Abstract
Background and Aims
Of the many threats to global food security, one of the most pressing is the increased incidence of extreme weather events. In addition to extreme rainfall, a combination of global sea level rise and storm surge is likely to result in frequent episodes of seawater flooding in arable systems along low-lying coasts. Our aim was to elucidate the effects of simulated seawater and freshwater flooding on the survival, growth and reproductive potential of four cultivars of the important seed crop, Brassica napus [canola, or oilseed rape (OSR)].
Methods
Established plants were exposed to 24 or 96 h freshwater or seawater root zone immersion (with a no immersion ‘control’). Initial post-treatment performance over 7 weeks was quantified using dry weight biomass. A second group of plants, cultivated until maturity, were used to quantify reproductive yield (siliqua and seed number, and seed size) and subsequent progeny performance (germination and seedling growth).
Results
Oilseed rape growth and reproductive responses were unaffected by freshwater, but seawater negatively affected growth and siliqua number for all cultivars, and seed mass for two (‘Agatha’ and ‘Cubic’). In addition to impacts on crop yield, the growth of seedlings cultivated from seed collected from maternal plants subjected to seawater immersion was also reduced.
Conclusions
Our results demonstrate the potential impact of seawater inundation on coastal cropping systems; although OSR may survive acute saline flooding, there are longer term impacts on growth and yield for some cultivars. The threat may necessitate changes in land-use practice and/or the development of salt-tolerant cultivars to maintain economically viable yields. In addition, by evidencing a hitherto unknown effect on reproductive performance (i.e. reduced seed yield) and subsequent seedling growth, our study highlights an important potential impact of coastal flooding on plant community dynamics for (semi-) natural habitats.
Keywords: Flooding, food security, osmotic stress, salinity, sea-level rise, storm surge
INTRODUCTION
Food security and climate change are two of the most pressing environmental issues facing the planet (Godfray et al., 2010; IPCC, 2014). When combined, however, the increased food demand imposed by a growing human population coupled with global-scale shifts in temperatures, precipitation and sea-level rise will inevitably impose significant demands on agriculture (Parry et al., 2005; Lobell et al., 2008). Much of the environmental threat from climate change stems from acute extreme events rather than simply longer term chronic change alone (Rahmstorf and Coumou, 2011; Vasseur et al., 2014; Parmesan and Hanley, 2015) and, of the former, flooding represents one of the greatest challenges. Future climate scenarios consistently predict major regional shifts in the intensity of extreme precipitation episodes across the globe and concomitant increases in large-scale regional flooding along river catchments (Li et al., 2013; Singh et al., 2013; IPCC, 2014). In addition to fluvial flooding, however, a combination of changes in sea-surface temperatures, weather patterns and sea-level rise is predicted to increase the frequency and severity of oceanic storm surges (Vousdoukas et al., 2016; Vitousek et al., 2017). The result will be that many low-lying coastal areas face an increased risk of seawater inundation over coming decades (Nicholls and Cazenave, 2010; Hoggart et al., 2014). When taken together, extreme precipitation, storm surge and sea-level rise are likely to cause significant flood risk to global agriculture and, as a result, understanding crop response to, and tolerance of, coastal flooding has become a research priority (Jackson and Ismail, 2015).
Although flooding can cause significant losses at any time of the crop production cycle, yields are most likely to suffer if flooding occurs during critical development stages (Rosenzweig et al., 2001; Parry et al., 2005). For the UK, for example, extreme winter and spring precipitation is projected to increase (Fowler et al., 2010) at a time when many arable crops are establishing ahead of summer harvest. This is one reason why the severe, but localized, 2014 winter floods that affected only a small portion of southern England (14 000 ha) caused economic losses estimated at £6.9 million in arable systems alone (ADAS, 2014). Globally, severe freshwater and seawater flooding has long been identified as a significant economic problem for agriculture, and one that poses an additional regional threat to human nutrition (Page and Williams, 1926; Mirza, 2002; Chau et al., 2015).
The impact of flooding on crop plants is well known, although research has almost exclusively focused on the effects of freshwater inundation. Flooding typically causes soil anoxia, as O2 is consumed without replacement, and the accumulation of various ions (Mn2+, Fe2+ and S2+) and compounds detrimental to plant growth, while submergence also denies plant tissues access to O2 and CO2 (Flowers and Colmer, 2008; Perata et al., 2011). All of these factors can limit crop growth and productivity significantly (Malik et al., 2001; Perata et al., 2011; Ren et al., 2014; Mutava et al., 2015). Due to its high salinity (typically about 35 gL–1 salt of which chloride and sodium contribute 19 g L–1 and 11 g L–1, respectively), seawater flooding imposes additional osmotic and ionic stresses; the former limits the plant’s ability to absorb water and the latter can result in toxicity through the accumulation of Na+ and Cl– in tissues (Munns and Tester, 2008). As a response, plants have adapted to avoid or lessen the impact of salt ions via the synthesis and accumulation of stress metabolites and the regulation of other tissue ions (i.e. K+) to exclude or compartmentalize Na+ and Cl– and re-establish homeostatic function (Maathuis and Amtmann, 1999; Munns and Tester, 2008).
From the perspective of agricultural production, even if crop plants survive freshwater or seawater flooding, any impact on growth or reproductive potential is of concern. Our understanding of the effects of salinity on crop performance is, however, focused largely on soil salinization in arid cropping systems (Pitman and Läuchli, 2002; Albacete et al., 2008; Flowers et al., 2010), and only a handful of studies have examined how agriculturally important species respond to short-duration immersion in seawater. White et al. (2014) report how immersion in seawater for only 24 h resulted in the accumulation of Na+ and Cl– in leaves of the pasture crop white clover (Trifolium repens). A concomitant increase in two key organic solutes (proline and sucrose) to re-establish osmotic balance was also observed, but nonetheless flooded plants showed reduced growth and flowering potential. Interestingly, White et al. (2014) also found variation between the responses of three different ecotypes to seawater immersion, suggesting that the relative tolerance of one ecotype could be of value in producing flood-tolerant cultivars for cultivation in low-lying coastal pastures. The study of Sun et al. (2015) is the only one to look at effects of seawater immersion on arable crops, with their experiment focusing on the responses of 1-month-old plants of ten crop plants (six of which were Brassicaceae) to 24 h of seawater immersion. All crops were negatively affected by immersion, with two (cucumber and Chinese greens) suffering complete mortality and the remainder showing reduced growth. However, since plants were harvested only 2 weeks after immersion treatments were imposed, it is unclear what potential any of the surviving crops had for long-term recovery or whether other key aspects of the crop production cycle (flowering and fruit/seed production) were affected by flooding.
The aim of this study was to determine how exposure to simulated seawater and freshwater flooding affected both immediate growth and longer term reproductive performance of the widely cultivated crop canola, or oilseed rape (OSR; Brassica napus L.). Beyond gaining an understanding of the short-term impact of flooding on mortality and growth of established plants, we also test the hypothesis that impacts on reproductive potential and subsequent seedling performance vary across four different cultivars. Elucidation of these responses is important for several reasons; OSR is grown primarily for seed yield and thus any impact of prolonged fluvial or seawater flooding on reproductive output has potential economic repercussions. In addition, establishing variation in growth and yield responses may help identify established genetic materials from which to develop flood/salinity-tolerant cultivars. A more general understanding of plant growth and reproductive responses to acute seawater flooding is also lacking in the literature (see Hanley et al., 2019), and no study to date has tested the hypothesis that progeny performance is affected by the exposure of parent plants to short-duration seawater immersion. Consequently, our study offers an important insight into the longer term impacts that this increasing environmental issue may have on natural, as well as agricultural, plant species and communities.
MATERIALS AND METHODS
Study species
Oilseed rape is a hybrid of B. rapa and B. oleracea (Chalhoub et al., 2014), wild forms of the latter being a coastal species with some natural salt tolerance (Snogerup et al., 1990). OSR is grown globally and its seeds are harvested to produce food and bioenergy oils, and for use as an animal feedstock. It accounts for 14 % (by area) of agricultural land use in the UK (Garthwaite et al., 2018), and major producers globally include the EU, Canada, India and China (USDA, 2015), regions likely to experience significant flooding events over coming decades (IPCC, 2014). The OSR cultivars used in these experiments were ‘Cracker’ (LS Plant Breeding, Impington, UK), ‘Agatha’, ‘Astrid’ and ‘Cubic’ (Grainseed Ltd., Eye, Suffolk, UK). All are commonly cultivated in the UK.
Plant cultivation
One hundred seeds of each cultivar were germinated in 90 mm diameter Petri dishes containing two layers of Whatman No. 1 filter paper moistened with 5 mL of distilled water, maintained in an incubator at 16.5 °C on a 12:12 h light:dark cycle. On germination (radicle emergence), 70 seedlings per cultivar were transplanted individually into 50 mm diameter, 50 mm deep plastic pots containing John Innes No. 2 potting compost and grown on in a naturally lit greenhouse with weekly watering with tap water, until early November 2014. At this time, seedlings possessing at least the first true leaf (equivalent to OSR growth stage 1.1; Sylvester-Bradley, 1985), were re-potted into larger 110 × 110 × 120 mm plastic pots containing John Innes No. 2 potting compost. Greenhouse temperatures during this cultivation period were: mean daily minimum = 10.4 °C (±0.4 °C s.e.); mean daily maximum = 25.7 °C (±1.0 °C).
Effect of immersion on growth
When the plants were 67 d old (early December 2014), 12 individuals from each cultivar (OSR growth stage 1.3–1.6; i.e. emergence of third–sixth true leaves) were allocated at random to one of five treatment groups. These were 24 or 96 h immersion in seawater (Sw) (collected from Plymouth Sound – electrical conductivity = 45.51 mS cm–1 at 16.2 °C); 24 or 96 h immersion in deionized water (Dw); or a no-immersion control treatment. Although river floodplains can experience much longer periods of immersion, sometimes extending to several months (Van Eck et al., 2004; Muchan et al., 2015), in this way we simulated the average 1 d long seawater flooding event reported for low-lying UK coastline habitats and extended the period to the maximum reported flood duration of 4 d (Environment Agency, 2014).
We recognize that inundation following coastal storm surge or fluvial flooding events would be likely to result in full shoot submergence but, by immersing to pot level (in large plastic tubs), our approach allowed us to separate the effect of ionic imbalance in the root zone rather than the impact of oxygen deficiency caused by full immersion that both treatments would impose. Immediately after immersion, the pots were allowed to drain fully before being arranged randomly on a wire mesh-topped bench inside the greenhouse; the wire mesh allowed free drainage and prevented cross-contamination between treatment groups. The pots were watered to capacity (with tap water) 48 h after seawater immersion.
Eight plants per treatment/cultivar combination were retained inside the greenhouse; the remainder were positioned outside, on adjacent elevated mesh. For both sets of plants, each individual was positioned at random, 20 cm apart from its nearest neighbour in 1 m long rows separated by 30 cm to simulate the recommended field density for OSR cropping systems in the UK (HGCA, 2014).
Greenhouse plants were watered twice weekly for a further 56 d; temperatures during this phase of the experiment were 4.9 °C (± 0.5) minimum and 18.0 °C (± 0.6) maximum. Surviving plants (growth stages 2.0–2.3; i.e. the plant has a rosette growth form and an extended stem with internodes) from each treatment/cultivar group were harvested at 113 d old (late January 2015), cleaned of any adhering compost and oven-dried at 50 °C for 24 h. A Levene’s test for homogeneity of variance across treatment and cultivar levels was negative (F19,189 = 2.896, P < 0.001) and biomass data were log10 transformed, resulting in a positive Levene’s test (F19,189 = 1.335, P = 0.165). Univariate general linear model (GLM) analyses were used to compare the results of experimental flooding on transformed biomass. Rather than classifying plants in different groups into two separate factors (water, Dw vs. Sw; and time, 24 h vs. 96 h), which would decrease the power of the tests, contrasts were employed to evaluate specific differences between treatment levels (control, Dw 24, Dw 96, Sw 24 and Sw 96). In addition to the F statistic and its probability, we report effect sizes (ηp2) and power at P < 0.05 (power0.05).
Effect of immersion on crop yield and progeny performance
The four individual plants from each treatment/cultivar group positioned on elevated outdoor standing were grown to reproductive maturity in order to assess the effects of immersion on seed yield. Plants were exposed to natural weather conditions and watered (to capacity with tap water) only following prolonged dry periods. Since individuals were randomly arranged with respect to treatment and cultivar, they were able to cross-pollinate and so resulting progeny were most probably hybrids between cultivars. Three plants (‘Agatha’ 24 h Dw; ‘Cracker’ 96 h Dw; and ‘Agatha’ 96 h Sw) died during this period. The main stems of plants were harvested in mid-June when most siliqua were fully ripened (growth stage 9.9). We quantified the total number of siliqua per plant and, from six (randomly selected) siliqua per plant, quantified seed number and mean individual seed mass per pod (averaging across all six replicate siliqua for each plant).
All seeds from each of the six sampled siliqua per plant were pooled. From these, 40 seeds were set to germinate in 90 mm diameter Petri dishes containing two layers of Whatman No. 1 filter paper moistened with 5 mL of deionized water, maintained in an incubator at 18 °C on a 12:12 h light:dark cycle. In addition to the three plants that died before harvest, a further two parent plants – ‘Cracker’ 96 h Dw and ‘Agatha’ 96 h Sw – failed to yield sufficient seeds for the germination/seedling growth trials. Petri dishes were checked daily for 14 d; on germination (appearance of the radicle), seedlings were counted and removed. Six seedlings from each Petri dish were retained, and planted into a 50 mm diameter pot containing John Innes No. 2 potting compost. These seedlings were grown in controlled conditions (15 ºC on a 12:12 h light:dark cycle with daily watering to capacity) until 14 d old when they were harvested and oven-dried (at 50 °C for 24 h) to determine dry weight biomass.
Levene’s tests of homogeneity of variances were significant for siliqua number (F19,57 = 2.621, P = 0.003), seed number (F19,56 = 2.962, P < 0.001) and seedling biomass (F19,498 = 2.291, P = 0.002), but did not show departure from homogeneity for seed mass (F19,56 = 1.264, P = 0.244). Logarithmic transformation of the former three homogenized the variance for siliqua number (F19,57 = 1.304, P=0.217), but not for seed number (F19,56 = 10.625, P < 0.001) and seedling mass (F19,497 = 3.289, P < 0.001). Consequently, we present the results of GLM’s hypothesis testing for (1) seed mass where the untransformed variable did not depart from homogeneous; (2) seed number and seedling mass where logarithmic transformation did not homogenize the variance; and (3) log siliqua number where logarithmic transformation resulted in variance homogeneity.
RESULTS
Effect of immersion on plant growth
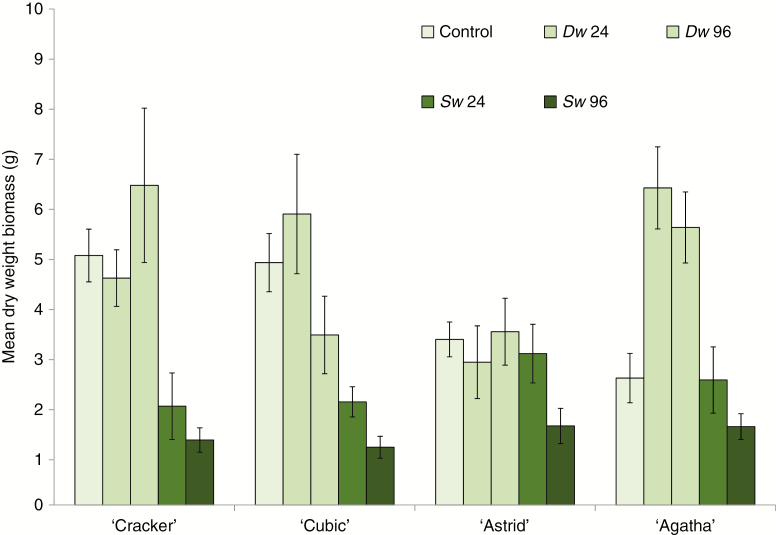
‘Treatment’ had a significant effect on adult plant biomass, with a moderate effect size (F4,189 = 17.71, P < 0.001; ηp2 = 0.273; power0.05 = 1; Fig. 1), and with the contrasts between control and each of the other four treatment levels significant only for Sw 96 (contrast = –0.339, s.e. = 0.066, P < 0.001). More specific comparison showed: (1) a negative contrast between both Sw treatments vs. control (contrast = –0.219, s.e.= 0.058, P < 0.001); (2) a small, but significant, positive contrast between Dw treatments vs. control (contrast = 0.114, s.e. = 0.057, P = 0.048); and (3) a then obvious positive contrast between Dw and Sw (contrast = 0.333, s.e = 0.045, P < 0.001), highlighting the smaller size of plants immersed in seawater. Plant biomass did not vary between cultivars, but the cultivar effect size was small (F3,189 = 1.85, P = 0.140; ηp2 = 0.029; power0.05 = 0.475). A significant ‘treatment × cultivar’ interaction with moderate effect size (F12,189 = 2.95, P = 0.001; ηp2 = 0.158; power0.05 = 0.989) emphasized several treatment- and cultivar-specific departures from the general trends described above (‘Astrid’ and ‘Agatha’, for example, were both tolerant of 24 h seawater immersion). No plants died during this first part of the experiment.
Fig. 1.
The effect of root zone immersion in deionized water (Dw) and seawater (Sw) on mean (± s.e.) total above-ground dry mass of four oilseed rape (Brassica napus) cultivars (‘Cracker’, ‘Cubic’, ‘Agatha’ and ‘Astrid’) 2 months after plants (67 d old; growth stage 1.3–1.6) were subject to transient immersion (24 or 96 h with a zero hour control). n = 8 for all treatment/cultivar combinations.
Effect of immersion on crop yield and progeny performance
The number of siliqua (Table 1) varied with ‘treatment’, with a small effect size (F4,57 = 5.974, P < 0.001; ηp2 = 0.295; power0.05 = 0.978) but not by ‘cultivar’ (F3,57 = 2.004, P = 0.124; ηp2 = 0.095; power0.05 = 0.489), and there was no interaction (F12,57 = 1.283, P = 0.254; ηp2 = 0.213; power0.05 = 0.643). The contrast analyses revealed a significant negative effect of Dw and Sw treatments compared with the control (contrast = –0.103, s.e. = 0.050, P = 0.046), specifically highlighting lower siliqua numbers in Sw vs. control (contrast = –0.141, s.e. = 0.055, P = 0.014). There was no variation between Dw and Sw (contrast = 0.76, s.e. = 0.046, P = 0.105) or Dw vs. control (contrast = –0.065, s.e. = 0.056, P = 0.250).
Table 1.
The effect of root zone immersion in deionized water (Dw) and seawater (Sw) on plant reproductive potential (crop yield) of four oilseed rape (Brassica napus) cultivars (‘Cracker’, ‘Cubic’, ‘Agatha’ and ‘Astrid’) 7 months after plants (growth stage 1.3–1.6) were subject to transient immersion (24 or 96 h with a zero hour control)
| Treatment | Number of siliqua per plant | Seeds per siliqua | Seed mass (mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Cracker’ | ‘Cubic’ | ‘Astrid’ | ‘Agatha’ | ‘Cracker’ | ‘Cubic’ | ‘Astrid’ | ‘Agatha’ | ‘Cracker’ | ‘Cubic’ | ‘Astrid’ | ‘Agatha’ | ||
| Control | Mean | 35.5 | 29.8 | 22.0 | 35.8 | 13.2 | 22.4 | 22.3 | 20.3 | 0.04 | 0.08 | 0.07 | 0.06 |
| s.e. | 8.6 | 2.8 | 2.4 | 4.8 | 2.7 | 1.2 | 1.0 | 1.2 | 0.009 | 0.009 | 0.004 | 0.005 | |
| 24 h Dw | Mean | 30.3 | 18.3 | 22.8 | 21.7 | 16.5 | 21.6 | 22.5 | 17.7 | 0.04 | 0.06 | 0.07 | 0.04 |
| s.e. | 3.4 | 2.7 | 6.2 | 3.3 | 0.6 | 1.0 | 2.0 | 1.0 | 0.005 | 0.003 | 0.01 | 0.004 | |
| 96 h Dw | Mean | 40.7 | 28.0 | 28.8 | 27.5 | 11.4 | 25.0 | 20.9 | 19.9 | 0.03 | 0.07 | 0.06 | 0.05 |
| s.e. | 13.2 | 3.6 | 2.7 | 5.0 | 4.9 | 0.5 | 1.1 | 1.4 | 0.02 | 0.004 | 0.005 | 0.004 | |
| 24 h Sw | Mean | 25.8 | 38.5 | 25.5 | 43.8 | 13.8 | 22.8 | 23.0 | 20.2 | 0.04 | 0.08 | 0.07 | 0.07 |
| s.e. | 6.0 | 11.1 | 0.9 | 5.9 | 1.6 | 0.8 | 0.7 | 1.5 | 0.004 | 0.006 | 0.008 | 0.006 | |
| 96 h Sw | Mean | 24.3 | 11.5 | 17.5 | 14.0 | 13.6 | 19.4 | 22.5 | 9.2 | 0.03 | 0.04 | 0.07 | 0.02 |
| s.e. | 6.2 | 3.2 | 3.0 | 5.1 | 3.2 | 2.0 | 1.2 | 4.7 | 0.01 | 0.006 | 0.006 | 0.01 |
n = 4 for all treatment/cultivar combinations except ‘Cracker’ 96 h Dw, and ‘Agatha’ 24 h Dw and 96 h Sw where n = 3.
Seed number per pod (Table 1) was not influenced by ‘treatment’ (F4,57 = 2.206, P = 0.080; ηp2 = 0.134; power0.05 = 0.612) and, while varying between cultivars (F3,57 = 20.265, P < 0.001; ηp2 = 0.516; power0.05 = 1), there was no interaction with ‘treatment’ (F12,57 = 1.31, P = 0.239; ηp2 = 0.216; power0.05 = 0.655). Mean individual seed mass (Table 1) did, however, vary according to ‘treatment’ (F4,57 = 5.456, P = 0.001; ηp2 = 0.277; power0.05 = 0.965) and ‘cultivar’ (F3,57 = 19.658, P < 0.001; ηp2 = 0.509; power0.05 = 1), although there was no significant interaction (F12,57 = 1.799, P = 0.070; ηp2 = 0.275; power0.05 = 0.822). While contrasts highlighted that Dw and Sw had an overall difference from control (contrast = –0.009, s.e. = 0.004, P = 0.036), this was driven primarily by variation between Sw and control (contrast = –0.010, s.e. = 0.004, P = 0.023), and not by the difference between Dw and control plants (contrast = –0.007, s.e. = 0.005, P = 0.122). Interestingly, however, there was no variation between Dw and Sw (contrast = 0.003, s.e. = 0.004, P = 0.373). These results emphasize the different intensity of effects on different cultivars, i.e. ‘Agatha’ and ‘Cubic’ showed the most marked negative responses in the 96 h Sw treatment (Table 1).
Germination was unaffected by any of the immersion treatments imposed on parent plants (data not shown); the lowest germination for any one cultivar/treatment group was the 76 % recorded for seeds produced by ‘Cracker’ 96 h Sw (with three of four Petri dishes for this group nonetheless attaining >85 % germination).
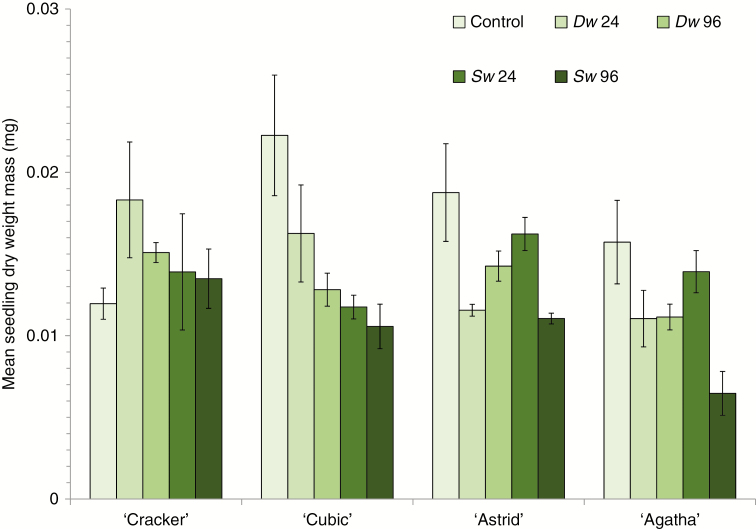
Finally, seedling mass (Fig. 2) differed between treatments, with a small effect size (F4,498 = 6.192 P < 0.001; ηp2 = 0.047; power0.05 = 0.988), but not among cultivars (F3,498 = 1.874 P = 0.133; ηp2 = 0.011; power0.05 = 0.486), and there was a significant ‘treatment × cultivar’ interaction also with a small effect size (F12,498 = 2.295 P = 0.008; ηp2 = 0.052; power0.05 = 0.959). Contrast analysis revealed significant differences between control and Dw and Sw together (contrast = –0.004, s.e. = 0.001, P < 0.001), between control and Dw (contrast = –0.003, s.e. = 0.001, P = 0.004) and between control and Sw (contrast = –0.005, s.e. = 0.001, P < 0.001), but not between Dw and Sw (contrast = 0.002, s.e. = 0.001, P = 0.089). The significant interaction between the two main factors highlights differences in the intensity and direction of cultivar responses. The most affected, ‘Cubic’, displayed reduced seedling growth in both the 24 and 96 h treatments, while progeny collected from ‘Cracker’ showed no response to Sw immersion (Fig. 2). Seedlings grown from ‘Astrid’ and ‘Agatha’ parents exhibited reduced growth in the 96 h Sw treatments only, although, given the low parental replication for ‘Agatha’ (only two plants survived to reproduction), the apparent negative response for seedlings collected from this cultivar should be taken with caution.
Fig. 2.
The effect of root zone immersion in deionized water (Dw) and seawater (Sw) on mean (± s.e.) seedling dry mass of four oilseed rape (Brassica napus) cultivars (‘Cracker’, ‘Cubic’, ‘Agatha’ and ‘Astrid’) of plants grown from seeds collected from parent plants subject to transient immersion (24 or 96 h with a zero hour control, at growth stage 1.3–1.6). n = 4 for all treatment/cultivar combinations except ‘Agatha’ 24 h Dw where n = 3, and ‘Cracker’ 96 h Dw and ‘Agatha’ 96 h Sw where n = 2.
DISCUSSION
Our results evidence substantial differences in OSR response to acute freshwater and seawater inundation; while the former exerted some impact on some of the responses examined here, the latter was more generally associated with reduced parent plant growth, seed yield and even progeny performance. As far as we are aware, this is the first time that the impacts of short-duration, acute, Sw flooding on several key growth and reproductive responses have been demonstrated across stages of the life cycle for any plant species. Indeed, the only previous study to examine this issue in arable crops, that of Sun et al. (2015), was limited to the immediate (2 week) post-immersion response, and while White et al. (2014) did look at growth and flowering in white clover over a 70 d post-immersion period, they did not consider seed yield or progeny performance. While there was some variation between cultivars, this study shows that even transitory immersion in seawater has significant, consistent and long-lasting impacts on OSR crop yield. Indeed, the fact that reduced reproductive output (most notably siliqua number) was manifest 6 months after maternal plants were exposed to Sw immersion highlights the potential long-term impacts of seawater flooding on arable cropping systems.
The consequences of reduced seed yields on agricultural crops such as OSR are obvious, but our results also raise the possibility that wild plant species could suffer reduced reproductive success as a result of the effect of acute seawater flooding stress on seed development. It is well established that the maternal plant environment alters phenotypic expression in progeny (Herman and Sultan, 2011) and as such the reduced growth of seedlings from maternal plants subjected to Sw immersion reported here is unsurprising. Nonetheless, and while there is evidence that salinity stress can induce similar shifts in maternal provisioning and seedling performance [for example as shown in the grass Cenchrus ciliaris; Ruiz and Taleisnik (2013)], the fact that parent immersion in seawater for only 4 d can negatively influence subsequent seedling growth highlights more generally the impact of coastal flooding on plant community dynamics. Put simply, even if parent plants (of any native species) survive prolonged flooding, their later ability to contribute to the recovering community could be compromised. We know of no study to have demonstrated this response. For two of our maternal cultivars (‘Cubic’ and ‘Agatha’) where 96 h Sw immersion reduced mean seed mass by ≥50 %, the most plausible explanation for the arrested growth of seedlings is that it resulted from reduced seed provisioning by the parent (Zas et al., 2013). We cannot, however, discount the possibility that other responses to salinity stress, including epigenetic changes, impact progeny performance and highlight this as a key area for future research.
From the perspective of arable plant species, progeny performance may be of minor importance however, since crops are routinely cultivated from commercially sourced seeds. Nonetheless, we demonstrated a consistent and marked reduction in siliqua number and seed mass for at least two cultivars (‘Cubic’ and ‘Agatha’) following 96 h Sw immersion, highlighting the significant economic impact that seawater flooding might have on coastal OSR crop yields. While a number of studies have shown that prolonged (i.e. one to several weeks) waterlogging reduces both OSR growth and yield (Cannell and Belford, 1980; Zhou and Lin, 1995; Xu et al., 2015), these studies looked at freshwater effects only. Although the relatively short-term Dw immersion treatments imposed here did not yield similar responses [but noting that we did not consider seed oil yield or content (Cannell and Belford, 1980; Xu et al., 2015)], all four cultivars exhibited much reduced plant growth, and later reduced siliqua number in the longer duration Sw treatment (see also Hanley et al., 2013). This highlights the challenge posed by salinity; even if a plant survives acute seawater immersion, it must prevent or alleviate damage caused by the accumulation of salt ions (Na+ and Cl–) in tissues. It is interesting that even the most prolonged immersion times (4 d) imposed here failed to induce mortality, a response that perhaps reflects an innate salinity tolerance due to OSR’s heritage in B. oleracea and this parent species’ natural affinity for maritime conditions (Snogerup et al., 1990). Salt tolerance is, however, often accomplished by the accumulation of stress metabolites and the regulation of tissue ions to exclude or compartmentalize the potentially damaging Na+ and Cl–. Nonetheless even if successfully achieved, as seems to be the case here, there are costs in terms of subsequent plant growth and reproductive performance (Munns and Tester, 2008; White et al., 2014).
At a time of human population growth and economic development, there are increasing demands on food supplies but, when coupled with unpredictable and probably ever more extreme climate events, global food security is far from assured (Godfray et al., 2010; IPCC, 2014). Flooding is widely recognized as one of the key threats to arable crops, but most research emphasis is placed on pluvial, freshwater flooding where the major negative impact comes from soil anoxia. In the UK, the threshold for crop viability under this scenario is 15 d (ADAS, 2014) but, as we show here, by virtue of the added effect of salinity, seawater flooding of only 4 d duration can impact OSR yield (while the same duration under freshwater does not). Historically much of the global agricultural salinization problem stems from poor irrigation coupled with excessive evaporation and/or deforestation in hot, dry climates (Vinod et al., 2013). Nonetheless, sea-level rise and the expected increase in frequency and severity of storm surges (Vousdoukas et al., 2016; Vitousek et al., 2017) are likely to increase the risk of seawater inundation to temperate coastal arable systems (Nicholls and Cazenave, 2010). Under these conditions, farmers face a choice between changing land-use practice, or cultivation of flood-tolerant crops. There is a rich literature documenting salt tolerance in crops grown in regions where the problems of soil salinization are long established; indeed, for one of the most important, namely rice, crops grown near to coasts are frequently subjected to seawater intrusions, and a genetic capacity for salt tolerance has been identified (Ganie et al., 2014). The impact of seawater immersion demonstrated here for OSR, coupled with the increasing risk of seawater flooding for coastal agriculture globally, underscores a new impetus for research into salt tolerance in a wider range of arable crop species (Jackson and Ismail, 2015).
ACKNOWLEDGEMENTS
We thank Jane Akerman and Tom Gove for technical assistance, and two anonymous referees for their comments on an earlier draft of the manuscript.
LITERATURE CITED
- ADAS 2014. Impact of 2014 winter floods on agriculture in England. Wolverhampton, UK: Agricultural Development and Advisory Service. [Google Scholar]
- Albacete A, Ghanem ME, Martínez-Andújar C, et al. 2008. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. Journal of Experimental Botany 59: 4119–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell RQ, Belford RK. 1980. Effects of waterlogging at different stages of development on the growth and yield of winter oilseed rape (Brassica napus L.). Journal of the Science of Food & Agriculture 3: 963–965. [Google Scholar]
- Chau VN, Cassells S, Holland J. 2015. Economic impact upon agricultural production from extreme flood events in Quang Nam, central Vietnam. Natural Hazards 7: 1747–1765. [Google Scholar]
- Chalhoub B, Denoeud F, Liu S, et al. 2014. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345: 950–953. [DOI] [PubMed] [Google Scholar]
- Environment Agency UK 2014. DataShare.http://www.geostore.com/environment-agency/. Accessed 4 April 2014.
- Flowers TJ, Colmer TD. 2008. Salinity tolerance in halophytes. New Phytologist 179: 945–963. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Gaur PM, Gowda CLL, et al. 2010. Salt sensitivity in chickpea. Plant, Cell & Environment 33: 490–509. [DOI] [PubMed] [Google Scholar]
- Fowler HJ, Cooley D, Sain SR, Thurston M. 2010. Detecting change in UK extreme precipitation using results from the climateprediction.net BBC climate change experiment. Extremes 13: 241–267. [Google Scholar]
- Ganie S, Karmakar J, Roychowdhury R, Mondal T, Dey N. 2014. Assessment of genetic diversity in salt-tolerant rice and its wild relatives for ten SSR loci and one allele mining primer of salT gene located on 1st chromosome. Plant Systematics & Evolution 300: 1741–1747. [Google Scholar]
- Garthwaite DG, Barker I, Ridley L, et al. 2018. Pesticide usage survey report 271: arable crops in the United Kingdom 2016. London: DEFRA. [Google Scholar]
- Godfray HCJ, Beddington JR, Crute IR, et al. 2010. Food security: the challenge of feeding 9 billion people. Science 327: 812–818. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Yip PYS, Hoggart S, Bilton DT, Rundle SD, Thompson RC. 2013. Riding the storm: the response of Plantago lanceolata to simulated tidal flooding. Journal of Coastal Conservation 17: 799–803. [Google Scholar]
- Hanley ME, Sanders SKD, Stanton H-M, Billington RA, Boden R. 2019.A pinch of salt: response of coastal grassland plants to simulated seawater inundation treatments. Annals of Botany. doi:10.1093/aob/mcz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JJ, Sultan SE. 2011. Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Frontiers in Plant Science 2: 102. doi: 10.3389/fpls.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HGCA 2014. Oilseed Rape Guide Number 55. Stoneleigh Park, Warwickshire, UK: HGCA. [Google Scholar]
- Hoggart SPG, Hanley ME, Parker DJ, et al. 2014. The consequences of doing nothing: the effects of seawater flooding on coastal zones. Coastal Engineering 87: 169–182. [Google Scholar]
- IPCC. 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Core Writing Team, Pachauri RK, Meyer LA, eds.). Geneva, Switzerland: IPCC. [Google Scholar]
- Jackson MB, Ismail AM. 2015. Introduction to the Special Issue: Electrons, water and rice fields: plant response and adaptation to flooding and submergence stress. AoB Plants 7: doi: 10.1093/aobpla/plv078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Zhang Q, Chen YD, Xu CY, Singh VP. 2013. Changing spatiotemporal patterns of extreme precipitation regimes in China during 2071–2100 based on Earth system models. Journal of Geophysical Research: Atmospheres 118: 12537–12555. [Google Scholar]
- Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL. 2008. Prioritizing climate change adaptation needs for food security in 2030. Science 319: 607–610. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A. 1999. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany 84: 123–133. [Google Scholar]
- Malik A, Colmer TD, Lambers H, Schortemeyer M. 2001. Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Australian Journal of Plant Physiology 28: 1121–1131 [Google Scholar]
- Mirza MQ. 2002. Global warming and changes in the probability of occurrence of floods in Bangladesh and implications. Global Environmental Change 12: 127–138. [Google Scholar]
- Muchan K, Lewis M, Hannaford J, Parry S. 2015. The winter storms of 2013/4 in the UK: hydrological responses and impacts. Weather 70: 55–61. [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salt tolerance. Annual Reviews in Plant Biology 59: 651–681. [DOI] [PubMed] [Google Scholar]
- Mutava RN, Prince SJK, Syed NH, et al. 2015. Understanding abiotic stress tolerance mechanisms in soybean: a comparative evaluation of soybean response to drought and flooding stress. Plant Physiology & Biochemistry 86: 109–120. [DOI] [PubMed] [Google Scholar]
- Nicholls RJ, Cazenave A. 2010. Sea-level rise and its impact on coastal zones. Science 328: 1517–1520. [DOI] [PubMed] [Google Scholar]
- Page HJ, Williams W. 1926. The effect of flooding with seawater on the fertility of the soil. Journal of Agricultural Science 16: 551–573. [Google Scholar]
- Parmesan C, Hanley ME. 2015. Plants and climate change: complexities and surprises. Annals of Botany 115: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M, Rosenzweig C, Livermore M. 2005. Climate change, global food supply and risk of hunger. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 2125–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perata P, Armstrong W, Laurentius AC, Voesenek J. 2011. Plants and flooding stress. New Phytologist 190: 269–273. [DOI] [PubMed] [Google Scholar]
- Pitman MG, Läuchli A. 2002. Global impact of salinity and agricultural ecosystems. In: Läuchli A, Lüttg U, eds. Salinity: environment – plants – molecules. Dordrecht, The Netherlands: Kluwer, 3–20. [Google Scholar]
- Rahmstorf S, Coumou D. 2011. Increase of extreme events in a warming world. Proceedings of the National Academy of Sciences, USA 108: 17905–17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren BZ, Zhang JW, Li X, et al. 2014. Effects of waterlogging on the yield and growth of summer maize under field conditions. Canadian Journal of Plant Science 94: 23–31. [Google Scholar]
- Rosenzweig C, Iglesias A, Yang X, Epstein PR, Chivian E. 2001. Climate change and extreme weather events: implications for food production, plant diseases, and pests. Global Change & Human Health 2: 90–104. [Google Scholar]
- Ruiz M, Taleisnik E. 2013. Field hydroponics assessment of salt tolerance in Cenchrus ciliaris (L.): growth, yield, and maternal effect. Crop Pasture Science 64: 631–639. [Google Scholar]
- Singh D, Tsiang M, Rajaratnam B, Diffenbaugh NS. 2013. Precipitation extremes over the continental United States in a transient, high-resolution, ensemble climate model experiment. Journal of Geophysical Research: Atmospheres 118: 7063–7086 [Google Scholar]
- Snogerup S, Gustafsson M, Von Bothmer R. 1990. Brassica sect. Brassica (Brassicaceae) I. Taxonomy and variation. Willdenowia 19: 271–365. [Google Scholar]
- Sun YP, Masabni J, Niu GH. 2015. Simulated seawater flooding reduces the growth of ten vegetables. Hortscience 50: 694–698. [Google Scholar]
- Sylvester-Bradley R. 1985. Revision of a code for stages of development in oilseed rape (Brassica napus L.). Annals of Applied Biology 10: 395–400. [Google Scholar]
- USDA 2015. Oilseeds: World Markets and Trade. Available at: http://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf. Accessed 9 September 2015.
- Van Eck WHJM, van de Steeg HM, Blom CWPM, de Kroon H. 2004. Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 grassland species. Oikos 107: 393–405. [Google Scholar]
- Vasseur DA, DeLong JP, Gilbert B, et al. 2014. Increased temperature variation poses a greater risk to species than climate warming. Philosophical Transactions of the Royal Society B: Biological Sciences 281: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KK, Krishnana SG, Babu NN, Nagarajan M, Singh AK. 2013. Improving salt tolerance in rice: looking beyond the conventional. In: Ahmed P, Azooz MM, Prasad MNV, eds. Salt stress in plants: signaling, omics and adaptations. New York: Springer, 219–260. [Google Scholar]
- Vitousek S, Barnard PL, Fletcher CH, Frazer N, Erikson L, Storlazzi CD. 2017. Doubling of coastal flooding frequency within decades due to sea-level rise. Scientific Reports 7: 1399. doi: 10.1038/s41598-017-01362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousdoukas MI, Voukouvalas E, Annunziato A, Giardino A, Feyen L. 2016. Projections of extreme storm surge levels along Europe. Climate Dynamics 47: 3171–3190. [Google Scholar]
- White AC, Colmer TD, Cawthray GR, Hanley ME. 2014. Variable response of three Trifolium repens ecotypes to soil flooding by seawater. Annals of Botany 114: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MY, Ma HQ, Zeng L, et al. 2015. The effect of waterlogging on yield and seed quality at the early flowering stage in Brassica napus L. Field Crop Research 180: 238–245. [Google Scholar]
- Zas R, Cendan C, Sampredo L. 2013. Mediation of seed provisioning in the transmission of environmental maternal effects in maritime pine (Pinus pinaster Aiton). Heredity 111: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WJ, Lin XQ. 1995. Effects of waterlogging at different growth stages on physiological characteristics and seed yield of winter rape (Brassica napus L.). Field Crop Research 44: 103–110. [Google Scholar]