Summary
Background
Hydroxychloroquine, a drug commonly used in the treatment of rheumatoid arthritis, has received much negative publicity for adverse events associated with its authorisation for emergency use to treat patients with COVID-19 pneumonia. We studied the safety of hydroxychloroquine, alone and in combination with azithromycin, to determine the risk associated with its use in routine care in patients with rheumatoid arthritis.
Methods
In this multinational, retrospective study, new user cohort studies in patients with rheumatoid arthritis aged 18 years or older and initiating hydroxychloroquine were compared with those initiating sulfasalazine and followed up over 30 days, with 16 severe adverse events studied. Self-controlled case series were done to further establish safety in wider populations, and included all users of hydroxychloroquine regardless of rheumatoid arthritis status or indication. Separately, severe adverse events associated with hydroxychloroquine plus azithromycin (compared with hydroxychloroquine plus amoxicillin) were studied. Data comprised 14 sources of claims data or electronic medical records from Germany, Japan, the Netherlands, Spain, the UK, and the USA. Propensity score stratification and calibration using negative control outcomes were used to address confounding. Cox models were fitted to estimate calibrated hazard ratios (HRs) according to drug use. Estimates were pooled where the I2 value was less than 0·4.
Findings
The study included 956 374 users of hydroxychloroquine, 310 350 users of sulfasalazine, 323 122 users of hydroxychloroquine plus azithromycin, and 351 956 users of hydroxychloroquine plus amoxicillin. No excess risk of severe adverse events was identified when 30-day hydroxychloroquine and sulfasalazine use were compared. Self-controlled case series confirmed these findings. However, long-term use of hydroxychloroquine appeared to be associated with increased cardiovascular mortality (calibrated HR 1·65 [95% CI 1·12–2·44]). Addition of azithromycin appeared to be associated with an increased risk of 30-day cardiovascular mortality (calibrated HR 2·19 [95% CI 1·22–3·95]), chest pain or angina (1·15 [1·05–1·26]), and heart failure (1·22 [1·02–1·45]).
Interpretation
Hydroxychloroquine treatment appears to have no increased risk in the short term among patients with rheumatoid arthritis, but in the long term it appears to be associated with excess cardiovascular mortality. The addition of azithromycin increases the risk of heart failure and cardiovascular mortality even in the short term. We call for careful consideration of the benefit–risk trade-off when counselling those on hydroxychloroquine treatment.
Funding
National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, NIHR Senior Research Fellowship programme, US National Institutes of Health, US Department of Veterans Affairs, Janssen Research and Development, IQVIA, Korea Health Industry Development Institute through the Ministry of Health and Welfare Republic of Korea, Versus Arthritis, UK Medical Research Council Doctoral Training Partnership, Foundation Alfonso Martin Escudero, Innovation Fund Denmark, Novo Nordisk Foundation, Singapore Ministry of Health's National Medical Research Council Open Fund Large Collaborative Grant, VINCI, Innovative Medicines Initiative 2 Joint Undertaking, EU's Horizon 2020 research and innovation programme, and European Federation of Pharmaceutical Industries and Associations.
Research in context.
Evidence before this study
We systematically searched PubMed, Embase, clinical trial registries (ClinicalTrials.gov, the International Clinical Trials Registry Platform Search Portal, and the Chinese Clinical Trial Registry), and preprint servers (bioRxiv and medRxiv) from inception until March 27, 2020 (appendix pp 126–30) for research articles in English, Chinese, Spanish, and Italian (see appendix p 126 for search terms). No contemporary large-scale evidence was found that investigated the real-world safety of hydroxychloroquine compared with other first-line disease-modifying antirheumatic drugs, especially in combination with macrolide antibiotics such as azithromycin, which have been proposed for use as a treatment for COVID-19. Systematic reviews that have informed European guidelines focused on severe adverse events associated with biological therapies with little high-level evidence focused on hydroxychloroquine. Severe cardiovascular adverse events, mostly lethal arrhythmias and heart failure, have been described in independent retrospective case series and case reports, and reported within the US Food and Drug Administration adverse events database.
Added value of this study
This study uses state-of-the-art methods to control for residual confounding and bias and shows comparable results across 14 international health databases. Hydroxychloroquine does not seem to confer increased risk when used in patients with rheumatoid arthritis without contraindications in the short term (up to 30 days) compared with sulfasalazine, but confers an increased risk of cardiovascular mortality when used long term. Short-term treatment with hydroxychloroquine plus azithromycin appears to be associated with elevated risk of cardiovascular mortality, angina, and heart failure compared with hydroxychloroquine plus amoxicillin.
Implications of all the available evidence
Short-term use of hydroxychloroquine appears to confer no increased risk in patients with rheumatoid arthritis without contraindications, but hydroxychloroquine in combination with azithromycin appears to be associated with serious cardiovascular adverse events and should therefore be used with caution.
Introduction
Hydroxychloroquine, which is most commonly used as the first-line treatment in patients with autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE), has gained extensive media coverage as a potential antiviral agent for use against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19.1, 2, 3, 4, 5 Unfortunately, the exponential generation of research into hydroxychloroquine has led to confusion in the rheumatological community regarding the safety implications of hydroxychloroquine within its traditional uses.
Early in the COVID-19 pandemic, publicity focused on a study from France6 showing faster recovery and reduction in viral load in patients treated with high-dose hydroxychloroquine plus azithromycin, a macrolide antibiotic, compared with patients receiving standard treatment available at the time. This report led to widespread use of high-dose hydroxychloroquine either alone or with azithromycin. Subsequently, serious cardiac adverse events associated with QT segment prolongation that could lead to potentially lethal arrhythmia and cardiovascular-related death were identified in patients taking hydroxychloroquine in several health-care centres in the USA and Brazil.7, 8, 9, 10 Because of these reports of increased risk, emergency authorisation of hydroxychloroquine by medicines regulators was retracted, statements cautioning against hydroxychloroquine use were released, and randomised trials were stopped.10, 11, 12, 13, 14, 15
European guidelines for the treatment of patients with rheumatoid arthritis contain little high-level evidence for the safety of hydroxychloroquine, and most systematic reviews of rheumatoid arthritis treatments have focused on biological therapies.16, 17 Before the COVID-19 pandemic, evidence for hydroxychloroquine safety was largely found in retrospective case series and case reports, or within pharmaceutical adverse events registers.18, 19, 20 Azithromycin and macrolides in general are also known to induce cardiotoxicity and to interact with other drugs that prolong QTc.21, 22, 23
The combination of minimal large-scale hydroxychloroquine safety studies before this pandemic, and the extensive research suggesting risks associated with hydroxychloroquine use that has been produced during 2020 is of great concern to both patients and clinicians. We therefore aimed to assess the safety of hydroxychloroquine alone compared with sulfasalazine and of hydroxychloroquine in combination with azithromycin (compared with hydroxychloroquine in combination with amoxicillin), in part to provide clarity for patients taking hydroxychloroquine for rheumatoid arthritis.
Methods
Study design and participants
In this multinational, retrospective study, new user cohort studies were used as recommended by methodological guidelines24 for observational drug safety research to estimate the safety of hydroxychloroquine alone or in combination with macrolide antibiotics in patients with rheumatoid arthritis. Sulfasalazine and amoxicillin were chosen as active comparators because they have similar indications as the target treatments (hydroxychloroquine and azithromycin, respectively). Participants were included if they had a history of rheumatoid arthritis (a condition occurrence or observation indicating rheumatoid arthritis any time before or on the same day as therapy initiation), were aged 18 years or older at the index event, and had at least 365 days of continuous observation time before the index event.
As a secondary analysis, a self-controlled case series was used to estimate the safety of hydroxychloroquine in the wider population, including patients without rheumatoid arthritis. For this analysis, all prevalent users of hydroxychloroquine were included, regardless of rheumatoid arthritis status or indication.
All data partners received approval or waiver from their institutional review boards in accordance with their institutional governance guidelines. The full study protocol is available online.
Data sources
Electronic health records (EHRs) and administrative claims data were mapped to the Observational Medical Outcomes Partnership common data model (version 5.0 or higher) and analysed in a distributed network as part of an international effort with the Observational Health Data Science and Informatics community, including 14 databases: IQVIA (Durham, NC, USA) Disease Analyzer Germany (ambulatory electronic medical record [EMR] from Germany); Japanese Medical Data Center Claims Database (Tokyo, Japan); Integrated Primary Care Information (IPCI; Rotterdam, Netherlands; primary care EMR); Information System for the Development of Research in Primary Care (SIDIAP; Barcelona, Spain; primary care EMR); Clinical Practice Research Datalink (CPRD; London, UK) and IQVIA UK (London, UK) Integrated Medical Record Data (IMRD; primary care EMRs); and IBM MarketScan (Somers, NY, USA) Commercial Claims and Encounters (CCAE), Optum (Eden Prairie, MN, USA) de-identified Clinformatics Data Mart Database (Clinformatics), Optum EHR (Optum de-identified Electronic Health Record dataset), IBM MarketScan Medicare Supplemental Database (MDCR), IBM MarketScan Multi-State Medicaid Database (MDCD), IQVIA Open Claims, US Department of Veterans Affairs (VA; Salt Lake City, UT, USA), and IQVIA US Ambulatory EMR (USA).
Self-controlled case series were done on a subset of these databases as a secondary analysis: CCAE, CPRD, Clinformatics, MDCD, MDCR, and VA. A description of these data sources is available in the appendix (pp 3–4).
Study period and outcomes
The study period started from Sept 1, 2000, and ended at the latest available date for all data sources in 2020. Follow-up for each of the cohorts started at an index date defined by the first dispensing or prescription of the target or comparator drug as described in the cohort definitions (appendix pp 5–8). Two periods were considered to define time at risk. For a short-term, intention-to-treat analysis, follow-up started 1 day after the index date and continued until the first of: outcome of interest, loss to follow-up, or 30 days after the index date to resemble the duration of COVID-19 treatment regimens.6 For a longer-term, on-treatment analysis, follow-up started 1 day after the index date and continued until the earliest of: outcome of interest, loss to follow-up, or discontinuation, with an added washout time of 14 days. Continued use of the same treatment was inferred by allowing up to 90-day gaps between dispensing or prescription records. Additional detail on the exposure cohorts is available in the appendix (pp 5–8).
For self-controlled case series, periods of persistent exposure to hydroxychloroquine were generated allowing up to 90-day gaps between dispensing or prescription records. Patients were followed up for their entire observation time (eg, from enrolment to disenrolment in each database), and rates of each of the outcomes calculated in periods of exposure and non-exposure time.
The proposed code lists for the identification of the study population and for the study exposures were created by clinicians with experience in the management of rheumatoid arthritis using ATLAS, and reviewed by four clinicians and one epidemiologist.25
16 severe adverse events were analysed. Hospital-based events, which are not available in primary care records (CPRD, IMRD, and SIDIAP), included gastrointestinal bleeding, acute renal failure, acute pancreatitis, myocardial infarction, stroke, transient ischaemic attack, and cardiovascular events (composite). Additionally, angina or chest pain, heart failure, cardiac arrhythmia, bradycardia, venous thromboembolism, end-stage renal disease, and hepatic failure were analysed from both primary and secondary care data. All-cause mortality outcomes were obtained only from data sources with reliable information on death date (CPRD, IMRD, IPCI, Clinformatics, SIDIAP, and VA) and cardiovascular mortality outcomes from sources with information on cardiovascular events preceding death (CPRD, IMRD, Clinformatics, and VA). All codes for the identification of the 16 proposed study outcomes were based on a previously published paper26 and are detailed in the appendix (pp 8–9). Face validity for each of the outcome cohorts was further reviewed and compared with previous clinical knowledge and existing literature.
A list of negative control outcomes was also assessed for which there is no known causal relationship with any of the drugs of interest. These outcomes were identified using a semi-automatic process based on data extracted from the literature, product labels, and spontaneous reports, and confirmed by manual review by three clinicians (JCEL, AP-U, and DP-A).27 A full list of the codes that were used to identify negative control outcomes and details on covariate and confounder identification are provided in the appendix (pp 10–11).
Statistical analysis
We used propensity score stratification (into quintiles) to adjust for observed confounders, using a large-scale regularised logistic regression fitted with a LASSO penalty and with the optimal hyperparameter determined through ten-fold cross-validation.28 Baseline patient characteristics were constructed for inclusion as potentially confounding covariates.29 Predictor variables included were based on all observed patient characteristics as available in each data source, including conditions, procedures, visits, observations, and measurements. We plotted the propensity score distribution and assessed covariate balance expressed as the standardised difference of the mean for every covariate before and after propensity score stratification. A standardised difference of more than 0·1 indicated a non-negligible imbalance between exposure cohorts.30 Cox proportional hazards models conditioned on the propensity score strata were fitted to estimate hazard ratios (HRs) according to treatment status. Negative control outcomes analyses and empirical calibration were used to minimise potential unresolved confounding, with calibrated HRs and 95% CIs estimated.31, 32
For self-controlled case series, safety of hydroxychloroquine therapy was assessed separately as a secondary analysis, regardless of indication, comparing exposed and unexposed time periods within the same individuals. The method is self-controlled in that it makes within-person comparisons of event rates during periods of hypothesised increased risk with other periods of baseline risk, which eliminates all time-invariant confounding. Because we do not compare between individuals, the self-controlled case series is robust to between-person differences, even including unmeasured differences (such as genetics). However, the method is vulnerable to time-varying confounders. To adjust for this confounding, we included many time-varying covariates in the models, including age, season, and other drug exposures. A conditional Poisson regression was used to fit the outcome model using the Cyclops package (version 2.0), with a hyperparameter selected through ten-fold cross-validation.33
Study diagnostics (power, propensity score distribution, covariate balance, and empirical null distribution) were evaluated by clinicians and epidemiologists to determine which database target comparator outcome analysis variants could produce unbiased estimates (appendix pp 104–18). Analyses with zero event outcomes or with confounder imbalances with standardised mean difference of more than 0·1 after stratification were excluded from analysis. All analyses were conducted for each database separately, with estimates combined in random-effects meta-analysis methods where the I2 value was less than 0·4.34 The standard errors of the database-specific estimates were adjusted to incorporate estimate variation across databases, where the across-database variance was estimated by comparing each database-specific result to that of an inverse-variance, fixed-effects meta-analysis. No meta-analysis was done where I2 for a given drug–outcome pair was 0·4 or more. Of note, when running analysis in a distributed network, it was not possible to link across datasets, and to know the extent of overlap between data.
Small cell counts (n) of less than five (and resulting estimates) are reported as <n to minimise risk of re-identification. For the cohort analysis, the CohortMethod package (version 3.1.0) was used as well as the Cyclops package (version 2.0) for propensity score estimation.33 All self-controlled case series were run using the self-controlled case series package.35 The full source code for analyses is available online.
This study is registered with the EU Post-Authorisation Studies Register, EUPAS34497.36
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit for publication. All authors had full access to aggregated data in the study, and the lead and senior authors (JCEL, JW, PRy, and DP-A) had final responsibility for the decision to submit for publication.
Results
956 374 hydroxychloroquine and 310 350 sulfasalazine users were identified, and 323 122 and 351 956 contributed to the analyses of combination therapy of hydroxychloroquine plus azithromycin compared with hydroxychloroquine plus amoxicillin, respectively. Participant counts in each data source are provided in the appendix (pp 13–65). Duration of hydroxychloroquine therapy in the long-term analysis varied between databases, and ranged from a median of 43 days (IQR 43–193) in IQVIA US Ambulatory EMR to 338 days (106–1507) in CPRD. Full details can be found in the power tab for each database online.
Compared with sulfasalazine, users of hydroxychloroquine were more likely to be female (eg, 82·0% vs 74·3% in CCAE) and less likely to have certain comorbidities such as Crohn's disease (0·6% vs 1·8% in CCAE) or psoriasis (3·0% vs 8·9% in CCAE; appendix pp 15–16). In CCAE, the mean baseline dose for hydroxychloroquine was 420 mg (SD 463), and 2·8% of patients had an estimated dose of more than 500 mg. All differences were minimised after propensity score stratification, with all reported analyses balanced on all identified confounders. For example, systemic corticosteroid use or a diagnosis of SLE in the year before hydroxychloroquine or sulfasalazine use before propensity score matching was imbalanced but was balanced through propensity score stratification. Full details of all of the variables used within the propensity score are available in the shiny application (population characteristics tab, searching for the variable within the raw setting). Similarly, users of combination hydroxychloroquine plus azithromycin differed from those of hydroxychloroquine plus amoxicillin, with a higher prevalence of acute respiratory disease among azithromycin users (eg, 62·5% vs 50·7% in CCAE; appendix p 43). Again, propensity score methods mitigated these differences, and comparison groups became balanced for all observed confounders after stratification. Detailed baseline characteristics for the two pairs of treatment groups after propensity score stratification in CCAE are detailed in table 1 for illustrative purposes, and a complete list of features for each database comparing before and after propensity score stratification are provided in the appendix (pp 13–65). Propensity score distribution plots and negative control outcome analyses can be found in the appendix (pp 104–118) in addition to all elements of the propensity model and Kaplan-Meier analyses.
Table 1.
Baseline characteristics of users of HCQ versus SSZ, and HCQ plus AZM versus HCQ plus AMX after propensity score stratification in the CCAE database
|
HCQ vs SSZ |
HCQ plus AZM vs HCQ plus AMX |
|||||
|---|---|---|---|---|---|---|
| HCQ (n=66 604) | SSZ (n=22 370) | Standardised mean difference | HCQ plus AZM (n=32 586) | HCQ plus AMX (n=32 496) | Standard mean difference | |
| Age, years | ||||||
| 15–19 | 0·6% | 0·6% | 0·00 | 0·5% | 0·5% | <0·00 |
| 20–24 | 1·8% | 2·0% | −0·01 | 1·4% | 1·4% | <0·00 |
| 25–29 | 2·5% | 2·7% | −0·01 | 2·2% | 2·2% | <0·00 |
| 30–34 | 4·5% | 4·4% | <0·00 | 4·0% | 3·9% | 0·01 |
| 35–39 | 7·1% | 7·1% | 0·00 | 6·8% | 6·7% | <0·00 |
| 40–44 | 9·7% | 9·5% | 0·01 | 9·3% | 9·3% | <0·00 |
| 45–49 | 13·6% | 13·4% | <0·00 | 13·2% | 13·3% | <0·00 |
| 50–54 | 18·2% | 18·1% | 0·01 | 18·1% | 18·0% | <0·00 |
| 55–59 | 20·8% | 20·8% | <0·00 | 21·5% | 21·8% | −0·01 |
| 60–64 | 19·4% | 19·8% | −0·01 | 21·1% | 21·1% | <0·00 |
| 65–69 | 1·8% | 1·6% | 0·01 | 2·0% | 2·0% | <0·00 |
| Sex | ||||||
| Female | 80·1% | 79·7% | 0·01 | 86·3% | 86·2% | 0·00 |
| Male | 19·9% | 20·3% | 0·01 | 13·7% | 13·8% | 0·00 |
| Medical history: general | ||||||
| Chronic obstructive lung disease | 4·3% | 4·5% | −0·01 | 5·0% | 5·2% | −0·01 |
| Depressive disorder | 13·3% | 13·5% | <0·00 | 14·7% | 14·8% | <0·00 |
| Diabetes | 13·6% | 13·8% | −0·01 | 13·2% | 13·1% | <0·00 |
| Hyperlipidaemia | 31·2% | 31·4% | <0·00 | 30·4% | 30·3% | <0·00 |
| Pneumonia | 4·0% | 4·0% | <0·00 | 5·7% | 5·5% | 0·01 |
| Renal impairment | 3·0% | 2·8% | 0·01 | 4·2% | 4·1% | <0·00 |
| Urinary tract infections | 11·6% | 11·5% | <0·00 | 14·0% | 13·9% | <0·00 |
| Medical history: cardiovascular disease | ||||||
| Atrial fibrillation | 1·4% | 1·3% | 0·01 | 1·7% | 1·8% | <0·00 |
| Cerebrovascular disease | 2·8% | 2·9% | −0·01 | 3·1% | 3·2% | −0·01 |
| Coronary arteriosclerosis | 4·4% | 4·6% | −0·01 | 5·0% | 4·9% | <0·00 |
| Heart disease | 15·5% | 15·4% | <0·00 | 17·8% | 17·9% | <0·00 |
| Heart failure | 1·9% | 2·0% | <0·00 | 2·5% | 2·4% | 0·01 |
| Ischaemic heart disease | 3·0% | 3·1% | −0·01 | 3·3% | 3·1% | 0·01 |
| Medication use | ||||||
| Agents acting on the renin–angiotensin system | 24·5% | 24·6% | <0·00 | 27·1% | 26·9% | <0·00 |
| Antidepressants | 36·3% | 36·5% | <0·00 | 43·0% | 42·8% | <0·00 |
| Drugs for obstructive airway diseases | 29·5% | 29·5% | <0·00 | 41·1% | 40·7% | 0·01 |
| Immunosuppressants | 43·4% | 43·6% | <0·00 | 51·1% | 51·2% | <0·00 |
| Opioids | 39·0% | 39·3% | −0·01 | 41·4% | 41·2% | <0·00 |
| Psycholeptics | 33·4% | 33·3% | <0·00 | 38·2% | 38·1% | <0·00 |
Percentages might not sum to 100% because of rounding. An example of one dataset is included. AMX=amoxicillin. AZM=azithromycin. CCAE=IBM Commercial Claims and Encounters. HCQ=hydroxychloroquine. SSZ=sulfasalazine.
Database-specific and subtotal (meta-analysis) counts and rates of key outcomes (cardiovascular mortality, all-cause mortality, chest pain or angina, and heart failure) observed in the prespecified 30-day intention-to-treat analysis are shown in Table 2, Table 3. Mortality risk was assessed only using databases with reliable death capture: Clinformatics, CPRD, IMRD, IPCI, SIDIAP, and VA. For the analysis of hydroxychloroquine versus sulfasalazine, four databases (Clinformatics, CPRD, IMRD, and VA) were used to analyse all-cause mortality (no events were seen in SIDIAP and IPCI), and three databases (Clinformatics, CPRD, and VA) were used to analyse cardiovascular mortality. Two databases were used to analyse all-cause mortality and cardiovascular mortality for hydroxychloroquine plus azithromycin versus hydroxychloroquine plus amoxicillin (Clinformatics and VA); no events were seen in the other datasets. Mortality rates ranged from 4·81 per 1000 person-years in hydroxychloroquine users in Clinformatics to 17·13 per 1000 person-years among hydroxychloroquine users in VA, with cardiovascular-specific mortality ranging from 3·43 per 1000 person-years in hydroxychloroquine users in VA to less than 4·25 per 1000 person-years in sulfasalazine users in the same data source. Database-specific counts and incidence rates for severe adverse events stratified by drug use are detailed in full in the appendix (pp 66–71).
Table 2.
Patient counts, event counts, and incidence rates of key outcomes according to HCQ versus SSZ use
|
30-day follow-up |
On-treatment follow-up |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCQ users | SSZ users | HCQ events | SSZ events | HCQ incidence rate (per 1000 person-years) | SSZ incidence rate (per 1000 person-years) | HCQ users | SSZ users | HCQ events | SSZ events | HCQ incidence rate (per 1000 person-years) | SSZ incidence rate (per 1000 person-years) | |
| Cardiovascular-related mortality | ||||||||||||
| Clinformatics | 51 280 | 17 389 | 16 | <5 | 3·85 | <3·54 | 51 280 | 17 389 | 234 | 25 | 4·39 | 2·00 |
| CPRD | NA | NA | NA | NA | NA | NA | 9127 | 11 398 | 7 | 25 | 0·39 | 0·94 |
| VA | 32 028 | 14 349 | 9 | <5 | 3·43 | <4·25 | 32 028 | 14 349 | 315 | 65 | 5·69 | 3·71 |
| Meta-analysis | 83 308 | 31 738 | 25 | <10 | 3·68 | <3·86 | 92 435 | 43 136 | 556 | 115 | 4·39 | 2·03 |
| All-cause mortality | ||||||||||||
| Clinformatics | 51 280 | 17 389 | 20 | 10 | 4·81 | 7·09 | 51 280 | 17 389 | 527 | 66 | 9·88 | 5·29 |
| CPRD | 9127 | 11 398 | 6 | 5 | 8·03 | 5·35 | 9127 | 11 398 | 253 | 386 | 14·02 | 14·56 |
| IMRD | 8851 | 8460 | <5 | 6 | <6·91 | 8·66 | 8851 | 8460 | 214 | 241 | 12·32 | 12·72 |
| VA | 32 028 | 14 349 | 45 | 17 | 17·13 | 14·45 | 32 028 | 14 349 | 1356 | 327 | 24·51 | 18·65 |
| Meta-analysis | 101 286 | 51 596 | <76 | 38 | <9·20 | 9·02 | NA | NA | NA | NA | NA | NA |
| Chest pain or angina | ||||||||||||
| AmbEMR | 57 140 | 15 268 | 122 | 31 | 26·04 | 24·76 | 57 140 | 15 268 | 451 | 112 | 24·44 | 19·89 |
| CCAE | 65 935 | 22 173 | 440 | 143 | 82·41 | 79·62 | 65 935 | 22 173 | 3354 | 810 | 55·00 | 58·80 |
| Clinformatics | 50 698 | 17 221 | 396 | 166 | 96·62 | 119·34 | 50 698 | 17 221 | 3185 | 829 | 66·13 | 72·48 |
| CPRD | 9114 | 11 388 | 10 | 17 | 13·40 | 18·22 | 9114 | 11 388 | 260 | 422 | 14·99 | 16·78 |
| DAGermany | 3884 | 5045 | <5 | 5 | <15·69 | 12·07 | 3884 | 5045 | 31 | 36 | 12·36 | 10·26 |
| IMRD | 8843 | 8452 | 9 | 10 | 12·45 | 14·46 | 8843 | 8452 | 235 | 293 | 14·00 | 16·25 |
| MDCD | 7982 | 2177 | 80 | 23 | 123·50 | 130·43 | 7982 | 2177 | 467 | 100 | 87·34 | 85·81 |
| MDCR | 15 690 | 5150 | 129 | 49 | 101·25 | 117·43 | 15 690 | 5150 | 1178 | 279 | 71·38 | 75·12 |
| OpenClaims | 617 628 | 182 776 | 2674 | 804 | 52·83 | 53·68 | 617 628 | 182 776 | 31 161 | 6198 | 38·59 | 38·11 |
| OptumEHR | 76 844 | 21 549 | 629 | 143 | 101·46 | 82·23 | NA | NA | NA | NA | NA | NA |
| VA | 31 824 | 14 276 | 130 | 54 | 49·89 | 46·20 | 31 824 | 14 276 | 1822 | 611 | 35·88 | 37·31 |
| Meta-analysis | 945 582 | 305 475 | <4624 | 1445 | <59·86 | 57·90 | 868 738 | 283 926 | 42 144 | 9690 | 40·36 | 37·07 |
| Heart failure | ||||||||||||
| AmbEMR | 57 383 | 15 305 | 42 | 10 | 8·92 | 7·96 | 57 383 | 15 305 | 182 | 53 | 9·76 | 9·37 |
| CCAE | 66 604 | 22 370 | 30 | 5 | 5·55 | 2·75 | 66 604 | 22 370 | 305 | 74 | 4·64 | 5·07 |
| Clinformatics | 51 204 | 17 356 | 84 | 25 | 20·23 | 17·76 | 51 204 | 17 356 | 915 | 207 | 17·55 | 16·90 |
| CPRD | 9126 | 11 397 | <5 | <5 | <6·69 | <5·35 | 9126 | 11 397 | 16 | 36 | 0·89 | 1·36 |
| DAGermany | 3885 | 5042 | <5 | <5 | <15·68 | <12·08 | 3885 | 5042 | 11 | 22 | 4·29 | 6·22 |
| IMRD | 8852 | 8460 | <5 | <5 | <6·91 | <7·22 | 8852 | 8460 | 15 | 21 | 0·86 | 1·11 |
| MDCD | 8072 | 2195 | 15 | <5 | 22·81 | <27·99 | 8072 | 2195 | 118 | 28 | 20·55 | 23·02 |
| MDCR | 15 808 | 5171 | 39 | 19 | 30·30 | 45·22 | 15 808 | 5171 | 586 | 141 | 33·13 | 36·29 |
| OpenClaims | 620 244 | 183 350 | 749 | 214 | 14·71 | 14·22 | 620 244 | 183 350 | 12 246 | 2246 | 14·36 | 13·22 |
| OptumEHR | 77 813 | 21 768 | 237 | 50 | 37·64 | 28·39 | NA | NA | NA | NA | NA | NA |
| VA | 31 895 | 14 307 | 56 | 17 | 21·42 | 14·49 | 31 895 | 14 307 | 897 | 296 | 16·75 | 17·42 |
| Meta-analysis | 950 886 | 306 721 | <1267 | <360 | <16·28 | <14·34 | 873 073 | 284 953 | 15 291 | 3124 | 13·85 | 11·43 |
AmbEMR=IQVIA Ambulatory EMR. CCAE=IBM Commercial Claims and Encounters. CPRD=Clinical Practice Research Datalink. DAGermany=IQVIA Disease Analyzer Germany. EMR=electronic medical record. HCQ=hydroxychloroquine. IMRD=IQVIA UK Integrated Medical Record Data. MDCD=IBM Multi-state Medicaid. MDCR=IBM Medicare Supplemental Database. NA=non-applicable (not reported because of failed diagnostics or on-treatment follow-up unavailable). OptumEHR=Optum de-identified Electronic Health Record. SSZ=sulfasalazine. VA=US Department of Veterans Affairs.
Table 3.
Patient counts, event counts, and incidence rates of key outcomes according to HCQ plus AZM versus HCQ plus AMX use
|
30-day follow-up |
On-treatment follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCQ plus AZM users | HCQ plus AMX users | HCQ plus AZM events | HCQ plus AMX events | HCQ plus AZM incidence rate (per 1000 person-years) | HCQ plus AMX incidence rate (per 1000 person-years) | HCQ plus AZM users | HCQ plus AMX users | HCQ plus AZM events | HCQ plus AMX events | HCQ plus AZM incidence rate (per 1000 person-years) | HCQ plus AMX incidence rate (per 1000 person-years) | |
| Cardiovascular-related mortality | ||||||||||||
| Clinformatics | 23 597 | 24 521 | 9 | 6 | 4·70 | 3·02 | 23 597 | 24 521 | 96 | 82 | 5·56 | 5·58 |
| VA | 6234 | 8005 | 46 | 18 | 90·60 | 27·49 | 6234 | 8005 | 157 | 115 | 14·60 | 10·20 |
| Meta-analysis | 29 831 | 32 526 | 55 | 24 | 22·70 | 9·08 | 29 831 | 32 526 | 253 | 197 | 9·03 | 7·59 |
| All-cause mortality | ||||||||||||
| Clinformatics | 23 597 | 24 521 | 17 | 17 | 8·88 | 8·55 | 23 597 | 24 521 | 268 | 276 | 15·56 | 18·85 |
| VA | 6234 | 8005 | 91 | 52 | 179·23 | 79·42 | 6234 | 8005 | 550 | 518 | 51·16 | 45·97 |
| Meta-analysis | 29 831 | 32 526 | 108 | 69 | 44·58 | 26·12 | 29 831 | 32 526 | 818 | 794 | 29·24 | 30·64 |
| CCAE | 32 610 | 32 507 | 13 | 11 | 4·92 | 4·17 | 32 610 | 32 507 | 117 | 94 | 4·33 | 4·33 |
| Clinformatics | 23 565 | 24 484 | 30 | 29 | 15·70 | 14·62 | 23 565 | 24 484 | 179 | 147 | 10·60 | 10·19 |
| MDCD | 3803 | 3808 | <5 | 6 | <16·21 | 19·40 | 3803 | 3808 | 29 | 27 | 11·46 | 13·46 |
| MDCR | 8119 | 9254 | 16 | 9 | 24·33 | 11·96 | 8119 | 9254 | 166 | 140 | 20·41 | 17·34 |
| Open Claims | 216 028 | 232 938 | 182 | 173 | 10·26 | 9·05 | 216 028 | 232 938 | 2065 | 1732 | 8·11 | 7·94 |
| OptumEHR | 18 477 | 16 424 | 26 | 20 | 17·35 | 15·01 | NA | NA | NA | NA | NA | NA |
| VA | 6203 | 7978 | 33 | 19 | 65·53 | 29·15 | 6203 | 7978 | 154 | 127 | 14·79 | 11·59 |
| Meta-analysis | 308 805 | 327 393 | <305 | 267 | <12·08 | 9·97 | 290 328 | 310 969 | 2710 | 2267 | 8·48 | 8·24 |
| Chest pain or angina | ||||||||||||
| AmbEMR | 13 093 | 12 028 | 32 | 21 | 29·80 | 21·29 | 13 093 | 12 028 | 142 | 119 | 25·69 | 25·31 |
| CCAE | 32 165 | 32 229 | 241 | 211 | 92·76 | 80·98 | 32 165 | 32 229 | 1402 | 1145 | 60·46 | 60·54 |
| Clinformatics | 23 206 | 24 254 | 244 | 203 | 130·28 | 103·70 | 23 206 | 24 254 | 1019 | 887 | 70·33 | 70·28 |
| MDCD | 3712 | 3764 | 30 | 37 | 99·97 | 121·56 | 3712 | 3764 | 129 | 113 | 60·05 | 63·39 |
| MDCR | 7991 | 9195 | 81 | 85 | 125·60 | 114·20 | 7991 | 9195 | 517 | 498 | 74·83 | 71·25 |
| OpenClaims | 214 494 | 231 851 | 1050 | 888 | 59·76 | 46·74 | 214 494 | 231 851 | 8348 | 7223 | 36·24 | 36·37 |
| OptumEHR | 18 039 | 16 191 | 218 | 134 | 150·01 | 102·42 | NA | NA | NA | NA | NA | NA |
| VA | 6121 | 7912 | 58 | 50 | 116·96 | 77·52 | 6121 | 7912 | 340 | 371 | 38·48 | 39·87 |
| Meta-analysis | 318 821 | 337 424 | 1954 | 1629 | 75·13 | 59·12 | 300 782 | 321 233 | 11 897 | 10 356 | 40·82 | 40·95 |
| Heart failure | ||||||||||||
| AmbEMR | 13 152 | 12 053 | 16 | 16 | 14·83 | 16·18 | 13 152 | 12 053 | 61 | 49 | 10·44 | 9·96 |
| CCAE | 32 586 | 32 496 | 30 | 23 | 11·36 | 8·73 | 32 586 | 32 496 | 177 | 126 | 6·58 | 5·82 |
| Clinformatics | 23 541 | 24 468 | 65 | 49 | 34·08 | 24·73 | 23 541 | 24 468 | 337 | 317 | 20·33 | 22·63 |
| MDCD | 3796 | 3795 | 16 | 9 | 52·08 | 29·21 | 3796 | 3795 | 65 | 48 | 26·26 | 24·83 |
| MDCR | 8085 | 9239 | 45 | 33 | 68·88 | 43·97 | 8085 | 9239 | 322 | 295 | 41·61 | 38·34 |
| OpenClaims | 215 732 | 232 725 | 472 | 370 | 26·68 | 19·38 | 215 732 | 232 725 | 4352 | 3714 | 17·50 | 17·43 |
| OptumEHR | 18 054 | 16 298 | 99 | 60 | 67·77 | 45·45 | NA | NA | NA | NA | NA | NA |
| VA | 6164 | 7959 | 79 | 31 | 158·53 | 47·73 | 6164 | 7959 | 280 | 229 | 28·17 | 21·64 |
| Meta-analysis | 321 110 | 339 033 | 822 | 591 | 31·32 | 21·32 | 303 056 | 322 735 | 5594 | 4778 | 17·58 | 17·44 |
AmbEMR=IQVIA Ambulatory EMR. AMX=amoxicillin. AZM=azithromycin. CCAE=IBM Commercial Claims and Encounters. CPRD=Clinical Practice Research Datalink. DAGermany=IQVIA Disease Analyzer Germany. EMR=electronic medical record. HCQ=hydroxychloroquine. IMRD=IQVIA UK Integrated Medical Record Data. MDCD=IBM Multi-state Medicaid. MDCR=IBM Medicare Supplemental Database. NA=non-applicable (not reported because of failed diagnostics or on-treatment follow-up unavailable). OptumEHR=Optum de-identified Electronic Health Record. VA=US Department of Veterans Affairs.
Least common outcomes among hydroxychloroquine users included bradycardia (eg, incidence rate 0·92 per 1000 person-years in CCAE) and end-stage renal disease (eg, less than 0·92 per 1000 person-years in CCAE), whereas most common outcomes were chest pain or angina (eg, 82·41 per 1000 person-years in CCAE; table 2) and composite cardiovascular events (eg, 17·96 per 1000 person-years in CCAE).
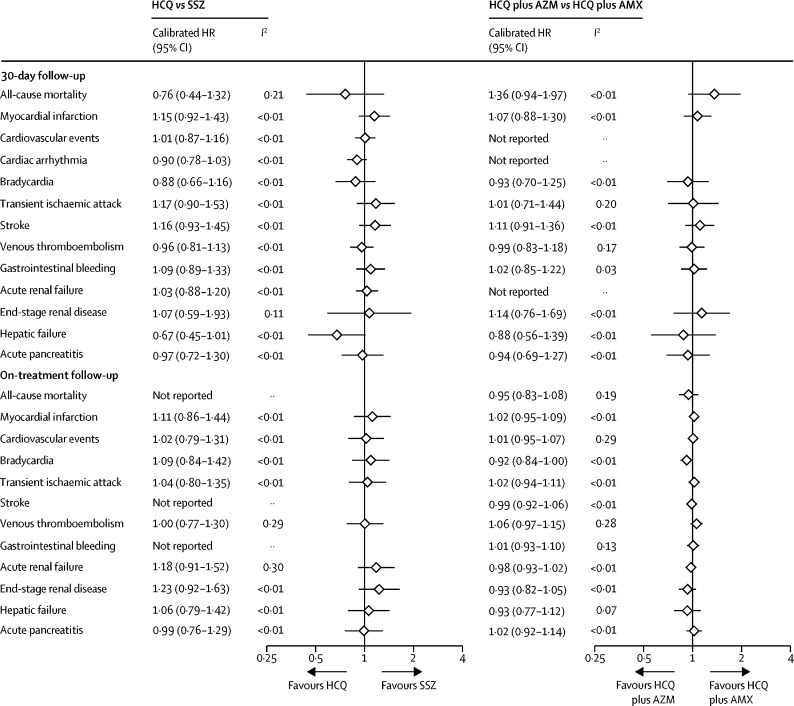
Database and outcome-specific HRs (uncalibrated and calibrated) are reported in full in the form of forest plots in the appendix (pp 72–103). None of the severe adverse events appeared to be consistently increased with the short-term use of hydroxychloroquine (vs sulfasalazine) in the 30-day intention-to-treat analyses (figure 1), with meta-analytic calibrated HRs ranging from 0·67 (95% CI 0·45–1·01) for hepatic failure to 1·17 (0·90–1·53) for transient ischaemic attack, and 1·36 (0·51–3·63) for cardiovascular mortality (figure 2). In our published study protocol, we decided a priori that meta-analytic estimates would only be reported if the I2 value was less than 0·4, indicating that there was low heterogeneity between the results included, and that it was appropriate for them to be pooled to produce this final result.36 For all-cause mortality in the on-treatment analysis, the I2 value was 0·71, indicating substantial heterogeneity between results and therefore a summary estimate was not reported. The same is true for gastrointestinal bleeding (I2=0·57) and stroke (I2=0·58) in the on-treatment analysis.
Figure 1.
Meta-analytic estimates for HCQ versus SSZ and HCQ plus AZM versus HCQ plus AMX new users during 30-day (intention-to-treat) and long-term (on-treatment) follow-up
AMX=amoxicillin. AZM=azithromycin. HCQ=hydroxychloroquine. HR=hazard ratio. SSZ=sulfasalazine.
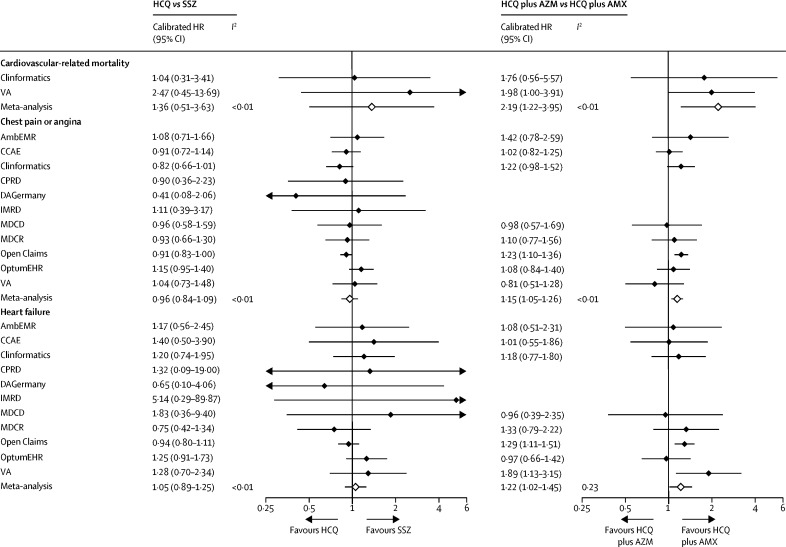
Figure 2.
Source-specific and meta-analytic-specific severe adverse event risk estimates for HCQ versus SSZ and HCQ plus AZM versus HCQ plus AMX new users during 30-day (intention-to-treat) follow-up
AmbEMR=IQVIA Ambulatory EMR. AMX=amoxicillin. AZM=azithromycin. CCAE=IBM Commercial Claims and Encounters. CPRD=Clinical Practice Research Datalink. DAGermany=IQVIA Disease Analyzer Germany. EMR=electronic medical record. HCQ=hydroxychloroquine. HR=hazard ratio. IMRD=IQVIA UK Integrated Medical Record Data. MDCD=IBM Multi-state Medicaid. MDCR=IBM Medicare Supplemental Database. OptumEHR=Optum de-identified Electronic Health Record. SSZ=sulfasalazine. VA=US Department of Veterans Affairs.
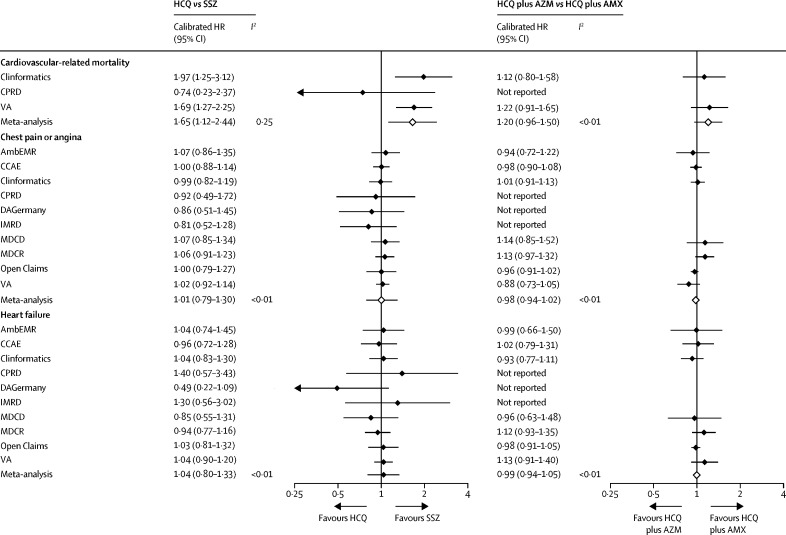
Similar findings were seen with the long-term (on-treatment) use of hydroxychloroquine versus sulfasalazine (figure 1; figure 3), with the exception of cardiovascular mortality, which appeared to be inconsistent in the available databases but increased overall in the hydroxychloroquine group when meta-analysed (pooled calibrated HR 1·65 [95% CI 1·12–2·44]).
Figure 3.
Source-specific and meta-analytic specific severe adverse event risk estimates for HCQ versus SSZ and HCQ plus AZM versus HCQ plus AMX new users during long-term (on-treatment) follow-up
AmbEMR=IQVIA Ambulatory EMR. AMX=amoxicillin. AZM=azithromycin. CCAE=IBM Commercial Claims and Encounters. CPRD=Clinical Practice Research Datalink. DAGermany=IQVIA Disease Analyzer Germany. EMR=electronic medical record. HCQ=hydroxychloroquine. HR=hazard ratio. IMRD=IQVIA UK Integrated Medical Record Data. MDCD=IBM Multi-state Medicaid. MDCR=IBM Medicare Supplemental Database. OptumEHR=Optum de-identified Electronic Health Record. SSZ=sulfasalazine. VA=US Department of Veterans Affairs.
Self-controlled case series analyses supported the findings of the main analysis, while looking at the effect of hydroxychloroquine use (on treatment vs off treatment) regardless of indication, and therefore including patients without rheumatoid arthritis (table 4; full results are given in the appendix pp 119–25).
Table 4.
Summary of self-controlled case series results for HCQ
|
CCAE |
Clinformatics |
CPRD |
JMDC Claims Database |
MDCD |
MDCR |
VA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calibrated incidence rate ratio | Calibrated 95% CI | Calibrated incidence rate ratio | Calibrated 95% CI | Calibrated incidence rate ratio | Calibrated 95% CI | Calibrated incidence rate ratio | Calibrated 95% CI | Calibrated incidence rate ratio | Calibrated 95% CI | Calibrated incidence rate ratio | Calibrated 95% CI | Calibrated incidence rate ratio | Calibrated 95% CI | |
| Myocardial infarction | ||||||||||||||
| Adjusted analysis* | 0·91 | 0·69–1·21 | 1·11 | 0·81–1·54 | NA | NA | NA | NA | NA | NA | 1·09 | 0·86–1·39 | 0·99 | 0·73–1·34 |
| Primary analysis | 0·92 | 0·70–1·22 | 1·02 | 0·74–1·40 | NA | NA | NA | NA | 0·89 | 0·70–1·15 | 0·99 | 0·78–1·26 | 1·00 | 0·74–1·36 |
| Acute pancreatitis events | ||||||||||||||
| Adjusted analysis* | NA | NA | 1·05 | 0·75–1·46 | NA | NA | 2·18 | 0·11–43·82 | 1·13 | 0·86–1·47 | 1·03 | 0·77–1·36 | 0·97 | 0·71–1·34 |
| Primary analysis | 0·90 | 0·68–1·20 | 1·05 | 0·75–1·46 | NA | NA | 2·11 | 0·14–31·50 | 1·12 | 0·86–1·46 | 0·99 | 0·74–1·32 | 0·98 | 0·71–1·35 |
| Acute renal failure | ||||||||||||||
| Adjusted analysis* | 0·88 | 0·67–1·16 | 0·96 | 0·58–1·59 | NA | NA | 1·33 | 0·31–5·71 | NA | NA | 1·08 | 0·85–1·37 | NA | NA |
| Primary analysis | 0·90 | 0·69–1·19 | 0·99 | 0·72–1·37 | NA | NA | 1·39 | 0·32–6·12 | 1 | 0·80–1·25 | 0·97 | 0·76–1·23 | 1·11 | 0·83–1·50 |
| Gastrointestinal bleeding | ||||||||||||||
| Adjusted analysis* | NA | NA | 1·13 | 0·82–1·55 | NA | NA | 0·25 | 0·03–2·44 | 0·95 | 0·75–1·20 | 1·02 | 0·81–1·30 | NA | NA |
| Primary analysis | 1·01 | 0·76–1·32 | 1·06 | 0·77–1·46 | NA | NA | 0·24 | 0·03–2·38 | 0·95 | 0·76–1·20 | 0·96 | 0·76–1·22 | 0·97 | 0·72–1·32 |
| Cardiac arrhythmia | ||||||||||||||
| Adjusted analysis* | 0·95 | 0·72–1·25 | 1·03 | 0·74–1·42 | 0·95 | 0·61–1·47 | 0·62 | 0·18–2·15 | 0·93 | 0·74–1·17 | 0·85 | 0·67–1·09 | 0·86 | 0·64–1·17 |
| Primary analysis | 0·95 | 0·72–1·26 | 1·03 | 0·74–1·43 | 0·95 | 0·61–1·48 | 0·58 | 0·17–1·98 | 0·93 | 0·74–1·17 | 0·86 | 0·67–1·10 | 0·85 | 0·63–1·15 |
| Bradycardia | ||||||||||||||
| Adjusted analysis* | NA | NA | 0·91 | 0·65–1·27 | 0·65 | 0·20–2·16 | 3·67 | 0·26–50·91 | NA | NA | 0·87 | 0·68–1·12 | 0·88 | 0·65–1·20 |
| Primary analysis | 0·72 | 0·54–0·96 | 0·92 | 0·67–1·28 | 0·68 | 0·21–2·18 | 3·69 | 0·26–51·54 | 0·74 | 0·55–0·99 | 0·87 | 0·68–1·12 | 0·93 | 0·68–1·26 |
| Chest pain or angina | ||||||||||||||
| Adjusted analysis* | 0·91 | 0·69–1·21 | 1·07 | 0·77–1·48 | 0·98 | 0·63–1·52 | 0·92 | 0·45–1·85 | 1·07 | 0·84–1·38 | 0·95 | 0·75–1·21 | NA | NA |
| Primary analysis | 0·91 | 0·69–1·21 | 1·06 | 0·76–1·47 | 0·98 | 0·63–1·52 | 0·91 | 0·45–1·84 | 1·07 | 0·84–1·36 | 0·94 | 0·74–1·20 | 0·98 | 0·73–1·33 |
| End-stage renal disease | ||||||||||||||
| Adjusted analysis* | 1·02 | 0·69–1·51 | NA | NA | 0·91 | 0·15–5·49 | NA | NA | NA | NA | 0·88 | 0·66–1·18 | 1·04 | 0·76–1·44 |
| Primary analysis | 1·03 | 0·76–1·39 | 1·26 | 0·90–1·76 | 0·91 | 0·15–5·31 | NA | NA | 1·24 | 0·93–1·64 | 0·88 | 0·66–1·19 | 1·02 | 0·74–1·40 |
| Heart failure | ||||||||||||||
| Adjusted analysis* | 0·99 | 0·75–1·29 | 1·15 | 0·83–1·58 | 1·20 | 0·69–2·09 | 1·02 | 0·50–2·10 | 0·95 | 0·75–1·20 | 1·12 | 0·88–1·42 | 1·03 | 0·76–1·39 |
| Primary analysis | 0·99 | 0·75–1·30 | 1·13 | 0·82–1·56 | 1·21 | 0·69–2·11 | 1·02 | 0·49–2·08 | 0·95 | 0·75–1·20 | 1·09 | 0·86–1·39 | 1·04 | 0·77–1·40 |
| Hepatic failure | ||||||||||||||
| Adjusted analysis* | 0·68 | 0·50–0·92 | NA | NA | NA | NA | 1·54 | 0·08–30·15 | 0·83 | 0·60–1·16 | 0·82 | 0·58–1·17 | NA | NA |
| Primary analysis | 0·64 | 0·47–0·88 | 0·73 | 0·52–1·02 | 0·09 | 0·01–1·35 | 1·48 | 0·07–33·23 | 0·77 | 0·55–1·07 | 0·81 | 0·57–1·15 | 0·79 | 0·56–1·11 |
| Stroke | ||||||||||||||
| Adjusted analysis* | NA | NA | 0·97 | 0·70–1·34 | NA | NA | 1·13 | 0·36–3·55 | 0·90 | 0·71–1·14 | 1·01 | 0·80–1·29 | 0·96 | 0·71–1·31 |
| Primary analysis | 0·80 | 0·61–1·06 | 0·90 | 0·65–1·24 | NA | NA | 1·14 | 0·36–3·59 | 0·85 | 0·67–1·08 | 0·93 | 0·73–1·18 | 0·98 | 0·72–1·34 |
| Cardiovascular events | ||||||||||||||
| Adjusted analysis* | NA | NA | 0·90 | 0·37–2·21 | NA | NA | 0·51 | 0·21–1·25 | 0·91 | 0·73–1·15 | 1·10 | 0·87–1·40 | NA | NA |
| Primary analysis | 0·86 | 0·66–1·14 | 0·95 | 0·69–1·31 | NA | NA | 0·50 | 0·20–1·25 | 0·86 | 0·68–1·08 | 1·02 | 0·80–1·29 | 1·08 | 0·80–1·46 |
| Transient ischaemic attack | ||||||||||||||
| Adjusted analysis* | 0·91 | 0·69–1·20 | 0·94 | 0·68–1·30 | NA | NA | NA | NA | 0·94 | 0·72–1·23 | 1 | 0·79–1·28 | NA | NA |
| Primary analysis | 0·92 | 0·70–1·21 | 0·93 | 0·68–1·29 | NA | NA | NA | NA | 0·92 | 0·71–1·20 | 0·97 | 0·76–1·24 | 1·20 | 0·88–1·65 |
| Venous thromboembolism | ||||||||||||||
| Adjusted analysis* | 0·79 | 0·54–1·15 | 0·86 | 0·62–1·18 | 0·70 | 0·45–1·09 | 1·51 | 0·62–3·67 | 0·88 | 0·71–1·10 | 0·76 | 0·60–0·96 | NA | NA |
| Primary analysis | 0·81 | 0·62–1·07 | 0·84 | 0·61–1·16 | 0·69 | 0·44–1·07 | 1·51 | 0·62–3·67 | 0·87 | 0·70–1·09 | 0·71 | 0·56–0·91 | 0·86 | 0·64–1·16 |
CCAE=IBM Commercial Claims and Encounters. CPRD=Clinical Practice Research Datalink. HCQ=hydroxychloroquine. JMDC=Japanese Medical Data Center. MDCD=IBM Multi-state Medicaid. MDCR=IBM Medicare Supplemental Database. NA=non-applicable (not reported because of failed diagnostics or on-treatment follow-up unavailable). VA=US Department of Veterans Affairs.
Adjusted for event-dependent observation.
All of the obtained database-specific and outcome-specific calibrated HRs for the association between short-term (on-treatment) use of hydroxychloroquine plus azithromycin versus hydroxychloroquine plus amoxicillin are depicted as forest plots in the appendix (pp 72–103). Three severe adverse events appeared to be increased with the short-term (30-day intention to treat) use of hydroxychloroquine plus azithromycin compared with hydroxychloroquine plus amoxicillin: chest pain or angina (meta-analytic calibrated HR 1·15 [95% CI 1·05–1·26]), heart failure (1·22 [1·02–1·45]), and cardiovascular mortality (2·19 [1·22–3·95]; figure 2).
Full results from each dataset, including power, attrition, and population characteristics are available online. This site also contains all of the cohort diagnostic tools that were examined before unblinding results and before a dataset was included in the meta-analyses. Each dataset was examined for the risk of observed confounding (within the propensity score model, propensity score distribution, and covariate balance with identified variables) or by unobserved confounding (assessing negative control variables within analysis of the risk of systematic error) before their inclusion. These diagnostic tools can be reviewed for each database for each outcome within the shiny application of R (version 3.61) in order to give full transparency of analysis.
Discussion
To our knowledge, this study is the largest ever analysis of the safety of hydroxychloroquine and hydroxychloroquine plus azithromycin worldwide, examining more than 950 000 hydroxychloroquine and more than 300 000 hydroxychloroquine plus azithromycin users, respectively. Short-term (up to 30 days) hydroxychloroquine treatment among patients with rheumatoid arthritis showed no excess risk of any of the considered severe adverse events compared with sulfasalazine. Short-term treatment is also proposed for COVID-19 therapy and might be informed by the experience of treatment in patients with rheumatoid arthritis. By comparison, long-term hydroxychloroquine therapy appears to be associated with a relative risk increase in cardiovascular-related mortality compared with a roughly equivalent rheumatoid arthritis therapy (sulfasalazine; calibrated HR 1·65 [95% CI 1·12–2·44]). Perhaps more worryingly, compared with hydroxychloroquine plus amoxicillin, significant risks were identified for the combination of hydroxychloroquine plus azithromycin even in the short term: increased risk of angina or chest pain (calibrated HR 1·15 [95% CI 1·05–1·26]) and heart failure (1·22 [1·02–1·45]), and a doubled risk of cardiovascular mortality in the first month of treatment (2·19 [1·22–3·94]).
A systematic review of reports on the toxicity of hydroxychloroquine has identified cardiac side-effects, including conduction disorders, heart failure, and ventricular hypertrophy resulting in 12·9% irreversible damage and 30% mortality.19, 20 Furthermore, interrogation of the US Food and Drug Administration Adverse Event Reporting System database identified 357 adverse events reported for chloroquine.18 20% of the events reported were cardiac and included arrhythmia, sudden cardiac death, or heart failure.
Our results suggest that long-term use of hydroxychloroquine leads to increased cardiovascular mortality, which might relate to cumulative effects of hydroxychloroquine leading to an increased risk of QT lengthening and potentially to sudden undetected torsade-de-pointes and cardiovascular death. Although long-term treatment with hydroxychloroquine is not expected for the management of COVID-19, some research suggests that the higher doses prescribed for COVID-19 than for rheumatoid arthritis can, even in the short term, lead to equivalent side-effects given the long half-life of hydroxychloroquine.19
In addition, QT lengthening is a known side-effect of all macrolides, including azithromycin, and physicians already use caution when prescribing macrolides concurrently with other medications that can interact to increase the QT interval.22, 23 In this study, a relative risk of 2·19 (95% CI 1·22–3·94) for cardiovascular death was seen even with short-term hydroxychloroquine plus azithromycin combination therapy, probably arising through their synergistic effects on QT length and subsequent induction of lethal arrhythmia. Considering that hydroxychloroquine and azithromycin are both contraindicated for use in patients with cardiac arrhythmias, this study assumes that clinicians are prescribing these medications for patients as per existing labelling advice. It is therefore concerning that cardiovascular effects were still seen in our study populations, possibly indicating that the true risks of these drugs are understated in the analysis.
It is important to identify potential sources of bias that could limit the study. The analyses are predicated on observing the presence of exposure, outcomes, and covariates in the data, or inferring their absence based on an assumption of complete data capture during a defined observation period during which a person is not expected to be lost to follow-up. In this regard, although there were no missing data that required imputation, each binary variable is subject to potential misclassification error, and the sensitivity and specificity of these variables in each database are unknown. Because of the nature of sudden cardiac death, capturing the true cause of cardiovascular-related mortality is difficult. Although we examined various aspects of cardiac complications as captured by diagnosis codes, the accuracy of evaluations of QT prolongation, ventricular tachycardia, or other arrhythmias would probably be improved with precise electrocardiogram measurements. Exposure misclassification can occur as a result of non-adherence or non-compliance with either treatment and thus could bias the results in either direction, and outcome misclassification might exist because of incomplete or incorrect recording of severe adverse events. Baseline covariates might also be subject to measurement error and, although observing balance on all baseline characteristics after propensity score adjustment provides reassurance that the risk of confounding has been reduced, there remains potential for confounding in any given source for differential misclassification. The consistency of findings across heterogeneous patient populations with disparate data capture processes mitigates this concern. Within the study design, use of routine health-care data in populations across four continents, and including all adults with rheumatoid arthritis was used to minimise selection bias. The self-controlled case series analysis was also added to investigate all users of hydroxychloroquine as an external validation of the hydroxychloroquine findings in the rheumatoid arthritis population via the new user design. To investigate systematic error, study diagnostics were evaluated before unblinding results through interrogation of negative controls.
We have taken into consideration that patients with rheumatoid arthritis taking hydroxychloroquine might also have further autoimmune conditions such as SLE and therefore generate the potential for confounding by indication. We also investigated the incidence of hyperlipidaemia, diabetes, venous thromboembolic disease, and coronary arteriosclerosis before unblinding because of the established evidence that hydroxychloroquine improves survival in patients with SLE through antilipidaemic and antithrombotic mechanisms of action and reduces the development of diabetes in patients with SLE and those with rheumatoid arthritis.28, 37, 38, 39 We ensured that, when investigating covariate balance after propensity score stratification and matching and before unblinding study results, we did not see unbalanced proportions of patients with a diagnosis of SLE between the groups. Negative control outcome analyses to assess for systematic error also did not identify any residual unobserved confounding in the propensity score analysis, adjusting for thousands of variables within the large-scale propensity score model. Although we have balanced for the coexistence of other conditions and medications through propensity scores, and we tested for residual unobserved confounding to ensure groups were balanced, no direct measure of severity of rheumatoid arthritis was drawn for patients at baseline. The cohort was made from patients who were new users of both hydroxychloroquine and sulfasalazine with a diagnosis of rheumatoid arthritis and without medication use in the previous 365 days, but the potential for differences in baseline rheumatoid arthritis severity not recorded in routinely collected data is also a limitation of the study.
Another criticism is the choice of sulfasalazine as an active comparator. Both hydroxychloroquine and sulfasalazine are second-line conventional synthetic disease-modifying antirheumatic drugs in the treatment of patients with rheumatoid arthritis, used in addition to, or instead of methotrexate. Although they are not fully equivalent to each other, and no drug can be an exact match, they are each the closest comparator treatment to the other. Appreciating they are not truly equivalent, we took care to ensure that propensity score stratification and negative control analysis for any systematic error ensured that the two groups were as balanced as possible to minimise confounding.
Another potential limitation in this study is the potential for patients to be included in more than one dataset in the USA. Although we ran meta-analyses, which assume populations are independent, we highlight that we are likely to have underestimated variance in our meta-analytic estimates. We also acknowledge the limitation that although 14 databases were used in total, mortality analysis was restricted to databases with good coverage of this outcome (ie CPRD, IMRD, IPCI, VA, and Clinformatics). Similarly, as we do not know the baseline risk of serious adverse events within this population, we cannot report absolute risk of these events in patients with rheumatoid arthritis, and this limitation must be acknowledged.
In this large-scale, international, real-world data network study, hydroxychloroquine appears to be largely safe for short-term use in patients with rheumatoid arthritis compared with sulfasalazine, but when used in combination with azithromycin, this therapy carries a relative risk of 2·19 for cardiovascular death compared with hydroxychloroquine combined with amoxicillin. The collective experience of almost a million patients builds our confidence in the evidence around the safety profile of hydroxychloroquine. In line with consensus expert guidance, our findings suggest that a cautious assessment of cardiovascular risk is needed before initiating high-dose hydroxychloroquine or hydroxychloroquine plus azithromycin combination therapy, and in long-term monitoring of patients with rheumatoid arthritis, especially those with cardiovascular risk factors.8
Data sharing
Open science is a guiding principle within Observational Health Data Sciences and Informatics. As such, we provide unfettered access to all open-source analysis tools used in this study via https://github.com/OHDSI/, as well as all data and results artefacts that do not include patient-level health information via http://evidence.ohdsi.org/Covid19EstimationHydroxychloroquine. Data partners contributing to this study remain custodians of their individual patient-level health information and hold either exemption from institutional review boards or approval for participation. All ethical approvals can be found in the appendix (p 130).
Acknowledgments
Acknowledgments
This research received partial support from the UK National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, US National Institutes of Health, US Department of Veterans Affairs, Janssen Research and Development, IQVIA, and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Korean Ministry of Health and Welfare (grant number HI16C0992 to SCY). Personal funding was provided by Versus Arthritis (21605; to JCEL), UK Medical Research Council (MRC) Doctoral Training Partnership (MR/K501256/1; to JCEL); MRC (MR/K501256/1 and MR/N013468/1; to AP-U) and Fundacion Alfonso Martin Escudero (to AP-U); Innovation Fund Denmark (5153-00002B) and the Novo Nordisk Foundation (NNF14CC0001; to BSK-H); Singapore Ministry of Health's National Medical Research Council Open Fund Large Collaborative Grant (NMRC/OFLCG/001/2017; to SMKS); VINCI (VA HSR RES 13-457; to SLD, MEM, and KEL); and NIHR Senior Research Fellowship (SRF-2018-11-ST2-004 to DP-A). The European Health Data and Evidence Network has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement number 806968. The Innovative Medicines Initiative 2 Joint Undertaking receives support from the EU's Horizon 2020 research and innovation programme and European Federation of Pharmaceutical Industries and Associations. The views and opinions expressed are those of the authors and do not necessarily reflect those of the NIHR senior Research Fellowship programme, NIHR, US Department of Veterans Affairs, or the US Government, UK National Health Service, or the UK Department of Health. We thank Catherine Hartley and Eli Harriss (Bodleian Health Care Libraries, University of Oxford, Oxford, UK), Runsheng Wang (Division of Rheumatology, Columbia University Irving Medical Center, New York, NY, USA), Joel Swerdel (Janssen Research and Development, Titusville, NJ, USA), Zeshan Ghory (IQVIA, Cambridge, MA, USA), Michael Kallfelz (Odysseus Data Services, Berlin, Germany), and Nigel Hughes (Janssen Research and Development, Beerse, Belgium). Finally, we acknowledge the tremendous work and dedication of the 350 participants from 30 nations in the March, 2020, Observational Health Data Sciences and Informatics COVID-19 Virtual Study-a-thon, without whom this study could not have been realised.
Contributors
OA, HA, PB, AVM, MTFA, TMA, PC, ACC, AD, DD, KF, LH, SKe, SKh, SKo, RM, PM, DRM, DN, FN, AO, AP-U, JX, SMKS, DV, HW, LZ, and JCEL searched the literature. JCEL, JW, GH, KK, TD-S, EB, JvdL, CR, JR, PRi, MSc, AGS, AS, MSp, MAS, MdW, SCY, PRy, and DP-A were involved in the study design and concept. JCEL, JW, MAS, GH, AVM, DV, FN, PRi, PRy, and DP-A were responsible for data interpretation, with assistance from JW, AO, JH, LH, GH, SKe, SKh, FN, RWP, AP-U, CR, PRi, AGS, and MAS. JCEL, JW, MMC, AD, SLDV, SF-B, CGL, KEL, RM, MEM, HM-S, MM, GAR, CR, JR, PRi, MSc, SS, AGS, AS, MSp, MAS, COT, DV, MdW, SCY, OZ, PRy, and DP-A analysed the data. JCEL, JW, PRy, and DP-A wrote the manuscript. The corresponding author confirms that all authors read and approved the final manuscript.
Declaration of interests
JW is an employee and shareholder at Janssen Research and Development. SLDV reports grants from Anolinx, Astellas Pharma, AstraZeneca Pharmaceuticals, Boehringer Ingelheim International, Celgene, Eli Lilly, Genentech, Genomic Health, Gilead Sciences, GlaxoSmithKline, Innocrin Pharmaceuticals, Janssen Pharmaceuticals, Kantar Health, Myriad Genetic Laboratories, Novartis International, Parexel International through the Western Institute for Biomedical Research, the non-profit corporation associated with the Department of Veterans Affairs (Salt Lake City, UT, USA; outside the submitted work). GH reports grants from the US National Library of Medicine and Janssen Research (outside the submitted work). BSK-H reports grants from the Innovation Fund Denmark (5153-00002B) and Novo Nordisk Foundation (NNF14CC0001; outside the submitted work). SKh is an employee of and stockholder in AstraZeneca. JvdL reports grants from the Innovative Medicines Initiative (during the conduct of the study). AVM is an employee of RTI Health Solutions, a unit of the independent, non-profit research organisation RTI international. DRM reports funding support from the Wellcome Trust, the UK National Institute for Health Research (NIHR), Scottish Chief Scientist Office, and Tenovus Scotland (outside the submitted work). MM and MdW report grants from the Innovative Medicines Initiative (during the conduct of the study). FN was an employee of AstraZeneca until September, 2019. GAR is an employee of Janssen Research and Development. PRi reports grants from the Innovative Medicines Initiative and Janssen Research and Development (during the conduct of the study). MSc is an employee of and shareholder in Janssen Research and Development. JH, JR, RM, PRy, MSc, and AGS are employees of Janssen Research and Development and shareholders in Johnson & Johnson. AS, LH, and MMC are employees of Janssen Research and Development. HM-S, KK, CR, COT, and SS are employees of IQVIA. MAS reports grants from the US National Science Foundation and US National Institutes of Health, and personal fees from Janssen Research and Development (during the conduct of the study). DV reports personal fees from Bayer (outside the submitted work) and is a full-time employee of Bayer. SCY reports grants from the Korean Ministry of Health and Welfare and the Korean Ministry of Trade, Industry and Energy (during the conduct of the study). DP-A reports grants from Amgen, UCB Biopharma, and Les Laboratoires Servier; personal fees (paid to his department) from Amgen and UCB Biopharma; and support for training programmes organised by his department from Innovative Medicines Initiative-funded European Health Data and Evidence Network and European Medical Information Framework consortiums, and Synapse Management Partners. All other authors declare no competing interests.
Supplementary Material
References
- 1.Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigerust DJ, Shepherd VL. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15:211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Cao R, Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautret P, Lagier JC, Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Mercuro NJ, Yen CF, Shim DJ. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. published online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTC in exploratory COVID-19 (coronavirus disease 2019) treatment. Circulation. 2020;141:e906–e907. doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 9.Chorin E, Dai M, Shulman E. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26:808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 10.Borba MGS, Val FFA, Sampaio VS. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RECOVERY Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on hydroxychloroquine, 5 June 2020. June 5, 2020. https://www.ox.ac.uk/news/2020-06-05-no-clinical-benefit-use-hydroxychloroquine-hospitalised-patients-covid-19
- 12.WHO “Solidarity” clinical trial for COVID-19 treatments update on hydroxychloroquine. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- 13.Hofschneider PH, Winter U, Lemmel E-M. Double blind controlled phase III multicenter clinical trial with interferon gamma in rheumatoid arthritis. Rheumatol Int. 1992;12:175–185. doi: 10.1007/BF00302149. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency COVID-19: reminder of the risks of chloroquine and hydroxychloroquine. April 23, 2020. https://www.ema.europa.eu/en/news/covid-19-reminder-risk-serious-side-effects-chloroquine-hydroxychloroquine
- 15.US Food and Drug Administration FDA revokes Emergency Use Authorization for chloroquine phosphate and hydroxychloroquine sulfate. June 15, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
- 16.Sepriano A, Kerschbaumer A, Smolen JS. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760–770. doi: 10.1136/annrheumdis-2019-216653. [DOI] [PubMed] [Google Scholar]
- 17.Costello R, David T, Jani M. Impact of adverse events associated with medications in the treatment and prevention of rheumatoid arthritis. Clin Ther. 2019;41:1376–1396. doi: 10.1016/j.clinthera.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Luo MH, Hu Q, Guirong X, Wu F, Wu B, Xu T. Data mining and safety analysis of drugs for novel coronavirus pneumonia treatment based on FAERS: chloroquine phosphate. Herald Med. 2020;39:505–512. [Google Scholar]
- 19.Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 20.Tselios K, Deeb M, Gladman DD, Harvey P, Urowitz MB. Antimalarial-induced cardiomyopathy: a systematic review of the literature. Lupus. 2018;27:591–599. doi: 10.1177/0961203317734922. [DOI] [PubMed] [Google Scholar]
- 21.Guo D, Cai Y, Chai D, Liang B, Bai N, Wang R. The cardiotoxicity of macrolides: a systematic review. Pharmazie. 2010;65:631–640. [PubMed] [Google Scholar]
- 22.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu ZK, Yuan J, Li M. Cardiac risks associated with antibiotics: azithromycin and levofloxacin. Expert Opin Drug Saf. 2015;14:295–303. doi: 10.1517/14740338.2015.989210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Network of Centres for Pharmacoepidemiology and Pharmacovigilance ENCePP guide on methodological standards in pharmacoepidemiology, revision 8. July, 2020. http://www.encepp.eu/standards_and_guidances/documents/GuideMethodRev8.pdf
- 25.Observational Health Data Sciences and Informatics . Observational Health Data Sciences and Informatics; 2020. The book of OHDSI. [Google Scholar]
- 26.Suchard MA, Schuemie MJ, Krumholz HM. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394:1816–1826. doi: 10.1016/S0140-6736(19)32317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voss EA, Boyce RD, Ryan PB, van der Lei J, Rijnbeek PR, Schuemie MJ. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform. 2017;66:72–81. doi: 10.1016/j.jbi.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace DJ, Gudsoorkar VS, Weisman MH, Venuturupalli SR. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol. 2012;8:522–533. doi: 10.1038/nrrheum.2012.106. [DOI] [PubMed] [Google Scholar]
- 29.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47:2005–2014. doi: 10.1093/ije/dyy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Robust empirical calibration of p-values using observational data. Stat Med. 2016;35:3883–3888. doi: 10.1002/sim.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med. 2014;33:209–218. doi: 10.1002/sim.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul. 2013;23:1–17. doi: 10.1145/2414416.2414791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Simpson SE, Madigan D, Zorych I, Schuemie MJ, Ryan PB, Suchard MA. Multiple self-controlled case series for large-scale longitudinal observational databases. Biometrics. 2013;69:893–902. doi: 10.1111/biom.12078. [DOI] [PubMed] [Google Scholar]
- 36.European Network of Centres for Pharmacoepidemiology and Pharmacovigilance EU PAS registration: hydroxychloroquine safety and potential efficacy as an antiviral prophylaxis in light of potential wide-spread use in COVID-19: a multinational, large-scale network cohort and self-controlled case series study. April 2, 2020. http://www.encepp.eu/encepp/viewResource.htm%3Fid=34498
- 37.Shinjo SK, Bonfá E, Wojdyla D. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum. 2010;62:855–862. doi: 10.1002/art.27300. [DOI] [PubMed] [Google Scholar]
- 38.Chen YM, Lin CH, Lan TH. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: a population-based cohort study. Rheumatology. 2015;54:1244–1249. doi: 10.1093/rheumatology/keu451. [DOI] [PubMed] [Google Scholar]
- 39.Wasko MC, Hubert HB, Lingala VB. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187–193. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Open science is a guiding principle within Observational Health Data Sciences and Informatics. As such, we provide unfettered access to all open-source analysis tools used in this study via https://github.com/OHDSI/, as well as all data and results artefacts that do not include patient-level health information via http://evidence.ohdsi.org/Covid19EstimationHydroxychloroquine. Data partners contributing to this study remain custodians of their individual patient-level health information and hold either exemption from institutional review boards or approval for participation. All ethical approvals can be found in the appendix (p 130).