Abstract
Background and aims
Sand dunes reduce the impact of storms on shorelines and human infrastructure. The ability of these ecosystems to provide sustained coastal protection under persistent wave attack depends on their resistance to erosion. Although flume experiments show that roots of perennial plants contribute to foredune stabilization, the role of other plant organs, and of annual species, remains poorly studied. Furthermore, it remains unknown if restored foredunes provide the same level of erosion resistance as natural foredunes. We investigated the capacity of three widespread pioneer foredune species (the perennial Ammophila arenaria and the annuals Cakile maritima and Salsola kali) to resist dune erosion, and compared the erosion resistance of Ammophila at natural and restored sites.
Methods
Cores collected in the field were tested in a flume that simulated a wave swash. A multi-model inference approach was used to disentangle the contributions of different below-ground compartments (i.e. roots, rhizomes, buried shoots) to erosion resistance.
Key Results
All three species reduced erosion, with Ammophila having the strongest effect (36 % erosion reduction versus unvegetated cores). Total below-ground biomass (roots, rhizomes and shoots), rather than any single compartment, most parsimoniously explained erosion resistance. Further analysis revealed that buried shoots had the clearest individual contribution. Despite similar levels of total below-ground biomass, coarser sediment reduced erosion resistance of Ammophila cores from the restored site relative to the natural site.
Conclusions
The total below-ground biomass of both annual and perennial plants, including roots, rhizomes and buried shoots, reduced dune erosion under a swash regime. Notably, we show that (1) annual pioneer species offer erosion protection, (2) buried shoots are an important plant component in driving sediment stabilization, and (3) management must consider both biological (plants and their traits) and physical (grain size) factors when integrating dunes into schemes for coastal protection.
Keywords: Erosion, sand dunes, root biomass, below-ground biomass, Ammophila arenaria, Cakile maritima, Salsola kali
INTRODUCTION
Coastal ecosystems, such as saltmarshes, mangroves and sand dunes, play a crucial role in the protection of coastlines and human infrastructure from storms (Costanza et al., 2008; Feagin et al., 2010; Salgado and Martinez, 2017). This coastal protection service will likely increase during this century because of the forecasted increase in storm frequency and strength (Woodruff et al., 2013) as well as the increments in human population living along coastal areas (Neumann et al., 2015). At the same time as protecting coastlines, however, these ecosystems are themselves vulnerable to storm damage from high waters, wind, wave attack and urbanization (Pries et al., 2008; Pye and Blott, 2008; Feagin et al., 2010; Calvão et al., 2013; Roberts et al., 2013). Therefore, great effort has been devoted to understanding the resistance of coastal ecosystems to erosion processes (e.g. Marani et al., 2011; Leonardi et al., 2016; Silva et al., 2016; Wang et al., 2017) and to successfully integrating them into management schemes for coastal protection (Bouma et al., 2014).
Coastal sand dunes form through the interaction of vegetation, wind and sediment supply at the water–land interface (Maun, 2009), often directly facing the full strength of storms (Feagin et al., 2010; Sigren et al., 2014). It is well known how storm surge and waves interact with the geomorphology of beaches and sand dunes (Saye et al., 2005; Pries et al., 2008; Pye and Blott, 2008; Roberts et al., 2013). For instance, wide beaches with gentle slopes suffer little or no erosion in comparison with beaches with steep slopes (Saye et al., 2005) and tall, wide dune systems are less likely to suffer wave overtopping and sediment loss from waves and storm surge generated by hurricanes (Pries et al., 2008). Recent observations also suggest that vegetation strengthens dunes’ resistance to storms. Vegetation cover was related to both greater dune volume and protection against property damage along a stretch of Texan coastline during Hurricane Ike (Sigren et al., 2018). Furthermore, during Hurricane Sandy, natural or managed dunes with established vegetation were reported to erode less than those consisting of bare sand (Bryant et al., 2017). The potential role of vegetation in reducing erosion is further supported by flume studies (Figlus et al., 2014; Silva et al., 2016; Maximiliano-Cordova et al., 2019). However, despite the crucial role of dunes in coastal protection and decades of vegetation planting for this purpose (e.g. Savage and Woodhouse, 1968; Feagin et al., 2010), researchers have only recently started to investigate the mechanisms behind the stabilizing effects of vegetation in these valuable coastal systems.
Plant traits and physical sediment properties are expected to play important roles in the resistance of coastal dunes to erosion from wave action. The below-ground biomass of plants has been identified as a key determinant of lateral resistance in salt marsh systems (Ford et al., 2016, De Battisti et al., 2019, Silliman et al., 2019) and emerging evidence from flume studies indicates that this may also be a key factor in coastal dunes, despite the sand sediment being much less cohesive; Feagin et al. (2019) show that total below-ground biomass increases initial stages of dune face erosion due to uprooting, but effectively reduces erosion following sediment excavation, while Bryant et al. (2019) report that below-ground biomass (as mimicked by coir fibres) mitigated erosion more strongly than above-ground biomass (wooden dowels). Yet, in natural dunes, biomass below the ground can consist of multiple plant organs (roots, rhizomes and buried shoots) with contrasting physical structures and, potentially, capacities to buffer against erosion. Indeed, roots (fine roots in particular) might be more important than below-ground biomass per se. Flume studies with grasses from garden plots and with trees from an Alpine habitat both indicate that higher erosion resistance is achieved by a dense, fine root system (De Baetes, 2006, 2007; Burylo et al., 2012). At the same time, sediment physical characteristics can also influence sediment stability, with higher grain sizes reducing cohesiveness (Schutten et al., 2005, Feagin et al., 2009). Overall, despite the emerging evidence for vegetation reducing erosion in sand dunes, there is limited evidence of the role of below-ground biomass and we do not know the extent to which roots are able to bind sediment in comparison with other plant organs, i.e. rhizomes and buried shoots. Addressing this gap will allow steps towards predicting which ecological (e.g. plant species, growth conditions) and management (e.g. natural versus restored dune) contexts offer the greatest erosion resistance.
Restoration of sand dunes is a widespread practice employed around the world for increasing coastal protection (Defra, 2007; Feagin et al., 2010), but the ability of restored dunes to provide coastal protection functions is poorly understood. Deployment of wood fences and plantation of perennial grasses (e.g. Ammophila arenaria) are common methods used for restoring damaged dunes or creating new ones (Defra, 2007; Hanley et al., 2013). Afterwards, planted grasses increase sediment deposition, ultimately leading to the formation of a new dune (Defra, 2007; Hanley et al., 2013). For example, the grass Ammophila is largely employed in dune stabilization for coastal protection around the world (Savage and Woodhouse, 1968; Pickart, 1997; Hilton, 2006), because its ability to increase and withstand sediment deposition (van der Putten et al., 1988, cited in Maun 2009). Surprisingly, the ability of Ammophila to stabilize the sediment under wave attack, at either natural or restored sites, is still untested.
Here, we address the contribution of pioneer vegetation to erosion resistance in the foredune, how this is mediated by plant species identity and plant organs, and how it is affected by sediment type and management context. We studied three functionally diverse species, Ammophila arenaria, Cakile maritima and Salsola kali, which grow commonly in temperate dunes. Ammophila is a perennial grass, commonly used in restoration projects, while Cakile and Salsola are annual forbs that grow at the seaward edge of the dune system on several continents. Core samples were collected from a natural sand dune in the UK and tested in a flume to establish differences in vegetation ability to stabilize the sediment and to identify the mechanism behind sediment stabilization. We hypothesized that: (1) pioneer vegetation, including both annual and perennial species, reduces erosion rates in sand dunes, with Ammophila having the strongest effect; (2) a developed root system binds the sediment and is thus more important than other below-ground compartments (i.e. rhizomes and buried shoots) in increasing sediment stability against swash attack; and (3) the presence of coarser sediment grain size increases erosion rates. Furthermore, we sampled cores with Ammophila from a restored site to evaluate the efficacy of restoration in terms of coastal protection and predicted that (4) as long as root systems are sufficiently developed, plants from the restored site will provide the same sediment stability as plants from the natural site.
MATERIALS AND METHODS
The annual forbs Cakile maritima and Salsola kali grow mainly on the driftline, are native in northwest Europe and the Mediterranean and have been introduced into the USA, Australia, and Mexico (Maun, 2009). Ammophila arenaria is a rhizomatous perennial, clonal species and it is the main dune builder in Europe and the Mediterranean; this species has also been introduced in the West Coast of USA, South Africa, Australia and New Zealand (Ranwell, 1972; Maun, 2009). Furthermore, in the USA native species of the genera Cakile and Ammophila (e.g. Cakile edentula and Ammophila breviligulata) are widespread in foredune habitats.
Core collection and erosion trial
In October 2018 in Swansea Bay, UK (Supplementary Data Figure S1), we collected ten samples of each of the three pioneer species Ammophila, Cakile and Salsola, and of bare sediment. At the natural site, cores (25 cm × 25 cm × 25 cm; Fig. 1A) of Ammophila were collected at the dune toe, a zone vulnerable to wave swash during storms (Sallenger, 2000; Bryant et al., 2017). Cores for Cakile and Salsola were collected 2–3 m seaward from the dune toe. Cores for each species were collected in monospecific stands to ensure that the below-ground biomass was representative of the species. Cores of bare sediment were collected in areas free of vegetation among the three species sampled to ensure that sediment had similar characteristics. Additionally, we collected ten cores for Ammophila at the dune toe of a restored dune situated roughly 1.5 km from the natural site. Cores, 50 in total, were tested for erosion within 1 d of collection. Sediment samples (~200 g) were collected next to each core at 0–10 cm depth for measuring sediment properties.
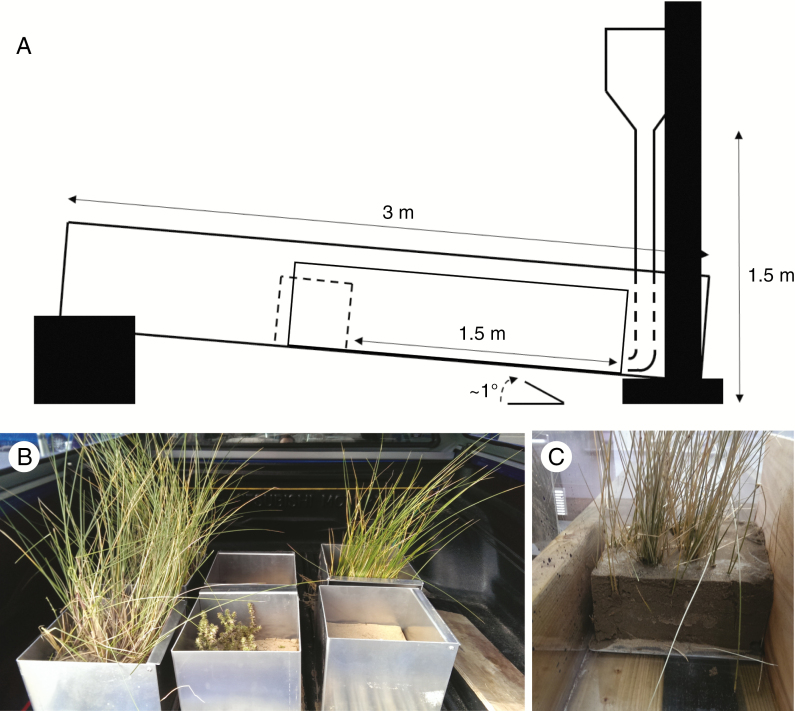
Fig. 1.
(A) Schematic representation of the flume used to experimentally expose sand dune cores to wave swash. The dashed square in the middle of the flume indicates the position of the samples (cores) during the erosion test, while the solid line indicates the position of the plywood sheet. The tank on the top of the flume at the right-hand side discharged 10 L of water per swash. (B) Examples of plants collected with cores. (C) Frontal view showing one sample with the plywood sheet.
Swash, produced by broken waves running up the shore, is one of the main mechanisms of sand dune erosion (Sallenger et al., 2000; Maun et al., 2009). To simulate a swash, we created a flume (3 m long, 35 cm wide and 35 cm in height; Fig. 1A), which allowed a rapid discharge of 10 L of water into the flume; the ~1° degree tilt of the flume allowed the water to flush back, simulating a back-swash. Water content in the sediment can alter the cohesiveness of the sediment (De Baets et al., 2006); thus, cores were saturated with 2 L of tap water prior to the erosion test. Cores were placed inside the flume 1.5 m from the water discharge pipe and exposed to six consecutive swashes. A sheet of plywood was inserted in the flume to match the smaller diameter on the cores (Fig. 1B, C). Cores were weighed to the nearest 0.01 kg before and after the swash test to measure the loss of sediment. The velocity of the water hitting the samples was measured by recording the time to cover 1.5 m (distance between the discharge pipe and the core sample) using a digital camera (Olympus TG5). This velocity (3.2 ms−1) was within the range that has been measured in the field (Hughes et al., 1997, Holland et al., 1998).
Vegetation and sediment properties
After the erosion test, we clipped the above-ground vegetation at the sediment surface to ensure it was isolated from the below-ground biomass (rhizomes, roots and buried shoots). We then washed off the sediment and distinguished the remaining material buried under the sand, i.e. below-ground shoots, rhizomes or tap roots, coarse roots (>1 mm) and fine roots (<1 mm). We chose the 1-mm cut-off to distinguish between coarse and fine roots because it is the typical threshold used in terrestrial studies (Freschet and Roumet, 2017). Total root biomass is the sum of the rhizomes, coarse roots and fine roots, while total below-ground biomass is the sum of the total root biomass with below-ground shoots. Sediment sub-samples of 20 (± 0.05) g of fresh material were oven-dried at 70 °C for 72 h, weighed and then sieved to ascertain sediment grain size. Sediment was composed entirely of fine sand (0.053–0.25 mm) and coarse sand (0.25–1 mm). As these were highly correlated, we used coarse sand (%) as an indicator of these two grain sizes in analyses.
Statistical analysis
The first phase of our analysis focused on cores extracted from the natural site only. To evaluate differences in plants’ below-ground properties, we used non-parametric Dunn’s tests (package FSA; Ogle et al., 2018). Then, to assess the effect of each species on sediment erosion, we used a linear model, with bare sediment set as the intercept and log-transformed sediment mass loss (to account for non-linearity) as the response. In order to assess which below-ground properties most parsimoniously explain sediment mass loss, we evaluated a set of a priori models (Supplementary Data Table S1) using the Akaike information criterion corrected for small sample size (Burnham et al., 2011) using the MUMin R package (Barton, 2018). These models recognize that erosion mitigation may arise from multiple plant organs (roots and buried shoots) but may be most parsimoniously viewed as arising from their summed biomass. Models accounted for potential differences between plant species, as well as the effects of coarse sand content. Finally, in recognition that root compartments may have different effects (e.g. fine roots forming extensive ‘nets’) we explored the contribution of each below-ground compartment (rhizome, coarse roots, fine roots and below-ground shoots) to sediment stabilization using a multiple regression. In the second phase of our analysis, we contrasted the natural and restored sites, focusing on Ammophila, the species common to both sites. We used the same approaches as described above to test for differences in below-ground properties and to identify of the most parsimonious model explaining sediment erosion. All the analyses were performed in R (R Core Team, 2018).
RESULTS
Natural site
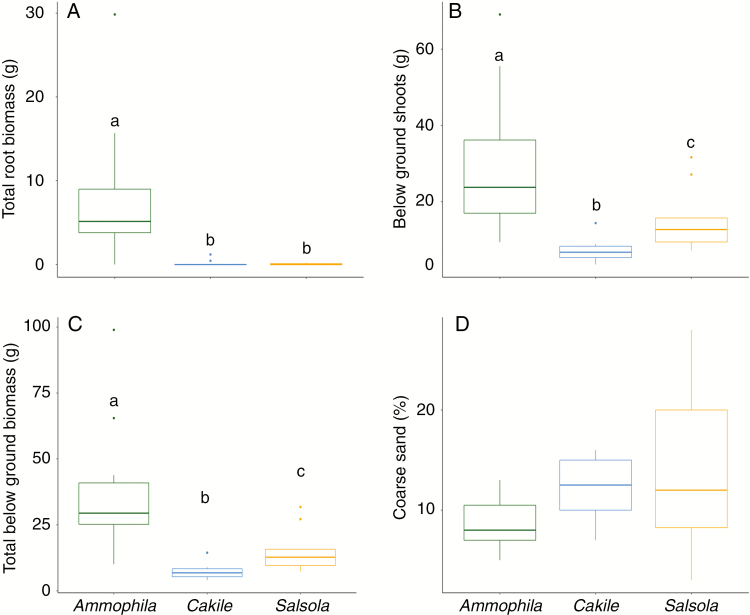
The three species differed in their biological parameters, with Ammophila having, as expected, the highest total root biomass, below-ground shoot biomass and total below-ground biomass (Fig. 2A–C). The two annual forbs, Salsola and Cakile, had similar total root biomass, but Salsola had higher below-ground shoot biomass and total below-ground biomass (Fig. 2A–C). Interestingly, for both annual species, below-ground shoot biomass constituted a high proportion of total below-ground biomass. Cores of all three species had similar coarse sand content (Fig. 2D).
Fig. 2.
Biological parameters (A–C) and sediment grain size (D) in species’ cores. Letters above the boxes indicate significant differences (P < 0.05) based on post hoc Tukey tests.
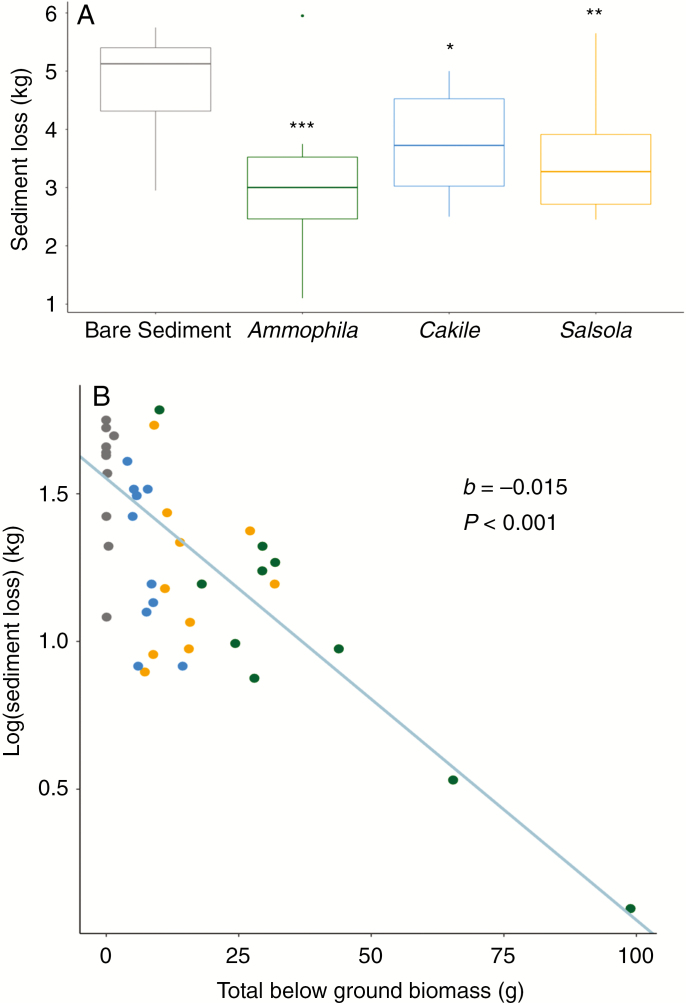
Despite the forbs having very low root biomass, all three species significantly lowered core sediment loss with respect to the bare sediment (Fig. 3A). Reduction in sediment loss was greater for Ammophila (36 %) than Salsola (27 %) and Cakile (23 %), although these differences were not statistically significant (Table 1; note that these differences are not significant even when the outlier for A. arenaria is removed). The two most parsimonious models in explaining sediment erosion both included total below-ground biomass and species as predictors (Table 2), with total below-ground biomass effectively reducing sediment erosion (Fig. 3C, Table 3). Interestingly, coarse sand was included in the second-best model but did not have a significant effect on sediment loss (Table 3). Species did not significantly affect sediment loss in either model (Table 3), implying that species identity per se plays a minor role in sediment stabilization; instead, any differences between species are likely to be mediated by their total below-ground biomass. When exploring the effect of below-ground compartments on sediment stabilization, we found no statistically significant effect of rhizomes (b = −0.0001; t = −0.093 P = 0.927), coarse roots (b = −0.002; P = 0.482) or fine roots (b = −0.001; P = 0.470), but a negative effect of below-ground shoots (b = −0.017, t = −6.198, P < 0.001) on sediment loss.
Fig. 3.
Effect of species (A; R2 = 0.28) and total below-ground biomass (B; R2 = 0.59) on sediment loss in natural sand dunes. In A, asterisks indicate statistical significance of bare sediment against, Cakile *P < 0.5, Salsola **P <0.01, and Ammophila ***P < 0.001. In (B) Ammophila, is shown as dark green circles, Cakile as ultramarine circles, Salsola as yellow circles and the bare sediment control as black circles. N = 40.
Table 1.
Differences in sediment loss (Dunn’s test, Holm method) between species from the natural site
| Species comparison | Mean difference | Lower confidence limit | Upper confidence limit | Adjusted P value |
|---|---|---|---|---|
| Cakile–Ammophila | 0.655 | 0.552 | 1.892 | 0.559 (0.207) |
| Salsola–Ammophila | 0.420 | 0.787 | 1.627 | 0.922 (0.437) |
| Cakile–Salsola | 0.235 | 1.442 | 0.972 | 0.559 (0.546) |
N = 30.
P values are adjusted for multiple comparisons; values in parentheses are for the model without the outlier for A. arenaria.
Table 2.
Ranking of models designed for testing the effect of sand and below-ground organs for sediment stabilization at the natural site
| Model | d.f. | Log likelihood | AICc | Weight |
|---|---|---|---|---|
| TotBLW + species | 6 | 2.885 | 8.8 | 0.341 |
| Sand + TotBLW + species | 7 | 4.268 | 9.0 | 0.310 |
| Sand + BLW shoots + species | 7 | 3.549 | 10.4 | 0.151 |
| BLW shoots + species | 6 | 1.904 | 10.7 | 0.128 |
| Sand + TotRB + BLW shoots + species | 8 | 4.304 | 12.0 | 0.067 |
| TotRB + species | 6 | −1.818 | 18.2 | 0.003 |
| Sand + TotRB + species | 7 | −1.558 | 20.6 | 0.000 |
| Sand + species | 6 | −8.215 | 22.2 | 0.000 |
Sand, coarse sand; TotRB, total root biomass (rhizomes + coarse roots + fine roots); BLW, shoots buried under the sediment; TotBLW, total below-ground biomass (shoots + rhizomes + coarse roots + fine roots); species, A. arenaria, S. kali, C. maritima; AICc, Akaike information criterion corrected for small sample size.
Table 3.
Summary table of the best and second-best model explaining sediment loss
| Model | Parameter | Coefficient (s.e.) | t | P | R 2 |
|---|---|---|---|---|---|
| Sediment loss ~ TotBLW + species | TotBLW | −0.015 (0.076) | −5.132 | <0.001 | 0.59 |
| Ammophila | 0.042 (0.003) | 0.276 | 0.784 | ||
| Cakile | −0.161 (0.154) | −1.473 | 0.150 | ||
| Salsola | −0.111 (0.116) | −0.955 | 0.346 | ||
| Sediment loss ~ sand + TotBLW + species | Sand | −0.012 (0.008) | −1.560 | 0.128 | 0.62 |
| TotBLW | −0.016 (0.003) | −5.428 | <0.001 | ||
| Ammophila | 0.032 (0.151) | 0.212 | 0.834 | ||
| Cakile | −0.154 (0.107) | −1.437 | 0.160 | ||
| Salsola | −0.082 (0.115) | −0.709 | 0.483 |
Significant parameters are reported in bold.
N = 40.
TotBLW, total below-ground biomass.
Natural versus restored site
At the restored site Ammophila had lower root biomass, but similar below-ground shoot biomass and total below-ground biomass compared with Ammophila from the natural site (Fig. 4A–C). At the restored site, cores of this species also had greater coarse sand content (Fig. 4D). Contrary to expectations, despite having comparable total below-ground biomass, erosion rates were higher at the restored site than the natural one (Fig. 3B). The best model explaining erosion rates in Ammophila (natural versus restored site; Table 4) showed that coarse sand and total below-ground biomass increased and decreased sediment loss, respectively (Fig. 5A, B). Interestingly, all samples from the restored site had a high percentage of coarse sand in the sediment and higher erosion than the natural site (Fig. 5A); thus, this difference in grain size explains the higher erosion rate at the restored site, despite the similar total below-ground biomass.
Fig. 4.
Differences in biological parameters of Ammophila between the natural (green symbols) and the restored (blue symbols) site. *P < 0.5 and ***P < 0.001 for restored versus natural site; N = 20.
Table 4.
Ranking of models designed for testing the effect of sand and below-ground organs for sediment stabilization in Ammophila arenaria with samples from the natural and restored sites
| Model | d.f. | Log likelihood | AICc | Weight |
|---|---|---|---|---|
| Sand + TotBLW | 4 | −26.051 | 62.8 | 0.519 |
| Sand + BLW shoots | 4 | −26.894 | 64.5 | 0.223 |
| Sand + TotRB + BLW shoots | 5 | −25.523 | 65.3 | 0.144 |
| Sand + TotRB | 4 | −28.074 | 66.8 | 0.069 |
| Sand | 3 | −30.251 | 68.0 | 0.038 |
| TotRB | 3 | −31.886 | 71.3 | 0.007 |
| TotBLW | 3 | −35.279 | 78.1 | 0.000 |
| BLW shoots | 3 | −36.114 | 79.7 | 0.000 |
Sand, coarse sand; TotRB, total root biomass (rhizomes + coarse roots + fine roots); BLW shoots, shoots buried under the sediment; TotBLW, total below-ground biomass (shoots + rhizomes + coarse roots + fine roots); AICc, Akaike information criterion corrected for small sample size.
Fig. 5.
Differences in sediment loss for natural and restored Ammophila cores (A) as explained by differences in coarse sand content (B) and total below-ground biomass (C); multiple R2 = 0.65. In (B), points represent the effect of coarse sand on sediment loss when total below-ground biomass is held constant (median). In (C) points represent the effect of total below-ground biomass on sediment loss when coarse sand is held constant (median). N = 20. Ammophila from natural and restored site represented respectively in green and blue.
DISCUSSION
This study demonstrates the importance of plant below-ground biomass in stabilizing the sediment in foredunes under attack from wave swash. Importantly, we showed here for the first time that not only roots, but all the plant below-ground biomass is important for sediment stabilization, with buried shoots having a key role. This result explains how annual plants also reduce erosion rates despite lacking a well-developed root system, indicating that the role of annual pioneer vegetation for sediment stabilization in foredunes has been under-appreciated. Our study supports the strong role that vegetation plays in sediment stabilization in coastal ecosystems and provides insights into erosion processes in coastal dunes.
The grass Ammophila has been largely employed in dune stabilization and in restoration projects for coastal protection purposes around the world (Savage and Woodhouse, 1968; Pickart, 1997; Hilton, 2006). Indeed, we found here that Ammophila was the best species in reducing erosion rates because of its large below-ground biomass (up to 100 g of roots per core; Fig. 3B). This is in accordance both with recent work in coastal dunes indicating an important role of below-ground biomass (Feagin et al., 2019) and with studies in terrestrial systems and salt marshes, where a dense root system strongly reduces topsoil and lateral erosion (De Baetes et al., 2006, 2007; Burylo et al., 2012; Ford et al., 2016).
However, we showed here that annual forbs (Cakile and Salsola) can also effectively reduce sediment erosion in sand dunes (although to a lesser extent than Ammophila). This result is of particular interest because the below-ground biomass of these two forbs was almost entirely composed of buried shoots (Fig. 2A). Therefore, this result indicates that not only roots, but the entire portion of the plant embedded in the sediment is important in dune stabilization. This point is further supported by (1) the analysis of the effect of below-ground compartments on erosion, where only below-ground shoots had a significant effect (reducing erosion rates), and (2) the observation that Ammophila from the restored site lacked a well-developed root system but its buried shoots still contributed to sediment stability (Fig. 5B). In terrestrial plants, Bardgett et al. (2014) suggested that roots with a wide diameter could increase sediment stability because they push sediment particles aside, compressing and holding the sediment together. Thus, the same mechanism may operate with buried shoots, which have a wider diameter than roots, possibly explaining the mechanism behind the enhanced sediment stability.
In contrast with our expectation, we found that erosion rates of Ammophila cores from the restored site were higher than those from the natural site. Comparison between the two sites indicates that this difference is most likely driven by high coarse sand content in cores from the restored site. Unlike silt-clay sediment, sandy sediment is less cohesive and more prone to erosion (Schutten et al., 2005) and studies in both salt marshes (Feagin et al., 2009; Ford et al., 2016; Lo et al., 2017; De Battisti et al., 2019) and sand dunes (Overton et al., 1994) have demonstrated that, with increasing sediment grain size, erosion rates also increase. Interestingly, a common practice for beach nourishment and dune creation is to adopt dredged, coarser sediment from the lower beach (or off-shore sediment) (Defra, 2007). For instance, beach nourishment along the Florida coast after hurricanes Matthew and Irma has employed coarse sand and shell fragments (J. N. Griffin, pers. comm.). Although in the restored site no allochthonous sediment material was employed, our study supports previous findings that coarser sediment reduces sediment stability and thus highlights that restoration projects should choose the type of sediment carefully.
We found here that Ammophila plants from the restored site lacked an extensive root biomass, despite having grown for roughly 2 years (restoration started in 2016; D. Hill, Swansea City Council). Several factors could explain this apparent lack of root development: (1) a temporal effect, i.e. younger plants in the restored site may have a less developed root system with respect to older clones from the natural site; (2) our cores sampled sediment and roots down to 25 cm depth, and thus dense roots might be buried deeper in the sediment, perhaps reflecting a compensatory vertical growth following sediment deposition; or (3) sediment granulometry affecting plant development. Nevertheless, as stated above, buried shoots still provided below-ground biomass that acted to reduce erosion. Future studies should focus on the effect that different restoration schemes (e.g. using dredged sediment versus local sediment) have on plants’ growth and physiology and their effect on biomass allocation to plant organs (e.g. above ground versus below ground).
In sand dune restoration, the focus on coastal protection in some cases has led managers to destroy natural dunes and to plant monocultures of perennial species with an extensive root system, which are better suited for sediment stabilization (e.g. Casuarina equisetifolia in India; Feagin et al., 2010). However, we showed here that annual species such as Salsola and Cakile, in addition to the widely recognized Ammophila, were also able to effectively stabilize the sediment. Although the effect of these forbs was lower in comparison with Ammophila, our results highlight that more species should be explored for their coastal protection capacity (see also Feagin et al., 2019). Annual pioneer species grow at lower elevation on the beach than Ammophila, occupying a different zone and forming embryo dunes in front of the foredune (Ranwell, 1972; Maun, 2009). An observational study in the USA has shown that foredunes reduce the impacts of waves on the first dune ridge (Pries et al., 2008). Thus, an embryo dune in front of a restored dune might reduce the impact of storms on the latter, potentially increasing the overall resistance of dunes to storms. Overall, restoration projects employing different species that occupy different dune zones might provide better resistance against waves with respect to systems that employ only a single species (e.g. Ammophila) or those occupying the same zone.
Lastly, we stress that multiple mechanisms, beyond those captured in our flume study, are involved in sand dune erosion. Our flume study reflects the particular case where the foredune’s edge is affected by the wave swash. Although this is an important mechanism of dune erosion, waves can directly hit the dune scarp or even overtop the dune itself (Figlus et al., 2014; Sigren et al., 2014; Silva et al., 2016). In these cases, physical (wave energy and period), bio-geomorphological (dune height and width) and biological (vegetation cover, architecture) factors will influence the net erosion of dunes (Pries et al., 2008; Silva et al., 2016; Charbonneau et al., 2017; Maximiliano-Cordova et al., 2019). Notwithstanding, a strength of our approach is that by extracting intact vegetated cores from dunes we used plants that have grown in situ, under natural conditions. In contrast to previous studies that employed vegetation grown in greenhouses (e.g. Figlus et al., 2014; Silva et al., 2016; Feagin et al., 2019), our approach allowed us to capture the natural deposition of sediment over the vegetation, pinpointing the pivotal role of buried shoots in sediment stabilization.
In conclusion, our study shows the strong effect that plants play in foredune stabilization, adding to a growing body of evidence on the importance of vegetation for coastal protection. In particular, we showed that not only roots, but all the structures buried under the sediment, especially buried shoots, play a crucial role in sediment stabilization. Furthermore, we highlight that employing a coarse sediment can be deleterious for sediment stability and thus for achieving restoration goals in terms of coastal protection. Crucially, our results also point to the effectiveness of annual plants in decreasing erosion, suggesting that management and restoration schemes could benefit from maintaining or planting a diverse array of species.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: The study sites. Ten cores of the perennial grass Ammophila were collected from each site, while ten cores for the annual forbs Salsola and Cakile and ten for the bare sediment were collected in the natural site only. Table S1: A priori models designed for testing the effect of sand and belowground traits for sediment stabilisation.
FUNDING
Funding to support this work was provided by Welsh Government and HEFCW through the Sêr Cymru National Research Network for Low Carbon, Energy and Environment RESILCOAST project and by the EU ERDF, SEACAMS project.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Deborah Hill from the Swansea City Council for providing permission and for sampling, and Laura Cappelatti, Tom Fairchild and Jessica Knoop, for field assistance.
LITERATURE CITED
- Bardgett RD, Mommer L, De Vries FT. 2014. Going underground: root traits as drivers of ecosystem processes. Trends in Ecology & Evolution 29: 692–699. [DOI] [PubMed] [Google Scholar]
- Barton K. 2018. MuMIn: multi-model inference. R package version 1.42.1. https://CRAN.R-project.org/package=MuM. [Google Scholar]
- Bouma TJ, van Belzen J, Balke T, et al. 2014. Identifying knowledge gaps hampering application of intertidal habitats in coastal protection: opportunities & steps to take. Coastal Engineering 87: 147–157. [Google Scholar]
- Bryant DB, Bryant MA, Grzegorzewski AS. 2017. Erosion of coastal foredunes: a review on the effect of dune vegetation. ERDC/CHL CHETN-I-94. Vicksburg, MS: US Army Research and Development Center. [Google Scholar]
- Bryant DB, Bryant MA, Sharp JA, Bell GL, Moore C. 2019. The response of vegetated dunes to wave attack. Coastal Engineering 152: 103506. [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65: 23–35. [Google Scholar]
- Burylo M, Rey F, Mathys N, Dutoit T. 2012. Plant root traits affecting the resistance of soils to concentrated flow erosion. Earth Surface Processes and Landforms 37: 1463–1470. [Google Scholar]
- Calvão T, Pessoa M, Lidon F. 2013. Impact of human activities on coastal vegetation-A review. Emirates Journal of Food and Agriculture 25: 926–944. [Google Scholar]
- Costanza R, Pérez-Maqueo O, Martinez ML, Sutton P, Anderson SJ, Mulder K. 2008. The value of coastal wetlands for hurricane protection. Ambio 37: 241–248. [DOI] [PubMed] [Google Scholar]
- Charbonneau BR, Wootton LS, Wnek JP, Langley JA, Posner MA. 2017. A species effect on storm erosion: invasive sedge stabilized dunes more than native grass during Hurricane Sandy. Journal of Applied Ecology 54: 1385–1394. [Google Scholar]
- De Baets S, Poesen J, Gyssels G, Knapen A. 2006. Effects of grass roots on the erodibility of topsoils during concentrated flow. Geomorphology 76: 54–67. [Google Scholar]
- De Baetes S, Poesen J, Knapen A, Galindo P. 2007. Impact of root architecture on the erosion-reducing potential of roots during concentrated flow. Earth Surface Processes and Landforms 32: 1323–1345. [Google Scholar]
- De Battisti D, Fowler MS, Jenkins SR, et al. 2019. Intraspecific root trait variability along environmental gradients affects salt marsh resistance to lateral erosion. Frontiers in Ecology and Evolution 7: 150. [Google Scholar]
- Defra 2007. Sand dune processes and management for flood and coastal defence. R&D Technical Report FD1302/TR. Joint Defra/EA Flood and Coastal Erosion Risk Management R&D Programme. London: Department for Environment, Food and Rural Affairs. [Google Scholar]
- Feagin RA, Lozada-Bernard SM, Ravens TM, Möller I, Yeager KM, Baird AH. 2009. Does vegetation prevent wave erosion of salt marsh edges? Proceedings of the National Academy of Sciences of the USA 106: 10109–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin RA, Mukherjee N, Shanker K, et al. 2010. Shelter from the storm? Use and misuse of coastal vegetation bioshields for managing natural disasters. Conservation Letters 3: 1–11. [Google Scholar]
- Feagin RA, Furman M, Salgado K, et al. 2019. The role of beach and sand dune vegetation in mediating wave run up erosion. Estuarine, Coastal and Shelf Science 219: 97–106. [Google Scholar]
- Figlus J, Sigren JM, Armitage AR, Tyler RC. 2014. Erosion of vegetated coastal dunes. Coastal Engineering Proceedings 1: 20. [Google Scholar]
- Ford H, Garbutt A, Ladd C, Malarkey J, Skov MW. 2016. Soil stabilization linked to plant diversity and environmental context in coastal wetlands. Journal of Vegetation Science. https://doi.org/10.1111/jvs.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschet GT, Roumet C. 2017. Sampling roots to capture plant and soil functions. Functional Ecology 31: 1506–1518. [Google Scholar]
- Hanley M, Hoggart S, Simmonds D, et al. 2013. Shifting sands? coastal protection by sand banks, beaches and dunes. Coastal Engineering 87. https://doi.org/10.1016/j.coastaleng.2013.10.020. [Google Scholar]
- Hilton MJ. 2006. The loss of New Zealand’s active dunes and the spread of marram grass (Ammophila arenaria). New Zealand Geographer 62: 105–120. [Google Scholar]
- Holland KT, Sallenger AH, Raubenheimer B, Elgar S. 1998. Swash zone morphodynamics and sediment transport processes. In: 26th International Conference on Coastal Engineering, American Society of Civil Engineers, Copenhagen, Denmark. https://doi.org/10.1061/9780784404119.212. [Google Scholar]
- Hughes MG, Masselink G, Brander RW. 1997. Flow velocity and sediment transport in the swash zone of a steep beach. Marine Geology 138: 91–103. [Google Scholar]
- Leonardi N, Ganju NK, Fagherazzi S. 2016. A linear relationship between wave power and erosion determines salt-marsh resilience to violent storms and hurricanes. Proceedings of the National Academy of Sciences 113: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo VB, Bouma TJ, van Belzen J, Van Colen C, Airoldi L. 2017. Interactive effects of vegetation and sediment properties on erosion of salt marshes in the Northern Adriatic Sea. Marine Environmental Research 131: 32–42. [DOI] [PubMed] [Google Scholar]
- Marani M, D’Alpaos A, Lanzoni S, Santalucia M. 2011. Understanding and predicting wave erosion of marsh edges. Geophysical Research Letters 38. https://doi.org/10.1029/2011GL048995 [Google Scholar]
- Maun AM. 2009. The biology of coastal sand dunes. Oxford: Oxford University Press. [Google Scholar]
- Maximiliano-Cordova C, Salgado K, Martínez ML, et al. 2019. Does the functional richness of plants reduce wave erosion on embryo coastal dunes? Estuaries and Coasts. https://doi.org/10.1007/s12237-019-00537-x. [Google Scholar]
- Neumann B, Vafeidis AT, Zimmermann J, Nicholls RJ. 2015. Future coastal population growth and exposure to sea-level rise and coastal flooding - a global assessment. PLOS ONE 10: e0118571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle DH, Wheeler P, Dinno A. 2018. FSA: fisheries stock analysis R package version 0.8.22 https://github.com/droglenc/FSA.
- Overton MF, Pratikto WA, Lu JC, Fisher JS. 1994. Laboratory investigation of dune erosion as a function of sand grain size and dune density. Coastal Engineering 23: 151–165. [Google Scholar]
- Pickart AJ. 1997. Control of European beachgrass (Ammophila arenaria) on the West Coast of the United States. In: California Exotic Pest Plant Council, 1997 Conference Proceedings https://www.cal-ipc.org/wp-content/uploads/2017/12/1997_symposium_proceedings1934.pdf. [Google Scholar]
- Pries AJ, Miller DL, Branch LC. 2008. Identification of structural and spatial features that influence storm-related dune erosion along a barrier-island ecosystem in the Gulf of Mexico. Journal of Coastal Research 4: 168–175. [Google Scholar]
- Pye K, Blott S. 2008. Decadal-scale variation in dune erosion and accretion rates: an investigation of the significance of changing storm tide frequency and magnitude on the Sefton coast, UK. Geomorphology 102: 652–666. [Google Scholar]
- R Core Team 2018. R: A language and environment for statistical computing.R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/ [Google Scholar]
- Ranwell DS. 1972. Ecology of salt marshes and sand dunes. London: Chapman and Hall. [Google Scholar]
- Roberts TM, Wang P, Puleo JA. 2013. Storm-driven cyclic beach morphodynamics of a mixed sand and gravel beach along the Mid-Atlantic Coast, USA. Marine Geology 346: 403–421. [Google Scholar]
- Salgado K, Martinez ML. 2017. Is ecosystem-based coastal defense a realistic alternative? Exploring the evidence. Journal of Coastal Conservation 21: 837–848. [Google Scholar]
- Sallenger 2000. Storm impact scale for barrier islands. Coastal Research 16: 890–895. [Google Scholar]
- Savage RP, Woodhouse WW. 1968. Creation and stabilization of coastal barrier dunes. In: Proceedings of the 11th Conference on Coastal Engineering American Society of Civil Engineers, 671–700. [Google Scholar]
- Saye SE, van der Wal D, Pye K, Blott SJ. 2005. Beach–dune morphological relationships and erosion/accretion: an investigation at five sites in England and Wales using LIDAR data. Geomorphology 72: 128–155. [Google Scholar]
- Schutten J, Dainty J, Davy AJ. 2005. Root anchorage and its significance for submerged plants in shallow lakes. Journal of Ecology 93: 556–571. 10.1111/j.1365-2745.2005.00980.x [DOI] [Google Scholar]
- Sigren JM, Figlus J, Armitage A. 2014. Coastal sand dunes and dune vegetation: restoration, erosion, and storm protection. Shore and Beach 82: 5–12 [Google Scholar]
- Sigren JM, Figlus J, Highfield W, Feagin RA, Armitage A. 2018. The effects of coastal dune volume and vegetation on storm-induced property damage: analysis from Hurricane Ike. Journal of Coastal Research 341: 164–173. [Google Scholar]
- Silliman BR, He Q, Angelini C. 2019. Field Experiments and Meta-analysis Reveal Wetland Vegetation as a Crucial Element in the Coastal Protection Paradigm. Current Biology, https://doi.org/10.1016/j.cub.2019.05.017 [DOI] [PubMed] [Google Scholar]
- Silva R, Martínez ML, Odériz I, Mendoza E, Feagin RA. 2016. Response of vegetated dune–beach systems to storm conditions. Coastal Engineering 109: 53–62. [Google Scholar]
- Wang H, Wal D van der, Li X, et al. 2017. Zooming in and out: scale dependence of extrinsic and intrinsic factors affecting salt marsh erosion. Journal of Geophysical Research: Earth Surface 122: 1455–147. [Google Scholar]
- Woodruff JD, Irish JL, Camargo SJ. 2013. Coastal flooding by tropical cyclones and sea-level rise. Nature 504: 44–52. https://doi.org/10.1038/nature12855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.