Abstract
Background
A subset of patients with severe COVID-19 develop a hyperinflammatory syndrome, which might contribute to morbidity and mortality. This study explores a specific phenotype of COVID-19-associated hyperinflammation (COV-HI), and its associations with escalation of respiratory support and survival.
Methods
In this retrospective cohort study, we enrolled consecutive inpatients (aged ≥18 years) admitted to University College London Hospitals and Newcastle upon Tyne Hospitals in the UK with PCR-confirmed COVID-19 during the first wave of community-acquired infection. Demographic data, laboratory tests, and clinical status were recorded from the day of admission until death or discharge, with a minimum follow-up time of 28 days. We defined COV-HI as a C-reactive protein concentration greater than 150 mg/L or doubling within 24 h from greater than 50 mg/L, or a ferritin concentration greater than 1500 μg/L. Respiratory support was categorised as oxygen only, non-invasive ventilation, and intubation. Initial and repeated measures of hyperinflammation were evaluated in relation to the next-day risk of death or need for escalation of respiratory support (as a combined endpoint), using a multi-level logistic regression model.
Findings
We included 269 patients admitted to one of the study hospitals between March 1 and March 31, 2020, among whom 178 (66%) were eligible for escalation of respiratory support and 91 (34%) patients were not eligible. Of the whole cohort, 90 (33%) patients met the COV-HI criteria at admission. Despite having a younger median age and lower median Charlson Comorbidity Index scores, a higher proportion of patients with COV-HI on admission died during follow-up (36 [40%] of 90 patients) compared with the patients without COV-HI on admission (46 [26%] of 179). Among the 178 patients who were eligible for full respiratory support, 65 (37%) met the definition for COV-HI at admission, and 67 (74%) of the 90 patients whose respiratory care was escalated met the criteria by the day of escalation. Meeting the COV-HI criteria was significantly associated with the risk of next-day escalation of respiratory support or death (hazard ratio 2·24 [95% CI 1·62–2·87]) after adjustment for age, sex, and comorbidity.
Interpretation
Associations between elevated inflammatory markers, escalation of respiratory support, and survival in people with COVID-19 indicate the existence of a high-risk inflammatory phenotype. COV-HI might be useful to stratify patient groups in trial design.
Funding
None.
Introduction
COVID-19, the disease caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with severe respiratory compromise and mortality of up to 21% in hospitalised patients.1 Outcomes are especially poor in patients requiring advanced respiratory support,2, 3 with recent UK data reporting a mortality of 54·4% in this group.4 Clinical deterioration often occurs 7–10 days after the onset of symptoms, in association with declining viral titres,5 suggesting that pathology is driven by inflammation rather than direct viral injury. Inflammatory markers are often substantially elevated in patients with severe COVID-19.3, 6, 7 Uncontrolled, self-perpetuating, and tissue-damaging inflammatory activity (hyperinflammation) has also been described previously in the pathogenesis of other human coronavirus infections.8
The term cytokine storm syndrome encompasses a number of overlapping hyperinflammatory clinical syndromes, including haemophagocytic lymphohistiocytosis (HLH), macrophage activation syndrome, macrophage activation-like syndrome in sepsis, and cytokine release syndrome.9, 10 The reported sensitivity of these syndromes to cytokine-directed therapy has fuelled speculation11, 12, 13 that negative outcomes in a proportion of people with severe COVID-19 could also be ameliorated by immunomodulation of a hyperinflammatory response.14, 15, 16, 17
Many trials investigating the use immunomodulatory or immunosuppressive drugs in COVID-19 are now underway. Immunosuppression during infection incurs risk, and the risk–benefit balance should be carefully accounted for in trial design. Notably, insufficient stratification has been shown to obscure positive results, such as in previous trials of sepsis.18 Therefore, an improved understanding of hyperinflammation in COVID-19 is needed to inform trial design and ensure robust clinical research.19, 20
Research in context.
Evidence before this study
We searched PubMed on May 23, 2020, for studies published in English using search terms (“novel coronavirus” OR “2019 novel coronavirus” OR “2019-nCoV” OR “COVID-19”) AND (“hyperinflammation” OR “cytokine storm” OR “cytokine release” OR “HLH”). Four papers described differences in C-reactive protein and other inflammatory laboratory parameters in relation to outcome, one including longitudinal monitoring. One study compared longitudinal changes in ferritin, D-dimer, and other tests between patients who survived or died, and a prospective cohort study of critically ill patients found an independent association between biomarkers of inflammation and in-hospital mortality. Numerous commentaries and review articles have discussed hyperinflammatory syndromes in COVID-19 and the therapeutic potential of immunomodulation such as interleukin-6 blockade. Reservations have been expressed concerning the appropriate selection criteria for intervention and the potential risks of compromising anti-viral immunity and risk of secondary bacterial infection.
Added value of this study
A phenotype of COVID-19-associated hyperinflammation (COV-HI) defined by measurement of readily available routine clinical parameters (C-reactive protein and ferritin concentrations) was observed among a proportion of people with COVID-19 admitted to one of two UK hospital trusts. In the total cohort, meeting the COV-HI criteria on admission was associated with a higher mortality (40%) than that in those who did not meet the criteria (26%). Among patients eligible for full escalation of treatment, 37% fulfilled the COV-HI criteria at admission, and 62% of these patients required escalation of respiratory support by day 3. In total, 74% of patients eligible for escalation of respiratory support met the criteria by the day they required the respiratory support.
Implications of all the available evidence
COV-HI is associated with adverse outcomes. A more detailed definition is achievable and desirable through further research and validation to develop a prediction model. Further research into this phenotype could facilitate targeted trials of intervention with immunomodulation and help to identify patients likely to require escalation of care.
The diagnostic criteria for hyperinflammation are incompletely defined, especially in the context of COVID-19. Early studies of patients with COVID-19 established independent associations between biomarkers of inflammation (such as C-reactive protein, interleukin [IL]-6, and ferritin) and severe disease (ie, requiring respiratory support or resulting in death).2, 3, 6, 21 Subsequent prospective studies have confirmed these associations in large cohorts of people admitted to hospital with COVID-19,22, 23 and critical illness has been proposed to be more strongly associated with high levels of inflammatory biomarkers (C-reactive protein concentration >200 mg/L and D-dimer concentration >2500 mg/L) than with age or comorbidity.22 This evidence has formed the basis for initial predictive models and decision aids for patients at risk of a poor outcome.24
We agreed an operational definition of COVID-19-associated hyperinflammation (COV-HI) based on emerging evidence from the literature,3, 13, 16, 19, 25 from clinical observation, and from extensive discussion with members of a UK group of specialists in hyperinflammation, the HLH Across Specialty Collaboration (HASC). Given that viruses are known to cause secondary HLH in a subgroup of patients, we also proposed to investigate the usefulness of the HLH diagnostic tool, the H-score, in the context of SARS-CoV-2 infection.26
In this study, we aimed to test and assess hyperinflammation in COVID-19 by evaluating longitudinal associations between hyperinflammatory biomarkers, disease severity, and survival. We applied the proposed COV-HI criteria and the H-score to our cohort and sought to determine whether it was possible to identify patients with hyperinflammation on admission to hospital, or who developed hyperinflammation during their admission, and how hyperinflammation was related to any deterioration in clinical status. We did not seek to develop an outcome prediction model based on hyperinflammation from this initial cohort because our sample size calculations indicated that this would require 500–1000 patients.
Methods
Study design and participants
We did a retrospective longitudinal cohort study of consecutive patients admitted to one of two National Health Service Foundation Trusts, University College London Hospitals or Newcastle upon Tyne Hospitals, for the treatment of community-acquired COVID-19-related illness (according to the WHO definition, WHO Department of Communications 2020). Patients were considered for inclusion if they were aged 18 years or older and had a positive PCR result for SARS-CoV-2. The only exclusion criterion was the use of home non-invasive ventilation. Patients were followed up until death or hospital discharge, or for a minimum of 28 days from admission.
The study protocol received ethical approval from the Yorkshire & The Humber–Leeds West Research Ethics Committee (reference 20/YH/0138), as well as approval from the Health Research Authority (IRAS ID 282626) and Health and Care Research Wales on April 15, 2020. The study was registered with the Clinical Trials Gateway (NCT04385069), the National Institute for Health Research portfolio (ID45542), and the Health Research Authority website.
Laboratory tests
SARS-CoV-2 was detected in nasal and throat swabs by use of RT-PCR.27 We recorded routine blood tests done as part of the usual clinical care of each patient which, where available, included full blood count, coagulation profile, renal and liver function, and C-reactive protein, ferritin, D-dimer, lactate dehydrogenase, and troponin concentrations. At both sites, all available patient data were collected daily until escalation of respiratory support, death, or discharge from hospital. After escalation of respiratory support, data collection continued daily at the London site and at least twice weekly at the Newcastle site.
Clinical data and definitions
Comorbidity was recorded with use of the Charlson Comorbidity Index28 from patient medical records, and was categorised as none, single comorbidity, or multiple or severe comorbidities. Patients were designated as not for escalation if any of the following statements were found in medical records within 24 h of admission: a community do not attempt cardiopulmonary resuscitation (DNA-CPR) order or a hospital treatment escalation plan of DNA-CPR; an order for ward-based care only; not for non-invasive ventilation; or not for mechanical ventilation. In most cases these statements were highly overlapping, with the exception of a small number of patients who received non-invasive ventilation in a ward-based setting or who received escalated support but had advance directives not for cardiopulmonary resuscitation (both included in the subgroup for escalation). A 24 h limit was chosen to identify those initially too frail or sick for escalation and to avoid including patients for whom decisions were made later in the disease course, after initial support and interventions had failed.
Respiratory support was categorised as follows: supplemental oxygen when delivered by nasal cannulae or face mask; non-invasive ventilation when non-invasive pressure support was supplied by face mask; and intubation when ventilation was delivered by endotracheal or tracheostomy tube. Escalation of respiratory support was defined as a transition from supplemental oxygen to either non-invasive ventilation or intubation.
We derived an operational definition of hyperinflammation from the literature on secondary HLH in sepsis, the H-score, and emerging reports that indicators of hyperinflammation (including C-reactive protein and ferritin) were significantly associated with poor outcomes in COVID-19.3, 13, 16, 19, 25
Hyperinflammation as a categorical variable was defined as any of the following: a C-reactive protein concentration greater than 150 mg/L; a doubling of C-reactive protein concentration within 24 h from a concentration of greater than 50 mg/L; or a ferritin concentration of greater than 1500 μg/L. These cutoffs were agreed from a literature review,3, 13, 16, 19, 25 clinical observation, and extensive discussion with members of HASC, including a subset of the authors of this Article (RST, JJM, MC, PM, BG, MN, MB, and MS).
From the clinically available information collected longitudinally during the study, we calculated the H-score, as defined by Fardet and colleagues,26 but modified this score to omit assessment of organomegaly and bone marrow cytology, which are difficult to ascertain in patients with COVID-19. The new possible total score was 264, and we used midpoint score of 132 as a cutoff for secondary HLH, following Kyriazopoulou and colleagues' model.29
Data management
Demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records using a bespoke web-based REDCap database,30 developed by TD, BCL, and MC at the Newcastle Joint Research Office. The data dictionary is available on request. Data were entered by members of the research or clinical care teams using a protocol jointly developed by the two centres. Anonymised data were downloaded from REDCap for statistical analysis by CC and JW. Outliers (implausible values) for variables and dates were identified and clarified with the data entry teams.
Statistical analysis
We did not plan any predictive modelling in this study as sample size calculations indicated the need for 500–1000 patients. Instead we pragmatically decided to analyse the first tranche of data when it was complete and 28 days of follow-up had occurred after the last patient's entry to the cohort.
We reported continuous variables as median (IQR) and categorical variables as n (%). Escalation-free survival was estimated with use of Kaplan-Meier curves, with death or escalation to non-invasive ventilation or intubation as events, and surviving patients censored at day 28. Scatter plots of daily results were plotted against time for longitudinal analysis. Missing data in baseline categorical values were included as a separate category. Missing daily values were imputed only for the repeated measures multivariate model below, by carrying forward results from the previous day, reflecting the reality of the information that would be available to a clinician at any given time point. If more than one result was recorded on a given day, the most deviated result was selected at data entry. The moving average for each variable was overlaid using a locally estimated scatterplot smoothing (LOESS) curve showing 95% CIs for the estimated daily mean. Depending on the analysis, time was centred on the date of first symptoms or the date of escalation of respiratory support. The other parameters in the LOESS models used default settings with a polynomial of degree 2, interpolation on a cell size of 0·2, and a Gaussian (fitted least squares) kernel.
To interrogate factors associated with escalation of respiratory support or in-hospital mortality, we fitted a Cox proportional hazards model with time-varying covariates for the repeated laboratory results, with time measured from day of symptom onset. A forward step-wise model-building approach was used from an initial model including three a-priori variables: hyperinflammation (as defined above), age, and sex. Additional variables—lymphocyte count, comorbidity (Charlson Comorbidity Index), and steroids or immunosuppressants on admission—were selected for inclusion if they improved model fit, as measured by a reduction in the Akaike Information Criterion. Where appropriate, we log-transformed variables to fit into the model. Age on hospital admission was included as a linear variable but was also tested for a departure from this linear trend.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Data were collected from 269 consecutive patients aged 18 years or older who were admitted to the study hospitals between March 1 and March 31, 2020, who had a positive swab for SARS-CoV-2 on PCR, and who were followed up until death or discharge for a maximum of 28 days. Baseline demographics of the cohort are summarised in the appendix (p 1). The median age of the cohort was 71 years (IQR 57–83), 167 (62%) were men and 102 (38%) women. 170 (63%) were white, and 69 (26%) were of Black, Asian, or other minority ethnic backgrounds. Except for ethnicity, no significant differences in patients' baseline characteristics were seen between the hospital trusts. Additionally, the proportions of patients deemed not fit for escalation, rates of escalation to non-invasive ventilation or intubation, and overall mortality did not differ significantly between hospital trusts. The date of symptom onset preceding admission was recorded in most records; in the 15 (6%) cases for which this information was missing, the date of admission was used as the date of symptom onset instead.
91 patients (34%) had treatment plans indicating ineligibility for escalation of care above supplemental oxygen, of whom 50 (55%) survived until the end of the 28-day follow-up (appendix p 2). On average, these patients were older and had higher Charlson Comorbidity Index scores than those eligible for escalation of respiratory support (appendix p 3). Of the 178 (66%) eligible for escalation, 137 (77%) survived. However, of the 90 patients in this group who received non-invasive ventilation or intubation, only 51 (57%) remained alive at the end of follow-up, compared with 86 (98%) of the 88 who did not require escalation of respiratory support (appendix p 2).
Baseline laboratory results of the cohort are summarised in the appendix (p 4). In patients who were eligible for escalation and subsequently died, lymphocyte counts were lower and C-reactive protein concentrations higher than in those who were eligible for escalation and survived. Few data were missing for routine blood tests. Of the inflammatory markers, only C-reactive protein concentration was recorded reliably (90% of patients), whereas ferritin, D-dimer, lactate dehydrogenase, and other measures were only reported for up to 50% of patients at admission (appendix p 4).
To synchronise the course of COVID-19 in patients presenting at different stages in their illness, we plotted mean daily blood test values versus elapsed time since the onset of symptoms (figure 1 ). As expected, a higher fraction of inspired oxygen was required by patients who required non-invasive ventilation or intubation than by patients who did not require escalation of respiratory support (figure 1B). C-reactive protein trajectories differed between patients not eligible for escalation and those who received oxygen without escalation compared with those escalated to non-invasive ventilation or mechanical ventilation (figure 1C). Peak mean C-reactive protein concentration was higher in those who required intubation, peaking at 267 mg/L on day 13, compared with an initial peak on admission of 227 mg/L and second peak of 150 mg/L on days 12–15 in the group requiring non-invasive ventilation only. As shown in figure 1A and the appendix (p 6), the early trends in C-reactive protein were a genuine reflection of the mean values of all patients, with the possibility of survivor bias from loss to discharge or death arising only around the time that C-reactive protein values peaked.
Figure 1.
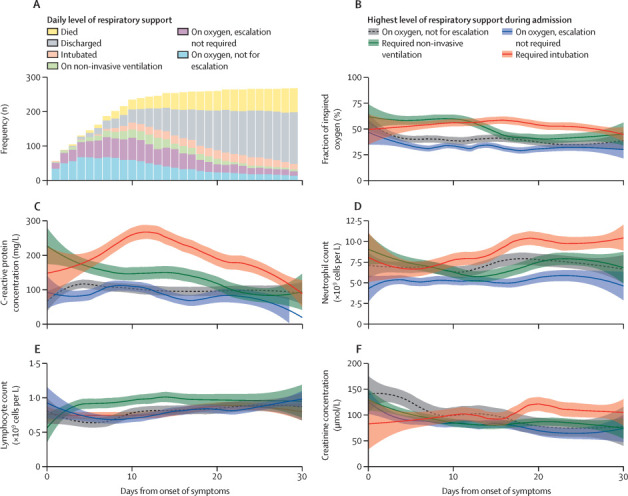
Longitudinal laboratory findings and escalation of respiratory support in the full cohort (n=269)
(A) Daily numbers of patients by level of respiratory support, centred on day of symptom onset (discussed in the appendix [p 6]). (B–F) Laboratory results plotted against time from symptom onset. Locally estimated scatterplot smoothing curves were fitted from mean daily worst values for all patients, stratified by highest level of respiratory support required during admission, with patients who were ineligible for cardiopulmonary resuscitation or escalation of support beyond ward-based care included as a separate category. Shaded areas are 95% CIs. Missing data were imputed from the last value carried forward. Other parameters in the models used default settings, with a span of 50%, polynomial of degree 2, interpolation on a cell size of 0·2, and a Gaussian (fitted least squares) kernel.
Creatinine concentrations and neutrophil and lymphocyte counts also varied between the groups (figure 1D-F), with the highest neutrophil counts seen late in the disease process in patients requiring mechanical ventilation (figure 1D).
Among the 90 patients whose care was escalated, the daily means of sequential tests were compared in survivors and non-survivors, synchronising their clinical course at the point of escalation (figure 2 ). Although the trajectories of C-reactive protein concentration (figure 2C), neutrophil count (figure 2D), and lymphocyte count (figure 2E) were similar between these two patient groups before escalation of respiratory support, patients who eventually died had a delayed and higher peak of C-reactive protein, a greater degree of neutrophilia, and more marked lymphopenia after treatment escalation compared to those who survived. The most noticeable changes in these variables occurred in the first 5 days after escalation, before the possibility of bias from loss to death or discharge substantially increased (appendix p 6).
Figure 2.
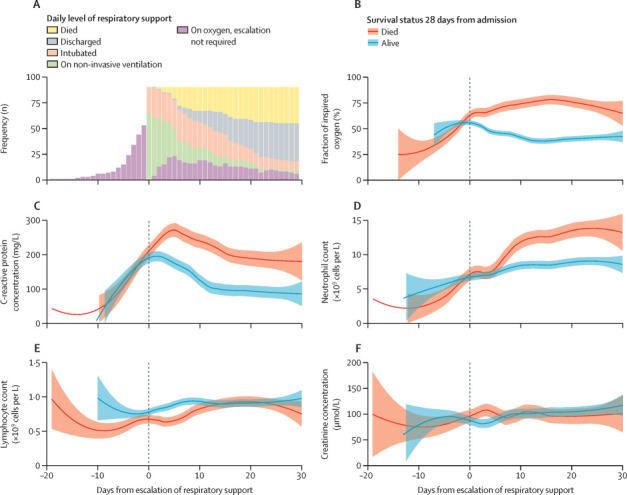
Longitudinal laboratory findings and survival in patients given escalated respiratory support (n=90)
(A) Daily numbers of patients by level of respiratory support, centred on day of increased respiratory support (discussed in the appendix [p 6]). (B–F) Laboratory results plotted against time from escalation of respiratory support. Locally estimated scatterplot smoothing curves were fitted from worst daily mean values for all patients given escalated respiratory support, stratified by overall survival within 28 days of admission. Shaded areas are 95% CIs. Missing data were imputed from the last value carried forward. Other parameters in the models used default settings, with a span of 50%, polynomial of degree 2, interpolation on a cell size of 0·2, and a Gaussian (fitted least squares) kernel.
The criteria for meeting the COV-HI definition at admission were C-reactive protein greater than 150 mg/L or ferritin concentration greater than 1500 μg/L, as a doubling of C-reactive protein was not possible with single measurements. Of the whole cohort, 90 (33%) patients met the COV-HI criteria on admission; of these, 74 (82%) met the criteria on the basis of their C-reactive protein concentration (13 of whom also met the definition on the basis of ferritin), whereas 16 (18%) met the definition on the basis of ferritin concentration alone (appendix p 9). Although patients with COV-HI on admission were younger (median age 66 years [IQR 57–80] vs 71 years [56–83]) and had fewer comorbidities (median Charlson Comorbidity Index score 1 [0–2] vs 2 [0–3]) than those without COV-HI on admission (n=179 [67%]), a higher proportion of them died within the follow-up period (36 [40%] patients with COV-HI vs 46 [26%] patients without COV-HI; appendix p 8). Of the 91 patients who were not eligible for escalation, 25 met the COV-HI (27%) criteria on the day of admission, of whom 17 (68%) died by 28 days (table 1 ).
Table 1.
Summary of patient mortality
| Patients, n (%) | Died by end of follow-up, n | Crude mortality, % | |
|---|---|---|---|
| Not eligible for escalation (n=91) | |||
| Hyperinflammation | 25 (27%) | 17 | 68% |
| No hyperinflammation | 60 (66%) | 22 | 37% |
| Unrecorded | 6 (7%) | .. | .. |
| Eligible for escalation (n=178) | |||
| Hyperinflammation | 65 (37%) | 19 | 29% |
| No hyperinflammation | 95 (53%) | 19 | 20% |
| Unrecorded | 18 (10%) | .. | .. |
Patients were stratified according to eligibility for treatment escalation at admission and whether they met the criteria for hyperinflammation at admission (C-reactive protein concentration >150 mg/L or ferritin concentration >1500 μg/L).
Among the 178 eligible for escalation, 65 (37%) met the criteria for COV-HI on admission, of whom 19 (29%) subsequently died (table 1). Among eligible patients, escalation-free survival was worse in those with COV-HI at admission, after adjusting for age, sex, and Charlson Comorbidity Index score, compared to those without COV-HI at admission (likelihood ratio test p<0·0001; figure 3A ). Admission C-reactive protein concentrations and ferritin concentrations (where recorded) were higher in patients eligible for escalation who died than in those who survived (appendix p 4).
Figure 3.
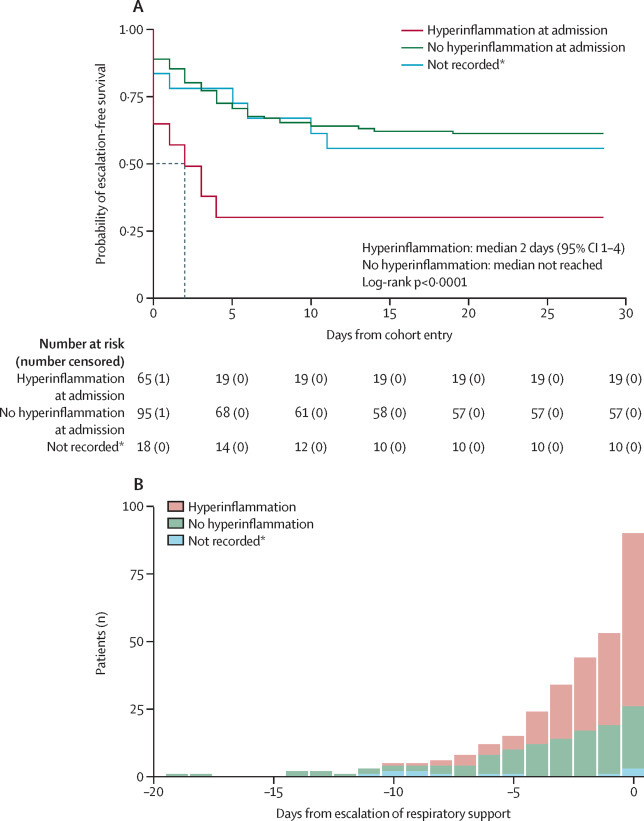
Hyperinflammation and escalation-free survival
(A) Kaplan-Meier plot of escalation-free survival (considering death or escalation of respiratory support as a combined endpoint) in patients eligible for respiratory support (censored at 28 days), plotted from the first day of data collection for each patient. Patients were stratified by whether they met the criteria for hyperinflammation (C-reactive protein >150 mg/L or ferritin >1500 μg/L) on the day of cohort entry (ie, admission to hospital). Predicted median survival is indicated by the dashed line for the group with hyperinflammation only. (B) Daily proportion of patients meeting the criteria for hyperinflammation (C-reactive protein concentration >150 mg/L or doubling within 24 h from >50 mg/L, or ferritin concentration >1500 μg/L) from the day of symptom onset until the day that respiratory support was escalated. 74% of the total population for whom escalation could be considered met the criteria for hyperinflammation by the time of escalation. *Patients without C-reactive protein or ferritin concentration recorded on the day of admission were included as a missing category.
40 (62%) of the 65 patients who met the criteria for COV-HI at admission and who were eligible for escalation required escalation of respiratory support by day 3 and three patients had died (median escalation-free survival 2 days [95% CI 1–4]; figure 3A). Among the 95 (53%) eligible patients who did not meet the COV-HI criteria at baseline and the 18 (10%) patients with missing C-reactive protein and ferritin data, fewer than 50% of patients reached the combined endpoint of escalation of respiratory support or death before day 28, and it was therefore not possible to calculate median escalation-free survival in these groups.
The association between increased C-reactive protein or ferritin concentration that met the predefined cutoff values for hyperinflammation and the need for next-day escalation was significant on a daily basis (hazard ratio 2·24 [95% CI 1·62–2·87]) after adjustment for age, sex, and comorbidity (table 2 ; appendix pp 10–11). In the fitted time-varying Cox model, there was no clear systematic pattern in the residuals for the key predictors, and the fitted line was approximately horizontal. Therefore, given the number of data points, the proportional hazards assumption was appropriate (Schoenfeld residual plots and log[–log] plots are shown in the appendix [p 12]). Within this model, there was no evidence to support a direct association of escalation with study site (p=0·95) and no evidence for an interaction by site of the association between COV-HI and escalation (p=0·80). Notably, 67 (74%) of the 90 patients who progressed to elevated respiratory care of patients who were eligible for treatment escalation met the criteria for COV-HI by the time they needed respiratory support (figure 3B), and 45 (50%) of these met the criteria on the day of admission.
Table 2.
Time-varying multivariable proportional hazards analysis of factors associated with next-day escalation of support or death among patients eligible for escalation of respiratory support
| Hazard ratio (95% CI) | ||
|---|---|---|
| Hyperinflammation* | ||
| No | 1 (ref) | |
| Yes | 2·24 (1·62–2·87) | |
| Age, years† | 1·04 (1·02–1·06) | |
| Sex | ||
| Female | 1 (ref) | |
| Male | 2·48 (1·70–3·26) | |
| Charlson Comorbidity Index | ||
| No comorbidity | 1 (ref) | |
| Single comorbidity | 0·93 (0·04–1·81) | |
| Multiple or severe comorbidities | 1·13 (0·39–1·88) | |
| Steroids or immunosuppressants on admission | ||
| No | 1 (ref) | |
| Yes | 0·83 (0·03–1·63) | |
Analysis included only patients who had either ferritin or C-reactive protein concentration measured at study enrolment and complete data for other included measurements (n=127 patients with 684 observations). Additional information is provided in the appendix (p 6).
Defined as C-reactive protein concentration greater than 150 mg/mL or ferritin concentration greater than 1500 μg/L.
Age was included as a linear variable, and there was no evidence within the model for a departure from a linear trend (p=0·48 [likelihood ratio test comparing linear age covariate or 20-year age bands] and p=0·68 [compared with quadratic transformation]).
Insufficient data were collected to determine an H-Score in most patients in this cohort, reflecting a lack of awareness of the score and clinical practice in a pandemic. Of the 47 patients who had sufficient data to calculate an H-Score, the median was well below the cutoff value indicating secondary HLH in the proposed modified score (appendix p 7).
Discussion
By longitudinal observation of a cohort of patients admitted with COVID-19 to two hospital trusts in the UK, this study supports the concept that a proportion of patients have a hyperinflammatory phenotype (COV-HI) and that meeting the criteria for this phenotype is associated with a poor clinical outcome. The C-reactive protein trajectories differed between patients with severe disease (defined by death or requiring ventilatory support) and those whose disease followed a milder course.
In patients who were eligible for escalation of care, we found an independent association between patients meeting the COV-HI criteria and the need for ventilatory support or death, after accounting for age, sex, and comorbidity. Furthermore, 33% of the entire cohort met the COV-HI criteria upon presentation, and 74% of those who went on to need ventilatory support met the COV-HI criteria before escalation was required. Our work builds on, and contributes to, the evidence enabling risk prediction models for people with COVID-19.
Meeting the COV-HI criteria at admission was associated with a mortality of 68% in patients not eligible for escalated respiratory support and 29% in patients who were eligible. The reason for this difference cannot be extrapolated from our data, but might relate to confounders such as increased frailty or multimorbidity in those with a ceiling of care, or might suggest that respiratory support can improve outcomes in patients with a hyperinflammatory response to COVID-19.
The thresholds we used to define COV-HI were in line with, or more stringent than, those reported elsewhere.3, 16, 19, 25 The use of C-reactive protein and ferritin values, measurable by readily available and inexpensive tests, were routinely and reliably collected in the majority of patients. By contrast, in this real-world study, some of the key data required for calculating an H-Score were only available for a minority of patients. Thus, the H-Score could not be determined in most patients, and we could not draw firm conclusions on its potential use in identifying at-risk patients. In the few patients for whom an H-Score could be calculated, the result did not suggest a high probability of secondary HLH.
An emerging body of evidence shows that hyperinflammation in COVID-19 is distinct31 from other recognised hyperinflammatory states, and a wider debate surrounds the definition and aetiopathogenesis of cytokine storm syndromes. C-reactive protein appears to be an important marker of poor outcome in COVID-19 infection in particular, and most patients who met the COV-HI criteria in this study did so on the basis of C-reactive protein criteria. By contrast, in other conditions leading to pneumonia or acute respiratory distress syndrome (ARDS), published studies have not shown C-reactive protein to be prognostically useful in predicting deterioration.32, 33
Elevated ferritin has been used in risk models for the hyperinflammatory subtype of sepsis (macrophage activation-like syndrome), defined as a ferritin concentration greater than 4420 ng/mL.29 Although we showed median ferritin levels in this cohort of people with COVID-19 infection were substantially lower than those in macrophage activation-like syndrome in sepsis, an ongoing study with a larger cohort will hope to address the question of whether there are subtypes of hyperinflammatory disease caused by SARS-CoV-2 infection defined by different biomarker thresholds. Previous work in sepsis and ARDS34 could help to inform that analysis.
The highly elevated C-reactive protein results in our study raise the question of superadded bacterial infection, but a recent review of the literature supports our clinical experience that very little bacterial (or fungal) infection was seen.35 We also found a late spike in C-reactive protein associated with raised neutrophils in the group requiring mechanical ventilation, which might represent ventilator-associated pneumonia. This potential phenomenon would not affect the validity of our proposed COV-HI definition, because in our methodology the definition was applied before escalation to respiratory support.
In contrast to many previous studies, which relied on single data points, we estimated the association between longitudinal, repeated measurements of laboratory markers and clinical outcomes. The main limitation of this dataset is that last value carried forward was used to impute missing data in our modelling, which is a known source of potential bias. This method was the only option available to us for the purposes of modelling risk of escalation (to be able to include the longitudinal repeated measurements) and partly reflects the real-world situation when a doctor is assessing a patient clinically. Of note, we had complete follow-up until 28 days post-admission including out of hospital deaths, which is a longer and more complete follow-up than many recent predictive studies in patients with COVID-19.36
The emerging literature supports our findings that inflammatory markers are strongly associated with critical illness and mortality22 in people with COVID-19, and suggests that clinicians should measure such markers routinely. Such emerging evidence also mandates further study to inform the understanding of inflammation and hyperinflammation in COVID-19. While some prognostic models for COVID-19 have been published or made available as preprints, all the models included in a previous systematic review and meta-analysis were found to be at high risk of bias.36 We did not attempt to create a prognostic score using our data; instead, we estimated the association between hyperinflammation and outcome. This study was not appropriately powered to examine thresholds via a derivation and validation approach, or to define a risk prediction model. However, using C-reactive protein and ferritin, simple biomarkers associated with hyperinflammation, we have identified a potential clinical phenotype (COV-HI) associated with poor outcome in severe cases of COVID-19.
The presence of markers of hyperinflammation in this study was associated with the need for escalation of respiratory support and the risk of death in people with COVID-19. These findings support the concept that a high-risk inflammatory phenotype (COV-HI) exists and might be associated with increased mortality. The COV-HI criteria need to be validated in a larger cohort but have the potential to be developed as an easy bedside risk-assessment tool and could be important for patient stratification and optimal trial design.
Data sharing
The study protocol and data dictionary are available upon request submitted to the corresponding author. Requests for access to the individual participant data that underlie the results reported in this Article can be made to the access committee via the corresponding author. De-identified data will be available from the date of publication for 36 months, subject to approval of a proposal with a signed data access agreement.
Acknowledgments
Acknowledgments
MC is supported by the NIHR Newcastle Biomedical Research Centre. ECJ is supported by grants from the MS Society (grant number 076), Lupus UK, The Rosetrees Trust (M409), and The Dunhill Medical Trust (RPGF1902\117). MN is supported by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre (grant number BRC525/III/CC). The Centre for Adolescent Rheumatology Versus Arthritis at University College London, UCLH, and Great Ormond Street Hospital (GOSH) is supported by grants from Versus Arthritis (grant numbers 21593, 20164, and 21226), Great Ormond Street Hospital Children's Charity (GOSCC), and the NIHR Biomedical Research Centres at GOSH and UCLH. JJM would like to acknowledge the helpful discussions with Prof David Moore, consultant in infectious diseases and tropical medicine at UCLH.
Contributors
JJM, RST, MC, JW, CC, ECJ, PM, TL, and ADS designed the study. AL, TL, MN, BG, EK, LM-G, KEW, GAR, LSR, EM, HJ'B, MG, HP, EH, AS, CA, ISvdL, KFB, CJAD, ATH, BCL, TR, and AL acquired study data. CC, JW, JP, JJM, MC, and RST analysed the data. JJM, RST, MC, JW, CC, MN, BG, PM, and ECJ wrote the manuscript. MB, MM, MS, and BW reviewed the manuscript. All authors approved the final version.
Declaration of interests
PM is a Medical Research Council–GlaxoSmithKline EMINENT clinical training fellow, which includes project funding outside the submitted work, and co-funding from the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre. CJAD reports grants from Wellcome Trust, during the conduct of the study. ADS reports grants and personal fees from AstraZeneca, Bayer, Chieisi, Teva, Forest Labs, Grifols, and Novartis, outside the submitted work. MS reports grants and advisory board fees from NewB, grants from the Defence Science and Technology Laboratory, Critical Pressure, Apollo Therapeutics, advisory board and speaker fees (paid to his institution) from Amormed, Biotest, GE, Baxter, Roche, and Bayer, and honorarium for chairing a data monitoring and safety committee from Shionogi. MC reports personal fees from Mallinkrodt, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centred, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intensive Care National Audit & Research Centre COVID-19 report. May 22, 2020. https://www.icnarc.org/our-audit/audits/cmp/reports
- 5.Joynt GM, Wu WKK. Understanding COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020;20:635–636. doi: 10.1016/S1473-3099(20)30237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69:1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 10.Carter SJ, Tattersall RS, Ramanan AV. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology (Oxford) 2019;58:5–17. doi: 10.1093/rheumatology/key006. [DOI] [PubMed] [Google Scholar]
- 11.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cron RQ, Chatham WW. The rheumatologist's role in COVID-19. J Rheumatol. 2020;47:639–642. doi: 10.3899/jrheum.200334. [DOI] [PubMed] [Google Scholar]
- 14.Chaidos A, Katsarou A, Mustafa C, Milojkovic D, Karadimitris A. Interleukin 6-blockade treatment for severe COVID-19 in two patients with multiple myeloma. Br J Haematol. 2020;190:e9–e11. doi: 10.1111/bjh.16787. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bovet M, Wadsack D, Kosely F, Zink W, Zahn R. Fatal outcome in a COVID-19 patient despite IL 6 blockage in cytokine storm. Research Square. 2020 doi: 10.21203/rs.3.rs-26470/v1. published online May 7. (preprint) [DOI] [Google Scholar]
- 18.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.England JT, Abdulla A, Biggs CM, et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100707. published online May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King A, Vail A, O'Leary C, et al. Anakinra in COVID-19: important considerations for clinical trials. Lancet Rheumatol. 2020;2:e379–e381. doi: 10.1016/S2665-9913(20)30160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XH, Lu SH, Chen J, et al. Clinical characteristics of foreign-imported COVID-19 cases in Shanghai, China. Emerg Microbes Infect. 2020;9:1230–1232. doi: 10.1080/22221751.2020.1766383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang W, Yao J, Chen A, et al. Early triage of critically ill COVID-19 patients using deep learning. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 27.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017;15:172. doi: 10.1186/s12916-017-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoeboer SH, Oudemans-van Straaten HM, Groeneveld AB. Albumin rather than C-reactive protein may be valuable in predicting and monitoring the severity and course of acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new onset fever. BMC Pulm Med. 2015;15:22. doi: 10.1186/s12890-015-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajwa EK, Khan UA, Januzzi JL, Gong MN, Thompson BT, Christiani DC. Plasma C-reactive protein levels are associated with improved outcome in ARDS. Chest. 2009;136:471–480. doi: 10.1378/chest.08-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bos LD, Schouten LR, van Vught LA, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72:876–883. doi: 10.1136/thoraxjnl-2016-209719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa530. published online May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369 doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol and data dictionary are available upon request submitted to the corresponding author. Requests for access to the individual participant data that underlie the results reported in this Article can be made to the access committee via the corresponding author. De-identified data will be available from the date of publication for 36 months, subject to approval of a proposal with a signed data access agreement.


