Abstract
Background
Evidence from anatomical, pharmacological, and genetic studies supports a role for the neuropeptide melanin concentrating hormone system in modulating emotional and cognitive functions. Genome-wide association studies revealed a potential association between the melanin concentrating hormone receptor (MCHR1) gene locus and schizophrenia, and the largest genome-wide association study conducted to date shows a credible genome-wide association.
Methods
We analyzed MCHR1 and pro-melanin concentrating hormone RNA-Seq expression in the prefrontal cortex in schizophrenia patients and healthy controls. Disruptions in the melanin concentrating hormone system were modeled in the mouse brain by germline deletion of MCHR1 and by conditional ablation of melanin concentrating hormone expressing neurons using a Cre-inducible diphtheria toxin system.
Results
MCHR1 expression is decreased in the prefrontal cortex of schizophrenia samples (false discovery rate (FDR) P < .05, CommonMind and PsychEncode combined datasets, n = 901) while pro-melanin concentrating hormone is below the detection threshold. MCHR1 expression decreased with aging (P = 6.6E-57) in human dorsolateral prefrontal cortex. The deletion of MCHR1 was found to lead to behavioral abnormalities mimicking schizophrenia-like phenotypes: hyperactivity, increased stereotypic and repetitive behavior, social impairment, impaired sensorimotor gating, and disrupted cognitive functions. Conditional ablation of pro-melanin concentrating hormone neurons increased repetitive behavior and produced a deficit in sensorimotor gating.
Conclusions
Our study indicates that early disruption of the melanin concentrating hormone system interferes with neurodevelopmental processes, which may contribute to the pathogenesis of schizophrenia. Further neurobiological research on the developmental timing and circuits that are affected by melanin concentrating hormone may lead to a therapeutic target for early prevention of schizophrenia.
Significance Statement.
This study is the first to our knowledge to connect the downregulation of MCHR1 mRNA in the brains of schizophrenia patients to a causal link between the germline MCHR1 deletion in mice and social deficits as well as deficits in sensorimotor gating and multiple cognitive tasks that resemble schizophrenia symptoms.
Here we show that MCHR1 mRNA levels are significantly lower in the prefrontal cortex of subjects with schizophrenia using a large sample of 901 subjects. There is a widespread cortical expression of MCHR1, increasing after birth and then showing a strong decrease of expression with aging. There is also a decreased expression of MCHR1 in the PFC from female compared with male brains. In human brain we showed single nuclei coexpression of SST and MCHR1 were enriched above sparse expression in 90% of nuclei examined.
We also showed that disruptions in the MCH system in the mouse brain by complete deletion of MCHR1 and by conditional ablation of MCH expressing neurons using a Cre-inducible diphtheria toxin (iDTR) system led to behavioral abnormalities mimicking schizophrenia-like phenotypes. Our study indicates that the disruption of the MCH system interferes with neurodevelopmental processes, which may contribute to the pathogenesis of schizophrenia, suggesting the MCH as a therapeutic target for early prevention and/or treatment of schizophrenia.
Background
The hypothalamic neuropeptide melanin-concentrating hormone (MCH) is a cyclic peptide originally discovered in fish to induce paling of the skin. In mammals, MCH is expressed almost exclusively in the lateral hypothalamus (LH) and zona incerta (ZI) but projects throughout the central nervous system (Bittencourt et al., 1992), indicating that it has a broad range of functions. The MCH system has been implicated as a regulator of energy homeostasis and food intake (Qu et al., 1996; Rossi et al., 1997) but also of sleep, stress, mood, aggression, reward, and cognition (Roy et al., 2006, 2007; Chung et al., 2009, 2011; Blouin and Siegel, 2013; Fraigne and Peever, 2013; Takase et al., 2014).
MCH exerts its action through interacting with 1 G protein-coupled receptor, MCHR1 (Bachner et al., 1999; Chambers et al., 1999; Saito et al., 1999, 2000; Shimomura et al., 1999), which has a widespread distribution in the brain. The abundance of MCHR1 in the frontal cortex, amygdala, nucleus accumbens, septum, and hippocampus (Knigge et al., 1996; Saito et al., 2001) provides an anatomical basis for an MCH role in the modulation of emotional and cognitive functions (Adamantidis et al., 2005). MCHR1 sites in the hippocampus, which controls spatial memory, have been shown to modulate cellular mechanisms underlying learning, such as N-methyl-D-aspartate receptor (NMDA) receptor-dependent production of nitric oxide (Varas et al., 2002b) and facilitation of synaptic transmission (Monzon et al., 2001; Varas et al., 2002a). MCH was shown to have a facilitatory effect on CA1 glutamatergic synaptic transmission and long-term synaptic plasticity (Pachoud et al., 2010). This effect was supported by the fact that the long-term synaptic depression and long-term synaptic potentiation, 2 cellular models of learning and memory, are strongly impaired in MCHR1 knockout mice (Pachoud et al., 2010). Also, AMPA and NMDA receptors are downregulated in the mutant mice compared with their controls (Adamantidis et al., 2005; Pachoud et al., 2010; Sears et al., 2010).
The largest genome-wide association study (GWAS) to date of schizophrenia (SZ) has shown a credible locus harboring the MCHR1 gene on chr:22q13.2 (Pardinas et al., 2018). Candidate gene studies have revealed nominal associations between single nucleotide polymorphisms (SNPs) located in the MCHR1 gene and schizophrenia (Severinsen et al., 2006; Demontis et al., 2012). There are no reported associations of SZ and PMCH to our knowledge.
Human genetic association studies and the findings of animal anatomical, physiological, and pharmacological studies led us to hypothesize a possible causal effect of MCH system dysfunction in the development of schizophrenia. Therefore, the primary aims of this study were to determine whether MCHR1 and PMCH mRNA levels are altered in the brains of schizophrenia patients and to investigate whether the germline deletion of the MCH receptor (MCHR1 KO) or the conditional ablation of MCH neurons (MCH cKO) produces behavioral responses in mice that mimic symptoms of schizophrenia.
Methods
Human Study of MCH System Expression
PsychEncode and CommonMind Studies
PsychEncode (Brain-GVEX study) and CommonMind RNA-Seq datasets were jointly analyzed using data directly downloaded from Synapse.org (Project PI, MPV). Only controls and patients diagnosed with SZ were included, resulting in a combined total of 901 dorsolateral prefrontal cortex samples (544 controls, 357 SZ) (Table 1). The RNA-Seq data were processed by TruSeq adapters removed using cutadapt, transcript abundances were quantified using Salmon (Patro et al., 2017) and the number of reads summarized at the gene level using Gencode version 29. Genes were required to be expressed above a threshold of 5 counts per million (CPM) in at least 10 patients, resulting in 18 595 genes for the combined dataset. Analysis of differential gene expression was performed using limma/voom by fitting a weighted least squares model to log-transformed CPM values (Law et al., 2014). In addition to psychiatric diagnosis, the covariates included in the model were age (binned at ≥90), sex, postmortem interval, RNA Integrity Number, and brain bank. P values were based on a moderated t statistic, and false discovery rates for multiple testing were calculated using the Benjamini-Hochberg method.
Table 1.
The analysis of MCHR1 in the combined dataset from PsychEncode and CommonMind included a total of 901 subjects
| Control | 544 |
| F | 213 |
| CMC MSSM | 79 |
| CMC Penn | 19 |
| CMC Pitt | 24 |
| PENC BSHRI | 76 |
| PEC SMRI Array | 9 |
| PEC SMRI Consortium | 5 |
| PEC SMRI Extra | 1 |
| M | 331 |
| CMC MSSM | 85 |
| CMC Penn | 18 |
| CMC Pitt | 60 |
| PENC BSHRI | 108 |
| PEC SMRI Array | 25 |
| PEC SMRI Consortium | 8 |
| PEC SMRI Extra | 8 |
| PEC SMRI New | 19 |
| SCZ | 357 |
| F | 122 |
| CMC MSSM | 47 |
| CMC Penn | 35 |
| CMC Pitt | 13 |
| PEC SMRI Array | 8 |
| PEC SMRI Consortium | 3 |
| PEC SMRI Extra | 8 |
| PEC SMRI New | 8 |
| M | 235 |
| CMC MSSM | 101 |
| CMC Penn | 23 |
| CMC Pitt | 44 |
| PEC SMRI Array | 26 |
| PEC SMRI Consortium | 8 |
| PEC SMRI Extra | 23 |
| PEC SMRI New | 10 |
| Grand Total | 901 |
CMC, Common Mind Consortium; F, Female; M, Male; PEC, PsychENCODE Consortium; SMRI, Stanley Medical Research Institute; BSHRI, Banner Sun Health Research Institute.
Details on PsychEncode and CommonMind collections can be found at https://www.synapse.org/#!Synapse:syn4590909.
Capstone Collection
To obtain cross-sectional expression of MCHR1 from prenatal to the elderly brain, we examined data available from Capstone collection (Gandal et al., 2018). The developmental dataset modeled differential expression across age in 320 control subjects across the lifespan from prenatal to over 90 years of age (data available to MPV through access at https://www.synapse.org/#!Synapse:syn12080241). We examined the expression of MCHR1 across the lifespan using binned data.
Allen Institute For Brain Science
Single-nuclei exon-mapping read counts for 15 928 cells from the middle temporal gyrus were obtained from the Allen Institute’s web portal (https://celltypes.brain-map.org/rnaseq), and differential gene expression tests between the 4 major cell classes were performed on log-transformed CPM values using nonparametric statistics due to the majority of nuclei showing zero expression level of MCHR1.
Animal Study of MCH System
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine and were performed in compliance with national and institutional guidelines for the care and use of laboratory animals.
MCHR1 Knockout Mice
Ten- to 12-week-old male and female wild-type and MCHR1 knockout (MCHR1 KO) mice were used for the behavioral experiments. MCHR1 KO mice were generated as previously described (Marsh et al., 2002). MCHR1 KO mice were backcrossed to a BL6 background for 9 or more generations, and then littermates were separately bred to generate the wild-type and MCHR1 KO mice.
MCH Conditional Knockout (MCH cKO) Mice
PmchCre/+;iDTR/+ mice were generated as described previously (Alhassen et al., 2019). Briefly, (Pmch-Cre) mice (Jackson Laboratories, Bar Harbor, ME) that express Cre-recombinase (Cre) under the MCH promoter (Kong et al., 2010) were crossed with homozygous inducible diphtheria toxin receptor iDTR/+ mice (from Dr Satchidinanda Panda and originally generated in the laboratory of Dr Ari Waisman) (Buch et al., 2005; Sanathara et al., 2018). The resulting iDTR+PmchCre+ (iDTR+/Cre+) and their control littermate iDTR+PmchCre−(iDTR+/Cre−) mice were injected twice in 4 days with the diphtheria toxin (DT) (16 µg/kg, i.p.).
Behavioral Assays
Locomotion and Stereotypy Assays
Locomotor activity and stereotypy were carried out as described previously (Alachkar et al., 2018). Mice were placed into a 40- × 40-cm locomotion test chamber (Med Associates, Inc.) and allowed to habituate to the chamber for 30 minutes. The horizontal, vertical, and stereotypic counts were recorded for 1 hour and analyzed using Activity Monitor 5 software (Med Associates, Inc.). The animal is recorded as exhibiting stereotypy when it breaks the same beam (or set of beams) repeatedly. This typically occurs during grooming and head bobbing.
Marble Burying
Marble burying was carried out as described previously (Sanathara et al., 2018). Mice were placed in a 30- × 18- × 12-cm polypropylene box containing 24 glass marbles (1.5-cm diameter) evenly spaced on the surface of 3 cm deep fresh bedding. After the end of the 30-minute sessions, which were video recorded, the number of marbles buried was counted by observers who were blind to the animal genotype or sex.
Three-Chamber Sociability Test
The sociability assay was carried out as described previously using the 3-chambers apparatus (manufactured by carpentry facility, University of California, Irvine, CA) that has empty wire-mesh containment cups placed in the middle of the side chambers (Alachkar et al., 2018). The tested mice were placed in the middle chamber and allowed exploration for 5 minutes with the dividing doors closed. A control mouse was placed inside the containment cup in one of the side chambers. The dividing doors were then removed between the chambers, and the test mouse was allowed to roam freely between all chambers for a total time of 10 minutes. Tests were video-recorded and analyzed by ANY-MAZE software (Stoelting Co.). The duration of direct contacts between the subject mice with both cups was scored individually.
Novel Object Recognition and Location-Dependent Object Recognition Assays
The novel object recognition (NOR) and location-dependent object recognition assays were carried out as described previously (Alachkar et al., 2018). Mice were habituated to the experimental apparatus (manufactured by carpentry facility, University of California, Irvine) for 10 min/d for 3 consecutive days. In the training phase, mice were allowed to explore 2 identical objects for 10 minutes. Twenty-four hours later, the retention test was conducted. In the NOR retention test, mice were allowed to explore 1 familiar and 1 novel object for 5 minutes. The time spent exploring the 2 objects was recorded. In location-dependent object recognition retention tests, mice were allowed to explore 1 familiar object placed in the same location as during the training phase and another familiar object placed in a novel location for 5 minutes. Time spent with objects in the 2 locations was recorded. The relative exploration time was expressed by a discrimination index: [D.I. = (Tnovel − Tfamiliar)/ (Tnovel + Tfamiliar) × 100%]. Tests were video recorded and analyzed by ANY-MAZE software (Stoelting Co.).
Prepulse Inhibition (PPI) Assay
The PPI assay was measured using the startle chambers (San Diego Instrument, Inc.) with a high-frequency loudspeaker that produced background noise of 65 dB as well as the various acoustic stimuli (Alachkar et al., 2018). A total of 65 readings are recorded at 1-millisecond intervals beginning at the stimulus onset. Average amplitude is used as the measure of startle.
During the test, mice were placed in the startle chambers for 5 minutes acclimation with 65-dB background noise. The PPI session consisted of 5 different trials: no-stimulus trials, 3 prepulse trials, and startle trials. No-stimulus trials consist of background noise only (65 dB). Startle trials consist of a 40-millisecond-duration startle stimulus at 120 dB (p120). Prepulse trials consist of a 20-millisecond-duration prepulse at 68 dB (pp3), 71 dB (pp6), or 77 dB (pp12), a 100-millisecond inter-stimulus interval, followed by a 40-millisecond-duration startle stimulus at 120 dB. The level of PPI is calculated as a percentage score for each acoustic prepulse intensity: % PPI = 100-([(startle response for prepulse+pulse trials)/ (startle response for pulse-alone trials)] × 100).
Contextual Fear Conditioning Assay
The Contextual Fear Conditioning Assay was carried out as previously described (Alachkar et al., 2018). During the training session, mice were placed in the conditioning chamber (TSE Systems, Inc.) for 2.5 minutes before receiving a 0.7-mA foot shock lasting for 2 seconds. After an additional 30 seconds in the chamber, mice were returned to their home cages. Freezing behavior prior to and after the shock (pre-and post-shock sessions) was measured. Twenty-four hours after training, mice were placed back into the same chamber (same conditioned context: wallpaper with striped pattern) in the absence of shock for 5 minutes, and their freezing behavior during this period (retention session) was measured. Freezing behavior was scored as freezing (1) or not (0) within an interval of 5 seconds, and the percentage of freezing behavior was calculated as 100 × (the number of intervals of freezing/ total intervals).
Immunohistochemistry
Verification of DT-Induced Ablation of MCH
At the end of all behavioral experiments, iDTRPmchCre animals were perfused transcardially with 4% paraformaldehyde, and coronal brain sections (20 mm) through the LH were cut. The MCH-neuron ablation was verified by visualizing MCH neurons using rabbit polyclonal anti-MCH antibody (1:150 000, antibody courtesy of W. Vale, Salk Institute, La Jolla, CA) as previously described (Sanathara et al., 2018). A goat anti-rabbit secondary antibody (1:500; ThermoFisher) was used to visualize MCH immunoreactivity.
Data Analysis
Animal study: GraphPadPrism (GraphPad Software, Inc.) was used for statistical analysis. Data are presented as means ± SEM. Results for the locomotor activity, stereotypic behavior, and marble burying as well as NOR and NOL assays were analyzed using Student unpaired t test. Social behavior, PPI, and fear conditioning data were analyzed using 2-way ANOVA followed by the Bonferroni post hoc comparison, and P < .05 was considered statistically significant.
RESULTS
Human Gene Expression
In the combined dataset (901 total subject;, the demographics for the SZ and control groups are shown in Table 1), there was a significant increase in age in control subjects (68.6 y ± 19.0 SD) compared with the age of subjects with SZ (61.7 y ± 19.1 SD, P < .0001) (Table 2). The PMI in subjects with SZ was increased compared with controls by 2-fold from 12 hours in controls to over 25 hours in subjects with SZ (P < .0001) (Table 2). The analysis of expression for combined dataset in the PFC thus included important demographic covariates (age, sex, PMI, RNA integrity number (RIN), brain bank).
Table 2.
The analysis of MCHR1 in the combined dataset (901 total subjects, each group shown in Table 1 for SZ and controls) showed a significant increase in age in control subjects and increased PMI in subjects with SZ
| Brainbank | Diagnosis | N | M | F | Age_mean | Age_sd | RIN_mean | RIN_sd | PMI_mean | PMI_sd |
|---|---|---|---|---|---|---|---|---|---|---|
| PEC BSHRI | Control | 184 | 108 | 76 | 83.05 | 7.80 | 6.96 | 0.93 | 3.03 | 1.86 |
| PEC SMRI Array | Control | 34 | 25 | 9 | 43.82 | 7.35 | 8.08 | 0.50 | 29.47 | 13.05 |
| PEC SMRI Array | SCZ | 34 | 26 | 8 | 42.53 | 8.59 | 7.99 | 0.62 | 31.56 | 15.74 |
| PEC SMRI Consortium | Control | 13 | 8 | 5 | 49.62 | 10.56 | 7.62 | 0.94 | 23.46 | 8.93 |
| PEC SMRI Consortium | SCZ | 11 | 8 | 3 | 41.82 | 12.46 | 7.25 | 0.70 | 38.36 | 13.95 |
| PEC SMRI Extra | Control | 9 | 8 | 1 | 45.78 | 14.81 | 7.00 | 0.66 | 27.67 | 14.19 |
| PEC SMRI Extra | SCZ | 31 | 23 | 8 | 40.84 | 12.02 | 7.09 | 0.75 | 46.42 | 31.46 |
| PEC SMRI New | Control | 19 | 19 | 0 | 51.42 | 8.69 | 8.16 | 0.62 | 30.11 | 18.38 |
| PEC SMRI New | SCZ | 18 | 10 | 8 | 46.00 | 8.92 | 7.66 | 0.80 | 46.39 | 35.25 |
| CMC MSSM | Control | 164 | 85 | 79 | 73.20 | 16.28 | 7.52 | 0.86 | 10.87 | 7.58 |
| CMC MSSM | SCZ | 148 | 101 | 47 | 72.38 | 12.45 | 7.13 | 0.80 | 23.79 | 15.57 |
| CMC Penn | Control | 37 | 18 | 19 | 67.14 | 15.04 | 7.46 | 0.70 | 13.42 | 7.51 |
| CMC Penn | SCZ | 58 | 23 | 35 | 78.74 | 11.05 | 7.35 | 0.77 | 13.57 | 7.66 |
| CMC Pitt | Control | 84 | 60 | 24 | 48.19 | 14.06 | 8.52 | 0.45 | 18.97 | 5.38 |
| CMC Pitt | SCZ | 57 | 44 | 13 | 48.11 | 13.03 | 8.17 | 0.63 | 20.04 | 8.42 |
| Total | Control | 544 | 331 | 213 | 68.64 | 19.09 | 7.53 | 0.97 | 12.06 | 11.22 |
| Total | SCZ | 357 | 235 | 122 | 61.68 | 18.96 | 7.44 | 0.85 | 25.82 | 19.74 |
| Total | 901 | 566 | 335 | P < .0001 | P = .153 | P < .0001 |
CMC, Common Mind Consortium; PEC, PsychENCODE Consortium; SMRI, Stanley Medical Research Institute; BSHRI, Banner Sun Health Research Institute.
Details on those collections can be found here: https://www.synapse.org/#!Synapse:syn4590909
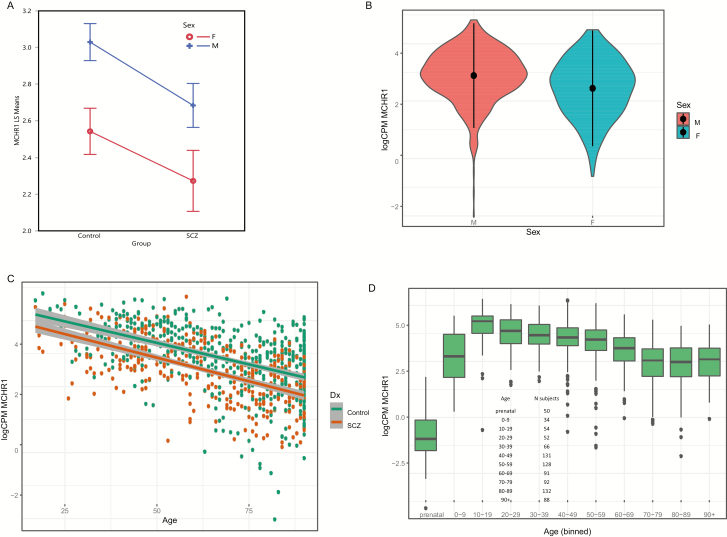
The subjects with SZ showed a significant decrease in MCHR1 expression (P value = 2.9E-04 FDR-adjusted; Table 3), and the sex effect was parallel in both subjects with SZ and controls (Figure 1A). However, the level of MCHR1 was significantly decreased in female cortical samples compared with male cortical samples (FDR P value = 1.6E-02; Figure 1B). Further, a highly significant decline in expression of MCHR1 was found beginning in adults (Figure 1C). The separate fit of age by diagnosis showed a highly significant decrease in both controls (P = 2.38E-35; R-squared = 0.247, adjusted R-squared = 0.246) and subjects with SZ (P = 1.16E-36; R-squared = 0.363, R-squared adjusted = 0.361). When the groups were combined, the P value remained highly significant (R-squared = .245, adjusted R-squared = 0.244, P value = 6.6E-57) for the fit of age and expression of MCHR1.
Table 3.
The combined analysis of expression in the PFC included covariates (age, sex, PMI, RIN, brain bank) and a significant decrease in MCHR1 expression was found (FDR P value = 2.9E-04)
| Ensemble Gene ID | Gene Symbol | Log2 difference | Average Expression (cpm) | t | P value | Adjusted P value |
|---|---|---|---|---|---|---|
| ENSG00000128285.4 | MCHR1 | −0.212 | 2.77 | −4.21 | 2.73E-05 | 2.91E-04 |
There is a low expression of PMCH in human cortex; therefore, data was not included in analysis.
Figure 1.
(A) The main effect of MCHR1 expression (least square means, y-axis) and group (x-axis) showed a significant decrease in subjects with schizophrenia (FDR P value = 2E-04) and the decreased expression was parallel across sex. Females showed a decreased expression of MCHR1 (FDR P value = 1.6E-02). F = female; M = male. (B) Violin plot of MCHR1 expression by sex, with mean ±SD superimposed for control and schizophrenia samples shown in Table 1. Females showed a decreased expression of MCHR1 (FDR P value = 1.6E-02). (C) Scatter plot for age and sex using the 901 schizophrenia and control PFC samples (Table 1, demographics) showing a significant age-related decrease in expression of MCHR1. Multiple R-squared = 0.2452, adjusted R-squared = 0.2443, P value (2-sided) t test for linear regression coefficient of MCHR1 expression and age of death P = 6.6E-57. Note that data are binned for all subjects ages >90 years. (D) Box plot for MCHR1 expression across the lifespan. Capstone control prefrontal cortex data obtained from (https://www.synapse.org/#!Synapse:syn12080241). The number of subjects in each age bin is shown in the inset.
The prenatal expression of MCHR1 was compared with postnatal expression across the lifespan (Figure 1D). The data from Capstone control prefrontal cortex was plotted, and the prenatal expression was decreased compared with the pooled postnatal decades (Mann-Whitney U test, P = 2.52E-31). PMCH mRNA expression was too low to be reliably determined and was excluded from analysis. The peptide MCH is expressed only in the hypothalamus compared with the cortex (Allen Brain Atlas, 141-fold higher in the hypothalamus compared with cortex).
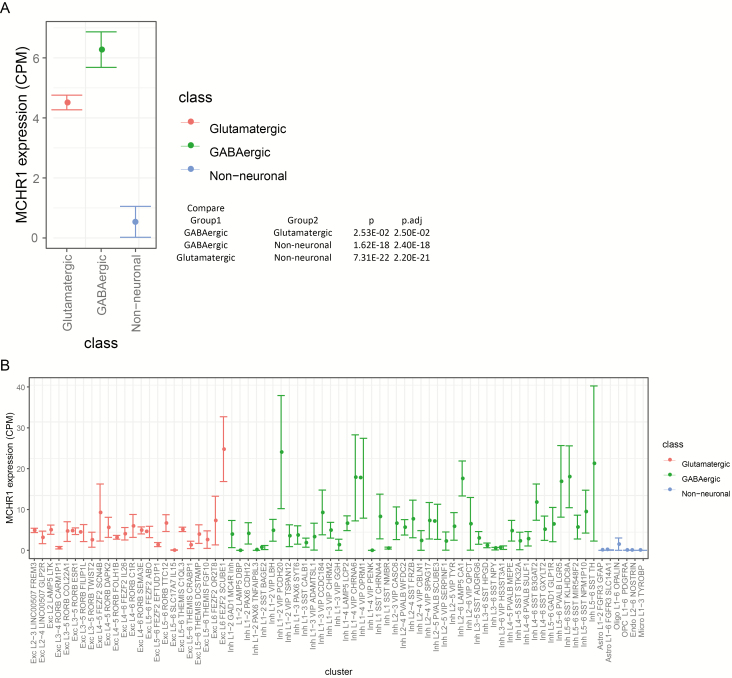
Cortical expression of MCHR1 was investigated at the resolution of single nuclei RNA-Seq using Allen Institute data. The middle temporal gyrus nuclei (Hodge et al., 2018) showed the highest expression of MCHR1 in GABAegic cell types compared with glutamatergic cells (P = .025) and non-neuronal cells (P = 2.40E-18; Figure 2A). There was some overlap of expression with HTR2A; however, this was in only a minority of cells that had non-zero expression (data not shown). MCHR1 expression is distributed in 22 excitatory cell types, and 33 inhibitory types (Figure 2B) as judged by the SEM bar remaining above zero CPM expression level. MCHR1-positive nuclei were detected in 9.1% of nuclei (1429/15 603) and exclusively in neurons (Figure 2B).
Figure 2.
(A) Single nuclei RNA-Seq data from human medial temporal gyrus showing cell class differential expression of MCHR1 (data publicly available at the Allen Institute for Brain Science). The mean ±SEM for single nuclei RNA-Seq expression of MCHR1 is shown for 3 cell types (no-class is omitted). The GABAergic cell type showed a significantly higher expression of MCHR1 compared with glutamatergic or non-neuronal cell types shown in the inset table listing P values for each comparison. (B) Box plot of cell cluster by MCHR1 expression for 3 classes of nuclei. There were 1429 positive MCHR1 expressing cells of 15 603 nuclei included in this figure, thus an estimate is that about 9.1% of all cells express MCHR1 in medial temporal gyrus.
To obtain eQTL data, we examined data available from brainseq.org (Jaffe et al., 2018). There is a strong eQTL to MCHR1 in the first exon of MCHR1 (Benjamini-Hochberg FDR P = 1.04E-06); the eQTL associated SNP (rs133073, chr22:41075695:C:T) decreased expression in prefrontal cortex.
Animal Study
Behavioral Responses in MCHR1 KO
Based on the human results, we asked whether the germline deletion of MCHR1 produces in mice any behavioral phenotypes that are related to schizophrenia.
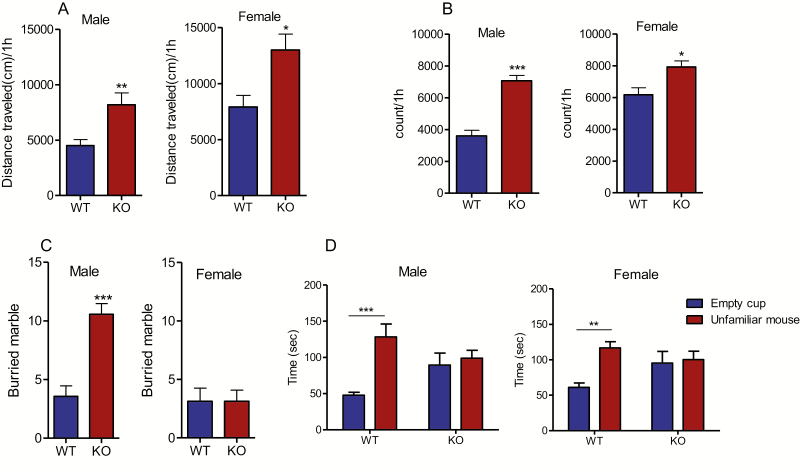
Both male and female MCHR1 KO mice exhibited an increase in their locomotor activities (male: P < .05, female: P < .01; Figure 3A) as well as a greater level of stereotypic behaviors (male: P < .01, female: P < .05; Figure 3B).
Figure 3.
Effect of MCHR1 germline deletion on locomotor activity, stereotypy, marble burying, and social interaction. (A) Distance mice travelled in 60 minutes of locomotion test: male: t = 3.28, P = .005 (n = 9 and 7 for wild type and MCHR1 KO, respectively), female: t = 2.95, P = .011. Unpaired student t test: WT vs MCHR1 KO, *P < .05, **P < .01 (n = 8 and 7 for wild-type and MCHR1 KO, respectively). (B) Stereotypic counts in 60 minutes of locomotion test: male: t = 6.858, P = .0001 (n = 9 and 7 for wild-type and MCHR1 KO, respectively), female: t = 2.96, P < = .011. Unpaired student t test: wild type vs MCHR1 KO,*P < .05, ***P < .001 (n = 8 and 7 for wild type and MCHR1 KO, respectively). (C) Number of marbles buried in 30 minutes: male: t = 5.41, P < .001, female: t = 0.0, P = 1.00. Unpaired student t test wild type vs MCHR1 KO, ***P < .001 (n = 8). (D) Male: Time mice spent interacting with empty cup and control mice in social interaction test: 2-way ANOVA, genotype effect: F1,38 = 0.21, P = .65, genotype effect × cup interaction F1,38 = 6.783, P = .01. Bonferroni post hoc test: empty cup vs unfamiliar mouse, wild type: t = 4.08, P < .001, KO: t = 0.51, P > .05.***P < .001 (n = 10 and 11 for wild type and MCHR1 KO, respectively). (E) Female: Time mice spent interacting with empty cup and control mice in social interaction test: 2-way ANOVA, genotype effect: F1,46 = 0.6378, P = .43, genotype effect × cup interaction: F1,46 = 5.2, P = .027. Bonferroni post hoc test: empty cup vs unfamiliar mouse, wild type: t = 2.387, P < .01, KO: t = 1.72, P > .05. **P < .01 (n = 13 and 12 for wild type and MCHR1 KO, respectively).
In the marble burying assay, as expected in light of our recent publication (Sanathara et al., 2018), male MCHR1 KO mice buried more marbles than the wild-type mice (P < .001; Figure 3C), indicating an increase in repetitive behavior. Female MCHR1 KO mice, however, buried a number of marbles comparable with the wild-type mice (P > .05; Figure 3C).
In the social interaction assay, male and female wild-type mice displayed more interaction with the unfamiliar mice than the empty cup (P < .001 and P < .01 for male and female mice, respectively; Figure 3D). However, the times that male and female MCHR1 KO mice spent with the empty cup and with the unfamiliar mouse were not significantly different (P > .05; Figure 3D), indicating social deficits in these animals.
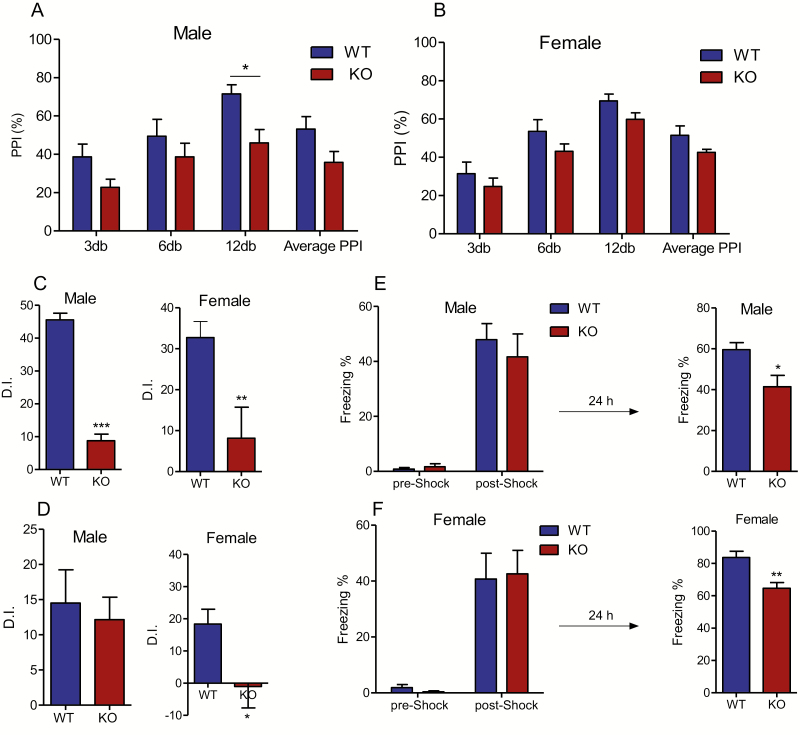
The MCHR1 KO male mice exhibited a significant decrease in PPI ratio compared with the wild-type males (P < .05; Figure 4A), suggesting an impairment in sensorimotor gating function. MCHR1 deletion produced a decrease in PPI in female mice (2-way ANOVA, genotype effect [F1,55 = 6.8, P = .01]; prepulse intensity effect [F1,55 = 20.66, P < .0001]); however, Bonferroni test revealed no significant difference in PPI values between MCHR1 KO and wild-type mice at any decible intensity (P > .05; Figure 4B).
Figure 4.
Effect of MCHR1 germline ablation on prepulse inhibition and different types of memory. (A) Prepulse inhibition ratio in male: 2-way ANOVA, genotype effect (F1,48 = 14.55, P = .0004), prepulse intensity effect (F3,48 = 6.27, P = .001) and genotype x prepulse intensity (F3,48 = 0.447, P = .72) followed by Bonferroni post hoc test: WT vs KO, *P < .05 (n = 7). (B) Prepulse inhibition ratio in female, 2-way ANOVA, genotype effect (F1,56 = 6.8, P = .006), prepulse intensity effect (F3,56 = 20.66, P < .0001), and genotype × prepulse intensity (F3,56 = 0.067, P = .98) followed by Bonferroni post hoc test: wild type vs KO, P > .05 (n = 8). (C) Discrimination index in the NOR assay: male: t = 12.83, P = .0001. Unpaired student t test: wild type vs MCHR1 KO, *** P < .001 (n = 7 and 6 for wild type and MCHR1 KO, respectively), female: t = 3.02, P = .007. Unpaired student t test: wild type vs MCHR1 KO, *** P < .001 (n = 12 and 10 for wild type and MCHR1 KO, respectively). (D) Discrimination index in the location-dependent object recognition assay: male: t = 0.28, P = .178, Unpaired student t test: WT vs MCHR1 KO (n = 8), female: t = 2.65, P = .0.015; Unpaired student t test: wild type vs MCHR1 KO, *P < .05 (n = 12). (E) Male: Percentage of the freezing behavior in contextual fear conditioning assay (n = 8). Training/Conditioning: 2-way ANOVA, genotype effect (F1,28 = 0.2789, P = .6), stage effect (F1,28 = 72.20, P < .0001), test: unpaired t test (t = 2.8, P = .012), WT vs KO: *P < .05. (F) Female: Percentage of the freezing behavior in contextual fear conditioning assay (n = 9). Training/Conditioning: 2-way ANOVA, genotype effect (F1,32 = 0.0009, P = .97), stage effect (F1,32 = 41.79, P < .0001), Test: Unpaired t test (t = 3.6, P = .002), **P < .01.
In the novel object recognition assay, which measures neutral memory, both male and female wild-type mice spent more time exploring the new object. However, MCHR1 KO mice failed to recognize the old object vs the novel object 24 hours after the initial presentation of the training objects (P < .001 and P < .01 respectively for discrimination index in male and female MCHR1 KO compared with wild-type) (Figure 4C). Similar results were found in the novel location recognition assay, which measures spatial memory. The female MCHR1 KO group displayed a deficit in discriminating the objects in the old and new location and displayed lower discrimination index compared with the wild-type female mice (P < .001) (Figure 4D); however, the male MCHR1 KO mice displayed similar discrimination index to the wild-type group (P > .05) (Figure 4D).
In the contextual fear conditioning assay, all groups barely exhibited freezing behavior (no difference between treatments, P > .05; Figure 4E) before the foot-shock stimulus during the training session. All groups exhibited a comparable increase in freezing behavior percentage immediately after the foot-shock stimulus (P > .05). In the retention session, however, the male and female MCHR1 KO mice exhibited significantly lower freezing behavior compared with the corresponding wild-type groups (P < .05, male, P < .01, female) (Figure 4E).
Behavioral Responses in Mice With Conditional MCH Ablation
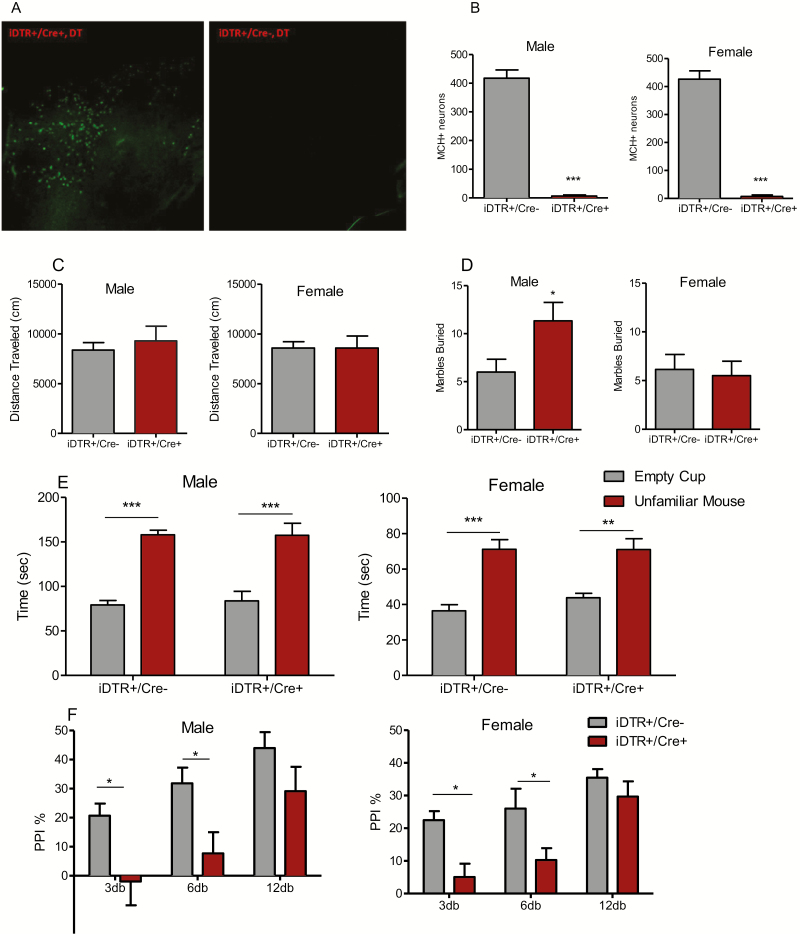
The number of MCH immunoreactive neurons from each section was compared between DT-injected iDTR+/PmchCre+ (MCH cKO) mice and iDTR+/PmchCre− littermates. Only animals that displayed over 90% loss of MCH neurons were included in the behavioral analyses (Figure 5A–B).
Figure 5.
Behavioral phenotype of conditional ablation MCH neurons. (A) MCH immunoreactivity in the lateral hypothalamus of iDTR+/Cre− and iDTR+/Cre+ mice following DT administration. (B) Counts of MCH immunoreactive neurons in DT-treated iDTR+/Cre- and iDTR+/Cre+ mice. Unpaired t test (male: t = 13.67, P < .0001, female: t = 10.72, P < .0001), ***P < .0001. Data are presented as mean ± SEM. (C) Distance mice travelled in 60 minutes of locomotion test, unpaired student t test: male: t = 0.589, P = .567 (n = 6); female: t = 0.001, P = .99 (n = 8). Data are presented as mean ± SEM. (D) Number of marbles buried in 30 minutes: male: t = 2.27, P = .046 (n = 6), female: t = 0.3, P = .76. Unpaired student t test iDTR+/Cre- vs iDTR+/Cre+, *P < .05 (n = 8). (E) Time mice spent interacting with empty cup and control mice in social interaction test: male: genotype effect, 2-way ANOVA, F1,36 = 0.039, P = .84. Bonferroni post hoc test: empty cup vs unfamiliar mouse, iDTR+/Cre−: t = 5.945, P < .001, iDTR+/Cre+: t = 5.553, P < .001 (n = 10); female: genotype effect, 2-way ANOVA, F1,36 = 0.5725, P = .45. Bonferroni post hoc test: empty cup vs unfamiliar mouse, iDTR+/Cre−: t = 5.702, P < .001, iDTR+/Cre+: t = 3.634, P < .001. **P < .01, ***P < .001 (n = 12 and 8 for iDTR+/Cre− and iDTR+/Cre+, respectively). Data are presented as mean ± SEM. (F) Prepulse inhibition ratio: male: 2-way ANOVA, genotype effect (F1,54 = 15.22, P = .0003), prepulse intensity effect (F2,54 = 9.049, P = .0004) followed by Bonferroni post hoc test: iDTR+/Cre− vs iDTR+/Cre+, *P < .05 (n = 7); female, 2-way ANOVA, genotype effect (F1,39 = 14.68, P = .0005), prepulse intensity effect (F2,39 = 11.23, P < .0001). Bonferroni post hoc test: iDTR+/Cre− vs iDTR+/Cre+, *P < .05 (n = 8 and 7 for iDTR+/Cre− and iDTR+/Cre+, respectively).
Both male and female MCH cKO mice displayed normal locomotor activity (Figure 5C) as well as normal social interaction (i.e., interacted more with an unfamiliar mouse than with an empty cup) (Figure 5D). However, similar to the results of MCHR1 KO mice, male but not female MCH cKO mice showed increased repetitive behavior in the marble burying assay (Figure 5E), and both male and female MCH cKO mice exhibited deficits in PPI (Figure 5F).
Discussion
This study is the first to our knowledge to connect the downregulation of MCHR1 mRNA in the brains of schizophrenia patients to an eQTL, further providing a causal link between the germline MCHR1 deletion in mice and deficits in social behavior, sensorimotor gating, and multiple cognitive tasks that resemble schizophrenia symptoms. MCHR1 mRNA levels are significantly lower in the prefrontal cortex of subjects with schizophrenia using a large sample of 901 subjects. There is a widespread cortical expression of MCHR1 positive nuclei mainly in neurons and a strong increase of expression with postnatal development compared with prenatal expression; however, expression peaks in adolescence and significantly decreased during subsequent decades of life. There is also a decreased expression of MCHR1 in the PFC from female compared with male brains. The human developmental data suggest that the effect of PMCH on the cortex might be delayed until the MCH receptor begins to be more highly expressed during early postnatal development. Indeed, PMCH mRNA also occurs in very low levels prenatally until 10–12 days of postnatal life when a dramatic increase occurs, reaching its peak at the weaning time (Breton et al., 1993). This increase in PMCH, which concurs with the crucial period of life that marks sucking-weaning transition, indicates a role for MCH system in the reorganization in neuronal processes that are involved in drinking and feeding behaviors at the weaning period.
Significant genetic evidence for the involvement of the MCHR1 locus in schizophrenia susceptibility came from the largest GWAS of SZ conducted to date (Pardinas et al., 2018). The reduced MCHR1 expression in schizophrenia found in RNA-Seq analysis of DLPFC provides functional evidence that corroborates the genetic association results, raising the question whether MCHR1 SNPs may act as an expression quantitative trait loci (eQTLs), thus affecting the RNA expression of the receptor. A significant eQTL in exon 1 of the MCHR1 gene was found in a large expression database (Jaffe et al., 2018), suggesting that minor allele carriers may indeed show reduced MCHR1 expression.
The MCH system has been implicated in modulating sleep, emotional behaviors, and learning and memory (Monzon et al., 1999; Varas et al., 2002b; Adamantidis et al., 2005). Since deficits in these functions are prominent in psychiatric disorders such as schizophrenia, depression, and autism, it is plausible to correlate the dysfunction of the MCH system with the pathophysiology of these disorders. While no study measured MCH in schizophrenia, MCH was measured in Alzheimer’s patients and revealed elevated levels in the CSF compared with healthy persons. Interestingly, the CSF-MCH levels were found to correlate with T-tau and P-tau as well as to the severity of cognitive impairment in the Alzheimer’s patients (Schmidt et al., 2013). Consequently, we investigated whether the genetic deletion of MCHR1 and/or the conditional ablation of MCH neurons are associated with schizophrenic-like phenotype in mice. While it is difficult to recapitulate the human psychopathological phenotypes of schizophrenia in rodents, a number of behavioral patterns have been validated and historically accepted as translational models between rodents and humans. Locomotor hyperactivity, augmented stereotypic, and repetitive behaviors are widely used to evaluate the positive symptoms of schizophrenia (Kokkinidis and Anisman, 1980; Hoffman, 1992; Lipska and Weinberger, 1994; Mueser and McGurk, 2004; Manahan-Vaughan et al., 2008). The 3-chamber social behavior assay is used to model some negative symptoms in mice (social withdrawal) (Moy et al., 2004).
The germline deletion of MCHR1 receptors significantly increased locomotor activity and stereotypic behavior in male and female mice in agreement with previous reports (Marsh et al., 2002; Astrand et al., 2004; Sanathara et al., 2018). However, conditional ablation of MCH neurons in adult mice did not affect locomotor activity. The locomotor phenotype discrepancy between the 2 knockout models is interesting and could be explained by the actions of additional neurotransmitters co-expressed in MCH-neurons or might indicate differential roles of MCHR1 vs MCH during development.
Interestingly, the augmented repetitive behavior (as measured by marble burying) in male MCHR1 KO and MCH cKO mice that is in agreement with our recent report (Sanathara et al., 2018) was not seen in female MCHR1 KO mice. The sex difference in the effect of MCH signaling deficits on repetitive behavior is of particular interest and points at a sexually dimorphic role for MCH signaling in schizophrenia. In humans, there was a significant decrease of MCHR1 expression in adult female cortex in both controls and patients with SZ. Evidence from anatomical, physiological, and pharmacological studies, in addition to human genetic studies, supports the dimorphic role of MCH in regulating various behaviors. Anatomical studies revealed a sexually dimorphic expression of MCH in rodent brain; MCH is expressed in the LH and medial zona incerta in both male and female animals, but only female rats express MCH in the latero-dorsal tegmental nucleus (Rondini et al., 2007), and only lactating dams express MCH in the preoptic area, the periventricular nucleus, and the anterior aspects of the paraventricular nucleus of the hypothalamus (Knollema et al., 1992; Rondini et al., 2010). The orexigenic effect of MCH has been demonstrated to be lower in female rats compared with male rats (Santollo and Eckel, 2008), and there are sex differences in the response of MCH neurons to glucose (Mogi et al., 2005), which is attributed to the higher circulating levels of estradiol in females compared with males. In support of this view, estrogen was shown to decrease food intake and body weight through inhibiting MCH neurons (Santollo and Eckel, 2008). MCHR1 genetic association with schizophrenia also seems to be sex-specific, with 6 SNPs found to be associated with schizophrenia in males but not females (Severinsen et al., 2006; Demontis et al., 2012).
Abnormal social interaction and communication are the main features of the negative symptoms of schizophrenia (Ellenbroek and Cools, 2000; Neill et al., 2014; Wilson and Koenig, 2014). Here, we found that germline deletion of MCHR1 but not ablation of MCH neurons affected social behavior in mice in the 3 chambers sociability test. Considering that in mice the essential role of olfaction in sociability, these results align well with our recent report that germline deletion of MCHR1 but not adult conditional ablation of MCH neurons produced deficits in olfaction functions (Alhassen et al., 2019). We previously showed that disruption of the MCH system during developmental stage but not adulthood produced impairment in behaviors typically mediated by olfactory functions such as olfaction habituation/dishabituation and maternal behaviors, particularly pups retrieval and interaction with pups (Alachkar et al., 2016; Alhassen et al., 2019). Therefore, we propose that the neuronal circuit that includes innervations of olfactory structures by the MCH neurons is crucial for the integration of chemosensory signals throughout life, and this MCH-olfactory circuit plays a critical role in the development of social behavior during the early stages of life.
Cognitive deficits are commonly evaluated using a wide range of assays such as the novel object recognition (neutral memory), novel location recognition (spatial memory), and fear conditioning (emotional memory) (Ozawa et al., 2006; O’Tuathaigh et al., 2007; Kelly et al., 2009; Brzozka et al., 2010). MCHR1 KO mice showed, to some extent, deficits in all cognitive assays tested. Both male and female MCHR1 KO mice displayed impairment in novel object recognition, an assay based on the tendency of rodents to explore a novel object more than a familiar one. On the other hand, in the novel location recognition assay, we found impairment in spatial memory in female MCHR1 KO mice, with a trend for impairment observed in male MCHR1 KO mice. Emotional memory, assessed by contextual fear conditioning test, was impaired in male and female MCHR1 KO mice. It could be claimed that locomotor hyperactivity seen in MCHR1 KO might mask the freezing behavior in the contextual fear conditioning test in these animals. However, this possibility is excluded given the comparable freezing scores displayed by MCHR1 KO and wild-type mice immediately after the foot-shock.
Impairment of prepulse inhibition of startle, which is present in patients with schizophrenia, reflects an inability to filter nonrelevant sensory information (Swerdlow et al., 2006). MCHR1 deletion and ablation of MCH neurons produced profound deficits in PPI, although the PPI deficits produced by germline deletion of MCHR1 were more pronounced in male than female mice. The congruent PPI deficit phenotypes in both MCH and MCHR1 knockout mouse models suggest that preattentive information processing is critically dependent on the intact functions of the MCH system.
CONCLUSION
MCH alterations affect many domains included in the Research Domain Criteria that overlap with schizophrenia (cognitive systems, social preferences, sensorimotor system) (https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/definitions-of-the-rdoc-domains-and-constructs.shtml). The expression of MCHR1 is downregulated in the frontal cortex of patients with schizophrenia, decreased with age beginning in adults, and decreased in the female frontal cortex. The absence of intact MCH function in mice leads to behavioral deficits that mimic Research Domain Criteria constructs of schizophrenia including augmented repetitive behavior, social and cognitive deficits, and abnormal pre-attentive information. These results support prior work implicating the MCHR1 locus with schizophrenia susceptibility and connect the downregulation of MCHR1 mRNA in the brains of schizophrenia patients to an eQTL providing a potentially causal link to deficits in sensorimotor gating, social interaction, and multiple cognitive tasks in patients with schizophrenia.
Acknowledgments
This work was supported by the Eric L. and Lila D. Nelson Chair in Neuropharmacology (Olivier Civelli), Science Without Borders/CAPES Scholarship (Lucas Pauluk), and School of Medicine Research grant (Amal Alachkar). For the PsychEncode data deposited as part of the Capstone, the data were generated as part of the PsychENCODE Consortium, supported by: U01MH103392, U01MH103365, U01MH103346, U01MH103340, U01MH103339, R21MH109956, R21MH105881, R21MH105853, R21MH103877, R21MH102791, R01MH111721, R01MH110928, R01MH110927, R01MH110926, R01MH110921, R01MH110920, R01MH110905, R01MH109715, R01MH109677, R01MH105898, R01MH105898, R01MH094714, P50MH106934, U01MH116488, U01MH116487, U01MH116492, U01MH116489, U01MH116438, U01MH116441, U01MH116442, R01MH114911, R01MH114899, R01MH114901, R01MH117293, R01MH117291, R01MH117292 awarded to Schahram Akbarian (Icahn School of Medicine at Mount Sinai), Gregory Crawford (Duke University), Stella Dracheva (Icahn School of Medicine at Mount Sinai), Peggy Farnham (University of Southern California), Mark Gerstein (Yale University), Daniel Geschwind (University of California, Los Angeles), Fernando Goes (Johns Hopkins University), Thomas M. Hyde (Lieber Institute for Brain Development), Andrew Jaffe (Lieber Institute for Brain Development), James A. Knowles (University of Southern California), Chunyu Liu (SUNY Upstate Medical University), Dalila Pinto (Icahn School of Medicine at Mount Sinai), Panos Roussos (Icahn School of Medicine at Mount Sinai), Stephan Sanders (University of California, San Francisco), NenadSestan (Yale University), Pamela Sklar (Icahn School of Medicine at Mount Sinai), Matthew State (University of California, San Francisco), Patrick Sullivan (University of North Carolina), Flora Vaccarino (Yale University), Daniel Weinberger (Lieber Institute for Brain Development), Sherman Weissman (Yale University), Kevin White (University of Chicago), Jeremy Willsey (University of California, San Francisco), and Peter Zandi (Johns Hopkins University).
For CommonMind, data were generated as part of the CommonMind Consortium supported by funding from Takeda Pharmaceuticals Company Limited, F. Hoffmann-La Roche Ltd and NIH grants R01MH085542, R01MH093725, P50MH066392, P50MH080405, R01MH097276, RO1-MH-075916, P50M096891, P50MH084053S1, R37MH057881, AG02219, AG05138, MH06692, R01MH110921, R01MH109677, R01MH109897, U01MH103392, and contract HHSN271201300031C through IRP NIMH. Brain tissue for the study was obtained from the following brain bank collections: the Mount Sinai NIH Brain and Tissue Repository, the University of Pennsylvania Alzheimer’s Disease Core Center, the University of Pittsburgh NeuroBioBank and Brain and Tissue Repositories, and the NIMH Human Brain Collection Core. CMC Leadership: Panos Roussos, Joseph Buxbaum, Andrew Chess, Schahram Akbarian, Vahram Haroutunian (Icahn School of Medicine at Mount Sinai), Bernie Devlin, David Lewis (University of Pittsburgh), Raquel Gur, Chang-Gyu Hahn (University of Pennsylvania), Enrico Domenici (University of Trento), Mette A. Peters, Solveig Sieberts (Sage Bionetworks), Thomas Lehner, Stefano Marenco, Barbara K. Lipska (NIMH).
Interest Statement: None.
References
- Adamantidis A, Thomas E, Foidart A, Tyhon A, Coumans B, Minet A, Tirelli E, Seutin V, Grisar T, Lakaye B (2005) Disrupting the melanin-concentrating hormone receptor 1 in mice leads to cognitive deficits and alterations of NMDA receptor function. Eur J Neurosci 21:2837–2844. [DOI] [PubMed] [Google Scholar]
- Alachkar A, Alhassen L, Wang Z, Wang L, Onouye K, Sanathara N, Civelli O (2016) Inactivation of the melanin concentrating hormone system impairs maternal behavior. Eur Neuropsychopharmacol 26:1826–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alachkar A, Wang L, Yoshimura R, Hamzeh AR, Wang Z, Sanathara N, Lee SM, Xu X, Abbott GW, Civelli O (2018) Prenatal one-carbon metabolism dysregulation programs schizophrenia-like deficits. Mol Psychiatry 23:282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhassen L, Phan A, Alhassen W, Nguyen P, Lo A, Shaharuddin H, Sanathara N, Civelli O, Alachkar A (2019) The role of Olfaction in MCH-regulated spontaneous maternal responses. Brain Res 1719:71–76. [DOI] [PubMed] [Google Scholar]
- Astrand A, Bohlooly-Y M, Larsdotter S, Mahlapuu M, Andersén H, Tornell J, Ohlsson C, Snaith M, Morgan DG (2004) Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am J Physiol Regul Integr Comp Physiol 287:R749–R758. [DOI] [PubMed] [Google Scholar]
- Bächner D, Kreienkamp H, Weise C, Buck F, Richter D (1999) Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1). FEBS Lett 457:522–524. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE (1992) The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319:218–245. [DOI] [PubMed] [Google Scholar]
- Blouin AM, Siegel JM (2013) Relation of melanin concentrating hormone levels to sleep, emotion and hypocretin levels. Sleep 36:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton C, Presse F, Hervieu G, Nahon JL (1993) Structure and regulation of the mouse melanin-concentrating hormone mRNA and gene. Mol Cell Neurosci 4:271–284. [DOI] [PubMed] [Google Scholar]
- Brzózka MM, Radyushkin K, Wichert SP, Ehrenreich H, Rossner MJ (2010) Cognitive and sensorimotor gating impairments in transgenic mice overexpressing the schizophrenia susceptibility gene Tcf4 in the brain. Biol Psychiatry 68:33–40. [DOI] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A (2005) A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2:419–426. [DOI] [PubMed] [Google Scholar]
- Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, Dytko GM, Foley JJ, Martin J, Liu WS, Park J, Ellis C, Ganguly S, Konchar S, Cluderay J, Leslie R, Wilson S, Sarau HM (1999) Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature 400:261–265. [DOI] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O (2009) The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A 106:6772–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Verheij MM, Hesseling P, van Vugt RW, Buell M, Belluzzi JD, Geyer MA, Martens GJ, Civelli O (2011) The melanin-concentrating hormone (MCH) system modulates behaviors associated with psychiatric disorders. Plos One 6:e19286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Nyegaard M, Christensen JH, Severinsen J, Hedemand A, Hansen T, Werge T, Mors O, Børglum AD (2012) The gene encoding the melanin-concentrating hormone receptor 1 is associated with schizophrenia in a Danish case-control sample. Psychiatr Genet 22:62–69. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR (2000) Animal models for the negative symptoms of schizophrenia. Behav Pharmacol 11:223–233. [DOI] [PubMed] [Google Scholar]
- Fraigne JJ, Peever JH (2013) Melanin-concentrating hormone neurons promote and stabilize sleep. Sleep 36:1767–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, et al. (2018) Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362:6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, et al. (2019) Conserved cell types with divergent features in human versus mouse cortex. Nature 573:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DC. (1992) Typical and atypical neuroleptics antagonize MK-801-induced locomotion and stereotypy in rats. J Neural Transm Gen Sect 89:1–10. [DOI] [PubMed] [Google Scholar]
- Jaffe AE, Straub RE, Shin JH, Tao R, Gao Y, Collado-Torres L, Kam-Thong T, Xi HS, Quan J, Chen Q, Colantuoni C, Ulrich WS, Maher BJ, Deep-Soboslay A, Cross AJ, Brandon NJ, Leek JT, Hyde TM, Kleinman JE, Weinberger DR; BrainSeq Consortium (2018) Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nat Neurosci 21:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Stein JM, Vecsey CG, Favilla C, Yang X, Bizily SF, Esposito MF, Wand G, Kanes SJ, Abel T (2009) Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing Galphas, a G-protein subunit genetically linked to schizophrenia. Mol Psychiatry 14:347,398–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knigge KM, Baxter-Grillo D, Speciale J, Wagner J (1996) Melanotropic peptides in the mammalian brain: the melanin-concentrating hormone. Peptides 17:1063–1073. [DOI] [PubMed] [Google Scholar]
- Knollema S, Brown ER, Vale W, Sawchenko PE (1992) Novel hypothalamic and preoptic sites of prepro-melanin-concentrating hormone messenger ribonucleic acid and peptide expression in lactating rats. J Neuroendocrinol 4:709–717. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, Anisman H (1980) Amphetamine models of paranoid schizophrenia: an overview and elaboration of animal experimentation. Psychol Bull 88:551–579. [PubMed] [Google Scholar]
- Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Brüning JC, Lowell BB (2010) Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab 12:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, Smyth GK (2014) voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR (1994) Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology 10:199–205. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U (2008) A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus 18:125–134. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, et al. (2002) Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A 99:3240–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi K, Funabashi T, Mitsushima D, Hagiwara H, Kimura F (2005) Sex difference in the response of melanin-concentrating hormone neurons in the lateral hypothalamic area to glucose, as revealed by the expression of phosphorylated cyclic adenosine 3’,5’-monophosphate response element-binding protein. Endocrinology 146:3325–3333. [DOI] [PubMed] [Google Scholar]
- Monzon ME, de Souza MM, Izquierdo LA, Izquierdo I, Barros DM, de Barioglio SR (1999) Melanin-concentrating hormone (MCH) modifies memory retention in rats. Peptides 20:1517–1519. [DOI] [PubMed] [Google Scholar]
- Monzón ME, Varas MM, De Barioglio SR (2001) Anxiogenesis induced by nitric oxide synthase inhibition and anxiolytic effect of melanin-concentrating hormone (MCH) in rat brain. Peptides 22:1043–1047. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3:287–302. [DOI] [PubMed] [Google Scholar]
- Mueser KT, McGurk SR (2004) Schizophrenia. Lancet 363:2063–2072. [DOI] [PubMed] [Google Scholar]
- Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM (2014) Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur Neuropsychopharmacol 24:822–835. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CM, Babovic D, O’Sullivan GJ, Clifford JJ, Tighe O, Croke DT, Harvey R, Waddington JL (2007) Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience 147:18–27. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M (2006) Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry 59:546–554. [DOI] [PubMed] [Google Scholar]
- Pachoud B, Adamantidis A, Ravassard P, Luppi PH, Grisar T, Lakaye B, Salin PA (2010) Major impairments of glutamatergic transmission and long-term synaptic plasticity in the hippocampus of mice lacking the melanin-concentrating hormone receptor-1. J Neurophysiol 104:1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardiñas AF, et al. ; GERAD1 Consortium; CRESTAR Consortium (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 50:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C (2017) Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E (1996) A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380:243–247. [DOI] [PubMed] [Google Scholar]
- Rondini TA, Rodrigues Bde C, de Oliveira AP, Bittencourt JC, Elias CF (2007) Melanin-concentrating hormone is expressed in the laterodorsal tegmental nucleus only in female rats. Brain Res Bull 74:21–28. [DOI] [PubMed] [Google Scholar]
- Rondini TA, Donato J Jr, Rodrigues Bde C, Bittencourt JC, Elias CF (2010) Chemical identity and connections of medial preoptic area neurons expressing melanin-concentrating hormone during lactation. J Chem Neuroanat 39:51–62. [DOI] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O’Shea D, Miyoshi T, Ghatei MA, Bloom SR (1997) Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology 138:351–355. [DOI] [PubMed] [Google Scholar]
- Roy M, David NK, Danao JV, Baribault H, Tian H, Giorgetti M (2006) Genetic inactivation of melanin-concentrating hormone receptor subtype 1 (MCHR1) in mice exerts anxiolytic-like behavioral effects. Neuropsychopharmacology 31:112–120. [DOI] [PubMed] [Google Scholar]
- Roy M, David N, Cueva M, Giorgetti M (2007) A study of the involvement of melanin-concentrating hormone receptor 1 (MCHR1) in murine models of depression. Biol Psychiatry 61:174–180. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O (1999) Molecular characterization of the melanin-concentrating-hormone receptor. Nature 400:265–269. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Civelli O (2000) Melanin-concentrating hormone receptor: an orphan receptor fits the key. Trends Endocrinol Metab 11:299–303. [DOI] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O (2001) Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol 435:26–40. [DOI] [PubMed] [Google Scholar]
- Sanathara NM, Garau C, Alachkar A, Wang L, Wang Z, Nishimori K, Xu X, Civelli O (2018) Melanin concentrating hormone modulates oxytocin-mediated marble burying. Neuropharmacology 128:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Eckel LA (2008) The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol Behav 93:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FM, Kratzsch J, Gertz HJ, Tittmann M, Jahn I, Pietsch UC, Kaisers UX, Thiery J, Hegerl U, Schönknecht P (2013) Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer’s disease. Plos One 8:e63136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, Aghajanian GK, DiLeone RJ (2010) Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci 30: 8263–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsen JE, Als TD, Binderup H, Kruse TA, Wang AG, Vang M, Muir WJ, Blackwood DH, Mors O, Børglum AD (2006) Association analyses suggest GPR24 as a shared susceptibility gene for bipolar affective disorder and schizophrenia. Am J Med Genet B Neuropsychiatr Genet 141B:524–533. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Mori M, Sugo T, Ishibashi Y, Abe M, Kurokawa T, Onda H, Nishimura O, Sumino Y, Fujino M (1999) Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun 261:622–626. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL (2006) Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry 63:1325–1335. [DOI] [PubMed] [Google Scholar]
- Takase K, Kikuchi K, Tsuneoka Y, Oda S, Kuroda M, Funato H (2014) Meta-analysis of melanin-concentrating hormone signaling-deficient mice on behavioral and metabolic phenotypes. Plos One 9:e99961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas M, Pérez M, Monzón ME, de Barioglio SR (2002a) Melanin-concentrating hormone, hippocampal nitric oxide levels and memory retention. Peptides 23:2213–2221. [DOI] [PubMed] [Google Scholar]
- Varas M, Pérez M, Ramírez O, de Barioglio SR (2002b) Melanin concentrating hormone increase hippocampal synaptic transmission in the rat. Peptides 23:151–155. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Koenig JI (2014) Social interaction and social withdrawal in rodents as readouts for investigating the negative symptoms of schizophrenia. Eur Neuropsychopharmacol 24:759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]