional and Institutional Animal Care and Use Committee review boards (University of Cincinnati). Time pregnant CD1-ICR mice (Charles River) were used for all experiments. In utero electroporation was performed as previously described.10,11 Briefly, approximately 1 μL of concentrated DNA mixtures (1 μg/μL for each plasmid) containing 0.05% Fast Green (Sigma) was injected into the fourth ventricle of embryos using a pulled glass capillary pipette. Successfully injected embryos were electroporated by applying 5 square pulses (45 V, 50 ms pulses with 950 ms intervals) with the positive electrode of a 3 mm tweezer electrode directed toward the lower rhombic lip of the fourth ventricle (BTX/Harvard Bioscience). Embryos were returned to the abdominal cavity and incision sutured, and the female monitored until fully recovered. Electroporated pups were monitored for development of neurologic symptoms related to tumor burden, including altered gait, lethargy, hydrocephalus, and weight loss. Mice presenting symptoms were euthanized according to recommendations provided by the veterinary staff.
Tissue Collection and Immunostaining
Brains were collected and processed as previously described.12 Briefly, brains were rapidly dissected in ice-cold phosphate buffered saline (PBS) and then fixed in 4% paraformaldehyde overnight. Fixed brains were washed in PBS and then incubated at 4°C in 30% sucrose/PBS solution overnight before embedding in tissue freezing media. Free-floating sections 50 μm thick were made using a cryostat (Leica). Free-floating sections were transferred to blocking solution (PBS + 0.5% Triton X-100 + 10% normal donkey serum) at room temperature for 30–60 minutes before adding specific combinations of antibodies. Primary antibodies were incubated at 4°C overnight. The next day, sections were washed in PBS and then transferred to blocking solution containing the appropriate secondary antibodies (1:500) and incubated at 4°C overnight. Finally, sections were washed in PBS followed by a 10 minute incubation in Hoechst (1:1000 in PBS) and washed in PBS again before being mounted onto slides (Fisher, Superfrost) and coverslipped (Prolong Gold Antifade, ThermoFisher). Primary antibodies used were: eGFP (Aves, #GFP1020), Olig2 (Millipore, #Ab9610), Gfap (Cell Signaling, #12389), Sox9 (Millipore, #Ab5535), Ki67 (Cell Signaling, #9129), CD31 (BD Biosciences, #550274), collagen IV (BioRad, #161115), desmin (Cell Signaling, #5332), nestin (DSHB #Rat401), Pdgfra (ThermoFisher #14-1402-82), and Hoechst (ThermoFisher). Corresponding secondary antibodies used were all purchased from Jackson ImmunoResearch. Images were acquired on a confocal microscope (Nikon A1), and image analysis was performed in Image J (National Institutes of Health). Vascular quantification was performed as previously described.12
For histopathology, 10% formalin fixed brains were transferred to 70% ethanol before paraffin embedding. Sections 5 μm thick were prepared on a microtome (Leica), and processed for hematoxylin-eosin (H&E) staining. Immunohistochemistry (IHC) for noted antigens was performed at the CCHMC pathology core using the automated Ventana Symphony and BenchMark stainers. Antibodies included: H3F3A K27M (Abcam, #Ab190631) and H3.3-trimethyl (Cell Signaling, #9733).
RNA Preparation, Whole Transcriptome Sequencing, and Analysis
Freshly isolated tumors were snap frozen, and total RNA isolated using the NucleoSpin RNA kit (Macherey-Nagel). RNA quality control was performed on a bioanalyzer (BioRad) to ensure the quality of each sample submitted. For isolation of polyA RNA, a NEBNext Poly(A) mRNA Magnetic Isolation Module (New England BioLabs) was used for polyA RNA purification with a total of 1 µg good quality total RNA as input. The SMARTer Apollo NGS library prep system (Takara) was used for automated polyA RNA isolation. For RNA sequencing library preparation, the library for RNA-seq was prepared by using the NEBNext Ultra II Directional RNA Library Prep Kit (New England BioLabs). After indexing via PCR enrichment (8 cycles), the amplified libraries together with the negative control were cleaned up for quality control analysis. To study differential gene expression, individually indexed and compatible libraries were proportionally pooled (~25 million reads per sample in general) for clustering in the cBot system (Illumina). Libraries at the final concentration of 15 pM were clustered onto a single-read flow cell using the Illumina TruSeq SR Cluster Kit v3, and sequenced to 51 bp using the TruSeq SBS Kit v3 on the Illumina HiSeq system. Sequence reads were aligned to the reference genome using the TopHat aligner, and reads aligning to each known transcript were counted using Bioconductor packages for next-generation sequencing data analysis. The differential expression analysis between different sample types was performed using the negative binomial statistical model of read counts as implemented in the edgeR Bioconductor package. Transcriptional profiles were interrogated with iGEAK (Interactive Gene Expression Analysis Kit for microarray and RNA-seq data), an R (v3.3.2) and JavaScript-based open-source desktop application.13 Differential gene expression heatmaps and Gene Set Enrichment Analysis (GSEA) files were generated and downloaded for analysis. GSEA 3.0 was used to analyze GSEA files. Functional enrichment of differentially expressed gene lists between tumor groups was performed with gProfiler using default settings. All RNA-seq files are deposited in Gene Expression Omnibus as GSE128807.
Results
Targeted Transfection of Embryonic Brainstem Neural Stem Cells by In Utero Electroporation
We utilized IUE to directly transfect brainstem neural stem and progenitor cells lining the floor of the fourth ventricle. Stably integrating PiggyBac DNA plasmids expressing green fluorescent protein (GFP) were injected into the fourth ventricle of mice at embryonic day 13.5 (e13.5) and electroporated toward the brainstem. GFP-positive embryos were collected at 24 hours post surgery and at postnatal day 21 (p21). Examination of whole mount hindbrains and coronal sections 24 hours post surgery showed brainstem-specific GFP expression, with GFP-positive cells clustered on one side of the brainstem due to the unidirectional transfection pattern of electroporation (Fig. 1A–C). GFP-positive cells displayed neural stem and progenitor cell morphologies, including nestin-positive radial glial-like processes that spanned between the fourth ventricle and ventral pial surface (Supplementary Fig. 1A–C). A subset of GFP-positive cells was also co-labeled with the oligodendrocyte specification transcription factor Olig2 (Fig. 1C, Supplementary Fig. 1D, E). Oligodendrocyte precursor cells (OPCs) have been identified in the developing hindbrain as early as e12.5.14,15 Co-labeling non-electroporated e13.5 brainstem sections with Olig2 and Pdgfra revealed double-positive OPCs scattered throughout the brainstem, with a subset in close or direct contact with the ventricular surface (Supplementary Fig. 1F, G). At postnatal day 21 (p21), GFP-positive neurons, astrocytes, and oligodendrocytes could be visualized throughout the brainstem, including in the pons and medulla regions, demonstrating the widespread migration and potential of transfected brainstem cells to generate all 3 major cell lineages of the central nervous system (Fig. 1D–I).
Fig. 1.
IUE targeted transfection of brainstem neural stem and progenitor cells. (A, B) Brightfield and GFP images of whole mount of dorsal brainstem 24 hours post IUE with empty vector GFP plasmid. Scale bar, 1 mm. (C) Coronal brainstem section 24 hours post IUE stained with GFP, Olig2, and Hoechst. Scale bar, 100 μm. (D, E) Brightfield and GFP images of ventral whole brain image of GFP IUE at p21. Scale bar, 2.5 mm. (F) Sagittal image of electroporated brainstem at p21 showing widespread GFP expression. Scale bar, 500 μm. (G–I) Merged projection images of GFP (green), Hoechst (blue) and (G) NeuN, (H) Olig2, or (I) Sox9 (magenta). Scale bar, 10 μm.
Generation of Spontaneous DIPG Mouse Models by IUE
To evaluate the ability of different genetic alterations to promote brainstem gliomagenesis, we performed IUE with defined combinations of plasmids to increase Pdgfra activation, inactivate Trp53, and model H3.3K27M mutations. IUE of PDGFB ligand + dominant negative Trp53 (DNp53)9 + H3.3K27M or H3.3WT resulted in rapid glioma formation, with pups displaying tumor-related symptoms, including hydrocephalus, domed skulls, and neurologic deficits (median survival = 19.5 days and 20 days post IUE for H3.3K27M and H3.3WT combinations, respectively) (Fig. 2A). Dense GFP-positive tumor regions were commonly seen near the ventral and dorsal brainstem regions, with additional GFP-positive cells displaying widespread infiltration of the hindbrain (Fig. 2B, C). IUE of constitutively active PdgfraD842V + DNp53 + H3.3K27M or H3.3WT also resulted in the development of fully penetrant gliomas, but showed distinct differences compared with PDGFB-driven DIPG models. Mice uniformly developed head tilts as the first symptom of tumor burden, with no hydrocephalus or domed skulls noted. H3.3K27M status also significantly shortened the latency to neurologic symptom presentation (median survival = 49 days and 84.5 days post IUE for H3.3K27M and H3.3WT combinations, respectively) (Fig. 2A). GFP-positive cells were dispersed throughout the brainstem, with dense areas commonly seen in the dorsal and ventral brainstem regions. GFP-positive cells could also be found in the cerebellar white matter and along hindbrain pial surfaces (Fig. 2B, D). IUE of PdgfraWT + DNp53 + H3.3K27M resulted in a subset of mice that developed neurologic symptoms prompting euthanasia (21%) by >200 days post IUE (Fig. 2A). Symptoms differed between mice, including head tilts, hind limb paralysis, and general lethargy. GFP expression patterns also varied, but most showed regions of focal and diffuse GFP signal throughout the brainstem (Fig. 2B, C and Supplementary Fig. 2A, B). While the remaining mice did not develop neurologic symptoms by the study endpoint, all displayed diffuse GFP expression in the brainstem at the time of collection (Supplementary Fig. 2C, D). IUE of DNp53 + H3.3K27M at e13.5 did not result in the development of neurologic symptoms by >200 days, and only a few brains displayed small regions of weak GFP expression at the time of collection (Fig. 2A).
Fig. 2.
Generation of DIPG mouse models by brainstem-targeted IUE. (A) Kaplan–Meier survival curves for indicated combinations of plasmids electroporated (***P < 0.0001, log-rank test). (B) Brightfield and GFP merged whole brain images demonstrating the brainstem location of GFP-positive tumors in each IUE condition. Scale bar, 2.5 mm. (C) Hindbrain sagittal sections of PDGFB (left), PdgfraD842V (middle), and PdgfraWT (right) tumors showing GFP-positive tumor cells spread throughout the brainstem. Hoechst (blue) and GFP (green). Scale bar, 1 mm.
Spontaneous DIPG Mouse Models Display a Range of Glioma Histopathology
To define pathological features of IUE-generated DIPG mouse models, we performed histologic analysis and IHC staining for glioma-associated antigens. PDGFB + DNp53 + H3.3K27M tumors (referred to as PDGFB tumors from here on) were classified as grade IV, with regions of pseudopalisading necrosis, vascular proliferation, and hemorrhage (Fig. 3A, B). PdgfraD842V + DNp53 + H3.3K27M tumors (referred to as PdgfraD842V tumors from here on) were characterized as grade III high-grade gliomas, with a high degree of cellular pleomorphism but no areas of necrosis or overt vascular proliferation (Fig. 3A, C). PdgfraD842V tumors displayed histologic features of anaplastic astrocytoma, and sometimes displayed a giant cell component (Fig. 3C), a characteristic previously described in human DIPG cases, as well as in other mouse glioma models.16,17 PdgfraWT + DNp53 + H3.3K27M tumors (referred to as PdgfraWT tumors from here on), including those from non-symptomatic mice, all displayed neoplastic features ranging from high grade to low grade. Tumors from mice with neurologic symptoms were all classified as high grade, with one presenting as grade IV with a prominent loose myxoid matrix component (Fig. 3A, D and Supplementary Fig. 3A–D). Tumor cells were highly pleomorphic, and perineuronal satellitosis, a common hallmark of DIPG, was present across multiple samples (Fig. 3D and Supplementary Fig. 3E, F). Other features, such as leptomeningeal spread and invasion along the vasculature, were also observed across tumors (Supplementary Fig. 3G, H). IHC staining with an H3 K27M mutant antibody was specific to tumors expressing H3.3K27M and corresponded with loss of H3K27 trimethylation (H3K27me3) staining, a hallmark of K27M mutant tumors (Fig. 3E). A decrease in H3K27 trimethylation was also confirmed by western blot of cell lines established from PdgfraD842V + DNp53 + H3.3K27M or PdgfraD842V + DNp53 + H3.3WT tumors (Supplementary Fig. 4).
Fig. 3.
PDGFB, PdgfraD842V, and PdgfraWT DIPG mouse models display distinct histopathology. (A) Histopathology grading of each IUE condition. (B–D) Representative images of H&E stained sections from (B) PDGFB + DNp53 + H3.3K27M, (C) PdgfraD842V + DNp53 + H3.3K27M, and (D) PdgfraWT + DNp53 + H3.3K27M tumors. Black arrowhead points to giant cells found in PdgfraD842V tumors. (E) IHC staining for mutant H3K27M protein (top row) and H3K27 trimethylation (bottom row) in PDGFB + DNp53 + H3.3WT, PDGFB + DNp53 + H3.3K27M, PdgfraD842V + DNp53 + H3.3WT, PdgfraD842V + DNp53 + H3.3K27M, and PdgfraWT + DNp53 + H3.3K27M tumors. Scale bars, 50 μm.
Positive Immunostaining for Glioma Markers in Spontaneous Mouse Models of DIPG
PDGFB, PdgfraD842V, and PdgfraWT GFP-positive tumor cells were regularly co-labeled with the glioma-associated marker Olig2 (Fig. 4A–C). Expression of glial fibrillary acidic protein (GFAP) appeared restricted mainly to GFP-negative reactive astrocytes in each tumor model (Fig. 4A–C). Ki67-positive proliferative tumor cells were present in all IUE conditions (Fig. 4A–C). PdgfraWT tumors displayed a range of Ki67 staining, with low-grade tumors showing only scattered Ki67-positive cells throughout the brainstem, which was not observed in control brainstems (Supplementary Fig. 5A, B). The presence of H3.3K27M or H3.3WT did not alter overall proliferation rates in PDGFB + DNp53 tumors (% Ki67-positive cells: 30.2 ± 5.7 vs 38.2 ± 2.5, respectively) and resulted in a small but significant increase in PdgfraD842V + DNp53 tumors (6.7 ± 0.8% vs 4.1 ± 0.6, respectively) (Supplementary Fig. 5C).
Fig. 4.
Shared expression of glioma antigens in PDGFB and PdgfraD842V DIPG mouse models. (A) PDGFB + DNp53 + H3.3K27M, (B) PdgfraD842V + DNp53 + H3.3K27M, and (C) PdgfraWT + DNp53 + H3.3K27M tumor samples co-stained for Hoechst (blue), GFP (green), and either Olig2, Gfap, or Ki67 (magenta). Scale bar, 10 μm.
Defining Gene Expression Differences Between DIPG Mouse Models
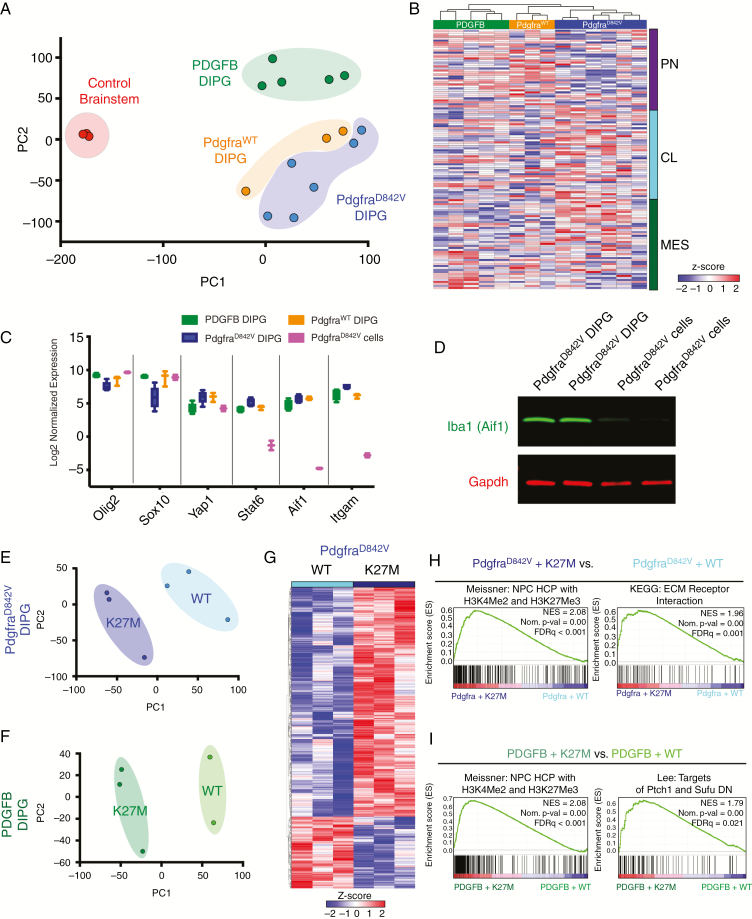
To understand molecular differences between PDGFB, PdgfraD842V, and PdgfraWT DIPG mouse models we performed genome-wide transcriptional profiling (RNA-seq) of freshly dissected tumor samples and control brainstems. Principle-component analysis (PCA) visualization revealed PDGFB tumors segregated from PdgfraD842V and PdgfraWT tumors, which were positioned adjacent to each other (Fig. 5A). We performed unsupervised hierarchical clustering with genes associated with the proneural, classical, and mesenchymal subtypes.18 PDGFB DIPG models displayed high expression of proneural genes, agreeing with previous studies that have shown that PDGFB expression promotes proneural subtype glioblastoma formation.19,20 PdgfraWT DIPG models clustered between PDGFB and PdgfraD842V tumors, showing a mix of proneural and mesenchymal gene expression. PdgfraD842V DIPG models showed higher expression of genes associated with classical and mesenchymal subtypes (Fig. 5B). GSEA comparing PDGFB, PdgfraWT, and PdgfraD842V DIPG models showed a similar shift from proneural to mesenchymal associated signatures, respectively (Supplementary Fig. 6A). Variation in the overall composition of the tumor microenvironment can influence transcriptional subtype designation.18 To determine if the mesenchymal profile of PdgfraD842V tumor samples represented the diffuse nature of these tumors or directly reflected differences in tumor cells, we examined the expression of key proneural (Olig2, Sox10), mesenchymal (Yap1, Stat6), and mesenchymal/microglial (Aif1, Itgam) subtype genes in cell lines derived from PdgfraD842V driven tumors. Compared with PdgfraD842V tumor samples, PdgfraD842V tumor cell lines showed increased expression of proneural genes and decreased mesenchymal genes (Fig. 5C). Decreased Aif1 expression was verified at the protein level by western blot of PdgfraD842V + DNp53 + H3.3K27M primary tumors and derived cell lines (Fig. 5D).
Fig. 5.
Transcriptional differences between PDGFB vs PdgfraD842V and H3.3WT vs H3.3K27M DIPG mouse models. (A) PCA of all samples based on transcriptome-wide expression. (B) Unsupervised hierarchical clustering of all samples based on the Verhaak glioma subtype 50 gene signatures. (C) Comparison of subgroup-related gene expression across PDGFB, PdgfraD842V, PdgfraWT tumors, and PdgfraD842V tumor cell lines. (D) Western blot of PdgfraD842V tumors and PdgfraD842V tumor cell lines for Aif1 and Gapdh. (E) PCA of PdgfraD842V tumors showing H3.3K27M induced transcriptional changes. (F) PCA of PDGFB tumors showing H3.3K27M induced transcriptional changes. (G) Heatmap of differentially expressed genes between H3.3WT vs H3.3K27M PdgfraD842V tumors. (H) GSEA analysis of H3.3WT vs H3.3K27M PdgfraD842V tumors displaying enrichment of specific gene sets in each group. (I) GSEA analysis of H3.3WT vs H3.3K27M PDGFB tumors displaying enrichment of specific gene sets in each group.
H3K27M mutations result in global changes in cellular H3K27 trimethylation, altering cellular programs to promote an undifferentiated stem/progenitor cell state.7,21–24 To determine how H3.3K27M versus H3.3WT expression altered gene expression profiles in our PDGFB and PdgfraD842V tumors, we examined each group individually. Following PCA visualization of PDGFB or PdgfraD842V tumor samples, H3.3K27M expressing tumors segregated from H3.3WT tumors (Fig. 5E, Supplementary Fig. 6C). Analyses of differentially expressed genes within PdgfraD842V tumors revealed a total of 1173 significantly (log2 fold change >1 or <−1, P < 0.05) upregulated (890) and downregulated (283) genes (Fig. 5G, Supplementary Table 1), while PDGFB tumors displayed 914 significantly upregulated (438) and downregulated (476) genes (Supplementary Table 1). Among the most highly enriched gene sets for both PdgfraD842V and PDGFB + H3.3K27M tumors was “Meissner NPC HCP with H3K4me2 and H3K27me3,” suggesting altered expression of genes normally trimethylated at K27 in neural progenitor cells (Fig. 5H, I). Within PdgfraD842V + H3.3K27M tumors, significantly enriched gene sets included extracellular matrix receptor interactions, and PDGFB + H3.3K27M tumors showed enrichment in sonic hedgehog signaling (Fig. 5H, I). Examination of the differentially expressed gene lists revealed approximately 200 common genes up- or downregulated by H3.3K27M in PDGFB and PdgfraD842V DIPG mouse models, which could represent tumor cell intrinsic or extrinsic changes. Shared upregulated genes included those involved in glial identity (Gfap, Aqp4), gap junctions (Gja1), insulin signaling (Igf2bp3), and Axl signaling (Gas6), while the tumor suppressor cyclin-dependent kinase inhibitor 2A (p16) was downregulated in both H3.3K27M conditions (Supplementary Table 1).
PDGFB Expression Induces Non-Cell-Autonomous Vascular Alterations in Tumors
Among the top gene sets enriched in PDGFB compared with PdgfraD842V or PdgfraWT DIPG mouse models were those related to tumor angiogenesis and extracellular matrix remodeling (Fig. 6A, Supplementary Fig. 6B), correlating with vascular difference seen in tumor pathohistology. Exogenous expression of PDGFB can promote Pdgfra-positive/Olig2-positive OPC growth,25 which we could identify in PDGFB pre-neoplastic lesions, but not in PdgfraD842V lesions (Supplementary Fig. 7). But the influence of PDGFB expression on other potentially responsive cell types is not well characterized. Based on publicly available single cell sequencing databases,26,27 Pdgfra is also expressed by vascular associated fibroblast-like cells, and Pdgfrb is expressed by arterial endothelial cells, vascular associated fibroblast-like cells, pericytes, and vascular smooth muscle cells (Supplementary Table 2). To investigate potential non-autonomous differences, we examined the endothelial and pericyte content within PDGFB and PdgfraD842V DIPG models. PDGFB tumors contained areas of morphologically abnormal blood vessels commonly found in glioblastoma, with an increased vascular density compared with control brainstem or PdgfraD842V tumors (% CD31 area, control brainstem = 10.68 ± 0.5; PDGFB = 16.07 ± 0.8; PdgfraD842V = 12.0 ± 0.4) (Fig. 6C). These regions were regularly associated with aberrant pericyte and extracellular matrix coverage, as seen with desmin and collagen IV staining, respectively (Fig. 6D). PdgfraD842V tumor vasculature, on the other hand, was nearly indistinguishable from the normal brainstem vascular architecture, with only small regions of some tumors displaying dilated vessels (Fig. 6B). PdgfraD842V tumor pericyte and extracellular matrix coverage of vessels also appeared unchanged compared with normal brain regions (Fig. 6D). Thus, exogenous expression of PDGFB by tumor cells likely functions through cell autonomous and non-autonomous mechanisms to promote tumorigenesis, stimulating vascular remodeling and angiogenesis through paracrine activation of Pdgfra- and Pdgfrb-expressing cell types.
Fig. 6.
PDGFB expression induces non-cell-autonomous vascular alterations. (A) GSEA of vascular associated gene sets in PDGFB tumors. (B) Immunofluorescent staining for GFP (green) and CD31 (red) in control brainstem, PDGFB, and PdgfraD842V tumors. (C) Quantification of vascular density (% area CD31) (n = 10 control brainstem, n = 8 PDGFB DIPG, n = 9 PdgfraD842V DIPG. ***P < 0.0001, unpaired 2-tailed t-test). (D) Immunofluorescent staining of control brainstem, PDGFB, and PdgfraD842V tumors with Hoechst (blue), CD31 (red), and desmin (top, cyan), or collagen IV (bottom, cyan). Scale bar, 50 μm.
Discussion
In this study, we describe a rapid and efficient platform to generate spontaneous DIPG mouse models in their native anatomical location. In addition, we provide data highlighting developmental, pathohistological, and molecular disparities of DIPG mouse models created by introducing different oncogenic combinations. A number of glioma mouse models have been developed,28–30 but few provide the ability to direct in vivo regional development of brainstem-specific tumors. Our data build on recent cortical IUE glioma studies8,31 demonstrating the capability of brainstem IUE to reliably generate regionally defined gliomas. We also define the developmental timeframe and differences of tumor-related symptoms in IUE models driven by specific genetic combinations. We note that PdgfraWT and PdgfraD842V brainstem glioma models rarely display necrotic foci found in genetically similar8 or identical (data not shown) cortical models, suggesting regional differences in tumor growth or microenvironment interactions. This should be considered when selecting preclinical models (spontaneous or orthotopic), as differences in tumor growth and vascular integrity could impact drug efficacy and pharmacokinetic results. Another difference compared with the recent Pathania study,8 which performed IUE at e12.5, is the inability of H3.3K27M + DNp53 to generate tumors at e13.5. Whether there is a limited developmental window of susceptibility or differences in the ability to target certain cell populations with IUE will be of great interest. The flexibility of the IUE modeling platform will provide an isogenic system to investigate how temporally and regionally defined cellular programs cooperate with H3.3K27M and other recently identified recurrent genetic mutations or alterations, including PPM1D, FGFR1, and CCND2,2 to drive specific gene expression programs important for DIPG pathogenesis.
A minimally disrupted blood–brain barrier is a hallmark of DIPG1 and is commonly seen in some patient derived xenograft models.32 Developing animal models that faithfully recapitulate this feature will be highly important, especially when performing preclinical studies to determine drug penetration and efficacy. We show that PDGFB-induced DIPGs result in vascular remodeling and angiogenesis, while PdgfraD842V DIPGs display minimal vascular alterations. Beside Pdgfra-positive tumor cells and OPCs, Pdgfra and Pdgfrb expression is restricted to vascular associated cells, and PDGFB is a known mitogen of fibroblasts, vascular smooth muscle cells, and pericytes.33,34PDGFB is not commonly amplified or overexpressed in pediatric gliomas,2 and even within our PdgfraD842V tumors, Pdgfb expression levels are comparable to control brainstem levels (data not shown). PDGFA amplifications are found in adult glioblastomas, and forced expression induces the development of proneural gliomas in mice that display histologic and molecular differences compared with PDGFB glioblastoma models,20 likely reflecting the vascular and stromal specific effects of widespread PDGFB expression.
In summary, we have presented the efficient generation of genetically defined DIPG mouse models using in utero electroporation. We demonstrate differences in tumor development caused by specific oncogenic combinations, and characterize pathological and molecular changes. The ability to rapidly and flexibly manipulate gene expression within the intact developing CNS, in a spatially and temporally specific manner, offers opportunities to examine newly identified mutations in brain tumor pathogenesis. IUE DIPG mouse models will provide a strong complement to many of the recently generated patient derived DIPG cell lines, and their immune competent status will allow for new therapies to be tested in immunocompetent preclinical models.
Supplementary Material
Acknowledgments
We would like to thank Dr Joseph LoTurco (University of Connecticut) for kindly providing the PiggyBac plasmids, and Dr Q. Richard Lu (CCHMC) for providing the H3F3A K27M plasmid. We would also like to thank members of the pathology core at CCHMC and the Genomics, Epigenomics, and Sequencing Core at UC (supported in part by CEG grant NIEHS P30-ES006096) for their assistance.
Funding
This work was supported by the University of Cincinnati/Cincinnati Children’s Hospital Medical Center (UC/CCHMC) CTSA CT2 award, Peer Review Cancer Research Program, Department of Defense (#CA171185), and start-up funds provided by UC/CCHMC (to TNP).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Authorship statement
SKP and RMH conducted experiments and contributed to data analysis and writing of the manuscript. XW, FE-R, and HB performed sectioning, staining, imaging, and data analysis. KC assisted with bioinformatics analysis. CF performed pathological reviews of tumor models and provided guidance on histologic stains. TNP conceived and supervised the project, performed IUE surgeries, planned experiments, wrote the manuscript, and secured funding.
References
- 1. Warren KE. Beyond the blood:brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front Oncol. 2018; 8:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017; 32(4):520–537 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filbin MG, Tirosh I, Hovestadt V, et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science. 2018; 360(6386):331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paugh BS, Zhu X, Qu C, et al. Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res. 2013; 73(20):6219–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones C, Karajannis MA, Jones DT, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. 2017; 19(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becher OJ, Hambardzumyan D, Walker TR, et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010; 70(6):2548–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Funato K, Major T, Lewis PW, Allis CD, Tabar V. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science. 2014; 346(6216):1529–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pathania M, De Jay N, Maestro N, et al. H3.3(K27M) cooperates with Trp53 loss and PDGFRA gain in mouse embryonic neural progenitor cells to induce invasive high-grade gliomas. Cancer Cell. 2017; 32(5):684–700 e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowman T, Symonds H, Gu L, Yin C, Oren M, Van Dyke T. Tissue-specific inactivation of p53 tumor suppression in the mouse. Genes Dev. 1996; 10(7):826–835. [DOI] [PubMed] [Google Scholar]
- 10. Phoenix TN, Temple S. Spred1, a negative regulator of Ras-MAPK-ERK, is enriched in CNS germinal zones, dampens NSC proliferation, and maintains ventricular zone structure. Genes Dev. 2010; 24(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson G, Parker M, Kranenburg TA, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012; 488(7409):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phoenix TN, Patmore DM, Boop S, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016; 29(4):508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi K, Ratner N. iGEAK: an interactive gene expression analysis kit for seamless workflow using the R/shiny platform. BMC Genomics. 2019; 20(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miguez A, Ducret S, Di Meglio T, et al. Opposing roles for Hoxa2 and Hoxb2 in hindbrain oligodendrocyte patterning. J Neurosci. 2012; 32(48):17172–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005; 45(1):55–67. [DOI] [PubMed] [Google Scholar]
- 16. Chow LM, Endersby R, Zhu X, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011; 19(3):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buczkowicz P, Hawkins C. Pathology, molecular genetics, and epigenetics of diffuse intrinsic pontine glioma. Front Oncol. 2015; 5:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017; 32(1):42–56 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lei L, Sonabend AM, Guarnieri P, et al. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One. 2011; 6(5):e20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ozawa T, Riester M, Cheng YK, et al. Most human non-GCIMP glioblastoma subtypes evolve from a common proneural-like precursor glioma. Cancer Cell. 2014; 26(2):288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cordero FJ, Huang Z, Grenier C, et al. Histone H3.3K27M represses p16 to accelerate gliomagenesis in a murine model of DIPG. Mol Cancer Res. 2017; 15(9):1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harutyunyan AS, Krug B, Chen H, et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat Commun. 2019; 10(1):1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larson JD, Kasper LH, Paugh BS, et al. Histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression. Cancer Cell. 2019; 35(1):140–155 e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silveira AB, Kasper LH, Fan Y, et al. Correction to: H3.3 K27M depletion increases differentiation and extends latency of diffuse intrinsic pontine glioma growth in vivo. Acta Neuropathol. 2019; 137(6):1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006; 26(25):6781–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeisel A, Hochgerner H, Lonnerberg P, et al. Molecular architecture of the mouse nervous system. Cell. 2018; 174(4):999–1014 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saunders A, Macosko EZ, Wysoker A, et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018; 174(4):1015–1030 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling adult gliomas using RCAS/t-va technology. Transl Oncol. 2009;2(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25(1):55–57. [DOI] [PubMed] [Google Scholar]
- 30. Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, et al. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat Commun. 2015;6:7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim GB, Rincon Fernandez Pacheco D, Saxon D, et al. Rapid generation of somatic mouse mosaics with locus-specific, stably integrated transgenic elements. Cell. 2019; 179(1):251–267 e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plessier A, Le Dret L, Varlet P, et al. New in vivo avatars of diffuse intrinsic pontine gliomas (DIPG) from stereotactic biopsies performed at diagnosis. Oncotarget. 2017; 8(32):52543–52559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998; 125(9):1591–1598. [DOI] [PubMed] [Google Scholar]
- 34. Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010; 116(22):4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.