Abstract
Background
The impact of activating alterations in non–small cell lung cancer (NSCLC) (epidermal growth factor receptor [EGFR] mutation/anaplastic lymphoma kinase [ALK] translocation) in prognosticating patients with brain metastasis (BM) is not well defined. This study was sought to identify this impact in NSCLC patients with BM accounting for the known validated variables.
Methods
Among 1078 NSCLC-BM patients diagnosed/treated between January 1, 2000 and December 31, 2015, three hundred and forty-eight with known EGFR/ALK status were analyzed. Overall survival (OS) and intracranial progression-free survival (PFS) were measured from the time of BM.
Results
Ninety-one patients had either ALK (n = 23) alterations or EGFR (n = 68) mutation and 257 were wild type (WT; negative actionable mutations/alterations). Median age of EGFR/ALK+ NSCLC BM patients was 60 years (range 29.8–82.6 y) and ~50% (n = 44) had Karnofsky performance status (KPS) score >80. Median number of BM was 2 (1 to ≥99). The median OS for the ALK/EGFR+ NSCLC BM was 19.9 versus 10.1 months for the WT (P = 0.028). The number of BM in the EGFR/ALK+ group did not impact OS (BM = 1 with 21.1 months vs 2–3 with 19.1 months and >3 with 23.7 months, P = 0.74), whereas fewer BM in the WT cohort had significantly better OS (BM = 1 with 13.8 mo, 2–3 with 11.0 mo and >3 with 8.1 mo; P = 0.006) with the adjustment of age, KPS, symptoms from BM and synchronicity.
Conclusions
Number of BM does not impact outcomes in the EGFR/ALK+ NSCLC patients, implying that targeted therapy along with surgery and/or radiation may improve OS irrespective of the number of BM. Number of BM, extracranial metastasis (ECM), and KPS independently affected OS/PFS in WT NSCLC BM, which was consistent with the known literature.
Keywords: actionable mutations, ALK, EGFR, NSCLC, number of brain metastases, radiosurgery, targeted therapy, wild type
Importance of the Study.
The disease-specific graded prognostic assessment (DS-GPA) tool for NSCLC BM includes age, KPS, ECM, and number of BM. There is a paucity of literature in addressing the role of activating mutation/rearrangement status in NSCLC BM. Our study found significant prognostic differences between EGFR/ALK+ and WT patient cohorts with respect to these prognostic variables. The number of BM affected OS/PFS only in the WT cohort but had no impact in the EGFR and ALK positive patients. Recently, Sperduto et al revised their original DS-GPA scale to include genetic and molecular data, called Lung-molGPA, and interestingly, number of BM is still a prognostic factor in this patient cohort; however, no statistically significant correlation is demonstrated in their study. Results of our study provide insight into this interaction between the number of BM and genetic alterations and its overall impact on survival.
Key Points.
Number of brain metastases does not impact outcomes in EGFR/ALK+ NSCLC patients.
Known BM prognostic variables affected OS/PFS in wild-type NSCLC BM only.
Brain metastasis (BM) is a serious neurological sequela of systemic malignancies that affects quality of life and overall survival (OS) in this patient population. BM is the most common malignant brain tumor and the incidence rate varies from 8.3 to 14.3 per 100 000 based on population registry analysis.1,2 More than 50% of brain metastases occur in lung cancer patients,3 followed by those with diagnoses of breast cancer, melanoma, renal cancer, colorectal cancer, and cancer of unknown primary.4,5 BM is associated with a median survival that ranges from 3 to 48 months based on the type of cancer, known subtype, and the treatment modality.6–9 Given this wide variation in median survival among patients with BM, various models have been postulated to help predict prognosis in these patients.10 Recursive partitioning analysis, one of the early prognostic indices, did not consider the site of primary malignancy causing BM as a factor affecting survival in patients with BM.11 To address this limitation, a new disease-specific graded prognostic assessment (DS-GPA) tool was suggested that categorized patients based on primary malignancies.12 For lung cancer patients with brain metastases, age, performance status, extracranial metastases (ECM), and number of brain metastases were reported as significant factors that impact OS. According to this DS-GPA scale, patients with multiple BM (≥4) have inferior outcomes compared with single or limited BM (≤3) in patients with non–small cell lung cancer (NSCLC).6 More recently, this scale has been updated to include molecular markers in patients with NSCLC.13 However, there is a paucity of studies evaluating the role of driver mutations/alterations in NSCLC patients with BM.13
The incidence of tumor associated epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement varies from 10% (in the USA) to 35% (in East Asia) and 5–7%, respectively, in patients with NSCLC.14–18 The aim of our study was to evaluate the prognostic significance of different patient, tumor, and treatment characteristics in NSCLC patients with BM, for whom molecular profiling data were available. We also evaluated the impact of these alterations (EGFR mutation and ALK rearrangement) on outcomes of NSCLC BM patients, especially with respect to variables that were prognostic in the DS-GPA scale, such as number of BM.
Materials and Methods
Clinical Data and Treatment Characteristics
Following approval of the institutional review board at the Cleveland Clinic, patients with NSCLC BM and known mutational/rearrangement status treated between 2000 and 2015 were included in this retrospective study. Demographics, clinical characteristics (Karnofsky performance status [KPS], symptoms), tumor characteristics (presence/absence of ECM, number of BM, and synchronicity of BM with primary tumor), and treatment characteristics (radiation techniques, surgery, chemotherapy, targeted therapy, and temporal relationship to the local treatment for BM) were collected in an online database (REDCap). Patients with negative EGFR mutation and ALK rearrangement were labeled as wild type (WT) for the analysis.
Stereotactic radiosurgery (SRS) was delivered through Gamma Knife (Elekta Instruments). All patients underwent brain MRI (T1-weighted with gadolinium contrast) for evaluating the number of metastases and planning for SRS. Since 2007, the Gamma Knife model has been upgraded from Model B to Model C to Model 4C and Perfexion. The dose prescribed to the peripheral margin was typically chosen based on the maximum dimension for each lesion according to the Radiation Therapy Oncology Group 90-05 protocol.19 Whole brain radiation therapy (WBRT) was usually given at 30–37.5 Gy in 10–15 fractions. Erlotinib, gefitinib, and afatinib were the small-molecule tyrosine kinase inhibitors (TKIs) used in treating patients with EGFR mutation, whereas crizotinib and ceritinib were the TKIs used in patients with ALK rearrangement.
Mutational Analysis
EGFR mutation was tested using real-time PCR, and ALK rearrangement was tested using interphase fluorescence in situ hybridization (FISH). For EGFR mutation, real-time PCR was used to evaluate specific mutations, deletions, and insertions in the tyrosine kinase domain of the EGFR gene. Eight reactions using 30 primers were used to target specific regions of exons 18–21 as well as WT sequence. FISH for the ALK gene (2p23) involving a dual color, break-apart probe—consisting of a mixture of 2 FISH DNA probes hybridizing to the centromeric and telomeric sides on the ALK (2p23) gene (Abbott Molecular)—was used to detect the presence of a translocation involving ALK (2p23).
Outcome Variables and Endpoints
The primary outcome was OS, while the secondary outcome was progression-free survival (PFS). For the purpose of this study, intracranial progression-free survival was referred to as PFS. OS and PFS were calculated from the time of first brain metastasis. Radiographic data, including CT and MRI scans, were reviewed to determine intracranial patterns of failure. Local failures were diagnosed with a range of modalities, including serial imaging, MR perfusion to evaluate cerebral blood volume within the treated lesion, 2-fluoro-2-deoxy-D-glucose avidity via PET, and surgical pathology, as determined by the treating physician. Recurrence and response after treatment were assessed by the treating physicians using established criteria.20 In case of disagreement or if imaging was not clear, serial imaging follow-up was ordered or multidisciplinary tumor board review was used to identify local failures. Similarly, radiation necrosis was diagnosed using an institutional algorithm.21 Date of death was identified using electronic health records or an online database (Social Security Death Index). The date of last follow-up was considered for survival analysis if date of death was not available.
Statistical Analysis
Categorical data were analyzed as frequency counts and percentages, whereas measured data were evaluated using medians and ranges. Time-to-event data were summarized using Kaplan–Meier estimates and analyzed using log-rank tests and Cox proportional hazards models. Univariable and multivariable analyses were performed for variables such as clinical factors and mutational status of patients. A two-tailed P-value of <0.05 was considered significant. All analyses were performed using SAS v9.4.
Results
Patient Demographics
A total of 1078 patients treated at our institution from 2000 to 2015 for NSCLC BM were reviewed. Of these, 348 patients with known molecular mutational/rearrangement status were identified and included in our analysis. Patient characteristics are listed in Table 1. Among 348 patients, 91 (26%) were positive for a mutation/rearrangement (68 [75%] had an activating mutation in the EGFR and 23 [25%] had ALK rearrangement) and 257 (74%) were WT (no known identifiable actionable mutations). There were more smokers in the WT cohort (P < 0.001), whereas there was an equal distribution of smokers and nonsmokers in the EGFR/ALK+ cohort. Fifty percent (44/91) in the EGFR/ALK+ cohort had KPS > 80. Median number of BM was 2 (1–99) and the majority of patients had a single metastasis in both groups (37% in the EGFR/ALK+ and 46% in the WT cohort). There was a significant difference in terms of presence of ECM (71% of EGFR/ALK+ cohort vs 59% of WT, P = 0.04), symptoms from BM (63% of EGFR/ALK+ cohort vs 72% of WT, P = 0.36), and synchronicity in relation to the diagnosis of primary tumor between the 2 groups (57% of EGFR/ALK+ cohort vs 62% of WT, P < 0.001).
Table 1.
Patient and treatment characteristics
| Clinical Factors | EGFR/ALK+ NSCLC BM Cohort (n = 91) | Wild Type Cohort (n = 257) | P |
|---|---|---|---|
| Age, y, median (range) | 60.0 (29.8,82.6) | 60.8 (37.3,92.8) | 0.059 |
| Sex n (%) | 0.57 | ||
| Male | 38 (42) | 117 (45) | |
| Female | 53 (58) | 142 (55) | |
| Smoking status n (%) | <0.001 | ||
| Smokers | 47 (52) | 233 (91) | |
| Nonsmokers | 44 (48) | 24 (9) | |
| KPS n (%) | 0.90 | ||
| <70 | 9 (10) | 28 (12) | |
| 70–80 | 35 (40) | 109 (46) | |
| >80 | 44 (50) | 99 (42) | |
| No. of brain metastases # median (range) and n (%) | 2.0 (1.00, ≥99$) | 2.0 (1.00, ≥99$) | 0.23 |
| 1 | 34 (37) | 118 (46) | |
| 2–3 | 24 (27) | 70 (28) | |
| >3 | 33 (36) | 66 (26) | |
| Extracranial metastases (ECM) n (%) | 0.04 | ||
| No | 26 (29) | 104 (41) | |
| Yes | 65 (71) | 152 (59) | |
| Clinical symptoms n (%) | 0.036 | ||
| Asymptomatic | 33 (37) | 68 (28) | |
| Symptomatic | 56 (63) | 178 (72) | |
| Synchronous n (%) | <0.001 | ||
| No | 39 (43) | 97 (38) | |
| Yes | 52 (57) | 160 (62) | |
| First-line treatment n (%) | |||
| SRS ± surgery | 30 (33) | 98 (39) | 0.48 |
| WBRT ± surgery | 39 (43) | 125 (50) | 0.40 |
| WBRT + SRS | 15 (17) | 16 (5) | 0.005 |
| Other | 7(7) | 18(6) | |
| TKI, target therapy n (%) | 0.0001 | ||
| Yes | 45 (33) | 0 (0.0) | |
| No | 91(67) | 257(100) | |
| Timing of targeted therapy n (%) | |||
| ALK+ (N = 23) | EGFR+ (N = 68) | ||
| Before BM diagnosis | 4 (17) | 11 (17) | |
| After BM diagnosis | 15 (65) | 48 (73) | |
| as first line | 7 (47) | 38 (79) | |
| at progression | 8 (53) | 10 (21) | |
| Both | 0 (0.0) | 6 (9) | |
| Never | 4 (17) | 1 (1) |
#Evaluated by MRI brain with gadolinium contrast.
$≥99 denote at least 99 brain metastases, which can be physically counted on MRI brain with contrast.
Treatment Characteristics
Thirty-three percent of patients in the EGFR/ALK+ group and 39% of patients in the WT cohort were treated with SRS ± surgery as an upfront treatment. Whereas WBRT ± surgery was used as an upfront treatment in 43% of patients in the EGFR/ALK+ group and 50% of patients in the WT cohort. Fifteen patients (17%) in the EGFR/ALK+ cohort received a combination of SRS plus WBRT compared with 6% in the WT cohort (P < 0.005) (Table 1). Thirty-three percent of patients in the EGFR/ALK+ cohort received targeted treatment after the diagnosis of brain metastases as first-line therapy compared with none in the WT group (Table 1). Seventeen percent of patients received some form of targeted therapy before the diagnosis of BM in the EGFR/ALK+ groups. In the EGFR+ group alone, most patients (79%) received targeted therapy in the first-line setting, whereas only one fifth (21%) received it at disease progression. In contrast, half of the patients (50%) in the ALK+ group received targeted therapy in the first-line setting and the remainder at disease progression. There were 6 patients in the EGFR+ group who received targeted therapy before the diagnosis of BM and this therapy was continued after diagnosis of BM along with local treatment for BM. Four patients in the ALK+ group (17%) and one in the EGFR+ group (1%) did not receive any targeted therapy, due to either rapid progression of systemic disease or patient’s choice of palliative care (Table 1).
Progression-Free Survival and Overall Survival
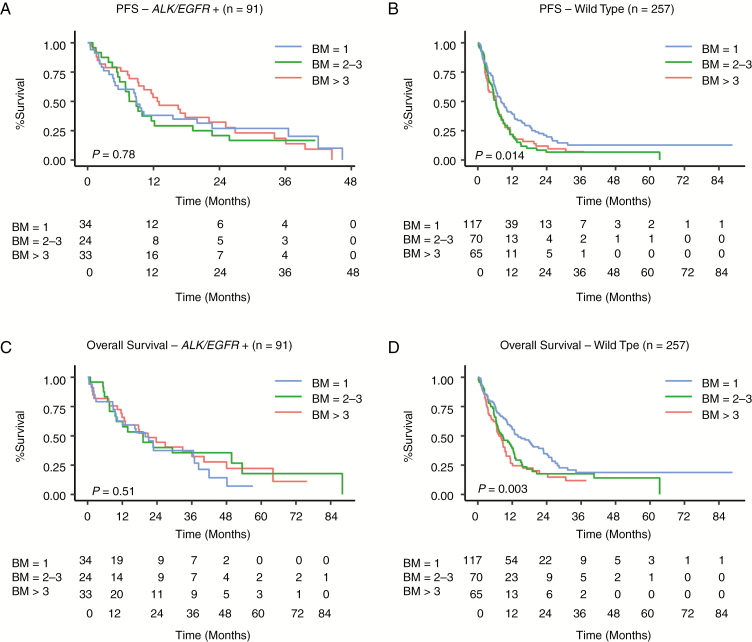
On univariate analysis, patients with poor performance status and those with presence of ECM had worse PFS in the EGFR/ALK+ group and WT group. However, the number of BM (1 BM vs 2–3 BM vs >3 BM) had no impact on PFS in the EGFR/ALK+ cohort (P = 0.78), whereas it was a statistically significant prognostic factor in the WT group (P = 0.014; Supplementary Table 1, Fig. 1A, B. The univariate analysis on PFS suggested that patients with a higher number of brain metastases in the WT cohort had a higher risk of disease progression.
Fig. 1.
Univariate analysis showing (A, B) PFS and (C, D) OS in the EGFR/ALK+ and WT cohorts, respectively.
The median OS for the ALK and EGFR positive NSCLC BM was 19.9 months compared with 10.1 months for the WT cohort (P = 0.028). When assessed independently, the median OS for the EGFR+ group was 19.1 months compared with 48.0 months in the ALK+ group (P = 0.13). The median OS for the ALK/EGFR + group of patients who received targeted therapy after the diagnosis of BM had significantly better OS and PFS compared with the patients who received targeted therapy prior to the diagnosis of BM (hazard ratio [HR], 0.39 [0.22–0.68], P = 0.0009, and HR 0.52 [0.30–0.89], P = 0.017) respectively). The presence of ECM negatively impacted OS in the WT but not in the EGFR/ALK+ cohort (Supplementary Table 1A and Fig. 1C, D). ECM was highly correlated with number of BM (P = 0.027) in the WT cohort but not in the EGFR/ALK+ group (P = 0.64) (Supplementary Table 1B). Based on the fact that ECM may be a cofounding factor for BM in the WT NSCLC, we designed 2 multivariate analysis models (one excluding ECM and one including ECM) to elucidate a prognostic model for those patients who present with BM alone and for those who present with both BM and ECM.
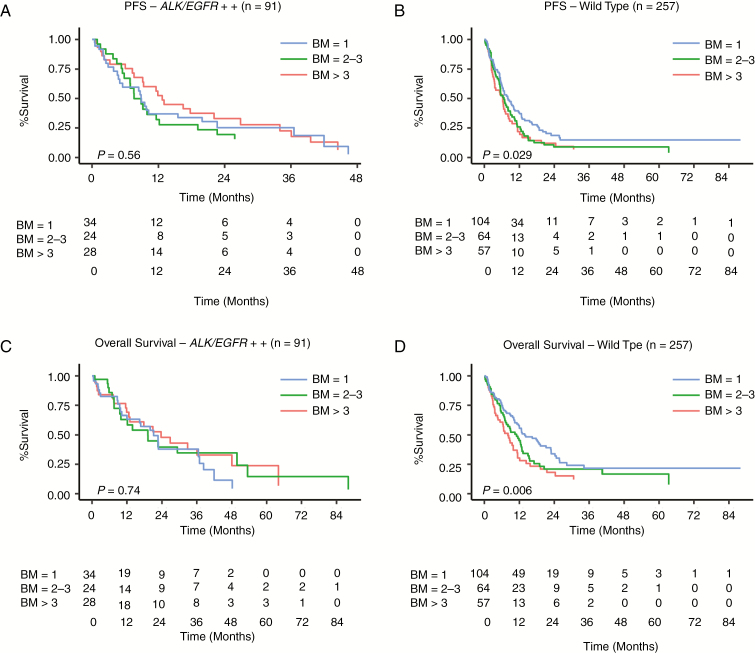
PFS and OS excluding ECM. On multivariate analysis, the number of brain metastases (1 BM vs 2–3 BM vs >3 BM) had no statistically significant impact on PFS or OS in the EGFR/ALK+ cohort, whereas it did in the WT group (Table 2, Fig. 2A–D). In the WT cohort, patients with single BM had significantly longer OS and PFS compared with those with 2–3 or >3 BM. However, there was no significant difference in OS/PFS in patients with 2–3 BM or >3 BM (Table 2, Fig. 2B and D, and Supplementary Table 2). Also, the estimated median survival in patients with a single BM in the WT cohort (Supplementary Table 2) was significantly higher than that of patients with >3 brain metastases (OS: 13.8 mo vs 8.1 mo, respectively, P = 0.007); however, no significant difference in OS was observed between 2–3 and >3 brain metastases (11 mo vs 8.1 mo, P = 0.38).
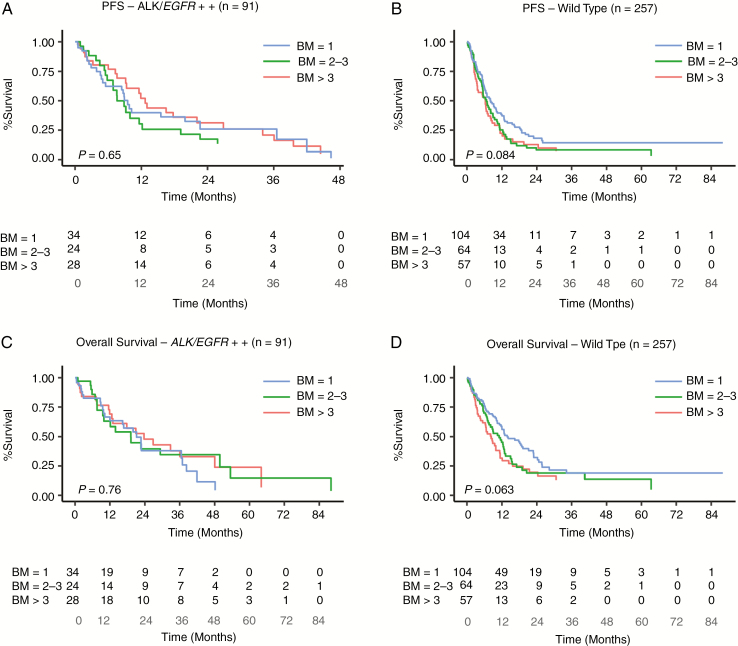
PFS and OS including ECM. On multivariate analysis, the number of BM did not impact PFS/OS in patients in the EGFR/ALK+ cohort but did marginally in the WT group (P = 0.08 and 0.06, respectively) (Tables 2 & 3 and Fig. 3A–D). After including ECM in the analysis, the number of BM (single, 2–3, or >3) had no significant impact on PFS/OS in patients in either the EGFR/ALK+ or WT cohorts. However, there was a trend toward significance (P = 0.09) in patients in the WT group with single BM and improved estimated median OS (Supplementary Table 3) which was in contrast to the EGFR/ALK+ group that retained insignificance regardless of presence or absence of ECM in the model. In the WT cohort, the previously significant impact of number of BM on OS/PFS became less significant after including ECM in the model. This was attributed to the statistically significant association between the number of BM and presence of ECM in the WT but not in the EGFR/ALK+ cohort (P = 0.027 vs 0.64).
Table 2.
Multivariable analysis excluding ECM
| PFS | EGFR/ALK+ NSCLC | P | Wild Type | P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (<50 vs 50–60 vs >60) | 0.83 (0.60, 1.14) | 0.24 | 0.91 (0.73, 1.14) | 0.42 |
| KPS (<70 vs 70–80 vs >80) | 0.65 (0.43, 1.00) | 0.05 | 0.73 (0.59, 0.92) | 0.006 |
| Brain mets (1 vs 2–3 vs >3) | 0.92 (0.69, 1.22) | 0.56 | 1.22 (1.02, 1.46) | 0.029 |
| Symptomatic | 1.11 (0.64, 1.92) | 0.71 | 1.05 (0.74, 1.49) | 0.80 |
| Synchronous | 0.75 (0.45, 1.24) | 0.26 | 0.79 (0.58, 1.07) | 0.12 |
| OS | ||||
| Age (<50 vs 50–60 vs >60) | 1.28 (0.88, 1.86) | 0.20 | 1.05 (0.84, 1.33) | 0.65 |
| KPS (<70 vs 70–80 vs >80) | 0.35 (0.22, 0.55) | <0.001 | 0.70 (0.56, 0.89) | 0.004 |
| Brain mets (1 vs 2–3 vs >3) | 0.95 (0.69, 1.31) | 0.74 | 1.31 (1.08, 1.59) | 0.006 |
| Symptomatic | 0.87 (0.48, 1.60) | 0.66 | 0.97 (0.67, 1.40) | 0.85 |
| Synchronous | 0.69 (0.40, 1.19) | 0.18 | 0.83 (0.60, 1.15) | 0.25 |
Fig. 2.
Multivariate analysis showing (A, B) PFS and (C, D) OS in the EGFR/ALK+ and WT cohorts, respectively, excluding patients with ECM.
Table 3.
Multivariable analysis including ECM
| PFS | EGFR/ALK+ NSCLC | P | Wild Type | P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (<50 vs 50–60 vs >60) | 0.81 (0.59, 1.13) | 0.21 | 0.92 (0.74, 1.15) | 0.48 |
| KPS (<70 vs 70–80 vs >80) | 0.59 (0.38, 0.92) | 0.02 | 0.72 (0.58, 0.90) | 0.004 |
| Brain mets (1 vs 2–3 vs >3) | 0.93 (0.70, 1.25) | 0.65 | 1.17 (0.98, 1.41) | 0.084 |
| Symptomatic | 1.06 (0.60, 1.87) | 0.84 | 1.13 (0.79, 1.61) | 0.50 |
| Synchronous | 0.72 (0.43, 1.18) | 0.19 | 0.78 (0.57, 1.06) | 0.11 |
| ECM (yes/no) | 0.51 (0.30, 0.87) | 0.014 | 1.41 (1.03, 1.94) | 0.031 |
| OS | ||||
| Age (<50 vs 50–60 vs >60) | 1.28 (0.88, 1.88) | 0.20 | 1.06 (0.84, 1.34) | 0.62 |
| KPS (<70 vs 70–80 vs >80) | 0.35 (0.22, 0.55) | <0.001 | 0.69 (0.54, 0.87) | 0.002 |
| Brain mets (1 vs 2–3 vs >3) | 0.95 (0.69, 1.31) | 0.76 | 1.20 (0.99, 1.46) | 0.063 |
| Symptomatic | 0.87 (0.48, 1.60) | 0.66 | 1.09 (0.75, 1.59) | 0.65 |
| Synchronous | 0.69 (0.40, 1.18) | 0.18 | 0.80 (0.58, 1.11) | 0.18 |
| ECM (yes/no) | 0.97 (0.54, 1.74) | 0.91 | 1.95 (1.37, 2.77) | <0.001 |
Fig. 3.
Multivariate analysis showing (A, B) PFS and (C, D) OS in the EGFR/ALK+ and WT cohorts, respectively, including patients with ECM.
Discussion
BM occurs in up to 40% of patients with systemic cancers based on clinical and autopsy studies.4,9,22–24 The management of BM has changed dramatically in the past few years and the majority of patients are being treated with SRS as part of multimodality.7,25–31 The blanket approach of treating >4 BM with WBRT is less common these days. Yamamoto et al32 reported that SRS without WBRT is suitable for patients with up to 10 BM, and OS in these patients was not different than in those with 2–4 BM. With improvement in radiation techniques along with availability of newer chemotherapeutic agents/targeted therapies that can reach better concentration in cerebrospinal fluid,33 it is likely that a higher number of BM would be amenable to treatment.34 The number of BM is a “moving target” in terms of management strategies and is a matter of contentious debate. In a classical study of 4259 patients,6 age, KPS, presence of ECM, and number of BM were identified as prognostic markers in patients with NSCLC and small cell lung cancer. However, there is a paucity of literature addressing the activating mutation/rearrangement status of BM and outcomes in patients with NSCLC.13,35–37
The results of our study suggest significant prognostic differences between EGFR/ALK+ and WT patient cohorts with NSCLC and BM. We found that the number of BM affected OS and PFS only in the WT cohort and had no impact in patients with positive actionable activating molecular alterations (EGFR and ALK respectively). In the WT patient cohort, OS was affected by the presence of ECM and there was a strong correlation between the number of BM and presence of ECM. We also noted significant differences in OS in patients (WT cohort) with single versus 2–3/ >3 BM compared with those with 2–3 BM versus >3 BM. We noted that the number of BM only tended to impact OS/PFS after excluding ECM in the model in patients with WT NSCLC BM. This finding can be attributed to the fact that the number of BM and the presence of ECM are correlated, thus reducing the significance of impact of number of BM on OS/PFS after including ECM in the analysis for the WT cohort.
Recently, Sperduto et al13 revised their original DS-GPA scale to include genetic and molecular data, called Lung-molGPA. This revised scale was based on their earlier multi-institutional retrospective study of 1521 patients with known alteration status in 816 patients (54%).13 They reported a median survival from first treatment of BM for the EGFR/ALK negative, EGFR+, ALK+, KRAS+, and unknown mutation status to be 14, 23 (P < 0.01), 45 (P < 0.001), 12 (P = 0.84), and 12 (P = 0.12) months, respectively.13 Interestingly, they described that the number of BM was still a prognostic factor in this patient cohort; however, no statistically significant correlation was demonstrated in their study.13 Results of our study provide insight into this interaction between the number of BM and genetic alterations and its overall impact on survival. Also, we tested the different DS-GPA variables among patients with positive actionable genetic alterations (EGFR/ALK) and without it (WT).
In this recently revised prognostic scale,13 patients with EGFR/ALK+ lung adenocarcinoma were given higher scores and those with Lung-molGPA scores of 3.5 to 4.0 had a longer median survival (46.8 mo) compared with those with scores of 0–1.0 (6.9 mo), 1.5–2.5 (13.7 mo), and 2.5–3.5 (26.5 mo).13 Concordantly, we have also identified activating mutational status (ALK rearrangement and EGFR mutation) as a possible positive prognostic indicator for NSCLC BM (median OS 19.9 mo vs 10.1 months for EGFR/ALK+ vs WT BM, respectively [P = 0.028]). This finding is similar to a median OS of 25.7 months in patients with EGFR positive BM compared with 3.8 months in patients with WT NSCLC BM.37 Interestingly, this study reported increased incidence of synchronous BM in patients with mutation positive NSCLC compared with those with WT. Also, the difference in OS was seen only in patients with synchronous BM compared with those with metachronous BM (14.6 mo EGFR MT vs 2.5 mo EGFR WT; P = 0.2). However, we noted increased incidence of synchronous BM in patients with WT BM compared with the EGFR/ALK+ group (62% vs 57%, respectively; P < 0.05), though there was no impact of this variable on OS/PFS in either of these cohorts. This difference can be attributed to the epidemiological variation in the frequency of mutations/rearrangements (Korean population vs USA population) and smaller sample size in the previous study.37
Another study reported improved OS in patients with mutation-positive NSCLC BM compared with those with WT BM (19.9 mo vs 7.1 mo; P < .001) in early onset BM.35 However, no such difference was noted in those with late onset (≥6 mo) BM after diagnosis.35 Eichler et al36 identified EGFR mutational status as an independent predictor of improved survival in NSCLC patients, with a median PFS of 12.7 months and median OS of 14.5 months.
Yuan et al35 reported single late onset BM and chemotherapy as positive predictors of survival in patients with WT NSCLC BM and systemic treatment only as a positive predictor in those with mutation-positive BM. In concordance, on multivariate analysis, we found that presence of ECM, KPS, and number of BM (tended toward significance) significantly affected OS/PFS in the WT cohort, whereas only KPS correlated with OS/PFS in the EGFR/ALK+ cohort. In our study, number of BMs had no correlation with either OS or PFS in the EGFR/ALK+ cohort (P = 0.51 and 0.78 respectively). However, number of BMs showed correlation with both OS and PFS in the WT cohort (P = 0.003 and 0.014 respectively). Our study demonstrated prognostic benefit of EGFR/ALK+ patients with NSCLC BM.
Implications for Revised GPA
Our study, as well as others,13,38 has shown that mutational status has an impact on the outcome of patients with NSCLC BM. EGFR/ALK+ NSCLC BM represents a diverse patient population with different prognostic factors and favorable outcome. More importantly, our study shows that the number of BM does not impact OS in EGFR and ALK positive NSCLC compared with the WT NSCLC brain metastases. Number of BM, presence of ECM, and KPS were independent predictors of OS/PFS in patients with WT NSCLC BM as seen in other studies.
Implications for Clinical Management and Role of TKIs
Various studies have shown that patients with EGFR/ALK+ NSCLC BM treated with TKIs have improved disease control and survival.38–40 Shaw et al40 reported that patients with ALK+ NSCLC BM, if not treated with an ALK inhibitor, crizotinib, had survival similar to those with WT patients, thus demonstrating the importance of ALK inhibitors in these patients. Also, Johung et al38 reported improved outcomes (OS: 49.5 mo and PFS: 11.5 mo) following a combination of radiation therapy (SRS/WBRT) and TKIs in patients with ALK rearranged NSCLC. With respect to the timing of targeted therapy, our results are consistent with recently published studies.13,38,41 In our study, EGFR-mutant and ALK+ patients who received TKIs prior to diagnosis of BM had significantly worse outcomes compared with those who received TKIs after the diagnosis of BM, which can be attributed to the selection of TKI-resistant niche in the brain along with the possibility of an aggressive disease.
The effect of crizotinib on CNS disease is poorly understood.42,43 Other TKI agents such as certinib44 and alectinib45 have been investigated in patients with crizotinib-resistant ALK+ tumors. Second-generation ALK inhibitors such as brigatinib have been shown to have improved CNS penetration with improved responses in patients with crizotinib treated NSCLC, with the highest intracranial PFS in patients treated with 180 mg once daily.46 Availability of these second-generation ALK inhibitors made it possible to treat these patients with multiple BMs/ECMs and reduce the disease burden to the level that can be treated with stereotactic radiation, while keeping WBRT as a reserve in these patients.
Results of our study showed that patients with actionable mutations/alterations were likely to respond better to new-generation TKIs with good CSF penetration (second generation) irrespective of number of BMs/ECMs compared with those with WT BMs. Therefore number of BMs had impact on prognosis in patients with WT BMs by increasing the burden of CNS disease with increasing number of BMs and dependence on radiation treatment only, till we have chemotherapeutic options available for this cohort of patients. Number of BM has traditionally been considered a bad prognostic factor and patients with a large number of BM were likely to be treated with WBRT, which has long-term deleterious effects; the results of our study challenge this practice in patients with actionable mutations/alterations.
Limitations
The potential limitations of our study include its single institutional retrospective design, non-homogeneous management strategies, lack of randomization, unknown kras mutational status, and variability in the extent of follow-up. Though it is known that patients with ALK+ NSCLC BM have a more favorable prognosis than those with EGFR+ NSCLC BM,38 we grouped them together, since both of these alterations can be treated with targetable agents and have better prognosis than the wild type. Nevertheless, our results will provide a reference to prognosticate the outcomes based on the mutational status and number of BM in patients with NSCLC. We acknowledge that we have not compared our results with the Lung-molGPA scale as described by Sperduto et al,13 which is based on number of BM (being a significant negative prognostic factor); however, this would be the focus of our future studies.
Conclusion
The number of BM does not impact OS in EGFR/ALK+ NSCLC, implying that targeted therapy along with surgery and/or radiation may improve OS regardless of the number of BM, whereas lack of activating actionable molecular alterations negates use of targeted treatment and would convey poor prognosis. Number of BM, presence of ECM, and KPS were independent predictors of OS/PFS in patients with wild type NSCLC BM.
Funding
There was no funding received for this project.
Conflict of interest statement.
Manmeet Ahluwalia: Receipt of grants/research supports: Astrazeneca, Abbvie, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Merck.Receipt of honoraria or consultation fees: Elsevier, Wiley, Astrazeneca, Abvvie, VBI Vaccines, Flatiron, Varian Medical Systems, Prime Education, Bayer, karyopharm, Tocagen, Forma therapeutics.Stock shareholder: Doctible, Mimivax.
Samuel T Chao: Honorarium from Varian Medical Systems.
Nathan Pennell: Consulting/advisory for BMS, Merck, Astrazeneca, Eli Lilly.
Alireza Mohammadi: consultant for Monteris Medical Company.
Rupesh Kotecha: Advisory board/honorarium/speaking engagements: Novocure, Accuracy Inc., Elekta AB.
John Suh: Consultant for Abbvie.
Michael Vogelbaum: Indirect Equity and Royalties: Infuseon Therapeutics.
Honoraria: Celgene, Tocagen, Blue Earth Diagnostics.
No other authors report conflicts of interest.
Authorship statement. S.K.B. designed the research, analyzed the data, and wrote the paper. M.S., V.A.V., P.S., R.K., S.T.C., J.H.S., L.A., A.M.Z., M.A.V., G.H.B., and N.A.P. helped with interpretation of the results and edited the manuscript. S.K.B., V.A.V., and P.S collected clinical data. X.J. helped with statistics. M.S.A. designed the study and wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Paul Elson for his advice on statistics. This study was an oral abstract at ASCO 2016, in Chicago.
References
- 1. Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology. 1985;35(2):219–226. [DOI] [PubMed] [Google Scholar]
- 2. Counsell CE, Collie DA, Grant R. Incidence of intracranial tumours in the Lothian region of Scotland, 1989-90. J Neurol Neurosurg Psychiatry. 1996;61(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Venur VA, Karivedu V, Ahluwalia MS. Systemic therapy for brain metastases. Handb Clin Neurol. 2018;149:137–153. [DOI] [PubMed] [Google Scholar]
- 4. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 5. Wen P, Black P, Loeffler J. Cancer: principles and practice of oncology (ed 6). In: DeVita V, Hellman S, Rosenberg SA, eds. Metastatic Brain Cancer. Philadelphia, PA: Lippincott, WIlliams, and Wilkins; 2001. [Google Scholar]
- 6. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 7. Soon YY, Tham IW, Lim KH, Koh WY, Lu JJ. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. 2014(3):CD009454–CD009454. doi:10.1002/14651858.CD009454.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 9. Schüttrumpf LH, Niyazi M, Nachbichler SB, et al. Prognostic factors for survival and radiation necrosis after stereotactic radiosurgery alone or in combination with whole brain radiation therapy for 1-3 cerebral metastases. Radiat Oncol. 2014;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venur VA, Ahluwalia MS. Prognostic scores for brain metastasis patients: use in clinical practice and trial design. Chin Clin Oncol. 2015;4(2):18. [DOI] [PubMed] [Google Scholar]
- 11. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. [DOI] [PubMed] [Google Scholar]
- 12. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. 2017;3(6):827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balasubramanian SK, Chao ST, Mohammadi AM, Angelov L, Elson P, Ahluwalia MS. Outcomes of patients with EGFR-positive non–small cell lung cancer brain metastases. J Natl Compr Cancer Netw. 2016;14(5S):e-1–e-30. [Google Scholar]
- 15. Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21(10):2227–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. [DOI] [PubMed] [Google Scholar]
- 17. Riely GJ, Helena AY. EGFR: the paradigm of an oncogene-driven lung cancer. Clin Cancer Res. 2015;21(10):2221–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. [DOI] [PubMed] [Google Scholar]
- 19. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. [DOI] [PubMed] [Google Scholar]
- 20. Lin NU, Lee EQ, Aoyama H, et al. ; Response Assessment in Neuro-Oncology (RANO) group Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–e278. [DOI] [PubMed] [Google Scholar]
- 21. Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–457. [DOI] [PubMed] [Google Scholar]
- 22. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–540. [DOI] [PubMed] [Google Scholar]
- 23. Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249(10):1357–1369. [DOI] [PubMed] [Google Scholar]
- 24. Arvold ND, Lee EQ, Mehta MP, et al. Updates in the management of brain metastases. Neuro Oncol. 2016;18(8):1043–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. 2012:Cd006121. doi:10.1002/14651858.CD006121.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunter GK, Suh JH, Reuther AM, et al. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):1394–1398. [DOI] [PubMed] [Google Scholar]
- 28. Trifiletti DM, Lee CC, Kano H, et al. Stereotactic radiosurgery for brainstem metastases: an international cooperative study to define response and toxicity. Int J Radiat Oncol Biol Phys. 2016;96(2):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahluwalia MS, Chao ST, Parsons MW, et al. Phase II trial of sunitinib as adjuvant therapy after stereotactic radiosurgery in patients with 1-3 newly diagnosed brain metastases. J Neurooncol. 2015;124(3):485–491. [DOI] [PubMed] [Google Scholar]
- 30. Kotecha R, Vogel S, Suh JH, et al. A cure is possible: a study of 10-year survivors of brain metastases. J Neurooncol. 2016;129(3):545–555. [DOI] [PubMed] [Google Scholar]
- 31. Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor–naïve epidermal growth factor receptor–mutant non–small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi:10.1200/jco.2016.69.7144 [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. [DOI] [PubMed] [Google Scholar]
- 33. Thapa B, Lauko A, Desai K, Venur VA, Ahluwalia MS. Novel systemic treatments for brain metastases from lung cancer. Curr Treat Options Neurol. 2018;20(11):48. [DOI] [PubMed] [Google Scholar]
- 34. Venur VA, Ahluwalia MS. Targeted therapy in brain metastases: ready for primetime? Am Soc Clin Oncol Educ Book. 2016;35:e123–e130. [DOI] [PubMed] [Google Scholar]
- 35. Yuan R, Yamada A, Weber B, Ho C. Radiographic patterns and survival of patients with early and late brain metastases in EGFR wild type and mutant non small cell lung cancer. J Neurooncol. 2016;127(3):525–533. [DOI] [PubMed] [Google Scholar]
- 36. Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12(11):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baek MY, Ahn HK, Park KR, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med. 2018;33(1):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johung KL, Yeh N, Desai NB, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2016;34(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mak KS, Gainor JF, Niemierko A, et al. Significance of targeted therapy and genetic alterations in EGFR, ALK, or KRAS on survival in patients with non-small cell lung cancer treated with radiotherapy for brain metastases. Neuro Oncol. 2015;17(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rothenstein JM, Letarte N. Managing treatment-related adverse events associated with Alk inhibitors. Curr Oncol. 2014;21(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaw AT, Gandhi L, Gadgeel S, et al. ; study investigators Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Camidge DR, Kim DW, Tiseo M, et al. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol. 2018;36(26):2693–2701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.