Pilocytic astrocytoma (PA) is clinically considered and classified as a grade I, non-malignant tumor per the World Health Organization guidelines for brain and other central nervous system (CNS) tumors.1 Despite this classification, for the purposes of cancer registration reports within North America and by the International Agency for Research on Cancer and International Association of Cancer Registries, these tumors have historically been reported as malignant tumors.2,3 As a member of the cancer registration community, the Central Brain Tumor Registry of the United States (CBTRUS) has followed this practice in its reporting of primary tumors of the brain and other CNS locations, including its annual statistical reports, unless otherwise stated. This practice does not correlate with clinical classification assignment for PAs, and presents a challenge to correctly report population-based incidence and survival patterns associated with these tumors.
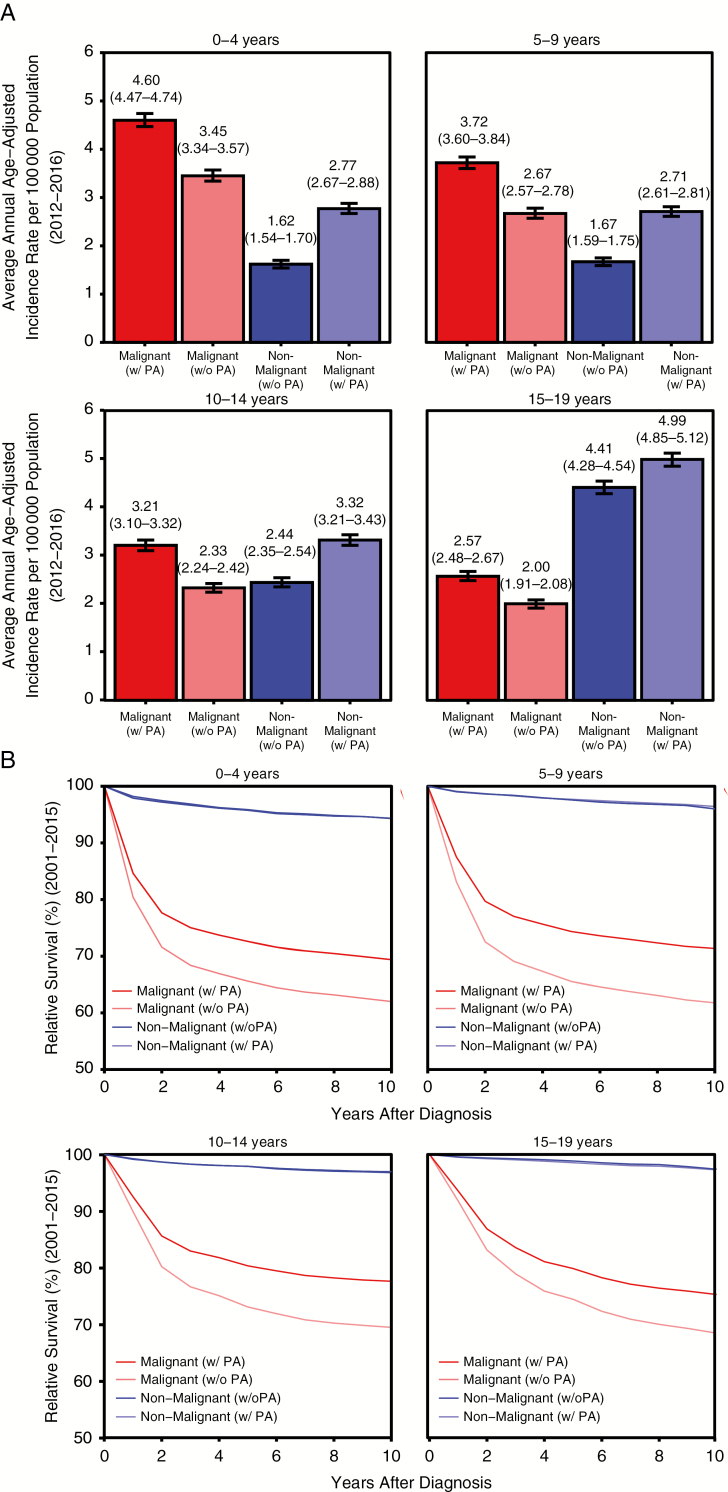
PAs are one of the most commonly diagnosed histologies of primary brain and other CNS tumors in children less than 20 years old, and have very high survival after diagnosis at most ages. When these tumors are classified in children ages 0–19 years as malignant, the average annual incidence rate (AAIR) of malignant tumors is 3.51 per 100 000 and 2.54 per 100 000 population for non-malignant tumors (Fig. 1A). After reclassification of PAs to non-malignant, the malignant tumor AAIR in this age group decreases 26% to 2.60 per 100 000 and the non-malignant tumor AAIR increases 36% to 3.45 per 100 000, resulting in non-malignant tumors being more common than malignant tumors among children. This pattern can be observed within all pediatric age subgroups (Fig. 1A), with the exception of children 0–4 years old, where malignant tumors remain the most common.
Fig. 1.
Effect of reclassification of PA from malignant to non-malignant by pediatric age groups and behavior for diagnoses in patients aged 0–19 years on (A) average annual incidence from 2012–2016 and (B) relative survival after diagnosis from 2004–2015.
Reclassification of PA also has a significant effect on estimates of relative survival (RS) after diagnosis in children 0–19 years old (Fig. 1B). With the current classification of PA as malignant, 5-year RS in malignant tumors is 76.0% and 5-year RS in non-malignant tumors is 97.9%. When PA is reclassified as a non-malignant tumor, 5-year RS in malignant tumors decreases by nearly 10% to 68.9%, while there is no substantial change in 5-year RS for non-malignant tumors (97.7%). The largest change in survival was in children 5–9 years old, where 5-year RS in malignant tumors decreased 12%, from 74.4% to 65.5% (Fig. 1B).
In adults 20 years and older, reclassification of PA has a smaller effect on AAIR and RS. When PA is classified as malignant, adult AAIR of malignant tumors is 8.52 per 100 000 and non-malignant AAIR is 21.88 per 100 000. When PA is classified as non-malignant, malignant AAIR decreases to 8.39 per 100 000 (decrease of 2%) and non-malignant AAIR increases to 22 per 100 000 (increase of 0.05%). The effect of reclassification on survival statistics in adults is also small. Reclassification of these tumors as non-malignant results in a decrease of 5-year RS for malignant tumors from 29.5% to 28.6% (3% decrease), while 5-year RS remains stable at 91.6%.
As the classification of primary brain tumors becomes increasingly refined through incorporation of molecular features into histologic classification,1 increased scrutiny of accuracy and completeness of reporting these tumors is warranted. Due to the heterogeneity of outcomes with varying brain and other CNS tumor histologies, reclassifying PA from malignant to non-malignant would have a significant effect on incidence and survival estimates. Using the classification of these tumors that is most clinically relevant (ie, non-malignant) increases the value of population-level cancer statistics for both public health surveillance and use of these statistics for guiding clinical decisions.
Funding
Q.T.O. is supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas (RP160097T). Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under contract no. 2016-M-9030, the American Brain Tumor Association, The Sontag Foundation, Novocure, Abbvie, the National Cancer Institute (NCI) under contract no. HHSN261201800176P, the Musella Foundation, National Brain Tumor Society, the Children’s Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in kind donations. The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the CDC or the NCI.
Conflict of interest statement. The authors declare that they have no conflict of interest.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Rous B. Histological groups. In: Bray F, Colombet M, Ferlay J, et al. , eds. Cancer Incidence in Five Continents Volume XI. Lyon, France: IARC; 2019. http://ci5.iarc.fr/CI5-XI/Pages/Chapter4.aspx. [Google Scholar]
- 3. Surveillance Epidemiology and End Results (SEER) Program. Solid tumor rules: non-malignant CNS 2019. https://seer.cancer.gov/tools/solidtumor/Non_Malignant_CNS_STM.pdf. [Google Scholar]