Abstract
In 1874, Karl Kahlbaum described catatonia as an independent syndrome characterized by motor, affective, and behavioral anomalies. In the following years, various catatonia concepts were established with all sharing the prime focus on motor and behavioral symptoms while largely neglecting affective changes. In 21st century, catatonia is a well-characterized clinical syndrome. Yet, its neurobiological origin is still not clear because methodological shortcomings of hitherto studies had hampered this challenging effort. To fully capture the clinical picture of catatonia as emphasized by Karl Kahlbaum, 2 decades ago a new catatonia scale was developed (Northoff Catatonia Rating Scale [NCRS]). Since then, studies have used NCRS to allow for a more mechanistic insight of catatonia. Here, we undertook a systematic review searching for neuroimaging studies using motor/behavioral catatonia rating scales/criteria and NCRS published up to March 31, 2019. We included 19 neuroimaging studies. Studies using motor/behavioral catatonia rating scales/criteria depict cortical and subcortical motor regions mediated by dopamine as neuronal and biochemical substrates of catatonia. In contrast, studies relying on NCRS found rather aberrant higher-order frontoparietal networks which, biochemically, are insufficiently modulated by gamma-aminobutyric acid (GABA)-ergic and glutamatergic transmission. This is further supported by the high therapeutic efficacy of GABAergic agents in acute catatonia. In sum, this systematic review points out the difference between motor/behavioral and NCRS-based classification of catatonia on both neuronal and biochemical grounds. That highlights the importance of Kahlbaum’s original truly psychomotor concept of catatonia for guiding both research and clinical diagnosis and therapy.
Keywords: catatonia, Kahlbaum, neuroimaging, Northoff Catatonia Rating Scale, CT, MRI, SPECT
Introduction
In 1874, Karl Kahlbaum systematically described a total of 21 psychotic patients presenting not only with hallucinations, formal thoughts disorders, and delusions, but also with flamboyant psychomotor abnormalities.1,2 In the traits of his observations, Kahlbaum developed the concept of catatonia, which he conceived an independent nosological entity characterized by hypokinetic and hyperkinetic motor phenomena (rigor, dyskinesia, festination, counteracting, posturing, catalepsy, etc.), affective symptoms (aggression, anxiety, flat affect, affect incontinence, etc.), and disorders of behavior (autism, mutism, echolalia, etc.).3–9 When Emil Kraepelin first reported on catatonia in 1896, he considered this syndrome to be—similar to Karl Kahlbaum’s concept—an independent diagnostic entity. Later on in 1899, when Kraepelin separated dementia praecox from manic-depressive illness, catatonia was attributed to one of the subtypes of dementia praecox, thereby limiting catatonia to motor and behavioral symptoms.10–12 Catatonia and its characteristic symptoms were no longer accepted as a disease on its own, but as irreversible transition into dementia praecox. Kraepelin also spoke of the fact that there are no objective neuropathological findings that would point to a different pathogenesis of catatonia and dementia praecox.5,10,11 Later on, Eugen Bleuler incorporated catatonic motor disorders (motility issues, stupor, mutism, stereotypies, mannerism, negativism, etc.) in his new concept of dementia praecox called “schizophrenia.” 13 Since that time, the concept of catatonia has been made almost invisible to clinicians and closely related to that of dementia praecox and (after 1908) schizophrenia, characterized mainly by motor and behavioral symptoms, thus neglecting affective alterations.3,5,14 In contrast, earlier description of catatonia being associated with affective psychosis like depression and mania and numerous works by contemporary American authors15–18 reporting catatonia in affective disorders remain in the background overshadowed by the predominant motor (and behavioral) characterization of catatonia as subtype of schizophrenia.19
This tendency has also prevailed in modern nosology and symptomatologic classification of catatonia (see DSM-IV-TR and ICD-10) that emphasize catatonic motor signs. In the last 2 decades, however, there has been a paradigm shift in modern research.20–24 Catatonia gained importance as an independent syndrome and has also been described in other psychiatric diseases such as affective disorders16, autism25, and organic mental disorders26 (eg, infective and immune causes).27 This is also visible in DSM-5 where catatonia can be used as a specifier to better characterize an underlying psychiatric disease. However, despite detailed recommendations provided by internationally acknowledged experts,6 DSM-5 does not provide a separate diagnostic code for catatonia besides “catatonia not otherwise specified”. In contrast to ICD-10, however, catatonia in DSM-5 can be associated with other mental disorders or specific medical conditions. This situation might change in the ICD-11, where catatonia could be recognized an independent diagnostic entity.28 Until then, the prime characterization of catatonia by motor (and behavioral) symptoms remains—this is for instance well manifested in catatonia criteria and rating assessments such as Rosebush criteria29, Rogers Catatonia Scale (RCS)30, Modified Rogers Scale (MRS)31,32, Bush-Francis Catatonia Rating Scale (BFCRS)33,34, Braunig Catatonia Rating Scale (BCRS)35, or KANNER (“Katatonia, Autism, Neuropsychiatric and Neuromovement Examination Rating”) Scale36. Although these rating scales have contributed to better understanding of the neurobiological origin of catatonia, they paid only little attention to the clinical importance of affective symptoms as originally emphasized by Karl Kahlbaum in 1874.3
One possible reason why affective symptoms have not been considered as a part of catatonia in either clinical practice or neurobiological research up to this point is that they were seen as a genuine part of schizophrenia spectrum disorders (SSD) or affective psychoses themselves.23 To overcome this neglect of affective symptoms in psychopathologic evaluation of catatonia, 20 years ago, the senior author (G.N.) developed the Northoff Catatonia Rating Scale (NCRS).3 The NCRS offers clinicians and scientists the possibility not only to assess the severity of motor and behavioral symptoms, but also to determine whether and to what extent affective symptoms are present and are specific to catatonia as distinguished from those associated with either schizophrenia or affective psychosis. The consideration of affective symptoms as being intrinsic to catatonia was empirically supported mainly by the following findings: (1) affective symptoms were significantly higher in catatonia patients than in psychiatric controls including both those with schizophrenia and affective psychosis3; (2) affective symptoms in NCRS were significantly associated with general catatonic symptoms as reflected in NCRS total score37; (3) some affective symptoms on the perceptual-experiential level were specific to catatonia like the inability to control emotions and affects which, in turn, as experienced by the patients, strongly affected their behavioral and motor functions38; (4) catatonia is present in affective disorders15,39; (5) anxiety measured by psychopathologic scales was not significantly associated with affective scores in catatonia scales3,40; and (6) there is an outstanding and almost immediate effect of gamma-aminobutyric acid (GABA)-ergic agents, eg, lorazepam that rapidly relieves affective symptoms in catatonic patients.41
Symptomatically, catatonia is characterized by a specific constellation of motor, behavioral, and affective symptoms (rather than being merely motor syndrome) with all 3 being closely intertwined in clinical presentation—this was well pointed out by Kahlbaum2 and has been taken as basis for the NCRS and subsequent neurobiological investigations. To characterize and point out the truly psychomotor nature of catatonia in the original sense of Kahlbaum, we here proceed in 4 main parts. First, we systematically review the neuroimaging evidence for catatonia-related structural and functional brain abnormalities. In particular, we elucidate the differences between neuroimaging studies using motor/behavioral catatonia rating scales/criteria such as RCS30, MRS31,32, or BFCRS33,34 and NCRS. Second, we use the identified brain regions and networks to create an update of the catatonia model presented in 2000.23 Third, we point out associated biochemical changes that mainly focus on dopamine and GABAergic, glutamatergic and dopaminergic transmission. Fourth, we highlight existing limitations, gaps in knowledge, and fertile avenues for future neuroimaging and clinical research on catatonia in psychiatric disorders as well as the relevance of such truly psychomotor concept for clinical diagnosis and therapy of catatonia. Finally, this systematic review advocate that catatonia should be included as an independent disease entity in future classification systems because it has its own specific pathophysiology.
Methods
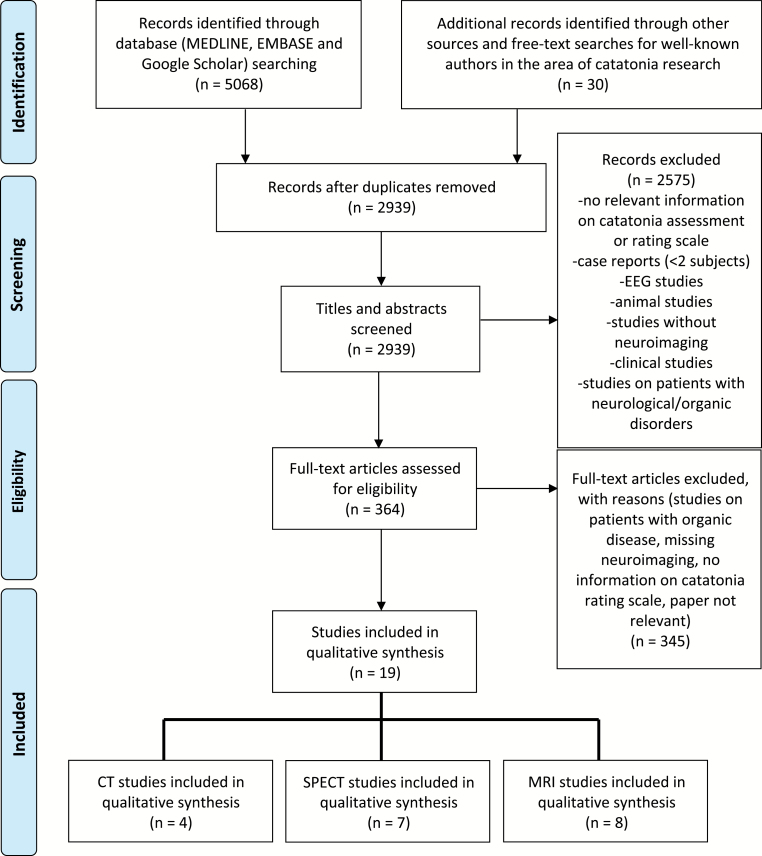
The systematic literature search and study selection approach followed PRISMA guidelines (figure 1; different sources: January 1, 1960 to March 31, 2019). A detailed information on the literature search and study selection approach is provided in supplementary material.
Fig. 1.
PRISMA flow chart of included neuroimaging studies on catatonia in mental disorders.
Systematic Literature Search Results
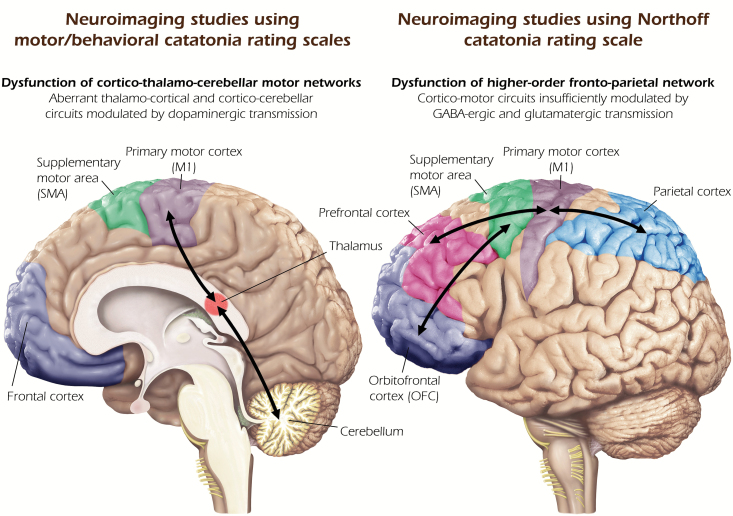
We identified 4 CT42–45 studies, 7 SPECT46–52 studies, and 8 MRI53–59 studies that were included in this systematic review. Ten studies used either the DSM-III/-IV-TR classification system, strict criteria for catatonia, or a motor/behavioral catatonia rating scale (eg, MRS or BFCRS) in psychiatric patients (table 1) and depicted cortical and subcortical motor regions as neuronal substrates of catatonia (figure 2, left side). Nine studies used NCRS in psychiatric patients (table 2) and found aberrant higher-order frontoparietal networks insufficiently modulated by GABAergic and glutamatergic transmission underlying catatonia (figure 2, right side).
Table 1.
CT, SPECT, and MRI Studies Using Motor/Behavioral Catatonia Rating Scales in Mental Disorders
| Study | Sample characteristics | Motor assessment | Neuroimaging method | Important findings |
|---|---|---|---|---|
| Joseph et al45 | CAT: n = 5, age = 65.4 y; M:F = 2:3 | CAT had all mutism, akinesia, and catalepsy plus at least 2 of the 4 symptoms such as negativism, stereotyping, posturing, and mannerisms. | CT scans on Siemens Somatom 2 with a 256 × 256 matrix and 8-mm-thick slices | Catatonic patients showed atrophy of posterior fossa structures (vermis and brainstem). |
| SP: n = 5, age = 66 y; M:F = 2:3 | ||||
| Wilcox44 | CAT: n = 17 | Criteria for CAT were mutism, motor rigidity, irrational behavior, and the absence of frank neurological or metabolic disease | CT scans on a Picker-600 CT Scanner with a resolution power in the 1-mm range or better | Catatonia may be associated with cerebellar atrophy. |
| SP: n = 30 with noncatatonic SZ, n = 20 with psychotic affective disorder, n = 15 nonpsychiatric controls (matched for age and gender) | ||||
| Wilcox43 | CAT: n = 30 patients (8 bipolar—32.58 y, 11 schizophrenia—32.31 y, 6 schizoaffective—37.39 y, and 5 major depression—33.29 y of age), M:F = 16:14 | Criteria for CAT were mutism, presence of bizarre motor activity including posturing, decrease in motor activity, or catatonic excitement characterized by intense bursts of agitated stereotypy. | CT scans on a Picker-600 CT Scanner with a resolution power in the 1-mm range or better | CAT patients with schizophrenia had larger ventricular/brain ratios compared with nonschizophrenic CAT patients. |
| Ebert et al52 | CAT: N = 2 (48 and 24 y old) | DSM-III-R schizophrenia diagnosis | rCBF studied via SPECT with 99mTcHMPAO | Temporal hypoperfusion as a neural correlate of aberrant sensory input in catatonia |
| SP: N = 10 | ||||
| HC: n = 8 | ||||
| Satoh et al111 | CAT: N = 6, age = 29.7 ± 8.6 y; M:F = 3:3; DOI: 0.5–20 y | 6 schizophrenia patients who currently or at an earlier time had received a DSM-III-R subtype diagnosis of catatonic schizophrenia (symptoms included catatonic stupor or excitement, posturing, mannerisms, and negativism). | rCBF studied via SPECT with IMP | Low rCBF, as indexed by IMP perfusion, of the dorsal parietal lobe was found in CAT compared with SP. |
| SP: N = 13, age = 31.4 ± 11.6 y; M:F = 10:3; all patients medicated | ||||
| Galynker et al51 | CAT: n = 1, age = 40 y; female; DOI: 25 y | One schizoaffective patient presenting with severe psychomotor retardation, negativism, and waxy flexibility | rCBF studied via SPECT with 99mTcHMPAO | The patient with catatonia showed decreased perfusion in the left parietal and left motor cortices when compared with healthy controls. |
| HC: n = 8, age = 36.1 y, M:F = 5:3 | ||||
| Escobar et al49 | CAT: N = 9, age = 28.3 ± 5.8 y; M:F = 8:1; 5 patients with schizophrenia and 4 patients with mood disorder | Diagnosis of catatonia was done clinically, according to DSM-IV criteria. The severity was assessed with Modified Rogers Scale. | rCBF studied via SPECT with 99mTcHMPAO | No SPECT differences between the 2 diagnostic groups. Significant increase in rCBF in parietal, temporal, and occipital regions in patients with mood disorder and no significant changes in schizophrenia patients |
| Walther et al56 | CAT: N = 15, age = 35.9 ± 12.7 y; M:F = 11:4; education: 12.0 ± 4.0 y; CPZ: 461.3 ± 346.4; DAE: 153.4 ± 143.9 mo | Bush-Francis Catatonia Rating Scale. | 3 Tesla MRT, SPM8 (VBM toolbox) | Excited and retarded catatonia differed in SMA perfusion. Higher catatonia scores were associated with higher SMA perfusion. |
| SP: N = 27; age = 37.1 ± 10.6; M:F = 17:10; education: 14.2 ± 3.1 y; CPZ: 373.6 ± 359.4; DAE: 126.4 ± 127.2 mo | ||||
| HC: N = 41, age = 38.6 ± 13.6 y; M:F = 25:16; education: 14.1 ± 2.7 y | ||||
| Walther et al58 | SP: N = 46, age = 38.0 ± 11.5 y; M:F = 30:16; education: 13.5 ± 3.1 y; CPZ: 444.6 ± 337.0; DOI: 12.1 ± 12.4 y | Bush-Francis Catatonia Rating Scale and Modified Rogers Scale | 3 Tesla MRT, Functional Connectivity Toolbox (CONN, version 17.c) | Thalamocortical (bilateral M1 to left thalamus) hyperconnectivity was linked to catatonia in schizophrenia. |
| HC: N = 44, age = 38.0 ± 13.6 y; M:F = 25:16; education: 14.1 ± 2.7 y |
CAT, catatonic patients; SP, schizophrenic patients/other mental disorders; HC, healthy controls; IMP , 123I-iodoamphetamine; DOI, duration of illness; CPZ, chlorpromazine equivalent dose; OLZ, Olanzapine equivalent dose; CT, computer tomography; rCBF, regional cerebral blood flow; OFC, orbitofrontal cortex; SMA, supplementary motor area; M, male; F, female; y, years; mo, months.
Fig. 2.
Relevant neural circuits underlying catatonia in mental disorders. Summary of findings from neuroimaging studies using motor/behavioral catatonia rating scales (left). Summary of findings from neuroimaging studies using Northoff Catatonia Rating Scale (right).
Table 2.
CT, SPECT, and MRI Studies Using NCRS in Mental Disorders
| Study | Sample characteristics | Motor assessment | Neuroimaging method | Important findings |
|---|---|---|---|---|
| Northoff et al48 | CAT: N = 10, age = 41.6 ± 5.3 (21–63) y; M:F = 4:6 | 10 patients with catatonia according to criteria by Lohr and Wiesniewski (1987), Rosebush et al (1990), BFCRS, and NCRS | rCBF and benzodiazepine receptor distribution were studied via SPECT with 123I-iomanezil | The study found significant relations of motor and affective catatonic symptoms with left upper frontal and right lower prefrontal cortical iomazenil binding as well as with right lower parietal rCBF. |
| SP: n = 10, age = 40.8 ± 4.9 y | ||||
| HC: N = 20, age = 40.1 ± 6.2 y | ||||
| Northoff et al53 | CAT: N = 2, age = 27 and 31 y; M:F = 1:1 | 2 patients with catatonia according to Lohr and Wiesniewski (1987), Rosebush et al (1990), and DSM-III-R (APA, 1987) | 1.5 Tesla MRT, task: sequential finger opposition, AFNI | CAT showed higher activity reduction in the contralateral primary sensory-motor cortex during sequential finger opposition after lorazepam application when compared with HC. |
| DOI: 2 and 6 mo | ||||
| HC: N = 2, age = 27 and 31 y | ||||
| Northoff et al47 | CAT: N = 10, age = 41.6 ± 5.3 (21–63) y; M:F = 4:6 | 10 patients with catatonia according to criteria by Lohr and Wisniewski, Rosebush Catatonia Scale, BFCRS, NCRS | rCBF studied via SPECT with Tc-99mECD | CAT showed decreased rCBF in right prefrontal and parietal cortex compared with psychiatric and healthy controls as well as significantly poorer performance in visual–spatial abilities associated with right parietal function. |
| SP: n = 10, age = 40.8 ± 4.9 y | ||||
| HC: N = 20, age = 40.1 ± 6.2 y | ||||
| Northoff et al54 | CAT: N = 10, age = 41.6 ± 5.3 y; M:F = 5:5; education: 9.5 ± 1.8 y; CPZ: 180.2 ± 177.5; DAE: 5.1 ± 4.2 y | NCRS, Rosebush Catatonia Scale, Lohr Catatonia Symptom Scale, and the Northoff Self-estimation Scale | 3 Tesla MRT, ROI-Analysis, SEM, CoCoMac; task: affective/motor paradigm using positive and negative stimuli from the International Affective Picture System (IAPS) | Affective and behavioral catatonic symptoms were associated with activity changes in OFC. Motor catatonic symptoms were associated with prefrontal cortex activity. |
| SP: N = 10, age = 40.8 ± 4.9 y; M:F = 5:5; education: 9.9 ± 1.8 y; CPZ: 167.0 ± 153.2 | ||||
| De Tiege et al50 | CAT: n = 1, age = 14, female, NCRS total score = 19 | NCRS | Positron emission tomography, SPM99, masking analysis | Patient with catatonia showed a decrease of metabolism in a large prefrontal area, the right anterior cingulate, the right medial prefrontal and dorsolateral cortices when compared with HC. |
| HC: n = 29, age = 32, M:F = 13:16 | ||||
| Scheuerecker et al57 | CAT: N = 12, age = 36.2 ± 9.5 y; M:F = 5:7; CPZ = no information available | NCRS, Rosebush Catatonia Scale, Lohr Catatonia Symptom Scale, and the Northoff Self-estimation Scale | 1.5 Tesla MRT, SPM5 | CAT showed reduced activity during self-initiated movements in the right superior frontal gyrus, both middle frontal gyrus, inferior frontal gyrus, and parietal cortex compared with HC. |
| DAE: no information available | ||||
| HC: N = 12, age = 35.8 ± 9 y; M:F = 5:7 | ||||
| Richter et al55 | CAT: N = 6, age = 41.6 ± 5.3 y; M:F = 1:5; education: information not available; CPZ: 198.3 ± 188.3 | NCRS, Rosebush Catatonia Scale, Lohr Catatonia Symptom Scale and the Northoff Self-estimation Scale | 3 Tesla MRT, KHOROS 2.1 with the extension KHORFU, task: emotional stimulation using the International Affective Picture System (IAPS) | CAT showed higher activity reduction in OFC, middle prefrontal cortex and premotor cortex after lorazepam application. |
| DAE: 6 ± 3.58 y | ||||
| HC: N = 6, no further information available | ||||
| Hirjak et al67 | CAT: N = 25, age = 39.1 ± 11.4 y; M:F = 14:11; education: 13.4 ± 2.3 y; OLZ: 17.4 ± 8.1; DAE: 13.6 ± 11.9 y | NCRS, Abnormal Involuntary Movement Scale, Simpson and Angus Scale, and Barnes Akathisia Rating Scale | 3 Tesla MRT, Freesurfer (cortical thickness, area, and gyrification) | CAT showed reduced cortical area in OFC and reduced LGI in temporal gyrus when compared with SP. CAT showed hypergyrification in OFC compared with SP. |
| SP: N = 22; age = 40.1 ± 11.8; M:F = 11:11; education: 13.3 ± 3.3 y; OLZ: 16.3 ± 10.5; DAE: 8.5 ± 9.5 y | ||||
| Hirjak et al59 | CAT: N = 24, age = 38.9 ± 11.6 y; M:F = 14:10; education: 13.4 ± 2.4 y; OLZ: 17.1 ± 8.18; DAE: 13.38 ± 12.15 y | NCRS, Abnormal Involuntary Movement Scale, Simpson and Angus Scale, and Barnes Akathisia Rating Scale | 3 Tesla MRT, SPM12, CAT12, data-driven fusion method called multiset canonical correlation analysis + joint independent component analysis (mCCA + jICA) | NCRS behavioral scores were associated with a joint structural and functional system that predominantly included cerebellar and prefrontal/cortical motor regions. NCRS affective scores were associated with frontoparietal intrinsic neural activity. |
| SP: N = 22; age = 40.1 ± 11.8; M:F = 11:11; education: 13.3 ± 3.3 y; OLZ: 16.3 ± 10.5; DAE: 8.5 ± 9.5 y |
CAT, catatonic patients; SP, schizophrenic patients/other mental disorders; HC, healthy controls; DOI, duration of illness; CPZ, chlorpromazine equivalent dose; OLZ, Olanzapine equivalent dose; OFC, orbitofrontal cortex; SMA, supplementary motor area; M, male; F, female; BFCRS, Bush-Francis Catatonia Rating Scale; NCRS, Northoff Catatonia Rating Scale; M, male; F, female; y, years; mo, months.
Discussion
This systematic review sought to contrast the findings from 19 neuroimaging studies using motor/behavioral catatonia rating scales/criteria and NCRS. Three main findings emerged: (1) To date, the vast majority of neuroimaging studies on catatonia investigated patients with SSD. (2) Neuroimaging studies using motor/behavioral catatonia rating scales/criteria have identified different cortico-subcortical brain circuits involving the dorsolateral prefrontal and cingulate cortex, insula, thalamus, precentral gyrus, supplementary motor area (SMA), and cerebellum as important neuronal substrates of catatonia in SSD. Biochemically, these cortical areas and their subcortical-cortical loops are mediated mainly by dopamine, which also has been found to be abnormal in SSD60,61 and parkinsonism62. (3) Neuroimaging studies using NCRS proposed a neurophysiological model of catatonia in SSD including rather right hemispheric neural network abnormalities that include the medial and lateral orbitofrontal (OFC), prefrontal, and posterior parietal cortex. Biochemically, these regions’ changes in catatonia are also associated with changes in GABAA receptors, as it has been shown in various studies.23,53,55
Neuroimaging Studies Using Motor/Behavioral Catatonia Rating Scales/Criteria
As early as 1985, Joseph et al45 examined 5 catatonic patients (4 patients had affective disorder and 1 patient had temporal lobe epilepsy) who showed atrophy of cerebellar vermis and brainstem when compared with psychiatric controls. Later, Wilcox found that significantly more catatonia patients (29%) showed cerebellar atrophy when compared with patients with noncatatonic schizophrenia, affective psychosis, and nonpsychiatric individuals (P < .05 in each category).44 In another study, Wilcox showed that patients with catatonic schizophrenia have significantly larger ventricular/brain ratios than patients with nonschizophrenic catatonia (P < .05).43 Northoff et al corroborated these CT studies by showing a relationship between catatonic syndrome and significant enlargements in almost all cerebrospinal fluid spaces, especially in the left frontotemporal area.42 These 4 CT studies42–45 were complemented by a number of SPECT studies in catatonic syndrome: Ebert et al52 showed abnormal regional cerebral blood flow (rCBF) in the limbic system and hippocampus of 2 patients with catatonic syndrome compared with 8 healthy and 10 psychiatric controls. The authors postulated that temporal hypoperfusion might be caused by the loss of sensory input in catatonia.52 Another study also focused on rCBF in 6 patients who currently or at an earlier time had received a DSM-III-R diagnosis of catatonic schizophrenia (eg, stupor or excitement, posturing, mannerisms, and negativism) using SPECT with 123I-iodoamphetamine (IMP).46 These patients showed reduced IMP perfusion in the dorsal part of the parietal lobe.46 Later on, similar rCBF alterations were found in one schizoaffective patient presenting with severe catatonic symptoms.51 This patient showed decreased rCBF in the frontal, posterior temporal, and parietal cortices, as well as in the motor cortex when compared with healthy controls.51 The authors concluded that hypoperfusion in frontoparietal regions might be associated with catatonic state rather than the diagnosis of catatonic schizophrenia.46
What all the studies mentioned above have in common is the fact that they could not identify any catatonia-specific alterations of the basal ganglia; most likely due to the inadequate imaging method. As suggested by earlier histopathological studies,63,64 dysfunction of subcortical structures such as basal ganglia, thalamus, cerebellum, and brainstem might lead to aberrant execution of learned motor plans.64 However, it took another 30 years for the first fMRI study to prove the involvement of subcortical regions in the pathogenesis of catatonia. Walther et al found that the catatonia is associated with functional connectivity between the left thalamus and bilateral M1.58 Furthermore, Walther et al also examined resting-state rCBF and gray matter in SSD patients with and without catatonia according to BFCRS.56 SSD patients with catatonia showed significant SMA and ventral premotor area hyperperfusion when compared with SSD patients without catatonia. Interestingly, the SMA perfusion differed between excited and retarded (hypokinetic) catatonia.56 In addition, there was an increased gray matter volume in the cerebellar region VIIb in catatonic patients.56 Furthermore, newer studies, which may have used more sensitive neuroimaging techniques, have also identified much more precise alterations in subcortical regions (eg, thalamus and cerebellum).56,58
On the one side, studies using motor/behavioral rating scales found altered structure as well as aberrant perfusion of motor regions in the parietal lobe. On the other side, differences in perfusion findings between earlier and newer rCBF studies in premotor and motor areas can be explained by different catatonic states (excited vs. retarded).56 This is also in line with a recent review on structure and neural mechanisms of catatonia conducted by Walther et al65 that conceptualized catatonia as a psychomotor syndrome based on disorder of cerebral motor network. In conclusion, the use of either DSM-based catatonia diagnosis or motor/behavioral rating scales/criteria primarily depicts brain regions within the cerebello-thalamo-motor circuit as neuronal substrates of catatonia, with the regions appearing to be affected differently depending on the symptoms’ quality.
Neuroimaging Studies Using NCRS
In one of the first studies, which used NCRS, 10 akinetic patients with catatonia, 10 psychiatric controls (similar diagnosis but without catatonia), and 20 healthy controls were investigated with SPECT 2 hours after injection of 123I-Iomazenil.48 Catatonic patients showed significantly lower Iomazenil binding with significant right-left alterations in the left sensorimotor cortex.48 Furthermore, there was a significantly reduced rCBF and significant right-left alterations in the right lower prefrontal and parietal cortex in catatonic patients. However, the specific cortical activation and connectivity patterns between frontal, parietal, and temporal cortical areas in catatonic patients were not examined until 2004. In 2004, Northoff et al examined 10 akinetic catatonic patients in a postacute state and compared them with 10 psychiatric controls without catatonia during an emotional-motor stimulation using fMRI.54 Catatonic patients showed aberrant OFC activation and functional connectivity to the premotor cortex in negative and positive emotions compared with healthy controls. Georg Northoff’s team succeeded in replicating these results in another patient sample consisting of 3 schizophrenia and 3 bipolar patients.55 The authors were also able to show that lorazepam induced higher signal decreases in OFC.55 This said, application of lorazepam can compensate for the dysfunction of the OFC and lead to better emotional regulation in catatonia. This observation may explain the effectiveness of lorazepam in treating catatonia, especially intense and uncontrollable anxieties and at the same time the so-called “scared stiffness”.66 Recent MRI study supported the concept that OFC alterations might lead to catatonia by showing reduced surface area in the right medial OFC and left superior parietal gyrus in catatonia according to NCRS.67 Furthermore, catatonic patients showed hypergyrification in the left medial OFC and rostral anterior cingulate cortex. This study illuminates the neurodevelopmental hypothesis of catatonia by showing an association between markers of early cortical development and catatonia in SSD.67 Another study suggested coaltered structure/function interactions in cerebellar, prefrontal/cortical motor regions, and frontoparietal areas underlying catatonia in SSD.59 Overall, multiple lines of evidence from above-mentioned rCBF and MRI studies suggest that alterations of the parietal cortex lead to disturbed motor attention and preparation of movements. The majority of catatonic patients are not aware of their motor symptoms whereas they might experience intense and overwhelming emotional experience.38
What may seem little surprising is the fact that the neuroimaging studies published so far did not identify an association between affective symptoms and dysfunctions of the limbic system (eg, hypothalamus, amygdala, mammillary body, hippocampus, etc.) typically involved in emotional processing. Nevertheless, we believe that the affective domain and its dysfunction play a major role in the pathogenesis of catatonia. There are important reasons that seem to support this notion: First, catatonia has been associated with limbic and temporal lobe lesions.68 Second, in numerous historical reports, intense affects such as fear and anxiety have been listed as triggers of absolute tonic immobility.69 Therefore, a subgroup of patients might become catatonic (motor, behavioral, and affective) in reaction to either an internal cognitive/mental event (like delusion, hallucination, or disturbing sadness) or external environmental event (like personally relevant life events or stressful environment the patients are living in). Still, we need further MRI studies on larger populations to investigate motor, behavioral, and affective phenotypes/clusters separately. Third, numerous studies have proven the administration of GABAA agonists (eg, lorazepam) as a relevant and often life-saving therapeutic option in catatonia.70 Strong D2 antagonists (eg, antipsychotics) might increase the GABAB activity and lead to the development of antipsychotic-induced catatonia.71 Catatonia might be caused by an abnormal balance between GABAA and GABAB receptors.72 Few authors reported that catatonia might be induced by benzodiazepine withdrawal as well.73,74 These findings show that anxiolytic medication in the sense of GABAA agonists can modulate catatonic affective symptoms. This justifies the observation that a massive affective dysregulation manifests itself as a physical-motor symptomatology either in the sense that the patients become akinetic or hyperkinetic. Both forms of catatonia can be traced back to the disbalance between GABA and dopaminergic transmission. This supports the notion that the affective domain is a central part of the complex pathomechanism of catatonia. Fourth, both previous and current MRI studies identified an association between catatonic affective symptoms and functional abnormalities in brain regions responsible for processing of negative stimuli. Northoff et al found decreased density of GABAA receptors in the left sensorimotor cortex in akinetic catatonia.48 More recently, Hirjak et al59 have shown that catatonic affective symptoms are associated with intrinsic neural activity in frontoparietal network, which consists of the superior, middle, medial, and inferior frontal cortices which also contain the OFC. The role of the OFC is complex, because of its manifold interconnections with amygdala, hippocampus, thalamus, cingulate/medial prefrontal, premotor, and parietal areas.54,59 Basically, OFC is responsible for decision making, mood regulation, and impulse control.37,75,76 In catatonia, aberrant OFC-prefrontal/parietal cortical connectivity reflects disrupted “horizontal modulation” of cortico-cortical relation, which might lead to disturbed affective/mood regulation (eg, fear), and consequently impulsive behavior and/or aggressiveness.54,77
Taken together, neuroimaging studies using NCRS have revealed more higher-order cortical changes within cortico-motor circuits than the neuroimaging studies using motor/behavioral catatonia rating scales did.2,23 The data suggest neural changes both in primary cognitive and affective regions, such as OFC and prefrontal cortex, and in parietal cortex—this renders catatonia truly psychomotor rather than merely motor. Therefore, neuroimaging studies using NCRS has provided scientific evidence for a truly psychomotor concept of catatonia from the perspective of symptoms, nosology, and neurobiology.
Dopamine vs GABA: Different Biochemical Substrates of Motor and Affective Symptoms
There is preliminary evidence that more severe catatonic signs in SSD patients (n = 1,095) are associated with the loss-of-function allele of a myelin-specific gene (copy number polymorphism [CNP] rs2070106-AA) (AA = individuals homozygous for the A allele).78 Interestingly, the same study found that healthy individuals carrying CNP rs2070106-AA showed white matter alterations in terms of higher axial diffusivity in frontotemporal and subcortical brain regions.78 The authors concluded that these MRI findings indicate low-grade inflammation in healthy AA individuals. Furthermore, this study showed that 2′-3′-cyclic nucleotide 3′-phosphodiesterase (Cnp) null mutant mice showed neuroinflammation associated with catatonic signs at age of 8 weeks. This finding determines the causality between reduced expression of Cnp (loss-of-function genotype), secondary low-grade inflammation/neurodegeneration leading to microgliosis and to catatonic signs (eg, bar test).78,79
Another study on mice could show that D2-receptor-deficient mouse show severe motor abnormalities such as aberrant locomotor-initiation and spontaneous catatonia.80 Furthermore, late-onset loss of D2-receptors leads to aberrant execution of previously learned movement sequences.80 These results once again underline the fundamental role of dopaminergic neurotransmission in motor skill learning and motor cortex synaptic plasticity.80,81 In addition, GABAergic neurotransmission plays an important role in catatonia as well. Earlier animal studies demonstrated induction and reversal of akinesia/posturing after injection of GABAA agonists into motor cortex.82–84 What conclusions can be drawn from basic animal research and neuroimaging studies in humans with regard to the pathogenesis of catatonia as a psychomotor syndrome?
First, the cognitive control of negative emotions is modulated by GABAA receptors in various prefrontal regions, such as the OFC and medial prefrontal cortex (PFC).85,86 Alterations of GABAergic neurotransmission/inhibition in the OFC and PFC together with hyperdopaminergic neurotransmission can lead to psychotic states and impaired processing of negative stimuli associated with the experience of anxiety.87–89 In line with this, catatonic patients showed increased levels of dopaminergic metabolites homovanillic and vanillic acid.90,91 Therefore, the administration of antipsychotics seems to be rather controversial because it can lead to drug-induced catatonia.92,93
Second, the disturbed emotional processing can lead to fluctuations in response inhibition due to activation/deactivation of the sensorimotor network comprising the inferior frontal gyrus, PFC, inferior parietal lobule, and pre-SMA. On the one side, lack of behavioral inhibition can lead to motor and behavioral catatonic symptoms such as mannerisms, echolalia/echopraxia, grimacing, perseveration, and compulsive behavior. On the other side, excessive motor inhibition might cause rigidity, akinesia, posturing, “Gegenhalten,” mutism, and stupor. In particular, switching between retarded and excited catatonia may be grounded in the hypoactivity and hyperactivity of dopaminergic transmission.94 The great clinical heterogeneity of catatonic symptoms can be explained by the constantly changing activity of different neurotransmitter systems and brain networks.72 In particular, the GABAergic, glutamatergic (in particular the NMDA receptors) and serotonergic systems (ie, upregulation of 5-HT1a and downregulation of 5-HT2a receptors95) may be involved in catatonia. This assumption is supported by the good efficacy of benzodiazepines modulating GABAergic system and clozapine because of its broad receptor profile.96 Benzodiazepines induce anxiolysis through positive allosteric modulation at the GABAA receptor in prefrontal areas and might help to release the affective symptoms.70 Furthermore, administration of a GABAA receptor agonist lorazepam might directly ameliorate GABAergic deficits in premotor and primary motor cortices (M1).23,41,97 Because GABA is one of the main inhibitory neurotransmitter within the M1, the application of lorazepam or electroconvulsive therapy (ECT) might increase the excitability of GABA-related inhibitory circuits in the motor cortex.66,80,97 This assumption was also supported by a case report that showed a successful amelioration of catatonic symptoms following ECT and at the same time an increase of intracortical inhibition (ICI) (possibly due to an increases in GABAergic transmission) as determined by double-pulse transcranial magnetic stimulation.98 This said, the application of lorazepam or ECT can balance the ICI of the partially overactivated motor regions—as we can observe in catatonia56,58 or dystonia99—and normalize motor and behavioral disturbances.100 From an immunological perspective, GABAergic modulation can also suppress the response of the immune system by reduction of proinflammatory cytokines and often help to relieve an infection-induced (viral meningitis or encephalitis, syphilis, systemic bacterial infection, etc.) catatonic syndrome.27,101 Furthermore, the glutamatergic hypofunction (often caused by N-methyl-d-aspartate receptor encephalitis) might also lead to (autoimmune) catatonia.27 This said, catatonia can be caused by an imbalance of excitatory glutamatergic neurons and by inhibitory GABAergic neurons.27,102
In sum, supported on biochemical grounds, motor symptoms in catatonia seem to be modulated by changes in dopamine, whereas affective symptoms may be mainly associated with GABA and glutamate changes. This is supported by the outstanding therapeutic effects of GABAergic (lorazepam) and glutamatergic (amantadine)103 agents on affective and motor catatonic symptoms.104 We thus conceive catatonia as truly psychomotor syndrome that combines both GABAergic and glutamatergic higher-order cortical and dopaminergic motor subcortical-cortical changes in a unique way.48,54,55
Limitations
Several limitations apply to the body of neuroimaging research on catatonia and thus also to the present systematic review. Among the main methodological obstacles are ethical aspects of catatonia, dispersion of study samples, missing consensus on the catatonia definition, overlapping patients’ samples, and a wealth of different (partially older and less accurate) neuroimaging techniques, respectively. For instance, the MRI scanning of patients with severe catatonic symptoms is only rarely acceptable from an ethical point of view. Another limitation is the limited availability of patients with catatonic subtype of schizophrenia. Therefore, we strongly acknowledge a paradigm shift when investigating the pathomechanisms of catatonia, ie, from a restricted motor–behavioral view to a broader psychomotor framing that focuses on the interaction between affective, motor, and behavioral features. Future studies should focus on the assessment of catatonic symptoms using appropriate scales, neuroimaging, genetic and biochemical methods in all mental disorders. Another limitation of our review may be the exclusion of clinical trials on the treatment of catatonia because a number of different rating scales yielded similar results across different cohorts of patients, questioning the specificity of affective domain. However, the present systematic review examined whether the putative pathomechanisms of catatonic syndrome depends on the clinical rating scale used.
Concluding Remarks
For about 30 years, scientific research on motor abnormalities in general and the catatonic symptoms in particular is experiencing a renaissance. A number of authors is going back to the origin of catatonia emphasizing its psychomotor nature (increasingly including affective symptoms) in a truly literal sense as it was understood by Kahlbaum and formalized 20 years ago in terms of a rating scale by Northoff.3,40,105 For the majority of catatonia rating scales, high inter-rater reliability (correlation coefficient or Cohen’s kappa) was estimated: BCRS35 (r > .83), RCS30 (r = .81), MRS31,32 (Cohen’s kappa = 0.87), BFCRS33,34 (r = .93), and NCRS (r = .80–.96) (for further details, see Sienaert et al106). However, only Northoff et al3 reported on the association between NCRS and Rosebush criteria,29 BFCRS, and MRS (all P < .0001; r = .72–.88). Although these scales define no severity criteria of catatonia,106 Northoff et al3 were able to demonstrate that NCRS total score of >7 separates patients with from patients without catatonia with a sensitivity and specificity of 100%.
Although the BFCRS have fewer items (n = 23), it includes some symptoms that NCRS classifies as “affective” (eg, combativeness, impulsivity, excitement, staring, and ambitendency) as well. Accordingly, there is an overlap between the rating scales in terms of catatonic symptoms, even though some are not defined the same way or named the same way. The different terminologies may have contributed to the different results of the MRI studies. In the future, the clinically relevant, but somehow artificial differentiation between the individual symptom domains and the different catatonia rating scales require a standardization based on empirical evidence and consensus of international scholars to capture affective, motor and behavioral catatonic symptoms of an individual patient as one dimension. Such an initiative would make it possible to better compare the findings of individual studies and achieve further meta-analytical evidence on both neurobiology and treatment of catatonia.
To date, however, the majority of studies examined patients suffering from SSD with little attention being paid to the other mental disorders.48,54 Therefore, we strongly advocate transnosologic neuroimaging studies on catatonia that accurately map the neurobiological link between hypokinetic and hyperkinetic motor symptoms, behavioral anomalies, and affective alterations as emphasized by Kahlbaum. Taken in such transnosologic way, the catatonic syndrome can be conceived a paradigmatic example of the recently introduced RDoC-nosological characterization that emphasizes syndromes over categories. Future transdiagnostic MRI studies that examine catatonic symptoms need to show that if the criteria of catatonia are met, there are clearly delineated structural and functional brain abnormalities underlying catatonic symptoms. This was also the aim of recent 2 MRI studies59,67 in which we did not compare patients with healthy volunteers but SSD patients with and without catatonia. A transnosologic approach will decisively help to distinguish catatonia from SSD as well as other mental disorders and establish catatonia as an independent diagnostic entity.
Nevertheless, in addition to numerous differences between the individual studies, there are also many overlaps between the identified brain regions. What became evident in all mentioned studies is that regions of the motor system are—regardless the diagnosis—important neuronal substrates of catatonia. It may be that all studies—independent of the methodology—represent the final stage of a catatonic development, ie, they might point to a motor system deficit as a consequence of aberrant affective/mood regulation (eg, fear).
Moreover, catatonia paradigmatically exemplifies that no symptom domains occurs in isolation. The clinical, nosological, and neurobiological studies reviewed here and Kahlbaum’s original description clearly demonstrate that catatonia is not a purely a motor disorder but a truly psychomotor syndrome in the literal sense of the terms, eg, “psycho” and “motor” that combines GABAergic and glutamatergic higher-order cortical and dopaminergic motor subcortical-cortical changes. This applies not only to SSD, but also to other psychiatric disorders. For instance, depression and mania are not purely affective syndromes as they include strong cognitive and psychomotor changes, a constellation that can be traced to the relationship between the brain’s different networks.107,108 Finally, the psychomotor rather than motor characterization of catatonia carries important clinical implications for diagnosis and therapy. Diagnostically, we need to search for constellations of motor, behavioral, and affective symptoms rather than just for the presence of single motor and/or behavioral symptoms something which is often overlooked in clinical practice. Therapeutically, if lorazepam fails, one may want to apply and use drugs that target affective symptoms such as ketamine or the somewhat analogous drug amantadine, as probed earlier in catatonia.109,110
In conclusion, we demonstrate here that it is sometimes worth getting back to the historical origins as, in our case, Kahlbaum’s truly transnosologic and literally psychomotor concept of catatonia. After the loss of its psychomotor and transnosologic features in the 20th century, we can now come back to Kahlbaum’s original description of catatonia by supporting and extending it using our novel imaging, genetic, and pharmacological tools in humans and mice. This, as we indicated, carries major neuroscientific and clinical implications, which ultimately will lead us to better diagnosis and therapy of catatonia in all its complexity.
Funding
This work was supported by the German Research Foundation (DFG) (grant number DFG HI 1928/2-1 to D.H. and WO 1883/6-1 to R.C.W.). The DFG had no further role in the study design; collection, analysis and interpretation of data; writing of the report; and in the decision to submit the paper for publication. GN is grateful for financial support from PSI and CIHR in Canada.
Supplementary Material
Acknowledgments
The authors thank Frank Geisler for his support in creating figure 2. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kahlbaum K. The clinico-diagnostic perspective in psychopathology. 1878. Hist Psychiatry. 2007;18(70 Pt 2):233–245. [DOI] [PubMed] [Google Scholar]
- 2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin, Germany: Verlag August Hirschwald; 1874. [Google Scholar]
- 3. Northoff G, Koch A, Wenke J, et al. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14(3):404–416. [DOI] [PubMed] [Google Scholar]
- 4. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;127(441):1–47. [DOI] [PubMed] [Google Scholar]
- 5. Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010;36(2):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Francis A, Fink M, Appiani F, et al. Catatonia in diagnostic and statistical manual of mental disorders, fifth edition. J ECT. 2010;26(4):246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1–3):256–262. [DOI] [PubMed] [Google Scholar]
- 8. Kirby GH. The catatonic syndrome and its relation to manic-depressive insanity. J Nerv Ment Dis. 1913;40:694–704. [Google Scholar]
- 9. Lange J. Katatonische Erscheinungen im Rahmen Manisch-Depressiver Erkrankungen. Berlin, Germany: Springer; 1922. [Google Scholar]
- 10. Kreapelin E. Psychiatrie. Ein Lehrbuch für Studierende und Ärzte. Vol 6. Auflage (2 Bde). Leipzig, Germany: Barth; 1899. [Google Scholar]
- 11. Berrios GE, Markova IS. Historical and conceptual aspects of motor disorders in the psychoses. Schizophr Res. 2018 Oct;200:5-11. doi: 10.1016/j.schres.2017.09.008. Epub 2017 Sep 21. [DOI] [PubMed] [Google Scholar]
- 12. Ungvari GS, Caroff SN, Gerevich J. The catatonia conundrum: evidence of psychomotor phenomena as a symptom dimension in psychotic disorders. Schizophr Bull. 2010;36(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bleuler E. Dementia praecox oder Gruppe der Schizophrenien. In: Aschaffenburg G, ed. Handbuch der Psychiatrie (Spezieller Teil, 4. Abteilung, 1. Hälfte). Leipzig, Germany: Deuticke; 1911. [Google Scholar]
- 14. Krüger S, Bräunig P. Catatonia in affective disorder: new findings and a review of the literature. CNS Spectr. 2000;5(7):48–53. [DOI] [PubMed] [Google Scholar]
- 15. Abrams R, Taylor MA, Coleman Stolurow KA. Catatonia and mania: patterns of cerebral dysfunction. Biol Psychiatry. 1979;14(1):111–117. [PubMed] [Google Scholar]
- 16. Taylor MA, Abrams R. Catatonia. Prevalence and importance in the manic phase of manic-depressive illness. Arch Gen Psychiatry. 1977;34(10):1223–1225. [DOI] [PubMed] [Google Scholar]
- 17. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):I8–13. [DOI] [PubMed] [Google Scholar]
- 18. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875–1876. [DOI] [PubMed] [Google Scholar]
- 19. Kirby G. The catatonic syndrome and its relation to manic-depressive insanity. J Nerv Ment Dis. 1913;40:694–704. [Google Scholar]
- 20. Heckers S, Tandon R, Bustillo J. Catatonia in the DSM – shall we move or not? Schizophr Bull. 2010;36(2):205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26–30. [DOI] [PubMed] [Google Scholar]
- 22. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull. 2017. doi: 10.1093/schbul/sbx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Northoff G. Brain imaging in catatonia: current findings and a pathophysiologic model. CNS Spectr. 2000;5(7):34–46. [DOI] [PubMed] [Google Scholar]
- 24. Ungvari GS. Catatonia in DSM 5: controversies regarding its psychopathology, clinical presentation and treatment response. Neuropsychopharmacol Hung. 2014;16(4):189–194. [PubMed] [Google Scholar]
- 25. Hefter D, Topor CE, Gass P, Hirjak D. Two sides of the same coin: a case report of first-episode catatonic syndrome in a high-functioning autism patient. Front Psychiatry. 2019;10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doran E, Sheehan JD. Acute catatonia on medical wards: a case series. J Med Case Rep. 2018;12(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rogers JP, Pollak TA, Blackman G, David AS. Catatonia and the immune system: a review. Lancet Psychiatry. 2019. Jul;6(7):620-630. doi: 10.1016/S2215-0366(19)30190-7. Epub 2019 Jun 10. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reed GM, First MB, Kogan CS, et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry. 2019;18(1):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry. 1990;51(9):357–362. [PubMed] [Google Scholar]
- 30. Rogers D. Catatonia: a contemporary approach. J Neuropsychiatry Clin Neurosci. 1991;3(3):334–340. [DOI] [PubMed] [Google Scholar]
- 31. Lund CE, Mortimer AM, Rogers D, McKenna PJ. Motor, volitional and behavioural disorders in schizophrenia. 1: Assessment using the Modified Rogers Scale. Br J Psychiatry. Mar 1991;158:323–327, 333-326. [DOI] [PubMed] [Google Scholar]
- 32. McKenna PJ, Lund CE, Mortimer AM, Biggins CA. Motor, volitional and behavioural disorders in schizophrenia. 2: The ‘conflict of paradigms’ hypothesis. Br J Psychiatry. 1991;158:328–336. [DOI] [PubMed] [Google Scholar]
- 33. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137–143. [DOI] [PubMed] [Google Scholar]
- 34. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. [DOI] [PubMed] [Google Scholar]
- 35. Bräunig P, Krüger S, Shugar G, Höffler J, Börner I. The catatonia rating scale I – development, reliability, and use. Compr Psychiatry. 2000;41(2):147–158. [DOI] [PubMed] [Google Scholar]
- 36. Carroll BT, Kirkhart R, Ahuja N, et al. Katatonia: a new conceptual understanding of catatonia and a new rating scale. Psychiatry (Edgmont). 2008;5(12):42–50. [PMC free article] [PubMed] [Google Scholar]
- 37. Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. [DOI] [PubMed] [Google Scholar]
- 38. Northoff G, Krill W, Eckert J, Russ M, Pflug B. Major differences in subjective experience of akinetic states in catatonic and parkinsonian patients. Cognit Neuropsychiatry. 1998;3:161–178. [Google Scholar]
- 39. Starkstein SE, Petracca G, Tesón A, et al. Catatonia in depression: prevalence, clinical correlates, and validation of a scale. J Neurol Neurosurg Psychiatry. 1996;60(3):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Northoff G. Katatonie. Einführung in die Phänomenologie, Klinik und Pathophysiologie eines psychomotorischen Syndroms. Stuttgart, Germany: Enke Verlag; 1997. [Google Scholar]
- 41. Northoff G, Wenke J, Demisch L, Eckert J, Gille B, Pflug B. Catatonia: short-term response to lorazepam and dopaminergic metabolism. Psychopharmacology (Berl). 1995;122(2):182–186. [DOI] [PubMed] [Google Scholar]
- 42. Northoff G, Waters H, Mooren I, et al. Cortical sulcal enlargement in catatonic schizophrenia: a planimetric CT study. Psychiatry Res. 1999;91(1):45–54. [DOI] [PubMed] [Google Scholar]
- 43. Wilcox JA. Structural brain abnormalities in catatonia. Neuropsychobiology. 1993;27(2):61–64. [DOI] [PubMed] [Google Scholar]
- 44. Wilcox JA. Cerebellar atrophy and catatonia. Biol Psychiatry. 1991;29(7):733–734. [DOI] [PubMed] [Google Scholar]
- 45. Joseph AB, Anderson WH, O’Leary DH. Brainstem and vermis atrophy in catatonia. Am J Psychiatry. 1985;142(3):352–354. [DOI] [PubMed] [Google Scholar]
- 46. Satoh K, Suzuki T, Narita M, et al. Regional cerebral blood flow in catatonic schizophrenia. Psychiatry Res. 1993;50(4):203–216. [DOI] [PubMed] [Google Scholar]
- 47. Northoff G, Steinke R, Nagel DCzerwenka C, et al. Right lower prefronto-parietal cortical dysfunction in akinetic catatonia: a combined study of neuropsychology and regional cerebral blood flow. Psychol Med. 2000;30(3):583–596. [DOI] [PubMed] [Google Scholar]
- 48. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Escobar R, Rios A, Montoya ID, et al. Clinical and cerebral blood flow changes in catatonic patients treated with ECT. J Psychosom Res. 2000;49(6):423–429. [DOI] [PubMed] [Google Scholar]
- 50. De Tiége X, Bier JC, Massat I, et al. Regional cerebral glucose metabolism in akinetic catatonia and after remission. J Neurol Neurosurg Psychiatry. 2003;74(7):1003–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galynker II, Weiss J, Ongseng F, Finestone H. ECT treatment and cerebral perfusion in Catatonia. J Nucl Med. 1997;38(2):251–254. [PubMed] [Google Scholar]
- 52. Ebert D, Feistel H, Kaschka W. Left temporal hypoperfusion in catatonic syndromes: a SPECT study. Psychiatry Res. 1992;45(4):239–241. [DOI] [PubMed] [Google Scholar]
- 53. Northoff G, Braus DF, Sartorius A, et al. Reduced activation and altered laterality in two neuroleptic-naive catatonic patients during a motor task in functional MRI. Psychol Med. 1999;29(4):997–1002. [DOI] [PubMed] [Google Scholar]
- 54. Northoff G, Kötter R, Baumgart F, et al. Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull. 2004;30(2):405–427. [DOI] [PubMed] [Google Scholar]
- 55. Richter A, Grimm S, Northoff G. Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol. 2010;25(1):55–62. [DOI] [PubMed] [Google Scholar]
- 56. Walther S, Schäppi L, Federspiel A, et al. Resting-state hyperperfusion of the supplementary motor area in Catatonia. Schizophr Bull. 2017;43(5):972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scheuerecker J, Ufer S, Käpernick M, et al. Cerebral network deficits in post-acute catatonic schizophrenic patients measured by fMRI. J Psychiatr Res. 2009;43(6):607–614. [DOI] [PubMed] [Google Scholar]
- 58. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hirjak D, Rashidi M, Kubera KM, et al. Multimodal MRI data fusion reveals distinct patterns of abnormal brain structure and function in catatonia. Schizophr Bull. 2019. Jun 8. pii: sbz042. doi: 10.1093/schbul/sbz042. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Joyce JN, Gurevich EV. D3 receptors and the actions of neuroleptics in the ventral striatopallidal system of schizophrenics. Ann N Y Acad Sci. 1999;877:595–613. [DOI] [PubMed] [Google Scholar]
- 61. Baumann B, Bogerts B. The pathomorphology of schizophrenia and mood disorders: similarities and differences. Schizophr Res. 1999;39(2):141–148; discussion 162. [DOI] [PubMed] [Google Scholar]
- 62. Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17(1):51–72. [DOI] [PubMed] [Google Scholar]
- 63. Stevens JR. Neuropathology of schizophrenia. Arch Gen Psychiatry. 1982;39(10):1131–1139. [DOI] [PubMed] [Google Scholar]
- 64. Taylor MA. Catatonia: a review of a behavioral neurologic syndrome. Neuropsychiatry Neuropsychol Behav Neurol. 1990;3(1):48–72. [Google Scholar]
- 65. Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019. Jul;6(7):610-619. doi: 10.1016/S2215-0366(18)30474-7. Epub 2019 Jun 10. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moskowitz AK. “Scared stiff”: catatonia as an evolutionary-based fear response. Psychol Rev. 2004;111(4):984–1002. [DOI] [PubMed] [Google Scholar]
- 67. Hirjak D, Kubera KM, Northoff G, Fritze S, Bertolino AL, Topor CE, Schmitgen MM, Wolf RC. Cortical contributions to distinct symptom dimensions of Catatonia. Schizophr Bull. 2019. Feb 7. doi: 10.1093/schbul/sby192. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ahuja N. Organic catatonia: a review. Indian J Psychiatry. 2000;42(4):327–346. [PMC free article] [PubMed] [Google Scholar]
- 69. Shorter E, Fink M.. The Madness of Fear: A History of Catatonia. New York, NY: Oxford University Press; 2018. [Google Scholar]
- 70. Ungvari GS, Leung CM, Wong MK, Lau J. Benzodiazepines in the treatment of catatonic syndrome. Acta Psychiatr Scand. 1994;89(4):285–288. [DOI] [PubMed] [Google Scholar]
- 71. Graham KT, Carroll BT. Dopamine in catatonia. J Clin Psychopharmacol. 2001;21(6):624–625. [DOI] [PubMed] [Google Scholar]
- 72. Carroll BT, Thomas C, Tugrul KC, Coconcea C, Goforth HW. GABA(A) versus GABA(B) in catatonia. J Neuropsychiatry Clin Neurosci. 2007;19(4):484. [DOI] [PubMed] [Google Scholar]
- 73. Rosebush PI, Mazurek MF. Catatonia after benzodiazepine withdrawal. J Clin Psychopharmacol. 1996;16(4):315–319. [DOI] [PubMed] [Google Scholar]
- 74. Deuschle M, Lederbogen F. Benzodiazepine withdrawal-induced catatonia. Pharmacopsychiatry. 2001;34(1):41–42. [DOI] [PubMed] [Google Scholar]
- 75. Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55(1):30–40. [DOI] [PubMed] [Google Scholar]
- 76. Bellani M, Cerruti S, Brambilla P. Orbitofrontal cortex abnormalities in schizophrenia. Epidemiol Psichiatr Soc. 2010;19(1):23–25. [PubMed] [Google Scholar]
- 77. Gansler DA, McLaughlin NC, Iguchi L, et al. A multivariate approach to aggression and the orbital frontal cortex in psychiatric patients. Psychiatry Res. 2009;171(3):145–154. [DOI] [PubMed] [Google Scholar]
- 78. Janova H, Arinrad S, Balmuth E, et al. Microglia ablation alleviates myelin-associated catatonic signs in mice. J Clin Invest. 2018;128(2):734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hagemeyer N, Goebbels S, Papiol S, et al. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med. 2012;4(6):528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bello EP, Casas-Cordero R, Galiñanes GL, et al. Inducible ablation of dopamine D2 receptors in adult mice impairs locomotion, motor skill learning and leads to severe parkinsonism. Mol Psychiatry. 2017;22(4):595–604. [DOI] [PubMed] [Google Scholar]
- 81. Rioult-Pedotti MS, Pekanovic A, Atiemo CO, Marshall J, Luft AR. Dopamine promotes motor cortex plasticity and motor skill learning via PLC activation. PLoS One. 2015;10(5):e0124986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hikosaka O, Tanaka M, Sakamoto M, Iwamura Y. Deficits in manipulative behaviors induced by local injections of muscimol in the first somatosensory cortex of the conscious monkey. Brain Res. 1985;325(1–2):375–380. [DOI] [PubMed] [Google Scholar]
- 83. Kurata K, Hoffman DS. Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol. 1994;71(3):1151–1164. [DOI] [PubMed] [Google Scholar]
- 84. Kubota K. Motor cortical muscimol injection disrupts forelimb movement in freely moving monkeys. Neuroreport. 1996;7(14):2379–2384. [DOI] [PubMed] [Google Scholar]
- 85. Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. [DOI] [PubMed] [Google Scholar]
- 87. Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. [DOI] [PubMed] [Google Scholar]
- 88. Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12(9):1016–1022. [DOI] [PubMed] [Google Scholar]
- 89. Tanaka S. Dysfunctional GABAergic inhibition in the prefrontal cortex leading to “psychotic” hyperactivation. BMC Neurosci. 2008;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gjessing LR. Catecholamines and antipsychotic drugs in periodic catatonia. J Psychiatr Res. 1974;11:71. [DOI] [PubMed] [Google Scholar]
- 91. Northoff G, Demisch L, Wenke J, Pflug B. Plasma homovanillic acid concentrations in catatonia. Biol Psychiatry. 1996;39(6):436–443. [DOI] [PubMed] [Google Scholar]
- 92. Duggal HS, Singh I. Drug-induced catatonia. Drugs Today (Barc). 2005;41(9):599–607. [DOI] [PubMed] [Google Scholar]
- 93. Herrmann N, Lieff SJ. Drug-induced catatonia. Can J Psychiatry. 1988;33(7):633–634. [DOI] [PubMed] [Google Scholar]
- 94. Koch A, Reich K, Wielopolski J, et al. Catatonic dilemma in a 33-year-old woman: a discussion. Case Rep Psychiatry. 2013;2013:542303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5(7):26–33. [DOI] [PubMed] [Google Scholar]
- 96.Lander M, Bastiampillai T, Sareen J. Review of withdrawal catatonia: what does this reveal about clozapine? Transl Psychiatry 2018 Jul 31;8(1):139. doi: 10.1038/s41398-018-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Di Lazzaro V, Oliviero A, Meglio M, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111(5):794–799. [DOI] [PubMed] [Google Scholar]
- 98. Dresler T, Giani AS, Reinsberger C, Scheuerpflug P, Stöber G, Fallgatter AJ. Electroconvulsive therapy resolves cortical inhibition and manneristic omissions in a chronic catatonic patient. J Neural Transm (Vienna). 2010;117(10):1209–1212. [DOI] [PubMed] [Google Scholar]
- 99. Lizarraga KJ, Gorgulho A, Chen W, De Salles AA. Molecular imaging of movement disorders. World J Radiol. 2016;8(3):226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mooney RA, Cirillo J, Byblow WD. GABA and primary motor cortex inhibition in young and older adults: a multimodal reliability study. J Neurophysiol. 2017;118(1):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Prud’homme GJ, Glinka Y, Wang Q. Immunological GABAergic interactions and therapeutic applications in autoimmune diseases. Autoimmun Rev. 2015;14(11):1048–1056. [DOI] [PubMed] [Google Scholar]
- 102. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555–577; discussion 578–604. [DOI] [PubMed] [Google Scholar]
- 103. Hervey WM, Stewart JT, Catalano G. Treatment of catatonia with amantadine. Clin Neuropharmacol. 2012;35(2):86–87. [DOI] [PubMed] [Google Scholar]
- 104. Taylor SF, Grove TB, Ellingrod VL, Tso IF. The fragile brain: stress vulnerability, negative affect and GABAergic neurocircuits in psychosis. Schizophr Bull. 2019 May 31. pii: sbz046. doi: 10.1093/schbul/sbz046. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Oldham M. A diversified theory of catatonia. Lancet Psychiatry. 2019 Jul;6(7):554-555. doi: 10.1016/S2215-0366(19)30212-3. Epub 2019 Jun 10. [DOI] [PubMed] [Google Scholar]
- 106. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1–3):1–9. [DOI] [PubMed] [Google Scholar]
- 107. Northoff G, Magioncalda P, Martino M, Lee HC, Tseng YC, Lane T. Too fast or too slow? time and neuronal variability in bipolar disorder-a combined theoretical and empirical investigation. Schizophr Bull. 2018;44(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Martino M, Magioncalda P, Yu H, et al. Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naïve patients with schizophrenia. Schizophr Bull. 2018;44(2):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997;62(4):404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Northoff G, Lins H, Böker H, Danos P, Bogerts B. Therapeutic efficacy of N-methyl D-aspartate antagonist amantadine in febrile catatonia. J Clin Psychopharmacol. 1999;19(5):484–486. [DOI] [PubMed] [Google Scholar]
- 111. Satoh K, Narita M, Someya T, Fukuyama H, Yonekura Y. Functional brain imaging of a catatonic type of schizophrenia: PET and SPECT studies. Jpn J Psychiatry Neurol. 1993;47(4):881–885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.